Abstract
In intertemporal choices between immediate and delayed rewards, people tend to prefer immediate rewards, often even when the delayed reward is larger. This is known as temporal discounting. It has been proposed that this tendency emerges because immediate rewards are more emotionally arousing than delayed rewards. However, in our previous research, we found no evidence for this but instead found that arousal responses (indexed with pupil dilation) in intertemporal choice are context-dependent. Specifically, arousal tracks the subjective value of the more variable reward option in the paradigm, whether it is immediate or delayed. Nevertheless, people tend to choose the less variable option in the choice task. In other words, their choices are reference-dependent and depend on variance in their recent history of offers. This suggests that there may be a causal relationship between reference-dependent choice and arousal, which we investigate here by reducing arousal pharmacologically using propranolol. Here, we show that propranolol reduces reference-dependence, leading to choices that are less influenced by recent history and more internally consistent.
Keywords: intertemporal choice, temporal discounting, emotional arousal, propranolol, pupil dilation
Introduction
We often face decisions involving trade-offs between smaller, immediate gains and larger long-term benefits. In these intertemporal choices (Strotz, 1956; Laibson, 1997), people generally prefer immediate rewards to delayed rewards, sometimes even when the delayed reward is larger (a phenomenon known as temporal discounting). One proposed explanation for temporal discounting is that choosing long-term gains over immediate ones requires one to overcome ‘hot’ affective responses to the immediate reward (Metcalfe and Mischel, 1999; Berns et al., 2007). One important component of any affective response is physiological arousal (Scherer, 2005), raising the question of whether arousal in response to immediate reward is the factor that drives impulsive choice. If this were the case, then we would expect that, in choices between immediate and delayed gains, immediate rewards would elicit more emotional arousal. However, few studies have measured or manipulated emotional arousal (i.e. sympathetic nervous system response) during intertemporal choice. Utilizing pharmacological intervention to modulate emotional arousal, here we ask for the first time whether such arousal has a causal role in intertemporal choice.
Given the relative lack of research on emotion in intertemporal choice, there are mixed views on what its role might be. Taking the view that immediate gains are more emotionally arousing than delayed gains, it would follow that arousal in response to immediate rewards would be increased relative to that in response to delayed rewards, regardless of their relative subjective values. Another possibility is that both immediate and delayed rewards are similarly emotionally arousing, to the extent that their values increase relative to some reference point. In a recent study, we measured arousal (pupil dilation responses) while people made intertemporal choices (Lempert et al., 2015). We found that arousal did not correlate with the value of immediate rewards preferentially. Rather, the relationship between arousal and value depended on the recent choice sets. Pupil dilation increased as the subjective value of the more variable reward increased relative to its (time averaged) expected subjective value, whether that reward was immediate or delayed (see also Lempert et al., 2016). In other words, immediate and delayed rewards elicit comparable levels of emotional arousal. The reward that elicits more arousal is simply the one that changes more from trial to trial. Therefore, the choice task structure influenced emotional arousal.
The choice task structure also influenced choices. When delayed rewards are more variable (‘Delay Vary’), people are more likely to select immediate rewards (i.e. their temporal discounting rates are higher). Similarly, when immediate rewards are more variable (‘Immediate Vary’), people are more likely to choose delayed rewards (i.e. their discount rates are lower). This suggests that the more stable reward in a series of choices becomes the ‘default’ option. We refer to this behavioral phenomenon as reference-dependence, since the discount rate depends on the current frame of reference, or task condition. Taken together, these results suggest that emotional arousal may play a role in tracking recent history and biasing decision-making based on the task environment. However, these findings were purely correlational, so it is also possible that reference-dependence in arousal and in choice are not causally related.
Here, we test for a causal role of emotional arousal in intertemporal choice by blunting arousal pharmacologically using propranolol, a beta-adrenergic receptor antagonist. Previous studies have shown that propranolol can diminish the impact of emotional arousal on cognitive functions, such as memory, attention and decision-making, without overt impacts on alertness (Phelps, 2006; Sokol-Hessner et al., 2015). If the role of emotional arousal is to shift choices based on context, then we would observe a reduction in reference-dependence on propranolol. Though less supported by prior efforts of our group and others to date, should immediate rewards be inherently more emotionally arousing than delayed rewards, we would expect propranolol to decrease temporal discounting by decreasing arousal.
