Abstract
Horizontal gene transfer is a key process in the evolution of bacteria and also represents a source of genetic variation in eukaryotes. Among elements participating in gene transfer, thousands of small (<10 kb) mobile bacterial plasmids that replicate by the rolling circle mechanism represent a driving force in the spread of antibiotic resistances. In general, these plasmids are built as genetic modules that encode a replicase, an antibiotic-resistance determinant, and a relaxase that participates in their conjugative mobilization. Further, they control their relatively high copy number (∼30 copies per genome equivalent) by antisense RNAs alone or combined with a repressor protein. We report here that the MobM conjugative relaxase encoded by the promiscuous plasmid pMV158 participates in regulation of the plasmid copy number by transcriptional repression of the antisense RNA, thus increasing the number of plasmid molecules ready to be horizontally transferred (mobilization) and/or vertically inherited (replication). This type of crosstalk between genetic modules involved in vertical and horizontal gene flow has not been reported before.
INTRODUCTION
Transfer of genetic information between individuals is achieved by two mechanisms: vertical, from parent to siblings, and horizontal (HGT) between individuals of the same or different species. Whereas the former ensures the maintenance of the identity of species, the latter is a driving force that strongly participates in evolutionary and adaptive processes (1). HGT plays a key role in the spread of multiple genetic traits, more importantly the resistance to antibiotics (2,3), and participates actively in the successful adaptation of bacteria to new niches (4). Further, HGT raises a fascinating issue when considering that prokaryotic mobile genetic elements (MGE) influence not only bacterial lifestyle (5) but also evolution of eukaryotic complex organisms (6), since there is growing evidence of gene transfer from bacteria to eukaryotes (reviewed in (7)).
In the bacterial kingdom, HGT is mainly mediated by different types of MGE that constitute the mobilome (8). Among the MGE, bacterial plasmids represent up to 20% of the commonly shared bacterial genes (the so-called plasmidome) that move through HGT processes (2,9). Plasmids are self-replicative DNA entities that can spread independently of the host chromosome. To have an independent lifestyle, plasmids tend to minimize the genetic load to their hosts but maintaining their own fitness. To do so, plasmids may undergo processes of ‘domestication’ (10) or co-evolution that may render them well adapted to the host features, such as codon usage (11). Reduction in genetic load by plasmids can be achieved by keeping a strict control on their copy number, which is done by negatively acting plasmid-encoded elements that operate on the positive player, the initiator. In some plasmids, the negative control elements can be either a short-lived antisense RNA that pairs with the messenger RNA (mRNA) encoding the initiator or, in addition to the antisense RNA, a repressor protein that regulates synthesis of the initiator at the transcriptional level (12–14). In some other plasmids, the initiator is a stable protein that also controls plasmid copy number negatively by mediating inhibitory plasmid pairing, which becomes effective following replication initiation (15). When entering into a new host, plasmids can over-replicate so that their average copy number (Nav) overshoots before the cell division occurs, as shown by repopulation experiments (16). Over-replication happens because of the lag in accumulation of the negative regulatory elements, and it may take a few generations until the Nav value is reached (17–20).
In addition to the direct control of Nav exerted by the regulatory elements that limit the initiation of replication frequency, growing evidence shows that even simple systems like plasmids may employ multi-model control mechanisms leading to stable inheritance of the plasmid molecules. Firstly, the partition (par) genes (21,22) that will take part in the segregation of the newly replicated plasmid molecules at the time of cell division by mitosis-like mechanisms (23). And, secondly, the stability (stb) genes that participate in either stable inheritance (24) or positioning of plasmid molecules in the cell poles most likely facilitating conjugation (25). Mechanisms linking replication and segregation of chromosomes have also been reported, thus broadening the instances of crosstalk within bacterial modules (26).
All the above and other findings have contributed to the notion that plasmids are built as gene modules integrated by two parts: the ‘backbone’ and the ‘accessory’ genes (27). These latter would include those genes that the replicons will be picking up along their colonization of different hosts, and that will provide selective advantage to the host, such as antibiotic- and heavy metal-resistance genes, and biodegradation pathways (28). Within the bacterial plasmidome (29), there are thousands of plasmids replicating by the rolling circle mechanism (RCR-plasmids) that have a small size (<10 000 bp), a high copy number (Nav ∼ 20–30) and lack both partitioning genes and toxin–antitoxin systems that could contribute to their stable inheritance. RCR-plasmids are supposed to have random partition at cell division and to replicate stochastically (30). However, mechanisms to inactivate the initiator protein to restrain the onset of replication by limiting re-initiation do exist, adding another level of complexity to the control of replication (31,32).
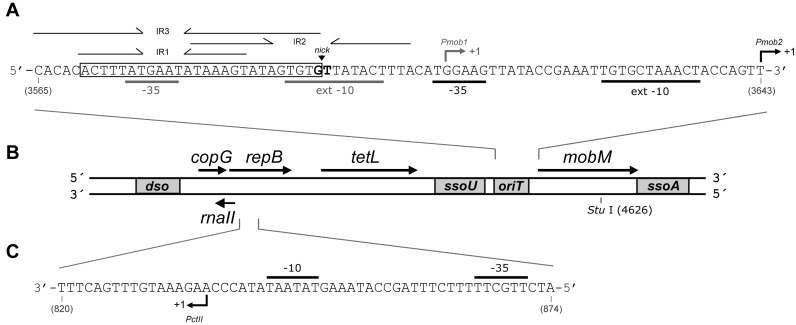
We have been studying the streptococcal broad host range RCR-plasmid pMV158 for a number of years (33). This plasmid consists of three modules that are involved in: (i) initiation of leading strand replication and its control; (ii) resistance to tetracycline (tetL-type determinant) and (iii) conjugative mobilization (Figure 1B). Further, pMV158 has two origins of lagging strand synthesis (ssoU and ssoA) that are flanking the mobilization cassette and are known to play a role in the plasmid promiscuity (34,35). Copy number control of pMV158 is achieved by the combination of two plasmid components: an antisense RNA, RNAII and a transcriptional repressor protein, CopG (36). RNAII limits synthesis of the initiator protein RepB by pairing with the translation initiation signals of the repB mRNA (37), whereas CopG represses transcription of the copG–repB operon by binding to the Pcr promoter and hindering the binding of, or displacing the already bound, RNA polymerase (38). This dual mechanism to control the Nav of pMV158 is shared with theta-replicating plasmids of the Inc18 family, such as pIP501 or pAMβ1 (12,39,40). These plasmids are considered as ‘auxiliary’ plasmids that provide the machinery (coupling protein and the T4 secretion system) for the mobilization of pMV158 (41). Although pMV158 is not conjugative, it can be mobilized among a number of bacterial species by the activity of the plasmid-encoded MobM protein, which is the prototype of the MOBV1 family of relaxases (33,42,43). MobM initiates plasmid transfer by binding to its cognate origin of transfer, oriT, where it introduces a nick that cleaves the phosphodiester bond at the dinucleotide 5΄pG/T-3΄ (coordinates 3595–3596) (44). In addition to its role as initiator of transfer, MobM was shown to regulate its own synthesis by binding to its promoter, thus hindering the binding of the RNA polymerase (45).
Figure 1.
Genetic map of plasmid pMV158. Only relevant features are indicated. (A) Nucleotide sequence (coding strand) of the region that includes the origin of transfer (oriT), the promoters of the mobM gene (Pmob1 and Pmob2) and the corresponding transcription initiation sites (arrows). The three inverted repeats (IR1, IR2, IR3), the minimal oriT sequence (coordinates 3570–3595) and the nicking site (nick) are indicated, as well as the −10 and −35 boxes of the Pmob1 and Pmob2 promoters used in Lactococcus lactis (72) and in Escherichia coli (45), respectively. (B) Genes encoded by the three pMV158 modules: (i) copG, repB and rnaII, involved in replication and its control; (ii) tetL, a type L tetracycline-resistance determinant and (iii) mobM, involved in mobilization. The origins for leading-strand (dso) and lagging-strand (ssoU, ssoA) synthesis are boxed, as well as the oriT. The position of the single StuI restriction site (coordinate 4626) is indicated. (C) Nucleotide sequence (non-coding strand) of the region (coordinates 820–874) that includes the promoter of the rnaII gene (PctII). The −10 and −35 sequences, and the transcription start site (arrow) (46) are indicated.
