Abstract
For decades, the prognosis for patients with advanced-stage non–small cell lung cancer (NSCLC) was bleak, with chemotherapy offering limited benefit and much toxicity. Now, with mutational testing, new generations of targeted therapies, and emerging immunotherapies, the treatment horizon for these patients has greatly expanded. In this article, the authors review molecular targets, biomarkers, as well as immune checkpoint inhibitors, which are having a major impact on the management of this patient population.
Molecular targets, biomarkers, their treatments, and immunotherapy have changed the treatment paradigm for non–small cell lung cancer (NSCLC) from chemotherapy ("one-size-fits-all") approach to specific recommendations for patients based on the presence or absence of gene mutations ("personalized medicine"). For decades, chemotherapy—with modest response rates, survival rates measured in weeks/months, and significant toxicities—was the mainstay of treatment for patients with advanced-stage lung cancer. The current trend is to treat patients based on specific pathology (squamous cell carcinoma or nonsquamous cell carcinoma), and the presence (or not) of gene mutations.
Biomarkers have various functions including diagnostic, monitoring, staging, predictive, and prognostic values (Grande, Viale, & Yamamoto, 2010). Predictive markers determine the particular therapy for select patients (Aggarwal, Somaiah, & Simon, 2010; Grande et al., 2010). Prognostic markers forecast those tumors that are likely to recur (lead to death) regardless of therapy (Kreamer, Eaby-Sandy, Sherry, & Stonehouse-Lee, 2011).
Somatic genome alterations, known as "driver mutations," are the most useful predictive markers for determining the efficacy of targeted therapy (Sequist & Neal, 2015). Driver mutations are usually transformative, meaning they initiate the change from a noncancerous cell to a malignant cell (Sequist & Neal, 2015). Driver mutations pass on a reliance (oncogene addiction) on cancer cells to continuously receive signals from the driver to survive (signal transduction; Sequist & Neal, 2015). Normal cellular mechanisms, which regulate cell growth, differentiation, and cell death, no longer function. Epidermal growth factor receptor (EGFR), Kirsten rat sarcoma viral oncogene homolog (KRAS), anaplastic lymphoma kinase (ALK), and ROS1 are driver mutations.
EPIDERMAL GROWTH FACTOR RECEPTOR
Epidermal growth factor receptor (EGFR) is the most common driver mutation in NSCLC, specifically adenocarcinomas (Lynch et al., 2004; Paez et al., 2004; Pao et al., 2004). This mutation belongs to the HER/ErbB family of receptor tyrosine kinases, which includes EGFR 2 (HER2/neu/ErbB2), EGFR 3 (HER3/ErbB3), and EGFR 4 (HER4/ErbB4; da Cunha Santos, Shepherd, & Tsao, 2011). Found on normal cells, EGFR is a transmembrane (has both extracellular and intracellular components), ligand-binding receptor (Kreamer et al., 2011). In normal cellular functions and pathways, EGFR has a significant role in cellular proliferation and differentiation (Yano et al., 2003).
During normal cellular activity, dimerization (ligands binding to extracellular receptors) and autophosphorylation occur, thus initiating an intracellular cascade of downstream signals resulting in normal cell growth, differentiation, and cell death (Kreamer et al., 2011). In malignant cells, dysregulation of the intracellular (tyrosine kinase) activity of EGFR may be caused by EGFR protein overexpression, EGFR gene mutations, and/or increased gene copy number (da Cunha Santos et al., 2011; Ciradello & Tortora, 2008), resulting in uncontrolled cellular proliferation, invasion, and inhibition of apoptosis (Kreamer et al., 2011).
EGFR Mutations
Mutations in EGFR occur in approximately 15% of white and African American patients with NSCLC; 30% of NSCLC of Asian ethnicity; and are associated with adenocarcinoma histology, female gender, and nonsmoking status (Massarelli et al., 2013; Cote et al., 2011; Reinersman et al., 2011; Shigematsu et al., 2005; Tokumo et al., 2005). Mutations in EGFR exist in the first four exons (18–21) of the tyrosine kinase domain of EGFR (See Table; Kreamer et al., 2011). The most common mutations involve point mutations in exon 18, insertions or deletions in exon 19, insertions/duplications and point mutations in exon 20, and point mutations in exon 21 (Massarelli et al., 2013). Point mutations in exon 18, predominantly G719, account for approximately 4% to 5% of EGFR mutations and are less sensitive to EGFR tyrosine kinase inhibitors (TKIs; Massarelli et al., 2013; Sharma, Bell, Settleman, & Haber, 2007).
Table.
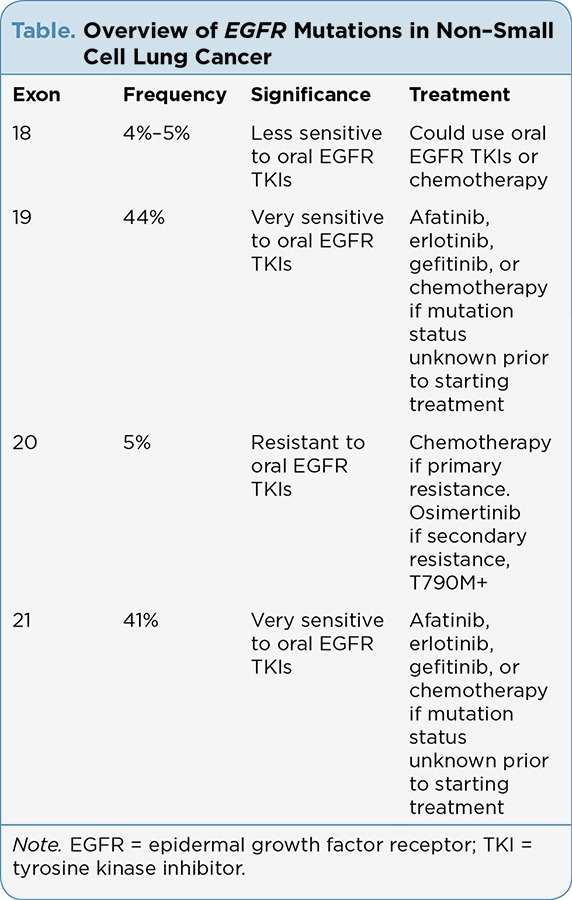
Overview of EGFR Mutations in Non–Small Cell Lung Cancer
The most common EGFR mutations are in exons 19 and 21 and account for 44% and 41% of all EGFR activation mutations, respectively, and are sensitive to treatment with EGFR TKIs (Massarelli et al., 2013). Mutations in EGFR in exon 19 include in-frame deletions, which frequently encompass L747 and E749; L858R is the most common point mutation for exon 21 (Massarelli et al., 2013). In-frame insertions and point mutations in exon 20 account for 5% of EGFR mutations (Zhang, Stiegler, Boggon, Kobayashi, & Halmos, 2010) and are resistant to EGFR TKIs (Massarelli et al., 2013).
