Abstract:
During cardiac surgery, myocardial protection is performed using diverse cardioplegic (CP) solutions with and without the presence of blood. New CP formulations extend ischemic intervals but use high-volume, crystalloid-based solutions. The present study evaluated four commonly used CP solutions and their effect on hemodilution during cardiopulmonary bypass (CPB). Records from 16,670 adult patients undergoing cardiac surgery with CPB between February 2016 and January 2017 were reviewed. Patients were classified into one of four groups according to CP type: 4–1 blood to crystalloid (4:1), microplegia (MP), del Nido (DN) and histidine-tryptophan-ketoglutarate (HTK). Covariate-adjusted estimates of group differences were calculated using multivariable logistic and linear mixed effects regression models. The primary end point was intraoperative transfusion of allogeneic red blood cells (RBCs), with a secondary end point of intraoperative hematocrit change. Among all patients, 8,350 (50.1%) received 4:1, 4,606 (27.6%) MP, 3,344 (20.1%) DN, and 370 (2.2%) HTK. Both 4:1 and MP were more likely to be used in patients undergoing coronary revascularization surgery, whereas DN and HTK were seen more often in patients undergoing valve surgery (p < .001). The highest volume of crystalloid CP solution was seen in the HTK group, 2,000 [1,754, 2200], whereas MP had the lowest, 50 [32, 67], p < .001. Ultrafiltration usage was as follows: HTK—84.9%. DN—83.7%, MP—40.1%, and 4:1—34.0%, p < .001. There were no statistically significant differences on the primary outcome risk of intraoperative RBC transfusion. However, statistically significant differences among all but one of the pair-wise comparisons of CP methods on hematocrit change (p < .05 or smaller), with MP having the lowest predicted drift (−7.8%) and HTK having the highest (−9.4%). During cardiac surgery, the administration of different CP formulations results in varying intraoperative hematocrit changes related to the volume of crystalloid solution administered.
Keywords: myocardial preservation, cardioplegia, microplegia, del Nido cardioplegia, hemodilution
Preservation of myocardial tissue during cardiac surgery involves a complex series of interventions that include the adequate delivery of beneficial chemicals, temperature management of the target tissue, and the removal of toxic substances that are generated by metabolic processes. The changing acuity of patients undergoing cardiac surgery with cardiopulmonary bypass (CPB) has further challenged the efforts of myocardial protection and has resulted in the reevaluation of previously accepted techniques that had been shown to be efficacious (1,2). Great variation exists in preservative techniques that transcend both geographical locations as well as among surgeons within the same institution (3). Traditionally, many centers in the United States use oxygenated blood from the extracorporeal circuit as the primary delivery solution, often using a ratio of four-parts blood to one-part crystalloid (4). The development of automated cardioplegia (CP) delivery systems has reduced the use of crystalloid-based carriers facilitating a more concentrated end product, which decreases the volume of asanguineous solution necessary for standard blood CP formulations (5).
Recently, myoprotective methods that have been shown to be effective in reducing the need for CP dosing have received special attention. At a minimum, they are used to lengthen the time between doses of CP, which may lower cross-clamp times by reducing the number of surgical interruptions necessary for delivery (6–9). The CP solutions used to arrest the heart are either based upon hyperkalemic intracellular formulations (del Nido, DN) or on extracellular hyponatremic concentrations [{histidine-tryptophan-ketoglutarate, HTK} Custodiol® Essential Pharmaceuticals, Ewing, NJ] (8,10–12). Both of these have been shown to effectively preserve the myocardium at extended ischemic intervals. However, they are crystalloid-based solutions and require greater asanguineous volumes then are normally seen with either standard 4–1 (4:1) blood to crystalloid ratio, or with low crystalloid volume microplegia (MP) formulations. The latter use blood as the primary delivery source with small volumes of concentrated solutes added to achieve chemical arrest (13–15). Although several studies have shown non-inferiority of the HTK and DN to other types of CP solutions, little research has been published on the impact of these higher crystalloid volumes on intraoperative blood management (12,16).
The purpose of this study was to evaluate the effect of various CP solutions on intraoperative red blood cell (RBC) transfusion requirements, and on fluctuations to intraoperative hematocrit in adult patients undergoing cardiac surgery with CPB.
MATERIALS AND METHODS
We reviewed cardiac surgical procedures where CPB was used from the SpecialtyCare Operative Procedure rEgistry (SCOPE), conducted between February 2016 and January 2017 at 171 hospitals throughout the United States. The SCOPE registry was established in 2011 as a quality improvement database for collecting intraoperative data from cardiac surgical procedures. It serves several purposes focused on performance improvement. It is used to standardize electronic data recording of specific perioperative quality indicators; create reporting tools including dashboards and written reports; and facilitate benchmarking of performance at multiple levels including the clinician, the hospital, and geographical region. More than 100 quality indicators are used for data analytics, and each indicator is regularly reviewed by an advisory board who use the best available evidence to assess which data points should be acquired. The system uses proprietary software1 that records demographic and operative data for every case, with more than 1.5 million cases populating the registry with one quarter of them being cardiac surgery cases. Data validation is assured by monthly auditing of random case records (a minimum of three records for each perfusionist per month) with the results analyzed and reported to the individual clinicians. Deviations are reviewed and corrections made to the central database. Managers use various dashboards to assess clinician and hospital performance as well as review the accuracy of the data. Institutional ethics review board approval was obtained for the use of data from the SCOPE registry (Protocol # 012,017, Schulman IRB, Cincinnati, OH).