In the present work, we report a novel mechanism that further regulates the Nav of plasmid pMV158. It is mediated by the MobM conjugative relaxase, which participates in the copy number control circuit by binding to the promoter region of the rnaII gene. Binding of MobM to this newly discovered site leads to a reduction in the synthesis of the antisense RNAII. As a consequence, the translational repression of the initiator RepB is relieved. The genetic organization and DNA sequence of the oriT region and the rnaII gene are conserved in plasmids of the pMV158 family (14). Our results reveal that control of the pMV158 copy number is further exerted by crosstalk between plasmid modules involved in replication and mobilization processes, a finding that, to our knowledge, has not been reported before.
MATERIALS AND METHODS
Bacterial strains and growth conditions
Streptococcus pneumoniae 708 was used as the host for plasmid pMV158 and its derivatives (46). Escherichia coli strains BL21(DE3) (47) and JM109(DE3) (Promega) were used for protein overproduction and β-galactosidase assays, respectively. Proteins MobM and MobMN199 were overproduced in E. coli and purified as described (48). Streptococcus pneumoniae cells were grown in AGCH medium as reported (49). Escherichia coli cells were grown in tryptone–yeast extract (TY) medium (Pronadisa). In the case of plasmid-harbouring cells, the medium was supplemented with tetracycline (1 and 2 μg/ml for pMV158 and pMP220 derivatives, respectively) or ampicillin (100 μg/ml for plasmids pET5 and pLGM2). Bacteria were grown at 37°C.
Plasmids and oligonucleotides
Plasmids and oligonucleotides used in this work are listed in Tables 1 and 2, respectively. Plasmid pMVT was constructed following a two-step polymerase chain reaction (PCR) strategy (Supplementary Figure S1). The first step involved: (i) the use of oligonucleotides 4SacI and C2/3 as primers and plasmid pMV158 as template, obtaining a 5420-bp DNA fragment that lacks the oriT and the promoters of mobM (Pmob1 and Pmob2) (45) and (ii) the use of oligonucleotides 1SacI and 2/C3 as primers and plasmid pAST (49) as template, obtaining a 290-bp DNA fragment that contains the transcriptional terminators T1T2 of the E. coli rrnB ribosomal RNA operon (50). In the second step, both DNA fragments were mixed and used as template for PCR amplification. Oligonucleotides 1SacI and 4SacI (both including a SacI restriction site; Table 2) were used. Given the partial overlapping between oligonucleotides C2/3 and 2/C3, a 5670-bp DNA fragment was obtained. After digestion with SacI, the reaction mixture was diluted and incubated with T4 DNA ligase (New England Biolabs) to favour self-ligation of the molecules. A similar PCR strategy was used for the construction of plasmid pMVT-PfcsK. In this case, oligonucleotides 1SacI and 2/C3 were mixed with plasmid pAST-PfcsK (49), obtaining a 407-bp DNA fragment that includes the T1T2 transcriptional terminators and the fucose-inducible promoter PfcsK (49,51).
Table 1. Plasmids.
| Name | Descriptiona | Reference |
|---|---|---|
| pMV158 | Originally isolated from Streptococcus agalactiae MV158. TcR | (53) |
| pAS | Terminator-probe vector based on pMV158. TcR | (49) |
| pAST | Promoter-probe vector based on pMV158. TcR | (49) |
| pAST-Pfcsk | pAST derivative. It carries the fucose-inducible promoter PfcsK. TcR | (49) |
| pMVT | pMV158 derivative. It lacks oriT and carries a promoter-less mobM gene. TcR | This work |
| pMVT-PfcsK | pMV158 derivative. It lacks oriT and carries the fusion gene PfcsK::mobM. TcR | This work |
| pLGM2 | pET5 derivative. It carries mobM fused to the ϕ10 promoter of phage T7. ApR | (44) |
| pMP220 | Cloning vector with a promoter-less lacZ gene. TcR | (52) |
| pΔORI | pMP220 derivative. Insertion of promoter Pmob2 upstream of the lacZ gene. TcR | This work |
| pRNAII | pMP220 derivative. Insertion of promoter PctII upstream of the lacZ gene. TcR | This work |
aApR, TcR: resistance to ampicillin and tetracycline, respectively.
Table 2. Oligonucleotides used.
| Name | Sequence (5΄-3΄)a | Coordinatesb |
|---|---|---|
| FP-rnaII | GCACTCCAAGCGTTTCAAGTTTCAGCTC | 946–919 |
| IR1+8 | ACTTTATGAATATAAAGTATAGTGTG | 3570–3595 |
| PctII | TCTTTAGCCATAAAGTATAATATA | 863–840 |
| 1SacI | ATAAGAGCTCATGCGAGAGTAGGGAACTGC | N.A. |
| 2/C3 | AAAGACACACGAGGCTCGCCCGGGGATCCTCTA | N.A. |
| 4SacI | attagagctCGACCAAAACCATAAAACCTT | 3541–3521 |
| C2/3 | ccccgggcgagcCTCGTGTGTCTTTCGGTAATC | 3683–3703 |
| PE-tet | GAGGGCAGACGTAGTTTATAGG | 1717–1696 |
| PE-mobM | CCCTCCTTAAGACTTGTCTTTCG | 3725–3703 |
| PE-cop-rep | CCGATTCACTTAATGTTATCGTCA | 691–668 |
| PE-rnaII | TGCTGGCAGGCACTGGC | 802–818 |
| M13-forward | GTAAAACGACGGCCAGT | N.A. |
| RNAII-F | AGTCGCTTGGAATTCATTCAGGATA | 916–892 |
| RNAII-R | CAGGCACTGGCTGCAGTCAAACATT | 808–832 |
| ΔORI-F | AGTATAGTGTGTTAGAATTCACATGGAAGTTATA | 3585–3618 |
| ΔORI-R | CAGACGAGCCGCTGCAGTCTATTGCT | 3690–3665 |
aBase changes that generate restriction sites (in bold) are underlined. Tail sequences are indicated in lower case letters.
bCoordinates are given with respect to pMV158 sequence (GenBank acc. no. NC_010096), except for 1SacI and 2/C3 (N.A., do not apply).
In addition to the E. coli plasmids constructed in this work (see below), we used plasmid pET5 (Novagen) and plasmid pLGM2 (44), which are pET5 derivatives that carry the mobM gene under control of the ϕ10 promoter of phage T7. The IncP broad-host range vector pMP220 has single restriction sites for EcoRI and PstI. These sites are located upstream of the promoter-less lacZ gene (52). To construct plasmid pRNAII (Table 1), a 109-bp region of pMV158 that contains the PctII promoter was PCR-amplified with the oligonucleotides RNAII-F (EcoRI site) and RNAII-R (PstI site). Plasmid pMP220 and the PCR-amplified DNA were further digested with EcoRI and PstI. Digested DNAs were mixed and treated with T4 DNA ligase. Ligation mixtures were used to transform E. coli JM109(DE3) cells. Transformants resistant to tetracycline (2 μg/ml) were selected. To construct plasmid pΔORI, a 106-bp region of pMV158 that contains the Pmob2 promoter was PCR-amplified with the oligonucleotides ΔORI-F (EcoRI site) and ΔORI-R (PstI site). The PCR-amplified DNA was digested with both enzymes EcoRI and PstI, and the digestion product was then inserted into pMP220.
Plasmids pMV158 (53) and pAS (49) were purified from S. pneumoniae by two consecutive CsCl gradients as previously described (54). For small-scale preparations of plasmid DNA, the High Pure Plasmid Isolation Kit (Roche Applied Science) was used. The suspension buffer of this kit was supplemented with 50 mM glucose and 0.1% deoxycholate in pneumococcus. All PCR amplifications were performed with the Phusion High-Fidelity DNA polymerase (Thermo Scientific). Plasmid constructions were confirmed by dye-terminator sequencing (Secugen, CIB, Spain).
Electron microscopy
Supercoiled pMV158 DNA (8 nM) was incubated with protein MobM (375 nM) at 30°C for 30 min. The reaction buffer contained 20 mM Tris–HCl, pH 7.6, 1 mM dithiothreitol (DTT), 1 mM ethylenediaminetetraacetic acid (EDTA), 1% glycerol and 50 mM NaCl. When indicated, the reaction buffer was supplemented with 15 mM MgCl2. MobM–pMV158 complexes were fixed with 0.3% glutaraldehyde for 15 min at the same temperature. Then, pMV158 was linearized with StuI. Reaction mixtures were diluted 10-fold in buffer GA (10 mM triethanolamine chloride, pH 7.5, 10 mM MgCl2), adsorbed onto freshly cleaved mica, positively stained with 2% uranyl acetate, rotary shadowed with Pt/Ir and covered with a carbon film as described (55). Micrographs of the carbon film replica were taken using a Philips CM100 (FEI Company, Hillsboro, Oregon) electron microscope at 100 kV. The contour lengths of the DNA regions between the MobM-binding site and the DNA ends were measured on projections of 35-mm negatives using a digitizer (LM4; Brüchl, Nüremberg, Germany).
