Abstract
Among violent offenders with schizophrenia, there are 2 sub-groups, one with and one without, conduct disorder (CD) and antisocial personality disorder (ASPD), who differ as to treatment response and alterations of brain structure. The present study aimed to determine whether the 2 groups also differ in Theory of Mind and neural activations subsuming this task. Five groups of men were compared: 3 groups of violent offenders—schizophrenia plus CD/ASPD, schizophrenia with no history of antisocial behavior prior to illness onset, and CD/ASPD with no severe mental illness—and 2 groups of non-offenders, one with schizophrenia and one without (H). Participants completed diagnostic interviews, the Psychopathy Checklist Screening Version Interview, the Interpersonal Reactivity Index, authorized access to clinical and criminal files, and underwent functional magnetic resonance imaging while completing an adapted version of the Reading-the-Mind-in-the-Eyes Task (RMET). Relative to H, nonviolent and violent men with schizophrenia and not CD/ASPD performed more poorly on the RMET, while violent offenders with CD/ASPD, both those with and without schizophrenia, performed similarly. The 2 groups of violent offenders with CD/ASPD, both those with and without schizophrenia, relative to the other groups, displayed higher levels of activation in a network of prefrontal and temporal-parietal regions and reduced activation in the amygdala. Relative to men without CD/ASPD, both groups of violent offenders with CD/ASPD displayed a distinct pattern of neural responses during emotional/mental state attribution pointing to distinct and comparatively successful processing of social information.
Keywords: social cognition, psychotic disorders, conduct disorder, functional magnetic resonance imaging, types of violent offenders
Introduction
Robust evidence shows that schizophrenia is associated with an increased risk of aggressive behavior, violent and nonviolent criminality.1 Among violent offenders with schizophrenia, there are at least 2 sub-groups, one with a history of antisocial behavior since childhood as indicated by a diagnosis of conduct disorder (CD) prior to age 15, and a second group with no childhood history of antisocial behavior who begin engaging in aggressive behavior as illness onsets.2 These 2 sub-groups differ as to predictors of violent behavior, engagement and response to antipsychotic medication3 and other treatments,4 and persistence of violent behavior.2,5 Further, those with a history of CD show structural brain abnormalities similar to persons with CD in addition to those typical of schizophrenia.6 These striking differences led us to hypothesize that neural mechanisms underlying violent behavior in the 2 groups differ.
Social cognition has been shown to be an important correlate of violent behavior among persons with schizophrenia7 and thought to be independent of neuro-cognition.8 One component of social cognition is Theory of Mind (ToM). Affective ToM is the ability to understand the emotions of others and cognitive ToM refers to the ability to understand others’ thoughts, actions and intentions. Persons with schizophrenia show poor performance on both kinds of ToM tasks.9 They are also impaired in recognizing emotions in the faces of others,10 particularly anger, fear, and sadness, even in early stages of illness (clinical high risk),11 and this impairment has been linked to violence.12 However, results of studies of ToM among men with schizophrenia are contradictory. Violent offenders, as compared to non-offenders, are reported to display better cognitive and affective ToM,13 similar cognitive ToM coupled with poorer affective ToM,14 and difficulty in recognizing fearful and angry facial expressions.12 Previous studies did not distinguish sub-types of violent offenders with schizophrenia. Non-mentally ill boys with CD and adults with psychopathy present deficits in affective ToM but not cognitive ToM.15,16 Based on this evidence, we hypothesized that affective and cognitive ToM would distinguish 2 groups of violent offenders with schizophrenia: those with early onset, persistent antisocial behavior, indexed by diagnoses of CD prior to age 15 and antisocial personality disorder (ASPD) would be expected to show deficient affective ToM; those with no history of antisocial behavior who began engaging in aggressive behavior as illness onset would be expected to show deficient affective and cognitive ToM.
Neural networks underlying affective and cognitive ToM are inter-related but distinct. Functional magnetic resonance imaging (fMRI) has shown that cognitive ToM primarily engages the dorsomedial prefrontal cortex, the dorsal anterior cingulate cortex, and the dorsal striatum, and affective ToM primarily engages the ventromedial and orbitofrontal cortices, the ventral striatum, and areas mediating emotional experience associated with affective ToM including the ventral anterior cingulate cortex, the amygdala, and the anterior insula.17,18 Together with the temporo-parietal junction (TPJ), both the area around the superior temporal sulcus and the posterior cingulate/precuneus, which are involved in representing and distinguishing self from others, contribute to the larger mentalizing network.19
Assessing impairment in ToM among persons with schizophrenia depends to some extent on the specific task properties, but is usually associated with reduced activation of the neural network underlying ToM.20 Similarly, reduced activation accompanies deficient affective ToM among males with psychopathic traits.21
Furthering understanding of the neural mechanisms underlying violent behavior of persons with schizophrenia has the potential to inform the development of treatments that specifically target mechanisms promoting violence. The present study used fMRI to determine whether activation patterns during a ToM task (ie, the Reading the Mind in the Eyes Test [RMET])22 differed among 2 groups of violent offenders with schizophrenia. Five groups of men were compared: violent offenders with schizophrenia who presented CD prior to age 15 and ASPD in adulthood; violent offenders with schizophrenia and no history of antisocial behavior prior to illness onset; men with schizophrenia and no violence; violent offenders with life-long antisocial behavior and no severe mental illness; and healthy men. Given that (1) intact amygdala functioning seems to be necessary to succeed on the RMET23 and (2) psychopathic relative to healthy men did not perform poorly on the RMET, Richell et al24 suggested that other perhaps cortical regions may compensate during development for reduced amygdala functioning. We therefore hypothesized that CD/ASPD, both with and without schizophrenia, would be associated with less activation in the affective ToM network, and with increased activation in cortical structures associated with cognitive ToM. Moreover, we aimed to explore whether the 2 groups of violent offenders with a life-time history of antisocial behavior, displayed reduced activity in areas mediating the emotional experience previously associated with affective ToM and the pathophysiology of severe antisociality, the bilateral amygdala, anterior insula, and anterior cingulate. Finally, we conducted exploratory analyses to determine whether group differences were associated with psychopathic traits.