Here, subjects participated in a 2 day, placebo-controlled, double-blind, within-subjects study. The procedure was identical on the two test days, except that all participants received an orally administered propranolol pill at one session and a matched placebo pill at the other, with the order counterbalanced. Participants completed an intertemporal choice task on each day, when the bioavailability of the medication was approximately at its peak. The intertemporal choice task had three conditions in which we manipulated the variability of choice options (Delay Vary, Immediate Vary and All Vary). We expected that, on placebo, discount rate would be higher in the Delay Vary compared with the Immediate Vary condition, consistent with our previous work showing that people are more likely to select the more stable reward in the paradigm. We also expected that, if arousal mediates the effect of task condition on discount rate, then discount rates would be more similar between the Immediate Vary and Delay Vary task conditions on propranolol.
Materials and methods
Experimental design
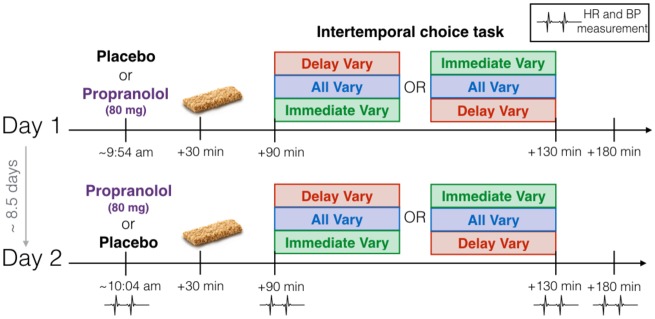
We screened 46 participants to obtain a final sample of ∼30, to ensure adequate power based on our previous study. We anticipated, based on prior research (Lempert et al., 2015; Sokol-Hessner et al., 2015), that a few participants would be excluded for medical reasons or if a discount rate could not be fit to their choice data with adequate confidence. Participants completed a medical screening before being invited to participate in the study (see full list of exclusion and inclusion criteria in Supplementary Material). Of these, seven did not meet the minimum blood pressure or heart rate requirements, and two did not meet electrocardiogram (ECG) criteria. Therefore, 37 subjects (22 F; mean age = 27.8 years; s.d. = 6.6) participated in a 2 day double-blind within-subjects design, with all subjects completing. The two test days were separated by an average of 8.57 days (range: 5–25 days). The procedure was identical on the two test days, except that all participants received an orally administered propranolol pill (80 mg) at one session and a matched placebo pill at the other, with the order counterbalanced (Figure 1). Participants were asked to refrain from eating for 3 h prior to each session and for 30 min following pill administration. Each session began with pill administration, which was followed 30 min later by consumption of a granola bar, to facilitate medication absorption and minimize participant variability in propranolol bioavailability. All test sessions began during the morning, and an effort was made to schedule a given participant’s sessions at the same time on both days (mean time of pill administration = 9:54 AM on Day 1 and 10:04 AM on Day 2). Participants were blind to the order in which they received the two treatments (when asked to guess at the end of the study, they were correct 38% of the time; chance = 50%; two-sided binomial test P = 0.19). The task began 90 min after pill administration, and task instructions, practice trials and the task itself required ∼40 min. After the participants completed the task on the second day, they filled out three questionnaires (for questionnaire details and results, see Supplementary Material). Participants left after 180 min. As part of the safety protocol, blood pressure and heart rate were assessed at four time points: at pill administration (0 min), before the task (+90 min), after the task (∼+130 min) and 180 min after pill administration. These measurements were either taken by a registered nurse or experimenter supervised by the nurse. The nurse obtained vital signs at time points 0 and 180 min to maintain experimenter blinding. The study was approved by the Institutional Review Board at the Nathan Kline Institute, and informed consent was obtained from all participants.
Fig. 1.
Timeline of experimental procedure. At the outset, participants received propranolol (80 mg) at one session and placebo at the other, with the order counterbalanced. Thirty minutes later, they consumed a granola bar. The task began 90 min after pill administration and required ∼40 min. Participants saw the same task order (Delay Vary First or Immediate Vary first) on both days. They left after 180 min. Blood pressure (BP) and heart rate (HR) were assessed at four time points: at pill administration (0 min), before the task (+90 min), after the task (∼+130 min) and 180 min after pill administration.
Intertemporal choice task
At each session, participants performed an intertemporal choice task while pupillometric data were collected. On each trial, participants were presented with a choice screen showing two options: a monetary reward available today (e.g. ‘$10 today’) and a monetary reward of larger magnitude available after a delay (e.g. ‘$20 in 30 days’). They had 6 s to press a button, indicating which option they preferred. The immediate and delayed reward options switched sides of the screen randomly. After the allotted 6 s, a fixation point appeared for 1 s, followed by an outcome screen (3 s), which showed the participants what they had just chosen. After a 3 s intertrial interval, the next choice screen appeared.
