Abstract
Background Quiescence is a fundamental feature of plant life, which enables plasticity, renewal and fidelity of the somatic cell line. Cellular quiescence is defined by arrest in a particular phase of the cell cycle, typically G1 or G2; however, the regulation of quiescence and proliferation can also be considered across wider scales in space and time. As such, quiescence is a defining feature of plant development and phenology, from meristematic stem cell progenitors to terminally differentiated cells, as well as dormant or suppressed seeds and buds. While the physiology of each of these states differs considerably, each is referred to as ‘cell cycle arrest’ or ‘G1 arrest’.
Scope Here the physiology and molecular regulation of (1) meristematic quiescence, (2) dormancy and (3) terminal differentiation (cell cycle exit) are considered in order to determine whether and how the molecular decisions guiding these nuclear states are distinct. A brief overview of the canonical cell cycle regulators is provided, and the genetic and genomic, as well as physiological, evidence is considered regarding two primary questions: (1) Are the canonical cell cycle regulators superior or subordinate in the regulation of quiescence? (2) Are these three modes of quiescence governed by distinct molecular controls?
Conclusion Meristematic quiescence, dormancy and terminal differentiation are each predominantly characterized by G1 arrest but regulated distinctly, at a level largely superior to the canonical cell cycle. Meristematic quiescence is intrinsically linked to non-cell-autonomous regulation of meristem cell identity, and particularly through the influence of ubiquitin-dependent proteolysis, in partnership with reactive oxygen species, abscisic acid and auxin. The regulation of terminal differentiation shares analogous features with meristematic quiescence, albeit with specific activators and a greater role for cytokinin signalling. Dormancy meanwhile appears to be regulated at the level of chromatin accessibility, by Polycomb group-type histone modifications of particular dormancy genes.
Keywords: Dormancy, meristem, quiescence, cell cycle, chromatin, differentiation, proliferation, hormone, reactive oxygen species, ubiquitin-dependent proteolysis, mitosis, branching
PHYSIOLOGICAL STATES OF QUIESCENCE
Multicellular life requires spatial and developmental control of cell proliferation. As plants have no fixed plan, they have evolved particularly flexible and opportunistic means to regulate cell division and fate, in response to nutritional availability and abiotic and biotic stress. Higher plant form also accommodates many specialized cell, tissue and organ types, which are defined by a lineage of cell differentiation. In addition, many plants have evolved mechanisms to calibrate growth and reproductive cycles to seasonal rhythms in the availability of light and temperature. These spatial, developmental and ecological features underpin the physiology of quiescence in plants (Considine and Considine, 2016).
At the organ and plant levels, the functional units of higher plant development are the meristems, principally the root and shoot apical meristems (RAM, SAM), but also cambium and intercalary meristems (Esau, 1965). The core of meristems is a small population of pluripotent stem cells, which are replenished and maintained by a reservoir of quiescent cells: the organizing centre (OC) and quiescent centre (QC) in the SAM and RAM, respectively (Verdeil et al., 2007; Aichinger et al., 2012). The micro-environment or niche of the stem cells in the SAM and RAM aid in the maintenance of their pluripotency. The niche defines non-cell-autonomous regulation of cell identity and fate. It is widely accepted that the slow cycling of QC and OC cells serves to ensure fidelity of DNA replication (Cruz-Ramírez et al., 2013; Heyman et al., 2014). Thus, quiescence within meristem organization has essential functions to preserve genome integrity, ensuring that the foundation of cell lineage is faithful to the parent genome. However, quiescence may also refer to cells of additional physiological states, notably dormant meristems (Considine and Considine, 2016) or differentiated cells (Polyn et al., 2015). Thus, we define three modes of quiescence in this review:
Meristematic quiescence, which refers to a transient state of repressed cell division in undifferentiated cells, whereby proliferation resumes immediately upon relief from the suppressing condition. This could equally be termed embryonic quiescence but for simplicity we refer to the meristem. This definition includes quiescence of the QC and OC cells, as quiescence is maintained by non-cell-autonomous regulation; targeted cell ablation studies have demonstrated that QC cells can change fate, or conversely that stem cells can adopt QC identity (van den Berg et al., 1997). It also includes suppression of quiescent shoot meristems (buds) by apical dominance or source/sink relations (paradormancy; Lang et al., 1987) and non- or post-dormant seeds and buds under inadequate growth conditions (ecodormancy; Lang et al., 1987; Considine and Considine, 2016).
Dormancy, a unique mode of quiescence, which is entrained in the life history of the plant, and requires quantitative conditioning to enable the transition to growth competence (i.e. to meristematic quiescence).
Terminal differentiation, or cell cycle exit (Polyn et al., 2015). Although differentiated cells still maintain the ability to endocycle, and cell identity can be reprogrammed, enabling pluripotency or totipotency (de Veylder et al., 2007; Verdeil et al., 2007), the literature commonly refers to differentiated cells as quiescent, i.e. non-mitotic. Understanding the molecular decision to cease division may help to define the more reversible modes of meristematic quiescence and dormancy.
Two further modes that we exclude from this review for simplicity are senescence and stress-induced quiescence, such under hypoxia (Bailey-Serres and Voesenek, 2008; Pucciariello and Perata, 2013). Rather, we focus on the developmental context.
In this review, we explore the regulation of the cell cycle with particular focus on cellular decisions to enter and maintain quiescence in each of the three modes we have defined, in order to highlight the importance of cell cycle regulation in plant survival, and consider whether distinct sets of cell cycle regulators suppress cell division in these quiescent states. Thus, the primary motivations for this review were two questions: (1) Are the canonical cell cycle regulators superior or subordinate in the regulation of quiescence? (2) Are these three modes of quiescence regulated by distinct molecular controls? In addressing these questions, we draw on knowledge from the root meristem, particularly the QC and meristem transition zone, as well as the seed and shoot meristems. For reference, we briefly summarize the canonical cell cycle regulators, but refer readers to previous comprehensive reviews for further detail, particularly on the synthesis and mitotic phases (Inzé and de Veylder, 2006; de Veylder et al., 2007). Gutierrez (2016) also provided a timely overview of the history and progress of cell cycle research in plants, Sablowski (2016) updated the relationship between the cell cycle and cell growth, and Verdeil et al. (2007) summarized the regulation of stem cell potency. Of more direct relevance to this review, Considine and Considine (2016) examined the physiology of quiescence and dormancy, Polyn et al. (2015) summarized the cell cycle decisions during transition to differentiation, and Heyman et al. (2014) summarized the evidence that QC cells serve as founder stem cells in the root apical meristem, providing a very useful summary of relevant actors on cell cycle decisions at the interface between quiescence and proliferation. We seek primarily to add to these reviews, rather than duplicate; however, it is necessary to give an introduction to the canonical cell cycle, to provide context for comparing nuclear states. Figure 1 describes the regulation of the canonical plant cell cycle, Figure 2 describes the regulation of dormancy and quiescence by plant hormones and chromatin modification, and Figure 3 provides a concise summary of the principal regulatory features governing the three quiescent states of G1 (i–iii).
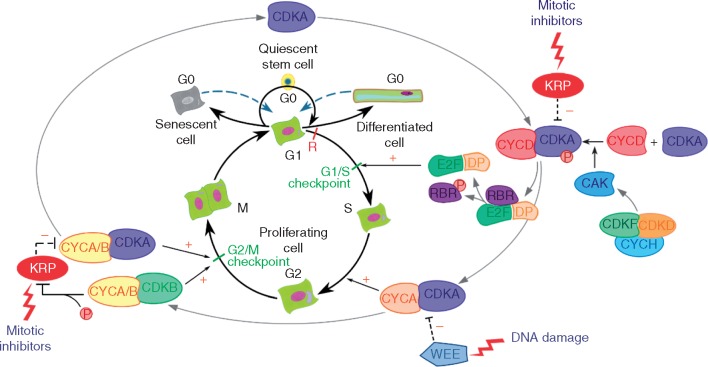
Fig. 1.
Canonical cell cycle regulation in plants. The cell cycle comprises four principal phases: DNA synthesis (S), mitosis (M) and two intervening gap phases (G1, G2), plus a theoretical quiescent phase (G0). Growth-promoting factors promote formation of the CYCD/CDKA complex, which, when activated by CDKF and CDKD in association with CYCH (CAK pathway), causes phosphorylation of RBR, thus activating the E2F/DP complex, which encourages the transcription necessary to cause G1/S transition. CYCAs are synthesized during the S phase, which, in complex with CDKAs, promotes transition to G2. CYCA/B in complex with CDKA and CDKB under the regulation of the CAK pathway acts at the G2/M check-point to regulate G2/M transition. WEE1 kinase suppresses the G2/M transition in response to DNA damage. In the absence of growth-promoting conditions, cells restrict growth in G0 but resume growth when favourable conditions become available. Differentiated and/or senescent G0 cells are rarely capable of re-entering the cell cycle. In animals, it has been proposed that cells can only enter G0 prior to the restriction point (R), but after passing R are committed to the cell cycle; as yet the evidence for this restriction point is lacking in plants. Mitotic inhibitors are capable of inactivating the CYC/CDK complexes by stimulating CKIs like KRP, causing cell cycle arrest at the check-points. KRP can be inactivated by CDKB kinase activity, causing an increase in CDK activity during mitosis. + and – indicate promotion and inhibition of the pathways, respectively. For simplification, P indicates either ATP (when it is used as substrate) or phosphate (when it is linked to a molecule). CDKs, cyclin-dependent kinases; CYCs, cyclins; CKI, cyclin-dependent kinase inhibitor; KRP, kip-related proteins; RBR, retinoblastoma-related protein.
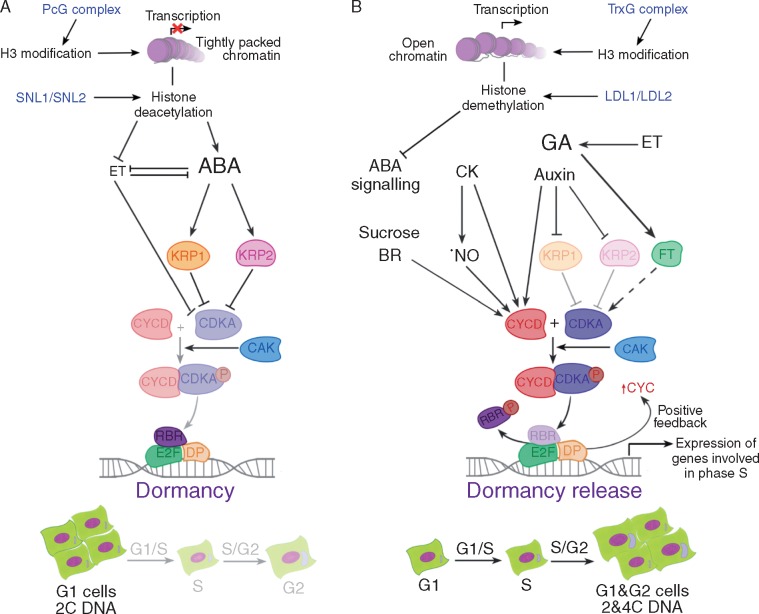
Fig. 2.
