Sexually transmitted disease rates are rising rapidly among men who have sex with men, yet sexually transmitted disease screening for people living with HIV is well below the Centers for Disease Control and Prevention recommendations, especially for chlamydia and gonorrhea.
Abstract
Background
Men who have sex with men with HIV have high sexually transmitted infection (STI) incidence. Thus, the Centers for Disease Control and Prevention (CDC) recommends at least yearly STI screening of HIV-infected individuals.
Methods
We calculated testing rates for syphilis, chlamydia, and gonorrhea among HIV-positive Californians with Medicare or Medicaid insurance in 2010. Logistic regressions estimated how testing for each bacterial STI relates to demographic and provider factors.
Results
Fewer than two-thirds of HIV-positive Medicare and fewer than three-quarters of Medicaid enrollees received a syphilis test in 2010. Screenings for chlamydia or gonorrhea were less frequent: approximately 30% of Medicare enrollees were tested for chlamydia or gonorrhea in 2010, but higher proportions of Medicaid enrollees were tested (45%–46%). Only 34% of HIV-positive Medicare enrollees who were tested for syphilis were also screened for chlamydia or gonorrhea on the same day. Nearly half of Medicaid enrollees were tested for all 3 STIs on the same day. Patients whose providers had more HIV experience had higher STI testing rates.
Conclusions
Testing rates for chlamydia and gonorrhea infection are low, despite the increase in these infections among people living with HIV and their close association with HIV transmission. Interventions to increase STI testing include the following: prompts in the medical record to routinely conduct syphilis testing on blood drawn for viral load monitoring, opt-out consent for STI testing, and provider education about the clinical importance of STIs among HIV-positive patients. Last, it is crucial to change financial incentives that discourage nucleic acid amplification testing for rectal chlamydia and gonorrhea infections.
The Centers for Disease Control and Prevention1 estimates that 1.9 million new cases of bacterial sexually transmitted infections (STIs; primary and secondary [P&S] syphilis, chlamydia, gonorrhea) were diagnosed in the United States in 2015. After years of decline, rates of STI have been growing. The national rate of reported P&S syphilis was 4 times greater in 2015 than it had been in 2000.1 Reported new chlamydia diagnoses grew by 90% between 2000 and 2015.1 Rates of gonorrhea infection per 100,000 population reached a historic low in 2009 but increased 26% by 2015.1
Bacterial STIs are prevalent among people living with HIV (PLWH), reflecting high rates among men who have sex with men (MSM), who account for 78% of HIV transmissions nationally.2 Men who have sex with men now account for 60% of all P&S syphilis cases and 82% of the cases among men. Between 2007 and 2013, the number of new cases of syphilis among MSM grew from approximately 5000 per year to 8200 per year, an increase of 64%.3 Black MSM had more than twice the rates of incident P&S syphilis as compared with all MSM. Furthermore, P&S rates are growing particularly among young (13–24 years), black MSM living in the largest metropolitan areas, who reported twice the number of syphilis diagnoses in 2008 as they had in 2004.4
Among MSM, the HIV-infected have the highest prevalence and experienced the greatest increase in syphilis diagnosis.5 Among MSM visiting Sexually Transmitted Disease Surveillance Network clinics, prevalence of P&S syphilis was 10.3% among HIV-positive men and 2.6% among HIV-negative MSM.1 HIV-positive MSM accounted for 82% of the syphilis cases among men in 2015.1 The high rates of syphilis among HIV-positive MSM are a particular health concern6 because syphilis infection increases HIV viral load and decreases CD4 in HIV-infected persons, thereby increasing the chance that PLWH transmit the virus.7–10
To detect asymptomatic STIs, the Centers for Disease Control and Prevention (CDC) recommends at least annual STI testing for MSM rather than symptom-driven testing.11 Men who have sex with men are more likely than other men to be tested for STIs at least once annually (26.2% vs 15.6%), yet STI testing is far from universal, even among HIV-positive MSM, who have the highest rates of STIs.1 Among people in treatment of HIV monitored in the Medical Monitoring Project, only 43% of sexually active PLWH received a test for gonorrhea, 43% for chlamydia, and 65% for syphilis in calendar 2013.2,12,13 Screening rates were even lower in a sample of commercially insured HIV-positive patients aged 18 to 64 years: 22.2% were tested for chlamydia, 21.9% for gonorrhea, and 51.1% for syphilis.14 Hoover et al.15 found relatively high annual rates of screening for syphilis (66%–76%) in large HIV clinics in 2004 to 2006, perhaps attributable to the fact that 7 of the 8 sites studied were Ryan White clinics, which are required to screen for syphilis. These clinics had lower rates of testing for chlamydia and gonorrhea (2.3%–18%), and most of these tests did not include rectal testing.