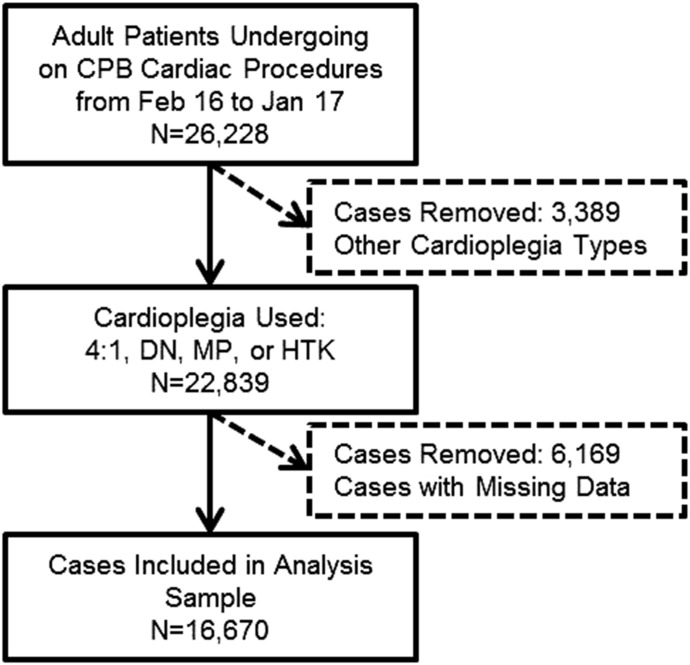
Figure 1 depicts all sample inclusion and exclusion criteria. All surgical patients above 18 years of age who underwent an on-CPB cardiac procedure, used one of four cardioplegia solutions of interest, and had complete data on all variables were included. Four distinct groups were identified based on the primary CP solution used: 4:1, MP, DN, and HTK. Protocols for administering CP were hospital- and surgeon-specific and were not standardized throughout the study groups. The operative procedures from which data were obtained includes isolated coronary artery bypass grafting (CABG), aortic surgery, isolated aortic valve (AV) surgery, combined AV Mitral Valve (MV) surgery, AV + CABG surgery, isolated MV surgery, MV surgery + CABG, and non-elective procedures.
Figure 1.
Sample selection criteria.
The primary end point was intraoperative transfusion of allogeneic RBCs. The secondary end point was the hematocrit change while on CPB, as defined by the change in hematocrit between the immediate post-heparinization period of CPB to the nadir hematocrit on CPB. Additional hematocrit change values were calculated and descriptively assessed: operating room entry to nadir CPB, operating room entry and nadir intraoperative, and post-heparinization and nadir intraoperative.
Statistical Analysis
Descriptive statistics and summary statistics were calculated for each CP group as count and percentage for categorical variables, mean and standard deviation for continuous and normally distributed variables, or median and interquartile range for heavily skewed continuous variables. Group differences were assessed using chi-squared tests, Welch’s anova, and the Kruskal–Wallis rank sum test, respectively.
We assessed the primary end point, transfusion of RBC units, and the secondary end point, hematocrit drift post-heparinization to nadir on CPB, using a multivariable logistic and linear mixed effects models, respectively. Mixed effects regression models account for bias arising from fact that patients receiving care in the same hospital and from the same surgeon tend to have correlated outcomes. In addition to random intercept controls for surgeon and hospital, the model included controls for a number of confounding variables: age, gender, estimated circulating blood volume (calculated using the Nadler formula), first hematocrit upon entry to the operating room, hematocrit immediately after heparinization, diabetes status, type of procedure, procedure acuity (elective vs. non-elective), reoperation, net priming volume in the extracorporeal circuit, asanguineous volume added by perfusion while on CPB, urine output on CPB, ultrafiltration volume, total volume administered by anesthesia, cross-clamp time, and total CPB time. Patients receiving intraoperative RBC transfusions were excluded from the regression analysis of hematocrit drift, the secondary outcome, as the specific timing of these transfusions relative to the hematocrit change could not be assured. Including these patients may have artificially elevated the nadir hematocrit, falsely influencing the results of the contribution of CP type on hemodilution.
All analyses were completed using the R statistical computing environment in conjunction with the “data.table,” “tableone,” “ggplot2,” “lme4,” “sjplot,” and “multcomp” packages (17–23).
RESULTS
A total of 16,670 procedures were identified with the following distribution of cases by CP technique: 4:1 (8,350), MP (4,606), DN (3,344), or HTK (370). Demographic statistics are shown in Table 1. There was a higher tendency to use both 4:1 and MP for isolated CABG procedures, whereas DN and HTK were more likely to be used for AV and/or MV surgeries. For non-elective procedures, the least likely solution to be used was DN.
Table 1.
Clinical characteristics of patients in four cardioplegia groups.
| 4:1 | MP | DN | HTK | p-Value | |
|---|---|---|---|---|---|
| Observations | 8,350 | 4,606 | 3,344 | 370 | |
| Female gender—n (%) | 2,391 (28.6) | 1,217 (26.4) | 1,057 (31.6) | 117 (31.6) | <.001 |
| Patient age (years)—mean (SD) | 65.2 (10.8) | 65.8 (10.8) | 66.9 (11.1) | 64.8 (11.7) | <.001 |
| Body surface area (m2)—mean (SD) | 2.01 (.25) | 1.99 (.24) | 1.97 (.24) | 2.02 (.26) | <.001 |
| Nadler est. blood volume (L)—mean (SD) | 5.2 (1.0) | 5.2 (.9) | 5.1 (1.0) | 5.3 (1.0) | <.001 |
| First Hct in OR (%)—mean (SD) | 36.1 (5.7) | 36.3 (5.7) | 36.0 (5.5) | 36.8 (5.2) | .022 |
| Post heparinization Hct (%)—mean (SD) | 33.9 (5.7) | 33.7 (5.8) | 33.3 (5.6) | 33.3 (5.2) | <.001 |
| Diabetic patient—n (%) | 2,928 (35.1) | 1,849 (40.1) | 1,003 (30.0) | 114 (30.8) | <.001 |
| Procedure type | <.001 | ||||
| Isolated CABG—n (%) | 5,696 (68.2) | 3,245 (70.5) | 1,527 (45.7) | 138 (37.3) | |
| Aortic surgery—n (%) | 305 (3.7) | 120 (2.6) | 145 (4.3) | 18 (4.9) | |
| AV surgery + CABG—n (%) | 621 (7.4) | 337 (7.3) | 371 (11.1) | 35 (9.5) | |
| Combined AV/MV Surgery—n (%) | 109 (1.3) | 80 (1.7) | 94 (2.8) | 10 (2.7) | |
| Isolated AV surgery—n (%) | 845 (10.1) | 467 (10.1) | 705 (21.1) | 96 (25.9) | |
| Isolated MV surgery—n (%) | 528 (6.3) | 226 (4.9) | 360 (10.8) | 55 (14.9) | |
| MV surgery + CABG—n (%) | 246 (2.9) | 131 (2.8) | 142 (4.2) | 18 (4.9) | |
| Non-elective procedure—n (%) | 1,777 (21.3) | 1,316 (28.6) | 575 (17.2) | 119 (32.2) | <.001 |
| Reoperation—n (%) | 273 (3.3) | 134 (2.9) | 146 (4.4) | 11 (3.0) | .004 |
AV, aortic valve; CABG, coronary artery bypass graft; DN, del Nido; Est, estimated; Hct, hematocrit; HTK, histidine-tryptophan-ketoglutarate; MP, microplegia; MV, mitral valve; OR, operating room; SD, standard deviation; 4:1, 4–1 blood to crystalloid.
