Abstract
Background.
Advances from glioma stemlike cell (GSC) research, though increasing our knowledge of glioblastoma (GBM) biology, do not influence clinical decisions yet. We explored the translational power of GSC-enriched cultures from patient-derived tumorspheres (TS) in predicting treatment response.
Methods.
The relationship between TS growth and clinical outcome was investigated in 52 GBMs treated with surgical resection followed by radiotherapy and temozolomide (TMZ). The effect on TS of radiation (6 to 60 Gy) and of TMZ (3.9 μM to 1 mM) was related with patients’ survival.
Results.
Generation of TS was an independent factor for poor overall survival (OS) and poor progression-free survival (PFS) (P < .0001 and P = .0010, respectively). Growth rate and clonogenicity of TS predicted poor OS. In general, TS were highly resistant to both radiation and TMZ. Resistance to TMZ was stronger in TS with high clonogenicity and fast growth (P < .02). Shorter PFS was associated with radiation LD50 (lethal dose required to kill 50% of TS cells) >12 Gy of matched TS (P = .0484). A direct relationship was found between sensitivity of TS to TMZ and patients’ survival (P = .0167 and P = .0436 for OS and PFS, respectively). Importantly, values for TMZ half-maximal inhibitory concentration <50 μM, which are in the range of plasma levels achieved in vivo, identified cases with longer OS and PFS (P = .0020 and P = .0016, respectively).
Conclusions.
Analysis of TS holds translational relevance by predicting the response of parent tumors to radiation and, particularly, to TMZ. Dissecting the clonogenic population from proliferating progeny in TS can guide therapeutic strategies to a more effective drug selection and treatment duration.
Keywords: cancer stem cells, glioblastoma, radiotherapy, temozolomide, treatment outcome
The cancer stem cell (CSC) theory for glioblastoma (GBM) postulates that a small population of tumor cells showing stemlike features (glioma stemlike cells, GSCs) may represent the actual tumorigenic core of GBM. These cells, which are functionally defined by their ability to self-renew, to grow as tumorspheres (TS) in culture, and to initiate tumor formation in vivo,1 are believed to be responsible for the perpetuation of the tumor. Conversely, their dividing progeny may only contribute to tumor bulk having lost the clonogenic capacity. This hierarchical model has recently been revisited, suggesting a more dynamic view, where the stemlike phenotype is partly regulated by the environment with possible dedifferentiation of nontumorigenic tumor cells toward CSCs.2 In spite of this, the CSC theory still provides a framework that explains events like therapy resistance and tumor recurrence and that identifies the CSCs as the main target for successful therapeutic intervention.
Though research into the CSC concept provided many important new insights into the biology of GBM, to date the translational impact of these advances has been modest and no data derived from GSC research influence clinical decisions. However, we and others showed that the ability of GBM to grow as floating TS under stem-cell culture conditions is an unfavorable prognostic factor for the donor patient.3–5 Several in vitro and in vivo studies demonstrated that GSCs are highly resistant to most common therapies, including radiation and temozolomide (TMZ).6,7 However, recent studies analyzing either brain gliomas after radiation schedules that enrich the CSC population8 or the percentage of GSCs in patient-matched primary and recurrent GBMs9 suggest that the relationship between tumor cells with stemlike features and patient outcome might be more complex than previously thought. Defining the relationship between resistance of GSCs to radiation and TMZ and clinical outcome is essential to establish the applicability and clinical relevance of GSC analysis as readout in therapeutic intervention for GBM.10 Here, we analyzed a large panel of TS and related the resistance to radiation and TMZ with survival of donor patients, all of whom underwent postoperative radiotherapy and TMZ cycles.11 Our aim is to determine whether TS may represent a valuable tool for predicting the response to current therapies and, ultimately, for developing personalized treatments.
Materials and Methods
Clinical Material
Glioblastoma samples were harvested from 176 consecutive patients who underwent craniotomy at the Institute of Neurosurgery, Catholic University, Rome (Supplementary Table S1, Supplementary Figs S1–S2, and Appendix). In all cases, World Health Organization grade IV GBM was diagnosed.12 Patients were 20 to 80 years old at diagnosis (median, 59 y); 116 were men and 60 were women. Immunohistochemical patterns, epidermal growth factor receptor variant III (EGFRvIII) expression, O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation, and isocitrate dehydrogenase 1 or 2 (IDH1/IDH2) mutation of tumors were assessed (Appendix).3,9,13,14 Overall survival (OS) was calculated from the date of surgery when a diagnosis of GBM was established until death. Progression-free survival (PFS) was determined from the date of surgery until progression or death. Median follow-up was 15 months.
Patient Treatment
After surgery, the patients received radiotherapy and concomitant TMZ followed by 6 cycles of adjuvant TMZ according to the Stupp protocol (Appendix).11,15 All the recurrent cases (n = 9) had received radiotherapy and TMZ following primary surgery.