DNase I footprinting assays
DNase I footprinting on supercoiled DNA was done essentially as reported (56,57). Binding reactions (45 μl) contained 20 mM Tris–HCl, pH 7.6, 1 mM EDTA, 1 mM DTT, 1% glycerol, 50 mM NaCl, protein MobM (1 or 2 μM) and supercoiled pAS DNA (1 μg) were purified by two consecutive CsCl gradients. Reaction mixtures were incubated at 24°C for 25 min. Then, 5 μl of a solution that contained 0.3 units of DNase I (Roche Applied Science), 10 mM MgCl2 and 5 mM CaCl2 was added to slightly cleave the supercoiled DNA, and to generate a series of fragments that contain random 5΄- and 3΄-ends. After 1 min at 24°C, DNase I digestion was stopped by the addition of 100 μl of stop buffer (1% sodium dodecyl sulphate (SDS), 200 mM NaCl, 20 mM EDTA, pH 8.0). DNA samples were extracted with phenol/chloroform and precipitated with ethanol. The denatured DNase I-digested DNA was annealed to a 5΄-end labelled specific oligonucleotide that was extended by Sequenase™ Version 2.0 DNA Polymerase (USB), thus copying the hybridized strands to the 5΄-end generated by the DNase I digestion. The source of radioactivity is provided by the 5΄-labelled oligonucleotide so that the only radioactive strands produced have the 5΄-end of the primer and a variable 3΄-end corresponding to a cleavage site in the original supercoiled DNA (56). Specifically, the FP-rnaII primer was radioactively labelled at the 5΄-end using [γ-32P]-ATP (PerkinElmer) and T4 polynucleotide kinase (New England Biolabs). Samples were analysed by sequencing gel (7 M urea, 8% polyacrylamide) electrophoresis. The loading buffer contained 80% formamide, 1 mM EDTA, 10 mM NaOH, 0.1% Bromophenol Blue and 0.1% Xylene Cyanol. A sequence marker was obtained by sequencing plasmid pAS with the 32P-labelled FP-rnaII oligonucleotide. With this technique, the radioactive bands are generated by extension of the labelled primer and not by DNase I digestion of a linear DNA labelled at the 5΄-end of one strand (usual method for DNase I footprinting on linear double-stranded DNA); consequently radioactive material does not accumulate at the top of the sequencing gels (57).
Electrophoretic mobility shift assays (EMSA)
Competitive electrophoretic mobility shift assays (EMSA) using protein MobMN199 and different oligonucleotides were carried out as reported (48). Reaction mixtures contained the Cy5-labelled IR1+8 oligonucleotide (2 nM), the indicated concentration of non-labelled competitor DNA (oligonucleotide PctII or IR1+8) and protein MobMN199 (80 nM). Reactions were incubated at 25°C for 20 min. Free and bound DNAs were separated by electrophoresis on native polyacrylamide (10%) gels. Fluorescent DNA was detected with a Typhoon scanner system (Molecular Dynamics) and quantified using the Quantity One software (Bio-Rad).
Western blots
Pneumococcal cells carrying plasmid pMVT or pMVT-PfcsK were exponentially grown in media containing 0.3% sucrose and the indicated concentration of fucose to an optical density at 650 nm (OD650) of 0.6. The protocol used to prepare whole-cell extracts was described (49). Total proteins were separated by SDS-polyacrylamide (12%) gel electrophoresis. Immunoblot polyvinylidene difluoride (PVDF) membranes (Bio-Rad) and polyclonal antibodies against MobM were used. Antigen–antibody complexes were detected as reported (58).
Total RNA preparations and primer extension assays
Streptococcus pneumoniae cells harbouring plasmid pMVT-PfscK were grown to an OD650 of 0.4 in media that contained or not contained fucose (1%). The RNeasy mini Kit (QIAGEN) was used to isolate total RNA. Cultures were processed as specified by the supplier. The integrity of rRNAs was checked by agarose gel electrophoresis. RNA concentration was determined using a NanoDrop ND-1000 Spectrophotometer (Bio-Rad). Primer extension assays were performed with oligonucleotides 32P-labelled at the 5΄-end as reported (58). Primer extension reactions were carried out using the same amount of total RNA. Then, the same volume of each reaction mixture was loaded onto the sequencing gel. Dideoxy-mediated chain termination sequencing reactions were run in the same gel. Labelled reaction products were visualized using the Fujifilm Image Analyzer FLA-3000. The intensity of the bands was quantified using the Quantity One software (Bio-Rad).
β-Galactosidase assays
Escherichia coli JM109(DE3) cells carrying the indicated plasmids were grown to an OD600 of 0.4. Then, IPTG (1 mM final concentration) was added to the cultures to induce the expression of the mobM gene located on plasmid pLGM2. As controls, cultures without Isopropyl-β-D-1-Thyogalactopyranoside (IPTG) were used. β-Galactosidase activity was measured as reported (45) using a Thermo Scientific Varioskan Flash instrument. All experiments were independently repeated five times (n = 5), and β-galactosidase activity was calculated in Miller units (MU) (59).
Total DNA preparations and plasmid copy number determinations
Total DNA from pneumococcal cells harbouring the indicated plasmid was isolated as described (35,46). Samples were analysed by agarose (0.8%) gel electrophoresis. Tris/Borate/EDTA buffer containing ethidium bromide (1 μg/ml) was used. After electrophoresis, the plasmid copy number was determined as reported (35,46). The intensity of the bands was quantified using a Gel-Doc system and the Quantity One software (Bio-Rad). All experiments were repeated five times (n = 5).
Bioinformatics and statistics
DNA structures were predicted using the Mfold (3.2) program (http://mfold.rna.albany.edu) (60). The statistical analyses of the data were done by employment of the SigmaPlot program (SYSTAT, USA).
RESULTS
Binding of MobM to supercoiled pMV158 DNA visualized by Electron microscopy (EM) analyses
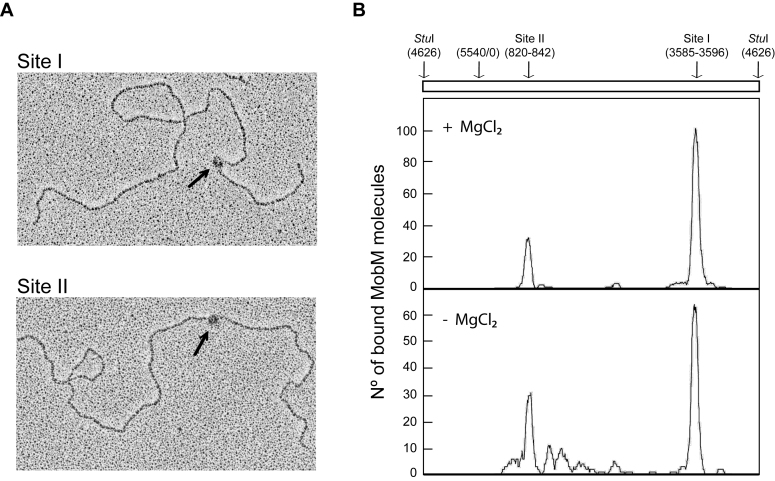
As an approach to analyse the interaction of MobM with its natural target, the supercoiled pMV158 DNA, we used electron microscopy. Specifically, MobM was incubated with supercoiled pMV158 DNA (5540 bp) in the absence (binding conditions) or in the presence of Mg2+ (binding and nicking conditions). MobM–pMV158 complexes were fixed with glutaraldehyde and then treated with the StuI restriction endonuclease, which cuts pMV158 DNA at coordinate 4626 (linearization of pMV158, Figure 1) (55). Electron micrographs of such complexes are shown in Figure 2A. To determine the MobM-binding sites, the contour lengths of the DNA regions between MobM positions and DNA ends were measured. Figure 2B shows the distribution of the MobM positions on the pMV158 DNA. In the absence of Mg2+, 51% of the 166 complexes measured showed a MobM-binding site around coordinate 3585 (site I), which fits well with the location of the minimal oriT sequence (3570–3595) defined previously using single-stranded DNAs (Figure 1A) (48). Moreover, 32% of such 166 complexes showed a potential MobM-binding site (site II) around coordinate 842. Both MobM-binding sites were also detected in the presence of Mg2+. Specifically, 67 and 20% of the 177 complexes examined showed a MobM-binding site around coordinates 3596 (site I) and 820 (site II), respectively. Therefore, in addition to the oriT sequence (site I being the major binding site), MobM seemed to interact with a region located around coordinates 820–842 of plasmid pMV158 (Figure 1C). This region is located just adjacent to the −10 element of the PctII promoter, and includes the transcription initiation site (coordinate 836) of the gene that encodes the antisense RNAII. This unexpected result was the first indication that MobM could have a secondary binding site within the module that controls the replication of plasmid pMV158.
