Abstract
While previous studies have found evidence for detrimental effects of metals on neurodevelopment, the long-term effects on mental health remain unclear. The objective was to explore the effect of early metal exposure on risk of psychotic disorder and on symptom severity following illness onset. Through the use of validated tooth-biomarkers, we estimated pre- and postnatal exposure levels of essential elements (copper, magnesium, manganese, and zinc) and elements associated with neurotoxicity (lead, arsenic, lithium, and tin). We found consistently higher levels of lithium in patients compared to controls. Higher levels of magnesium and lower levels of zinc were associated with more severe psychopathology over 20 years after metal exposure. The results show promise for the use of teeth biomarkers in examining early environmental risk for psychosis and underscore the relevance of studying metal exposure during critical neurodevelopmental periods.
Keywords: environmental exposure, neurodevelopmental disorders, psychosis, tooth, trace elements
Introduction
The neurodevelopmental model posits that psychotic disorders may be a consequence of interactions between early neurological abnormalities and later maturational events.1 Metal exposure is of particular interest in this context because low-level exposure is ubiquitous, and unlike other environmental factors studied in schizophrenia (eg, immigration and preterm birth), it is modifiable. Early life low-level exposure to metal toxicants, such as lead, arsenic, and tin, has well-documented adverse effects on neurodevelopment.2–4 Even essential nutritive elements (eg, zinc, copper, manganese) may be detrimental to healthy neurodevelopment in excess as well as deficiency.5–7
For this proof-of-concept study, we investigated whether it was possible to detect effects of early exposure to neurotoxicants and nutritive elements with an established influence on neurotransmitter, neuropsychological, and behavioral processes similar to those found in psychotic disorders.2,3,5,6,8–21 For example, an excess of both copper and lithium has been linked with psychotic behavior14,22; Wilson’s disease, a genetic disorder that causes excessive copper accumulation, is often initially misdiagnosed as schizophrenia.22 Patients with schizophrenia have been found to have lower levels of zinc than healthy controls,9,23 and higher delta-aminolevulinic acid levels (marker of prenatal lead exposure) were associated with increased risk of schizophrenia.17
Remarkably, despite the strong relationship between early metal exposure and abnormal neurodevelopment, biological plausibility, and importance for the neurodevelopmental model, the impact of early life exposure to metals has not been studied systematically. Metal exposure has been generally measured after disease onset using blood or urine assays that do not uncover historical exposure during fetal and early childhood critical developmental windows. Even when exposure was measured during pregnancy, it tended to be done indirectly (eg, through maternal blood samples) and without accurate measurements of exposure timing or changes in exposure over time.17
It is now possible to overcome these limitations by objectively reconstructing the timing and dose of early life (pre- and postnatal) environmental metal exposure. Through the use of biomarkers in shed deciduous teeth, we are able to derive detailed temporal profiles of exposure to multiple metals. In the present study, we were specifically interested in exploring and generating hypotheses about the association between metal exposure and psychotic symptom severity in patients diagnosed with schizophrenia and the differential exposure levels of patients and unaffected siblings (a genetically high-risk control sample).
Methods
To determine the effects of timing and dose of prenatal and early childhood exposure to metals associated with neurotoxicity lead, arsenic, tin, and lithium and essential elements manganese, copper, zinc, and magnesium on the development and severity of psychotic disorders/experiences, we analyzed shed deciduous teeth from 20 individuals with a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, diagnosis of schizophrenia and 5 unaffected siblings of patients with psychosis, ascertained from the referrals to the Academic Medical Center in Amsterdam from 2014 to 2017. Nine patients took part in an earlier, smaller explorative analysis.24
Deciduous teeth (ie, “baby teeth”), kept by parents and voluntarily provided to researchers, were evaluated using the biomarker method of laser ablation inductively coupled plasma mass spectrometry, generating integrated, longitudinal 1- to 2-week metal exposure estimates in utero and during early childhood. For full description and validation, see articles.10,25–30 The time-varying difference between early-life (−4 to 6 months) metal concentrations, as measured in the tooth biomarker, and case/control designation was evaluated using a distributed lag model (DLM).31 Severity of positive symptoms, negative symptoms, and general psychopathology were assessed by the Positive and Negative Symptom Scale (PANSS).32 We restrict the data from −120 days before birth to 40 days postnatally due to sparse data at later time points. In the analysis, a critical window is defined as a time point in the DLM model when the 95% confidence band (shaded) does not cross the 0 line of no association. A P value below .05 was considered statistically significant. The vertical bars represent Holm-Bonneferroni corrections for multiple comparisons, and because the sample size is small, associations are not likely to remain significant after this stringent adjustment. However, we prefer to take a conservative approach and display this limitation clearly in the results. In all analyses, sex and biological age were adjusted for.