Cell Culturing
Tumorsphere cultures were established from tumor specimens through mechanical dissociation and culturing in Dulbecco’s modified Eagle’s medium/F12 serum-free medium containing 2 mM glutamine, 0.6% glucose, 9.6 g/mL putrescine, 6.3 ng/mL progesterone, 5.2 ng/mL sodium selenite, 0.025 mg/mL insulin, and 0.1 mg/mL transferrin sodium salt (Sigma-Aldrich), supplemented with epidermal growth factor and basic fibroblast growth factor.3,16 Under these conditions, tumor cells grow as spheroid clusters expressing stem-cell markers, such as cluster of differentiation (CD)133, sex determining region Y-box 2 (Sox2), Musashi, and nestin. CD133 expression of TS was detected by anti-CD133–phycoerythrin (AC133-PE) antibody or PE-conjugated mouse immunoglobulin (Ig)G1 isotype control antibody (Miltenyi Biotec). The expression of Sox2 was analyzed by PerCP-Cy 5.5 mouse anti-Sox2 or PerCP-Cy 5.5 mouse IgG1 isotype control (Becton Dickinson [BD]). Viable cells were identified using 7-amino actinomycin D (Sigma-Aldrich). Cells were analyzed with a FACSCanto flow cytometer (BD). The MGMT promoter methylation of TS was assessed after DNA extraction from cell pellets with the Wizard genomic DNA purification kit (Promega).14
Apart from sphere generation in vitro, the stemlike phenotype of TS cells was assessed by self-renewal capacity, coexpression of astrocytic and neuronal markers, and generation of xenografts histologically mimicking the parent tumor (Appendix).1,3 Subtype classification of TS was performed with the RT2 Profiler PCR Arrays (Qiagen) (Supplementary Table S2; Appendix).17 Human neural progenitor cells (HNPCs) from developing human brain were purchased from Lonza. Adult neural progenitor cells from human olfactory bulb (NS5) were isolated, as previously described.18 U87MG and T98G cell lines were purchased from American Type Culture Collection.19 Tumorsphere, U87MG, and T98G cells were validated by short tandem repeat DNA fingerprinting (Cell ID System, Promega). Profiles were compared against publicly available databases to confirm authenticity (Biological Bank and Cell Factory, IST). To assess clonogenicity, viable cells were dispensed at different densities (1-3-10 cells/well) in 96-well plates by fluorescence activated cell sorting (FACS Aria, BD). After 10–14 days, wells with growing clones were enumerated, and results were analyzed by extreme limiting dilution assay (ELDA) software.20 Cells were used within 6 months after receipt or resuscitation.
Exposure of TS to Radiation and TMZ and Assessment of Cytotoxicity
Tumorsphere cells were plated at a density of 2.5 × 104 cells/mL, in triplicate, in 96-well plates. For evaluation of radiation, TS were exposed either to single doses of acute cesium-137 (137Cs) gamma irradiation (from 10 to 60 Gy) or to 6 Gy fractioned radiation (6-18-42-60 Gy). Dose rate was 0.8 Gy/min. Temozolomide was purchased from Sigma-Aldrich and dissolved in dimethyl sulfoxide (DMSO). Twenty-four, 48, 72, 96 hours, and 7 days after radiation or TMZ exposure, ATP levels were measured as a surrogate of cell viability using CellTiter-Glo (Promega). Mean of raw luminescence values (LD) from triplicate wells treated with vehicle (DMSO 0.2%; mLC) was used as reference to interpolate percent viability from wells treated with drugs (VD) according to the formula: VD = (LD/mLC) × 100.
Tumorspheres were simultaneously exposed to TMZ (3.9 μM–1 mM) and to single doses of 137Cs gamma irradiation (10–60 Gy). Seventy-two hours and 7 days after TMZ and radiation, cell viability was assessed.
Gene Expression Profiling of TS
Using the Affymetrix platform, the expression of 357 genes involved in radio- and chemoresistance was assessed in TS (Appendix).21
Western Blot
Tumorspheres were immunoblotted for checkpoint proteins ATM, ATR, Rad17, Chk1 and Chk2, and for the MGMT protein (Appendix).
Statistical Analysis
Survival curves were plotted using the Kaplan–Meier method and analyzed using the log-rank test. Cox analysis was used for determination of hazard ratios (HRs) and 95% confidence intervals. Multivariate analysis for survival was performed using the Cox proportional hazards model. Comparison of continuous variables between groups was performed using Student’s t-test. Comparison of categorical variables between groups was performed by the chi-square statistic using Fisher’s exact test when appropriate. Correlation between continuous variables was evaluated using regression analysis and the Spearman correlation test. All P-values are based on 2-tailed tests and differences were considered significant when P < .05. StatView v5.0 was used (SAS Institute).