Regulation of hormone control of the G1/S transition during dormancy by chromatin regulators. (A) In the dormant state most of the cells have 2C DNA, so it is considered that there is strong regulation of the G1/S transition. RBR mediates the repression of genes involved in the S phase. Abscisic acid (ABA) induces the activity of CDKA inhibitors, KRP1 (ICK1) and KRP2 (ICK2), inhibiting G1/S transition. Although it is suggested that ethylene (ET) and ABA are antagonistic, the signalling cascade induced by ET has also been suggested to repress CDKA activity. The histone deacetylation mediated by SNL1 and SNL2 is suggested to promote this ABA–ethylene antagonism. This modification would be relevant in the dormant state. (B) Histone demethylation, mediated by LDL1 and LDL2, would have implications for dormancy release by reducing ABA signalling (by repressing the expression of ABA2, ABI3 and ABI5). Cytokinins (CK) induce CYCD both by themselves and by inducing nitric oxide (•NO) accumulation. GA represses the CDK inhibitors and promotes CYC and FT, which is suggested to induce CDK. Moreover, ET induces gibberellic acid (GA) signalling. In such a situation the complex CYCD/CDKA is able to phosphorylate RBR, allowing the expression of genes involved in S phase. Hence the non-dormant state is characterized by the presence of a greater G1:G2 ratio. CAK, CDK-activating kinases; CDK, cyclin dependent kinases; CYC, cyclins; LDL, lysine-specific demethylase like; RBR, retinoblastoma related protein; SNL, SIN3-like.
Fig. 3.
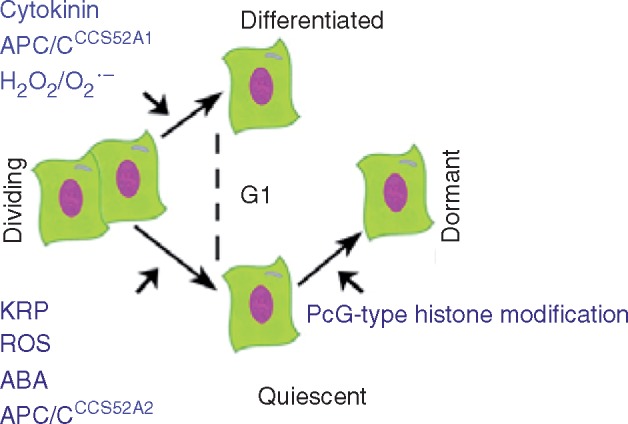
Major distinguishing regulators defining the decision of a dividing cell to quiesce, differentiate or enter dormancy. Regulated proteolysis of transcription factors and other unknown targets by APC/C E3 ligase, activated by CCS52A2, operates in partnership with an oxidized cellular state (ROS), abscisic acid (ABA) signalling and cyclin-dependent kinase inhibitors (KRP) to define meristematic quiescence. In a similar way, regulated proteolysis by the APC/C, activated by CCS52A1, regulates the commitment to differentiation in partnership with the regulated balance of H2O2 and O2•− and cytokinin signalling. By contrast, dormancy is regulated at the level of chromatin accessibility, by the action of PcG-type histone modifications. Further details are given in the text.
CANONICAL CELL CYCLE CONTROL
Mitosis is defined by a sequence of regulated and coordinated cyclic events or phases that recur through subsequent cell divisions. It facilitates the duplication of cellular material and their subsequent division between daughter cells. Similar to all other eukaryotes, the plant cell cycle includes two principal phases – the DNA synthesis (S) and mitosis (M) phases and two intervening gap phases – G1 and G2 (Fig. 1; Inzé and de Veylder, 2006; de Veylder et al., 2007). The S phase comprises DNA replication and synthesis of histone proteins required to package genomic DNA, while the M phase involves the segregation of genetic material to two daughter cells through nuclear (karyokinesis) and cell (cytokinesis) division. The gap phases serve to monitor intra- and extracellular conditions and DNA fidelity, enabling check-point decisions and DNA repair (Waterworth et al., 2011). The G1 phase primes the cell for the subsequent DNA replication, through the synthesis of mRNA, histone proteins and the enzymes required for replication. The G2 phase involves synthesis of RNA and proteins required for mitosis. A third, somewhat ethereal gap phase termed G0 defines nuclear quiescence (Fig. 1).
Primary cell cycle regulators
The cell cycle is governed by the activity of protein kinases called cyclin-dependent kinases (CDKs) and their regulatory partners, called cyclins (CYCs; Inzé and de Veylder, 2006; de Veylder et al., 2007). These cell cycle regulatory proteins are highly conserved among eukaryotes at the molecular level. The activation of CDKs requires phosphorylation by CDK-activating kinases (CAKs) and their inactivation involves cyclin-dependent kinase inhibitors (CKIs), known as kip-related proteins (KRPs) in plants (Fig. 1). The G1/S and G2/M transitions are known to be key regulatory check-points for cell division, with the cell cycle not ensuing without appropriate cell developmental progress. In plants, the A-type CDKs (CDKAs) act upon the G1/S and G2/M phase transitions and during the S phase, while the B-type CDKs (CDKB) act on the G2/M transition and during the M phase (Fig. 1). The D-type CYCs (CYCD) operate in partnership with the CDKAs during the G1/S transition, while A-type CYCs (CYCA) act during the S phase, and with B-type CYCs (CYCB) during the G2/M transition. In this way, phase-specific activities are determined by the corresponding partnerships. Proteolysis by the ubiquitin–proteasome system, involving the 26S proteasome, ubiquitin-carrying enzymes (E2) and ubiquitin–protein ligases (E3), ensures unidirectional cycling of cells through the proliferation cycle (reviewed by Marrocco et al., 2010). The anaphase-promoting complex/cyclosome (APC/C) and Skp1/Cullin/F-box protein (SCF)-related complex are the E3 ubiquitin ligases involved in targeting cell cycle proteins for proteolysis.
G1/S transition
The G1 phase functions to prepare the nuclei for S phase and is the principal medium of cross-talk between environmental signals and cell cycle activation/progression (Inzé and de Veylder, 2006). The presence of growth-promoting factors such as sucrose, auxin and cytokinin induces the expression of genes coding for CDKA and CYCD, promoting the formation of the CDKA/CYCD complex. Activation of the CDKA/CYCD complex requires phosphorylation by the CAK pathway, comprising CDKF and CDKD associated with CYCH (Fig. 1). Meanwhile mitotic inhibitors, such as abscisic acid (ABA), can inactivate the CDKA/CYCD complex by inducing KRPs, resulting in cell cycle arrest at the G1/S check-point and subsequent accumulation of cells at G1. Low-energy homeostasis also induces KRPs, leading to G1 arrest; however, this relationship is not yet clear as the energy sensor SUCROSE NON-FERMENTING 1-RELATED KINASE1 (SnRK1) can directly phosphorylate KRPs, leading to their inhibition (Guérinier et al., 2013). Arrest at G1 is commonly observed in inactive or dormant tissues or organs under conditions unfavourable for growth (discussed further below). The retinoblastoma-related protein (RBR) in plants is a primary phosphorylation target of the CDKA/CYCD, and, in partnership with the E2F/DIMERIZATION PARTNER (DP) transcription factors, exerts considerable influence over the G1/S phase transition (Wildwater et al., 2005). Cell-specific silencing of RBR triggers QC division (Cruz-Ramírez et al., 2013). The Arabidopsis RBR binds three types of E2F (E2Fa–c), with differential activities on the cell cycle. E2Fa and E2Fb promote the G1/S transition, while E2Fc represses it. The Arabidopsis E2F isoforms form heterodimers with two DPs, primarily DPa, to promote nuclear localization. Based on this, the G1/S transition is elicited by the CDKA/CYCD complex through two parallel pathways: (1) phosphorylation of CDKA/CYCD causes degradation of the E2Fc/DP–RBR transcriptional repressor complex by SCF E3 ubiquitin–protein ligase; and (2) phosphorylation of RBR in the E2Fa–b/DP–RBR complex activates the E2Fa–b/DP complex, enabling transcriptional activation of the cis-acting E2F box motif of S phase genes and subsequent entry into S phase of DNA replication (Fig. 1). Energy signalling also converges on E2F, as Arabidopsis TARGET OF RAPAMYCIN1 kinase (TOR1) directly phosphorylates and activates E2Fa, promoting G1/S phase progression (Xiong et al., 2013). Interestingly, E2Fa also positively influences the commitment to differentiate, with the finding that proliferating cells retain a fraction of E2Fa in complex with RBR, preventing premature differentiation (Magyar et al., 2012), and providing a clue to the distinction between meristematic quiescence and terminal differentiation (discussed further below).
G2/M transition
The G2/M transition check-point ensures that only cells with replicated DNA enter mitosis. During this transition, cyclins CYCA, CYCB and likely CYCD form complexes with CDKA and CDKB, which is transcriptionally regulated by the E2F pathway (Fig. 1; de Veylder et al., 2007). The activity of these individual complexes is regulated by the CAK pathway, comprising CDKF, CDKD and CDKH. The WEE1 kinase regulates the activity of CDKs through phosphorylation and suppresses the cell cycle in response to DNA damage (Fig. 1). Initial reports suggested that a plant homologue of CELL DIVISION CYCLE 25 (CDC25)-related kinase may have a similar function to that in animals, activating the CYC/CDK complex and consequently triggering the G2/M transition. However, this hypothesis was challenged by evidence demonstrating that neither mutant nor CDC25-overexpressing plants have altered cell cycle progression, suggesting that the plant CDC25 homologue does not play a particular role in regulating the cell cycle (Dissmeyer et al., 2009). Finally, APC/C-mediated degradation of the complex completes mitosis (Marrocco et al., 2010).
Definition of G0
Various authors have used the term G0 to describe meristematic quiescence or terminal differentiation; however, whether G0 is a distinct nuclear state or merely a prolonged state of G1 is ill-defined. The G0 state (non-cycling cells) in plants was proposed on the basis of observations by Clowes (1971), who found that the QC of maize roots contained a heterogeneous population of slowly cycling and non-cycling cells, as demonstrated by thymidine pulse-labelling experiments (Clowes, 1971). The concept of G0 is more widely accepted in mammalian cells, as proposed by Pardee (1974), who suggested a restriction point (R; Fig. 1) early in G1 that governs cell fate: G1 cells can enter G0 before reaching R but must commit to the cell cycle once they pass R. Somatic mammalian cells seem capable of entering a reversible (quiescent cells) or irreversible (senescent and differentiated cells) G0 state from G1 before R and, in rare cases, the irreversibly arrested cells have also been shown to re-enter the cell cycle by supressing certain tumour suppressors, such as retinoblastoma protein (RB) and tumour protein P53 (Pardee, 1974; Cheung and Rando, 2013). The existence of this early-G1 restriction point (R) in plant cells is yet to be demonstrated, as opposed to the familiar late-G1 restriction point (or G1 restriction point) analogous to yeast START, which is a cell size/growth check-point.
Hence cell cycle progression is regulated by CDK activity and regulated ubiquitin-dependent proteolysis of the cell cycle master regulators, with fine-tuning provided by hormone and sucrose signalling. In the remainder of the review we consider the current knowledge of the cell cycle state in meristematic quiescence, dormancy and terminal differentiation. We particularly focus on the molecular functions that act on the maintenance of G1 and the G1/S transition. Table 1 summarizes the literature demonstrating that meristematic organs, whether quiescent or dormant, are predominantly arrested in the G1(/G0) phase.
Table 1.