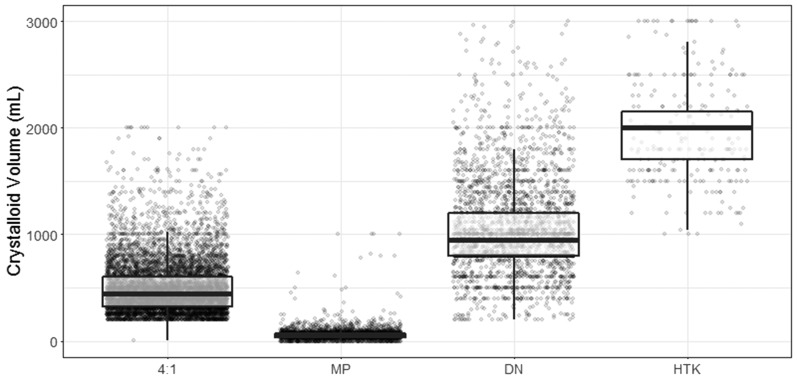
Operative data and crystalloid volumes administered are shown in Table 2. The 4:1 group had the highest net prime volume when compared with the other groups, and the highest urine output during CPB. Patients receiving HTK CP had significantly higher crystalloid volumes added during CPB than any other group. Utilization of ultrafiltration was more than two times higher in the DN and HTK groups than those receiving either 4:1 or MP. When MP was used, there was significantly less ultrafiltration volume removed than any other CP technique. Both CPB and cross-clamp times were lowest in the DN group. The distributions of total crystalloid CP volumes are shown in Figure 2. The highest volume was seen in HTK patients, followed by DN, 4:1, and MP. Total blood2 CP volumes presented as median and interquartile range were as follows: 4:1 (n = 7,210), 2,200 [1,600, 3,000]; MP (n = 3,603), 3,090 [2,074, 4,637]; DN (n = 2,322), 1,200 [1,000, 1,550]; and HTK (n = 370), 0 [0, 0], p < .001.
Table 2.
Operative and volume characteristics by cardioplegia type.
| 4:1 | MP | DN | HTK | p-Value | |
|---|---|---|---|---|---|
| Observations | 8,350 | 4,606 | 3,344 | 370 | |
| Net prime volume (mL)—median [IQR] | 760 [625, 900] | 660 [510, 820] | 655 [500, 900] | 675 [525, 875] | <.001 |
| CPB added crystalloid (mL)—median [IQR] | 283 [82, 700] | 314 [105, 700] | 225 [60, 550] | 915 [343, 1,260] | <.001 |
| Urine output on CPB (mL)—median [IQR] | 250 [130, 400] | 200 [125, 350] | 200 [110, 350] | 230 [135, 350] | <.001 |
| Ultrafiltration used—n (%) | 2,842 (34.0) | 1,895 (41.1) | 2,799 (83.7) | 314 (84.9) | <.001 |
| Ultrafiltration volume when used (mL)—median [IQR] | 1,500 [1,000, 2,300] | 1,200 [700, 1,800] | 2,000 [1,300, 3,000] | 2,900 [2,205, 3,600] | <.001 |
| Anesthesia crystalloid volume (mL)—median [IQR] | 1,500 [1,100, 2,000] | 1,500 [1,000, 1,900] | 1,000 [1,000, 1,550] | 1,300 [1,000, 1,700] | <.001 |
| CPB time (minutes)—median [IQR] | 100 [77, 133] | 103 [77, 138] | 91 [69, 122] | 104 [81, 132] | <.001 |
| Cross clamp time (minutes)—median [IQR] | 73 [55, 98] | 77 [54, 105] | 66 [48, 90] | 82 [64, 100] | <.001 |
CPB, cardiopulmonary bypass; DN, del Nido; HTK, histidine-tryptophan-ketoglutarate; IQR, interquartile range; MP, microplegia; 4:1, 4–1 blood to crystalloid.
Figure 2.
Distribution of crystalloid solution volume given during cardioplegia. Administration by cardioplegia type. Boxplot interpretation: line at middle of box represents median, top and bottom of box are 25th and 75th percentiles, respectively. “Whiskers” extend to 1.5 times the interquartile range above the 75th and below the 25th percentiles. Dots represent individual data points.