Results
Sample characteristics
The shed deciduous teeth of 20 patients with a psychotic disorder and 5 controls (whose siblings had schizophrenia) were analyzed. The patient group was comprised of 18 (90%) males and 2 (10%) females (mean age = 24.35, SD = 3.78). The control group was comprised of one (20%) male and four (80%) females (mean age = 28, SD = 8.40). Ninety-one percent of the sample was never married/never lived together with a partner. IQ measures were available for 11 patients (mean IQ = 99.1; SD = 11.3; range = 75–112) and 5 controls (mean IQ = 114; SD = 19.2; range 87–132). Twenty-one out of 23 participants reported being Dutch (data were missing on two individuals).
The patient group mean had mean PANSS positive subscale = 15.06(SD = 8.90), mean PANSS negative subscale = 15.53(SD = 6.99), mean PANSS general psychopathology subscale score = 31.82(SD = 11.51), and mean PANSS total score = 62.41(SD = 25.07).
Early metal dysregulation and psychosis
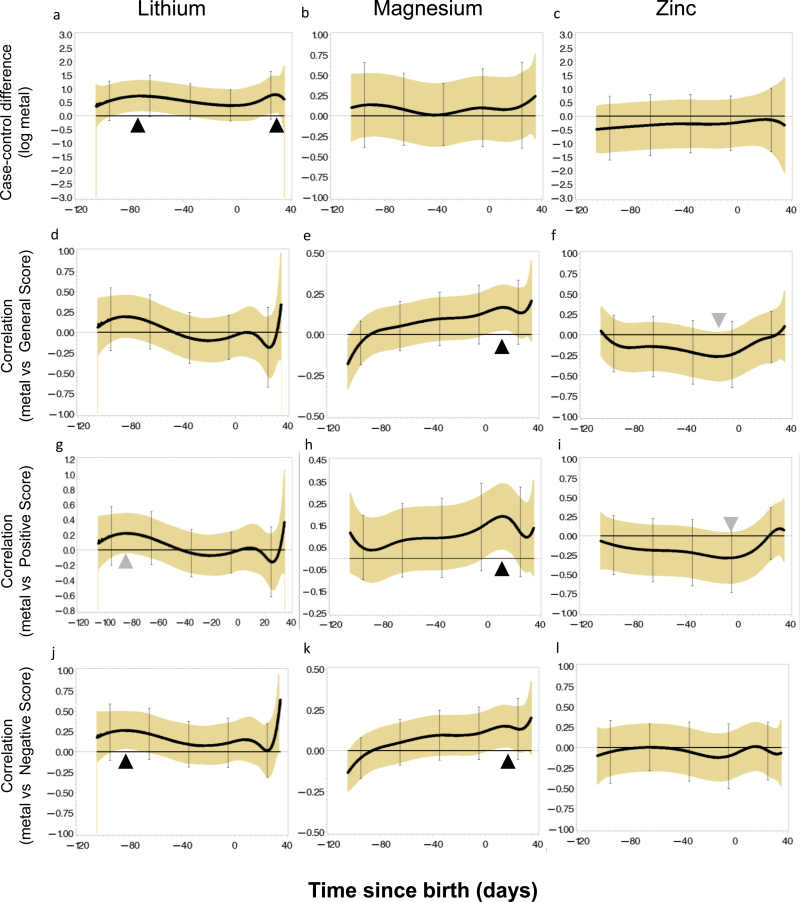
Patients had consistently higher lithium levels than controls, a trend that reached statistical significance from about −12 to 4 weeks before birth and the first month after birth (figure 1a). Patients had consistently higher concentrations of magnesium and lower concentrations of zinc compared to controls, although these results were not statistically significant.
Fig. 1.
Association of lithium, magnesium and zinc uptake with schizophrenia diagnosis and severity measures. Lithium and magnesium uptake were consistently higher in cases (A) and associated with increased severity (B–D) but only at distinct developmental periods (black arrowheads). Zinc was generally lower in cases and inversely associated with severity, approaching significance at specific time points (gray arrowheads).