Summary of physiological data on cell cycle phases determined in cells of dormant or quiescent organs
| Type of growth cessation | Predominant phase | Organ | Species | Observation | Reference |
|---|---|---|---|---|---|
| Dormancy | G1 (98 %), G2 (1 %) | Cotyledon | Hordeum vulgare | Absence of 4C nuclei entering the first cell cycle post-dormancy in dormant leaf demonstrated by cytophotometry and [3H]-thymidine labelling. | Ahmed and Kamra, 1976 |
| Dormancy | G1, G2 | Embryo | Triticum durum | Different G1/G2 nuclei ratios in distinct meristems due to water content sensitivity revealed by microspectrophotometry. | Avanzi et al., 1969 |
| Dormancy | G1 | Seeds | Arabidopsis thaliana, Brassica oleracea | Absence of DNA replication in dormant seeds and the activation of microtubular cytoskeleton assembly at the start of germination observed by flow cytometry, β-Glucuronidase (GUS) assays, mRNA in situ hybridization and in vivo Green Fluorescent Protein (GFP) localization. | Barrôco et al., 2005 |
| Dormancy | G1 (90 %), G2 (10 %) | Seed | Pisum sativum | DNA synthesis more sensitive to dehydration than mitosis. DNA synthesis ceased prior to commencement of mitosis in ripening seeds. | Bogdanov et al., 1967 |
| Dormancy | G1 | Embryo | Vicia faba | DNA synthesis more sensitive to dehydration than mitosis, causing DNA synthesis to stop at a water content of 75 % of fresh weight, whereas mitosis continued up to a water content of 65 % of fresh weight during ripening. | Brunori, 1967 |
| Dormancy | G1 | Embryo | Allium cepa | Pure G1 (2C) population observed by microspectrophotometry. | Brunori and Ancora, 1968 |
| Dormancy | G1 | Embryo | Pinus pinea, Lactuca sativa | Pure G1 (2C) population observed by DNA cytophotometry. | Brunori and D'Amato, 1967 |
| Dormancy | G1 (70 %) | Tubers | Solanum tuberosum | Indirect regulation of cell division by post-transcriptional regulation of genes controlling the cell cycle in dormant meristems revealed by immunoblotting. | Campbell et al., 1996 |
| Dormancy | G1 | Seeds | Lycopersicon esculentum | Low 4C DNA content in primary dormant seeds shown by absence of DNA synthesis and BrdU incorporation and lack of accumulation of β-tubulin and formation of microtubular cytoskeleton. | de Castro et al., 2001 |
| Dormancy | G1 | Seeds | Hordeum vulgare | Temperature greatly influenced the status of the cell cycle. | Gendreau et al., 2012 |
| Dormancy | G1 (45 %) | Terminal buds | Pseudotsuga menziesii | Absence of mitotic activity and distinct cytohistological zonation in dormant apex. | Owens and Molder, 1973 |
| Quiescence | G1 | Roots | Zea mays | Predominance of 2C cells and presence of cells between 2C and 4C as opposed to 4C as predicted earlier, observed by microdensitometry. | Clowes, 1968 |
| Quiescence | G1 (50 %), G2 (50 %) | Roots | Zea mays | 2C and 4C cells were found in a 1:1 ratio, indicating a higher proportion of G2 cells, observed by microspectrophotometry. | Conger and Carabia, 1976 |
| Quiescence | G1 (80 %) | Shoots | Zea mays | 2C and 4C cells were found in a 5:1 ratio, indicating a low proportion of G2 cells, observed by microspectrophotometry. | Conger and Carabia, 1976 |
| Quiescence | G1* | Roots | Arabidopsis thaliana | Lateral root initiation is controlled by auxin through transcriptional regulation of KIP-RELATED PROTEIN2 (KRP2), which regulates the G1/S transition in the pericycle. | Himanen et al., 2002 |
Indirect evidence.
MERISTEMATIC QUIESCENCE
Root and shoot apical meristems
Meristematic quiescence is exemplified by the QC and OC cells of the root and shoot apical meristem, respectively. We also propose that the physiological state of the QC and OC is reflected at the organ level in quiescent buds and non-dormant seeds and proleptic buds. Several key molecular regulators of the QC and OC and of stem cell identity are homologous (reviewed by Aichinger et al., 2012; Heyman et al., 2014). As considerably more is known of the regulation of its identity and rate of proliferation, this section largely focuses on the QC of the RAM. We introduce QC physiology and identity before summarizing the current knowledge of molecular and biochemical regulators of the cell cycle in the QC, with particular attention to the convergence of the regulators of identity and the canonical cell cycle. We also introduce recent research demonstrating the centrality of redox and oxygen signalling in regulating quiescence and proliferation. We then extend this to summarize knowledge in relation to the cell cycle controls in quiescent buds.
The QC comprises a reserve of pluripotent stem cell progenitors at the core of the RAM, which is established early post-embryogenesis. The QC cells are mitotically less active than the surrounding stem cells, their cell cycle duration being 3- to 6-fold longer. The size of the QC varies between species, e.g. from approximately four to eight cells in Arabidopsis (van den Berg et al., 1997) to 500–1200 cells in maize (Jiang et al., 2003). In species with closed-type meristems the QC is anatomically constrained by the columella, cortical/endodermal and stelar stem cells, while in species with open meristems the QC cells are anatomically interspersed with the stem cells at the core of the RAM (Heimsch and Seago, 2008). The QC cells are considerably more resistant to mutagens than surrounding stem or meristem cells, and divide asymmetrically to form the stem cells. According to the ‘mortal strand’ hypothesis, mitotic segregation of chromosomes is not random but serves to preserve the genetic identity of the QC, reducing the risk that any mutation during the S phase affects the integrity of the QC genome for future division (Charville and Rando, 2013). Thus the low rate of cell division in the QC, together with an elevated expression of DNA repair genes, confers greater resistance to DNA mutagens such as bleomycin, zeocin and UV-B radiation (Cruz-Ramírez et al., 2013).
The developmental identity of the QC and stem cells is defined by a network of three converging pathways (Aichinger et al., 2012; Heyman et al., 2014): (1) the polar auxin gradient (proximal to distal), driving expression of the PLETHORA (PLT) gene family, which in turn positively regulates auxin transport, reinforcing the gradient; (2) the radial signalling of the SCARECROW (SCR)–SHORTROOT (SHR) module; and (3) the polar signalling (distal to proximal) of CLV3/EMBRYO SURROUNDING REGION (CLE) peptides via the ARABIDOPSIS CRINKLY 4 (ACR4) receptor-like kinase. Together, these pathways converge on QC markers, notably the homeodomain transcription factor WUSCHEL RELATED HOMEOBOX 5 (WOX5). These signalling interactions are gated by plasmodesmata, which define symplastic fields (Daum et al., 2014).
Although considerable genetic and biochemical research has been undertaken to identify the networks defining non-cell-autonomous regulation of meristem cell identity, at least in Arabidopsis, exactly how they function in relation to cell cycle control, i.e. quiescence versus proliferation, remains to be elucidated. Identification of targets of PLT is still under way; however, a recent study indicated that the PLT family largely act redundantly to promote expression of a number of cell cycle genes, although auxin functions were primary targets in the QC (Santuari et al., 2016). This is consistent with their expression maxima in stem cells (Galinha et al., 2007) and the positive feedback loop in auxin transport and PLT expression. In contrast, a loop involving the SCR/SHR complex, CYCDs and RBR constrains the formative division in the stem cell initials of the cortex/endodermis lineage (Cruz-Ramírez et al., 2012). In stem cells, SCR and SHR act sympathetically with auxin to promote division via transcriptional activation of CYCDs. In the QC, SCR abundance is restrained by direct interaction with RBR. QC-specific silencing of RBR confirmed its function in repressing QC division, and further showed that this depletion of RBR heightened the sensitivity of QC cells to DNA damage (Cruz-Ramírez et al., 2013), which is in agreement with the observations cited above. SCR also transcriptionally activates the QC marker WOX5 (Sarkar et al., 2007), which in turn maintains quiescence, at least in part by transcriptional repression of CYCDs (Forzani et al., 2014). Hence tight spatial control of these interactions enables division in stem cells and quiescence in the QC.
Less is known of how the regulatory networks that define cell identity and fate in the SAM, such as WUSCHEL (WUS) and CLAVATA3 (CLV3), directly regulate the cell cycle. However, Gaamouche et al. (2010) showed that expression of a dominant negative allele of CDKA in the shoot apex of Arabidopsis (driven by a SHOOT MERISTEMLESS (STM) promoter) restricted cell division and promoted premature differentiation. Nevertheless, these effects were coupled to the localization of STM, which is largely peripheral to the OC and stem cell niche.
Regulated ubiquitin-dependent proteolysis is central to the maintenance of quiescence in the QC and OC, specifically through the APC/C, an E3 ubiquitin ligase (Marrocco et al., 2010; Heyman et al., 2014). The APC/C is activated by CELL CYCLE SWITCH52 (CCS52) proteins, of which there are two A-type isoforms in Arabidopsis, which appear to have subtle but important differences with regard to the cell cycle and display different expression patterns (Vanstraelen et al., 2009). Activity of the APC/CCCS52A2 appears largely confined to the QC and columella of the root (Vanstraelen et al., 2009) and the vegetative and reproductive SAM (Liu et al., 2012). By contrast, CCS52A1 is localized to the transition zone (root) and implicated in the onset of the endocycle (see below). In ccs52a2 mutants there is increased proliferation of the QC and failure to retain QC identity post-germination (Vanstraelen et al., 2009), and similarly there is disordered SAM organization, bifurcation and premature termination of inflorescence shoots (Liu et al., 2012). Interestingly, the effect of the ccs52a2 mutant on SAM identity genes was dependent on developmental state. In ccs52a2 seedlings there were increases in the expression level of WUS and the expression zone of WUS and CLV3, resulting in an enlarged OC/stem cell niche (Liu et al., 2012). In contrast, inflorescence meristems were smaller and showed reduced expression of WUS and CLV3, perhaps predicting termination of the reproductive meristem.
Further functional studies of the APC/CCCS52A2 complex in the root have identified one of the primary targets as a Group X ETHYLENE RESPONSE FACTOR (ERF115; Heyman et al., 2013). Expression of the ERF115 is elevated early during mitosis and remains expressed throughout mitosis and G1 until early S phase. ERF115 is induced by a number of hormones, including ethylene, cytokinin, jasmonate and brassinosteroids, or by stem cell damage, with resulting increases in QC division (Heyman et al., 2013). Site-directed mutation of one or other D-box motifs that are recognized by the APC/C lead to constitutive stability, indicating that post-translational modification is required to target ERF115 to the proteasome, and that this is stabilized under stress conditions. One of the transcriptional targets of ERF115 is the PHYTOSULFOKINE 5 (PSK5) peptide. The PSK5 is a peptide growth factor that controls root and shoot growth (Heyman et al., 2013, 2014). The action of TYROSYLPROTEIN SULFOTRANSFERASE (TPST) is required for the sulphation-mediated activation of PSK5, in addition to the activation of root growth factor (RGF)/Golven (GLV) peptides, which affect levels of PLT involved in QC maintenance (Heyman et al., 2014). Hence a link to quiescence and cell identity has been established, although direct targets of the APC/CCCS52A2 involved in the canonical cell cycle remain to be resolved.
Silencing ERF115 does not completely attenuate brassinosteroid-dependent QC division (Heyman et al., 2013). A second stress-responsive protein of the QC is the BRAVO protein (BRASSINOSTEROIDS ASSOCIATED AT VASCULAR AND ORGANISING CENTRE; Vilarrasa-Blasi et al., 2014). BRAVO is a target of brassinosteroid signalling, which serves to repress BRAVO transcriptionally and post-translationally (Vilarrasa-Blasi et al., 2014). In developmental conditions, BRAVO represses QC division, while under stress-induced brassinosteroid signalling BRAVO is repressed, enabling activators of QC division such as ERF115 to accumulate and increase QC proliferation, e.g. to replace damaged stem cells. Nevertheless, direct targets of BRAVO with respect the cell cycle are yet to be determined (Vilarrasa-Blasi et al., 2014).