Figure 2.
Binding of MobM to supercoiled pMV158 DNA. Plasmid pMV158 was incubated with MobM at 30°C for 30 min. MobM–pMV158 complexes were fixed with glutaraldehyde and then pMV158 was linearized with StuI. (A) Electron micrographs of linear plasmid molecules with MobM bound to site I or site II (black arrows). (B) Distribution of MobM positions on the pMV158 DNA when the binding reactions were performed in the presence of MgCl2 or in its absence. The coordinates of the two main MobM-binding sites are indicated.
MobM binds to the PctII promoter region
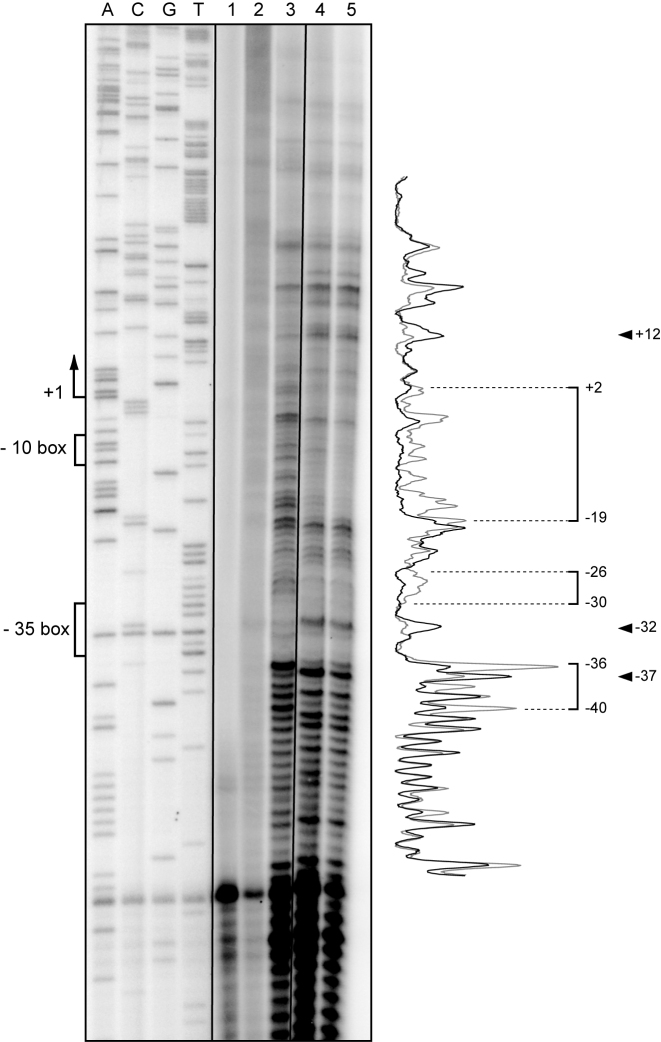
We have shown that MobM binds poorly to linear double-stranded DNA fragments that contain the oriT sequence (61). Thus, to confirm that MobM binds to a region of pMV158 that is located around coordinates 820–842, we performed DNase I footprinting experiments on supercoiled plasmid DNA. To favour the binding of MobM to such a region, we used a pMV158 derivative that lacks the oriT sequence (plasmid pAS) (49). After binding, the reaction mixtures were subjected to DNase I digestion. Specific cleavages and protected sites were detected by primer extension on the denatured DNase I-digested DNA using the 32P-labelled FP-rnaII oligonucleotide (Table 2). A sequence marker was obtained by sequencing plasmid pAS with the same 32P-labelled oligonucleotide. As shown in Figure 3, the region spanning coordinates 835 and 855 (positions +2 and −19 of the PctII promoter) was protected by MobM. MobM-mediated protections were also observed between coordinates 862 and 866 (positions −26 and −30). Additionally, coordinates 825, 868 and 873 (positions +12, −32 and -37) were more sensitive to DNase I cleavage. This analysis confirmed that MobM interacts with the replication module of plasmid pMV158. The site (site II) recognized by MobM was located between coordinates 835 and 866 (Figure 1C), which corresponds with the positions +2 and −30 of the PctII promoter.
Figure 3.
DNase I footprinting analysis on supercoiled pAS DNA. Plasmid pAS was incubated with MobM. Binding reactions were treated with DNase I and then subjected to primer extension using the 32P-labelled FP-rnaII oligonucleotide. The primer extension reactions were performed on plasmid pAS in the absence of both MobM and DNase I (lane 1), in the presence of MobM (2 μM) and absence of DNase I (lane 2), in the absence of MobM and presence of DNase I (lane 3), and in the presence of both MobM (1 and 2 μM) and DNase I (lanes 4 and 5, respectively). Dideoxy-mediated chain-termination sequencing reactions using pAS DNA and the FP-rnaII oligonucleotide were run in the same gel (lanes A, C, G and T). The −10 and −35 boxes of the PctII promoter and the MobM-protected regions are indicated with brackets. The indicated positions are relative to the transcription start site (position +1) of the PctII promoter. Arrowheads indicate positions that are more sensitive to DNase I cleavage. Densitometer scans corresponding to DNA without MobM (lane 3, grey line) and DNA with MobM (lane 5, black line) are shown.
The oriTpMV158 sequence contains three inverted repeats (IR1, IR2 and IR3) (Figure 1A). Using single-stranded DNA substrates and a truncated MobM protein (MobMN199; the first 199 residues), the minimal oriT sequence was previously delimited to a stretch of 26 nucleotides (coordinates 3570–3595) (48). This minimal origin includes the IR1 inverted repeat and was denominated IR1+8 (see Figure 4A). MobMN199 was used in such experiments because, unlike MobM, it lacks the ability to cleave single-stranded DNA. Searching for homologies, we found similarities between the minimal oriT sequence (coding strand of pMV158) and the PctII promoter region that spans positions −27 and −4 (non-coding strand; coordinates 863–840; Figure 4A). Prediction of possible hairpins within both sequences (oligonucleotides IR1+8 and PctII) showed that they could generate a stem–loop structure (Figure 4B). This observation suggested to us that protein MobMN199 could interact with oligonucleotide PctII, as it was shown that this truncated protein interacts with oligonucleotide IR1+8 (48). To test this hypothesis, a competitive gel retardation assay was performed (Figure 4C). The Cy5-labelled IR1+8 oligonucleotide (Cy5-IR1+8) was incubated with MobMN199 in the absence of competitor DNA or in the presence of a non-labelled competitor oligonucleotide, either PctII (24-mer) or IR1+8 (26-mer, positive control). In the absence of competitor DNA, a protein–DNA complex was detected (Figure 4C). This complex was also detected in the presence of 512 nM of an unspecific competitor oligonucleotide (48). On the contrary, its formation was impaired in the presence of PctII (128 nM) or IR1+8 (64 nM) (Figure 4C). Therefore, we can conclude that MobMN199 binds to the region that spans positions −4 and −27 of the PctII promoter on single-stranded DNA, and that the affinity of MobMN199 for oligonucleotide PctII is lower than for oligonucleotide IR1+8.
Figure 4.
Binding of MobMN199 to the PctII promoter on single-stranded DNA. (A) Sequence alignment of the minimal oriT sequence (IR1+8; coordinates 3570–3595) and the PctII promoter region spanning coordinates 863–840 (positions −27 to −4). Arrows illustrate inverted repeats. The −10 box of the PctII promoter is indicated. (B) Stem–loop structure predictions for oligonucleotides IR1+8 and PctII. (C) Competitive EMSA. Different concentrations of non-labelled competitor DNA (oligonucleotides PctII and IR1+8) were mixed with Cy5-labelled oligonucleotide IR1+8 (2 nM). Then, protein MobMN199 (80 nM) was added to the reaction mixtures. Samples were loaded onto a native polyacrylamide (10%) gel. The positions of free DNA (F) and bound DNA (C) are indicated.