Participants were told at the outset that one of their responses from the task on each test day would be randomly selected and that they would receive the amount they chose on that trial, at the delay specified. That is, if they chose the immediate reward on the randomly selected trial, they would receive the money in cash after the session; conversely, if they chose the larger, delayed reward, they would receive the money via Paypal (www.paypal.com) into their bank accounts. All participants registered for a Paypal account during the screening session of the study.
The intertemporal choice task had three conditions: ‘Delay Vary’, ‘Immediate Vary’ and ‘All Vary’. For half of the subjects, the Delay Vary condition came first, followed by the All Vary condition, followed by the Immediate Vary condition. For the other half, the order was reversed. Participants performed the task with the same condition order, on both test days, but the order of the trials within condition typically changed, since trial order was randomized. Thus, every participant saw a unique trial order in each session. There were 180 trials total, divided into six blocks with 30 trials each (60 trials/condition).
In the Delay Vary condition, there were two possible immediate rewards ($10 or $20), each paired with 15 possible delayed rewards of a higher magnitude to be received after 7, 30 or 100 days. $10 today was presented with $11, 15, 20, 25 and 30 after a delay, and $20 today was paired with $22, 30, 40, 50 and 60 after a delay. Each delayed reward magnitude was paired with each delay.
In the Immediate Vary condition, there were two possible delayed rewards: $45 in 30 days or $60 in 30 days. Each of these was presented with one of 15 immediately available rewards. $45 in 30 days was presented with immediate rewards in the set $(9, 11, 13, 15, 17, 19, 21, 23, 25, 28, 31, 34, 37, 40, 43), and $60 in 30 days was presented with immediate rewards in the set $(15, 18, 21, 24, 27, 30, 33, 36, 39, 42, 45, 48, 51, 54, 57).
In the All Vary condition, the variability of the immediate and delayed rewards was equated. The purpose of this condition in this experiment was to create a ‘baseline’ in between presentation of the Delay Vary and Immediate Vary conditions, to reduce carryover effects on behavior between these conditions. For half of the trials here, one of the choice options was an immediate reward of $10. The other option was one of 15 delayed rewards, each dollar amount in the range ($11–25), each available in 30 days. For the other half of the trials in this condition, one of the options was $30 in 30 days, and the other option was one of 15 immediately available rewards, each dollar amount in the range ($13–27). The paradigm was programmed with E-Prime 2.0 Stimulus Presentation Software (Psychology Software Tools Inc, Sharpsburg, PA).
Choice data analysis
To quantify each individual’s temporal discounting rate, we fit their choices to the hyperbolic model of temporal discounting (Mazur, 1987; Green and Myerson, 2004; Kable and Glimcher, 2007) and determined the best-fitting discount rate parameter k in each session (Propranolol/Placebo) as well as for each condition (Immediate Vary/Delay Vary/All Vary) in each session.
Specifically, each participant’s choice data were fit with the following logistic function using maximum likelihood estimation:
where P1 refers to the probability that the participant chose the delayed option, and P2 refers to the probability that the participant chose the immediate option. SV1 and SV2 refer to the participant’s estimated subjective value of the delayed and immediate options, respectively. β is used as a scaling factor, which represents the slope of the logistic function (roughly equivalent to the noisiness of participant choices). The subjective value of the options was estimated using a hyperbolic function:
where A is the amount of the option, D is the delay until its receipt and k is a discount rate parameter that varies across subjects. Higher k values represent relative preference for immediate reward. For immediate options, the subjective value was equal to its magnitude. All discount rates were log-transformed before statistical analyses were performed. See Table 1 for average discount rates across conditions in raw units.
Table 1.
Average discount rates across subjects
| Placebo |
Propranolol |
|||||
|---|---|---|---|---|---|---|
| Condition | Mean (SEM) | Delay at which 50% SV lost (days) | % SV remaining after 1 year | Mean (SEM) | Delay at which 50% SV lost (days) | % SV remaining after 1 year |
| Delay Vary | 0.040 (0.008) | 25 | 6.4 | 0.031 (0.007) | 32.26 | 8.12 |
| Delay Vary (low Effective Dose) | 0.041 (0.012) | 24.39 | 6.26 | 0.035 (0.009) | 28.57 | 7.26 |
| Delay Vary (high Effective Dose) | 0.038 (0.010) | 26.32 | 6.72 | 0.026 (0.009) | 38.46 | 9.53 |
| Immediate Vary | 0.027 (0.005) | 37.04 | 9.21 | 0.020 (0.003) | 50 | 12.05 |
| Immediate Vary (low Effective Dose) | 0.032 (0.009) | 31.25 | 7.89 | 0.020 (0.003) | 50 | 12.05 |
| Immediate Vary (high Effective Dose) | 0.020 (0.005) | 50 | 12.05 | 0.020 (0.005) | 50 | 12.05 |
| Overall | 0.030 (0.005) | 33.33 | 8.37 | 0.024 (0.004) | 41.67 | 10.25 |
| Overall (low Effective Dose) | 0.034 (0.008) | 29.41 | 7.46 | 0.025 (0.005) | 40 | 9.88 |
| Overall (high Effective Dose) | 0.025 (0.005) | 40 | 9.88 | 0.023 (0.006) | 43.48 | 10.64 |
Note: Average hyperbolic discount rates across subjects, the delays at which 50% of the subjective value (SV) of the delayed reward has decreased (implied by the average discount rates) and the percent SV remaining after 1 year (implied by the average discount rates; N = 26). We also include these values separately for participants with high and low effective dose of propranolol (median split).