Given the high rates of syphilis, gonorrhea, and chlamydia among PLWH, it is important to know whether people in treatment of HIV meet minimum CDC guidelines for annual screening for STIs. We examine the factors that relate to the odds of PLWH being tested for syphilis, chlamydia, and gonorrhea in 2010. This article extends the research on STIs among PLWH by examining a large sample of PLWH with Medicare or Medicaid insurance who received medical care in a variety of settings.
MATERIALS AND METHODS
Data
Medicare and Medicaid claims data for HIV-positive Californians were acquired through a confidential data use agreement with the Centers for Medicare and Medicaid Services (CMS). We distinguished Medicare beneficiaries enrolled solely in Medicare from those enrolled dually in both Medicare (the primary payer) and Medicaid (hereafter, Duals). Beneficiaries enrolled solely in Medicaid (Medicaid-only) were analyzed separately. We applied a case identification algorithm to create an analysis file of adult beneficiaries with verifiable HIV.16 The sample includes full-year fee-for-service enrollees, because managed care data lack diagnosis fields needed to confirm HIV status. Nearly half of the 6246 Medi-Cal enrollees who met the previously mentioned criteria (3104) were not included in the analysis sample because they visited a federally qualified or a rural health center and therefore lacked claims data to document procedures such as STI testing. There were 3142 individuals in the Medi-Cal analysis sample. None of the 11,465 Medicare enrollees were dropped for this reason.
Data acquisition was reviewed and approved by the CMS Privacy Board. Research was also approved by the UCLA Institutional Review Board.
Outcome Variables
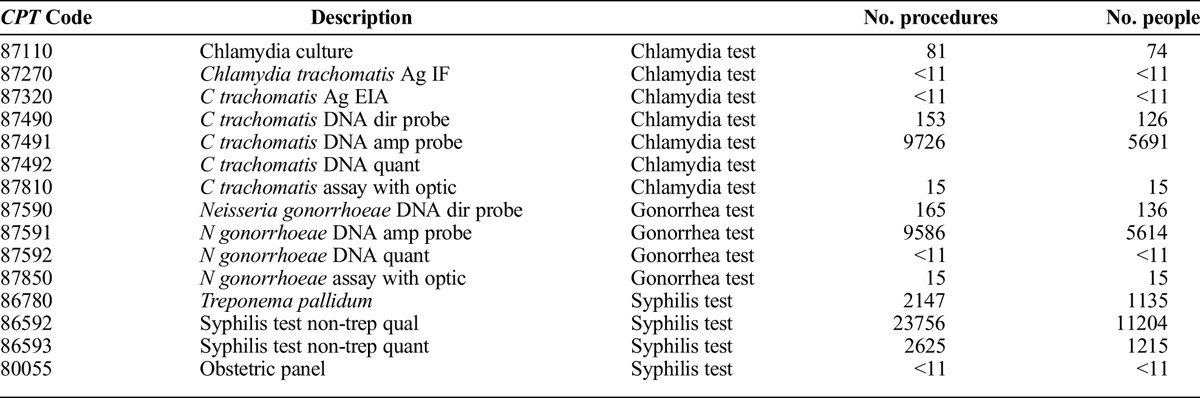
This article examines testing for 3 bacterial STIs (chlamydia, gonorrhea, and syphilis). Outpatient claim procedure codes shown in Appendix A were used to identify tests for each of the 3 STIs at least once during the year.
Predictor Variables
Enrollment data from CMS provided information on participant sex, age (<40, 40-54, and ≥55 years), and race and ethnicity (non-Hispanic white, African American, Hispanic, other). Reason for Medicare or Medicaid coverage was coded as “disability” or other (eg, aged). A provider's HIV patient case load predicts quality of care better than provider specialty17; thus, we measure an individual's access to an HIV specialist by whether he/she had an evaluation and management visit with any provider who treated 50 or more Medicare or Medicaid HIV patients in California in 2010.18
Statistical Analysis
Screening rates for each of the STIs were calculated by patient characteristics separately for Medicare and Medicaid-only enrollees. We also examined how frequently the STI screenings were performed at the same visit. We estimated logistic regressions to examine how testing for each of the bacterial STIs relates to demographic factors.