Redox and oxygen-dependent regulation of meristem quiescence
Reactive oxygen species (ROS) and redox signalling play important roles in the spatial cellular control of the balance of quiescence and proliferation in the RAM and SAM (Considine and Foyer, 2014). In the RAM, G1 arrest of QC cells is tightly coupled with oxidation of the principal soluble antioxidants ascorbate and glutathione, which is interdependent on the polar auxin maximum in the QC and surrounding stem cell initials. Accordingly, the ascorbate and glutathione pools are both highly oxidized in the QC, but highly reduced in the proximal meristem (Jiang et al., 2003). Addition of glutathione (Kerk et al., 2000) or an ascorbate precursor (de Tullio et al., 2010) stimulated S phase progression in the QC, while the addition of oxidized glutathione (GSSG) or ascorbate (dehydroascorbate) favoured G0/G1 arrest (Potters et al., 2004). In further support of the low redox buffering of the QC, levels of superoxide (O2•−) and hydrogen peroxide (H2O2) were considerably higher, and NADPH was undetectable in the QC in comparison with the proximal meristem (Jiang et al., 2003). Disturbing the auxin gradient by removing the root cap or inhibiting auxin transport increased proliferation in the QC and abolished the difference in redox status of the ascorbate and glutathione pools in the QC and proximal meristem cells, such that the QC pools became more reduced and meristem pools more oxidized (Jiang et al., 2003). Conversely, addition of an ascorbate precursor alters the polar auxin gradient (de Tullio et al., 2010), indicating the interdependence of the auxin and redox gradients (Considine and Foyer, 2014).
A number of studies of Arabidopsis mutants of synthetic pathways for redox metabolites and enzymes have described the central function of redox regulation of meristematic quiescence and proliferation (Considine and Foyer, 2014). In particular, mutants of γ-glutamyl-cysteine synthase (γECS), which catalyses the first committed step of glutathione synthesis (Vernoux et al., 2000), NADPH-thioredoxin reductase (NTR), which functions in the NADPH-dependent thioredoxin system (Reichheld et al., 2007), and GDP-l-galactose phosphorylase (GGP), a key regulatory enzyme of ascorbate synthesis (de Simone et al., 2017). A strong mutant allele of γECS (rml1) results in a root meristemless phenotype, with the RAM arrested in G1 (Vernoux et al., 2000), while weaker alleles show reduction in lateral root density (Considine and Foyer, 2014). A degree of redundancy between glutathione and NTR in the SAM has also been demonstrated, with double mutants of NTR (ntra ntrb) being hypersensitive to a reduction in glutathione, resulting in a shoot and root meristemless phenotype (Reichheld et al., 2007).
Intracellular cycles in redox state have been functionally related to the cell cycle, as previously demonstrated in animal cells (Menon and Goswami, 2007). In plant cell cultures, glutathione was shown to shuttle into the nucleus during G1, leaving the cytosol relatively oxidized (Diaz-Vivancos et al., 2010). Direct evidence that this reflects the state in planta, and hence that redox poise intimately regulates proliferation, was recently established using a redox-sensitive GFP reporter (roGFP), with additional cell identity and cell cycle reporters in synchronized RAM cells (de Simone et al., 2017). The cytosol and then nuclei were transiently oxidized during the G1 phase, followed by redox reduction in G2. Mild oxidation in the background of weak GGP mutants (vtc2) impaired the redox rhythms and reduced nuclear size. The vtc2 lines had ∼20 % of the wild-type concentration of ascorbate in the leaves but otherwise showed only a mild phenotype of slow growth. Hence this is consistent with the intracellular shuttling of glutathione. Together with the tight relationship between ROS and auxin functions in the meristem, this line of research promises to reveal a great deal more about the post-translational regulation of meristematic quiescence.
Three further studies indicated an important role for mitochondrial function in regulating ROS and redox signalling in relation to quiescence and cell fate. Loss of function of a mitochondrial NTPase (APP1) in Arabidopsis led to reduced activity of Complex I and reduced levels of ROS but increased proliferation of QC cells, and promoted differentiation of distal stem cells (Yu et al., 2016). Complex I is a key site of mitochondrial generation (Millar et al., 2001). The app1 phenotype was dependent on reduced ROS levels, as treatment with methyl viologen or H2O2 rescued the wild-type phenotype in app1 mutants (Yu et al., 2016). The increased QC proliferation was attributed to reduced expression of SCR and SHR, although genes coding for PLT, B-type cyclin and other prominent QC and stem cell transcription factors were unaltered. Consistent with these findings, the loss of function of an ATP-dependent mitochondrial protease (FTSH4) resulted in increased ROS levels in the SAM, leading to meristem termination at mildly elevated temperature (30 °C; Dolzblasz et al., 2016). The authors suggested that FTSH4 plays a role in regulating stem cell mitochondrial transmembrane potential, thus affecting the intracellular redox state, and that this underpinned the termination of stem cell fate. In addition, it was suggested that FTSH4 plays a role in redox regulation of auxin signalling. Thus, data on APP1 and FTSH4 in the RAM and SAM are consistent with a strong relationship between intracellular redox state and quiescence, proliferation and cell fate. However, the interrelationship between ROS/redox and hormone signalling in regulating the cell cycle is still not entirely clear. Firstly, a study of the ABA-overly-sensitive mutant (abo8-1) mutant, which affects mitochondrial ROS, found an alternative relationship between ROS and proliferation (Yang et al., 2014). The abo8-1 mutant is defective in a PPR protein responsible for the splicing of NAD4 intron 3 of mitochondrial Complex I, accumulates more ROS in the RAM and displays reduced proliferation, reduced meristem size and an altered auxin gradient (Yang et al., 2014). Reduced proliferation in abo8-1 was attributed to reduced expression of PLT and B-type cyclin genes, while glutathione supplementation could partially rescue the phenotype. Hence, while crosstalk between ROS and auxin is implicated in the abo8-1 phenotype, there was no simple inverse relationship between ROS and proliferation. Secondly, evidence suggested that the app1 phenotype was independent of auxin (Yu et al., 2016). Such inconsistencies in the phenotype of ROS and redox mutants have been observed in other physiological functions in meristems, e.g. the effect of mitochondrial versus plastid ROS on plasmodesmatal aperture (Stonebloom et al., 2012). Thus, it has been noted that the intracellular location, type and concentration of ROS or the redox gradient are variables that must be considered further. It would be interesting to follow the intracellular redox state in these and other mutants with the roGFP redox reporters targeted to several organelles.
It is widely accepted in mammalian tissues that stem cell identity, proliferation and fate are dependent on the cellular oxygen concentration, in addition to redox status and ROS-dependent signalling. Numerous studies on mammalian cells have indicated that the stem cell niche is maintained at a low oxygen partial pressure to avoid the deleterious effects of oxygen on proteins and DNA through ROS production (Mohyeldin et al., 2010). Evidence suggests that physiological hypoxia is central to the identity and fate of mammalian stem cells (Webster, 2007). Although direct measurement of the oxygen partial pressure in plant stem cell niches has yet to be performed, regulated transitions in oxygen status are mechanistically involved in vital developmental events such as seed germination, the skoto-photomorphogenic transition and meiosis (reviewed by Considine et al., 2017). The present evidence from tissue oxygen availability and mathematical models (Armstrong and Armstrong, 2014) and biochemical and molecular signatures (Jiang et al., 2010) suggests that the QC is also maintained in a hypoxic state, to preserve genome integrity and pluripotency. However, this idea requires validation. The development and calibration of cellular oxygen reporters, analogous to the roGFP redox reporter, will greatly enhance understanding of the role of oxygen in cell quiescence, proliferation and fate.
Axillary shoot meristems/buds
The proliferation of shoot apical meristems and axillary buds is highly regulated and organized, similarly to the root, albeit with the additional input of light. We refer here to non-dormant buds, otherwise known as paradormant buds (Lang et al., 1987), where outgrowth of quiescent shoots is regulated by the balance of hormone (Shimizu-Sato et al., 2009) and sugar signalling (Barbier et al., 2015a), and external inputs such as light quality (Leduc et al., 2014). For detailed description of the roles of light, hormone and sugar signalling in axillary bud outgrowth we refer to recent reviews (Shimizu-Sato et al., 2009; Leduc et al., 2014; Barbier et al., 2015a). As seen in quiescent root meristems, cells of quiescent buds are predominantly arrested in the G1 phase (Table 1). Early studies examining bud outgrowth following decapitation in pea identified rapid changes in expression of genes coding for CDC2 kinase and CYCB, as well as histones H2A and H4, ribosomal protein rpL27 and rpL34, and a Mitogen-Activated Protein Kinase (MAPK), belonging to a family of highly conserved serine/threonine protein kinases involved in basic cellular processes (Devitt and Stafstrom, 1995). Nevertheless, genetic manipulation of canonical cell cycle regulators has rarely resulted in alteration of the overall developmental behaviour, despite change in mitotic activity (Grandjean et al., 2004). One of the more elegant examples, however, was ectopic differentiation induced by transient expression of RBR in the shoot apical meristem (Wyrzykowska et al., 2006), which is consistent with data from the RAM (see above).
In Arabidopsis, BRANCHED1 (BRC1), a homologue of the tillering repressor TEOSINTE BRANCHED1 (TB1) of sorghum, appears to be a major hub of signals regulating axillary bud quiescence and outgrowth (branching). BRC1 is required for axillary bud quiescence in response to far-red light or apical dominance (González-Grandío et al., 2013). BRC1-dependent genes include several canonical cell cycle regulators functioning in S phase and cytokinesis, as well as ribosomal proteins. These transcripts are repressed in wild-type plants under far-red-rich conditions or increased following decapitation, supporting the findings in pea. In addition, ABA response genes are positively regulated by BRC1, indicating that BRC1 is also upstream of ABA function in branching control, while cytokinin, auxin, strigolactone, light quality and sugar signalling are upstream of BRC1. BRC1 and TB1 are members of the TCP (CINCINNATA-like, TEOSINTE BRANCHED1-CYCLOIDEA-PCF) family of transcription factors. Class I TCP members generally promote cell division, while class II members, including BRC1 and TB1, are repressors. Another class II TCP member, TCP4 of Arabidopsis, was shown to arrest budding yeast at the G1/S transition when ectopically expressed (Aggarwal et al., 2011).
Hormones also regulate branching through activation of NADPH oxidases (RESPIRATORY BURST OXIDASE HOMOLOGUE; RBOH) to produce O2•− and consequently H2 (Considine and Foyer, 2014). RBOH have a prominent role in ROS generation and act as a central signalling hub incorporating several signal transduction pathways in plants. Silencing one or two homologues of RBOH in tomato resulted in up to a 10-fold increase in axillary bud outgrowth, associated with reduced H2O2 and altered auxin and cytokinin synthesis and transport (Chen et al., 2016), which is consistent with findings in Arabidopsis roots (Li et al., 2015), but contrary to the effects on non-dormant pea seeds (Barba-Espin et al., 2011) and dormant grapevine buds. The nature of these differential effects, whether genetic, developmental or concentration-dependent, remains to be explored.