Treatment of EGFR-Mutant Disease
Many factors are involved in treatment decisions for patients with NSCLC. Pathology, histology, the presence or absence of gene mutations, and the timing of when this information is known (results of gene-mutation testing) are paramount in the treatment of NSCLC. Other concerns are patient age, performance status, hepatic and renal function, comorbidities, and whether the patient has started systemic (chemotherapy) treatment.
The National Comprehensive Cancer Network (NCCN) has established guidelines for the treatment of NSCLC. Patients with untreated (i.e., have not started first-line chemotherapy), advanced-stage NSCLC with a sensitizing EGFR mutation (known prior to the initiation of treatment) should start treatment with an oral EFGR TKI (afatinib [Gilotrif], erlotinib, or gefitinib; NCCN, 2016). For patients whose mutation status (sensitizing EGFR mutation) is known after they have started chemotherapy, the NCCN recommends either completing the planned number of chemotherapy treatments or interrupting treatment (chemotherapy) and starting afatinib, erlotinib or gefitinib (NCCN, 2016).
The NCCN has recommendations for patients with disease progression on afatinib or erlotinib. If the patient has oligometastatic disease (one site of metastasis), continue the oral EGFR TKI and give local treatment (e.g., radiation therapy) for the metastasis (NCCN, 2016). If the patient develops widespread metastatic disease, discontinue the oral EGFR TKI and start chemotherapy (NCCN, 2016).
Oral EGFR TKIs
Afatinib: Afatinib is an oral irreversible ErbB family blocker that blocks signaling from EGFR (ErbB1), EGFR 2 (HER2/ErbB2), EGFR 4 (ErbB4), and transphorylation of ErbB3 (Sequist et al., 2013; Solca et al., 2012). Afatinib was approved by the US Food and Drug Administration (FDA) in July 2013 for first-line treatment of patients with metastatic NSCLC whose tumors express EGFR mutations with exon 19 deletions or exon 21 (L858R; National Cancer Institute [NCI], 2013a). The FDA’s approval is based on the results of the LUX-Lung 3 randomization clinical trial of afatinib vs. cisplatin plus pemetrexed (Alimta) in patients with advanced-stage, EGFR-mutant adenocarcinoma of the lung (NCI, 2013a).
Erlotinib: Erlotinib is a potent and selective oral EGFR TKI that reduces HER1/EGFR autophosphorylation, inhibits epidermal growth factor–dependent cell proliferation, and blocks cell-cycle progression at the G1 phase (Perez-Soler et al., 2004; Pollack et al., 1999). Erlotinib has three FDA indications for NSCLC. In 2004, erlotinib was approved by the FDA for second-line treatment of NSCLC, regardless of mutation status, based on results showing improvement in progression-free (PFS) and overall survival (OS), from a phase III clinical trial (Shepherd et al., 2013; Kreamer et al., 2011). The next FDA approval for erlotinib was in April 2010, for maintenance therapy for patients with stable disease after chemotherapy (Cappuzzo et al., 2010). In 2013, erlotinib was approved for first-line treatment of patients with NSCLC with EGFR exon 19 deletions or exon 21 (L858R) substitutions (NCI, 2013b). The first-line treatment approval was based on results of the phase III OPTIMAL randomization clinical trial of erlotinib vs. carboplatin/gemcitabine, which demonstrated improved PFS for patients who received erlotinib (Zhou et al., 2011).
Gefitinib: Gefitinib (Iressa) is another oral EGFR TKI that was "re-approved" by the FDA for first-line treatment of NSCLC with EGFR mutations in exon 19 deletions or exon 21 L858R substitution gene mutations (FDA, 2015b). This new approval is based on results from phase III and IV clinical trials (Mok et al., 2008; Douillard et al., 2014), showing improved objective response rates and PFS in patients with sensitizing EGFR mutations treated with gefitinib.
EGFR Resistance
Patients treated with oral EGFR TKIs eventually develop treatment resistance to these drugs (Gibbons & Byers, 2014; da Cunha Santos et al., 2011). Repeat biopsies of tumors (from patients with initial sensitizing EGFR mutations) have identified secondary mutations including T790M, present in approximately 50% of EGFR-mutated patients (da Cunha Santos et al., 2011; Gibbons & Byers, 2014). The T790M mutation prevents binding of the TKI to the intracellular domain (Gibbons & Byers, 2014). Other mechanisms of drug resistance include bypass mechanisms, such as MET overexpression, alterations in other HER family proteins, downstream activations of the RAS or PI3K pathways, and transformation from NSCLC to small cell lung cancer (SCLC; Gibbons & Byers, 2014).
The presence of the T790M mutation previously posed treatment challenges for an oncology team. The treatment options for patients with the T790M mutation were discontinuation of the oral EFGR TKI drug and start chemotherapy; cetuximab (Erbitux) with afatinib; or, if eligible, participation in clinical trials with drugs that overcome the T790M mutation. There is an FDA approved agent, osimertinib [Tagrisso], specifically for patients with the T790M mutation (FDA, 2015d).
The combination of cetuximab and afatinib is an effective, yet toxic, treatment for patients with the T790M mutation (Gibbons & Byers, 2014; Jänne et al., 2015), based on results of a phase I clinical trial by Janjigian and colleagues (2014). The overall response rate is 29%, 32% for patients with the T790M mutation, and 25% for patients without the T790M mutation (Janjigian et al., 2014). The median PFS is 4.7 months, and the median duration of confirmed overall response is 5.7 months (Janjigian et al., 2014; Gibbons & Byers, 2014). The combination of cetuximab and afatinib is associated with high rates of toxicities, especially skin and gastrointestinal (Janjigian et al., 2014; Gibbons & Byers, 2014).
Two oral drugs, osimertinib and rociletinib (CO-1686), were evaluated in clinical trials for patients with T790M mutations. Results from the clinical trial by Jänne and colleagues (2015) demonstrated osimertinib has activity in patients with the T790M mutation, previously treated with an oral EGFR TKI. The response rate was 61% for evaluable patients with confirmed T790M mutation; 21% for evaluable patients without the T790M mutation (Jänne et al., 2015). The median PFS is 9.6 months for T790M-positive patients vs. 2.8 months for patients without the mutation (Jänne et al., 2015). In November 2015, the FDA approved osimertinib for EGFR-positive patients with T790M mutation who have disease progression on an oral TKI (FDA, 2015d). The most common side effects were rash, diarrhea, nausea, and decreased appetite (Jänne et al., 2015). The NCCN guidelines (2016) include osimertinib as a second-line treatment option for patients with the T790M mutation who have progressed on previous TKIs.
Rociletinib was another oral TKI agent targeting the T790M mutation. Results from a phase I/II clinical trial evaluating rociletinib demonstrated activity in patients with the T790M mutation (Sequist et al., 2015). Rociletinib has not been granted new drug approval by the FDA as updated data revealed lower response rates than initially reported (Broderick, 2016).
KRAS
In the United States, activating KRAS mutations are found in approximately 20% to 25% of patients with adenocarcinoma of the lungs (both white and African American) and are generally associated with smoking (Sequist & Neal, 2015; Cote et al., 2011; Reinersman et al., 2011). The RAS family of proteins is a central mediator of the mitogen-activated protein kinase (MAPK); signal transducer and activator of transcription (STAT); and phosphoinositide 3-kinase (PI3K) signaling pathways, which together control cell proliferation and apoptosis (Sequist & Neal, 2015). Oncogenic RAS mutations, most commonly those that correspond to missense substitutions in codons 12, 13, and 61, cause continual activity of RAS independent of upstream signals (Sequist & Neal, 2015).