Ethics Statement
All patients provided written informed consent allowing for clinical data collection and analysis and for tissue collection, culture, and processing, which was approved by the institutional ethics committee of the Università Cattolica del Sacro Cuore, Rome. The study was conducted in adherence to the principles outlined in the World Medical Association Declaration of Helsinki: Research Involving Human Subjects. Experiments involving animals were approved by the ethics committee of the Università Cattolica del Sacro Cuore, Rome.
Results
Generation of TS and Relationship with Clinical and Pathological Variables
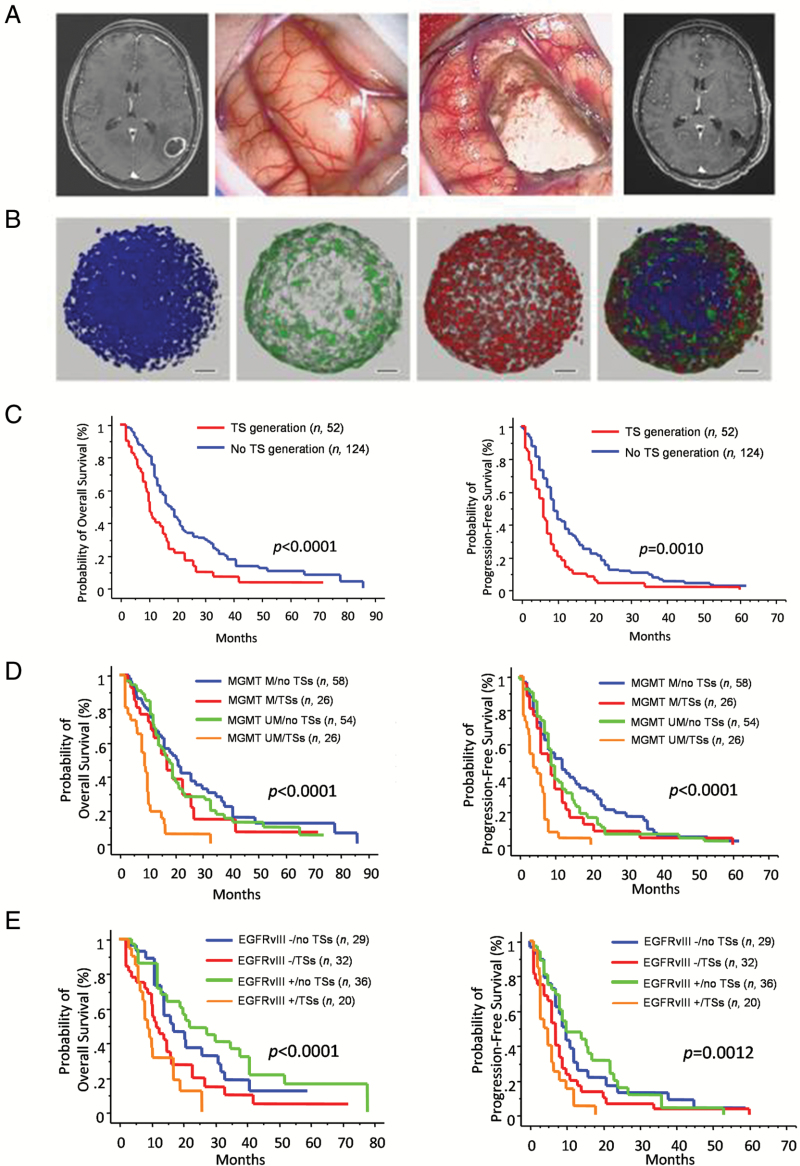
Of the 176 GBM tumors, 52 of them (29.5%) generated TS (Table 1; Appendix). The time required for establishing TS varied between 2 and 33 weeks (12.8 ± 7.7 wk, mean ± SD). In 15 patients, multiple specimens (n = 34) from the same tumor were obtained; 4 of such cases generated TS from each specimen, whereas in the remaining 11 cases, TS could not have arisen from any specimen. These findings confirm our previous observations that TS generation, more than being influenced by heterogeneity of GBM, can be regarded as an intrinsic tumor feature, possibly related to low requirements for survival and expansion of the tumorigenic cell fraction.3 Expression of the stem-cell markers CD133 and Sox2 was 26.7 ± 4.8% (mean ± SEM; range 0.2%–95.8%) and 65.9 ± 4.6% (range 9.3%–95.2%) of TS cells, respectively. There were no significant differences between TS generating tumors and those tumors that did not generate TS relative to patient age and sex, symptom duration, and tumor location (Table 1). Conversely, median OS and PFS were significantly shorter in tumors that generated TS compared with those tumors that did not (P < .0001 and P = .0010, respectively; log-rank test) (Table 1 and Supplementary Table S3). Among the pathological variables, we found a nonsignificant trend for tumors without EGFRvIII expression to generate TS (P = .0936, Fisher’s exact test; Table 1). This finding is consistent with our previous results suggesting that, from a hierarchic point of view, the EGFR mutation takes place at the stage of late progenitors concurrent with increase of proliferation.9 Generation of TS was not related with Ki67 and MGMT promoter methylation by parent tumors. IDH1/2 was mutated in 10/11 (90.9%) and in 2/18 (11.1%) secondary and primary GBMs, respectively. The single case of secondary GBM that generated TS showed mutated IDH1/2.
Table 1.
Generation of TS from 176 glioblastoma tumors
| Estimate | TS Generation | P | |
|---|---|---|---|
| Yes | No | ||
| N | 52 (29.5%) | 124 (70.5%) | |
| Age, y | |||
| Median | 59.5 | 58.5 | .1259* |
| Mean ± SD | 60.5 ± 10.7 | 57.3 ± 13.6 | |
| Sex | |||
| Male | 38 (73.1%) | 78 (62.9%) | .2259# |
| Female | 14 (26.9%) | 46 (37.1%) | |
| Symptom duration (mo) | 5.1 ± 7.9 | 7.4 ± 15.8 | .3194* |
| Tumor location | |||
| Frontal | 14 (26.9%) | 42 (33.9%) | .3968ç |
| Temporal | 15 (28.8%) | 43 (34.7%) | |
| Parietal | 15 (28.8%) | 26 (21.0%) | |
| Occipital | 6 (11.5%) | 7 (5.6%) | |
| Other | 1 (1.9%) | 3 (2.4%) | |
| Multicentric | 1 (1.9%) | 3 (2.4%) | |
| De novo vs secondary GBM | |||
| De novo | 51 (98.1%) | 114 (91.9%) | .1781# |
| Secondary | 1 (1.9%) | 10 (8.1%) | |
| Primary surgery vs surgery for recurrence | |||
| Primary surgery | 43 (82.7%) | 97 (78.2%) | .5465# |
| Surgery for recurrence | 9 (17.3%) | 27 (21.8%) | |
| Tumor resection | |||
| Total | 39 (75%) | 94 (75.8%) | .9999# |
| Partial | 13 (25%) | 30 (24.2%) | |
| Patient survival (median, mo) | |||
| Overall | 10.5 | 18 | <.0001$ |
| Disease-free | 5 | 8 | .0010$ |
| Ki67, % | 28.5 ± 14.0 | 29.5 ± 15.5 | .7149* |
| MGMT | |||
| Methylated | 26 (50%) | 58 (51.8%) | .8678# |
| Unmethylated | 26 (50%) | 54 (48.2%) | |
| EGFRvIII | |||
| Positive | 20 (38.5%) | 36 (55.4%) | .0936# |
| Negative | 32 (61.5%) | 29 (44.6%) | |
| p53 | |||
| Wt | 40 (76.9%) | 85 (68.5%) | .2818# |
| Mutant | 12 (23.1%) | 39 (31.5%) | |
| Phosphatase and tensin homolog | |||
| Normal | 25 (58.1%) | 39 (59.1%) | .9999# |
| Hypoexpressed | 18 (41.9%) | 27 (40.1%) | |
Student’s t-test;
Fisher’s exact test;
chi-square test;
Kaplan–Meier analysis (log-rank test).