RESULTS
Sample Characteristics
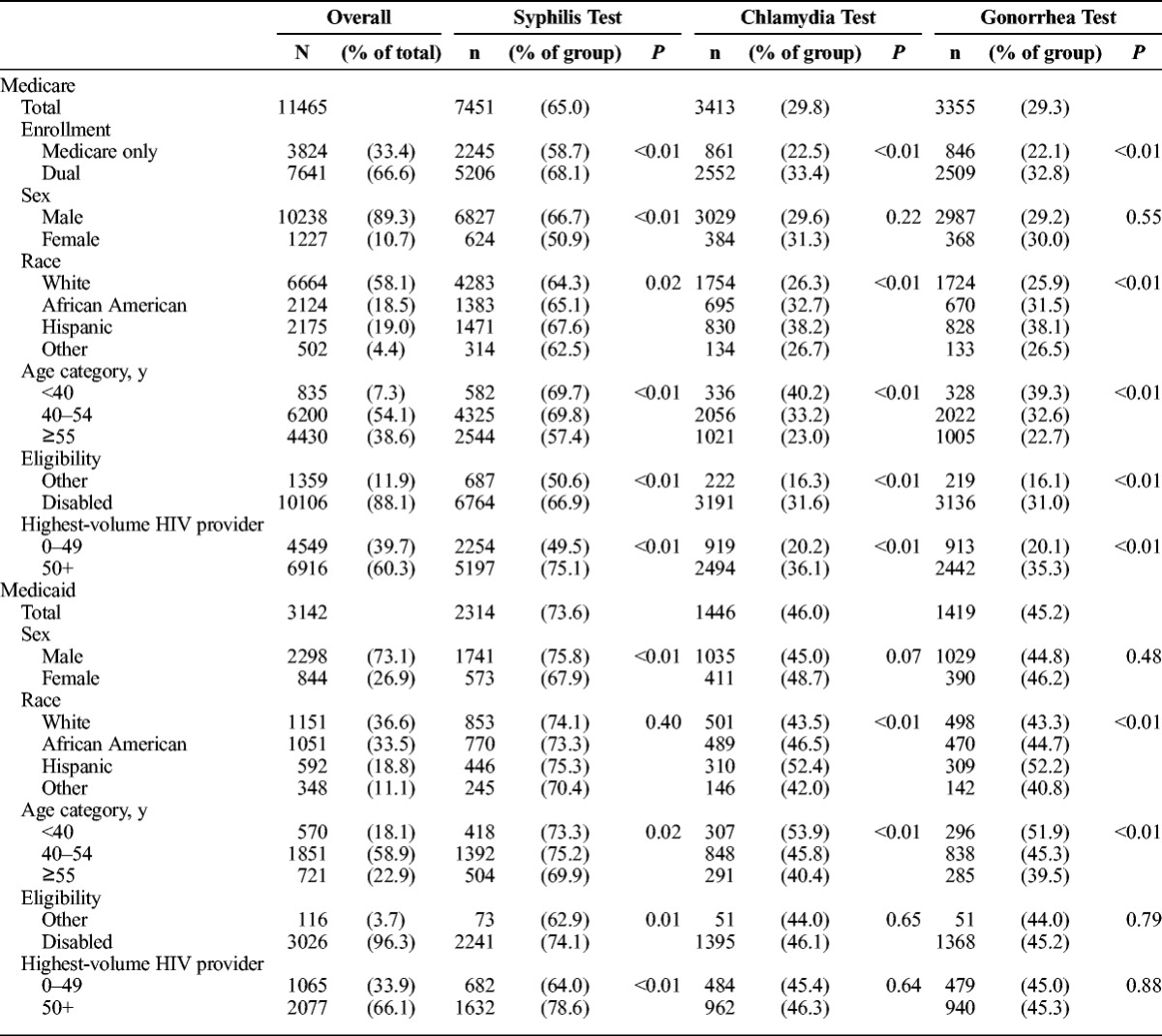
There were 11,465 PLWH covered by Medicare (10,238 men and 1227 women) in California in 2010 (Table 1). In addition, there were 3142 PLWH (2298 men and 844 women) meeting the sample inclusion criteria who were covered only by Medicaid. Consistent with the demographics of the 115,700 PLWH in California in 2010,19 most of both samples were male (89% of the Medicare sample and 73% of the Medicaid sample). Racial composition differed between the 2 groups. The Medicare sample was 58% white and 19% African American. The Medicaid-only sample was 37% white and 34% African American. Hispanics constituted 19% of each insurance group (Table 1). Younger PLWH (≤40 years) account for larger shares of the Medicaid (18%) than the Medicare samples (7%).