There are conflicting data regarding the presence of the KRAS mutation and response or resistance to certain therapies (Sequist & Neal, 2015; Kreamer et al., 2011). In the TRIBUTE clinical trial, a phase III randomization study comparing first-line treatment of platinum-based doublet chemotherapy with/without erlotinib, 55 patients (21%) tested positive for KRAS mutation (Sequist & Neal, 2015; Kreamer et al., 2011). The patients with KRAS mutation who received chemotherapy (carboplatin/paclitaxel) alone (without erlotinib) had a response rate of 23%, whereas the KRAS mutation–positive patients who received chemotherapy and erlotinib had a response rate of 8% as well as worse overall survival (Eberhard et al., 2005).
There is a suggestion that KRAS mutations may sensitize tumors to antifolates, such as pemetrexed, possibly by upregulation of a microRNA (mir-181c), which downregulates KRAS (Sequist & Neal, 2015). In a combined analysis of four adjuvant chemotherapy trials, patients with KRAS codon 12 mutation similarly benefitted from chemotherapy as patients with wild-type KRAS; however, the presence of codon 13 mutation appeared to be predictive of worse survival from adjuvant chemotherapy, although the sample size was small (Shepherd et al., 2013).
Treatment of KRAS-Mutant Disease
Treatment of KRAS mutation–positive patients remains a challenge. Multiple early efforts to identify specific RAS inhibitors that are clinically useful against KRAS-mutated lung cancer were unsuccessful (Sequist & Neal, 2015). Presently, the focus/target for treating KRAS-mutated lung cancers is against downstream effectors of activated KRAS (Sequist & Neal, 2015).
In a phase II clinical trial, 87 patients with previously treated KRAS-mutant NSCLC were randomized to receive treatment of docetaxel with selumetinib (an oral MEK inhibitor) vs. docetaxel with placebo; results from this study showed improved PFS in the selumetinib arm (5.3 months) compared with the placebo are (2.1 months; Jänne et al., 2013). There was also a trend toward improved OS of 9.4 months vs. 5.2 months in the combination arm of docetaxel plus selumetinib vs. docetaxel plus placebo (Jänne et al., 2013). Objective partial responses were seen in 16 of 43 patients (37%) treated with docetaxel plus selumetinib vs. none of 40 in the docetaxel plus placebo arm; however, there was also greater toxicity (more febrile neutropenia, diarrhea, nausea, vomiting, rash) in the docetaxel plus selumetinib arm (Jänne et al., 2013).
Other phase II clinical trials have looked at combinations of MEK inhibitors with oral EGFR TKIs and also other MEK inhibitors. One phase II randomization clinical trial evaluated selumetinib vs. selumetinib plus erlotinib. The result of this study indicated that there was no increased activity with the combination arm in patients with wild-type or KRAS-mutant NSCLC (Carter et al., 2013).
Trametinib (Mekinist), an oral MEK inhibitor FDA approved for treatment of melanoma, may have some activity in NSCLC, based on results of phase II clinical trials (Flaherty et al., 2012; Gandara et al., 2013; Blumenschein et al., 2015). More research is needed in this area of treating KRAS-mutant NSCLC. Currently, the treatment recommendations are for patients with KRAS-mutant NSCLC to receive standard-of-care chemotherapy or, if eligible, to participate in a clinical trial (Sequist & Neal, 2015).
ANAPLASTIC LYMPHOMA KINASE
Anaplastic lymphoma kinase is another driver mutation found in a small percentage (approximately 3% to 7%) of patients with NSCLC and in a smaller percentage of African American patients (approximately 1.7%; Pao & Girard, 2011; Soda et al., 2007; Choi et al., 2008; Koivunen et al., 2008; Horn & Pao, 2009; Araujo et al., 2015). Tumors that are ALK-positive contain the fusion of echinoderm microtubule-associated protein-like 4 gene and the ALK gene (EML4-ALK), which causes constitutive kinase activity; this activity is associated with uncontrolled cell growth and proliferation (Shaw & Solomon, 2014; Soda et al., 2007). The ALK protein is a receptor kinase in the insulin receptor superfamily (Luo & Lam, 2013).
Clinical features associated with ALK-positive tumors include light smoking history or never smokers, younger age of patients, and diagnosis of adenocarcinoma with signet ring or acinar histology (Takahashi et al., 2010). Tumors that are ALK-positive are sensitive to treatment with ALK-targeted therapy (Shaw & Solomon, 2014). There are first- and second-generation, FDA-approved treatments for patients with ALK-positive NSCLC.
Treatment of ALK-Positive NSCLC
Treatment recommendations from the NCCN for patients with ALK-positive NSCLC mirror those for EGFR-mutant NSCLC: If the tumor is ALK-positive and the patient has not started systemic treatment, begin treatment with an oral targeted therapy (NCCN, 2016). If results of mutation testing are known after starting systemic treatment (chemotherapy), stop chemotherapy and begin targeted treatment or complete the prescribed number of chemotherapy treatments and then begin treatment with targeted therapy (NCCN, 2016). The NCCN also recommends continuing oral targeted therapy even if the patient develops isolated metastases (one site or multiple metastases in only one site) and consider local treatment (radiation) for the metastases (NCCN, 2016).
Crizotinib: Crizotinib (Xalkori) is a multitargeted small molecule TKI and a potent inhibitor of ALK phosphorylation and signal transduction (Christensen et al., 2007). Crizotinib induces rapid tumor regression and objective responses in the majority of patients with ALK-positive tumors (Shaw et al., 2013).
Results from a phase III randomized clinical trial of crizotinib vs. single-agent chemotherapy (pemetrexed or docetaxel) for previously treated patients showed increased PFS for patients randomized to treatment with crizotinib (the median PFS was 7.7 months vs. 3 months) compared with those treated with chemotherapy (Shaw et al., 2013). Responses were achieved more rapidly on the crizotinib arm (6.3 weeks) than the chemotherapy arm (12.6 weeks) and were of longer duration (32 weeks vs. 24 weeks; Shaw et al., 2013).
Results from another clinical trial that randomized untreated (no systemic treatment) patients to crizotinib vs. platinum-based chemotherapy showed improved PFS for the crizotinib arm (10.9 months) vs. the chemotherapy arm (7 months) and a higher overall response rate (74%, crizotinib; 45%, chemotherapy; Mok et al., 2009).
Resistance to ALK Inhibitors: Patients treated with crizotinib eventually develop resistance to the drug. Causes of resistance include tumor acquisition of a secondary mutation within the ALK tyrosine kinase domain, amplification of the ALK fusion gene (which may occur alone or in combination with a secondary resistance mutation), or development of alternative or bypass signaling pathways (Shaw & Solomon, 2014).