Early metal exposure and psychotic symptom severity
To examine whether early life metal exposure also has a long-term effect on clinical severity in individuals diagnosed with a psychotic disorder, we subsequently correlated the tooth-matrix biomarkers with the severity of positive, negative, and general symptom scores of the PANSS in the patient sample (all assessed within the first 10 years of the illness).32 Higher magnesium levels in the first postnatal month were significantly associated with higher levels of general (r = 0.18), positive (r = 0.20), and negative (r = 0.15) PANSS scores. (figure 1e, 1h, 1k). As seen in figure 1g and 1j, elevated lithium levels at the beginning of the third trimester were associated with positive (r = 0.22) symptoms and significantly with negative (r = 0.28) symptoms. Lower zinc concentrations in the final prenatal weeks were associated with higher positive (r = −0.28) and general (r = −0.25) PANSS scores (figure 1i and 1f). None of the other metals showed significant associations with general symptom severity in this exploratory analysis. All distributions of metal exposure in the sample are summarized in table 1.
Table 1.
Distribution of Element Exposure
| Variable | Minimum | Maximum | 5th Pctl | Median | 95th Pctl |
|---|---|---|---|---|---|
| Zn | 0.0186311 | 0.1184584 | 0.0263683 | 0.0494432 | 0.0825733 |
| Cu | 0.000099816 | 0.0761735 | 0.0006245 | 0.0027339 | 0.0132552 |
| Li | 3.59E-06 | 0.001 | 2.426E-05 | 0.0001687 | 0.0004843 |
| Mg | 0.6369624 | 2.2494252 | 0.8319266 | 1.4670307 | 1.8343537 |
| Mn | 0.000069174 | 0.0571423 | 0.0016282 | 0.0047491 | 0.0092598 |
| Pb | 0.000049718 | 0.00626 | 0.0001787 | 0.000903 | 0.0029021 |
| Sr | 0.1855598 | 1.1205665 | 0.251711 | 0.4454806 | 0.7570979 |
| Sn | 6.97E-06 | 0.0016906 | 2.368E-05 | 0.0001714 | 0.0005196 |
Zn, zinc; Cu, copper; Li, lithium; Mg, magnesium; Mn, maganese; Pb, lead; Sr, strontium; Sn, tin.
Discussion
Among the body of literature investigating environmental factors that may increase the risk for psychotic disorders, few, if any, studies have examined early metal exposure in relation to the expression and severity of psychotic symptoms decades later. The proof-of-concept findings point to pre- and postnatal case-control differences for three metals (lithium, zinc, and magnesium). Taken together with results from a previous explorative study showing lower pre- and postnatal levels of manganese and copper in patients diagnosed with schizophrenia,24 these results suggest that early life metal dysregulation is a potential important contributor of risk to psychotic disorder.
Interestingly, levels of lithium were elevated in the patient group during two critical neurodevelopmental windows, and higher levels of lithium were consistently associated with higher symptom severity over 20 years later. There are several possible explanations for this finding. First, although we did not have data on parental medication use, it is possible that maternal medication may have contributed to elevated lithium concentrations in the patient group–especially because the effects of lithium levels on symptom severity were significant only during the prenatal period. However, lithium uptake does not necessarily indicate medication because lithium presents in other sources such as water and food.14 Medication use could instead be a proxy of increased genetic risk, and lithium levels alone may not have a direct causal effect on psychosis. However, we did find significant differences between lithium levels in patients and a genetically high-risk control group, potentially discounting this explanation. These elevated levels of lithium may also partly explain the association between magnesium and increased symptom levels, because lithium has been found to increase magnesium levels in the blood.33
A potential mechanism for metal dysregulation during fetal development is placental dysregulation. Recent evidence from human and animal studies suggests that under stress, placental transfer of nutrients to the fetus is disrupted, and factors such as prenatal stress and placental inflammation increase hyperactivity in offspring.34,35 In the case of monozygotic twins sharing a placenta, external stressors that affect only one twin may induce epigenetic modification; notably, in one study, fetal zinc deficiency induced epigenetic alterations in the gene coding for the metal transporter, metallothionein-2, which also regulates other metals.36
We urge caution in interpreting these findings due to the small sample size, and the diversity of the sample under study. Despite the small sample size, with few controls compared to patients, we were able to uncover significant associations between pre- and early postnatal metal concentrations and the presentation and severity of psychotic disorders two decades later, generating interesting hypotheses that could be further explored in future studies. The neurodevelopmental model posits that early life events may affect the risk of developing a psychotic illness, and the tentative results of this study point to a class of preventable public health relevant exposures that have not been investigated in reference to psychosis. Future larger studies are needed to extend the preliminary findings we report here. In these studies, it will be important to explore the impact that other environmental factors, such as low socioeconomic status, upbringing in rural vs urban neighborhoods (potentially associated with increased exposure to certain toxins), and (physical) health of the mother during pregnancy may have on the observed differences.
