Supplemental digital content is available in the text.
Key Words: Totally endoscopic cardiac surgery, Thoracoscopy, Atrial septal defect, Beating heart, Congenital heart disease
Abstract
Objective
The aim of the study was to investigate the effectivity and safety of totally endoscopic cardiac surgery without robotic assistance for atrial septal defect (ASD) closure on beating hearts.
Methods
Twenty-five patients (adults/children: 15/10) underwent ASD closure using nonrobotically assisted totally endoscopic approach on beating heart. Three 5-mm trocars and one 12-mm trocar were used, only the superior vena cava is snared, filling the pleural and pericardial cavities with CO2, and the heart was beating during the surgery. Twenty-three patients had isolated secundum ASD (2 of which had severe tricuspid regurgitation) and two patients had ASD combined with partial anomalous pulmonary venous connection. All ASDs were closed using artificial patch, continuous suture; tricuspid regurgitations were repaired and the anomalous pulmonary veins were drained to the left atrium.
Results
No postoperative complications or deaths occurred. Mean ± SD operation time and mean cardiopulmonary bypass time were 267.2 ± 44.6 and 156.1 ± 33.6 min, respectively. These patients were extubated within the first 5 hours, and the volume of blood drainage on the first day was less than 80 mL. Four days after surgery, patients did not need analgesics and were able to return to normal activities 1 week postoperatively.
Conclusions
Totally endoscopic operation for ASD closure on beating heart is safe, with short recovery period, and surgical scars are of high cosmetic value, especially in a woman and girl.
Atrial septal defect (ASD) is one of the most common congenital heart diseases (CHDs),1 which accounts for 6% to 10% of all CHDs. The disease often progresses silently and leads to heart failure and severe pulmonary hypertension. For the past 20 years, transcatheter closure has been the first choice in almost all countries all over the world with many advantages.2–4 However, there are many types of ASD that cannot be closed percutaneously, and there are increasing numbers of reports on long-term complications of transcatheter closure of ASD leading to reoperation or life-threatening conditions.5–7
Surgical treatment of ASD through median sternotomy or small thoracotomy with endoscopic support still shows some limitations.8–11 There were not many scientific reports on totally endoscopic surgery without robotic assistance for ASD closure, especially in small children.12,13 We report our first experience in applying totally endoscopic surgery techniques for the treatment of ASD.
PATIENTS AND METHODS
Patient Selection
From May 2016 to December 2016, 23 patients with isolated secundum ASD and two patients with ASD combined with partial anomalous pulmonary venous connection (p-APVC) were selected and underwent totally endoscopic repair without the aid of the robotic system on beating heart. Study subjects included both adults and children with the following inclusion criteria: (1) isolated secundum ASD, ASD associated with p-APVC, sinus venosus ASD, ASD combined with tricuspid regurgitation (TR); (2) body weight of 13 kg or more; (3) no previous history of operation on right lung; and (4) no atherosclerotic stenosis of pelvic-femoral arteries. Patients who were unable to meet all of these criteria were excluded from this study. Preoperative demographic indices (age, weight, skin area, defect size), characteristics of pathophysiology, and indications for surgery are presented in Table 1. Surgical technique was approved by Scientific board of the hospital and by patients' family.
TABLE 1.
Demographics and Preoperative Indices

Anesthesia
Patients were intubated with either single- or double-lumen endotracheal tube. The anesthetist placed the central venous catheter through left internal jugular vein (IJV) and inserted a needle, which would be subsequently used for guidewire introduction for superior vena cava (SVC) cannulation—in the right IJV, all procedures were performed under sterile condition.
Surgical Technique
The patient was positioned in the supine position with the right side of the body elevated to 20% to 300, two hands were placed along the body, patient's head were tilted to the left to expose the already placed intravenous needle. The surgeon marked the intended ports for trocar placement.