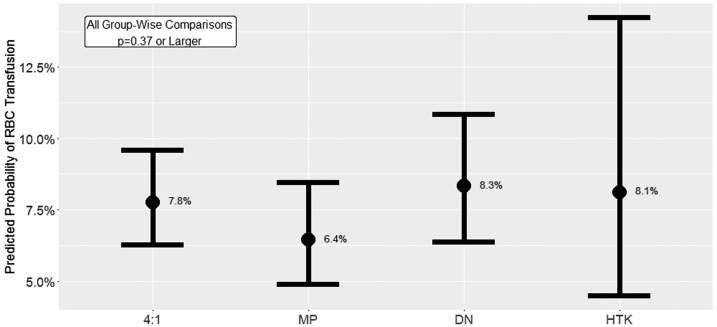
The effects of CP solution on hematocrit and RBC transfusion rates are shown in Table 3. There were no differences among groups in the observed nadir intraoperative or CPB hematocrit levels. The greatest change in hematocrit from operating room entry to either nadir CPB or nadir intraoperative values was seen in the HTK group. When examining hematocrit change using post-heparinization to the lowest level, the 4:1 group had the largest change compared with other groups. The MP group had the highest crude RBC transfusion rate followed by 4:1, DN, and HTK. Our final analysis of the primary outcome, a multivariable logistic mixed effects regression model evaluating the effect of CP type on intraoperative transfusion of one or more units of RBC, is shown in Figure 3 and Table A1. After controlling for numerous demographic and operative confounds, we found no statistically significant relationship between CP type and the likelihood of receiving an RBC transfusion.
Table 3.
Hematocrit and RBC transfusion by cardioplegia type.
| 4:1 | MP | DN | HTK | p-Value | |
|---|---|---|---|---|---|
| Observations | 8,350 | 4,606 | 3,344 | 370 | |
| Intraoperative RBC transfusion—n (%) | 1,635 (19.6) | 967 (21.0) | 579 (17.3) | 55 (14.9) | <.001 |
| Lowest CPB Hct (%)—mean (SD) | 25.3 (4.5) | 25.3 (4.7) | 25.3 (4.4) | 25.3 (4.5) | .920 |
| Lowest intraoperative Hct (%)—mean (SD) | 25.7 (4.6) | 25.9 (4.7) | 25.6 (4.4) | 25.6 (4.5) | .119 |
| Hematocrit drift values | |||||
| OR entry to nadir CPB (%)—mean (SD) | −10.3 (4.1) | −10.4 (4.3) | −10.4 (4.5) | −11.1 (4.1) | .006 |
| OR entry to nadir intraoperative (%)—mean (SD) | −10.8 (4.1) | −11.0 (4.3) | −10.8 (4.4) | −11.4 (4.1) | .001 |
| Post-heparin to nadir CPB (%)—mean (SD) | −8.1 (3.9) | −7.8 (4.1) | −7.7 (4.4) | −7.7 (4.1) | <.001 |
| Post-heparin to nadir intraoperative (%)—mean (SD) | −8.6 (3.9) | −8.4 (4.1) | −8.0 (4.3) | −8.0 (4.1) | <.001 |
RBC, red blood cells; CPB, cardiopulmonary bypass; DN, del Nido; Hct, hematocrit; HTK, histidine-tryptophan-ketoglutarate; MP, microplegia; OR, operating room; SD, standard deviation; 4:1, 4–1 blood to crystalloid.
Figure 3.
Model-predicted probability of RBC transfusion by cardioplegia type. Intervals depicted are 95% confidence intervals.
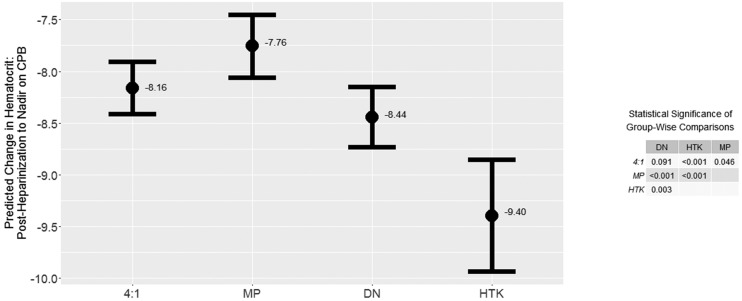
The final assessment of the secondary outcome, a multivariable linear mixed effects regression model evaluating the effect of CP type on hematocrit change between post-heparinization and nadir on CPB, is reported in Figure 4 and Table B1. After controlling for demographic and operative confounds, we found a statistically significant relationship between CP type and hematocrit change over this period. The small table included within Figure 4 reports p-values obtained from a post hoc Tukey’s honestly significance difference (HSD) test of multiple comparisons. Differences in hematocrit drift were significant at the p < .05 level or smaller among all possible comparisons between CP types, except for the 4:1 vs. DN which had a significance level of p = .091.
Figure 4.
Model-predicted hematocrit drift by cardioplegia type. Intervals depicted are 95% confidence. p-values reported for group-wise comparisons from post hoc Tukey’s honestly significance difference test.
DISCUSSION
Beating-heart surgery for treating individuals with coronary disease is achieved without the use of myocardial arrest, but its application in non-catheter-based surgery for most other cardiac lesions is limited. Instead, surgeons prefer to achieve electrical quiescence by the administration of a CP solution that induces a rapid arrest and relaxes the heart in a flaccid state amenable to traction and mobilization. Myocardial tissue is susceptible to ischemia in a time-dependent manner with injury occurring as a consequence both of the absence of perfusion and as an exacerbation with subsequent reperfusion. Re-dosing of CP throughout the ischemic period is thought to reduce the likelihood of sustaining irreversible injury related to several factors, which include the reappearance of electrical activity, the temperature of the myocardial tissue and the length of the ischemic interval.
Although preservation of the myocardium during cardiac surgery has been studied for over 60 years, there remains wide variability in the application of techniques with little standardization and an absence of agreement on optimizing strategies (3). There are many reasons why consensus has not occurred (24). These include a paucity of well-designed and controlled prospective outcome-driven studies, a lack of specific point of care markers for rapid assessment of injured myocardium, and the inherent biological diversity associated with patients presenting with non-uniform cardiovascular disease. The quest to identify optimum strategies for myocardial protection is predicated upon formulating solutions with constituents that lower energetic demands to sustain a period of ischemia, and preserve cellular mechanisms for a rapid restoration of aerobic metabolism. In addition, the delivery of these solutions must be precisely and safely administered by mechanical systems incorporating varying cannulation strategies, temperature regulation by the use of heat exchangers, and filtration to remove insoluble elements.