As is common practice in neuroeconomics research aimed at understanding within-subject dynamics (Tymula et al., 2012, 2013; Lempert et al., 2015), participants were excluded if a unique discount rate could not be compellingly fit for any condition on either day. This occurred for four of the subjects, two of whom selected all delayed rewards and two of whom selected all immediate rewards (thus, exclusions were balanced). However, given that our hypotheses centered on the Immediate Vary and Delay Vary conditions, we only included participants if a discount rate was fit for both the Delay Vary and Immediate Vary conditions on both days (leaving N = 26). Of the seven participants additionally excluded, two chose all immediate rewards in the Immediate Vary condition on placebo. The remaining five chose too inconsistently in the Immediate Vary condition, either on placebo (n = 3), on propranolol (n = 1) or in both medication conditions (n = 1). This occurred when an indifference point could not be calculated because there was too much stochasticity in participants’ choices (e.g. choosing $60 in 30 days over $40 today but also choosing $15 today over $60 in 30 days).
As a robustness check, we also fit the economically more standard exponential model to the data in each condition (Samuelson, 1937):
Here, the parameters are the same as in the hyperbolic model, and e is a mathematical constant (base of the natural logarithm). We performed the same statistical analyses described in the text, using the log-transformed discount rate from the exponential model.
Pupil diameter data collection
To collect pupil dilation data, we used Eye Link 1000 eye tracking equipment (SR Research Ltd, Mississauga, Ontario, CA). We sampled pupil diameter at 250 Hz throughout the task. Participants rested their chins on a chinrest. They were asked to minimize blinking and to focus their eyes on the screen. They were allowed to take breaks in between each block of the task, and the eye tracker was recalibrated between each block.
Pupil diameter data were analyzed using in-house software for Matlab 7.11 (MathWorks, Natick, MA). Eye-blinks were categorized as pupil dilation changes that transpired too quickly to represent actual pupil dilation. They were removed using linear interpolation (Henckens et al., 2009). One participant was dropped from pupil analyses because of unreliable eye tracking (for full pupil methods, analyses and results, see Supplementary Material).
Results
Propranolol reduces reference-dependence in intertemporal choice
As expected, propranolol significantly reduced peripheral arousal (heart rate and systolic blood pressure; see Supplementary Material). Due to propranolol’s high lipophilicity (i.e. its propensity to dissolve easily in fat), it can also cross the blood-brain barrier to influence cognition (Woods and Robinson, 1981). Because of this property, however, propranolol has dose-dependent pharmacokinetics (Borgström et al., 1981). The effective plasma dose depends on lipid content throughout the body. People with higher body mass index (BMI; kg/m2), then, will experience lower peak plasma concentration and greater distribution of the medication in fat (Bowman et al., 1986; Sokol-Hessner et al., 2015). We computed an Effective Dose score by taking the medication dose given to each participant (80 mg) and dividing it by each participant’s BMI. We entered this as a covariate in all analyses.
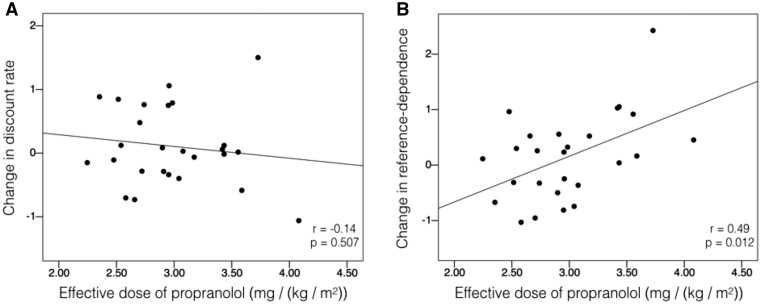
We first tested to see if propranolol changed overall discount rate. To this end, we fit the hyperbolic model to individuals’ intertemporal choices across all trials on each day. We ran a repeated-measures ANOVA on discount rate, with medication (Propranolol/Placebo) as a factor and Effective Dose as a covariate. There was neither a main effect of Medication on discount rate [F(1,24) = 0.63; P = 0.434; η2p = 0.026; η2p 90% CI (0, 0.186)] nor was there an Effective Dose × Medication interaction [F(1,24) = 0.46; P = 0.507; η2p = 0.019; η2p 90% CI (0, 0.170)]. In other words, propranolol did not reduce temporal discounting overall, and the effective dose of propranolol did not correlate with the change in discount rate between propranolol and placebo conditions (r = −0.14; P = 0.507; Figure 2A).