Recent reports have provided strong evidence that sink strength/sugar signalling, rather than auxin, is primary in regulating apical dominance (Mason et al., 2014; Barbier et al., 2015a, b). This provides further context for understanding the direct and indirect regulation of the cell cycle in axillary buds, including BRC1, but notably also via SnRK1 and HEXOKINASE1 (HK1; Barbier et al., 2015a), and TOR1 (Considine and Considine, 2016). Sucrose can directly activate cell cycle regulators, notably the CYCDs, promoting G1/S transition, but also regulators of G2/M transition (Barbier et al., 2015a).
Taking these findings together, axillary bud quiescence is a latent, opportunistic state. BRC1 appears to be a key hub between hormone, sugar and light signalling in regulating bud outgrowth, but the signalling relationship with the cell cycle and how redox signalling may interact remain to be explored. Another significant opportunity for future research is to translate knowledge of how proteolysis functions in regulating axillary bud outgrowth.
DORMANT SEEDS AND PERENNIAL BUDS
Dormancy is the eco-developmental quiescence of a meristematic or embryonic organ, whereby growth fails (is viable but incompetent) to respond to favourable conditions until sufficient entrainment by environmental cues (Considine and Considine, 2016). As with quiescent tissues, the cells of dormant meristems have largely been observed to be arrested in the G1 phase (Table 1). Fundamentally, two considerations must be borne in mind in relation to cell cycle control and dormancy: (1) it is widely accepted that, following release of dormancy in seeds, hydration and cell expansion drive germination sensu stricto before cell division resumes (Bewley, 1997); and (2) despite extensive quantitative trait locus (QTL) studies, particularly in Arabidopsis (Chahtane et al., 2016), a mechanistic relationship between most dormancy QTLs and canonical cell cycle regulators is yet to be established. This is despite some QTLs, such as DELAY OF GERMINATION1 (DOG1), accounting for >10 % of the natural variance in seed dormancy (Bentsink et al., 2006). While there is evidence of early activation of the cell cycle during dormancy release in buds and cambial meristem of several species (Considine and Considine, 2016), the lack of QTLs directly implicated in cell cycle control in dormancy may suggest that the cell cycle is subordinate to the major hubs of dormancy control, and quantitatively regulated. Although reverse genetics of some regulators of the canonical cell cycle have altered the dormancy phenotype (Table 2), chromatin accessibility regulated by histone modifications of dormancy-associated genes provides a more consistent path to regulation of the cell cycle during dormancy (Considine and Considine, 2016). For further comment on chromatin accessibility we refer authors to recent reviews on quiescence (Coller, 2007) and the cellular chromatin state (de la Paz Sanchez et al., 2015).
Table 2.
Summary of reverse genetic studies involving components of the canonical cell cycle causing altered dormancy or quiescence phenotypes
| Gene name | Description | Species | Allele | Dormancy-related phenotype | Reference |
|---|---|---|---|---|---|
| CYCD1;1 | D-type cyclin | Arabidopsis thaliana (Col. 0), Arabidopsis thaliana (L. er.) | cycd1;1 | Radicle emergence delayed during germination due to delayed cell cycle activation in the root apex. | Masubelele et al., 2005 |
| CYCD1;1oe | Germination and radicle emergence accelerated in overexpression lines. | ||||
| CYCD2;1 | D-type cyclin | Arabidopsis thaliana (Col. 0), Arabidopsis thaliana (L. er.) | CYCD2;1oe | Accelerated germination and radicle emergence in overexpression lines. | Masubelele et al., 2005 |
| CYCD3;1 | D-type cyclin | Arabidopsis thaliana (L. er.) | CYCD3;1oe | Germination delayed in overexpressing line. | Masubelele et al., 2005 |
| CYCD4;1 | D-type cyclin | Arabidopsis thaliana (Col. 0) | cycd4;1 | Radicle emergence deferred during germination due to delayed cell cycle activation in the root apex. | Masubelele et al., 2005 |
| CYC1 | Mitotic cyclin | Populus tremuIa (L.) × P. alba (L.) | Pcyc1At-gus | Expression of Pcyc1At-gus low in dormant axillary buds and downregulated by short days, which in poplar induces dormancy. | Rohde et al., 1997 |
| CDC2A | Cyclin-dependent kinase | Populus tremuIa (L.) × P. alba (L.) | Pcdc2a-gus | Expression of Pcdc2a-gus low in dormant axillary buds and downregulated by short days, which in poplar induces dormancy. | Rohde et al., 1997 |
| ICK1/KRP1 | Cyclin-dependent kinase inhibitor | Oryza sativa | KRP1oe | Germination ability of pollen affected in overexpression lines. | Barrôco et al., 2006 |
| ICK2/KRP2 | Cyclin-dependent kinase inhibitor | Arabidopsis thaliana (Col. 0) | ick2-1/krp2 ick2oe | Increase in density and number of lateral roots under normal conditions. Lateral root formation inhibited in overexpression lines. | Sanz et al., 2011 |
| ICK3/KRP5 | Cyclin-dependent kinase inhibitor | Arabidopsis thaliana (Col. 0) | ick3/krp5-1 | Loss of functional ICK3/KRP5 causes delayed germination. | Wen et al., 2013 |
| 1CK4/KRP6 | Cyclin-dependent kinase inhibitor | Arabidopsis thaliana (Col. 0) | krp6-1 | Rapid germination. | Nieuwland et al., 2016 |
| E2Fa | Transcription factor | Arabidopsis thaliana (Col. 0) | E2Fa/DPaoe | Overexpression of AtE2Fa induced ectopic cell divisions and DNA endoreduplication. Dry seeds contain low levels of E2Fa whereas their levels increase on imbibition. | de Veylder et al., 2002 |
| E2Fa | Masubelele et al., 2005 | ||||
| E2Fc | Transcription factor | Arabidopsis thaliana (Col. 0) | E2Foe | Overexpression of AtE2Fc reduces number of cell divisions. Dry seeds contain high levels of E2Fc; however, their levels decrease on imbibition. | del Pozo et al., 2002 |
| E2Fc | Masubelele et al., 2005 |
Nuclear quiescence is associated with a primarily heterochromatin state. Two evolutionarily conserved functions that regulate the chromatin state during quiescence and active growth are the Polycomb group (PcG) and Trithorax group (TrxG) complexes. Characteristically, the PcG complex functions to repress gene activity, associated with a heterochromatin state, by trimethylation of histone-3 lysine-27 (H3K27), dimethylation of H3K9 and ubiquitination of H2AK118/119, while the TrxG complex functions to antagonize PcG activities and promote a more euchromatic state of active transcription, by trimethylation of H3K4 and K3K36 (de la Paz Sanchez et al., 2015). Accordingly, the expression and physiological function of the major dormancy loci in seeds and perennial buds are demonstrated to be regulated by a balance of characteristic PcG and TrxG modifications. The major dormancy loci in seeds include DOG1 and those in perennial buds include DORMANCY ASSOCIATED MADS BOX1 (DAM1) and EARLY BUD-BREAK (EBB1). In several species, the expression of homologues of DOG1 (Müller et al., 2012; Molitor et al., 2014; Footitt et al., 2015) in seeds and DAM1 (Horvath et al., 2010; Leida et al., 2012; Saito et al., 2015) in perennial buds, increased trimethylation of H3K27 and decreased trimethylation of H3K4 coincide with the decline in gene expression and release of dormancy, and vice versa at the onset of dormancy (Considine and Considine, 2016). More recently, expression of EBB1, a member of the APETALA2/ETHYLENE RESPONSE FACTOR (AP2/ERF) family and positive regulator of bud burst (Yordanov et al., 2014), was also shown to be associated with trimethylation of H3K4 and, moreover, evidence was presented suggesting direct interaction between the EBB protein and the promoter region of D-type cyclins (Anh Tuan et al., 2016). The FLOWERING LOCUS T (FT) gene is a pivotal signal for photoperiod regulation of flowering, which has also been implicated in photoperiod (Böhlenius et al., 2006) and temperature control of bud dormancy (Rinne et al., 2011). Recent studies also implicate PcG-mediated repression of FT expression, which is disrupted by the photoperiod pathway to enable long-day flowering (Wang et al., 2014). It remains to be established whether similar features govern FT expression during dormancy transitions. Howe et al. (2015) identified a further set of candidates for chromatin regulation during dormancy onset and release. Hence these data, in dormant seeds and perennial buds, are consistent with a view that nuclear dormancy is regulated at the level of chromatin modification characteristic of the PcG and TrxG activities.
It is worthwhile considering the interface between hormone and nitric oxide (•NO) signalling and chromatin regulation. The regulatory crosstalk between ROS and the major hormone signalling pathways during dormancy release has been described in recent reviews (Diaz-Vivancos et al., 2013; Considine and Considine, 2016; Née et al., 2017). Cytokinins are involved in breaking dormancy and known to induce the expression of cyclins, both directly and by inducing •NO accumulation (Fig. 2). For example, treatment with a synthetic cytokinin (1-(α-ethylbenzyl)-3-nitroguanidine) terminated potato tuber dormancy, through the upregulated expression of genes coding for a D-type cyclin, along with histones H1B, H2A, H2B, H3 and H4, and the downregulation of an AGAMOUS-like MADS box transcription factor homologous to the Prunus persica DAM genes (Campbell et al., 2014). Transcripts homologous to Arabidopsis SWITCH2/SUCROSE NON-FERMENTABLE2 (SWI2/SNF2), involved in regulation of genome methylation state, and to ARGONAUTE4 (ARGO-4), involved in histone H3 methylation and structural alteration of chromatin, showed increased expression levels (Campbell et al., 2014). In addition, gibberellic acid (GA) has been shown to promote the expression of two B-type cyclins (CYCB2;1 and CYCB2;2) and a CDC2 protein kinase in rice (Sauter, 1997), as well as D-type cyclin (CYCD3) in leafy spurge (Horvath et al., 2005). In contrast, ABA may prevent cell division acting through the induction of KRP1, which inhibits CDKs (Wang et al., 1998). Olsen (2003) suggested that the opposite effects of GA and ABA in dormancy may be explained by KRP1, as its expression is induced by ABA but inhibited by GA (Fig. 2). Furthermore, although it has been suggested that ethylene and ABA are antagonistic (Corbineau et al., 2014), the signalling cascade induced by ethylene has also been suggested to repress CDK activity (Fig. 2). Recently, two Arabidopsis histone demethylases, LYSINE SPECIFIC DEMETHYLASE LIKE1 and 2 (LDL1 and LDL2), were shown to act redundantly in repressing seed primary dormancy (Zhao et al., 2015). Additionally, the two SIN3-LIKE1 proteins SNL1 and SNL2, members of the histone deacetylation complex, were suggested to regulate seed dormancy by mediating the ABA–ethylene antagonism (Wang et al., 2013). The effect of ABA may vary depending on the cell cycle state. For example, during mitosis the C2H2 zinc finger transcription factor family becomes phosphorylated at the conserved linker region, resulting in dissociation from mitotic chromatin (Rizkallah et al., 2011). Consequently, C2H2 zinc finger proteins that are usually induced by ABA, such as ARS1 in Arabidopsis (Baek et al., 2015), are not able to activate the transcription of the genes under its regulation and therefore ABA will not trigger that response in mitotic cells. In particular, these transcription factors are suggested to induce enzymatic antioxidant defence. If cells are in the G1 and G2 phases, ABA will promote antioxidant defence, controlling ROS levels. However, it is speculated that this may not be the case during M phase.