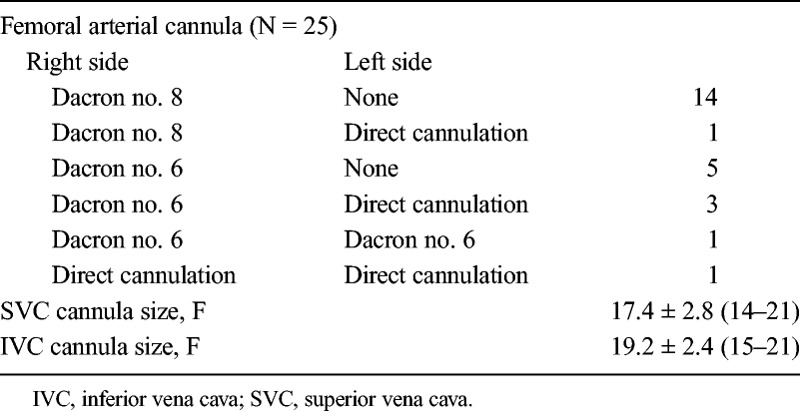
For setting up peripheral cardiopulmonary bypass (CPB), we created a 2-cm horizontal incision on the right inguinal fold, revealing common femoral artery and femoral vein. For patients weighing more than 15 kg, arterial cannula was set up indirectly in the common femoral artery through an artificial vessel (Knitted Dacron graft [Vascutek Terumo, Bangkok, Thailand] or expanded polytetrafluoroethylene [Vascutek Terumo, Bangkok, Thailand]) (Fig. 1A). Superior vena cava and inferior vena cava (IVC) cannulae were placed through the right IJV and femoral vein with Seldinger technique (Fig. 1B). Before setting up trocars, we tested the arterial line. Arterial pressure of less than 220 mm Hg with full flow was acceptable. If the arterial pressure was greater than 220 mm Hg, one additional femoral arterial cannula (2F–4F smaller than expected) was placed directly on the left side. For patients weighing less than 15 kg, we placed cannulae directly in both sides, using cannulae 2F–4F smaller than expected (Fig. 1C).
FIGURE 1.

Setting up peripheral CPB. A, Femoral artery cannula was established indirectly through artificial graft (Knitted Dacron graft [Vascutek Terumo, Bangkok, Thailand] or expanded polytetrafluoroethylene [Vascutek Terumo, Bangkok, Thailand]). B, SVC and IVC were drained through right IJV and femoral vein with Seldinger technique. C, Bilateral femoral arterial cannulae were placed directly in a 4-year-old boy.
Four trocars were placed in the previously marked positions, included the following: one 12-mm trocar at the fifth intercostal space in the anterior axillary line was the main surgical trocar (for surgical instruments: tissue forceps, needle holder, or electric surgical knife), one 5-mm trocar at the third intercostal space in the midaxillary line (for tissue forceps), one 5-mm trocar at the fifth intercostal space in the midaxillary line (for endoscopic camera), and one 5-mm trocar at the sixth intercostal space in the midaxillary line (for right heart sucker) (Fig. 2).
FIGURE 2.

Location of four trocars on the right chest wall. The green point (trocar 1 in C) was for the main surgical instruments (needle holder, scissor, electric scalpel). The yellow one (trocar 2 in C) was for forceps. The blue one (trocar 3 in C) was for camera. The red one (trocar 4 in C) was for right heart sucker.
The pericardium was opened 1.5 to 2 cm anterior and parallel to the phrenic nerve (Video 1, Supplemental Digital Content 1, http://links.lww.com/INNOV/A155). After snaring SVC (IVC was free) and placing patient in Trendelenburg position, right atrium was opened. Heart continuously beat during surgery. Blood flowed to operation field through the coronary sinus, the direct ostia on right atrium wall, and IVC ostium (Video 2, Supplemental Digital Content 2, http://links.lww.com/INNOV/A156). A right heart sucker through the trocar 4 (Fig. 2B) was used to remove blood from the surgical field and was used as a retractor to expose the defect. The anatomical landmarks that need to be identified during the surgery include the following: tricuspid valve orifice, coronary sinus ostium, IVC ostium, and the ostia of right pulmonary veins.
Air Embolism Prevention
Prevention of Air From LV to the Aorta
The pleural and pericardiac cavities were filled with CO2. Initially, CO2 was pumped at the rate of 0.5 L/min, followed by the adjustment of pumping rate to maintain the arterial pressure of CO2 ranging from 35 to 40 mm Hg. The mean arterial pressure was kept greater than 60 mm Hg for adults and 50 mm Hg for children. Right before the completion of ASD repair, the lung was briefly inflated to remove air.