Fig. 2.
Effective propranolol dose vs change in discount rate (discount rate in placebo condition minus propranolol condition) (A) and change in reference-dependence (Delay Vary minus Immediate Vary discount rate, on placebo minus on propranolol) (B). Effective dose = 80 mg divided by the participant’s BMI. There was no significant correlation between change in discount rate overall and effective propranolol dose (N = 26; r = −0.14; P = 0.507). As effective dose increased, the difference between Delay Vary and Immediate Vary discount rates decreased on propranolol relative to placebo (N = 26; r = 0.49; P = 0.012).
To examine if propranolol reduced reference-dependence, we fit the hyperbolic model separately for the Immediate Vary and Delay Vary conditions on each day. On placebo, participants had significantly higher discount rates in the Delay Vary than in the Immediate Vary condition (two-sided paired t-test: t25 = 3.11; P = 0.005). This replicates our previous work, showing that people are more biased to choose the immediate reward when it is more stable, and the delayed reward when it is more stable (Note: since delayed rewards offered in the Immediate Vary condition were on average larger than those offered in the Delay Vary condition, we attempted to control for the ‘magnitude effect’ in follow-up analyses; see Supplementary Material). This difference between conditions was weaker when individuals were given propranolol (t25 = 1.45; P = 0.16). To test for an interaction, we ran a 2 × 2 repeated-measures ANOVA, with Medication (Propranolol/Placebo) and Task Condition (Delay Vary/Immediate Vary) as factors and Effective Dose as a covariate. There was a significant Medication × Task Condition interaction [F(1,24) = 6.41; P = 0.018; η2p = 0.211; η2p 90% CI (0.021, 0.411)], indicating that propranolol decreased the effect of task condition on choice. Moreover, there was a significant Medication × Task Condition × Effective Dose interaction [F(1,24) = 7.43; P = 0.012; η2p = 0.236; η2p 90% CI (0.032, 0.434)], showing that effective dose moderated the effect of the medication on reference-dependence. Specifically, as the effective dose increased, the difference between the Delay Vary and Immediate Vary discount rates decreased on propranolol relative to placebo (r = 0.49; P = 0.012; Figure 2B). There were no significant main effects of Medication [F(1,24) = 0.04; P = 0.842] or Task Condition [F(1,24) = 0.003; P = 0.960], and all other interactions were non-significant (see Supplementary Material).
When replacing Effective Dose with another proxy of medication effectiveness—heart rate difference between conditions—we obtained similar results. We also obtained similar results when we fit discount rates using the exponential, rather than the hyperbolic, model (see Supplementary Material), showing that this result is robust to model specifications. Furthermore, using two non-parametric measures of discounting—percent of choices where delayed reward was chosen and ‘reward index’ (the extent to which the accumulated reward exceeds the amount that would be obtained by always choosing the immediate reward; Palombo et al., 2016)—these effects were significant as well (see Supplementary Material). This provides converging evidence that to the extent that propranolol diminished arousal, it diminished the effects of task environment on choice. Of note, there was no Medication × Task Condition interaction when effective dose was not entered as a covariate in the model, consistent with propranolol’s effects being dose-dependent (Sokol-Hessner et al., 2015).
These results remained significant after controlling for Task Order (Delay Vary first vs Immediate Vary first), Session Order (Propranolol first vs Placebo first), age and gender (see Supplementary Material).