The most common resistance mutations are the gatekeeper L1196M mutation and G1296A (Shaw & Solomon, 2014). One notable mutation is G1202R, as it confers high-level resistance to crizotinib and next-generation ALK inhibitors (Shaw & Solomon, 2014).
Another mode of crizotinib resistance is the development of alternative or "detour" signaling pathways, including abnormalities in EGFR, KIT, and insulin-like growth factor-1 receptor (IGFR-1) pathways (Shaw & Solomon, 2014). This last method of crizotinib resistance suggests, perhaps, evaluating combination (targeted) therapies to overcome resistance (Shaw & Solomon, 2014).
Ceritinb: Ceritinib (Zykadia) is a second-generation, FDA-approved ALK inhibitor for patients who are or have become resistant to crizotinib (Shaw & Solomon, 2014). Ceritinib is more potent than crizotinib (Shaw & Solomon, 2014). Based on results of clinical trials, the FDA approved ceritinib in April 2014 for patients whose tumors had progressed on, or were intolerant to, crizotinib (Shaw & Solomon, 2014).
Currently, there are clinical trials looking at other second-generation ALK-targeted therapies, such as alectinib (Alecensa; Shaw & Solomon, 2014). For patients with ALK-positive NSCLC, there are two FDA-approved drugs and ongoing clinical trials for other agents.
Alectinib: Alectinib is another second-generation ALK inhibitor with activity in crizotinib-resistant disease and brain metastases (Shaw & Solomon, 2014; Seto et al., 2013; Gadgeel et al., 2014; Gainor et al., 2015; Ou et al., 2015). Outcomes from the phase I/II clinical trial that evaluated the safety and activity of alectinib in patients with crizotinib-resistant NSCLC and brain metastases demonstrated alectinib’s efficacy in treating brain metastases (Gadgeel et al., 2014). Additionally, analysis of cerebral spinal fluid (CSF) from five patients showed drug concentrations of alectinib in the CSF (Gadgeel et al., 2014).
Findings from a phase II single-arm clinical trial confirmed alectinib has activity in treating brain metastases (Ou et al., 2015). In November 2015, the FDA approved alectinib for treatment of patients with ALK-positive NSCLC who progressed on or are intolerant of crizotinib (FDA, 2015c). The NCCN guidelines recommend either ceritinib or alectinib as second-line treatment for patients with ALK-positive NSCLC who progressed on crizotinib (NCCN 2016).
ROS1
ROS1 is a receptor tyrosine kinase of the insulin receptor family and a potent oncogenic driver (Shaw & Solomon, 2014; Gainor & Shaw, 2013). ROS1 rearrangements are believed to promote signal transduction leading to upregulation and activation of various intracellular pathways, resulting in promotion of cell survival and proliferation (Gainor & Shaw, 2013). ROS1 rearrangements have been found in 1% to 2% of patients with NSCLC and were associated with younger-age, never smokers, Asian ethnicity, and, advanced stage (Gainor & Shaw, 2013; Bergethon et al., 2012). The predominant histology for ROS1-positive NSCLC is adenocarcinoma, although ROS1 has been found (infrequently) in large cell and squamous cell histologies (Gainor & Shaw, 2013; Davies et al., 2012; Rimkunas et al., 2012).
Results from a phase I clinical trial showed that crizotinib had activity, with objective responses, duration of response, and PFS, in patients with ROS 1 positive NSCLC (Shaw et al., 2014). In March 2016, the FDA approved crizotinib for the treatment of ROS1-positive tumors (Briz, 2016).
IMMUNOTHERAPY
Programmed Cell Death Protein 1 (PD-1)/Programmed Cell Death Ligand 1 (PD-L1)
Immunotherapy is the newest treatment modality for NSCLC. The goals of immunotherapy for cancer are aiding the immune system to recognize cancer (cells) as foreign bodies, stimulate immune responsiveness, and relieve the inhibition of the immune system that allows for tumor growth (Gettinger, 2015).
Generating an effective antitumor immune response is a complex multistep process (Chen, Irving, & Hodi, 2012). First, the T cells (of the immune system) must be able to recognize cancer cells as foreign and then generate cytotoxic T lymphocytes (CTLs) to travel to and infiltrate tumors to bind to the cancer cells and kill them (Chen et al., 2012). Each step of the process must happen to derive clinical benefit (Chen et al., 2012).
Research and clinical data have shown the importance of one inhibitory ligand and receptor pair—PD-L1 and PD-1—in inhibiting the last step in the process: preventing the killing of cancer cells by CTLs (Chen et al., 2012). Tumors that express PD-L1 are able to inactivate the normal immune system’s response to killing cancer cells. Cytotoxic T lymphocytes become nonfunctional by engaging the inhibitory receptor PD-1 (Chen et al., 2012).
Expressed on the surface of T cells, PD-1, when activated, binds to PD-L1, triggering an inhibitory signal that results in reduced cytokine production and (reduced) proliferation of T cells (Carter et al., 2002; Freeman et al., 2000). And PD-L1 is upregulated in tumors through activation of key oncogenic pathways (PI3K, MAPK; Chen et al., 2012). It is through upregulation of PD-L1 expression that cancer cells evade detection by the host immune system and progress (Chen et al., 2012).
Treatment Targeting PD-L1
Antibodies that target PD-L1 act mainly by inhibiting the binding of PD-L1 to PD-1, thus freeing cancer-specific CTLs to mediate killing of cancer cells that express PD-L1 (Pardoll, 2012; Mellman & Nelson, 2008). There are now two FDA-approved monoclonal antibodies for treatment of NSCLC that target PD-1: nivolumab (Opdivo) and pembrolizumab (Keytruda; Sosman, 2015).
Nivolumab: Based on the results of two clinical trials, nivolumab received FDA approval (in March 2015) for treatment of NSCLC in patients with advanced squamous cell carcinoma previously treated with chemotherapy who had disease progression on or after treatment with platinum-based chemotherapy The results of the phase III randomized CheckMate 017 trial, nivolumab vs. docetaxel in previously treated patients with squamous cell carcinoma showed improved median survival of 9.3 months for the patients treated with nivolumab vs. 6 months for patients treated with docetaxel (Brahmer et al., 2015). The phase II single-arm clinical trial (CheckMate 063) showed nivolumab had activity (in terms of response and survival) in previously treated patients with squamous cell histology after at least two prior lines of chemotherapy (Rizvi et al., 2015).
In March 2015, the FDA approved nivolumab for the treatment of patients with advanced squamous cell carcinoma of the lungs (Gettinger, 2015). Results from the phase II single-arm CheckMate 063 study showed nivolumab had activity (in terms of response and survival) in previously treated patients with squamous cell histology (Rizvi et al., 2015). Outcomes from the phase III randomized CheckMate 017 study nivolumab vs. docetaxel revealed a survival benefit for the patients who received nivolumab (9.3 months vs. 6 months; Brahmer et al., 2015).