Prevention of Air Return to IVC and to Arterial Line
The position of IVC cannula was adjusted so that the tip of the cannula was 1 to 2 cm lower than Eustachian valve of the IVC to prevent air from returning to the IVC. Air vent block in arterial was used, and in recent patients, CAPIOX FX Advance Oxygenator (Terumo, Bangkok, Thailand) was used to prevent air from returning to arterial line.
All defects were closed with artificial patch, continuous suture (Fig. 3; Video 3, Supplemental Digital Content 3, http://links.lww.com/INNOV/A157). Anomalous pulmonary veins were drained to left atrium through ASD. The concomitant moderate to severe TR was repaired using posterior annuloplasty technique or placing a ring, depending on the annular size. Right atrial incision was closed in two layers. Heart was filled after removing SVC snare and placing patient in supine position. Bleeding was carefully controlled; the pericardium was closed. Cardiopulmonary bypass was taken off after placing one pericardial and one pleural drain through trocar 2 and 4. The remaining procedures were similar to normal video-assisted surgery.
FIGURE 3.

Totally endoscopic ASD closure technique. A, Right atrium is opened after filling pleural and pericardial cavities with CO2, the secundum ASD is at the tip of sucker. B, The ASD closure process with artificial patch, continuous suture. C, Completion of ASD closure.
Perioperative Management
Before surgical intervention, education and counseling were provided to all patients and their family on surgical techniques, advantages of this intervention, and potential complications. All patients were routinely examined for the status of pelvic-femoral arteries by Doppler ultrasonography before the operation. After the operation, patients were extubated as soon as possible. Bedside chest radiographic analysis and sometimes transthoracic ultrasound were performed to exclude lung complications (atelectasis, plural effusion). All patients were examined postoperatively with transthoracic echocardiography and vascular Doppler ultrasound before discharge.
Postoperative Follow-up
Follow-up appointments were scheduled at 1, 3, and 6 months after surgery. Surgeons checked scars, the symmetry between the two breasts, paresthesia around the incision area in the chest and the groins, satisfaction of families and patients about the location, and size of surgical scars. Patients were examined with transthoracic echocardiography and vascular Doppler ultrasound.
Statistical Analysis
Data are expressed as mean ± SD for quantitative variables and number and percentage for qualitative variables. Data are managed and analyzed with SPSS 15.0 software (SPSS Inc, Quarry Bay, Hongkong).
RESULTS
Totally endoscopic ASD repair was successfully performed in all patients. No hospital death occurred. No case needed to expand the incision or change to sternotomy. All patients were established peripheral circulation. Arterial cannula was placed indirectly via Dacron circuit in 24 patients (15 adults used circuit no. 8 and 9 children used circuit no. 6). Five of these 24 patients (including 1 adult and 4 children) needed an additional arterial line in left femoral artery. Bilateral femoral arterial cannulae were actively placed in the youngest girl weighing 13.5 kg. Arterial cannula was used for IVC cannulation in three small children. Details on extracorporeal circulation were presented in Table 2.
TABLE 2.
Parameters for Extracorporeal Circulation Establishment
The mean ± SD operation time and CPB time were 267.2 ± 44.6 minutes (range = 200–360 minutes) and 156.1 ± 33.6 minutes (range = 100–220 minutes), respectively. All patients did not need to use vasoactive drugs after stopping CPB and were extubated within the first 5 hours. The mean ± SD duration of intensive care unit stay was 18.8 ± 5.2 hours (range = 12–24 hours) and the mean ± SD drainage in the first 24 postoperative hours was 75.2 ± 44.7 mL. No postoperative neurological complications occurred. Patients did not need analgesics after 4 days and were able to perform almost all normal activities (except exertion) 7 days postoperatively. The mean ± SD postoperative hospital stay time was 9.2 ± 3.8 days.
Outpatients were divided into two groups: the first 15 patients who were operated on between May 2016 and September 2016 and the other group who was composed of the last 10 patients. Parameters during and after surgery are summarized in Table 3. Operation time and CPB time are significantly reduced in the last 10 patients compared with the first 15 patients (P = 0.0006 and 0.018, respectively). There were no significant differences in surgical outcomes and rate of complications.