Methods
Ethics
The study was approved by the Committee on Medical Ethics of the University of Duisburg-Essen, Germany, and performed in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All participants provided written informed consent after a detailed description of the study.
Participants
Three groups of male, violent offenders were recruited from forensic hospitals and prisons: 13 violent offenders with schizophrenia plus CD/ASPD (VSZ+CD/ASPD); and 16 violent offenders with schizophrenia and no CD/ASPD (VSZ); and 18 violent offenders with CD/ASPD and no severe mental illness (VCD/ASPD). The study also included 2 groups of non-offenders: 18 men with schizophrenia and no history of CD/ASPD were recruited from local hospitals (SZ); and 18 healthy (H) men with no DSM-IV diagnoses other than past substance use disorders were recruited through advertisements. Comparisons of the 5 groups of participants are presented in table 1.
Table 1.
Comparisons of Mean (±SD) Sociodemographic and Clinical Characteristics, Empathy, and Antisocial Behavior of 5 Groups of Participants
| H (n = 18) | VCD (n = 18) | SZ (n = 18) | VSZ-CD (n = 16) | VSZ+CD (n = 13) | Group Comparisons (ANOVA or chi-square) Bonferroni-Adjusted (P < .003) | ||
|---|---|---|---|---|---|---|---|
| F-Statistics | Post hoc Tukey Tests (Bonferroni) | ||||||
| Demographic characteristics | |||||||
| Age (y) | 36.3 ± 9.8 | 35.3 ± 9.3 | 37.8 ± 8.3 | 38.4 ± 9.0 | 34.4 ± 5.7 | 0.5, P = .712 | NA |
| Years of education | 9.9 ± 1.1 | 9.3 ± 0.8 | 9.8 ± 1.7 | 9.4 ± 1.4 | 9.2 ± 0.4 | 1.2, P = .321 | NA |
| Clinical characteristics | |||||||
| Age at schizophrenia onset | NA | NA | 22.7 ± 5.7 | 27.3 ± 7.6 | 22.6 ± 4.7 | 2.9, P = .066 | NA |
| Duration of illness | NA | NA | 15.1 ± 7.1 | 11.1 ± 7.1 | 11.8 ± 5.7 | 1.7, P = .195 | NA |
| PANSS positive syndrome | NA | NA | 7.3 ± 3.0 | 7.0 ± 3.6 | 7.2 ± 2.9 | 0.4, P = .957 | NA |
| PANSS negative syndrome | NA | NA | 19.8 ± 3.7 | 17.4 ± 5.8 | 16.1 ± 4.7 | 2.3, P = .108 | NA |
| PANSS cognitive syndrome | NA | NA | 8.2 ± 3.0 | 7.2 ± 2.5 | 6.8 ± 1.6 | 1.2, P = .302 | NA |
| PANSS hostile excitement | NA | NA | 9.7 ± 2.4 | 12.4 ± 3.1 | 14.3 ± 3.0 | 10.5, P < .001 | VSZ+CD, VSZ-CD > SZ |
| PANSS depression | NA | NA | 6.6 ± 1.3 | 9.4 ± 3.7 | 9.9 ± 3.7 | 5.7, P = .006 | VSZ+CD, VSZ-CD > SZ |
| CPZ score (mg/day) | NA | NA | 633 ± 395 | 599 ± 393 | 654 ± 258 | 0.1, P = .872 | NA |
| History alcohol disorders (%) | 22 | 61 | 33 | 13 | 85 | x 2 = 21.0, P < .001 | NA |
| History drug disorders (%) | 17 | 56 | 39 | 44 | 92 | x 2 = 19.8, P = .001 | NA |
| Premorbid IQ | 111 ± 11 | 105 ± 9 | 102 ± 10 | 107 ± 14 | 103 ± 11 | 1.2, P = .313 | NA |
| Empathy measures | |||||||
| IRI perspective taking | 15.9 ± 1.5 | 14.5 ± 2.8 | 14.4 ± 1.9 | 12.6 ± 2.2 | 13.6 ± 2.0 | 4.8, P = .003 | H > SZ, VSZ-CD, VSZ+CD; SZ > VSZ-CD |
| IRI empathic concern | 14.9 ± 2.4 | 15.1 ± 2.8 | 13.9 ± 3.0 | 13.4 ± 2.4 | 12.8 ± 3.5 | 2.0, P = .104 | NA |
| IRI distress | 9.0 ± 2.0 | 10.5 ± 3.3 | 13.2 ± 2.2 | 10.9 ± 2.3 | 10.6 ± 2.8 | 6.7, P < .001 | SZ > H, VCD, VSZ-CD, VSZ+CD |
| Antisocial behavior | |||||||
| Age at first conviction (violence) | NA | 19.4 ± 4.5 | NA | 31.1 ± 7.0 | 18.4 ± 3.9 | 23.6, P < .001 | VCD, VSZ+CD > VS-CD |
| Number of CD symptoms | 1.1 ± 0.8 | 6.3 ± 2.4 | 1.1 ± 0.7 | 1.1 ± 1.0 | 7.8 ± 2.5 | 60.5, P < .001 | VCD, VSZ+CD > H, SZ, VSZ-CD |
| Number of criminal convictions | 0 ± 0 | 6.8 ± 3.7 | 0 ± 0 | 1.8 ± 1.8 | 7.5 ± 6.0 | 23.5, P < .001 | VCD, VSZ+CD > H, SZ, VSZ-CD |
| PCL:SV total score | 4.4 ± 2.5 | 12.3 ± 2.0 | 3.3 ± 1.6 | 5.8 ± 2.6 | 13.8 ± 3.5 | 62.5, P < .001 | VCD, VSZ+CD > H, SZ, VSZ-CD |
| Factor 1 score | 2.8 ± 1.9 | 5.8 ± 1.7 | 1.6 ± 0.9 | 2.4 ± 1.5 | 6.8 ± 1.8 | 32.2, P < .001 | VCD, VSZ+CD > H, SZ, VSZ-CD |
| Factor 2 score | 1.7 ± 0.8 | 6.5 ± 1.0 | 1.8 ± 0.8 | 3.4 ± 1.4 | 7.0 ± 2.0 | 69.2, P < .001 | VCD, VSZ+CD > H, SZ, VSZ-CD; VSZ-CD > H, SZ |
Note: H, Nonviolent healthy subjects; VCD, Violent offenders with CD/ASPD and no schizophrenia; SZ, nonviolent schizophrenic patients; VSZ-CD, violent offenders with schizophrenia but no CD; VSZ+CD, Violent offenders with schizophrenia and CD; NA, Not applicable; PANSS, Positive and Negative Symptom Scale; IRI, Interpersonal Reactivity Index; CD, Conduct Disorder; PCL:SV, Psychopathy Checklist:Screening Version.