Pembrolizumab: Pembrolizumab is another monoclonal antibody approved by the FDA for treatment of NSCLC. Based on the results of a phase I clinical trial, pembrolizumab received breakthrough therapy designation for advanced NSCLC in late 2014 (Gettinger, 2015). Results of this clinical trial revealed a median duration of response of 12.5 months, PFS of 3.7 months, and OS of 12 months (Garon et al., 2015). Recently, the FDA approved pembrolizumab for treatment of NSCLC (FDA, 2015a). The 2016 NCCN guidelines recommend either nivolumab or pembrolizumab as preferred subsequent treatment for metastatic NSCLC (NCCN, 2016).
CONCLUSION
For many decades, the prognosis for advanced-stage NSCLC was not bright. Chemotherapy, the mainstay of treatment, had limited benefit and much toxicity. Now with mutation testing and appropriate treatments, there has been a trend toward improved PFS and OS. There is an array of treatments for mutant-positive NSCLC, with research evaluating newer generations of targeted therapies. Additionally, immunotherapy is now part of the treatment armament. The treatment horizon for advanced-stage NSCLC is greatly expanded, with more options available to patients and their oncology team.
Footnotes
The authors have no potential conflicts of interest to disclose.
References
- 1.Aggarwal Charu, Somaiah Neeta, Simon George R. Biomarkers with predictive and prognostic function in non-small cell lung cancer: ready for prime time? Journal of the National Comprehensive Cancer Network : JNCCN. 2010;8:822–832. doi: 10.6004/jnccn.2010.0059. [DOI] [PubMed] [Google Scholar]
- 2.Araujo Luiz H, Lammers Philip E, Matthews-Smith Velmalia, Eisenberg Rosana, Gonzalez Adriana, Schwartz Ann G, Timmers Cynthia, Shilo Konstantin, Zhao Weiqiang, Natarajan Thanemozhi G, Zhang Jianying, Yilmaz Ayse Selen, Liu Tom, Coombes Kevin, Carbone David P. Somatic Mutation Spectrum of Non-Small-Cell Lung Cancer in African Americans: A Pooled Analysis. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015;10:1430–1436. doi: 10.1097/JTO.0000000000000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergethon Kristin, Shaw Alice T, Ou Sai-Hong Ignatius, Katayama Ryohei, Lovly Christine M, McDonald Nerina T, Massion Pierre P, Siwak-Tapp Christina, Gonzalez Adriana, Fang Rong, Mark Eugene J, Batten Julie M, Chen Haiquan, Wilner Keith D, Kwak Eunice L, Clark Jeffrey W, Carbone David P, Ji Hongbin, Engelman Jeffrey A, Mino-Kenudson Mari, Pao William, Iafrate A John. ROS1 rearrangements define a unique molecular class of lung cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumenschein G R, Smit E F, Planchard D, Kim D-W, Cadranel J, De Pas T, Dunphy F, Udud K, Ahn M-J, Hanna N H, Kim J-H, Mazieres J, Kim S-W, Baas P, Rappold E, Redhu S, Puski A, Wu F S, Jänne P A. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC)†. Annals of oncology : official journal of the European Society for Medical Oncology. 2015;26:894–901. doi: 10.1093/annonc/mdv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer Julie, Reckamp Karen L, Baas Paul, Crinò Lucio, Eberhardt Wilfried E E, Poddubskaya Elena, Antonia Scott, Pluzanski Adam, Vokes Everett E, Holgado Esther, Waterhouse David, Ready Neal, Gainor Justin, Arén Frontera Osvaldo, Havel Libor, Steins Martin, Garassino Marina C, Aerts Joachim G, Domine Manuel, Paz-Ares Luis, Reck Martin, Baudelet Christine, Harbison Christopher T, Lestini Brian, Spigel David R. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broderick J M. Clovis ends development of rociletinib in lung cancer. 2016 Retrieved from http://www.onclive.com.
- 7.Briz L. FDA approves crizotinib for the treatment of ROS 1-positive NSCLC. 2016 Retrieved from http://www.cancertherapyadvisor.com/lung-cancer/lung-cancer-nsclc-crizotinib-fda-approval-treatment/article/482795/
- 8.Cappuzzo Federico, Ciuleanu Tudor, Stelmakh Lilia, Cicenas Saulius, Szczésna Aleksandra, Juhász Erzsébet, Esteban Emilio, Molinier Olivier, Brugger Wolfram, Melezínek Ivan, Klingelschmitt Gaëlle, Klughammer Barbara, Giaccone Giuseppe. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. The Lancet. Oncology. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 9.Carter C A, Rajan A, Szabo E, Khozin S, Thomas A, Brzezniak C E, Giaccone G. Two parallel randomized phase II studies of selumetinib and erlotinib in advanced non-small cell lung cancer selected by KRAS mutations [Abstract 8026]. Journal of Clinical Oncology (Meeting Abstracts) 2013;(Suppl) [Google Scholar]
- 10.Carter L, Fouser L A, Jussif J, Fitz L, Deng B, Wood C R, Carreno B M. PD-1: PD-L inhibitory pathway affects both CD 4+ and CD 8+ T cells and is overcome by IL-2. European Journal of Immunology. 2002;32(3):634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen Daniel S, Irving Bryan A, Hodi F Stephen. Molecular pathways: next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:6580–6587. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 12.Choi Young Lim, Takeuchi Kengo, Soda Manabu, Inamura Kentaro, Togashi Yuki, Hatano Satoko, Enomoto Munehiro, Hamada Toru, Haruta Hidenori, Watanabe Hideki, Kurashina Kentaro, Hatanaka Hisashi, Ueno Toshihide, Takada Shuji, Yamashita Yoshihiro, Sugiyama Yukihiko, Ishikawa Yuichi, Mano Hiroyuki. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer research. 2008;68:4971–4976. doi: 10.1158/0008-5472.CAN-07-6158. [DOI] [PubMed] [Google Scholar]
- 13.Christensen James G, Zou Helen Y, Arango Maria E, Li Qiuhua, Lee Joseph H, McDonnell Scott R, Yamazaki Shinji, Alton Gordon R, Mroczkowski Barbara, Los Gerrit. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Molecular cancer therapeutics. 2007;6:3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 14.Ciardiello Fortunato, Tortora Giampaolo. EGFR antagonists in cancer treatment. The New England journal of medicine. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 15.Cote Michele L, Haddad Ramsi, Edwards David J, Atikukke Govindaraja, Gadgeel Shirish, Soubani Ayman O, Lonardo Fulvio, Bepler Gerold, Schwartz Ann G, Ethier Stephen P. Frequency and type of epidermal growth factor receptor mutations in African Americans with non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:627–630. doi: 10.1097/JTO.0b013e31820a0ec0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Cunha Santos Gilda, Shepherd Frances A, Tsao Ming Sound. EGFR mutations and lung cancer. Annual review of pathology. 2011;6:49–69. doi: 10.1146/annurev-pathol-011110-130206. [DOI] [PubMed] [Google Scholar]