TABLE 3.
Comparison of Parameters During and After Operation of Two Patients' Groups

Posterior annuloplasty was performed in two patients immediately after ASD closure in the same procedure (Fig. 3). Echocardiography before discharge showed no TR in both patients. Two patients (19- and 4-year-old girls) had ASD combined with p-APVC, which was repaired with a patch that drained blood from right pulmonary veins to left atrium through ASD. No gradient was found at ostia of pulmonary veins and ASD on postoperative echocardiography. No vessel complications were recorded at 6 months after surgery. There was one case with a 3-mm residual ASD shunt in transesophageal echocardiography before discharge. The size of the defect did not increase at 6-month follow-up. All patients and their families were satisfied with the effectiveness and the cosmetic value of surgical scars.
DISCUSSION
The ASD is one of the most common CHDs with the incidence rate from 0.5 to 2.5 per 1000 births between 1945 and 2009, respectively.1 Atrial septal defect occurs with a female-to-male ratio of approximately 2:1 and is most diagnosed in adulthood—the age range that requires the surgery to be performed cosmetically. Therefore, improving the quality of ASD treatment will effectively contribute to community health care.
Nowadays, the ASD surgery with video assistance has been performed all around the world because of its simplicity, high efficiency, and nearly no complications. However, patients still experience thoracic incisions, pain after surgery, scars that are still long, and breasts' asymmetry. The endoscopic ASD repair with da Vinci robotic assistance (Intuitive Surgical, Sunnyvale, CA USA) overcomes all the previously mentioned disadvantages. However, training and operation cost so much that almost all hospital cannot afford them. The totally endoscopic ASD repair without robotic assistance has come into practice but has not been widely applied.
The major concern in operation on the beating heart is the prevention of air embolism, which occurs when air presents in the left ventricle and LV pressure is greater than that in the aorta root.14 We prevented this complication using following principles: (1) maintain high arterial line pressure,14,15 (2) keep LA and LV to be always filled with blood,16 sucker only sucked on a part of LA in certain stages, (3) insufflation of pericardial and pleural space by CO2,15,17 and deairing by inflating the lungs right before completing the ASD closure. Mo et al14 believed that high pressure of arterial line played important role in preventing air from the left heart going to the aorta. Many authors when performed mini-invasive cardiac surgery still try to remove air by traditional measures (using LA, LV, or aortic root vent).14,18 However, the placement of an aortic root vent sometimes causes postoperative bleeding.13 Carbon dioxide insufflation has been proven to be an alternative method for classical air removal ones by replacing gas in the surgical field and the strong ability to dissolve in water.19 Carbon dioxide rapidly dissolves air bubble in the heart and deairing time was three times shorter compared with classical method. In addition, the use of CO2 in operation on the beating heart has been shown to not be associated with any neurological complications.15 Thapmongkol et al performed ASD closure surgery for 63 patients on the beating heart. To prevent air embolism, they did not use neither CO2 nor aortic root vent.16
Most of the ASDs, which do not have indications for device closure, have large defects with short or without IVC edge. Inferior vena cava snare would make the inferior edge of the defects shriveled and lead to difficulties during suturing or risk of residual shunt. The technique in which IVC is not snared has been applied in some types of open heart surgeries such as Fontan procedure, reoperation, and other surgeries.20 We did not snare the IVC to facilitate the suturing of the defect. Bloodsucker was not only used for suctioning the blood in the surgical field but also used as a retractor to expose the defect during suturing. To prevent air embolism, we placed the IVC cannula so that the tip of the catheter is 1 to 2 cm lower than the level of Eustachian valve of the IVC combined with negative pressure suctioning. Corno et al20 noted that this technique of IVC cannulation never provokes reduction of their venous drainage nor air locks in the venous line. To prevent air from going to arterial line, we used CAPIOX FX Advance Oxygenator (Terumo, Bangkok, Thailand).
