Abstract
Although radical prostatectomy is a popular treatment modality for clinically localized prostate cancer, 10-year biochemical recurrence can reach 28%. Before salvage radiation therapy (SRT), prostate-specific antigen (PSA) values alone should be used cautiously in predicting SRT eligibility. A long, slow PSA rise may suggest locally confined disease still amenable to SRT; corresponding imaging to identify potential gross recurrence is useful. Patients with local disease may safely benefit from higher doses of radiation.
Keywords: Salvage radiation therapy, Prostate-specific antigen, Radical prostatectomy
Although radical prostatectomy is a popular treatment modality for clinically localized prostate cancer, 10-year biochemical recurrence can reach 28%.1 Before salvage radiation therapy (SRT), prostate-specific antigen (PSA) values alone should be used cautiously in predicting SRT eligibility. A long, slow postprostatectomy PSA rise should be evaluated with magnetic resonance imaging (MRI) to identify potential gross local recurrence, which may respond to SRT. Radiographically identified gross recurrence may benefit from higher than standard radiation doses.
The key to successful SRT following radical prostatectomy (RP) is distinguishing between local and distant recurrence. Unfortunately, no diagnostic test can accurately distinguish local from distant recurrence at low PSA levels when biochemical recurrence is first observed. Most clinicians use clinical correlates to prognosticate the effectiveness of SRT. Among other factors, a high PSA level prior to SRT has been correlated with poor control.2 However, a long, slow PSA rise, despite a high PSA value, may independently predict local recurrence and good biochemical control following SRT.3 A high PSA value may also be associated with clinically detectable gross disease within the prostate fossa. Patients with gross recurrent disease have been reported to have poorer outcomes following standard SRT doses when compared with their biochemical progression-only counterparts.4
Here we present two patients with biochemical recurrence after RP. Both present with high PSA values (some practitioners choose to forego referral for SRT with PSA values this high). Both patients had MRI demonstrating evidence of gross disease in the prostatic fossa and were treated with an integrated boost, as standard doses were thought to be insufficient.
Our case report emphasizes three key points. First, a high pre-SRT PSA value does not automatically equate with a poor SRT outcome. Second, patients with a long, slow PSA rise should be evaluated with MRI to identify potential gross recurrence. And third, radiographically identified recurrence may benefit from higher radiation doses.
Presentation
Patient 1 is a 59-year-old white man with post-RP pathology showing a margin-negative, pT2c, Gleason score 3 + 4 5 7. His preoperative PSA level was 7.1 ng/mL. His post-RP PSA level was 0.1 ng/mL, which rose over 11 years to a pre- SRT level of 1.59 ng/mL. Pre-SRT MRI showed a small soft-tissue lesion, measuring 1.7 × 0.7 × 1.2 cm, within the surgical bed at the level of the vesicoureteral anastomosis (Figure 1). The patient was treated with rotational arcbased intensity-modulated radiation therapy (IMRT) using 6 MV photons. Dose to the prostate fossa was 66.6 Gy in 37 fractions, and an integrated boost treated the nodule to 74 Gy in 37 fractions (Figure 2). The patient did not receive any hormone therapy.
Figure 1.
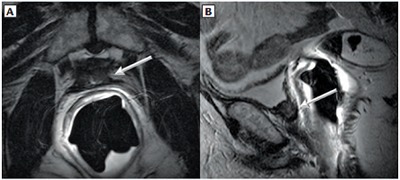
Patient 1, a 59-year-old man with biochemical recurrence after radical retropubic prostatectomy. Transaxial (A) and sagittal (B) T2-weighted magnetic resonance images without fat saturation. A soft-tissue signal-intensity nodule (arrows) measuring 1.7 × 0.7 × 1.2 cm was detected in the prostatectomy bed involving the neck of the urinary bladder.
Figure 2.
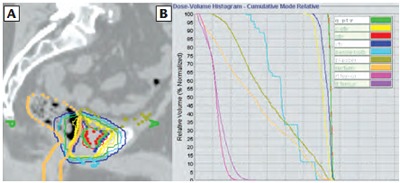
Patient 1 (A) dosimetry (saggital) and corresponding (B) dose volume histogram showing gross tumor volume (red) treated to 74 Gy. Gross disease planning tumor volume is displayed in green and standard prostate fossa planning tumor volume is shown in yellow.