No participant had used alcohol or drugs during the 6 months prior to brain scan, (98% for a minimum of 1 year, 83% for a minimum of 2 years) as confirmed by random urine screens in forensic hospitals and prisons or by self-reports. Past diagnoses of alcohol or drug abuse and/or dependence, however, characterized large proportions of participants in each group.
Measures
Clinical Assessment.
Diagnoses were confirmed using the Structural Clinical Interview for DSM-IV. Symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS)25 and 5 sub-scale scores computed26: hostile excitement, cognitive syndrome, negative syndrome, positive syndrome, and depression. A multiple choice vocabulary test27 was administered to estimate general or verbal intelligence that is thought to be stable across the course of schizophrenia. It is therefore often labeled as a measure of premorbid IQ.
Criminal Convictions.
Information was extracted from official criminal records. Violent crimes were defined as parts 13, 16–18, and 20 (murder, grievous bodily harm, robbery, contact sexual offences, violent crimes against personal liberty) of the German penal code; all other crimes were defined as nonviolent.
Psychopathic Traits.
The Psychopathy Checklist: Screening version (PCL:SV)28 was rated based on the interview and a file review. The PCL:SV includes 2 factors: Factor 1 assesses interpersonal and affective traits, and factor 2 antisocial behavior.
Empathy.
The validated and shortened German29 version of the interpersonal reactivity index (IRI)30 includes 4 scales. Cognitive ToM is assessed by the Perspective Taking (PT) scale, and affective ToM by the Empathic Concern (EC), Fantasy (F), and Personal Distress (PD) subscales. This latter scale measures distress in response to others’ distress.
ToM.
A simplified version of the RMET22 was completed in the scanner. The RMET involves mental state decoding from visual stimuli rather than mental state reasoning, which can occur in the absence of stimulus material.31 A previous study suggests that mental state decoding is a better predictor of social functioning than mental state reasoning. This may therefore also apply to socially deviant behavior, including interpersonal violence. Participants were shown a picture of a person’s eyes and asked which of 2 words, eg, concerned or angry, best described the person’s emotional/mental state (experimental condition) or to judge whether the eyes belonged to a male or female (control condition). Both conditions included the same 36 pictures, for a total of 72 stimuli presented in a block design with 12 blocks. Responses were made by pressing a button using the index and middle fingers of the right hand. Outcome measures were the number of errors in each condition. Details of the task procedure are provided elsewhere.32
Data Acquisition
All MR images were obtained using a 1.5 T MR (Sonata, Siemens) with a standard head coil. BOLD contrast images were acquired by applying an echo-planar acquisition technique (repetition time [TR] 3500 ms, time to echo [TE] 45 ms, flip angle 90°, field of view [FOV] 240 mm, matrix 64) with 38 transversal slices (thickness 3.8 × 3.8 × 3 mm) and a 0.3 mm slice gap. Six initial “dummy” scans were eliminated prior to the data analysis to account for T1 relaxation effects.
Image Processing
We used SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/) to analyze imaging data. Prior to second level statistical analyses, the images were realigned using sinc interpolation and normalized to the stereotactic template of the Montreal Neurological Institute (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach). Bilinear interpolation was applied for normalization to the MNI-template. Normalized images were smoothed with an isotropic Gaussian kernel of 9 mm FWHM. The cutoff for subjects showing translational head movement was set to 2 mm and for rotational movement to 2°. No subject had to be excluded. Single subject contrasts between experimental and control conditions were computed. The model consisted of a boxcar function convolved with the hemodynamic response function (HRF) and the corresponding temporal derivative. High-pass filtering with a cutoff frequency of 120sec. and low-pass filtering with the HRF were applied.
Statistical Analysis
We used the framework of the General Linear Model to perform a statistical group analysis on a voxel-by-voxel basis. To compare groups, single-subject contrast images were entered into a random effects model with subjects as the random factor. Significant signal changes for each contrast were assessed by means of t-statistics and maximum likelihood estimation. The resulting set of voxel values for each contrast constituted the statistical parametric map of the t-statistic. For the second-level group analyses, the resulting single-subject contrast images (emotional/mental state recognition > gender discrimination) were analyzed separately for all 5 groups using 1 sample t tests.
To test the extent to which regionally specific activation patterns differed across groups, ANOVA with group as the between-subject factor of interest were conducted. Statistical parametric maps were computed on a voxel-by-voxel basis to test for between-group differences in a whole brain approach. Post-hoc analyses depicting between-groups of interest (ie, VSZ+CD/ASPD vs H; VSZ+CD/ASPD vs VCD/ASPD; VSZ+CD/ASPD vs SZ; VSZ+CD/ASPD vs VSZ; VSZ vs H; VSZ vs SZ) were calculated using independent sample t tests. The results of analyses were considered significant at a threshold of P < .001 uncorrected at voxel-level (height threshold) and P < .05 corrected after Family-Wise-Error (FWE) at cluster-level (extent threshold). Activation differences in small structures such as the amygdala, the insula, and the anterior cingulate may not be detected when requiring cluster-level significance. Therefore, a region of interest (ROI) analysis was conducted using the automated anatomical labeling approach.33
Finally, Pearson correlations were computed to explore associations between behavioral task performance, brain activity, and clinical characteristics.