Taking all the above results together, we conclude that the conjugative relaxase MobM has a secondary binding site at the PctII promoter region, which is located within the pMV158 replication module.
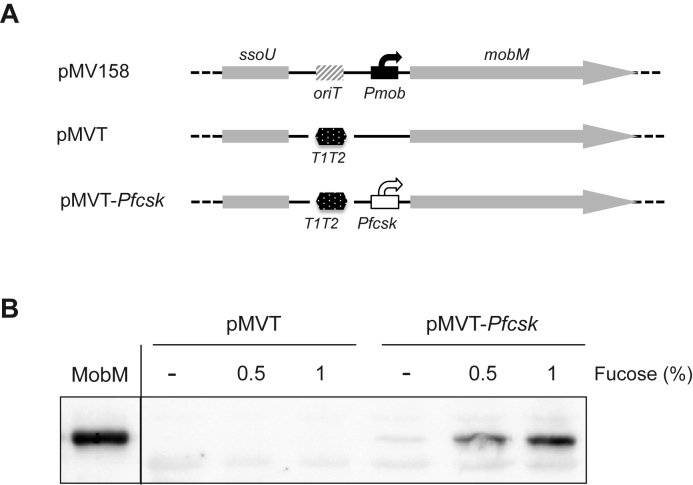
MobM represses transcription from the PctII promoter
The existence of the MobM-binding site II at promoter PctII suggested that MobM could repress transcription from such a promoter. To investigate this assumption, we constructed plasmid pMVT-PfcsK (Figure 5A). This pMV158 derivative lacks the oriT sequence (site I, the major binding site of MobM) and carries the mobM gene under control of the pneumococcal PfcsK promoter, which is induced by fucose (49,51). A western blot analysis demonstrated that pneumococcal cells harbouring plasmid pMVT-PfcsK synthesize higher levels of MobM in the presence of fucose (Figure 5B). This bacterial strain allowed us to analyse the effect of MobM on the transcription initiated at several plasmid promoters by primer extension experiments (Figure 6). Total RNA was isolated from cells carrying plasmid pMVT-PfcsK grown in the absence or presence of fucose. For complementary DNA (cDNA) synthesis, a mixture of four 5΄-radioactively labelled oligonucleotides was used. They annealed to the rnaII, copG–repB, mobM and tetL transcripts, respectively, generating cDNA products of 30, 56, 73 and 102 nt, respectively (Figure 6). As expected, the mobM cDNA product was mostly detected when bacteria were grown in the presence of fucose (lanes 6 and 8), with a nearly 20-fold increase compared to the uninduced cultures (compare lanes 2 and 6). A faint band corresponding to the copG–repB cDNA product was detected in the absence (lane 4) as well as in the presence of fucose (lane 8), as expected for a self-regulated operon in which the mRNA levels are kept fairly constant (62). Regarding tetL, its transcription is known to be constitutive (63) and independent of MobM (45). However, since the plasmid copy number augmented in the presence of fucose (see below), the amount of the tetL cDNA product was nearly 3-fold higher under such a condition (compare lanes 4 and 8). Then, we determined the relative amount of the rnaII cDNA product using the tetL cDNA product as internal control. Compared to cells grown without fucose (lane 4), the relative amount of the rnaII cDNA product was 2-fold lower in the presence of fucose (lane 8). This result indicated that an increase in the intracellular levels of MobM leads to a decrease in the transcription initiating at promoter PctII.
Figure 5.
Fucose-mediated induction of mobM expression in pneumococcal cells. (A) Relevant features of plasmids pMV158, pMVT and pMVT-PfcsK: ssoU, lagging strand replication origin; oriT, origin of transfer; T1T2, tandem transcriptional terminators T1 and T2 of the Escherichia coli rrnB rRNA operon; Pmob, promoters of the mobM gene; PfcsK, promoter induced by fucose. (B) Western blot analysis using antibodies against MobM. Pneumococcal cells carrying plasmid were grown in media containing 0.3% sucrose and the indicated concentration of fucose. Total proteins were separated by sodium dodecyl sulphate (SDS)-polyacrylamide (12%) gel electrophoresis. Pre-stained proteins (Invitrogen) were run in the same gel as molecular weight markers (not shown). Purified MobM (400 ng) was run in the same gel.
Figure 6.

Primer extension reactions on total RNA isolated from pneumococcal cells harbouring plasmid pMVT-PfcsK. Bacteria were grown in the absence or presence (1%) of fucose. As primers, different 5΄-radioactively labelled oligonucleotides were used: primer PE-tet (lanes 1 and 5); primer PE-mobM (lanes 2 and 6); primer PE-rnaII (lanes 3 and 7); a mix of the PE-tet, PE-mobM, PE-cop-rep and PE-rnaII primers (lanes 4 and 8). Primers were designed taking into account the transcription start site of the genes under study. Primer extension products were analysed by 8 M urea–8% polyacrylamide gel electrophoresis. Sequence ladders were used as DNA size markers (lanes A, C, G and T). They were prepared using M13 DNA and forward sequencing primer (Table 2). The sizes (in nucleotides) of the cDNA extension products are indicated on the right.
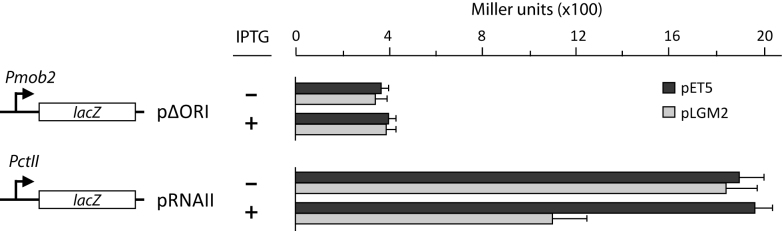
Plasmid pMV158 is able to replicate in the Gram-negative bacterium E. coli (54). In E. coli, gene mobM is transcribed from the Pmob2 promoter, which is located adjacent to oriT (Figure 1A). Binding of MobM to sequences of oriT reduces the activity of such a promoter (45). To know whether MobM is able to modulate the activity of the PctII promoter in E. coli, we constructed plasmid pRNAII (Table 1). This pMP220 (52) derivative carries the lacZ gene under control of the PctII promoter (Figure 7). We also constructed plasmid pΔORI (control plasmid), which carries the lacZ gene fused to a region that contains the Pmob2 promoter but lacks the oriTpMV158 (MobM-binding site). Both plasmids pRNAII (PctII::lacZ) and pΔORI (Pmob2::lacZ) were transferred separately to E. coli JM109(DE3) cells harbouring either plasmid pET5 (absence of mobM) or pLGM2 (presence of mobM). JM109(DE3) cells harbouring pLGM2 synthesizes MobM in the presence of IPTG (44,45). Under such a condition, the β-galactosidase activity (lacZ expression) was 2-fold lower in cells harbouring plasmids pLGM2 and pRNAII compared to cells harbouring pET5 and pRNAII. Such a reduction was not detected when bacteria were grown in the absence of IPTG (Figure 7). Moreover, no changes in lacZ expression were detected in cells carrying the Pmob2::lacZ transcriptional fusion (plasmid pΔORI) after induction of MobM synthesis. These results demonstrated that MobM decreases the activity of the PctII promoter. We conclude that in E. coli, like in S. pneumoniae, MobM functions as a repressor of the PctII promoter.
Figure 7.
β-Galactosidase assays. Relevant features of the pΔORI (fusion Pmob2::lacZ) and pRNAII (fusion PctII::lacZ) plasmids are indicated. Each plasmid was introduced into Escherichia coli JM109(DE3) harbouring either pLGM2 (mobM expression is induced by IPTG) or vector pET5 (lacking mobM). β-Galactosidase activity (Miller units, MU) was measured in bacteria growing in the absence (−) or in the presence (+) of IPTG. Each result represents the mean of five independent experiments (standard deviations are given).