Patient 2 is a 79-year-old Hispanic man with post-RP pathology showing bilateral seminal vesicle invasion, right capsule extension, positive margin, and Gleason score 3 + 4 = 7. His preoperative PSA level was 7.8 ng/mL. His post-RP PSA was 0.1 ng/mL, which then rose over 11 years, due to the patient declining workup, to a pre-SRT level of 6.5 ng/mL. Pre-SRT MRI showed a small soft-tissue lesion anterior to the rectum and posterior to the bladder anastomosis within the operative bed, measuring 1.0 × 0.6 × 0.6 cm (Figure 3). The patient was treated with rotational arc-based IMRT using 6 MV photons. Dose to the prostate fossa was 68.4 Gy in 38 fractions, and an integrated boost treated the nodule to 76 Gy in 38 fractions (Figure 4). This patient refused hormone therapy.
Figure 3.
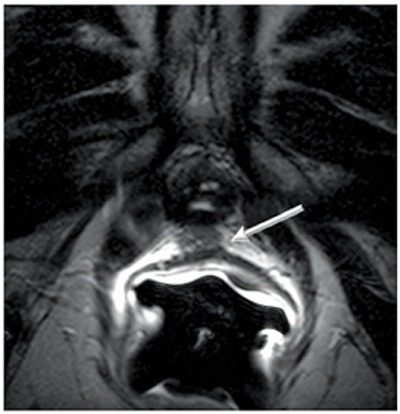
Patient 2, a 79-year-old man with biochemical recurrence after radical prostatectomy. Transaxial T2-weighted magnetic resonance image without fat saturation. A soft-tissue signal-intensity nodule (arrow) measuring 1.0 × 0.6 × 0.6 cm was detected in the prostatectomy bed between the rectum and the neck of the urinary bladder.
Figure 4.
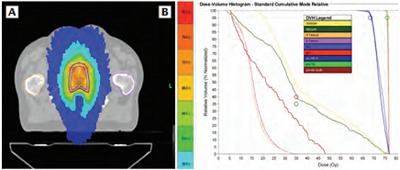
Patient 2, (A) dosimetry (axial) and corresponding (B) dose volume histogram showing gross tumor volume (red) treated to 76 Gy. Gross disease planning tumor volume is displayed in green and standard prostate fossa planning tumor volume is shown in blue. Hot-spot of 81.3 Gy.
Following completion of SRT, both patients had their PSA values fall to an undetectable level (Figure 5). Neither patient had any late toxicity other than erectile dysfunction, which was present prior to the start of SRT. Patient 2 developed a separate epidermal growth factor receptor-mutated non–small-cell lung cancer and died of that disease.
Figure 5.
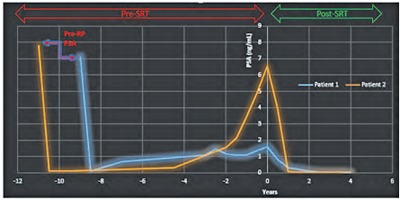
Post-SRT PSA history. The last PSA value before radical prostatectomy is labeled. Subsequent pre-SRT values are in years before SRT. PSA values at “time = 0” represent the last recorded pre-SRT value. Each value that follows is the corresponding PSA for the specified post-SRT year. Both pre-SRT values, 1.59 ng/mL (Patient 1) and 6.5 ng/mL (Patient 2), approach 0 after SRT. PSA, prostate-specific antigen; RP, radical prostatectomy; SRT, salvage radiation therapy.