Other than in the whole cohort (n = 176; Fig. 1), generation of TS did hold high prognostic power for poor patient survival also after excluding either recurrent tumors (n = 36) or recurrent tumors plus cases that did not complete the Stupp protocol (n = 78; for OS, P < .0001 and P = .0008, respectively; for PFS, P = .0002 and P = .0108, respectively; log-rank test) (Supplementary Table S3). Significantly poorer survival distinguished the TS generating tumors with either unmethylated MGMT promoter (OS = 9 mo, P < .0001, and PFS = 4 mo, P < .0001; log-rank test) or EGFRvIII expression (OS = 9 mo, P < .0001, and PFS = 3 mo, P = .0012; log-rank test) (Fig. 1 and Supplementary Table S3).
Fig. 1.
(A) Preoperative MR image showing a left temporal tumor (left panel). Intraoperative views before (center left panel) and after (center right panel) tumor removal. Postoperative (48 h) MR image (right panel). (B) Confocal immunofluorescence of TS expressing both stemness-related genes (Sox2, red) and differentiation markers (glial fibrillary acidic protein [GFAP], green). Nuclei stained with 4′,6′-diamidino-2-phenylindole (DAPI) (blue). Scale bars, 50 μm. (C–E) Kaplan–Meier graphs for OS and PFS of GBM patients according to TS generation (n = 176; C); TS generation combined with MGMT promoter methylation (MGMT methylated [M] vs MGMT unmethylated [UM]; D); TS generation combined with EGFRvIII expression (EGFRvIII+ vs EGFRvIII−; E).
In a multivariate analysis that included TS generation, age, postoperative KPS, extent of tumor resection, completion of the Stupp protocol, Ki67, MGMT promoter methylation, and expression of EGFRvIII, TS generation and completion of Stupp protocol emerged as significant independent prognosticators for OS (both P < .0001, Cox proportional hazards test), whereas postoperative KPS trended toward statistical significance (P = .0742, Cox proportional hazards test) (Supplementary Table S4A). Generation of TS, completion of the Stupp protocol, and postoperative KPS were significant independent prognosticators for PFS (P = .0019, P = .0005, and P = .0424, respectively; Cox proportional hazards test) (Supplementary Table S4B). Given that generation of TS selects GBM patients with very poor prognosis, in whom the Stupp regimen may be stopped due to rapid progression, we performed multivariate analysis for survival including only patients who completed the Stupp protocol (n = 132). Results showed that TS generation holds significance as an independent prognosticator of poor survival (P = .0035 and P = .0267 for OS and PFS, respectively; Cox analysis; Supplementary Table S5).
Clonogenicity of TS and Relationship with Patient Survival
In a previous study, we established a correlation between the time of GSC expansion, which was defined as the time required for primary cultures to generate 3 × 106 GSCs, and survival of donor patients.3 Here, we assessed the clonogenic potential of TS cells and its relationship with patients’ outcome. To this aim, we used the ELDA software, which provides an estimate of the frequency of clonogenic cells in a mixed population of clonogenic and nonclonogenic cells. Results are expressed as the reciprocal of clonogenic cell frequency (clonogenicity index, CI); therefore, higher CI values correspond to lower clonogenic potential. Using this method, we found quite variable CIs among TS, ranging from 1.5 to 138. We were unable to establish a direct relationship between CI of TS and patients’ survival. However, TS with CI <8.5 distinguished GBMs with poorer OS (9.7 ± 1.59 mo vs 15.03 ± 1.62 mo, P = .0242; unpaired Student’s t-test). The CI value of 8.5 was arbitrarily defined postulating that following a cell division by one self-renewing clonogenic cell, the nonclonogenic daughter cells proliferate faster, undergoing 3 cell divisions (Supplementary Fig. S3A). In this model, CI = 8.5 corresponds to a frequency of self-renewing cells equal to 11.8% of the TS cell population (2/17 × 100).
Expansion time did hold unfavorable weight for prognosis with a cutoff value of 14 weeks (P = .0143 and P = .0178 for OS and PFS, respectively; Fig. 2A and Supplementary Table S3). This cutoff value was calculated by assuming that 3 × 103 GBM cells, which were initially seeded in our cultures, proliferated with a median culture cell cycle time of 9.8 days,22 thus reaching the number of 3 million over 98 days (14 wk) after having done 10 cell replications. Combined analysis of clonogenicity and expansion time showed that TS with CI 1.5–8.5 and expansion time <14 weeks arose from tumors with significantly shorter OS (P = .0014; unpaired Student’s t-test) (Fig. 2B). Among TS with CI >8.5, expansion time <14 weeks predicted worse OS (Fig. 2B). In TS, the fraction of proliferating cells was about 3-fold higher than the clonogenic one (Fig. 2C). We did not find any relationship between CD133 and Sox2 expression by TS and either patient survival or clonogenicity (Supplementary Fig. S3B–D). There was a trend for the degree of expression of the 2 stemness markers to be related to each other (P = .0745; Spearman correlation test).
Fig. 2.
(A) Kaplan–Meier graphs for OS and PFS of GBM patients according to expansion time of TS (<14 wk vs ≥14 wk). (B) Graph showing OS according to expansion time (<14 wk vs ≥14 wk) and clonogenic index (1.5–8.5 vs >8.5) (** P < .01). (C) Ki67 staining of TS (left panel) in whole mount preparation (upper) and paraffin section (lower). Note the scarcely proliferating core of TS. Bar graph showing average clonogenic and proliferating cells in TS (right panel).
Based on the Phillips gene set (Supplementary Table S2),17 we were able to categorize our TS (n = 45) as proneural = 14 (31.1%), proliferative = 12 (26.7%), and mesenchymal = 19 (42.2%). We did not recognize morphological features that may identify any specific TS subtype (Supplementary Fig. S4A). However, comparing survivals of patients whose tumors generated TS of the 3 subtypes, the proneural one was associated with significantly better outcome (P < .0001 for both OS and PFS; log-rank test; Supplementary Fig. S4B). This result is consistent with a recent study by our group showing that GS-full (GSf) TS, which are reminiscent of the proneural-like subtype, arise from donor patients with slower clinical evolution, better performance status, and longer median OS than tumors generating GS-restricted (GSr) TS, which are reminiscent of the mesenchymal-like subtype.21
Response of TS to Radiation and Relationship with Outcome
To assess radiation effects, TS were exposed to increasing doses of acute irradiation ranging from 10 to 60 Gy. Results are presented as the Gy radiation dose necessary to kill 10% (lethal dose; LD10), 20% (LD20), and 50% (LD50) of TS cells (Fig. 3A and Supplementary Fig. S5). Overall, TS were highly resistant to single dose radiation. In 30/56 TS (43.6%), LD50 was higher than 60 Gy, corresponding to the cumulative dose administered to the patient brain during whole fractionated radiotherapy. Only 10/56 TS (17.9%) were more sensitive to radiation than U87MG and T98G GBM cells (Fig. 3A). Interestingly, most TS showed LD50 values far superior to those of human neural stem cells (HNPC and NS5), which ranged between 10 and 12 Gy. Tumorspheres that were established from different regions of the same tumor showed quite similar LD50 values (Supplementary Fig. S6A).