With experience in ASD repair surgery with video assisted on beating hearts for more than 80 cases, we performed totally endoscopic ASD repair without the assistance of the robotic system, beating heart since May 2016 with very good initial results (Fig. 4). All patients are followed up to 1 to 6 months after surgery. We performed surgery for 10 pediatric patients and 15 adult patients, and the smallest patient weighed 13.5 kg. To perform surgery for such small patients, we made some changes in the establishment of CPB (Table 2). Indirect femoral artery cannulation via Dacron vessel graft would help avoid lower extremities ischemia during operation as well as postoperative stenosis of iliac and femoral artery, especially in small children. Rosu et al21 only used indirect cannulation via Dacron graft in patients with femoral artery smaller than standard size to optimize blood flow and avoid leg ischemia. Bilateral femoral cannulation was only employed in cases with high arterial line pressure.21 We used indirect cannulation in all cases with the purposes as mentioned previously; when the pressure of the arterial line was too high, additional cannulation of the contralateral femoral artery would be used. Small children were the main subjects of bilateral cannulation because of small arteries and reactive spasm. The smallest patient who weighed 13.5 kg had direct bilateral cannulation, and because the arteries were too small, we could not perform end-to-side anastomosis between femoral artery and artificial graft. We think that the use of cannula 2F–4F smaller than the standard size was the key factor to avoid led ischemia during operation as well as arterial stenosis after surgery. With this new approach in setting up peripheral CPB, all these 10 small patients were operated on successfully without any vascular complications up to 6 months after surgery.
FIGURE 4.

Results of totally endoscopic ASD repair on beating heart without robotic assistance. A and B, A 24-year-old male patient right after operation. C, A 7-year-old girl 1 month after surgery. D, A 3-year-old girl before discharge.
The locations and use of trocars in our study are similar to those in totally endoscopic surgery with robotic assistance.19,22,23 The mean operation time (skin to skin) and CPB time of our report were 4 to 4.5 and 2 to 2.5 hours respectively, similar to reports of Argenziano et al,23 Wimmer-Greinecker et al,22 and Bonaros et al.24 Xiao et al19 performed ASD closure surgery on the beating heart for 106 patients with operation time comparable with that of our study but with shorter CPB time. Significant decreases were seen in operation and CPB time in the beating heart group of the report by Xiao et al.19 Bonaros et al24 showed that learning curve is associated with a rapid decrease in operation times. In our study, operation time and CPB time in 10 latter patients were significantly shorter compared with the first 15 patients; however, we need more time and patients to show that our learning curve is meaningful and stable.
There is no difference in postoperative restoration between totally endoscopic surgery and sternotomy. The volume of blood drainage was significantly reduced compared with that of thoracotomy and sternotomy. Usually, with video-assisted cardiac surgery, patients had limited movements of the right arm several weeks after surgery because of pain and contraction of thoracic muscles.8 The totally endoscopic approach through trocars from 5 to 12 mm in diameter on the chest wall reduces postoperative pain. We only give morphine on the first day in the intensive care unit and nonsteroidal analgesics for following days. On average, the patients did not need analgesics on the fourth day after operation. They could move their right hand normally 1 week after surgery.
Some surgeons reported postoperative right atelectasis with the incidence from 2.5% to 7.1% because of one-lung ventilation during surgery.12,25,26 We chose two-lung ventilation with single-lumen endotracheal tube because 40% of our patients were small children, and this method made CPB time last longer, but it helped prevent postoperative atelectasis. To decrease CPB time, recently, double-lumen endotracheal tube was only used for a young adult patient, and it resulted in 10-minute reduction in CPB time. We prevented atelectasis by endotracheal tube suction after reventilation and instructed the patient to breath effectively after the surgery.
However, there were two cases with large ASD near the coronary sinus ostium (the edge of the defect is the edge of coronary sinus), and the third-degree atrioventricular block occurred when we strongly pulled these stitches. We identified that the blockage was due to the pulling of the suture near the conducting system. The last stitches were removed, and the rhythm returned to normal sinus rhythm after 10 to 15 minutes. According to Mo et al14 and Pendse et al,27 the possibility of detecting arrhythmia during operation is one of the advantages of performing surgery on the beating heart.