To summarize, chromatin modification/accessibility and ROS/hormone-related signalling appear to be defining features governing dormancy, with little evidence that the degree of proteolytic control seen in meristematic quiescence is also central to dormancy. This may reflect the different degrees of latency and required response times, with proteolytic control relatively more rapid than gene expression. A heightened function for regulated chromatin accessibility in dormancy may also reflect the more hostile environmental and cellular conditions prevailing, and thus the need to protect the genome. Finally, it is worthwhile commenting on some areas of ambiguity. Although several modes of dormancy have been described in seeds, differing in physical or physiological behaviour, reports have occasionally failed to describe the physiological state of seeds or buds adequately, leading to some uncertainty over the distinction in cell cycle control between different modes of dormancy. One example is secondary dormancy, which takes place following prolonged exposure to mild stress conditions (Hilhorst et al., 2010). Thus, some data are omitted from Tables 1 and 2, because the description of physiological state was inadequate for a judgement to be made. The issue of secondary dormancy may also extend to perennial buds; although to date this has only been described in seeds, it is conceivable that secondary dormancy also occurs in buds of temperate perennials, e.g. buds that fail to burst for one or more years. Additionally, there is evidence that dormancy in temperate perennials develops with age, similarly to floral competence (Böhlenius et al., 2006), suggesting that caution should be used in interpreting studies with juvenile perennials. However, to date no study has directly investigated age-related control of the cell cycle in buds of temperate perennials.
SWITCH FROM CELLULAR PROLIFERATION TO DIFFERENTIATION
Higher plant cells proceed through lineages of differentiation following mitosis. In the absence of cytokinesis, however, cells continue replicating their DNA through endoreduplication (de Veylder et al., 2007; Polyn et al., 2015). The initiation of differentiation requires asymmetrical division of undifferentiated cells to form progeny cells with distinct cell fates. Various developmental processes, including the initiation of lateral roots and embryogenesis and the development of stomata and pollen, are regulated through asymmetrical divisions. Available evidence suggests the specific involvement of APC/C E3 ligase and regulation by cytokinin and the balance of O2•− to H2O2 to be important in the commitment to differentiation (Polyn et al., 2015; Fig. 3).
As indicated earlier, CCS52A1 regulates the onset of the endocycle through activation of the APC/C E3 ligase, in contrast to CCS52A2, which defines quiescence. In turn, CCS52A1 is regulated by cytokinins, acting via B-type ARABIDOPSIS RESPONSE REGULATOR (ARR) transcription factors (Takahashi et al., 2013). ARR2 directly upregulates expression of the CCS52A1, while further B-type ARRs promote expression of auxin signalling repressors. The APC/CCCS52A1 targets the mitotic A-type cyclin to the proteasome, thereby short-circuiting mitosis (Boudolf et al., 2009).
Fine-tuning of ROS levels has also been demonstrated to influence cell fate and the commitment to differentiation (reviewed by Considine and Foyer, 2014; Considine et al., 2017). Root cell proliferation and elongation are known to be regulated by O2•− and H2O2, respectively. Notably, the transcriptional repression of peroxidases in the Arabidopsis root transition zone by the UPBEAT1 (UPB1) transcription factor controls the balance of O2•− and H2O2, which appears to be central to the commitment to differentiate (Tsukagoshi et al., 2010). In turn, H2O2 influences the level of UPB1 through a feedback loop. Interruption of UPB1 activity causes delayed inception of differentiation due to disruption of ROS homeostasis. In addition, the MYB DOMAIN PROTEIN36 (MYB36) regulates transition from proliferation to differentiation during development of the root epidermis and later stages of lateral root primordium, in a manner related to UPB1 (Liberman et al., 2015; Fernández-Marcos et al., 2017). In myb36 mutants there is reduced expression of UPB1 along with additional cell divisions in the meristematic and elongation zones. Although the evidence is somewhat conflicting, the balance of O2•− and H2O2 also appears to be central to differentiation during leaf development. Arabidopsis KUODA1 (KUA1) modulates expansion but not proliferation during Arabidopsis leaf development through transcriptional regulation of peroxidase genes (Lu et al., 2014). In kua1-1 mutants there is reduced leaf cell size and increased activity of peroxidase. Taking these findings together, regulated proteolysis of canonical cell cycle activities, together with fine-tuning of ROS, serves to restrict cell cycle re-entry and promote the endocycle in the transition to terminal differentiation.
Of other components of the canonical cell cycle (Fig. 1), RBR, in partnership with E2Fc, and the KRPs appear to play key roles in regulating the commitment to differentiation. Repressing E2Fc in Arabidopsis resulted in increased expression of B-type cyclin in dividing and differentiated tissues, whereas its overexpression reduced the number of cells in the G2/M phase in the dividing areas (del Pozo et al., 2006). Degradation of E2Fc is regulated by an SCF E3 ligase with the F-box subunit SKP2A, rather than APC/C. The expression level of KRPs also appears to regulate the balance of proliferation and differentiation. For example, in the Arabidopsis root KRP5 is a positive regulator of the endocycle and cell expansion (Jégu et al., 2013; Wen et al., 2013), while in the shoot KRP3 appears to function similarly. However, strong overexpression of KRP2 resulted in reduced ploidy levels, whereas lines with weak overexpression showed elevated ploidy levels and reduced mitotic cycles in Arabidopsis leaves (Verkest et al., 2005).
CONCLUSIONS
Here we have considered the major regulators of the cell cycle and in turn how their function is regulated during three modes of quiescence in plants, each physiologically distinct but cytologically characterized by cell cycle arrest in the G1 phase: meristematic quiescence, dormancy and terminal differentiation. Our first question was whether the canonical cell cycle regulators are superior or subordinate in the regulation of quiescence. Although there is evidence for altered quiescence, dormancy or differentiation phenotypes in mutants of the canonical cell cycle regulators, by and large control appears to be superior, regulated at the level of the ubiquitin proteasome system and chromatin accessibility. We then questioned whether these three modes of quiescence were governed by distinct controls. As summarized in Fig. 3, the review found not only distinct controls but distinct levels of control, particularly between meristematic quiescence and dormancy. Meristematic quiescence is intrinsically linked to the non-cell-autonomous regulation of meristem identity and is particularly defined by regulated proteolysis, together with ROS/redox and hormone fine tuning. Consistently, the CCS52A2 activator of the APC/C E3 ligase together with auxin- and ABA-dependent signalling were identified as regulators of meristematic quiescence. In contrast, dormancy appears to act at a level superior, i.e. subsequent, to meristematic quiescence. Current literature points consistently to PcG-type histone modifications of particular dormancy genes in defining the decision to enter or exit dormancy. Whether or how this is regulated by ROS and hormone signalling poses an interesting question for future research, considering the consistent evidence of ROS and hormone functions in dormancy transitions. Meanwhile, terminal differentiation is regulated in a remarkably analogous manner to meristematic quiescence – via CCS52A1 and cytokinin signalling, together with ROS homeostasis. Although ROS–hormone and hormone–proteolysis crosstalk has been described in both meristematic quiescence and terminal differentiation, crosstalk between ROS or redox and proteolysis has yet to be established, but remains a plausible mechanism of fine-tuning cellular decisions. Further research building the relationships between the canonical cell cycle, meristem identity, proteolysis, ROS/redox, hormone, and chromatin accessibility promises to enrich the understanding of plant developmental and stress biology, including other important agricultural and ecological contexts of quiescence such as hypoxia, darkness, nutrient limitation and senescence.
ACKNOWLEDGEMENTS
The authors would like to thank Professor Christine Foyer for her comments in drafting the review, and also to apologize to authors of research papers we could not cite in the interest of brevity, particularly where these papers are adequately covered in the reviews we have cited. M.J.C. acknowledges research funding by the Australian Research Council (DP150103211), which also provided the salary of S.S. Y.V. and M.C. acknowledge the University of Western Australia for a postgraduate research scholarship and stipend, the Australian Grape and Wine Authority postgraduate top-up scholarship and the International Organisation of Vine and Wine (OIV) for a research travel grant.
LITERATURE CITED
- Aggarwal P, Padmanabhan B, Bhat A, Sarvepalli K, Sadhale PP, Nath U.. 2011. The TCP4 transcription factor of Arabidopsis blocks cell division in yeast at G1 → S transition. Biochemical and Biophysical Research Communications 410: 276–281. [DOI] [PubMed] [Google Scholar]
- Ahmed ZU, Kamra OP.. 1976. DNA content of dormant barley leaf nuclei and the rate of cell entry into S-phase and mitosis. Caryologia 29: 187–193. [Google Scholar]
- Aichinger E, Kornet N, Friedrich T, Laux T.. 2012. Plant stem cell niches. Annual Review of Plant Biology 63: 615–636. [DOI] [PubMed] [Google Scholar]
- Anh Tuan P, Bai S, Saito T, Imai T, Ito A, Moriguchi T.. 2016. Involvement of EARLY BUD-BREAK, an AP2/ERF transcription factor gene, in bud break in Japanese pear (Pyrus pyrifolia Nakai) lateral flower buds: expression, histone modifications and possible target genes. Plant and Cell Physiology 57: 1038–1047. [DOI] [PubMed] [Google Scholar]
- Armstrong W, Armstrong J.. 2014. Plant internal oxygen transport (diffusion and convection) and measuring and modelling oxygen gradients In: Van Dongen JT, Licausi F. eds. Low-oxygen stress in plants: oxygen sensing and adaptive responses to hypoxia. Vienna: Springer, 267–297. [Google Scholar]
- Avanzi S, Brunori A, D'Amato F.. 1969. Sequential development of meristems in the embryo of Triticum durum. A DNA autoradiographic and cytophotometric analysis. Developmental Biology 20: 368–377. [DOI] [PubMed] [Google Scholar]
- Baek D, Cha J-Y, Kang S, et al. 2015. The Arabidopsis a zinc finger domain protein ARS1 is essential for seed germination and ROS homeostasis in response to ABA and oxidative stress. Frontiers in Plant Science 6: 963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Voesenek LA.. 2008. Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology 59: 313–339. [DOI] [PubMed] [Google Scholar]
- Barba-Espín G, Diaz-Vivancos P, Job D, Belghazi M, Job C, Hernández JA.. 2011. Understanding the role of H2O2 during pea seed germination: a combined proteomic and hormone profiling approach. Plant, Cell & Environment 34: 1907–1919. [DOI] [PubMed] [Google Scholar]
- Barbier FF, Lunn JE, Beveridge CA.. 2015a. Ready, steady, go! A sugar hit starts the race to shoot branching. Current Opinion in Plant Biology 25: 39–45. [DOI] [PubMed] [Google Scholar]