Despite the existence of significant variation in the use of CP solutions, one common goal that is universally accepted is the quest to reduce the frequency of dosing necessary for myocardial protection (24). Many European hospitals use a hyponatremic solution that was originally described by Bretschneider in the early 1960s (10,11). This solution has more recently been applied to cardiac cases in the United States, although it has been used for organ transplantation and vascular surgery (25,26). Although many benefits have been ascribed to HTK in cardiac surgery, one of the more attractive features is its use in a single-dose administration, which is especially beneficial for minimally invasive procedures where access is limited (6,7,27). Because of its formulation, HTK has been shown to induce hyponatremia with drops in serum sodium of 15 mmol/L reported (28). However, the negative consequences of hyponatremia are most evident when concomitant hypotonicity is encountered, with cerebral tissue especially susceptible to the edema (29). Although hyponatremia does occur with HTK, the slight hypertonicity of the solution maintains an isotonic state, so it is rarely recommended to treat low systemic sodium levels (28).
An additional myoprotective solution that is gaining popularity is DN CP. It was initially used for protection of the pediatric heart and has since been expanded into the adult realm as well (8,9,16,30). When DN is compared with traditional blood CP, it has resulted in shorter cross-clamp and operating room times, lower intraoperative glucose levels, lower insulin requirements, less CP re-dosing, fewer post-ischemic defibrillatory shocks, and lower total CP volumes (16,31–34). Despite these promising results, some have questioned the application of a solution initially designed for pediatric patients for routine use in adult cardiac surgery (35,36). A lack of data from well-designed randomized trials fosters cautionary acceptance, along with a growing number of centers modifying the original formulation and administering it without published data to support its efficacy.3 Nevertheless, both HTK and DN have been shown to be non-inferior to traditional CP formulations that are blood based (34,37). Systematic reviews for both MP and HTK have not established superiority of either technique over standard blood and crystalloid formulations as myocardial preservation strategies (38,39).
We chose to examine the effects of various CP solutions on hemodilution and the resulting interventions that are used to limit iatrogenic anemia. The most commonly used CP techniques were 4:1 and MP. The 4:1 technique is often referred to as the Buckberg formula and has been in wide use since the late 1970s (4). It is the most often used solution reported in SCOPE across the United States. A move toward primary sanguineous CP supplemented with concentrated chemical elements, has shown less dependency on crystalloid carrier solutions, and has been referred to as MP (13–15). The use of MP has been shown to reduce hemodilution maintaining a higher level of all the formed elements found in blood, as well as delivering higher buffering capacity and better osmotic potential (39). Therefore, both 4:1 and MP may confer a specific blood management benefits over DN and HTK. In our study, we found significantly higher volumes of crystalloid solutions used in both the DN and HTK groups as compared with 4:1 and MP. However, the higher use of ultrafiltration during CPB resulted in the absence of statistical differences in nadir intraoperative and on-CPB hematocrits. Although the use of ultrafiltration has been shown to remove excess plasma water and reduce hemodilution, a recent study using registry data offers a cautionary note to its use (40). Paugh and associates found that patients who underwent ultrafiltration during CPB not only had a higher percentage or RBC transfusions, but had an increased risk for developing new onset of acute kidney injury if they presented with a low creatinine clearance rate and had high ultrafiltrate volumes.
Although there were higher transfusion rates of RBC seen in the MP group, once confounders were removed, the predicted probability of receiving a transfusion was not related to the type of CP used. The multivariable logistic regression analysis revealed the significant contribution of the hospital as a primary determinant of whether or not a patient received an RBC transfusion (Table A1). Other prominent factors included the crystalloid volumes added during CPB or by anesthesia as well as gender, age, CPB and cross-clamp time, first operating room hematocrit, and others.
Cardiac surgery with the use of CPB has historically resulted in significant use of allogeneic blood products related to several factors including the presurgical presence of anemia, the use of pre and intraoperative medications that alter hemostatic capacity, and the iatrogenic induction of hemodilution by anesthesia and perfusion management techniques. Significant efforts have been proposed to mitigate both the anemic and coagulation disturbances in an effort to preserve hemostatic function and reduce a patient’s likelihood of receiving allogeneic transfusions. The traditional formulations of HTK and DN cardioplegia necessitate the use of significant volumes of crystalloid solutions to be used as carrier vehicles for the delivery of CP constituents. This increased dependency on crystalloid-based CP has been shown to increase hemodilution and result in lower intraoperative hematocrits and greater transfusion rates (41,42). Eucher et al. (41) had described a means of reducing the CP induced hemodilution by sequestering the solution by the use of a retrograde coronary sinus CP cannula. A more recent study by Gunday and Bingol prospectively compared the effects of a crystalloid CP solution (St. Thomas II) with a blood-based CP technique (Calafiore) (42). Although significant differences were seen in nadir intraoperative hematocrit and transfusion of RBC and fresh-frozen plasma, the authors did not report on fluid balances or the use of ultrafiltration to mitigate these changes.
A recent study by Salinas et al. (30) compared DN with 4:1 blood CP in first-time CABG patients. Using propensity analysis, the authors were able to show a slight benefit in reduced transfusions for patients receiving DN CP. This was in contrast to our study where we were unable to show that the type of CP solution affected the risk of receiving a transfusion. However, the authors did not define whether transfusion was only for RBC, as was our study, or for all allogeneic products. They also reported the change in hematocrit defined as the initial operating room hematocrit minus the end-operative hematocrit at chest closure, which did not differ between groups. We chose to examine the change in hematocrit by using two different start points. We examined the operating room entry and post-heparinization hematocrits, with the latter taking into effect the drift in hematocrit occurring by anesthesia interventions before CPB. There were significant differences when we used nadir intraoperative and CPB hematocrits as end points to calculate the change. These differences were greatest when measuring the change from operating room entry to nadir CPB with 4:1, MP, and DN all at least .7% point lower than HTK CP. By using a multivariable linear mixed model to remove confounders, we found that the CP type can predict the change in hematocrit with MP resulting in the least change in hematocrit compared with other solutions. There was no difference in predicting the change between 4:1 and DN.