Results
Comparisons of Participants
As presented in table 1, the 5 groups were similar as to age, level of education, and premorbid IQ. The 3 groups with schizophrenia were similar as to age of illness onset, duration of illness, scores for positive, negative, and cognitive syndromes, and chlorpromazine-equivalent doses of antipsychotic medication, and differed on scores for hostile excitement and depression that were higher among VSZ+CD/ASPD than VSZ. The 2 groups of violent offenders with CD/ASPD, both those with and without schizophrenia, were similar as to numbers of CD symptoms, age at first violent conviction, number of convictions, and PCL Factor 1 scores, and proportions with histories of alcohol and drug use disorders. The VSZ+CD/ASPD and VCD/ASPD were first convicted for a violent offence at a younger age than the VSZ.
Self-Reported Empathy
On the perspective taking scale, the 3 groups with schizophrenia obtained lower scores than H, with the VSZ obtaining the lowest scores. On the distress scale, the SZ obtained the highest scores.
Behavioral Performance on Reading-the-Mind-in-the Eyes Test
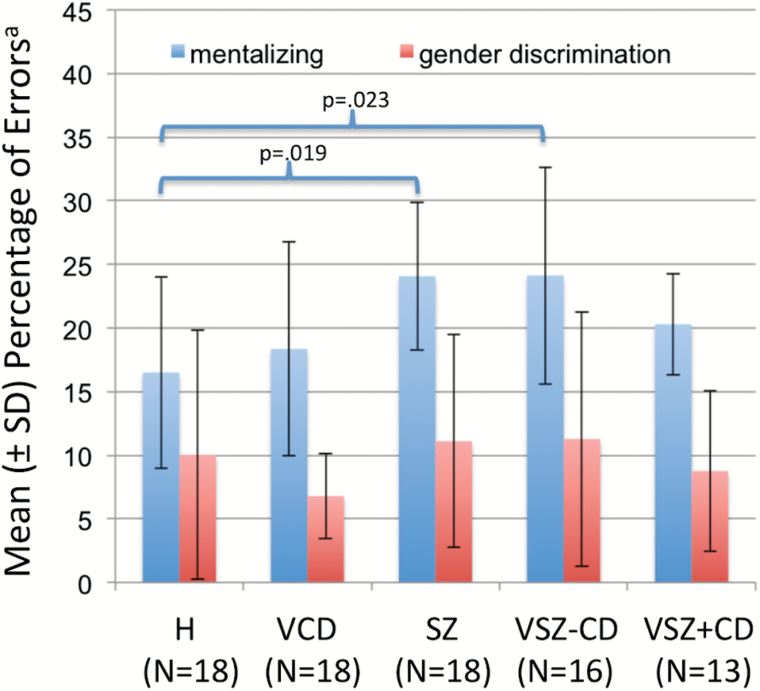
The condition-by-group ANOVA revealed a significant main effect of condition (F(1,78) = 89.7, P < .001) and a significant condition × group interaction (F(4,78) = 3.92, P = .006), indicating that: (1) participants made more errors on emotional/mental state attribution than on gender discrimination; and (2) the groups differed with respect to ToM performance. As presented in figure 1, post hoc tests revealed that SZ and VSZ participants performed more poorly than H, while the VCD/ASPD and VSZ+CD/ASPD made similar numbers of errors as H.
Fig. 1.
Theory of Mind performancea. aSignificant differences between test conditions (F = 89.7, df = 78, P < .001) and condition × group interaction (F = 3.9, df = 78, P = .006).
fMRI Results
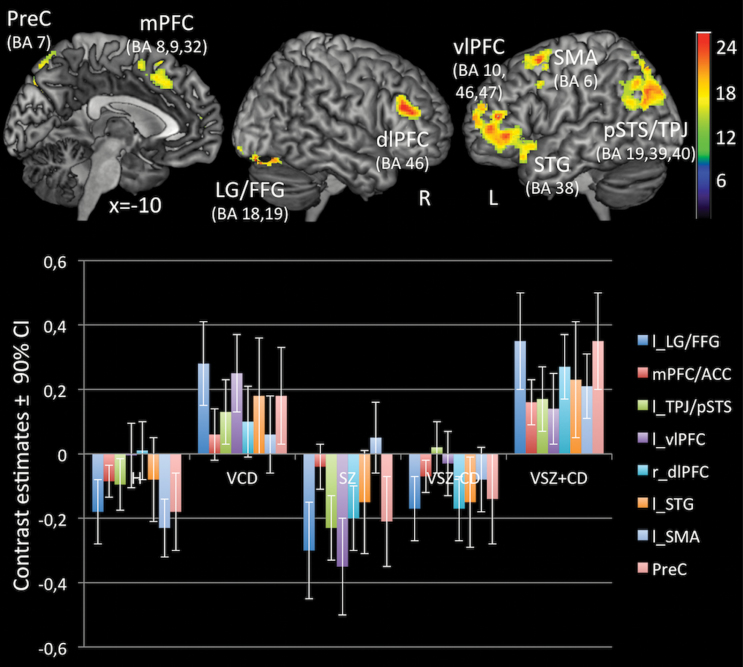
As illustrated in figure 2, the 1-factorial ANOVA revealed a significant main effect of group on brain activity during emotional/mental state attribution as compared to gender discrimination. There were between-group differences in activation patterns in the medial PFC (Brodman area; BA 8,9,32), left ventrolateral PFC (BA 10,46,47; extending into the superior temporal gyrus, STG, BA 38), left supplementary motor area (SMA, BA 6), right dorsolateral PFC (BA 46), left pSTS/TPJ (BA 39,40, extending into the left precuneus, BA 7), right precuneus (BA 7), and right lingual/fusiform gyrus (BA 18,19; for details, see figure legend). As presented in table 2, post-hoc analyses indicated that group differences in ToM related activation mainly reflected activation increases displayed by VSZ+CD/ASPD and VCD/ASPD as compared to VSZ, SZ, and H.
Fig. 2.