Discussion
Post-RP PSA levels should remain suppressed and undetectable. A detectable PSA level may be attributed to local recurrence at the prostatic fossa, metastasis, residual benign prostatic tissue, or some combination thereof. Although RP is a popular modality, 10-year biochemical recurrences of up to 28% are reported.1
Without SRT, the median time from PSA recurrence to metastasis is 8 years.5 SRT reduces prostate cancer-specific mortality compared with observation alone,3 and delays time to hormone administration and systemic progression.6 SRT can be curative in patients with biochemical recurrence following RP7; however, less than half of patients who receive a post-RP secondary therapy of some sort will receive SRT.8 It is important to note that all men may not benefit from SRT, particularly those with findings suggestive of systemic disease. In this setting, balancing potential benefits against therapeutic toxicities and underlying comorbidities can be challenging and significant differences in practice between urologist and radiation oncologist recommendations for post-RP radiotherapy exist.9
SRT reduces prostate cancer-specific mortality compared with observation alone, and delays time to hormone administration and systemic progression.
A short time to postoperative PSA failure, short PSA doubling time, and elevated PSA velocity prior to RP have all been associated with presumed subclinical metastatic disease.
SRT does not address subclinical disease outside of the prostate fossa, and efforts to identify subclinical systemic disease are challenging. Post-RP factors predictive of local or metastatic disease include PSA velocity, Gleason score, and pathologic stage.2 A short time to postoperative PSA failure, short PSA doubling time, and elevated PSA velocity prior to RP have all been associated with presumed subclinical metastatic disease.10,11 Frequently, clinicians weigh presenting post-RP PSA level heavily as part of this determination; our case report illustrates why this should be done with caution.
The ideal SRT dose to the prostatic fossa is still debated, along with the role of neoadjuvant androgen deprivation therapy. Technologic development has facilitated dose escalation, and doses have slowly been rising. Modest doses of 64 to 65 Gy may be insufficient for durable biochemical control.12,13 Doses above 65 Gy correlate with improved disease-free survival.14 Dose escalations to 70 Gy, versus 60 Gy, demonstrate improved 5-year biochemical control.15 Guidelines endorse a minimum of 64 to 65 Gy,16 but patients with gross disease probably require significantly higher doses, as displayed by the sustained biochemical response in our report.
Endorectal coil MR has the potential to capture a high proportion of suspected local recurrence after RP.17 Although no minimum PSA threshold for initiating MRI has been established, many clinicians will use PSA kinetics and value at presentation to guide decision making. Interestingly, Sella and colleagues17 reported that post-RP endorectal coil MRI confirmed local recurrence in 38% of their cohort when PSA levels were lower than 0.4 ng/mL at the time of imaging. Dynamic contrast-enhanced (DCE) MRI is used in combination with diffusion-weighted imaging for local detection after radiation therapy,18 whereas DCE-MRI alone can effectively increase detection utility in the post-RP setting.18 The radiographic confirmation of local disease by MRI here allowed for treatment with an additional integrated radiotherapy boost. Despite the escalated dose—higher than most published studies—neither patient demonstrated late urinary or rectal symptoms. Studies on dose escalation have shown similar results.19
Dynamic contrast-enhanced (DCE) MRI is used in combination with diffusion-weighted imaging for local detection after radiation therapy, whereas DCE-MRI alone can effectively increase detection utility in the post-RP setting.
Post-RP hormone therapy with SRT has been shown to improve overall survival.20 Of note, both treated patients did not receive hormone therapy. Our study suggests that pre-SRT PSA values alone should be used cautiously in predicting SRT eligibility. Instead, we advocate incorporating the pattern of PSA rise. A long, slow PSA rise may suggest locally confined disease still amenable to SRT; corresponding imaging to identify potential gross recurrence is useful. Patients with local disease may safely benefit from higher doses, here treated with an integrated boost up to 76 Gy.
Main Points.
Before salvage radiation therapy (SRT), prostate-specific antigen (PSA) values should be used cautiously in predicting SRT eligibility. Incorporating the pattern of PSA rise is recommended. A long, slow PSA rise may suggest locally confined disease still amenable to SRT.
Postprostatectomy PSA rise should be evaluated with magnetic resonance imaging (MRI) to identify potential gross local recurrence, which may respond to SRT. Although no minimum PSA threshold for initiating MRI has been established, many clinicians will use PSA kinetics and value at presentation to guide decision making.
Radiographically identified gross recurrence may benefit from higher than standard radiation doses.