- 17.Davies Kurtis D, Le Anh T, Theodoro Mariana F, Skokan Margaret C, Aisner Dara L, Berge Eamon M, Terracciano Luigi M, Cappuzzo Federico, Incarbone Matteo, Roncalli Massimo, Alloisio Marco, Santoro Armando, Camidge D Ross, Varella-Garcia Marileila, Doebele Robert C. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4570–4579. doi: 10.1158/1078-0432.CCR-12-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douillard J-Y, Ostoros G, Cobo M, Ciuleanu T, McCormack R, Webster A, Milenkova T. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. British journal of cancer. 2014;110:55–62. doi: 10.1038/bjc.2013.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eberhard David A, Johnson Bruce E, Amler Lukas C, Goddard Audrey D, Heldens Sherry L, Herbst Roy S, Ince William L, Jänne Pasi A, Januario Thomas, Johnson David H, Klein Pam, Miller Vincent A, Ostland Michael A, Ramies David A, Sebisanovic Dragan, Stinson Jeremy A, Zhang Yu R, Seshagiri Somasekar, Hillan Kenneth J. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 20.Flaherty Keith T, Robert Caroline, Hersey Peter, Nathan Paul, Garbe Claus, Milhem Mohammed, Demidov Lev V, Hassel Jessica C, Rutkowski Piotr, Mohr Peter, Dummer Reinhard, Trefzer Uwe, Larkin James M G, Utikal Jochen, Dreno Brigitte, Nyakas Marta, Middleton Mark R, Becker Jürgen C, Casey Michelle, Sherman Laurie J, Wu Frank S, Ouellet Daniele, Martin Anne-Marie, Patel Kiran, Schadendorf Dirk. Improved survival with MEK inhibition in BRAF-mutated melanoma. The New England journal of medicine. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 21.Freeman G J, Long A J, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz L J, Malenkovich N, Okazaki T, Byrne M C, Horton H F, Fouser L, Carter L, Ling V, Bowman M R, Carreno B M, Collins M, Wood C R, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadgeel Shirish M, Gandhi Leena, Riely Gregory J, Chiappori Alberto A, West Howard L, Azada Michele C, Morcos Peter N, Lee Ruey-Min, Garcia Linta, Yu Li, Boisserie Frederic, Di Laurenzio Laura, Golding Sophie, Sato Jotaro, Yokoyama Shumpei, Tanaka Tomohiro, Ou Sai-Hong Ignatius. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. The Lancet. Oncology. 2014;15:1119–1128. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 23.Gainor Justin F, Shaw Alice T. Novel targets in non-small cell lung cancer: ROS1 and RET fusions. The oncologist. 2013;18:865–875. doi: 10.1634/theoncologist.2013-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gainor Justin F, Sherman Carol A, Willoughby Kathryn, Logan Jennifer, Kennedy Elizabeth, Brastianos Priscilla K, Chi Andrew S, Shaw Alice T. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015;10:232–236. doi: 10.1097/JTO.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandara D R, Hiret S, Blumenschein G R, Barlesi F, Delford J P, Madelaine J, Leighl N B. Oral MEK1/MEK2 inhibitor trametinib (GSK1120212) in combination with docetaxel in KRAS-mutant and wild-type advanced non-small cell lung cancer: A phase 1/1b trial [Abstract 8028]. . Journal of Clinical Oncology (Meeting Abstracts) 2013;31(Suppl) [Google Scholar]
- 26.Garon Edward B, Rizvi Naiyer A, Hui Rina, Leighl Natasha, Balmanoukian Ani S, Eder Joseph Paul, Patnaik Amita, Aggarwal Charu, Gubens Matthew, Horn Leora, Carcereny Enric, Ahn Myung-Ju, Felip Enriqueta, Lee Jong-Seok, Hellmann Matthew D, Hamid Omid, Goldman Jonathan W, Soria Jean-Charles, Dolled-Filhart Marisa, Rutledge Ruth Z, Zhang Jin, Lunceford Jared K, Rangwala Reshma, Lubiniecki Gregory M, Roach Charlotte, Emancipator Kenneth, Gandhi Leena. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England journal of medicine. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 27.Gettinger S. 2015 Retrieved from http://www.uptodate.com.
- 28.Gibbons Don L, Byers Lauren Averett. A HER 1-2 punch: dual EGFR targeting deals resistance a deadly blow. Cancer discovery. 2014;4:991–994. doi: 10.1158/2159-8290.CD-14-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grande C, Viale P, Yamamoto D. Biomarkers in colorectal cancer: Implications for nursing practice. . Journal of the Advanced Practitioner in Oncology. 2010;1(4):245–255. [Google Scholar]
- 30.Herbst Roy S, Prager Diane, Hermann Robert, Fehrenbacher Lou, Johnson Bruce E, Sandler Alan, Kris Mark G, Tran Hai T, Klein Pam, Li Xin, Ramies David, Johnson David H, Miller Vincent A. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 31.Horn Leora, Pao William. EML4-ALK: honing in on a new target in non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4232–4235. doi: 10.1200/JCO.2009.23.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janjigian Yelena Y, Smit Egbert F, Groen Harry J M, Horn Leora, Gettinger Scott, Camidge D Ross, Riely Gregory J, Wang Bushi, Fu Yali, Chand Vikram K, Miller Vincent A, Pao William. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer discovery. 2014;4:1036–1045. doi: 10.1158/2159-8290.CD-14-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jänne Pasi A, Shaw Alice T, Pereira José Rodrigues, Jeannin Gaëlle, Vansteenkiste Johan, Barrios Carlos, Franke Fabio Andre, Grinsted Lynda, Zazulina Victoria, Smith Paul, Smith Ian, Crinò Lucio. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. The Lancet. Oncology. 2013;14:38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 34.Jänne Pasi A, Yang James Chih-Hsin, Kim Dong-Wan, Planchard David, Ohe Yuichiro, Ramalingam Suresh S, Ahn Myung-Ju, Kim Sang-We, Su Wu-Chou, Horn Leora, Haggstrom Daniel, Felip Enriqueta, Kim Joo-Hang, Frewer Paul, Cantarini Mireille, Brown Kathryn H, Dickinson Paul A, Ghiorghiu Serban, Ranson Malcolm. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. The New England journal of medicine. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 35.Koivunen Jussi P, Mermel Craig, Zejnullahu Kreshnik, Murphy Carly, Lifshits Eugene, Holmes Alison J, Choi Hwan Geun, Kim Jhingook, Chiang Derek, Thomas Roman, Lee Jinseon, Richards William G, Sugarbaker David J, Ducko Christopher, Lindeman Neal, Marcoux J Paul, Engelman Jeffrey A, Gray Nathanael S, Lee Charles, Meyerson Matthew, Jänne Pasi A. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:4275–4283. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreamer K, Eaby-Sandy B, Sherry V, Stonehouse-Lee S. Biomarkers in non-small cell lung cancer: Opportunity and challenge. . Journal of the Advanced Practitioner in Oncology. 2011;2(3):163–175. [Google Scholar]
- 37.Luo S Y, Lam D C. Oncogenic driver mutations in lung cancer. . Translational Respiratory Medicine. 2013;1(6):1–8. doi: 10.1186/2213-0802-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynch Thomas J, Bell Daphne W, Sordella Raffaella, Gurubhagavatula Sarada, Okimoto Ross A, Brannigan Brian W, Harris Patricia L, Haserlat Sara M, Supko Jeffrey G, Haluska Frank G, Louis David N, Christiani David C, Settleman Jeff, Haber Daniel A. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 39.Massarelli Erminia, Johnson Faye M, Erickson Heidi S, Wistuba Ignacio I, Papadimitrakopoulou Vassiliki. Uncommon epidermal growth factor receptor mutations in non-small cell lung cancer and their mechanisms of EGFR tyrosine kinase inhibitors sensitivity and resistance. Lung cancer (Amsterdam, Netherlands) 2013;80:235–241. doi: 10.1016/j.lungcan.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 40.Mellman Ira, Nelson W James. Coordinated protein sorting, targeting and distribution in polarized cells. Nature reviews. Molecular cell biology. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mok Tony S, Wu Yi-Long, Thongprasert Sumitra, Yang Chih-Hsin, Chu Da-Tong, Saijo Nagahiro, Sunpaweravong Patrapim, Han Baohui, Margono Benjamin, Ichinose Yukito, Nishiwaki Yutaka, Ohe Yuichiro, Yang Jin-Ji, Chewaskulyong Busyamas, Jiang Haiyi, Duffield Emma L, Watkins Claire L, Armour Alison A, Fukuoka Masahiro. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England journal of medicine. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 42.National Cancer Institute. FDA approval for afatinib dimaleate. . 2013a Retrieved from http://www.cancer.gov/about-cancer/treatment/drugs/fda-afatinibdimaleate.