- Barbier FF, Péron T, Lecerf M, et al. 2015b. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. Journal of Experimental Botany 66: 2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrôco RM, Van Poucke K, Bergervoet JHW, et al. 2005. The role of the cell cycle machinery in resumption of postembryonic development. Plant Physiology 137: 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrôco RM, Peres A, Droual AM, et al. 2006. The cyclin-dependent kinase inhibitor Orysa;KRP1 plays an important role in seed development of rice. Plant Physiology 142: 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentsink L, Jowett J, Hanhart CJ, Koornneef M.. 2006. Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proceedings of the National Academy of Sciences of the USA 103: 17042–17047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B.. 1997. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 390: 287–289. [DOI] [PubMed] [Google Scholar]
- Bewley JD. 1997. Seed germination and dormancy. Plant Cell 9: 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov YF, Liapunova NA, Sherudilo AI.. 1967. Cell population in pea embryos and root tip meristem. Microphotometric and autoradiographic studies. Tsitologiya 9: 569–576. [Google Scholar]
- Böhlenius H, Huang T, Charbonnel-Campaa L, et al. 2006. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312: 1040. [DOI] [PubMed] [Google Scholar]
- Boudolf V, Lammens T, Boruc J, et al. 2009. CDKB1;1 forms a functional complex with CYCA2;3 to suppress endocycle onset. Plant Physiology 150: 1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori A. 1967. Relationship between DNA synthesis and water content during ripening of Vicia faba seed. Caryologia 20: 333–338. [Google Scholar]
- Brunori A, Ancora G.. 1968. The DNA content of nuclei in the embryonic root apices of dry seeds of Allium cepa and their radiation response. Caryologia 21: 261–269. [Google Scholar]
- Brunori A, D'Amato F.. 1967. The DNA content of nuclei in the embryo of dry seeds of Pinus pinea and Lactuca sativa. Caryologia 20: 153–161. [Google Scholar]
- Campbell MA, Suttle J, Douches DS, Buell CR.. 2014. Treatment of potato tubers with the synthetic cytokinin 1-(α-ethylbenzyl)-3-nitroguanidine results in rapid termination of endodormancy and induction of transcripts associated with cell proliferation and growth. Functional & Integrative Genomics 14: 789–799. [DOI] [PubMed] [Google Scholar]
- Campbell MA, Suttle JC, Sell TW.. 1996. Changes in cell cycle status and expression of p34cdc2 kinase during potato tuber meristem dormancy. Physiologia Plantarum 98: 743–752. [Google Scholar]
- Chahtane H, Kim W, Lopez-Molina L.. 2016. Primary seed dormancy: a temporally multilayered riddle waiting to be unlocked. Journal of Experimental Botany 68: 857–869. [DOI] [PubMed] [Google Scholar]
- Charville GW, Rando TA.. 2013. The mortal strand hypothesis: non-random chromosome inheritance and the biased segregation of damaged DNA. Seminars in Cell & Developmental Biology 24: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-J, Xia X-J, Guo X, et al. 2016. Apoplastic H2O2 plays a critical role in axillary bud outgrowth by altering auxin and cytokinin homeostasis in tomato plants. New Phytologist 211: 1266–1278. [DOI] [PubMed] [Google Scholar]
- Cheung TH, Rando TA.. 2013. Molecular regulation of stem cell quiescence. Nature Reviews. Molecular Cell Biology 14: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes FAL. 1968. The DNA content of the cells of the quiescent centre and root cap of Zea mays. New Phytologist 67: 631–639. [Google Scholar]
- Clowes FAL. 1971. The proportion of cells that divide in root meristems of Zea mays L. Annals of Botany 35: 249–261. [Google Scholar]
- Coller HA. 2007. What's taking so long? S-phase entry from quiescence versus proliferation. Nature Reviews. Molecular Cell Biology 8: 667–670. [DOI] [PubMed] [Google Scholar]
- Conger BV, Carabia JV.. 1976. Microspectrophotometric determination of the 2C and 4C nuclear complement in the root and shoot of the dormant maize embryo. Environmental and Experimental Botany 16: 171–175. [Google Scholar]
- Considine MJ, Considine JA.. 2016. On the language and physiology of dormancy and quiescence in plants. Journal of Experimental Botany 67: 3189–3203. [DOI] [PubMed] [Google Scholar]
- Considine MJ, Foyer CH.. 2014. Redox regulation of plant development. Antioxidants & Redox Signaling 21: 1305–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine MJ, Diaz-Vivancos P, Kerchev P, et al. 2017Learning to breathe: developmental phase transitions in oxygen status. Trends in Plant Science 22: 140–153. [DOI] [PubMed] [Google Scholar]
- Corbineau F, Xia Q, Bailly C, El-Maarouf-Bouteau H.. 2014. Ethylene, a key factor in the regulation of seed dormancy. Frontiers in Plant Science 5: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Ramírez A, Díaz-Triviño S, Blilou I, et al. 2012. A bistable circuit involving SCARECROW-RETINOBLASTOMA integrates cues to inform asymmetric stem cell division. Cell 150: 1002–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Ramírez A, Díaz-Triviño S, Wachsman G, et al. 2013. A SCARECROW-RETINOBLASTOMA protein network controls protective quiescence in the Arabidopsis root stem cell organizer. PLoS Biology 11: e1001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G, Medzihradszky A, Suzaki T, Lohmann JU.. 2014. A mechanistic framework for noncell autonomous stem cell induction in Arabidopsis. Proceedings of the National Academy of Sciences of the USA 111: 14619–14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro RD, Bino RJ, Jing H-C, Kieft H, Hilhorst HWM.. 2001. Depth of dormancy in tomato (Lycopersicon esculentum Mill.) seeds is related to the progression of the cell cycle prior to the induction of dormancy. Seed Science Research 11: 45–54. [Google Scholar]
- de la Paz Sanchez M, Aceves-García P, Petrone E, et al. 2015. The impact of Polycomb group (PcG) and Trithorax group (TrxG) epigenetic factors in plant plasticity. New Phytologist 208: 684–694. [DOI] [PubMed] [Google Scholar]
- de Simone A, Hubbard R, De La Torre NV, et al. 2017. Redox changes during the cell cycle in the embryonic root meristem of Arabidopsis thaliana. Antioxidants & Redox Signaling, in press. doi:10.1089/ars.2016.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tullio MC, Jiang K, Feldman LJ.. 2010. Redox regulation of root apical meristem organization: connecting root development to its environment. Plant Physiology and Biochemistry 48: 328–336. [DOI] [PubMed] [Google Scholar]
- de Veylder L, Beeckman T, Beemster GTS, et al. 2002. Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa–DPa transcription factor. EMBO Journal 21: 1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Boniotti MB, Gutierrez C.. 2002. Arabidopsis E2Fc functions in cell division and is degraded by the ubiquitin-SCF(AtSKP2) pathway in response to light. Plant Cell 14: 3057–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Diaz-Trivino S, Cisneros N, Gutierrez C.. 2006. the balance between cell division and endoreplication depends on E2FC-DPB, transcription factors regulated by the ubiquitin-SCFSKP2A pathway in Arabidopsis. Plant Cell 18: 2224–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devitt M, Stafstrom J.. 1995. Cell cycle regulation during growth-dormancy cycles in pea axillary buds. Plant Molecular Biology 29: 255–265. [DOI] [PubMed] [Google Scholar]
- Diaz-Vivancos P, Dong Y, Ziegler K. et al. 2010. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant Journal 64: 825–838. [DOI] [PubMed] [Google Scholar]
- Diaz-Vivancos P, Barba-Espín G, Hernández J.. 2013. Elucidating hormonal/ROS networks during seed germination: insights and perspectives. Plant Cell Reports 32: 1491–1502. [DOI] [PubMed] [Google Scholar]
- Dissmeyer N, Weimer AK, Pusch S, et al. 2009. Control of cell proliferation, organ growth, and DNA damage response operate independently of dephosphorylation of the Arabidopsis Cdk1 homolog CDKA;1. Plant Cell 21: 3641–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzblasz A, Smakowska E, Gola EM, Sokołowska K, Kicia M, Janska H.. 2016. The mitochondrial protease AtFTSH4 safeguards Arabidopsis shoot apical meristem function. Scientific Reports 6: 28315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K. 1965. Plant anatomy, 2nd edn Tokyo: John Wiley & Sons. [Google Scholar]
- Fernández-Marcos M, Desvoyes B, Manzano C, et al. 2017. Control of Arabidopsis lateral root primordium boundaries by MYB36. New Phytologist 213: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S, Müller K, Kermode AR, Finch-Savage WE.. 2015. Seed dormancy cycling in Arabidopsis: chromatin remodelling and regulation of DOG1 in response to seasonal environmental signals. Plant Journal 81: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forzani C, Aichinger E, Sornay E, et al. 2014. WOX5 suppresses CYCLIN D activity to establish quiescence at the centre of the root stem cell niche. Current Biology 24: 1939–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaamouche T, de O. Manes C-L, Kwiatkowska D, et al. 2010. Cyclin-dependent kinase activity maintains the shoot apical meristem cells in an undifferentiated state. Plant Journal 64: 26–37. [DOI] [PubMed] [Google Scholar]
- Galinha C, Hofhuis H, Luijten M, et al. 2007. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449: 1053–1057. [DOI] [PubMed] [Google Scholar]
- Gendreau E, Cayla T, Corbineau F.. 2012. S phase of the cell cycle: a key phase for the regulation of thermodormancy in barley grain. Journal of Experimental Botany 63: 5535–5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Grandío E, Poza-Carrión C, Sorzano COS, Cubas P.. 2013. BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell 25: 834–850. edna. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean O, Vernoux T, Laufs P, Belcram K, Mizukami Y, Traas J.. 2004. In vivo analysis of cell division, cell growth, and differentiation at the shoot apical meristem in Arabidopsis. Plant Cell 16: 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérinier T, Millan L, Crozet P, et al. 2013. Phosphorylation of p27KIP1 homologs KRP6 and 7 by SNF1-related protein kinase-1 links plant energy homeostasis and cell proliferation. Plant Journal 75: 515–525. [DOI] [PubMed] [Google Scholar]
- Gutierrez C. 2016. 25 years of cell cycle research: what's ahead? Trends in Plant Science 21: 823–833. [DOI] [PubMed] [Google Scholar]
- Heimsch C, Seago JL.. 2008. Organization of the root apical meristem in angiosperms. American Journal of Botany 95: 1–21. [DOI] [PubMed] [Google Scholar]
- Heyman J, Cools T, Vandenbussche F, et al. 2013. ERF115 controls root quiescent center cell division and stem cell replenishment. Science 342: 860–863. [DOI] [PubMed] [Google Scholar]
- Heyman J, Kumpf RP, de Veylder L.. 2014. A quiescent path to plant longevity. Trends in Cell Biology 24: 443–448. [DOI] [PubMed] [Google Scholar]
- Hilhorst HWM, Finch-Savage WE, Buitink J, Bolingue W, Leubner-Metzger G.. 2010. Dormancy in plant seeds In: Lubzens E, Cerda J, Clark M. eds. Dormancy and resistance in harsh environments. Berlin: Springer, 43–67. [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, De Almeida Engler J, Inzé D, Beeckman T.. 2002. Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14: 2339–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath DP, Anderson JV, Jia Y, Chao WS.. 2005. Cloning, characterization, and expression of growth regulator CYCLIN D3-2 in leafy spurge (Euphorbia esula). Weed Science 53: 431–437. [Google Scholar]
- Horvath DP, Sung S, Kim D, Chao WS, Anderson JA.. 2010. Characterization, expression and function of DORMANCY ASSOCIATED MADS-BOX genes from leafy spurge. Plant Molecular Biology 73: 169–179. [DOI] [PubMed] [Google Scholar]
- Howe GT, Horvath DP, Dharmawardhana P, Priest HD, Mockler TC, Strauss SH.. 2015. Extensive transcriptome changes during natural onset and release of vegetative bud dormancy in Populus. Frontiers in Plant Science 6: 989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzé D, de Veylder L.. 2006. Cell cycle regulation in plant development. Annual Review of Genetics 40: 77–105. [DOI] [PubMed] [Google Scholar]
- Jégu T, Latrasse D, Delarue M, et al. 2013. Multiple functions of Kip-related protein5 connect endoreduplication and cell elongation. Plant Physiology 161: 1694–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Meng YL, Feldman LJ.. 2003. Quiescent centre formation in maize roots is associated with an auxin-regulated oxidizing environment. Development 130: 1429–1438. [DOI] [PubMed] [Google Scholar]