Foci and contrast estimates of brain regions, that distinguished between groups on Theory of Mind related brain activationa. aP < .05 corrected for multiple comparisons after Family-Wise-Error at cluster-level. Significant clusters of activation differences include the medial PFC (Brodman areas [BAs] 8,9,32; MNI: −10, 28, 40; k = 407; z = 4.26), left ventrolateral PFC (BAs 10,46,47; MNI: −44, 50, 6; k = 1095; z = 4.78), extending into the left superior temporal gyrus (STG, BA 38; MNI = −46, 14, −20; z = 4.52), left supplementary motor area (SMA, BA 6; MNI: −24, 12, 60; k = 413; z = 4.25), right dorsolateral PFC (BA 46; MNI: 48, 32, 24; k = 705; z = 4.64), left pSTS/TPJ (BA 39,40; MNI: −26, −66, 44; k = 1330; z = 4.69), the bilateral precuneus (BA 7, MNI: −2, −72, 56; k = 557; z = 4.63) and lingual/fusiform gyrus (BAs 18,19; MNI: 24, −84, −20; k = 812; z = 5.09). The color or b/w bar indicates F-value.
Table 2.
Between-Group Differences (Planned Comparisons) in Brain Activation (Mental State Attribution > Gender Discrimination) Pattern (PFWE < .05, Cluster-Level)
| Contrast/Brain Regions | Lat. | BA | Cluster Size | MNI Coordinate | z-Score | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| VSZ+CD vs. H | |||||||
| H > VSZ+CD | None | ||||||
| VSZ+CD > H | |||||||
| Medial PFC | L | 6,8,32 | 675 | −12 | 26 | 40 | 4.19 |
| Supplementary motor area | −24 | 12 | 60 | 4.27 | |||
| pSTS/TPJ | L | 7,19,39 | 237 | −30 | −66 | 34 | 4.25 |
| Precuneus | R/L | 7 | 206 | −2 | −72 | 56 | 3.88 |
| Ventrolateral PFC | L | 10 | 201 | −36 | 56 | 20 | 4.07 |
| Lingual/fusiform gyrus | L | 18,19 | 263 | −28 | −74 | −18 | 4.11 |
| VSZ+CD vs VCD | |||||||
| VCD > VSZ+CD | None | ||||||
| VSZ+CD > VCD | None | ||||||
| VSZ+CD vs SZ | |||||||
| SZ > VSZ+CD | None | ||||||
| VSZ+CD > SZ | |||||||
| Ventrolateral PFC | R | 10,46,47 | 629 | 44 | 32 | 22 | 4.34 |
| L | 44–46 | 592 | −46 | 44 | 14 | 4.00 | |
| Superior temporal gyrus | L | 38 | −46 | 14 | −20 | 3.98 | |
| pSTS/TPJ | L | 7,19,39 | 428 | −32 | −64 | 32 | 3.94 |
| Precuneus | L/R | 7 | 253 | −4 | −74 | 50 | 3.74 |
| Lingual/fusiform gyrus | R | 18,19 | 244 | 22 | −84 | 20 | 3.80 |
| VSZ+CD vs VSZ-CD | |||||||
| VSZ-CD > VSZ+CD | None | ||||||
| VSZ+CD > VSZ-CD | |||||||
| Precuneus/pSTS/TPJ | R/L | 7,39,40 | 845 | 18 | −74 | 42 | 4.72 |
| −12 | −69 | 40 | 4.43 | ||||
| Post. cingulate/Precuneus | L/R | 5,7,31 | 223 | −12 | −40 | 46 | 4.38 |
| 24 | −40 | 48 | 4.22 | ||||
| pSTS/TPJ | L | 40 | 386 | −50 | −38 | 48 | 4.18 |
| Dorsolateral PFC | R | 46 | 286 | 48 | 32 | 24 | 4.02 |
| Lingual/fusiform gyrus | L | 18,19 | 225 | −28 | −74 | −18 | 3.95 |
| R | 18,19 | 305 | 22 | −68 | −16 | 3.90 | |
| VSZ-CD vs H | |||||||
| H > VSZ-CD | None | ||||||
| VSZ-CD > H | None | ||||||
| VSZ-CD vs SZ | |||||||
| SZ > VSZ-CD | None | ||||||
| VSZ-CD > SZ | |||||||
| pSTS/TPJ | L | 39,40 | 257 | −34 | −70 | 28 | 3.95 |
| Ventrolateral PFC | L | 44,45 | 286 | −36 | 42 | −4 | 3.72 |
Note: BA, Brodman area; H, Nonviolent healthy subjects; VCD, Violent offenders with CD/ASPD and no schizophrenia; SZ, nonviolent schizophrenic patients; VSZ-CD, violent offenders with schizophrenia but no CD; VSZ+CD, Violent offenders with schizophrenia and CD; PFC, prefrontal cortex; pSTS/TPJ, posterior superior temporal sulcus at temporoparietal junction.
VSZ+CD/ASPD vs H.
Violent offenders with schizophrenia and a history of CD and ASPD, as compared to healthy non-offenders, displayed increased activity in the medial PFC including SMA (BA 6,8,32), left pSTS/TPJ (BA 7,19,39), left precuneus (BA 7), left vlPFC (BA 10) and lingual/fusiform gyrus (BA 18,19).
VSZ+CD/ASPD vs SZ.
Violent offenders with schizophrenia and a history of CD and ASPD, as compared to men with schizophrenia and no history of violent offending nor of CD, exhibited increased activation in the left pSTS/TPJ (BA 7,19,39), bilateral precuneus (BA 7), right lingual/fusiform areas (BA 18,19), and regions within the ventrolateral and dorsolateral PFC of both hemispheres (BA 10,46,47).
VSZ+CD/ASPD vs VSZ.
Violent offenders with schizophrenia and a history of CD and ASPD, as compared to violent offenders with schizophrenia and no CD, revealed significant increased activation in the bilateral precuneus including regions of the posterior cingulate (BA 5,7,31), left pSTS/TPJ (BA 39,40) right dlPFC (BA 46), and lingual/fusiform areas (BA 18,19).
VSZ vs SZ.
As compared to nonviolent men with schizophrenia, violent men with schizophrenia and no history of antisocial behavior showed increased activity in the left vlPFC (BA 44,45), and left pSTS/TPJ (BA 39).
VSZ+CD/ASPD vs CD.