Propranolol increases consistency in intertemporal choice
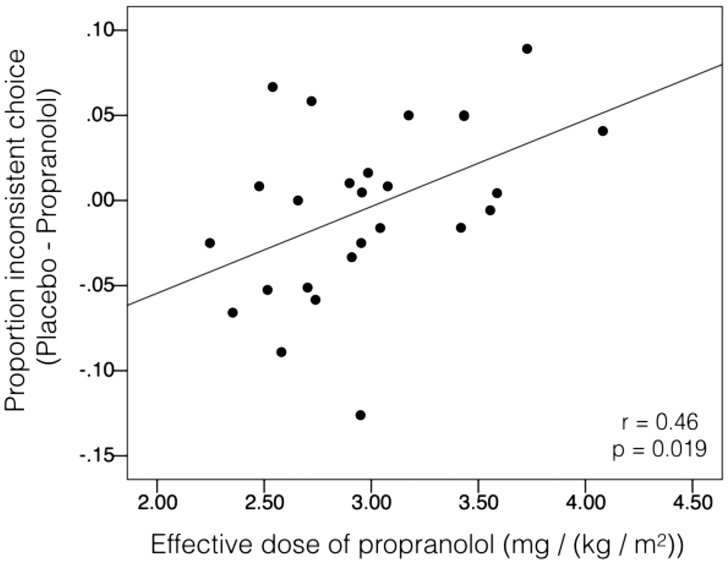
The tendency to choose the more stable reward in the current task condition is non-normative. A traditional economist would argue that the options presented on previous trials should have no bearing on choice in the current trial, and participants should always choose the same option when presented with a given choice. When they do not, they are said to be internally inconsistent. Using a procedure to approximately identify inconsistent choices, we tested to see if, by reducing reference-dependence, propranolol also reduced the proportion of inconsistent choices that participants made. Using the discount rate parameter fit across all choices in each session, we computed the subjective value of the delayed reward on each trial. Since this discount rate represents the discount rate across all task conditions, it most closely resembles the participant’s ‘true’ discount rate. When the subjective value of the delayed reward exceeds the value of the immediate reward, the model predicts that the participant will choose the delayed reward (and vice versa if the immediate reward value is larger than the subjective value of the delayed reward). A trial is labeled ‘inconsistent’, then, if the participant chooses the opposite of what the model would predict. We found that, when controlling for Effective Dose, there was a main effect of Medication on the proportion of inconsistent choices [F(1,24) = 6.50; P = 0.018; η2p = 0.213; η2p 90% CI (0.022, 0.413)]. Participants made more choices consistent with their true discount rate when on propranolol than on placebo. The Effective Dose × Medication interaction was also significant [F(1,24) = 6.29; P = 0.019; η2p = 0.208; η2p 90% CI (0.020, 0.408)], showing that the degree to which participants were more consistent on propranolol than on placebo correlated with Effective Dose of the medication (r = 0.46; P = 0.019; Figure 3). These results remained significant after controlling for Task Order [Main effect of Medication: F(1,23) = 5.88; P = 0.024; Medication × Effective Dose interaction: F(1,23) = 5.69; P = 0.026].
Fig. 3.
Effective dose of propranolol vs difference in proportion of inconsistent choices between medication conditions. Effective dose was operationalized as 80 mg divided by the participant’s BMI. An inconsistent choice is defined as a trial where participants chose the reward that the hyperbolic model predicted they would not choose on that trial (i.e. when they chose the reward with a lower subjective value). As the effective dose of propranolol increased, the proportion of inconsistent choices on propranolol compared with placebo decreased (N = 26; r = 0.46; P = 0.019).
In the earlier analysis, we assumed that participants made inconsistent choices because they were biased to select the more stable option in the task condition (immediate reward in Delay Vary condition and delayed reward in Immediate Vary condition). To test this assumption, we extracted the subset of choices for each participant where the stable option conflicted with their usually preferred option. For example, for a relatively patient participant (one who chose delayed rewards >50% of the time) their idiosyncratic ‘default’ option would be the delayed reward, but in the Delay Vary condition, the task-induced default option is the immediate reward. An impulsive participant, on the other hand, would have conflicting defaults in the Immediate Vary condition. On those trials where there was a conflict, when the participant made an inconsistent choice, it was more likely than chance to be because they chose the stable option in the task environment (mean = 0.75 of trials; s.d. = 0.23; two-sided t-test compared with mean = 0.5: t24 = 5.35; P < 0.001) and not because they chose their usual preferred option. They were also not more likely than chance to choose immediate rewards on those trials (mean = 0.51 of trials; s.d. = 0.34; t24 = 0.15; P = 0.88). This suggests that when participants are more consistent in their decision-making, it is because they ignore the irrelevant trial-to-trial variance of the options in the task.