Fig. 3.
(A) Bar graph showing the effect of radiation on 47 TS lines, including 6 lines from 3 patients, at 1 week after exposure to 60 Gy. Gray bars represent radiation doses required to kill 50% of TS cells (LD50). Red bars, LD50 of U87MG and T98G cells; green bars, LD50 of human neural stem cells. Each bar corresponds to the mean of triplicate experiments. Error bars, SD. (B) Bar graph comparing the effect of single-dose and fractionated radiation on a subset of 23 TS. Each bar corresponds to the mean of triplicate experiments. Inset, relationship between LD50 for single-dose and fractionated radiation (R2 = 0.9464; P < .0001). Error bars, SD. (C) Activation state of checkpoint proteins (ATM, ATR, Rad17, Chk1 and Chk2) in sensitive (148, 76, and 83.2) and resistant (67, 70, and 62) TS assessed before (−) and 1 h after (+) 3 Gy radiation. (D, E) Kaplan–Meier graphs for OS (D) and PFS (E) of GBMs according to LD50 of TS (<12 Gy vs ≥12 Gy).
To assess sensitivity to radiation under conditions closer to clinical ones, a subset of TS (n = 23) received radiations at 6 Gy fractions (6-18-42-60 Gy). Fractionation did not substantially change TS response to radiation (Fig. 3B). In 8/23 TS, neither single nor fractionated radiation gave LD50 effect at the maximum administered dose (60 Gy). In 12/23 TS, LD50 was achieved at doses that differed less than 10 Gy between single and fractionated radiation. Only in 3/23 TS was fractionation more efficient than single dose radiation by about 10 Gy.
The molecular mechanisms involved in resistance of TS to radiation were investigated by analyzing early DNA damage checkpoint responses in 3 sensitive and 3 resistant TS lines (Fig. 3C). Tumorspheres were exposed to 3 Gy radiation and the activation state of checkpoint responses was assessed before and 1 h after treatment. Cell lysates were immunoblotted for phosphorylated and total amounts of checkpoint proteins (ATM, ATR, Rad17, Chk1 and Chk2). Consistent with previous observations,6 we found that activating phosphorylation of ATM, Rad17, and to a lesser extent Chk2 was higher in resistant than in sensitive TS, indicating that the former show greater checkpoint activation response to DNA damage (Fig. 3C). Lack of functional wild-type (wt) p53 has been associated with resistance to fractionated irradiation due to a deficit in the G1-checkpoint.23 Then, we assessed the p53 status of TS by immunocytochemistry (Supplementary Fig. S6B) and found that of the 9 TS where fractionation was more efficient than single dose radiation, 7 had wt p53, whereas both TS where fractionation was less efficient than single dose radiation harbored mutant p53.
There was no direct relationship between sensitivity to radiation of TS and patients’ survival. However, by applying the cutoff value of 12 Gy, which corresponds to LD50 of human NS5 cells, we found a significant relationship whereby TS with LD50 >12 Gy arose from tumors with lower PFS (P = .0484, log-rank test; Fig. 3D, E and Supplementary Table S3).
Response of TS to TMZ and Relationship with Outcome
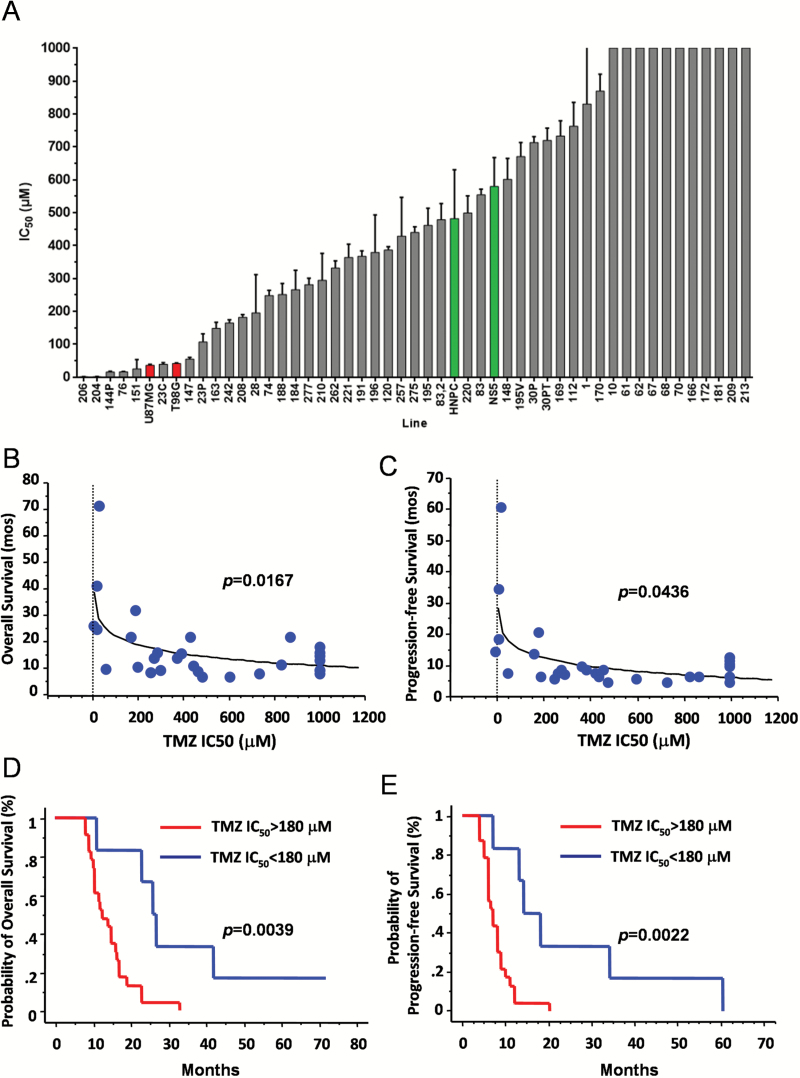
Sensitivity to TMZ was assessed by exposing TS to increasing concentrations of TMZ (3.9 μM–1 mM). Results are presented as half maximal inhibitory concentration (IC50). As for radiation or even more, TS were highly resistant to TMZ (Fig. 4A). Only 6/52 TS (11.5%) were as sensitive as or more sensitive to TMZ than U87MG and T98G cells. Tumorspheres established from the same tumor showed quite similar IC50 values (Supplementary Fig. S6C). Note that 16/52 TS (30.8%) showed TMZ IC50 >1000 μM (ie, more than 2-fold higher than IC50 of HNPC and NS5 cells). A direct relationship was found between response of TS to TMZ and patients’ survival (P = .0167 and P = .0436 for OS and PFS, respectively; Spearman correlation test; Fig. 4B, C). We then established a cutoff value for in vitro TMZ IC50 equal to 180 μM. Though this value