Funding
Drs. Arora and Reichenberg were supported by the National Institutes of Environmental Health Sciences (P30ES023515 to Drs Arora and Reichenberg; DP2ES025453 and R01ES026033 to Dr Arora). Dr Velthorst received support from the Netherland Organization for Scientific Research (NWO) VENI Grant No. 916-15-005 and the Beatrice and Samuel A. Seaver Foundation Foundation; Dr Velthorst, PhD, is a Seaver Faculty Scholar.
Acknowledgments
Conflict of interest: The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. [DOI] [PubMed] [Google Scholar]
- 2. Needleman HL, Gunnoe C, Leviton A et al. . Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N Engl J Med. 1979;300:689–695. [DOI] [PubMed] [Google Scholar]
- 3. Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27:210–216. [DOI] [PubMed] [Google Scholar]
- 4. Jenkins SM, Ehman K, Barone S Jr. Structure-activity comparison of organotin species: dibutyltin is a developmental neurotoxicant in vitro and in vivo. Brain Res Dev Brain Res. 2004;151:1–12. [DOI] [PubMed] [Google Scholar]
- 5. Zhang L, Bai R, Liu Y et al. . The dose-dependent toxicological effects and potential perturbation on the neurotransmitter secretion in brain following intranasal instillation of copper nanoparticles. Nanotoxicology. 2012;6:562–575. [DOI] [PubMed] [Google Scholar]
- 6. Koh JY, Choi DW. Zinc toxicity on cultured cortical neurons: involvement of N-methyl-D-aspartate receptors. Neuroscience. 1994;60:1049–1057. [DOI] [PubMed] [Google Scholar]
- 7. Guilarte TR. Manganese neurotoxicity: new perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Front Aging Neurosci. 2013;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guilarte TR, Chen MK. Manganese inhibits NMDA receptor channel function: implications to psychiatric and cognitive effects. Neurotoxicology. 2007;28:1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rahman A, Azad MA, Hossain I et al. . Zinc, manganese, calcium, copper, and cadmium level in scalp hair samples of schizophrenic patients. Biol Trace Elem Res. 2009;127:102–108. [DOI] [PubMed] [Google Scholar]
- 10. Arora M, Bradman A, Austin C et al. . Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environ Sci Technol. 2012;46:5118–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sandstead HH, Penland JG, Alcock NW et al. . Effects of repletion with zinc and other micronutrients on neuropsychologic performance and growth of Chinese children. Am J Clin Nutr. 1998;68:470S–475S. [DOI] [PubMed] [Google Scholar]
- 12. Salustri C, Barbati G, Ghidoni R et al. . Is cognitive function linked to serum free copper levels? A cohort study in a normal population. Clin Neurophysiol. 2010;121:502–507. [DOI] [PubMed] [Google Scholar]
- 13. Tsunoda M, Konno N, Nakano K, Liu Y. Altered metabolism of dopamine in the midbrain of mice treated with tributyltin chloride via subacute oral exposure. Environ Sci. 2004;11:209–219. [PubMed] [Google Scholar]
- 14. Schrauzer GN. Lithium: occurrence, dietary intakes, nutritional essentiality. J Am Coll Nutr. 2002;21:14–21. [DOI] [PubMed] [Google Scholar]
- 15. Nagaraja TN, Desiraju T. Regional alterations in the levels of brain biogenic amines, glutamate, GABA, and GAD activity due to chronic consumption of inorganic arsenic in developing and adult rats. Bull Environ Contam Toxicol. 1993;50:100–107. [DOI] [PubMed] [Google Scholar]
- 16. Guilarte TR, Opler M, Pletnikov M. Is lead exposure in early life an environmental risk factor for Schizophrenia? Neurobiological connections and testable hypotheses. Neurotoxicology. 2012;33:560–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Opler MG, Buka SL, Groeger J et al. . Prenatal exposure to lead, delta-aminolevulinic acid, and schizophrenia: further evidence. Environ Health Perspect. 2008;116:1586–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zuch CL, O’Mara DJ, Cory-Slechta DA. Low-level lead exposure selectively enhances dopamine overflow in nucleus accumbens: an in vivo electrochemistry time course assessment. Toxicol Appl Pharmacol. 1998;150:174–185. [DOI] [PubMed] [Google Scholar]