TABLE 1.
Demographics and STI Testing Among HIV-Positive Publically Insured Californians, 2010
STI Testing Rates
Rates of syphilis testing for Medicaid enrollees were higher than for Medicare enrollees in 2010 (74% vs 65%). Screenings for chlamydia or gonorrhea were much less frequent than for syphilis. Approximately 30% of Medicare enrollees were tested for chlamydia or gonorrhea in 2010, but higher proportions of Medicaid enrollees were tested (45%–46%; Table 1). Women were somewhat less likely than men to receive a syphilis test (51% of female Medicare enrollees and 68% of female Medicaid enrollees were tested for syphilis in 2010; P < 0.001 in both cases).
There was considerable overlap in testing for chlamydia or gonorrhea. In 97% of the cases, a test for chlamydia was accompanied by a test for gonorrhea on the same day. However, only 34% of Medicare PLWH enrollees tested for syphilis were screened for chlamydia or gonorrhea on the same day (a total of 38% within 30 days of a syphilis test.) However, nearly half of Medicaid enrollees who were tested for syphilis also received tests for gonorrhea and chlamydia on the same day (53% within a month of the syphilis test).
Multivariate Predictors of STI Testing
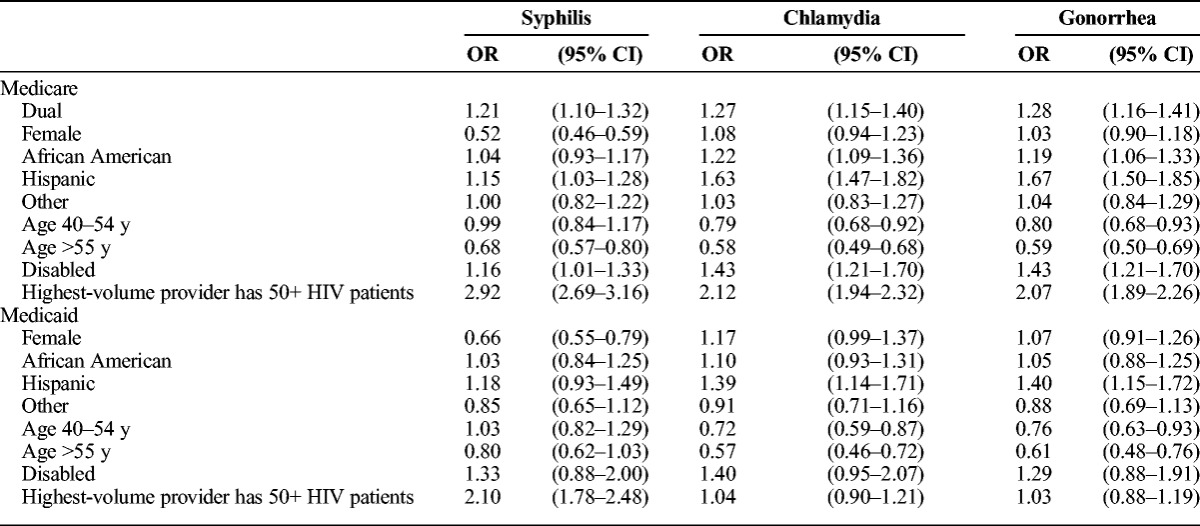
Table 2 presents logistic regression estimates of the effect of predictor variables on the odds of being tested for each of the 3 bacterial STIs. Women were less likely to be tested for syphilis than men in both Medicare (adjusted odds ratio [aOR], 0.52; 95% confidence interval [CI], 0.46–0.59) and Medicaid (aOR, 0.66; 95% CI, 0.55–0.79) samples. African American Medicare enrollees had significantly greater odds of being tested for chlamydia (aOR, 1.22; 95% CI, 1.09–1.36) and gonorrhea (aOR, 1.19; 95% CI, 1.06–1.33) compared with whites. Hispanic enrollees were more likely than whites to be tested for each of the bacterial STIs, with the exception of Medicaid testing for syphilis. Testing for chlamydia and gonorrhea was more likely for those younger than 40 years than for those 40 years or older. Medicare enrollees older than 55 years were also less likely to be tested for syphilis than those younger than 40 years. Medicare patients with access to providers with greater HIV caseloads were more likely to be tested for all 3 bacterial STIs than those without access to a high-volume HIV provider. Medicaid patients with access to large-volume HIV providers had greater odds of getting tested for syphilis compared with those with only low-volume providers, but this was not the case for chlamydia or gonorrhea.