We propose that propranolol diminishes choice of the stable option by interfering with the influence of recent history on choice. To test this directly, we ran a logistic regression to predict choice of the stable option on the current trial (1 = chose delayed reward in Immediate Vary or immediate reward in Delay Vary; 0 = chose immediate reward in Immediate Vary or delayed reward in Delay Vary). We included as independent variables the subjective values of the stable and variable rewards on the current trial as well as the expected subjective values of the stable and variable options (i.e. average value over the past 40 trials; see Supplementary Material). On placebo, after controlling for the subjective value of rewards on the current trial, the expected value of the stable option positively predicted choice of the stable option (Coeff. = 0.063; P < 0.001). The expected value of the variable reward did not influence choice (Coeff. = 0.002; P = 0.863). This suggests that participants are choosing not only based on the current trial values but they are also more likely to fall back on the stable reward if its value has been high in the past. A Wald test showed that the addition of the expected value terms significantly improved the model fit (χ2 = 15.79; P < 0.001). In contrast, on propranolol, there were no significant effects of the expected value of either the stable reward (Coeff. = 0.027; P = 0.142) or the variable reward (Coeff. = −0.003; P = 0.829) on choice. Here, a Wald test showed no significant improvement in model fit when including the expected value terms (χ2 = 2.37; P = 0.306). Thus, propranolol might diminish reference-dependence by reducing the impact of recent history on choice. This proposed mechanism is further supported by physiological (pupil) data. The pre-trial tonic pupil diameter tracks the expected value of the stable reward and predicts choice of the stable reward on placebo but not on propranolol (see Supplementary Material for pupil dilation results).
Discussion
Here, we have provided causal evidence that the role of emotional arousal in intertemporal choice is to shift choices based on environmental context.
We replicated our previous research (Lempert et al., 2015), showing that intertemporal choices are susceptible to the structure of the choice environment. When immediate rewards are relatively stable over time (e.g. always $10 or $20 today) and delayed rewards vary, individuals are more likely to select immediate rewards. When delayed rewards are relatively stable and immediate rewards vary, people are more likely to select delayed rewards. This behavior is not expected from a normative perspective, since the variability of the choice set is not expected to influence decisions. By pharmacologically manipulating arousal using propranolol (which blocks the effects of endogenously generated epinephrine and norepinephrine), we demonstrated that this effect is caused by neurohormonal factors mediating arousal. Individuals with a higher effective concentration of propranolol showed a smaller difference in discount rate between Delay Vary and Immediate Vary task conditions on propranolol compared with placebo. They also made choices that were more consistent with their ‘true’ discount rate when on the medication compared with placebo. By increasing reference-dependence—perhaps through maintaining a representation of the (task-irrelevant) recent history of offers—arousal leads to suboptimal behavior in this task.
Even though propranolol decreased the impact of task condition on discount rate, it did not change discount rate overall. It has been suggested that ‘hot’ affective responses to immediate rewards, which may include arousal, lead to increased temporal discounting. If this were the case, we would have observed a reduction in temporal discounting when arousal was reduced on propranolol. We cannot rule out that propranolol would reduce discount rate in a higher-powered study, however, since the effect was in the expected direction. We also cannot rule out the possibility that other components of the emotional response, besides arousal, do contribute to temporal discounting. Given that there is no evidence that immediate rewards are more arousing than delayed rewards in this paradigm per se (Lempert et al., 2015) and the growing evidence for a common subjective value signal for immediate and delayed rewards in the brain (Kable and Glimcher, 2007, 2010; Bartra et al., 2013), we think it is unlikely that propranolol would decrease discount rate in a larger sample.
A limitation of this study is that we did not measure risk preferences. If propranolol affects risk preferences, and hence the curvature of the utility function, it would affect the measured discount rate. However, we do not believe that propranolol changes risk preferences, for two reasons. First, a previous study investigating risky decision-making on propranolol with a similar population found no effect of propranolol on risk preferences, even when taking into account effective dose (i.e. BMI; Sokol-Hessner et al., 2015). Second, a change in risk aversion would lead to an overall change in discount rate, not to the task condition-dependent change in discounting that we observed here. Another potential concern is that transaction costs were not equated for immediate and delayed rewards. Immediate rewards were paid in cash, while delayed rewards were paid via Paypal. If propranolol alters attitudes toward those costs, then it would affect discount rate. Once again, we think this is unlikely because there was no overall change in temporal discounting observed between medication conditions. Finally, a change in trust preferences on the medication might lead to more or less impulsivity, since choosing delayed rewards requires some trust in the experimenter to fulfill their agreement to pay out one choice in the future. This is unlikely both because of our null result on overall discount rate but also because we did not observe an effect of Session (Day 1 / Day 2) on discount rate (t25 = −1.44; P = 0.162), and presumably, participants would be more trusting of the experimenter on the second day after their first bonus had already been determined (and, in some cases, paid out).
By focusing on intertemporal choice, we could directly test whether immediate rewards evoke more arousal than delayed rewards. This experiment provides further evidence that arousal responses during intertemporal choice depend on the task environment (see Supplementary Material) and provides novel evidence that arousal causes behavior to be biased by the task environment. Future research will show if this extends to other value-based decision-making tasks or other task structure manipulations (e.g. changes in average value, instead of variance). In addition, given the apparent dose-dependence of the effects seen here, we hope that future studies will directly test dose-dependence, perhaps by tailoring propranolol dosage based on participant weight, as is common practice in non-human animal research.