References
- 1.Han M, Partin AW, Zahurak M, et al. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–523. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 2.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–2041. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trock BJ, Han M, Freedland SJ, et al. Prostate cancerspecific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald OK, Schild SE, Vora S, et al. Salvage radiotherapy for men with isolated rising PSA or locally palpable recurrence after radical prostatectomy: do outcomes differ. Urology. 2004;64:760–764. doi: 10.1016/j.urology.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Pound CR, Partin AW, Epstein JI, Walsh PC. Prostatespecific antigen after anatomic radical retropubic prostatectomy: patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395–406. doi: 10.1016/s0094-0143(05)70386-4. [DOI] [PubMed] [Google Scholar]
- 6.Boorjian SA, Karnes RJ, Crispen PL, et al. Radiation therapy after radical prostatectomy: impact on metastasis and survival. J Urol. 2009;182:2708–2715. doi: 10.1016/j.juro.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 7.vander Kooy MJ, Pisansky TM, Cha SS, Blute ML. Irradiation for locally recurrent carcinoma of the prostate following radical prostatectomy. Urology. 1997;49:65–70. doi: 10.1016/S0090-4295(96)00371-8. [DOI] [PubMed] [Google Scholar]
- 8.Grossfeld GD, Stier DM, Flanders SC, et al. Use of second treatment following definitive local therapy for prostate cancer: data from the caPSURE database. J Urol. 1998;160:1398–1404. [PubMed] [Google Scholar]
- 9.Lavallée LT, Fergusson D, Mallick R, et al. Radiotherapy after radical prostatectomy: treatment recommendations differ between urologists and radiation oncologists. PLoS One. 2013;8:e79773. doi: 10.1371/journal.pone.0079773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partin AW, Pearson JD, Landis PK, et al. Evaluation of serum prostate-specific antigen velocity after radical prostatectomy to distinguish local recurrence from distant metastases. Urology. 1994;43:649–659. doi: 10.1016/0090-4295(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 11.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 12.Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325–1332. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 13.Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol Phys. 2000;48:369–375. doi: 10.1016/s0360-3016(00)00645-3. [DOI] [PubMed] [Google Scholar]
- 14.King CR, Spiotto MT. Improved outcomes with higher doses for salvage radiotherapy after prostatectomy. Int J Radiat Oncol Biol Phys. 2008;71:23–27. doi: 10.1016/j.ijrobp.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 15.Wong GW, Palazzi-Churas KL, Jarrard DF, et al. Salvage hypofractionated radiotherapy for biochemically recurrent prostate cancer after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2008;70:449–455. doi: 10.1016/j.ijrobp.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 16.Valicenti RK, Thompson I, Jr, Albertsen P, et al. American Society for Radiation Oncology/American Urological Association, authors. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. Int J Radiat Oncol Biol Phys. 2013;86:822–828. doi: 10.1016/j.ijrobp.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Sella T, Schwartz LH, Swindle PW, et al. Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology. 2004;231:379–385. doi: 10.1148/radiol.2312030011. [DOI] [PubMed] [Google Scholar]
- 18.Roy C, Foudi F, Charton J, et al. Comparative sensitivities of functional MRI sequences in detection of local recurrence of prostate carcinoma after radical prostatectomy or external-beam radiotherapy. AJR Am J Roentgenol. 2013;200:W361–W368. doi: 10.2214/AJR.12.9106. [DOI] [PubMed] [Google Scholar]
- 19.Orman AG, Pollack A, Stoyanova R, et al. A phase 3 randomized trial of MRI-mapped dose-escalated salvage radiotherapy postprostatectomy: the MAPS Trial, an initial dosimetric assessment. Int J Radiat Oncol Biol Phys. 2014;90(suppl):S413–S414. [Google Scholar]
- 20.Shipley WU, Seiferheld W, Lukka H, et al. Report of NRG Oncology/RTOG 9601, a phase 3 trial in prostate cancer: anti-androgen therapy (AAT) with bicalutamide during and after radiation therapy (RT) in patients following radical prostatectomy (RP) with pT2–3pN0 disease and an elevated PSA. Int J Radiat Oncol Biol Phys. 2016;94:3. [Google Scholar]