TABLE 2.
Logistic Regressions of Testing for STIs Among HIV Positive Publically Insured Californians, 2010
DISCUSSION
Responding to what has been called a “hidden epidemic”20 and the fact that many who are infected with STIs are asymptomatic and undiagnosed, the CDC recommends at least annual STI testing of sexually active PLWH.11 Nonetheless, we found that in 2010, fewer than two-thirds of HIV-positive Medicare and fewer than three-quarters of Medicaid enrollees received a test for syphilis. Rates were even lower for chlamydia and gonorrhea (~30% of Medicare enrollees and fewer than half of Medicaid enrollees). Women and older PLWH were significantly less likely to be tested for bacterial STIs. These findings have implications for both HIV providers and for health policy.
Implications for HIV Providers
Making STI screening (rather than symptom-driven STI testing) a routine part of medical care for PLWH is important because it would identify many STIs that are asymptomatic and that carry significant morbidity and, in rare cases, mortality, if left untreated. Not only can these STIs be secondarily transmitted, but they also increase HIV infectiousness. Providers often fail to identify patients who are at risk for STIs because they do not elicit information on sexual risk behaviors and patients frequently do not reveal sexual behaviors that would put them at risk for STIs.21 In particular, gonorrhea and chlamydia are often asymptomatic, so patients are less likely to report symptoms of the infection that would lead to testing. One study found that symptom- and sexual history–based testing identified only half of the chlamydia and gonorrhea cases identified through universal screening.21
Our analyses confirm that testing rates for chlamydia and gonorrhea infection are low, despite the increase in these infections in PLWH and their close association with HIV transmission.7–10 Gonorrhea and chlamydia had greater incidence compared with syphilis among HIV-negative MSM; however, among HIV-positive MSM, new syphilis were more frequently diagnosed (14.3%) than chlamydia (8.7%) or gonorrhea cases (10.4%).3 Even PLWH known to be at risk for STIs are not commonly tested. One study found that only one-quarter of patients diagnosed as having incident syphilis were screened for gonorrhea and chlamydia.22
“Opt-out” strategies have been effective in increasing HIV testing rates and could potentially also increase STI testing. Blood drawn for monitoring viral loads could be used to screen for syphilis at minimal additional cost and burden. This strategy could quickly increase the rate of syphilis testing because more than 90% of publicly insured patients living with HIV have been prescribed antiretroviral medications and 85% are regularly tested for CD4 and/or viral load.23 Increasing screening for gonorrhea and chlamydia will be more challenging because of the characteristics of the different tests. Syphilis tests are serologic, but accurate diagnoses of gonorrhea and chlamydia require rectal, urethral, and/or pharyngeal samples, which patients find more physically and emotionally undesirable.24 Indeed, urine testing is the most common testing modality, but it cannot detect rectal infections. A study of STI testing of MSM visiting sexually transmitted disease clinics found that 83.9% of the MSM were tested for urogenital gonorrhea and 81.4% for urogenital chlamydia, many fewer than received rectal tests for gonorrhea (50.4%) or chlamydia (45.9%). The authors found that 70% of extragenital gonorrhea infection and 85% of chlamydia infections occurred in MSM who had negative urethral test results at the same visit. Thus, these infections would not have been detected by urethral screening alone.25 Studies of STI testing among MSM have found that only 10% of first testing episodes included extragenital sites.26 Patient stigma, shame, fear of invasive sampling, and confidentiality concerns as well as clinicians' time pressures and lack of cultural competency act as barriers to testing in rectal sites. Self-collection of specimens, a method with “acceptable diagnostic accuracy,” is an alternative that would address both patient and provider issues.24 Self-collection of rectal swabs has been found as valid as clinician-collected samples and is equally or more acceptable to most patients.27
The greater frequency of STI testing among patients with access to more experienced HIV providers suggests that non-HIV specialists' lack of prioritization of STI testing or their discomfort in having anatomically specific discussions of sexual acts and collecting rectal samples could be barriers to their implementing routine screening. Indeed, an experiment that both educated providers about the importance of screening for gonorrhea, chlamydia, and syphilis and introduced a sexual risk assessment for patients resulted in significant increases in screening.28 Automated structural changes, such as including a prompt in the electronic medical record to screen HIV-positive patients for STIs at regular intervals, could help to overcome a multiple barriers to routinization of the CDC's STI screening recommendations.