In sum, manipulating arousal using propranolol allowed us to infer that emotional arousal has a causal role in reference-dependence in intertemporal choice. This augments our knowledge of the role of emotion in intertemporal choice and has implications for the design of decision-making tasks and for interventions to alter choice.
Funding
This work was supported by the National Institutes of Health Grant R01AG039283 awarded to E.A.P. and a National Science Foundation Graduate Research Fellowship to K.M.L.
Supplementary Material
Acknowledgements
The authors thank Peter Sokol-Hessner for advice on study design and analyses as well as Laura Panek for assistance with data collection.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Bartra O., McGuire J.T., Kable J.W. (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns G.S., Laibson D., Loewenstein G. (2007). Intertemporal choice–toward an integrative framework. Trends in Cognitive Sciences, 11(11), 482–8. [DOI] [PubMed] [Google Scholar]
- Borgström L., Johansson C.G., Larsson H., Lenander R. (1981). Pharmacokinetics of propranolol. Journal of Pharmacokinetics and Biopharmaceutics, 9(4), 419–29. [DOI] [PubMed] [Google Scholar]
- Bowman S.L., Hudson S.A., Simpson G., Munro J.F., Clements J.A. (1986). A comparison of the pharmacokinetics of propranolol in obese and normal volunteers. British Journal of Clinical Pharmacology, 21(5), 529–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L., Myerson J. (2004). A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin, 130(5), 769–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens M.J.A.G., Hermans E.J., Pu Z., Joëls M., Fernández G. (2009). Stressed memories: how acute stress affects memory formation in humans. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(32), 10111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. (2007). The neural correlates of subjective value during intertemporal choice. Nature Neuroscience, 10(12), 1625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. (2010). An “as soon as possible” effect in human intertemporal decision making: behavioral evidence and neural mechanisms. Journal of Neurophysiology, 103(5), 2513–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laibson D. (1997). Golden eggs and hyperbolic discounting. The Quarterly Journal of Economics, 112(2), 443–78. [Google Scholar]
- Lempert K.M., Glimcher P.W., Phelps E.A. (2015). Emotional arousal and discount rate in intertemporal choice are reference dependent. Journal of Experimental Psychology: General, 144(2), 366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempert K.M., Johnson E., Phelps E.A. (2016). Emotional arousal predicts intertemporal choice. Emotion, 16(5), 647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur J. (1987). An adjusting procedure for studying delayed reinforcement In: Commons M.L., Mazur J.E., Nevin J.A., editors. Quantitative Analyses of Behavior: Vol. 5: The Effect of Delay and of Intervening Events on Reinforcement Value, pp. 55–73, Hillsdale, NJ: Erlbaum. [Google Scholar]
- Metcalfe J., Mischel W. (1999). A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychological Review, 106(1), 3–19. [DOI] [PubMed] [Google Scholar]
- Palombo D.J., Keane M.M., Verfaellie M. (2016). Using future thinking to reduce temporal discounting: under what circumstances are the medial temporal lobes critical?. Neuropsychologia, 89, 437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A. (2006). Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology, 57, 27–53. [DOI] [PubMed] [Google Scholar]
- Samuelson P.A. (1937). A note on measurement of utility. The Review of Economic Studies, 4(2), 155. [Google Scholar]
- Scherer K.R. (2005). What are emotions? And how can they be measured? Social Science Information, 44(4), 695–729. [Google Scholar]
- Sokol-Hessner P., Lackovic S.F., Tobe R.H., Camerer C.F., Leventhal B.L., Phelps E.A. (2015). Determinants of propranolol’s selective effect on loss aversion. Psychological Science, 26(7), 1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotz R.H. (1956). Myopia and inconsistency in dynamic utility maximization. Review of Economic Studies, 23(3), 165–80. [Google Scholar]
- Tymula A., Rosenberg Belmaker L.A., Roy A.K., et al. (2012). Adolescents’ risk-taking behavior is driven by tolerance to ambiguity. Proceedings of the National Academy of Sciences of the United States of America, 109(42), 17135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymula A., Rosenberg Belmaker L.A., Ruderman L., Glimcher P.W., Levy I. (2013). Like cognitive function, decision making across the life span shows profound age-related changes. Proceedings of the National Academy of Sciences of the United States of America, 110(42), 17143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods P.B., Robinson M.L. (1981). An investigation of the comparative liposolubilities of β-adrenoceptor blocking agents. Journal of Pharmacy and Pharmacology, 33(1), 172–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.