is about 3 times higher than the plasma levels achieved in vivo,24 it corresponds to the minimally cytotoxic dose for in vitro radiosensitization of human GBM.25 By applying this cutoff, we recognized a group of TMZ-resistant TS (IC50 >180 μM) that arose from patients with shorter OS and PFS (P = .0039 and P = .0022, respectively; log-rank test; HR, 4.464; 95% confidence interval, 1.46–13.699, and HR, 4.878; 95% confidence interval, 1.567–15.152, respectively; Cox analysis) (Fig. 4D, E and Supplementary Table S3). After applying the 50-μM cutoff for TMZ IC50, which is in the range of plasma levels,24 we identified 6 cases (IC50 <50 μM) with longer OS and PFS (P = .0020 and P = .0016, respectively; log-rank test) (Supplementary Table S3).
Fig. 4.
(A) Bar graph showing the effect of TMZ at 1 week after exposure to the drug on 47 TS lines, including 8 lines established from 4 patients. Gray bars represent TMZ doses required to kill 50% of TS cells (IC50). Red bars, TMZ IC50 of U87MG and T98G cells; green bars, TMZ IC50 of human neural stem cells. Each bar corresponds to the mean of 3 different experiments. Error bars, SD. (B, C) X-Y graphs plotting the IC50 values to TMZ of TS with the OS (B) and PFS (C) of donor patients. (D, E) Kaplan–Meier graphs for OS (D) and PFS (E) of GBM patients according to TMZ IC50 of matched TS (<180 μM vs ≥180 μM). Analyses in panels B–E were conducted on TS-generating GBMs that completed both concomitant radio-chemotherapy and adjuvant chemotherapy according to Stupp protocol.
Several mechanisms contribute to resistance of GSCs against TMZ.26 Here, the MGMT methylation status of TS was MGMT methylated = 38/44 (86.4%) and MGMT unmethylated = 6/44 (13.6%) with a 47.7% (21/44) concordance rate between parental tumors and matched TS (Table 1). We then analyzed MGMT protein expression in 22 TS from 20 patients and related MGMT protein levels with TMZ IC50 (Supplementary Figs S7A–B). Tumorspheres with IC50 >180 μM had MGMT protein levels significantly higher than TS with IC50 <180 μM (P = .0112; unpaired t-test; Supplementary Fig. S7B). Unmethylated TS associated with poor patient survival (P = .0269, log-rank test; Supplementary Fig. S7C and Supplementary Table S3). Also, highly TMZ-resistant TS derived more frequently from recurrent GBMs than from initial ones. In fact, IC50 >1 mM was found in 6/9 (66.7%) and in 10/43 (23.3%) TS derived from recurrent and initial GBMs, respectively (P = .0176; Fisher’s exact test).
In general, the in vitro sensitivity of GSC-enriched patient-derived TS to TMZ was recapitulated in vivo (Supplementary Fig. S7D). Xenografts established from TS with in vitro IC50 <180 μM (TS# 23C and 163) either did not grow or grew only by 13% of their initial size during TMZ treatment and over one week after the last TMZ dose. Conversely, xenografts from TS with in vitro IC50 >180 μM (TS# 1, 61, and 83) continued to grow during TMZ treatment by 27%–52% of their initial size.
Interestingly, a direct relationship was found between resistance to radiation and that to TMZ (P = .0029; Spearman correlation test), indicating that highly radioresistant TS are likely to be also highly resistant to TMZ. The highest degrees of radio-chemoresistance were found in highly clonogenic and fast expanding TS (Fig. 5A). Plotting both radiation LD50 and TMZ IC50 against OS in an X-Y-Z diagram, it clearly appeared that TS with lower IC50 and LD50 were associated with longer OS (Fig. 5B). Gene expression profiling showed that the highest modulated genes between treatment (radiation and TMZ)-sensitive and treatment-resistant TS were those involved in hypoxia and DNA damage repair (Supplementary Fig. S8).
Fig. 5.
(A) Graph showing radiation LD50 and TMZ IC50 according to expansion time (<14 wk vs ≥14 wk) and clonogenic index (1.5–8.5 vs ≥8.5) of TS. (**P < .02). (B) X-Y-Z graph plotting radiation LD50 and TMZ IC50 of TS with OS of matched patients.