MobM increases the copy number of the pMV158 replicon
In pMV158, the antisense RNAII is transcribed from the PctII promoter (Figure 1). It inhibits the synthesis of the plasmid-encoded initiator RepB protein at the translational level (37). Thus, the copy number of plasmid pMV158 depends on the intracellular levels of the antisense RNAII (36). This regulation mechanism predicted that MobM-mediated repression of the PctII promoter should lead to an increase in the plasmid copy number. To test this prediction, pneumococcal cells carrying either plasmid pMVT (control plasmid) or pMVT-PfcsK were used (Figure 5A). Both pMV158 derivatives lack oriTpMV158 (major binding site of MobM). However, whereas plasmid pMVT carries a promoter-less mobM gene, plasmid pMVT-PfcsK carries the mobM gene under the control of the fucose-inducible promoter PfcsK. These plasmid-harbouring strains were grown in media containing or not containing fucose, which allowed us to determine the effect of MobM on the plasmid copy number. Total DNA from the different bacterial cultures was analysed by agarose gel electrophoresis (Figure 8), and the plasmid copy number per genome equivalent (Nav) was determined as previously reported (35,46). In the absence of fucose, the Nav value was similar for plasmids pMVT and pMVT-PfcsK (22 ± 3 and 23 ± 4, respectively). However, in the presence of fucose, the Nav value for plasmid pMVT-PfcsK showed a 10-fold increase compared to plasmid pMVT. These results demonstrate that MobM participates in the control of plasmid DNA replication. By binding to the PctII promoter, MobM reduces the intracellular level of the antisense RNAII and, consequently, increases the plasmid copy number.
Figure 8.
Effect of MobM on the plasmid copy number. Total DNA was isolated from pneumococcal cells harbouring either pMVT (promoter-less mobM) or pMVT-PfcsK (mobM expression is induced by fucose; see Figure 5). Cells were grown in media containing (+) or not containing (−) fucose. Total DNA extracts (3, 6 and 12 μl) were analysed by agarose (0.8%) gel electrophoresis in the presence of ethidium bromide (1 μg/ml). Chromosomal DNA (chr), open circular plasmid DNA (FII) and covalently closed circular plasmid DNA (FI) are indicated. The average copy number of the plasmids (Nav) is indicated.
DISCUSSION
We have shown here the existence of intramolecular crosstalk between the dispensable conjugative mobilization module of plasmid pMV158 and the essential plasmid module involved in replication. To our knowledge, this is the first instance of communication between such plasmid modules, one involved in vertical gene transfer (replication), and the other in horizontal gene transfer (mobilization). There are some examples of transcriptional repressor proteins dedicated to copy number control, such as CopG-like proteins in the pMV158 plasmid family (64) and CopR-like proteins in the Inc18 group of plasmids (40,65). There are also some examples of repressor proteins controlling the expression of transfer operons (tra), such as TrwA in R388 (66) and, perhaps, TraN in pIP501 (40). Furthermore, some plasmid-encoded transcriptional repressors can play a multiple role in the regulation of various operons, like the Omega protein of plasmid pSM19035 (67). However, our findings on MobM, a mobilization-dedicated relaxase, regulating the synthesis of the antisense RNAII constitute a novel mechanism for modulating plasmid copy number. Our results predict that plasmids with the pMV158 replicon and an intact mobM gene should have a higher copy number than those lacking a functional mobM gene. Indeed, this was the case when Nav was measured in some of the different hosts in which the plasmid replicates (Supplementary Table S1). The increase in copy number mediated by MobM would multiply the plasmid chances to be mobilized, as well as its vertical inheritance. Although higher plasmid copy numbers may result in increased genetic load to the host, this is not the case of pMV158 (68).
Additional examples of communications between plasmid modules have been reported. Firstly, there is a crosstalk between the kis–kid (toxin–antitoxin) auxiliary maintenance system and the replication module in plasmid R1 (69). Recent results supported that the antitoxin Kis is the switch that couples both systems; they also supported that a Kid-dependent reduction in the levels of the transcriptional repressor CopB increases the copy number of plasmid R1 (70). Secondly, in the theta-replicating E. coli plasmid R388 and the Streptomyces lividans RCR-plasmid pIJ101, operons that were thought to be unrelated to plasmid segregation and/or stable inheritance have been shown to communicate either with the plasmid conjugative machinery (25) or with the plasmid stability system (24). Interactions of the initiator of conjugation, protein TraI from plasmid R1, with two plasmid-encoded proteins involved in partitioning have also been reported (22). Finally, evidence of crosstalk between replication and segregation machineries in the Vibrio cholerae chromosome II (26) has provided a further mechanism that links these two apparently different processes. It would appear that as our understanding of the biological processes deepens, the examples of crosstalk between different machineries would be more frequent than previously envisaged.
Our present findings highlight that protein MobM plays a multi-task direct role on the pMV158 lifestyle since it: (i) initiates conjugative transfer by cleavage of supercoiled plasmid DNA at a specific dinucleotide within oriTpMV158; (ii) controls the expression of its own gene and (iii) is able to control the plasmid replication process by repressing the expression of the antisense RNAII. How general could be the latter role of MobM in plasmids of the MOBV1 family? Can we conclude that their corresponding Mob relaxases control the Nav value? A search for genetic organizations similar to that of pMV158 in RCR-plasmids showed that several of them have a genetic structure that could lead to inhibition of rep mRNA translation by an antisense RNA. This situation was found in plasmids pE194 from Staphylococcus aureus, pADB201 from Mycoplasma mycoides and pLB4 from Lactobacillus plantarum (71). Whether the mobilization relaxases codified by these plasmids are involved in the regulatory circuit that controls plasmid DNA replication would need further research (72).
A comprehensive model of the regulatory circuits that control the replication of pMV158 is shown in Figure 9. This model is likely valid for several members of the plasmid family. The main regulation is exerted at two levels: (i) transcriptional, by binding of the repressor protein CopG to the promoter that directs synthesis of the single copG–repB mRNA and (ii) post-transcriptional, by pairing of the antisense RNAII with the translation initiation signals of the repB mRNA (37). CopG and RNAII are trans-acting elements that act coordinately (62). CopG has a long half-life and regulates both its own synthesis and that of the initiator RepB; thus, CopG would maintain a nearly constant level of the copG–repB mRNA. On these mRNA molecules would operate the short-lived RNAII by sensing small fluctuations in the Nav of the plasmid and correcting them quickly (14,62). How would MobM fit within this control scenario? The MobM relaxase represses transcription from its own promoter, thus controlling the intracellular amount of MobM molecules (45). Additional plasmid-encoded elements able to control the synthesis of MobM have not been identified, neither antisense RNAs nor auxiliary proteins (our unpublished results). The main role of MobM would be to act as a relaxase when conjugal transfer is triggered by yet unknown signals. However, pMV158 encodes two DNA-relaxing proteins, RepB and MobM, and both of them need supercoiled plasmid DNA as substrate. This implies that replicating plasmid molecules would not be able to participate in transfer and conversely, plasmid molecules relaxed by MobM cannot replicate. We envisage that when the plasmid mobilization process is triggered, the number of supercoiled plasmid molecules available for the replicative machinery decreases in the donor cell. Under such a situation, the ability of MobM to repress the synthesis of the antisense RNAII, one of the elements that limit the initiation of replication frequency, would provide a safety mechanism by ensuring the maintenance of the Nav of the plasmid.
Figure 9.
Crosstalk between the replication and mobilization modules of plasmid pMV158. Regulatory circuits include proteins and the antisense RNAII. Protein RepB is the initiator of leading strand replication (blue) that interacts with its target DNA (the origin of replication, dso). The copG–repB mRNA encodes also the transcriptional regulatory protein CopG (purple) that binds to and represses transcription from the single Pcr promoter. The second control element is the antisense RNAII, which acts post-transcriptionally by pairing with the translation initiation signals of the essential repB mRNA. MobM (orange) is involved in the initiation of plasmid conjugative transfer (relaxase activity). In addition, it controls its own synthesis by binding to promoter Pmob and hindering the binding of the host RNA polymerase. Further, as reported here, MobM acts as a regulatory element in the plasmid replication control circuit, because it represses transcription of rnaII from promoter PctII. Positively regulated circuits are indicated by a + in green, whereas negatively regulated circuits are indicated by a − in red.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dhruba Chattoraj for his critical reading of the manuscript and Verónica Navarro-Martínez for technical help.
Author contributions: All authors designed research and analysed data. F.L.D, C.F.L, R.L. and A.B. performed experiments; M.E. wrote the first draft, A.B. the next draft and all authors worked on this last one until production of the final version of the paper.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Spanish Ministry of Economy and Competitiveness [BIO2013-49148-C2-2-R, BIO2015-69085-REDC]; AEI/FEDER, UE [BIO2016-76412-C2-2-R to A.B.]; Sara Borrell contract [CD13/00304 to F.L.D.]. Funding for open access charge: AEI/FEDER, UE [BIO2016-76412-C2-2-R to A.B.].