Violent offenders with schizophrenia and a history of CD and ASPD, as compared to violent offenders with CD, showed similar activation patterns.
VSZ vs H.
Violent offenders with schizophrenia and no history of CD showed activation patterns similar to H.
ROI-Analyses.
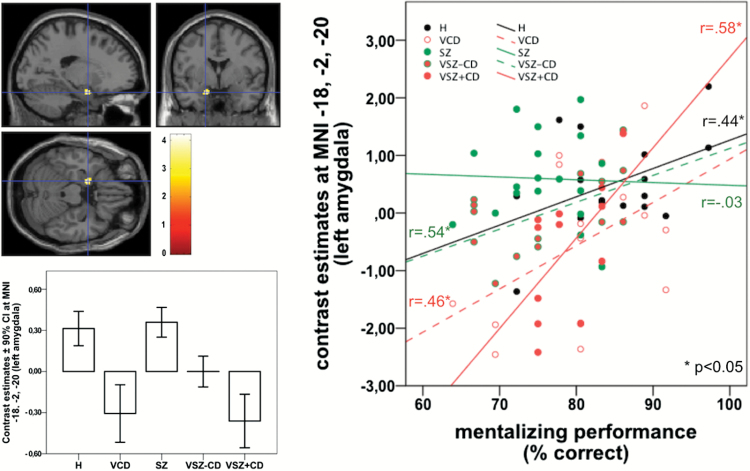
Group differences were only detected in the left amygdala. Violent offenders with CD/ASPD, both those with and without schizophrenia, showed significantly reduced ToM related activity relative to groups of nonviolent men while the VSZ-CD is intermediate (figure 3).
Fig. 3.
Location, and contrast estimates of in Theory of Mind related activation of the left amygdalaa, and associations between amygdala activation and Theory of Mind performance. aMNI coordinates: x = −18, y = −2, z = −20; cluster-size = 43 voxels; z value = 3.52; PFWE–SVC = .032. Scale bar depicts the uncorrected T-statistic.
Correlational Analyses.
As shown in supplementary table 1, behavioral task performance was positively associated with ToM related activity in the left mPFC (r = .333, P < .05), right dlPFC (r = .387, P < .05), left STG (r = .295, P < .05), and left SMA (r = .361, P < .05) and negatively associated with IRI personal distress (r = −.470, P < .01) and activation of the precuneus (r = −.313, P < .05). Amygdala activation was positively correlated with scores for personal distress (r = .293, P ≤ .05) and negatively correlated with PCL Factor 1 scores (r = −.609, P < .01). Moreover, as shown in the right panel of figure 3, ToM related amygdala responses were also positively associated with mentalizing performance within each group except SZ.
Discussion
This is the first study that directly compared ToM related neural activation patterns in 2 sub-groups of violent male offenders with schizophrenia, comparing them to violent offenders without schizophrenia who had a life-long history of antisocial behavior, to violent offenders with schizophrenia and no life-long history of antisocial behavior, and to healthy non-offenders. SZ and VSZ-CD/ASPD participants performed more poorly than H on the ToM task, while the VCD/ASPD and VSZ+CD/ASPD performed similarly to H. Moreover, ToM related neural activations in a network comprising prefrontal (medial PFC/ACC, left vlPFC, right dlPFC, left SMA) temporal (left STG), temporo-parietal regions (left pSTS/TPJ) parietal regions (precuneus) occipital regions (left LG/FFG) and the left amygdala differed between men with and without a life-long history of antisocial behavior regardless of the presence or absence of schizophrenia. These differences were not attributable to age, level of education, (premorbid) IQ, illness duration, positive or negative symptoms, or dose of antipsychotic medication.
The 2 groups of violent offenders with life-long antisocial behavior, VSZ+CD/ASPD and VCD/ASPD, showed increased activations in the medial PFC, vlPFC, left pSTS/TPJ, and precuneus thought to be associated with cognitive ToM, as compared to H, SZ, VSZ, and decreased activation in the left amygdala, a structure associated with affective ToM. ToM related activation in almost all of these areas was positively associated with emotional/mental state attribution performance in all schizophrenic groups (supplementary table 1). There was a linear relationship between emotional/mental state attribution performance and left amygdala activity in all groups except for SZ. This pattern of reduced activity in regions associated with affective ToM, accompanied by increased activity in areas associated with cognitive ToM may be interpreted to suggest that violent offenders with a life-long history of antisocial behavior compensate for their deficient empathy by using cognitive ToM. ToM accompanied by lower levels of activation in the left amygdala that was observed among the violent offenders with a life-long history of antisocial behavior, both those with and without schizophrenia as compared to H, SZ, and VSZ-CD, and the strong negative correlations between amygdala activation and PCL factor 1 scores, are consistent with results of studies of antisocial males with and without schizophrenia and high levels of psychopathic traits.15,34 The 2 groups of violent offenders with schizophrenia self-reported distress in response to others’ distress at levels similar then H and CD/ASPD and significantly less than SZ. These reports were positively correlated with amygdala activation and negatively correlated with activations in all other regions that distinguished between the groups. Intriguingly, the VSZ showed activations in the vlPFC, posterior superior temporal sulcus, and the temporal-parietal junction relative to non-offenders with schizophrenia. Future studies are needed to confirm this difference and relate it to clinical features of such individuals about whom so little is known.2 There is evidence showing that ToM impairment is related to the severity of symptoms in major depressive disorder.35 In the present study, not only the VSZ, but also the VSZ+CD/ASPD obtained higher PANSS scores for depression than the SZ group. However, there was no association between performance on the RMET and PANSS depression scores. Moreover, except in one region, the precuneous, we observed no association between PANSS depression scores and the ToM related activations that distinguished between groups. Activation in the precuneus was positively related with PANSS depression scores and negatively related to ToM performance.
Results of the present study indicate that the neural mechanisms underlying violent behavior differ in men with schizophrenia who present CD/ASPD and those with no antisocial behavior prior to illness onset. Among the violent offenders with schizophrenia, those with a life-long history of antisocial behavior showed reduced responsivity to emotions of others while being able to recognize and label others’ emotions, whereas those who began engaging in violence as illness onset showed heightened reactivity to emotions of others, as confirmed by the reactivity of the amygdala, and a reduced ability to label others’ emotions or mental states.