In the present study, we observed a skewed use of CP solutions related to the type of procedure that patients were undergoing. Both 4:1 and MP were seen most often in isolated CABG surgeries whereas DN and HTK were used more than twice as often for isolated aortic and mitral valve procedures. The use of HTK and DN CP solutions in surgeries that result in longer cross clamp times (multiple valve surgeries, combined procedures, and aortic operations) may actually result in lower total CP volumes being administered than standard 4:1 CP related to a reduced number of doses (12,31). In a recent meta-analysis of the use of MP the authors found that patients who received MP had lower volumes of CP than those receiving standard blood CP (39). In our study, the group with the highest total blood volume was MP with the 4:1 group receiving approximately 1 L less than MP and the DN group 2 L less. Although the lower volume in the DN group is explainable by less frequent dosing, it is unclear why the MP group was higher than the 4:1 CPB and as cross-clamp times were similar.
Limitations
There are several limitations to this study and include the analysis of data from a retrospective observational registry. This study was conducted using a national registry of data collected in a prospective, but non-randomized manner. Although the study comprised more than 16,000 cases during the study period, only 370 patients were in the HTK group. Protocols for CP administration were not standardized across centers. Therefore, differences in practice patterns do exist and although we attempted to minimize these by multivariable logistic regression, we realize that variability may exist. Furthermore, transfusion protocols varied both across and within individual hospitals, so the administration of RBC may have been biased by clinical decisions. The data analyzed were intraoperative and did not include events that occurred in the postoperative period. Therefore, we cannot comment on whether the patients received transfusions postoperatively, not on fluctuations in hematocrit that occurred after leaving the operating room.
CONCLUSIONS
Techniques for myocardial protection incorporate formulations that have varying chemical constituents, added to both sanguineous and asanguineous solutions, which are used to preserve tissue and maintain function in the post-ischemic period. The volume of crystalloid solution added during myocardial protection varies tremendously as a function of the cardioplegia type. The use of ultrafiltration ameliorates the effects of hemodilution and occurs more than twice as often as high crystalloid solutions, such as DN and HTK, are used. Ultrafiltration reduces the risk of RBC transfusion when high crystalloid-based solutions are used. After controlling for demographic and operative confounds, we found no statistically significant relationship between CP type and the likelihood of receiving an RBC transfusion. The change in hematocrit is affected by cardioplegia type such that patients receiving MP resulted in the lowest levels of intraoperative hemodilution. Future research should be conducted to determine how the application of intraoperative blood conservation measures would affect the results shown in this study.
APPENDIX A. LOGISTIC MIXED EFFECTS REGRESSION ESTIMATING ODDS OF RBC TRANSFUSION
Table A1.
Multivariable logistic mixed model full results.
| Odds of Intraoperative Transfusion ≥1 Unit Allogeneic Red Blood Cells | |||
|---|---|---|---|
| Odds Ratio Estimate | 95% Confidence Interval | p-Value | |
| Fixed effects | |||
| Intercept | .073 | .06–.09 | <.001 |
| Cardioplegia type = MP* | .818 | .59–1.14 | .231 |
| Cardioplegia type = DN* | 1.082 | .82–1.43 | .578 |
| Cardioplegia type = HTK* | 1.049 | .56–1.96 | .881 |
| Net CPB circuit priming volume | .976 | .92–1.04 | .435 |
| Perfusion volume added on CPB | 1.543 | 1.45–1.65 | <.001 |
| Urine output CPB | .973 | .92–1.03 | .37 |
| Anesthesia volume added on CPB | 1.291 | 1.21–1.38 | <.001 |
| Ultrafiltration volume | .943 | .88–1.01 | .106 |
| Total CPB time | 2.404 | 2.09–2.77 | <.001 |
| Cross-clamp time | .774 | .68–.88 | <.001 |
| Age | 1.288 | 1.22–1.36 | <.001 |
| Nadler total blood volume | .515 | .48–.56 | <.001 |
| Hct: first in OR | .478 | .43–.54 | <.001 |
| Hct: post-heparin | .371 | .33–.42 | <.001 |
| Female gender | 1.377 | 1.20–1.59 | <.001 |
| Diabetes | 1.166 | 1.04–1.31 | .009 |
| Non-elective procedure | 1.454 | 1.25–1.69 | <.001 |
| Reoperation | 2.095 | 1.61–2.73 | <.001 |
| Aortic surgery† | 1.856 | 1.38–2.50 | <.001 |
| AV surgery + CABG† | .945 | .77–1.16 | .588 |
| Combined AV/MV surgery† | .584 | .40–.86 | .006 |
| Isolated AV surgery† | .529 | .44–.64 | <.001 |
| Isolated MV surgery† | .549 | .43–.69 | <.001 |
| MV surgery + CAB† | .891 | .67–1.19 | .432 |
| Random effects | |||
| Variance attributable to surgeons | .279 | ||
| Variance attributable to hospitals | 1.056 | ||
| Number of surgeons | 450 | ||
| Number of hospitals | 171 | ||
| Intraclass correlation coefficient: surgeons | .06 | ||
| Intraclass correlation coefficient: hospitals | .228 | ||
| Observations | 16,670 | ||
All continuous variables have been scaled and centered before model estimation.
Effect defined relative to 4:1 CP.
Effect defined relative to CABG procedure.
APPENDIX B. LINEAR MIXED EFFECTS REGRESSION ESTIMATING HEMATOCRIT DRIFT
Table B1.