- Jiang K, Zhu T, Diao Z, Huang H, Feldman L.. 2010. The maize root stem cell niche: a partnership between two sister cell populations. Planta 231: 411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerk NM, Jiang K, Feldman LJ.. 2000. Auxin metabolism in the root apical meristem. Plant Physiology 122: 925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang GA, Early JD, Martin GC, Darnell RL.. 1987. Endo-, para-, and ecodormancy: physiological terminology and classification for dormancy research. HortScience 22: 371–377. [Google Scholar]
- Leduc N, Roman H, Barbier F, et al. 2014. Light signaling in bud outgrowth and branching in plants. Plants 3: 223–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leida C, Conejero A, Arbona V, et al. 2012. Chilling-dependent release of seed and bud dormancy in peach associates to common changes in gene expression. PLoS One 7: e35777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Sun L, Zhang L, et al. 2015. AtrbohD and AtrbohF negatively regulate lateral root development by changing the localized accumulation of superoxide in primary roots of Arabidopsis. Planta 241: 591–602. [DOI] [PubMed] [Google Scholar]
- Liberman LM, Sparks EE, Moreno-Risueno MA, Petricka JJ, Benfey PN.. 2015. MYB36 regulates the transition from proliferation to differentiation in the Arabidopsis root. Proceedings of the National Academy of Sciences of the USA 112: 12099–12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ye W, Li B, et al. 2012. CCS52A2/FZR1, a cell cycle regulator, is an essential factor for shoot apical meristem maintenance in Arabidopsis thaliana. BMC Plant Biology 12: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Wang T, Persson S, Mueller-Roeber B, Schippers JHM.. 2014. Transcriptional control of ROS homeostasis by KUODA1 regulates cell expansion during leaf development. Nature Communications 5: 3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar Z, Horváth B, Khan S, et al. 2012. Arabidopsis E2FA stimulates proliferation and endocycle separately through RBR-bound and RBR-free complexes. EMBO Journal 31: 1480–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco K, Bergdoll M, Achard P, Criqui M-C, Genschik P.. 2010. Selective proteolysis sets the tempo of the cell cycle. Current Opinion in Plant Biology 13: 631–639. [DOI] [PubMed] [Google Scholar]
- Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA.. 2014. Sugar demand, not auxin, is the initial regulator of apical dominance. Proceedings of the National Academy of Sciences of the USA 111: 6092–6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masubelele NH, Dewitte W, Menges M, et al. 2005. D-type cyclins activate division in the root apex to promote seed germination in Arabidopsis. Proceedings of the National Academy of Sciences of the USA 102: 15694–15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S, Goswami P.. 2007. A redox cycle within the cell cycle: ring in the old with the new. Oncogene 26: 1101–1109. [DOI] [PubMed] [Google Scholar]
- Millar AH, Considine MJ, Day DA, Whelan J.. 2001. Unraveling the role of mitochondria during oxidative stress in plants. IUBMB Life 51: 201–205. [DOI] [PubMed] [Google Scholar]
- Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A.. 2010. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7: 150–161. [DOI] [PubMed] [Google Scholar]
- Molitor AM, Bu Z, Yu Y, Shen W-H.. 2014. Arabidopsis AL PHD-PRC1 complexes promote seed germination through H3K4me3-to-H3K27me3 chromatin state switch in repression of seed developmental genes. PLOS Genetics 10: e1004091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Bouyer D, Schnittger A, Kermode AR.. 2012. Evolutionarily conserved histone methylation dynamics during seed life-cycle transitions. PLoS One 7: e51532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Née G, Xiang Y, Soppe WJJ.. 2017. The release of dormancy, a wake-up call for seeds to germinate. Current Opinion in Plant Biology 35: 8–14. [DOI] [PubMed] [Google Scholar]
- Nieuwland J, Stamm P, Wen B, Randall RS, Murray JAH, Bassel GW.. 2016. Re-induction of the cell cycle in the Arabidopsis post-embryonic root meristem is ABA-insensitive, GA-dependent and repressed by KRP6. Scientific Reports 6: 23586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JE. 2003. Molecular and physiological mechanisms of bud dormancy regulation. Acta Horticulturae 618: 437–453. [Google Scholar]
- Owens JN, Molder M.. 1973. A study of DNA and mitotic activity in the vegetative apex of Douglas fir during the annual growth cycle. Canadian Journal of Botany 51: 1395–1409. [Google Scholar]
- Pardee AB. 1974. A restriction point for control of normal animal cell proliferation. Proceedings of the National Academy of Sciences of the USA 71: 1286–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyn S, Willems A, de Veylder L.. 2015. Cell cycle entry, maintenance, and exit during plant development. Current Opinion in Plant Biology 23: 1–7. [DOI] [PubMed] [Google Scholar]
- Potters G, Horemans N, Bellone S, et al. 2004. Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiology 134: 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucciariello C, Perata P.. 2013. Quiescence in rice submergence tolerance: an evolutionary hypothesis. Trends in Plant Science 18: 377–381. [DOI] [PubMed] [Google Scholar]
- Reichheld J-P, Khafif M, Riondet C, Droux M, Bonnard G, Meyer Y.. 2007. Inactivation of thioredoxin reductases reveals a complex interplay between thioredoxin and glutathione pathways in Arabidopsis development. Plant Cell 19: 1851–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne PLH, Welling A, Vahala J, et al. 2011. Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-β-glucanases to reopen signal conduits and release dormancy in Populus. Plant Cell 23: 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizkallah R, Alexander KE, Hurt MM.. 2011. Global mitotic phosphorylation of C2H2 zinc finger protein linker peptides. Cell Cycle 10: 3327–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde A, Van Montagu M, Inzé D, Boerjan W.. 1997. Factors regulating the expression of cell cycle genes in individual buds of Populus. Planta 201: 43–52. [Google Scholar]
- Sablowski R. 2016. Coordination of plant cell growth and division: collective control or mutual agreement? Current Opinion in Plant Biology 34: 54–60. [DOI] [PubMed] [Google Scholar]
- Saito T, Bai S, Imai T, Ito A, Nakajima I, Moriguchi T.. 2015. Histone modification and signalling cascade of the dormancy-associated MADS-box gene, PpMADS13-1, in Japanese pear (Pyrus pyrifolia) during endodormancy. Plant, Cell & Environment 38: 1157–1166. [DOI] [PubMed] [Google Scholar]
- Santuari L, Sanchez-Perez G, Luijten M, et al. 2016. The PLETHORA gene regulatory network guides growth and cell differentiation in Arabidopsis roots. Plant Cell 28: 2937–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L, Dewitte W, Forzani C, et al. 2011. The Arabidopsis D-type cyclin CYCD2;1 and the inhibitor ICK2/KRP2 modulate auxin-induced lateral root formation. Plant Cell 23: 641–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar AK, Luijten M, Miyashima S, et al. 2007. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446: 811–814. [DOI] [PubMed] [Google Scholar]
- Sauter M. 1997. Differential expression of a CAK (cdc2-activating kinase)-like protein kinase, cyclins and cdc2 genes from rice during the cell cycle and in response to gibberellin. Plant Journal 11: 181–190. [DOI] [PubMed] [Google Scholar]
- Shimizu-Sato S, Tanaka M, Mori H.. 2009. Auxin–cytokinin interactions in the control of shoot branching. Plant Molecular Biology 69: 429. [DOI] [PubMed] [Google Scholar]
- Stonebloom S, Brunkard JO, Cheung AC, Jiang K, Feldman L, Zambryski P.. 2012. Redox states of plastids and mitochondria differentially regulate intercellular transport via plasmodesmata. Plant Physiology 158: 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kajihara T, Okamura C, et al. 2013. Cytokinins control endocycle onset by promoting the expression of an APC/C activator in Arabidopsis roots. Current Biology 23: 1812–1817. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H, Busch W, Benfey PN.. 2010. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143: 606–616. [DOI] [PubMed] [Google Scholar]
- Vanstraelen M, Baloban M, Da Ines O, et al. 2009. APC/CCCS52A complexes control meristem maintenance in the Arabidopsis root. Proceedings of the National Academy of Sciences of the USA 106: 11806–11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdeil JL, Alemanno L, Niemenak N, Tranbarger TJ.. 2007. Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends in Plant Science 12: 245–252. [DOI] [PubMed] [Google Scholar]
- Verkest A, Manes C-LDO, Vercruysse S, et al. 2005. The cyclin-dependent kinase inhibitor krp2 controls the onset of the endoreduplication cycle during Arabidopsis leaf development through inhibition of mitotic CDKA;1 kinase complexes. Plant Cell 17: 1723–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, et al. 2000. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Veylder L, Beeckman T, Inzé D.. 2007. The ins and outs of the plant cell cycle. Nature Reviews. Molecular Cell Biology 8: 655–665. [DOI] [PubMed] [Google Scholar]
- Vilarrasa-Blasi J, González-García M-P, Frigola D, et al. 2014. Regulation of plant stem cell quiescence by a brassinosteroid signalling module. Developmental Cell 30: 36–47. [DOI] [PubMed] [Google Scholar]
- Wang H, Qi Q, Schorr P, et al. 1998. ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. Plant Journal 15: 501–510. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gu X, Yuan W, Schmitz Robert J, He Y.. 2014. Photoperiodic control of the floral transition through a distinct polycomb repressive complex. Developmental Cell 28: 727–736. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cao H, Sun Y, et al. 2013. Arabidopsis paired amphipathic helix proteins SNL1 and SNL2 redundantly regulate primary seed dormancy via abscisic acid–ethylene antagonism mediated by histone deacetylation. Plant Cell 25: 149–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth WM, Drury GE, Bray CM, West CE.. 2011. Repairing breaks in the plant genome: the importance of keeping it together. New Phytologist 192: 805–822. [DOI] [PubMed] [Google Scholar]
- Webster KA. 2007. Hypoxia: life on the edge. Antioxidants & Redox Signalling 9: 1303–1308. [DOI] [PubMed] [Google Scholar]
- Wen B, Nieuwland J, Murray JAH.. 2013. The Arabidopsis CDK inhibitor ICK3/KRP5 is rate limiting for primary root growth and promotes growth through cell elongation and endoreduplication. Journal of Experimental Botany 64: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildwater M, Campilho A, Perez-Perez JM, et al. 2005. The RETINOBLASTOMA-RELATED gene regulates stem cell maintenance in Arabidopsis roots. Cell 123: 1337–1349. [DOI] [PubMed] [Google Scholar]
- Wyrzykowska J, Schorderet M, Pien S, Gruissem W, Fleming AJ.. 2006. Induction of differentiation in the shoot apical meristem by transient overexpression of a retinoblastoma-related protein. Plant Physiology 141: 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J.. 2013. Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature 496: 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhang J, He J, et al. 2014. ABA-mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in Arabidopsis. PLOS Genetics 10: e1004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanov YS, Ma C, Strauss SH, Busov VB.. 2014. EARLY BUD-BREAK 1 (EBB1) is a regulator of release from seasonal dormancy in poplar trees. Proceedings of the National Academy of Sciences of the USA 111: 10001–10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Tian H, Yue K, et al. 2016. A P-loop NTPase regulates quiescent center cell division and distal stem cell identity through the regulation of ROS homeostasis in Arabidopsis root. PLoS Genetics 12: e1006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Yang S, Liu X, Wu K.. 2015. Arabidopsis histone demethylases LDL1 and LDL2 control primary seed dormancy by regulating DELAY OF GERMINATION 1 and ABA signaling-related genes. Frontiers in Plant Science 6: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]