Clinical Implications
All people with schizophrenia require multiple interventions and support, including but not limited to antipsychotic medications, as shown most recently by the RAISE study.34 Results of the present study confirm and extend previous evidence showing that sub-groups of patients who engage in violent behavior present additional needs for treatment. Patients with schizophrenia who presented CD in childhood/adolescence require treatments aimed at reducing antisocial and aggressive behavior, including substance misuse.36,37 Findings from the present study showed that both groups of violent offenders with CD/ASPD as compared to men without CD/ASPD, both those with and without schizophrenia, displayed a distinct pattern of neural responses during emotional/mental state attribution that point to a more cognitive and less affective processing of social information. Additionally, reduced affective processing as reflected by reduced amygdala reactivity was associated higher levels of psychopathy. Consequently, developing interventions aimed at improving affective responsiveness or empathy might additionally contribute to reducing violence of this sub-group. However, these patients present not only a life-long history of antisocial and aggressive behavior, but also antisocial attitudes and ways of thinking that limit engagement with services, necessitating, perhaps, community treatment orders.38 Identifying such patients at first contact with psychiatric services and providing them with effective treatments for both schizophrenia and antisocial behavior has the potential to prevent violent crime. Many of these individuals already have committed crimes1,3,6 or as a recent meta-analysis reported, assaults prior to first contact with clinical services.33 Thus, general psychiatric services, and most particularly first episode services, may improve outcomes by identifying patients with prior CD and providing them with additional treatments targeting antisocial and aggressive behavior.
The results of the present study imply that forensic services would potentially reduce violent recidivism by providing their patients with schizophrenia with different treatments depending on whether or not they present CD/ASPD. While the latter group requires treatments as described above, violent offenders with schizophrenia and no history of CD/ASPD and a lower risk of re-offending, may require only treatments for schizophrenia and perhaps substance misuse. This conclusion is based on findings suggesting that the impairments of emotional/mental state attribution and the corresponding neural response pattern characterizing the violent offenders with schizophrenia who had no CD/ASPD resemble nonviolent men with SZ and thus seemed largely unrelated to their violent behavior. However, nonviolent men with SZ reported elevated distress in response to other’s distress.
Limitations and Strengths
The primary limitation of the study was the small sample size particularly in the VSZ+CD/ASPD group. Reduced power may have increased type II errors. For example, the effect sizes of ToM performance differences between VSZ+CD/ASPD and H (Cohen’s d = 0.749) as well as VSZ with or without CD/ASPD (d = 0.578) that both did not reach statistical significance laid in the medium range. However, particular with respect to the imaging findings, the small samples may also have negatively affected the likelihood that the statistically significant findings reflect a true effect (ie, a type I error), as for instance discussed in the framework of the positive predictive value.39 Another limitation of the study concerns the fact that we used a single ToM task which has been criticized for its reliability40 and taps affective but less cognitive ToM. Future studies would ideally combine measures of both aspects of ToM. Further, in the present study there was no measure of reactive and instrumental aggressive behavior that may be differentially associated with ToM. However, this was the first study that directly compared ToM related neural activation patterns of 2 groups of violent offenders with schizophrenia comparing them to non-mentally ill violent offenders, non-offenders with schizophrenia, and healthy men. All groups were similar as to age, education, and (premorbid) IQ. The groups with schizophrenia were similar as to the key features of schizophrenia, while the 2 groups with antisocial behavior were similar as to antisocial behavior, criminal convictions, and PCL scores.
Conclusions
Among men with schizophrenia who commit violent crimes, there are at least 2 sub-types who differ as to age of onset and persistence of antisocial behavior. The present study extends knowledge of their distinctiveness by showing that those with a life-long history of antisocial behavior resemble non-mentally ill offenders with a similar childhood onset of antisocial behavior as to relatively intact mentalizing performance and elevated cognition related but reduced affect related brain activation patterns as compared to men without CD/ASPD. The second sub-type of violent men with schizophrenia who show no antisocial behavior prior to illness onset were similar to nonviolent men with schizophrenia in presenting deficiencies in emotional/mental state attribution and a neural response pattern rather resembling healthy men. These findings add to prior evidence indicating the need for different interventions to reduce violence among these 2 types of offenders with schizophrenia.
Supplementary Material
Supplementary data are found at Schizophrenia Bulletin online.
Funding
Landschaftsverband Rheinland, Germany (B.S.).