Multivariable linear mixed model full results.
| Hematocrit Change: Postheparinization to Nadir on CPB | |||
|---|---|---|---|
| β Estimate | 95% Confidence Interval | p-Value | |
| Fixed effects | |||
| Intercept | −7.777 | −8.04 to −7.52 | <.001 |
| Cardioplegia type = MP* | .406 | .10 to .72 | .011 |
| Cardioplegia type = DN* | −.281 | −.52 to −.04 | .022 |
| Cardioplegia type = HTK* | −1.236 | −1.75 to −.72 | <.001 |
| Net CPB circuit priming volume | −.128 | −.18 to −.08 | <.001 |
| Perfusion volume added on CPB | −.728 | −.78 to −.67 | <.001 |
| Urine output CPB | .0431 | −.01 to .10 | .109 |
| Anesthesia volume added on CPB | −.181 | −.24 to −.13 | <.001 |
| Ultrafiltration volume | .598 | .54 to .66 | <.001 |
| Total CPB time | −.36 | −.47 to −.24 | <.001 |
| Cross-clamp time | −.04 | −.15 to .07 | .476 |
| Age | −.115 | −.16 to −.07 | <.001 |
| Nadler total blood volume | 1.031 | .98 to 1.08 | <.001 |
| Hct: first in O.R. | 1.054 | .96 to 1.14 | <.001 |
| Hct: post-heparin | −3.667 | −3.76 to −3.57 | <.001 |
| Female gender | −.586 | −.71 to −.47 | <.001 |
| Diabetes | −.159 | −.25 to −.07 | <.001 |
| Non-elective procedure | −.203 | −.32 to −.08 | <.001 |
| Reoperation | −.163 | −.41 to .09 | .204 |
| Aortic surgery† | .442 | .17 to .71 | .001 |
| AV surgery + CABG† | .026 | −.15 to .20 | .772 |
| Combined AV/MV surgery† | .890 | .53 to 1.25 | <.001 |
| Isolated AV surgery† | .632 | .50 to .77 | <.001 |
| Isolated MV surgery† | .795 | .61 to .98 | <.001 |
| MV surgery + CAB† | .104 | −.16 to .37 | .443 |
| Random effects | |||
| Residual variance | 5.432 | ||
| Variance attributable to surgeons | .204 | ||
| Variance attributable to hospitals | 1.845 | ||
| Number of surgeons | 434 | ||
| Number of hospitals | 170 | ||
| Intraclass correlation coefficient: surgeons | .027 | ||
| Intraclass correlation coefficient: hospitals | .247 | ||
| Observations | 13,434 | ||
All continuous variables have been scaled and centered before model estimation.
Effect defined relative to 4:1.
Effect defined relative to CABG procedure.
Footnotes
Case Documentation System, SpecialtyCare, Nashville, TN.
The observations in the 4:1, MP, and DN groups are reported lower here than in the tables because of a change in labeling of blood cardioplegia that occurred during the study.
In a recent review of the SCOPE, we found that modified del Nido was utilized in 2.4% of cases using del Nido cardioplegia: Conducted February 9, 2017, through April 13, 2017, of 785 cases with del Nido, 107 were using modified formulations.
REFERENCES
- 1.Yaffee DW, Williams MR. Cardiovascular surgery in the elderly. Semin Thorac Cardiovasc Surg. 2016;28:741–7. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi Y, Ohtani M, Hiraishi T, et al. “Initial, continuous and intermittent bolus” administration of minimally-diluted blood cardioplegia supplemented with potassium and magnesium for hypertrophied hearts. Heart Lung Circ. 2006;15:325–31. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson ZG, Yarborough DE, Jarvis BL, et al. Evidence-based medicine and myocardial protection—Where is the evidence? Perfusion. 2015;30:415–22. [DOI] [PubMed] [Google Scholar]
- 4.Follette DM, Mulder DG, Maloney JV, et al. Advantages of blood cardioplegia over continuous coronary perfusion or intermittent ischemia. Experimental and clinical study. J Thorac Cardiovasc Surg. 1978;76:604–19. [PubMed] [Google Scholar]
- 5.Sydzyik RT, Stammers AH, Zavadil DP, et al. Evaluation of a new generation cardioplegia administration system. J Extra Corpor Technol. 1997;29:145–53. [PubMed] [Google Scholar]
- 6.Matzelle SJ, Murphy MJ, Weightman WM, et al. Minimally invasive mitral valve surgery using single dose antegrade custodiol cardioplegia. Heart Lung Circ. 2014;23:863–8. [DOI] [PubMed] [Google Scholar]
- 7.Savini C, Murana G, Di Eusanio M, et al. Safety of single-dose histidine-tryptophan-ketoglutarate cardioplegia during minimally invasive mitral valve surgery. Innovations. 2014;9:416–20. [DOI] [PubMed] [Google Scholar]
- 8.Kim K, Ball C, Grady P, et al. Use of del Nido cardioplegia for adult cardiac surgery at the Cleveland Clinic: Perfusion implications. J Extra Corpor Technol. 2014;46:317–23. [PMC free article] [PubMed] [Google Scholar]
- 9.Charette K, Gerrah R, Quaegebeur J, et al. Single dose myocardial protection technique utilizing del Nido cardioplegia solution during congenital heart surgery procedures. Perfusion. 2011;27:98–103. [DOI] [PubMed] [Google Scholar]
- 10.Bretschneider HJ. Uberlebenszeit und Wiederbelebungszeit des Herzens bei Normo- und Hypothermie. Verh Dtsch Ges Kreislaufforsch. 1964;30:11–34. [PubMed] [Google Scholar]
- 11.Bretschneider HJ. Myocardial protection. Thorac Cardiovasc Surg. 1980;28:295–302. [DOI] [PubMed] [Google Scholar]
- 12.Mishra P, Jadhav RB, Mohapatra CK, et al. Comparison of del Nido cardioplegia and St. Thomas Hospital solution - two types of cardioplegia in adult cardiac surgery. Kardiochir Torakochirurgia Pol. 2016;13:295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCann UG 2nd, Lutz CJ, Picone AL, et al. Whole blood cardioplegia (minicardioplegia) reduces myocardial edema after ischemic injury and cardiopulmonary bypass. J Extra Corpor Technol. 2006;38:14–21. [PMC free article] [PubMed] [Google Scholar]
- 14.Francesco Onorati F, Santini F, Dandale R, et al. “Polarizing” microplegia improves cardiac cycle efficiency after CABG for unstable angina. Int J Cardiol. 2013;167:2739–46. [DOI] [PubMed] [Google Scholar]
- 15.Algarni KD, Weisel RD, Caldarone CA, et al. Microplegia during coronary artery bypass grafting was associated with less low cardiac output syndrome: A propensity-matched comparison. Ann Thorac Surg. 2013;95:1532–8. [DOI] [PubMed] [Google Scholar]
- 16.Ota T, Yerebakan H, Neely RC, et al. Short-term outcomes in adult cardiac surgery in the use of del Nido cardioplegia solution. Perfusion. 2016;31:27–33. [DOI] [PubMed] [Google Scholar]
- 17.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. Available at: https://www.R-project.org/.