CONCLUSIONS
The totally endoscopic ASD repair on beating heart without robotic assistance is a promising method, which can be deployed widely in the near future, and contributes to improving the quality of treatment of CHDs.
Supplementary Material
CLINICAL PERSPECTIVE
This is a small case series from Dr. Dang and his associates from Hanoi, Vietnam, reporting their experience in 25 patients with totally endoscopic beating heart atrial septal defect (ASD) repair. Twenty-three patients had secundum ASDs and two patients had ASDs combined with partial anomalous pulmonary venous return. They used peripheral cannulation and CO2 insufflation. They had no mortality or neurologic events. They had relatively long cardiopulmonary bypass and operative times; while these did improve over their small experience, they remained more than 2 hours for bypass time and almost 4 hours for total operative time in the second half of their series. Postoperative hospital stay was 9.2 ± 3.8 days.
This small case series demonstrated the feasibility of this approach. However, the long cardiopulmonary bypass times and postoperative length of stay make it difficult to see the advantages of their technique over more traditional ones. The potential for air embolism is also a limitation of performing ASD repair on the beating heart. In the era of transvenous device closure of ASDs, this type of approach will likely have a small role in the management of most patients. However, the authors are to be congratulated for attempting to minimize operative trauma. The clinical value of their approach will await larger studies and comparisons with other minimally invasive techniques.
Footnotes
A video clip is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.innovjournal.com).
Presented at the Annual Scientific Meeting of the International Society for Minimally Invasive Cardiothoracic Surgery, June 7–10, 2017, Rome, Italy.
Disclosure: The authors declare no conflicts of interest.
REFERENCES
- 1.van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–2247. [DOI] [PubMed] [Google Scholar]
- 2.Alobaidan M, Saleem A, Abdo H, Simpson J. Successful percutaneous closure of spiral atrial septal defect. Echo Res Pract. 2015;2:K7–K9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murakami T, Nakazawa G, Horinouchi H, et al. Transcatheter closure of atrial septal defect protects from pulmonary edema: septal occluder device gradually reduces LR shunt. Heart Vessels. 2017;32:101–104. [DOI] [PubMed] [Google Scholar]
- 4.Giardini A, Moore P, Brook M, Stratton V, Tacy T. Effect of transcatheter atrial septal defect closure in children on left ventricular diastolic function. Am J Cardiol. 2005;95:1255–1257. [DOI] [PubMed] [Google Scholar]
- 5.Jalal Z, Hascoet S, Baruteau AE, et al. Long-term complications after transcatheter atrial septal defect closure: a review of the medical literature. Can J Cardiol. 2016;32:e11–e18. [DOI] [PubMed] [Google Scholar]
- 6.Lambert V, Losay J, Piot JD, et al. Late complications of percutaneous closure of atrial septal defects with the Sideris occluder [in French]. Arch Mal Coeur Vaiss. 1997;90:245–251. [PubMed] [Google Scholar]
- 7.Tomar M, Khatri S, Radhakrishnan S, et al. Intermediate and long-term followup of percutaneous device closure of fossa ovalis atrial septal defect by the Amplatzer septal occluder in a cohort of 529 patients. Ann Pediatr Cardiol. 2011;4:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li WW, Lee RL, Lee TW, et al. The impact of thoracic surgical access on early shoulder function: video-assisted thoracic surgery versus posterolateral thoracotomy. Eur J Cardiothorac Surg. 2003;23:390–396. [DOI] [PubMed] [Google Scholar]
- 9.Nomori H, Horio H, Fuyuno G, Kobayashi R. Non-serratus-sparing antero-axillary thoracotomy with disconnection of anterior rib cartilage. Improvement in postoperative pulmonary function and pain in comparison to posterolateral thoracotomy. Chest. 1997;111:572–576. [DOI] [PubMed] [Google Scholar]