Response of TS to Serum-Induced Differentiation and to Combined Radiation and TMZ
To assess whether cell differentiation may affect radio- and chemosensitivity, TS with various degrees of radio- and chemosensitivity, namely the TS# 1, 10, 23C, 61, 76, 83, 163, and 213, were grown under serum conditions and then exposed either to increasing doses of single radiation or to increasing concentration of TMZ (Appendix). Culturing under serum conditions resulted in increased sensitivity to radiation only in TS# 83, whereas in the remaining 7 lines there were no substantial changes in radiosensitivity (Supplementary Fig. S9A). Concerning response to TMZ, the chemosensitive TS# 76, 23C, and 163 acquired resistance to TMZ after serum culturing. Conversely, chemoresistant TS did not show substantial changes of TMZ response by serum culturing (Supplementary Fig. S9B).
To investigate the response to combined treatment with TMZ and radiation, TS# 61 (LD50 >60 Gy and IC50 >1 mM), TS# 83 (LD50 <18 Gy and IC50 >180 μM), and TS# 23C (LD50 >60 Gy and IC50 <60 μM) were simultaneously exposed to increasing concentrations of TMZ (3.9 μM–1 mM) and to single acute radiation (10–60 Gy). Results showed that in the chemoresistant TS# 61 and 83, exposure to 10–20 Gy radiation increased sensitivity at lower doses of TMZ (Supplementary Fig. S10).
Discussion
This study reinforces previous evidence of the highly unfavorable prognostic value for TS generation in GBM patients, a result that after our first report has largely been confirmed by others.3–5 More specifically, TS generation predicts poor prognosis independently from age, postoperative KPS, and completion of postoperative radio-chemotherapy, all of which are significant prognosticators in our series.
The main question here addressed is whether TS may represent a valuable tool for obtaining data to be translated onto clinical settings. Two factors may limit the translational power of TS. The first is that, using our techniques, we were able to obtain TS from approximately one third of GBMs only, which implies that this paradigm can not be applied in a substantial fraction of patients. Although specific culturing, like hypoxic environment or cell debris removal, may enhance efficiency,3,27,28 establishment of TS from GBM has traditionally been difficult, yielding a sustainable culture beyond early passages in 50% of processed GBMs at best.29
The second issue that may hinder clinical relevance of TS analysis relates to cell heterogeneity, a concept that applies both to parent tumor and to tumor-derived TS. Glioblastoma is the archetype of heterogeneous cancer. A recent study that used single-cell RNA sequencing of primary GBMs revealed unthinkable diversity within each tumor.30 Then, intratumoral heterogeneity raises the hypothesis that diverse cell populations may generate distinct TS, each with its own oncogenic pathways. However, transcriptional diversity was lower in tumor-derived stemlike cells than in parental GBM cells,30 suggesting that changes of oncogenic signals are likely to occur in more differentiated progenitors. Furthermore, the 4 cases in which we established TS from different regions of the same tumor showed quite similar features in terms of resistance to radiation and TMZ, suggesting that at least these features are largely shared by most TS cells. Heterogeneity has also been demonstrated within individual GSC cultures, where mixed populations of CSCs and committed progenitors coexist, both of which are capable of forming TS.7,31
One major result of this study is that the sensitivity of TS to radiation and, particularly, to TMZ is linked with patients’ survival. More specifically, TS with radiation LD50 >12 Gy and TMZ IC50 >180 μM arose from tumors that clinically responded very poorly to the Stupp protocol. Importantly, values for TMZ IC50 <50 μM, which are in the range of plasma levels achieved in patients, identified responder cases. Most TS were found to be highly resistant to both radiation and TMZ and this involved mechanisms of early DNA damage checkpoint responses and MGMT expression. Increased resistance to TMZ of TS after serum culturing can be ascribed to the reduction of proliferation due to differentiation that gradually shifts terminally differentiated cells to a post-mitotic state.32 However, the serum-induced resistance to TMZ may also be related to adhesion and/or to pro-survival factors contained in serum.
Notably, the majority of TS showed radiation LD50 and TMZ IC50 far superior to those of human neural stem and precursor cells. On a translational basis, this would mean that killing GSCs by radiation and/or TMZ might imply destroying the rare brain cells endowed with some regenerative potentiality.
Two additional results reinforce the translational value of this study. The first one is that neural stem and precursor cells used as normal controls showed LD50 of 10–12 Gy after single shot radiation, a value that nicely approaches the maximum tolerated dose for adult brain and optic pathways on unfractionated radiosurgery.33 The second result is that in TS treated with combined radiation and TMZ, which mimics the clinical treatment regimen, low dose radiation (10–20 Gy) increased sensitivity to TMZ at low concentrations, a result that validates the efficacy of concomitant TMZ in the Stupp protocol.11
Our data on clonogenicity and expansion/proliferation of TS reveal a complex picture, where the canonical distinction between high clonogenic/slowly proliferating GSCs and low clonogenic/highly proliferating progenitors is not clearly recognizable. In spite of this, high clonogenicity and fast growth of TS are associated with significantly shorter OS and higher resistance to TMZ. Further efforts are necessary to define the role of proliferating nonclonogenic GSCs. A recent paper argues that, whereas clonogenic CSCs are necessary for tumor maintenance and progression, growth kinetics are best described by the non-stem compartment.34 A corollary of this result is that eradicating clonogenic GSCs may require treatments lasting longer than predicted patients’ survival. Current protocols for GBM patients are largely based on short-term clinical/radiological responses, where no response nearly always results in trial interruption. However, drugs causing short-term radiological decrease or even tumor remission may not affect GSCs at all. Conversely, therapies that do not induce radiological response may be able to eradicate clonogenic GBM cells in the long term. Tumorsphere analysis may help address these issues. Dissecting the clonogenic population from cell progenies and determining posttreatment changes in clonogenicity and proliferation rate of TS may guide therapeutic strategies in terms of both more appropriate drug selection and treatment duration.
In addition, these findings could reinforce the concept of a combined approach for future GBM therapy, whereas targeting the proliferating cell progenies for an initial extension of the lifespan could be associated with GSC targeting, for a more sustained effect.35
Supplementary Material
Supplementary data are available at Neuro-Oncology online.