- 19. Matzen TA, Martin RL. Magnesium deficiency psychosis induced by cancer chemotherapy. Biol Psychiatry. 1985;20:788–791. [DOI] [PubMed] [Google Scholar]
- 20. Nechifor M, Vaideanu C, Palamaru I, Borza C, Mindreci I. The influence of some antipsychotics on erythrocyte magnesium and plasma magnesium, calcium, copper and zinc in patients with paranoid schizophrenia. J Am Coll Nutr. 2004;23:549S–551S. [DOI] [PubMed] [Google Scholar]
- 21. Slutsky I, Abumaria N, Wu LJ et al. . Enhancement of learning and memory by elevating brain magnesium. Neuron. 2010;65:165–177. [DOI] [PubMed] [Google Scholar]
- 22. Bidaki R, Zarei M, Mirhosseini SM et al. . Mismanagement of Wilson’s disease as psychotic disorder. Adv Biomed Res. 2012;1:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vidović B, Dorđević B, Milovanović S et al. . Selenium, zinc, and copper plasma levels in patients with schizophrenia: relationship with metabolic risk factors. Biol Trace Elem Res. 2013;156:22–28. [DOI] [PubMed] [Google Scholar]
- 24. Modabbernia A, Velthorst E, Gennings C et al. . Early-life metal exposure and schizophrenia: a proof-of-concept study using novel tooth-matrix biomarkers. Eur Psychiatry. 2016;36:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hare D, Austin C, Doble P, Arora M. Elemental bio-imaging of trace elements in teeth using laser ablation-inductively coupled plasma-mass spectrometry. J Dent. 2011;39:397–403. [DOI] [PubMed] [Google Scholar]
- 26. Arora M, Austin C. Teeth as a biomarker of past chemical exposure. Curr Opin Pediatr. 2013;25:261–267. [DOI] [PubMed] [Google Scholar]
- 27. Arora M, Hare D, Austin C, Smith DR, Doble P. Spatial distribution of manganese in enamel and coronal dentine of human primary teeth. Sci Total Environ. 2011;409:1315–1319. [DOI] [PubMed] [Google Scholar]
- 28. Arora M, Kennedy BJ, Elhlou S et al. . Spatial distribution of lead in human primary teeth as a biomarker of pre- and neonatal lead exposure. Sci Total Environ. 2006;371:55–62. [DOI] [PubMed] [Google Scholar]
- 29. Arora M, Austin C, Sarrafpour B et al. . Determining prenatal, early childhood and cumulative long-term lead exposure using micro-spatial deciduous dentine levels. PLoS One. 2014;9:e97805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Austin C, Smith TM, Bradman A et al. . Barium distributions in teeth reveal early-life dietary transitions in primates. Nature. 2013;498:216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gasparrini A, Armstrong B, Kenward MG. Distributed lag non-linear models. Stat Med. 2010;29:2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 33. Makara-Studzińska M, Dąbrowski W, Kiełczykowska M, Musik I. Level of magnesium in patients with depression treated with lithium—pilotage research. Ann Agric Environ Med. 2013;20:111–115. [PubMed] [Google Scholar]
- 34. Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 2014;155:2635–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walker CK, Ashwood P, Hertz-Picciotto I. Preeclampsia, placental insufficiency, autism, and antiphospholipid antibodies-reply. JAMA Pediatr. 2015;169:606–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kurita H, Ohsako S, Hashimoto S, Yoshinaga J, Tohyama C. Prenatal zinc deficiency-dependent epigenetic alterations of mouse metallothionein-2 gene. J Nutr Biochem. 2013;24:256–266. [DOI] [PubMed] [Google Scholar]