- 43.National Cancer Institute. FDA approval for erlotinib hydrochloride. 2013b Retrieved from http://www.cancer.gov/about-cancer/treatment/drugs/fda-erlotinib-hydrochloride.
- 44.National Comprehensive Cancer Network. . NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Version 4.2016. . 2016 Retrieved from http://www.nccn.org.
- 45.Ou Sai-Hong Ignatius, Ahn Jin Seok, De Petris Luigi, Govindan Ramaswamy, Yang James Chih-Hsin, Hughes Brett, Lena Hervé, Moro-Sibilot Denis, Bearz Alessandra, Ramirez Santiago Viteri, Mekhail Tarek, Spira Alexander, Bordogna Walter, Balas Bogdana, Morcos Peter N, Monnet Annabelle, Zeaiter Ali, Kim Dong-Wan. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34:661–668. doi: 10.1200/jco.2015.63.9443. [DOI] [PubMed] [Google Scholar]
- 46.Paez J Guillermo, Jänne Pasi A, Lee Jeffrey C, Tracy Sean, Greulich Heidi, Gabriel Stacey, Herman Paula, Kaye Frederic J, Lindeman Neal, Boggon Titus J, Naoki Katsuhiko, Sasaki Hidefumi, Fujii Yoshitaka, Eck Michael J, Sellers William R, Johnson Bruce E, Meyerson Matthew. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (New York, N.Y.) 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 47.Pao William, Girard Nicolas. New driver mutations in non-small-cell lung cancer. The Lancet. Oncology. 2011;12:175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 48.Pao William, Miller Vincent, Zakowski Maureen, Doherty Jennifer, Politi Katerina, Sarkaria Inderpal, Singh Bhuvanesh, Heelan Robert, Rusch Valerie, Fulton Lucinda, Mardis Elaine, Kupfer Doris, Wilson Richard, Kris Mark, Varmus Harold. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardoll Drew M. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez-Soler R, Chachoua A, Hammond L A, Rowinsky E K, Huberman M, Karp D, Bonomi P. Perez-Soler, R., Chachoua, A., Hammond, L. A., Rowinsky, E. K., Huberman, M., Karp, D.,…Bonomi, P. (2004). Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. Journal of Clinical Oncology. 2004;22:3238–3247. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 51.Pfizer. Pfizer receives U.S. FDA breakthrough designation for Xalkori® (crizotinib) for the treatment of patients with ROS1-positive non-small cell lung cancer. 2015 Retrieved from http://www.pfizer.com/news/press-release-detail/
- 52.Pollack V A, Savage D M, Baker D A, Tsaparikos K E, Sloan D E, Moyer J D, Barbacci E G, Pustilnik L R, Smolarek T A, Davis J A, Vaidya M P, Arnold L D, Doty J L, Iwata K K, Morin M J. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. The Journal of pharmacology and experimental therapeutics. 1999;291:739–748. [PubMed] [Google Scholar]
- 53.Reinersman J Matthew, Johnson Melissa L, Riely Gregory J, Chitale Dhananjay A, Nicastri Anthony D, Soff Gerald A, Schwartz Ann G, Sima Camelia S, Ayalew Getinet, Lau Christopher, Zakowski Maureen F, Rusch Valerie W, Ladanyi Marc, Kris Mark G. Frequency of EGFR and KRAS mutations in lung adenocarcinomas in African Americans. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:28–31. doi: 10.1097/JTO.0b013e3181fb4fe2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rimkunas Victoria M, Crosby Katherine E, Li Daiqiang, Hu Yerong, Kelly Meghan E, Gu Ting-Lei, Mack Jennifer S, Silver Matthew R, Zhou Xinmin, Haack Herbert. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4449–4457. doi: 10.1158/1078-0432.CCR-11-3351. [DOI] [PubMed] [Google Scholar]
- 55.Rizvi Naiyer A, Mazières Julien, Planchard David, Stinchcombe Thomas E, Dy Grace K, Antonia Scott J, Horn Leora, Lena Hervé, Minenza Elisa, Mennecier Bertrand, Otterson Gregory A, Campos Luis T, Gandara David R, Levy Benjamin P, Nair Suresh G, Zalcman Gérard, Wolf Jürgen, Souquet Pierre-Jean, Baldini Editta, Cappuzzo Federico, Chouaid Christos, Dowlati Afshin, Sanborn Rachel, Lopez-Chavez Ariel, Grohe Christian, Huber Rudolf M, Harbison Christopher T, Baudelet Christine, Lestini Brian J, Ramalingam Suresh S. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. The Lancet. Oncology. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sequist L V, Neal J W. Personalized, genotype-directed therapy for advanced non-small cell lung cancer. 2015 Retrieved from http://www.uptodate.com.