Funding
This work was supported by the Ministero della Salute (N.ONC_ORD 15/07 to R.P.); Università Cattolica del Sacro Cuore (Fondi d’Ateneo Linea D1 to R.P. and L.M.L.); Associazione Italiana per la Ricerca sul Cancro (IG 2013 N.14574 to R.P., Start-up 6326 to L.R.V., IG 2014 N.15584 to L.R.V.).
Conflict of interest statement.
None disclosed.
Supplementary Material
References
- 1. Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 2. Vermeulen L, de Sousa e Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: clinical challenges and opportunities. Lancet Oncol. 2012;13(2):e83–e89. [DOI] [PubMed] [Google Scholar]
- 3. Pallini R, Ricci-Vitiani L, Banna GL, et al. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14(24):8205–8212. [DOI] [PubMed] [Google Scholar]
- 4. Laks DR, Masterman-Smith M, Visnyei K, et al. Neurosphere formation is an independent predictor of clinical outcome in malignant glioma. Stem Cells. 2009;27(4):980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kong BH, Moon JH, Huh YM, et al. Prognostic value of glioma cancer stem cell isolation in survival of primary glioblastoma patients. Stem Cells Int. 2014;2014:838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. [DOI] [PubMed] [Google Scholar]
- 7. Chen J, Li Y, Yu TS, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leder K, Pitter K, Laplant Q, et al. Mathematical modeling of PDGF-driven glioblastoma reveals optimized radiation dosing schedules. Cell. 2014;156(3):603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montano N, Cenci T, Martini M, et al. Expression of EGFRvIII in glioblastoma: prognostic significance revisited. Neoplasia. 2011;13(12):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia. 2015;17(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 12. Kleihues P, Burger PC, Aldape KD, et al. Glioblastoma. In: Louis DN, Ohgaki H, Cavenee WK, eds. WHO classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer; 2007:33–49. [Google Scholar]
- 13. Martini M, Pallini R, Luongo G, Cenci T, Lucantoni C, Larocca LM. Prognostic relevance of SOCS3 hypermethylation in patients with glioblastoma multiforme. Int J Cancer. 2008;123(12):2955–2960. [DOI] [PubMed] [Google Scholar]
- 14. Martini M, Cenci T, D’Alessandris GQ, et al. Epigenetic silencing of Id4 identifies a glioblastoma subgroup with a better prognosis as a consequence of an inhibition of angiogenesis. Cancer. 2013;119(5):1004–1012. [DOI] [PubMed] [Google Scholar]
- 15. Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 16. Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468(7325):824–828. [DOI] [PubMed] [Google Scholar]
- 17. Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. [DOI] [PubMed] [Google Scholar]
- 18. Casalbore P, Budoni M, Ricci-Vitiani L, et al. Tumorigenic potential of olfactory bulb-derived human adult neural stem cells associates with activation of TERT and NOTCH1. PLoS One. 2009;4(2):e4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. American Type Culture Collection. ATCC; c2014. http://www.atcc.org Accessed April 15, 2015
- 20. Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347(1-2):70–78. [DOI] [PubMed] [Google Scholar]
- 21. Marziali G, Signore M, Buccarelli M, et al. Metabolic/proteomic signature defines two glioblastoma subtypes with different clinical outcome. Sci Rep. 2016;6:21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furneaux CE, Marshall ES, Yeoh K, et al. Cell cycle times of short-term cultures of brain cancers as predictors of survival. Br J Cancer. 2008;99(10):1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haas-Kogan DA, Kogan SS, Yount G, et al. p53 function influences the effect of fractionated radiotherapy on glioblastoma tumors. Int J Radiat Oncol Biol Phys. 1999;43(2):399–403. [DOI] [PubMed] [Google Scholar]
- 24. Hammond LA, Eckardt JR, Baker SD, et al. Phase I and pharmacokinetic study of temozolomide on a daily-for-5-days schedule in patients with advanced solid malignancies. J Clin Oncol. 1999;17(8):2604–2613. [DOI] [PubMed] [Google Scholar]
- 25. Bobola MS, Kolstoe DD, Blank A, Silber JR. Minimally cytotoxic doses of temozolomide produce radiosensitization in human glioblastoma cells regardless of MGMT expression. Mol Cancer Ther. 2010;9(5):1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Messaoudi K, Clavreul A, Lagarce F. Toward an effective strategy in glioblastoma treatment. Part I: resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov Today. 2015;20(7):899–905. [DOI] [PubMed] [Google Scholar]
- 27. Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20(19):7370–7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gürsel DB, Beyene RT, Hofstetter C, et al. Optimization of glioblastoma multiforme stem cell isolation, transfection, and transduction. J Neurooncol. 2011;104(2):509–522. [DOI] [PubMed] [Google Scholar]
- 29. Günther HS, Schmidt NO, Phillips HS, et al. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2008;27(20):2897–2909. [DOI] [PubMed] [Google Scholar]
- 30. Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bez A, Corsini E, Curti D, et al. Neurosphere and neurosphere-forming cells: morphological and ultrastructural characterization. Brain Res. 2003;993(1–2):18–29. [DOI] [PubMed] [Google Scholar]
- 32. Villalva C, Cortes U, Wager M, et al. O6-methylguanine-methyltransferase (MGMT) promoter methylation status in glioma stem-like cells is correlated to temozolomide sensitivity under differentiation-promoting conditions. Int J Mol Sci. 2012;13(6):6983–6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leavitt JA, Stafford SL, Link MJ, Pollock BE. Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2013;87(3):524–527. [DOI] [PubMed] [Google Scholar]
- 34. Morton CI, Hlatky L, Hahnfeldt P, Enderling H. Non-stem cancer cell kinetics modulate solid tumor progression. Theor Biol Med Model. 2011;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Signore M, Pelacchi F, di Martino S, et al. Combined PDK1 and CHK1 inhibition is required to kill glioblastoma stem-like cells in vitro and in vivo. Cell Death Dis. 2014;5:e1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.