- 18.Dowle M, Srinivasan A Data.table: Extension of ‘data.frame’. 2016. Available at: https://CRAN.R-project.org/package=data.table.
- 19.Yoshida K, Bohn J Tableone: Create “Table 1” to describe baseline characteristics. 2015. Available at: https://CRAN.R-project.org/package=tableone.
- 20.Wickham H. ggplot2: Elegant graphics for data analysis. New York, NY: Springer Science & Business Media; 2009. [Google Scholar]
- 21.Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 22.Lüdecke D. sjPlot: Data visualization for statistics in social science. 2017. Available at: https://CRAN.R-project.org/package=sjPlot.
- 23.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–63. [DOI] [PubMed] [Google Scholar]
- 24.Durandy DY. Is there a rationale for short cardioplegia re-dosing intervals? World J Cardiol. 2015;7:658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tshomba Y, Kahlberg A, Melissano G, et al. Comparison of renal perfusion solutions during thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2014;59:623–33. [DOI] [PubMed] [Google Scholar]
- 26.Voigt MR, DeLario GT. Perspectives on abdominal organ preservation solutions: A comparative literature review. Prog Transplant. 2013;23:383–91. [DOI] [PubMed] [Google Scholar]
- 27.Vistarini N, Laliberté E, Beauchamp P, et al. del Nido cardioplegia in the setting of minimally invasive aortic valve surgery. Perfusion. 2017;32:112–7. [DOI] [PubMed] [Google Scholar]
- 28.Linder G, Zapletal B, Schwarz C, et al. Acute hyponatremia after cardioplegia by histidine-tryptophane-ketoglutarate – A retrospective study. J Cardiothorac Surg. 2012;7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diringer MN, Zazulia AR. Hyponatremia in neurologic patients: Consequences and approaches to treatment. Neurologist. 2006;12:117–26. [DOI] [PubMed] [Google Scholar]
- 30.Salinas GEG, Nutt R, Rodriguez-Araujo G. Del Nido cardioplegia in low risk adults undergoing first time coronary artery bypass surgery. Perfusion. 2017;32:68–73. [DOI] [PubMed] [Google Scholar]
- 31.Sorabella RA, Akashi H, Yerebakan H, et al. Myocardial protection using del Nido cardioplegia solution in adult reoperative aortic valve surgery. J Card Surg. 2014;29:445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mick SL, Robich MP, Houghtaling PL, et al. del Nido versus Buckberg cardioplegia in adult isolated valve surgery. J Thorac Cardiovasc Surg. 2015;149:626–36. [DOI] [PubMed] [Google Scholar]
- 33.Ramanathan R, Parrish DW, Armour TK, et al. Use of del Nido cardioplegia in adult cardiac surgery. Thorac Cardiovasc Surg. 2015;63:624–7. [DOI] [PubMed] [Google Scholar]
- 34.De Palo M, Guida P, Maestro F, et al. Myocardial protection during minimally invasive cardiac surgery through right mini-thoracotomy. Perfusion. 2017;32:245–52. [DOI] [PubMed] [Google Scholar]
- 35.Valooran GJ, Shiv Kumar Nair SK, Chandrasekharan K, et al. del Nido cardioplegia in adult cardiac surgery - scopes and concerns. Perfusion. 2016;31:6–14. [DOI] [PubMed] [Google Scholar]
- 36.Sinha P, Jonas RA. Time for a randomized prospective trial of single dose del Nido cardioplegia solution in adults. Perfusion. 2016;31:34–7. [DOI] [PubMed] [Google Scholar]
- 37.Kim JS, Jeong JH, Moon SJ, et al. Sufficient myocardial protection of del Nido cardioplegia regardless of ventricular mass and myocardial ischemic time in adult cardiac surgical patients. J Thorac Dis. 2016;8:2004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edelman JJB, Seco M, Dune B, et al. Custodiol for myocardial protection and preservation: A systematic review. Ann Cardiothorac Surg. 2013;2:717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong B, Ji B, Sun Y, et al. Is microplegia really superior to standard blood cardioplegia? The results from a meta-analysis. Perfusion. 2015;30:375–82. [DOI] [PubMed] [Google Scholar]
- 40.Paugh TA, Dickinson TA, Martin JR, et al. Impact of ultrafiltration on kidney injury after cardiac surgery: The Michigan experience. Ann Thorac Surg. 2015;100:1683–8. [DOI] [PubMed] [Google Scholar]
- 41.Eucher PM, Buche M, Broka S, et al. Retrieval of crystalloid cardioplegic solutions. Ann Thorac Surg. 1996;61:746–7. [DOI] [PubMed] [Google Scholar]
- 42.Gunday M, Bingol H. Is crystalloid cardioplegia a strong predictor of intra-operative hemodilution? J Cardiothorac Surg. 2014;9:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]