Conflict of interest statement. None declared.
REFERENCES
- 1. Syvanen M. Evolutionary implications of horizontal gene transfer. Ann. Rev. Genet. 2012; 46:341–358. [DOI] [PubMed] [Google Scholar]
- 2. Smillie C., Garcillan-Barcia M.P., Francia M.V., Rocha E.P.C., de la Cruz F.. Mobility of plasmids. Microbiol. Mol. Biol. Rev. 2010; 74:434–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lanza V.F., Tedim A.P., Martinez J.L., Baquero F., Coque T.M.. The plasmidome of Firmicutes: impact on the emergence and the spread of resistance to antimicrobials. Microbiol. Spectr. 2015; 3, doi:10.1128/microbiolspec.PLAS-0039-2014. [DOI] [PubMed] [Google Scholar]
- 4. Wozniak R.A.F., Waldor M.K.. Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat. Rev. Microbiol. 2010; 8:552–563. [DOI] [PubMed] [Google Scholar]
- 5. Bellanger X., Payot S., Leblond-Bourget N., Guédon G.. Conjugative and mobilizable genomic islands in bacteria: evolution and diversity. FEMS Microbiol. Rev. 2014; 38:720–760. [DOI] [PubMed] [Google Scholar]
- 6. Schaack S., Gilbert C., Feschotte C.. Promiscuous DNA: horizontal transfer of transposable elements and why it matters for eukaryotic evolution. Trends Ecol. Evol. 2010; 25:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lacroix B., Citovsky V.. Transfer of DNA from bacteria to eukaryotes. Mbio. 2016; 7, doi:10.1128/mBio.00863-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siefert J.L. Defining the mobilome. Methods Mol. Biol. 2009; 532:13–27. [DOI] [PubMed] [Google Scholar]
- 9. Boucher Y., Douady C.J., Papke R.T., Walsh D.A., Boudreau M.E., Nesbo C.L., Case R.J., Doolittle W.F.. Lateral gene transfer and the origins of prokaryotic groups. Ann. Rev. Genet. 2003; 37:283–328. [DOI] [PubMed] [Google Scholar]
- 10. Touchon M., Bobay L.-M., Rocha E.P.C.. The chromosomal accommodation and domestication of mobile genetic elements. Curr. Opin. Microbiol. 2014; 22:22–29. [DOI] [PubMed] [Google Scholar]
- 11. del Solar G., Alonso J.C., Espinosa M., Díaz-Orejas R.. Broad host range plasmid replication: an open question. Mol. Microbiol. 1996; 21:661–666. [DOI] [PubMed] [Google Scholar]
- 12. Brantl S. Antisense-RNA mediated control of plasmid replication—pIP501 revisited. Plasmid. 2015; 78:4–16. [DOI] [PubMed] [Google Scholar]
- 13. Chattoraj D. Control of plasmid replication by iterons: no longer paradoxical. Mol. Microbiol. 2000; 37:467–476. [DOI] [PubMed] [Google Scholar]
- 14. del Solar G., Espinosa M.. Plasmid copy number control: an ever-growing story. Mol. Microbiol. 2000; 37:492–500. [DOI] [PubMed] [Google Scholar]
- 15. Ramachandran R., Jha J., Paulsson J., Chattoraj D.. Random versus cell cycle-regulated replication initiation in bacteria: insights from studying Vibrio cholerae chromosome 2. Microbiol. Mol. Biol. Rev. 2017; 81, doi:10.1128/MMBR.00033-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Highlander S.K., Novick R.P.. Plasmid repopulation kinetics in Staphylococcus aureus. Plasmid. 1987; 17:210–221. [DOI] [PubMed] [Google Scholar]
- 17. del Solar G., Giraldo R., Ruiz-Echevarría M.J., Espinosa M., Díaz-Orejas R.. Replication and control of circular bacterial plasmids. Microbiol. Mol. Biol. Rev. 1998; 62:434–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nordström K., Austin S.. Mechanisms that contribute to the stable segregation of plasmids. Annu. Rev. Genet. 1989; 23:37–69. [DOI] [PubMed] [Google Scholar]
- 19. Novick R.P. Staphylococcal plasmids and their replication. Annu. Rev. Microbiol. 1989; 43:537–565. [DOI] [PubMed] [Google Scholar]
- 20. Novick R.P., Iordanescu S., Projan S.J., Kornblum J., Edelman I.. pT181 plasmid replication is regulated by a countertranscript-driven transcriptional attenuator. Cell. 1989; 59:395–404. [DOI] [PubMed] [Google Scholar]
- 21. Austin S.J., Nordström K.. Partition-mediated incompatibility of bacterial plasmids. Cell. 1990; 60:351–354. [DOI] [PubMed] [Google Scholar]
- 22. Gruber C.J., Lang S., Rajendra V.K.H., Nuk M., Raffl S., Schildbach J.F., Zechner E.L.. Conjugative DNA transfer is enhanced by plasmid R1 partitioning proteins. Front. Mol. Biosci. 2016; 3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Funnell B. ParB partition proteins: complex formation and spreading at bacterial and plasmid centromeres. Front. Mol. Biosci. 2016; 3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thoma L., Sepulveda E., Latus A., Muth G.. The stability region of the Streptomyces lividans plasmid pIJ101 encodes a DNA-binding protein recognizing a highly conserved short palindromic sequence motif. Front. Microbiol. 2014; 5:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guynet C., Cuevas A., Moncalian G., de la Cruz F.. The stb operon balances the requirements for vegetative stability and conjugative transfer of plasmid R388. PLoS Genet. 2011; 7:e1002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Venkova-Canova T., Baek J.H., FitzGerald P.C., Blokesch M., Chattoraj D.K.. Evidence for two different regulatory mechanisms linking replication and segregation of Vibrio cholerae Chromosome II. PLoS Genet. 2013; 9:e1003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fernandez-Lopez R., Garcillan-Barcia M.P., Revilla C., Lazaro M., Vielva L., de la Cruz F.. Dynamics of the IncW genetic backbone imply general trends in conjugative plasmid evolution. FEMS Microbiol. Rev. 2006; 30:942–966. [DOI] [PubMed] [Google Scholar]
- 28. Wellington E.M., Boxall A.B., Cross P., Feil E.J., Gaze W.H., Hawkey P.M., Johnson-Rollings A.S., Jones D.L., Lee N.M., Otten W. et al. . The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect. Dis. 2013; 13:155–165. [DOI] [PubMed] [Google Scholar]
- 29. Walker A. Welcome to the plasmidome. Nature Rev. Microbiol. 2012; 10:379. [DOI] [PubMed] [Google Scholar]
- 30. Reyes-Lamothe R., Tran T., Meas D., Lee L.N., Li A.M., Sherratt D.J., Tolmasky M.E.. High-copy bacterial plasmids diffuse in the nucleoid-free space, replicate stochastically and are randomly partitioned at cell division. Nucleic Acids Res. 2014; 42:1042–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Novick R.P. Contrasting lifestyles of rolling-circle phages and plasmids. Trends Biochem. Sci. 1998; 23:434–438. [DOI] [PubMed] [Google Scholar]
- 32. Rasooly A., Wang P.-Z., Novick R.P.. Replication-specific conversion of the Staphylococcus aureus pT181 initiator protein from an active homodimer to an inactive heterodimer. EMBO J. 1994; 13:5245–5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lorenzo-Díaz F., Fernández-López C., Garcillán-Barcia M.P., Espinosa M.. Bringing them together: Plasmid pMV158 rolling circle replication and conjugation under an evolutionary perspective. Plasmid. 2014; 74:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kramer M.G., del Solar G., Espinosa M.. Lagging-strand origins of the promiscuous plasmid pMV158: physical and functional characterization. Microbiology. 1995; 141:655–662. [DOI] [PubMed] [Google Scholar]
- 35. Lorenzo-Díaz F., Espinosa M.. Lagging strand DNA replication origins are required for conjugal transfer of the promiscuous plasmid pMV158. J. Bacteriol. 2009; 191:720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. del Solar G., Acebo P., Espinosa M.. Replication control of plasmid pLS1: the antisense RNA II and the compact rnaII region are involved in translational regulation of the initiator RepB synthesis. Mol. Microbiol. 1997; 23:95–108. [DOI] [PubMed] [Google Scholar]