- 57.Sequist Lecia V, Soria Jean-Charles, Goldman Jonathan W, Wakelee Heather A, Gadgeel Shirish M, Varga Andrea, Papadimitrakopoulou Vassiliki, Solomon Benjamin J, Oxnard Geoffrey R, Dziadziuszko Rafal, Aisner Dara L, Doebele Robert C, Galasso Cathy, Garon Edward B, Heist Rebecca S, Logan Jennifer, Neal Joel W, Mendenhall Melody A, Nichols Suzanne, Piotrowska Zofia, Wozniak Antoinette J, Raponi Mitch, Karlovich Chris A, Jaw-Tsai Sarah, Isaacson Jeffrey, Despain Darrin, Matheny Shannon L, Rolfe Lindsey, Allen Andrew R, Camidge D Ross. Rociletinib in EGFR-mutated non-small-cell lung cancer. The New England journal of medicine. 2015;372:1700–1709. doi: 10.1056/NEJMoa1413654. [DOI] [PubMed] [Google Scholar]
- 58.Sequist Lecia V, Yang James Chih-Hsin, Yamamoto Nobuyuki, O'Byrne Kenneth, Hirsh Vera, Mok Tony, Geater Sarayut Lucien, Orlov Sergey, Tsai Chun-Ming, Boyer Michael, Su Wu-Chou, Bennouna Jaafar, Kato Terufumi, Gorbunova Vera, Lee Ki Hyeong, Shah Riyaz, Massey Dan, Zazulina Victoria, Shahidi Mehdi, Schuler Martin. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 59.Seto Takashi, Kiura Katsuyuki, Nishio Makoto, Nakagawa Kazuhiko, Maemondo Makoto, Inoue Akira, Hida Toyoaki, Yamamoto Nobuyuki, Yoshioka Hiroshige, Harada Masao, Ohe Yuichiro, Nogami Naoyuki, Takeuchi Kengo, Shimada Tadashi, Tanaka Tomohiro, Tamura Tomohide. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. The Lancet. Oncology. 2013;14:590–598. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 60.Sharma Sreenath V, Bell Daphne W, Settleman Jeffrey, Haber Daniel A. Epidermal growth factor receptor mutations in lung cancer. Nature reviews. Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 61.Shaw Alice T, Kim Dong-Wan, Nakagawa Kazuhiko, Seto Takashi, Crinó Lucio, Ahn Myung-Ju, De Pas Tommaso, Besse Benjamin, Solomon Benjamin J, Blackhall Fiona, Wu Yi-Long, Thomas Michael, O'Byrne Kenneth J, Moro-Sibilot Denis, Camidge D Ross, Mok Tony, Hirsh Vera, Riely Gregory J, Iyer Shrividya, Tassell Vanessa, Polli Anna, Wilner Keith D, ä Pasi A. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. The New England journal of medicine. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 62.Shaw Alice T, Ou Sai-Hong I, Bang Yung-Jue, Camidge D Ross, Solomon Benjamin J, Salgia Ravi, Riely Gregory J, Varella-Garcia Marileila, Shapiro Geoffrey I, Costa Daniel B, Doebele Robert C, Le Long Phi, Zheng Zongli, Tan Weiwei, Stephenson Patricia, Shreeve S Martin, Tye Lesley M, Christensen James G, Wilner Keith D, Clark Jeffrey W, Iafrate A John. Crizotinib in ROS1-rearranged non-small-cell lung cancer. The New England journal of medicine. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaw A T, Solomon B. Anaplastic lymphoma kinase (ALK) fusion oncogene positive non-small cell lung cancer. . 2014 Retrieved from http://www.uptodate.com.
- 64.Shepherd Frances A, Domerg Caroline, Hainaut Pierre, Jänne Pasi A, Pignon Jean-Pierre, Graziano Stephen, Douillard Jean-Yves, Brambilla Elizabeth, Le Chevalier Thierry, Seymour Lesley, Bourredjem Abderrahmane, Le Teuff Gwénaël, Pirker Robert, Filipits Martin, Rosell Rafael, Kratzke Robert, Bandarchi Bizhan, Ma Xiaoli, Capelletti Marzia, Soria Jean-Charles, Tsao Ming-Sound. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvant chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2173–2181. doi: 10.1200/JCO.2012.48.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shigematsu Hisayuki, Lin Li, Takahashi Takao, Nomura Masaharu, Suzuki Makoto, Wistuba Ignacio I, Fong Kwun M, Lee Huei, Toyooka Shinichi, Shimizu Nobuyoshi, Fujisawa Takehiko, Feng Ziding, Roth Jack A, Herz Joachim, Minna John D, Gazdar Adi F. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. Journal of the National Cancer Institute. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 66.Soda Manabu, Choi Young Lim, Enomoto Munehiro, Takada Shuji, Yamashita Yoshihiro, Ishikawa Shunpei, Fujiwara Shin-ichiro, Watanabe Hideki, Kurashina Kentaro, Hatanaka Hisashi, Bando Masashi, Ohno Shoji, Ishikawa Yuichi, Aburatani Hiroyuki, Niki Toshiro, Sohara Yasunori, Sugiyama Yukihiko, Mano Hiroyuki. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 67.Solca Flavio, Dahl Goeran, Zoephel Andreas, Bader Gerd, Sanderson Michael, Klein Christian, Kraemer Oliver, Himmelsbach Frank, Haaksma Eric, Adolf Guenther R. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. The Journal of pharmacology and experimental therapeutics. 2012;343:342–350. doi: 10.1124/jpet.112.197756. [DOI] [PubMed] [Google Scholar]
- 68.Sosman J A. Immunotherapy of advanced melanoma with immune checkpoint inhibition. 2015 Retrieved from http://www.uptodate.com.
- 69.Takahashi Tsuyoshi, Sonobe Makoto, Kobayashi Masashi, Yoshizawa Akihiko, Menju Toshi, Nakayama Ei, Mino Nobuya, Iwakiri Shotaro, Sato Kiyoshi, Miyahara Ryo, Okubo Kenichi, Manabe Toshiaki, Date Hiroshi. Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene. Annals of surgical oncology. 2010;17:889–897. doi: 10.1245/s10434-009-0808-7. [DOI] [PubMed] [Google Scholar]
- 70.Tokumo Masaki, Toyooka Shinichi, Kiura Katsuyuki, Shigematsu Hisayuki, Tomii Kunitoshi, Aoe Motoi, Ichimura Kouichi, Tsuda Toshihide, Yano Masaaki, Tsukuda Kazunori, Tabata Masahiro, Ueoka Hiroshi, Tanimoto Mitsune, Date Hiroshi, Gazdar Adi F, Shimizu Nobuyoshi. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:1167–1173. [PubMed] [Google Scholar]
- 71.US Food and Drug Administration. FDA approves Keytruda for advanced non-small cell lung cancer. 2015a Retrieved from http://www.fda.gov/newsevents/newsroom/press announcements/cut 465444.htm.
- 72.US Food and Drug Administration. Gefitinib (Iressa). 2015b Retrieved from http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm454692.htm.
- 73.US Food and Drug Administration. FDA approves new oral therapy to treat ALK-positive lung cancer [alectinib]. . 2015c Retrieved from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm476926.htm.
- 74.US Food and Drug Administration. FDA approves new pill to treat certain patients with non-small cell lung cancer. 2015d Retrieved from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm472525.htm.
- 75.Yano S, Kondo K, Yamaguichi M, Richmond G, Hutchinson M, Wakeling A, Wadswoth P. Distribution and function of EGFR in human tissue and effect of EGFR tyrosine kinase inhibition. . Anticancer Research. 2003;23(5A):3639–3650. [PubMed] [Google Scholar]
- 76.Zhang Zhenfeng, Stiegler Amy L, Boggon Titus J, Kobayashi Susumu, Halmos Balazs. EGFR-mutated lung cancer: a paradigm of molecular oncology. Oncotarget. 2010;1:497–514. doi: 10.18632/oncotarget.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou C, Wu Y L, Chen G, Feng J, Liu X-Q, Wang C, You C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR-mutation-positive non-small-cell-lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. . Lancet Oncology. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]