- 10.Athanassiadi K, Bagaev E, Simon A, Haverich A. Lung herniation: a rare complication in minimally invasive cardiothoracic surgery. Eur J Cardiothorac Surg. 2008;33:774–776. [DOI] [PubMed] [Google Scholar]
- 11.Ishida R, Oiwa H, Honda K, et al. Evaluation of sternal deformity after pediatric minimally invasive cardiac surgery [in Japanese]. Kyobu Geka. 2004;57:111–114. [PubMed] [Google Scholar]
- 12.Wang F, Li M, Xu X, et al. Totally thoracoscopic surgical closure of atrial septal defect in small children. Ann Thorac Surg. 2011;92:200–203. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y, Chen H, Mohl W, Liu X, Si X. Totally endoscopic congenital heart surgery compared with the traditional heart operation in children. Wien Klin Wochenschr. 2013;125:704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mo A, Lin H, Wen Z, Lu W, Long X, Zhou Y. Efficacy and safety of on-pump beating heart surgery. Ann Thorac Surg. 2008;86:1914–1918. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhuri K, Storey E, Lee GA, et al. Carbon dioxide insufflation in open-chamber cardiac surgery: a double-blind, randomized clinical trial of neurocognitive effects. J Thorac Cardiovasc Surg. 2012;144:646–653. [DOI] [PubMed] [Google Scholar]
- 16.Thapmongkol S, Sayasathid J, Methrujpanont J, Namchaisiri J. Beating heart as an alternative for closure of secundum atrial septal defect. Asian Cardiovasc Thorac Ann. 2012;20:141–145. [DOI] [PubMed] [Google Scholar]
- 17.Landenhed M, Al-Rashidi F, Blomquist S, Höglund P, Pierre L, Koul B. Systemic effects of carbon dioxide insufflation technique for de-airing in left-sided cardiac surgery. J Thorac Cardiovasc Surg. 2014;147:295–300. [DOI] [PubMed] [Google Scholar]
- 18.Ma ZS, Dong MF, Yin QY, Feng ZY, Wang LX. Totally thoracoscopic closure for atrial septal defect on perfused beating hearts. Eur J Cardiothorac Surg. 2012;41:1316–1319. [DOI] [PubMed] [Google Scholar]
- 19.Xiao C, Gao C, Yang M, et al. Totally robotic atrial septal defect closure: 7-year single-institution experience and follow-up. Interact Cardiovasc Thorac Surg. 2014;19:933–937. [DOI] [PubMed] [Google Scholar]
- 20.Corno AF, Horisberger J, David J, von Segesser LK. Right atrial surgery with unsnared inferior vena cava. Eur J Cardiothorac Surg. 2004;26:219–220. [DOI] [PubMed] [Google Scholar]
- 21.Rosu C, Bouchard D, Pellerin M, Lebon JS, Jeanmart H. Preoperative vascular imaging for predicting intraoperative modification of peripheral arterial cannulation during minimally invasive mitral valve surgery. Innovations. 2015;10:39–43. [DOI] [PubMed] [Google Scholar]
- 22.Wimmer-Greinecker G, Dogan S, Aybek T, et al. Totally endoscopic atrial septal repair in adults with computer-enhanced telemanipulation. J Thorac Cardiovasc Surg. 2003;126:465–468. [DOI] [PubMed] [Google Scholar]
- 23.Argenziano M, Oz MC, Kohmoto T, et al. Totally endoscopic atrial septal defect repair with robotic assistance. Circulation. 2003;108 Suppl 1:II191–II194. [DOI] [PubMed] [Google Scholar]
- 24.Bonaros N, Schachner T, Oehlinger A, et al. Robotically assisted totally endoscopic atrial septal defect repair: insights from operative times, learning curves, and clinical outcome. Ann Thorac Surg. 2006;82:687–693. [DOI] [PubMed] [Google Scholar]
- 25.Liu G, Qiao Y, Zou C, et al. Totally thoracoscopic surgical treatment for atrial septal defect: mid-term follow-up results in 45 consecutive patients. Heart Lung Circ. 2013;22:88–91. [DOI] [PubMed] [Google Scholar]
- 26.Ma ZS, Dong MF, Yin QY, Feng ZY, Wang LX. Totally thoracoscopic repair of atrial septal defect without robotic assistance: a single-center experience. J Thorac Cardiovasc Surg. 2011;141:1380–1383. [DOI] [PubMed] [Google Scholar]
- 27.Pendse N, Gupta S, Geelani MA, et al. Repair of atrial septal defects on the perfused beating heart. Tex Heart Inst J. 2009;36:425–427. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