Supplementary Material
Acknowledgments
The authors thank Alexander Wormit for his assistance with data collection. They also thank the many individuals at the hospitals and prisons in Northrhine-Westfalia, Germany for making this research possible. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Wallace C, Mullen PE, Burgess P. Criminal offending in schizophrenia over a 25-year period marked by deinstitutionalization and increasing prevalence of comorbid substance use disorders. Am J Psychiatry. 2004;161:716–727. [DOI] [PubMed] [Google Scholar]
- 2. Hodgins S, Piatosa M, Schiffer B. Violence among people with schizophrenia: phenotypes and neurobiology. In: Miczek K, Meyer-Lindenberg A, eds. Neuroscience of Aggression. Heidelberg, Germany: Springer; 2013:329–368. [DOI] [PubMed] [Google Scholar]
- 3. Swanson JW, Swartz MS, Van Dorn RA, et al. Comparison of antipsychotic medication effects on reducing violence in people with schizophrenia. Br J Psychiatry. 2008;193:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hodgins S. Violent behaviour among people with schizophrenia: a framework for investigations of causes, and effective treatment, and prevention. Philos Trans R Soc Lond B Biol Sci. 2008;363:2505–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hodgins S, Tiihonen J, Ross D. The consequences of Conduct Disorder for males who develop schizophrenia: associations with criminality, aggressive behavior, substance use, and psychiatric services. Schizophr Res. 2005;78:323–335. [DOI] [PubMed] [Google Scholar]
- 6. Schiffer B, Leygraf N, Müller BW, et al. Structural brain alterations associated with schizophrenia preceded by conduct disorder: a common and distinct subtype of schizophrenia? Schizophr Bull. 2013;39:1115–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Reilly K, Donohoe G, Coyle C, et al. Prospective cohort study of the relationship between neuro-cognition, social cognition and violence in forensic patients with schizophrenia and schizoaffective disorder. BMC Psychiatry. 2015;15:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pickup GJ. Relationship between Theory of Mind and executive function in schizophrenia: a systematic review. Psychopathology. 2008;41:206–213. [DOI] [PubMed] [Google Scholar]
- 9. Brüne M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31:21–42. [DOI] [PubMed] [Google Scholar]
- 10. Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Addington J, Penn D, Woods SW, Addington D, Perkins DO. Facial affect recognition in individuals at clinical high risk for psychosis. Br J Psychiatry. 2008;192:67–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang DY, Liu AC, Lui SS, et al. Facial emotion perception impairments in schizophrenia patients with comorbid antisocial personality disorder. Psychiatry Res. 2016;236:22–27. [DOI] [PubMed] [Google Scholar]
- 13. Abu-Akel A, Abushua’leh K. ‘Theory of mind’ in violent and nonviolent patients with paranoid schizophrenia. Schizophr Res. 2004;69:45–53. [DOI] [PubMed] [Google Scholar]
- 14. Majorek K, Wolfkühler W, Küper C, Saimeh N, Juckel G, Brüne M. “Theory of mind” and executive functioning in forensic patients with schizophrenia. J Forensic Sci. 2009;54:469–473. [DOI] [PubMed] [Google Scholar]
- 15. Blair RJ. The neurobiology of psychopathic traits in youths. Nat Rev Neurosci. 2013;14:786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shamay-Tsoory SG, Harari H, Aharon-Peretz J, Levkovitz Y. The role of the orbitofrontal cortex in affective theory of mind deficits in criminal offenders with psychopathic tendencies. Cortex. 2010;46:668–677. [DOI] [PubMed] [Google Scholar]
- 17. Abu-Akel A, Shamay-Tsoory S. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia. 2011;49:2971–2984. [DOI] [PubMed] [Google Scholar]
- 18. Dvash J, Shamay-Tsoory SG. Theory of mind and empathy as multidimensional constructs: neurological foundations. Topics in Language Disorders 2014;34:282–295. [Google Scholar]
- 19. Schlaffke L, Lissek S, Lenz M, et al. Shared and nonshared neural networks of cognitive and affective theory-of-mind: a neuroimaging study using cartoon picture stories. Hum Brain Mapp. 2015;36:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLoS One. 2011;6:e25322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Decety J, Chen C, Harenski C, Kiehl KA. An fMRI study of affective perspective taking in individuals with psychopathy: imagining another in pain does not evoke empathy. Front Hum Neurosci. 2013;7:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- 23. Stone VE. The Role of the Frontal Lobes and the Amygdala in Theory of Mind. Oxford, UK: Oxford University Press; 1999. [Google Scholar]
- 24. Richell RA, Mitchell DG, Newman C, Leonard A, Baron-Cohen S, Blair RJ. Theory of mind and psychopathy: can psychopathic individuals read the ‘language of the eyes’? Neuropsychologia. 2003;41:523–526. [DOI] [PubMed] [Google Scholar]
- 25. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 26. Lindenmayer JP, Bernstein-Hyman R, Grochowski S. A new five factor model of schizophrenia. Psychiatr Q. 1994;65:299–322. [DOI] [PubMed] [Google Scholar]
- 27. Lehrl S. Mehrfachwahl-Wortschatz-Intelligenztest MWT-B. Balingen, Germany: Spitta Verlag; 2005. [Google Scholar]
- 28. Hart SD, Cox DN, Hare RD. The Hare Psychopathy Checklist: Screening Version. Toronto, ON, Canada: Multi-Health Systems; 1995. [Google Scholar]
- 29. Paulus C. The Saarbruecken Personality Questionnaire on Empathy: psychometric evaluation of the German version of the Interpersonal Reactivity Index. 2009. urn:nbn:de:bsz:291-psydok-23630.
- 30. Davis M. A multidimensional approach to individual differences in empathy. JSAS catalogue of Selected Documents in Psychology. 1980;10:85. [Google Scholar]
- 31. Bora E, Eryavuz A, Kayahan B, Sungu G, Veznedaroglu B. Social functioning, theory of mind and neurocognition in outpatients with schizophrenia; mental state decoding may be a better predictor of social functioning than mental state reasoning. Psychiatry Res. 2006;145:95–103. [DOI] [PubMed] [Google Scholar]
- 32. Schiffer B, Pawliczek C, Müller BW, Gizewski ER, Walter H. Why don’t men understand women? Altered neural networks for reading the language of male and female eyes. PLoS One. 2013;8:e60278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 34. Abu-Akel A, Heinke D, Gillespie SM, Mitchell IJ, Bo S. Metacognitive impairments in schizophrenia are arrested at extreme levels of psychopathy: the cut-off effect. J Abnorm Psychol. 2015;124:1102–1109. [DOI] [PubMed] [Google Scholar]
- 35. Bora E, Berk M. Theory of mind in major depressive disorder: a meta-analysis. J Affect Disord. 2016;191:49–55. [DOI] [PubMed] [Google Scholar]
- 36. Marion-Veyron R, Lambert M, Cotton SM, et al. History of offending behavior in first episode psychosis patients: a marker of specific clinical needs and a call for early detection strategies among young offenders. Schizophr Res. 2015;161:163–168. [DOI] [PubMed] [Google Scholar]
- 37. Hodgins S, Klein S. New clinically relevant findings about violence by people with schizophrenia [published online ahead of print September 7, 2016]. Can J Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Swartz MS, Bhattacharya S, Robertson AG, Swanson JW. Involuntary outpatient commitment and the elusive pursuit of violence prevention: a view from the United States [published online ahead of print October 24, 2016]. Can J Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. [DOI] [PubMed] [Google Scholar]
- 40. Jarrold C, Butler DW, Cottington EM, Jimenez F. Linking theory of mind and central coherence bias in autism and in the general population. Dev Psychol. 2000;36:126–138. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.