- 37. López-Aguilar C., Romero-López C., Espinosa M., Berzal-Herranz A., del Solar G.. The 5΄-tail of antisense RNAII of pMV158 plays a critical role in binding to the target mRNA and in translation inhibition of repB. Front. Genet. 2015; 6:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hernandez-Arriaga A.M., Rubio-Lepe T.S., Espinosa M., del Solar G.. Repressor CopG prevents access of RNA polymerase to promoter and actively dissociates open complexes. Nucleic Acids Res. 2009; 37:4799–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Le Chatelier E., Ehrlich S.D., Janniére L.. The pAMb1 CopF repressor regulates plasmid copy number by controlling transcription of the repE gene. Mol. Microbiol. 1994; 14:463–471. [DOI] [PubMed] [Google Scholar]
- 40. Grohmann E., Goessweiner-Mohr N., Brantl S.. DNA-binding proteins regulating pIP501 transfer and replication. Front. Mol. Biosci. 2016; 3:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Priebe S.D., Lacks S.A.. Region of the streptococcal plasmid pMV158 required for conjugative mobilization. J. Bacteriol. 1989; 171:4778–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garcillan-Barcia M.P., Francia M.V., de la Cruz F.. The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol. Rev. 2009; 33:657–687. [DOI] [PubMed] [Google Scholar]
- 43. Fernández-López C., Bravo A., Ruiz-Cruz S., Solano-Collado V., Garsin D.A., Lorenzo-Díaz F., Espinosa M.. Mobilizable rolling-circle replicating plasmids from Gram-positive bacteria: a low-cost conjugative transfer. Microbiol. Spectr. 2014; 2, doi:10.1128/microbiolspec.PLAS-0008-2013. [DOI] [PubMed] [Google Scholar]
- 44. Guzmán L.M., Espinosa M.. The mobilization protein, MobM, of the streptococcal plasmid pMV158 specifically cleaves supercoiled DNA at the plasmid oriT. J. Mol. Biol. 1997; 266:688–702. [DOI] [PubMed] [Google Scholar]
- 45. Lorenzo-Díaz F., Solano-Collado V., Lurz R., Bravo A., Espinosa M.. Autoregulation of the synthesis of the MobM relaxase encoded by the promiscuous plasmid pMV158. J. Bacteriol. 2012; 194:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. del Solar G., Espinosa M.. The copy number of plasmid pLS1 is regulated by two trans-acting plasmid products: the antisense RNA II and the repressor protein, RepA. Mol. Microbiol. 1992; 6:83–94. [DOI] [PubMed] [Google Scholar]
- 47. Studier F.W., Moffatt B.A.. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 1986; 189:113–130. [DOI] [PubMed] [Google Scholar]
- 48. Lorenzo-Díaz F., Dostál L., Coll M., Schildbach J.F., Menendez M., Espinosa M.. The MobM-relaxase domain of plasmid pMV158: thermal stability and activity upon Mn2+-and DNA specific-binding. Nucleic Acids Res. 2011; 39:4315–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ruiz-Cruz S., Solano-Collado V., Espinosa M., Bravo A.. Novel plasmid-based genetic tools for the study of promoters and terminators in Streptococcus pneumoniae and Enterococcus faecalis. J. Microb. Meth. 2010; 83:156–163. [DOI] [PubMed] [Google Scholar]
- 50. Brosius J., Dull T.J., Sleeter D.D., Noller H.F.. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J. Mol. Biol. 1981; 148:107–127. [DOI] [PubMed] [Google Scholar]
- 51. Chan P.F., O’Dwyer K.M., Palmer L.M., Ambrad J.D., Ingraham K.A., So C., Lonetto M.A., Biswas S., Rosenberg M., Holmes D.J. et al. . Characterization of a novel fucose-regulated promoter (PfcsK) suitable for gene essentiality and antibacterial mode-of-action studies in Streptococcus pneumoniae. J. Bacteriol. 2003; 185:2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spaink H.P., Okker R., Wijffelman C., Pees E., Lugtengerg B.. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRLIJI. Plant Mol. Biol. 1987; 9:27–39. [DOI] [PubMed] [Google Scholar]
- 53. Burdett V. Identification of tetracycline-resistant R-plasmids in Streptococcus agalactiae (group B). Antimicrob. Agents Chemother. 1980; 18:753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. del Solar G., Díaz R., Espinosa M.. Replication of the streptococcal plasmid pMV158 and derivatives in cell-free extracts of Escherichia coli. Mol. Gen. Genet. 1987; 206:428–435. [DOI] [PubMed] [Google Scholar]
- 55. Spiess E., Lurz R.. Electron microscopic analysis of nucleic acids and nucleic acid-protein complexes. Methods Microbiol. 1988; 20:293–323. [Google Scholar]
- 56. Gralla J.D. Rapid “footprinting" on supercoiled DNA. Proc. Natl. Acad. Sci. U.S.A. 1985; 82:3078–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tugores A., Brenner D.A.. A method for in vitro DNase I footprinting analysis on supercoiled templates. Biotechniques. 1994; 17:410–412. [PubMed] [Google Scholar]
- 58. Solano-Collado V., Espinosa M., Bravo A.. Activator role of the pneumococcal Mga-like virulence transcriptional regulator. J. Bacteriol. 2012; 194:4197–4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miller J.H. Experiments in Molecular Genetics. 1972; NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 60. Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003; 31:3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grohmann E., Guzmán L.M., Espinosa M.. Mobilisation of the streptococcal plasmid pMV158: interactions of MobM protein with its cognate oriT DNA region. Mol. Gen. Genet. 1999; 261:707–715. [DOI] [PubMed] [Google Scholar]
- 62. del Solar G., Acebo P., Espinosa M.. Replication control of plasmid pLS1: efficient regulation of plasmid copy number is exerted by the combined action of two plasmid components, CopG and RNA II. Mol. Microbiol. 1995; 18:913–924. [DOI] [PubMed] [Google Scholar]
- 63. Lacks S.A., López P., Greenberg B., Espinosa M.. Identification and analysis of genes for tetracycline resistance and replication functions in the broad-host-range plasmid pLS1. J. Mol. Biol. 1986; 192:753–765. [DOI] [PubMed] [Google Scholar]
- 64. del Solar G., Hernández-Arriaga A.M., Gomis-Rüth F.X., Coll M., Espinosa M.. A genetically economical family of plasmid-encoded transcriptional repressors in control of plasmid copy number. J. Bacteriol. 2002; 184:4943–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brantl S., Wagner E.G.H.. Dual function of the copR gene product of plasmid pIP501. J. Bacteriol. 1997; 179:7016–7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moncalian G., de la Cruz F.. DNA binding properties of protein TrwA, a possible structural variant of the Arc repressor superfamily. Biochim. Biophys. Acta. 2004; 1701:15–23. [DOI] [PubMed] [Google Scholar]
- 67. de la Hoz A.B., Ayora S., Sitkiewicz I., Pankiewicz R., Alonso J.C., Ceglowski P.. Plasmid copy-number control and better-than-random segregation genes of pSM19035 share a common regulator. Proc. Natl. Acad. Sci. U.S.A. 2000; 97:728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hernández-Arriaga A.M., Espinosa M., Del Solar G.. Fitness of the pMV158 replicon in Streptococcus pneumoniae. Plasmid. 2012; 67:162–166. [DOI] [PubMed] [Google Scholar]
- 69. Bravo A., Ortega S., de Torrontegui G., Díaz R.. Killing of Escherichia coli cells modulated by components of the stability system ParD of plasmid R1. Mol. Gen. Genet. 1988; 215:146–151. [DOI] [PubMed] [Google Scholar]
- 70. López-Villarejo J., Lobato-Márquez D., Díaz-Orejas R.. Coupling between the basic replicon and the Kis-Kid maintenance system of plasmid R1: Modulation by Kis antitoxin levels and involvement in control of plasmid replication. Toxins. 2015; 7:478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brantl S. Plasmid replication control by antisense RNAs. Microbiol. Spectr. 2014; 2, doi:10.1128/microbiolspec.PLAS-0001-2013. [DOI] [PubMed] [Google Scholar]
- 72. Farias M.E., Grohmann E., Espinosa M.. Expression of the mobM gene of the streptococcal plasmid pMV158 in Lactococcus lactis subsp. lactis. FEMS Microbiol. Lett. 1999; 176:403–410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.