Implications for Health Policy
Several policies raise financial hurdles to obtaining preventive screenings for chlamydia and gonorrhea. The US Preventive Services Task Force finds insufficient evidence of net benefits of chlamydia and gonorrhea screening in men.29 These screenings can be financially penalized because insurance reimbursement is often based on US Preventive Services Task Force evaluations.
Medicare will cover annual syphilis screening for “men and women at increased risk for STIs,” but chlamydia and gonorrhea screening seems to be covered only for pregnant women and women at increased risk for STIs.30 The high STI incidence rates among nonpregnant PLWH means that many infections will go undetected because of lack of symptoms and lack of full sexual histories.
The CDC recommends that laboratories test for gonorrhea and chlamydia using nucleic acid amplification tests (NAATs), which have greater sensitivity than culture, but the Food and Drug Administration (FDA) has not yet cleared NAAT screening for extragenital specimens.31,32 Although laboratories that have met all validation requirements for an off-label procedure may use them, most insurance plans, including Medicare, will not cover non–FDA-cleared tests. Medicare and Medi-Cal will nominally reimburse NAAT for diagnostic purposes but will not reimburse for rectal STI screenings using NAAT technology. Rates of pharyngeal and rectal infections among asymptomatic HIV-positive MSM are high, so the lack of FDA clearance of these tests may present a substantial barrier to identifying STIs in extragenital sites. A further hurdle is that Medicare will cover only STI screenings ordered by a primary care provider. More than half of providers to PLWH do not classify themselves as “primary care” providers; thus, it may be difficult for them to get reimbursed by CMS for STI screening.32
This study has a number of limitations. First, the data relate only to California and patients treated in fee-for-service settings other than federally qualified and rural health centers. Thus, findings may not generalize to other states, managed care settings, or safety-net providers. Second, the insurance claims data include only STI tests submitted for insurance reimbursement. However, we believe that lack of billing is not a major problem because most freestanding STI clinics, such as Planned Parenthood, do bill Medicare and Medicaid for their publicly insured patients. The claims data do not indicate whether the sample for gonorrhea and chlamydia testing was taken from an extragenital site and perhaps intentionally mislabeled for purposes of testing and/or billing. However, other studies suggest that extragenital testing is rare.15 The CDC guidelines apply to sexually active patients. Although we did not have data on sexual activity, more than 60% of PLWH are sexually active.13 Last, we were unable to assess treatment of STIs, relapses, and reinfections, important parameters in completing the understanding of the STI diagnostic and therapeutic landscape.
Our study also has a number of strengths. It uses STI testing data for bacterial STIs for large numbers of Medicare and Medicaid enrollees with HIV, a group with very high rates of STIs. It examines care for PLWH in a wide variety of settings and is not limited to a small sample or a single clinic, as in many prior analyses.
Increasing rates of condomless anal sex among MSM have led to increases in STIs at the same time that transmission of HIV has fallen as a result of antiretroviral therapy's effect on reducing HIV viral loads.1,2 Untreated STIs adversely affect the health of patients with HIV and increase the likelihood of transmitting the HIV virus to others. Incorporating STI testing as a routine part of HIV care and treatment visits would increase detection and treatment of STIs for PLWH. However, the lack of FDA approval for NAAT on rectal and pharyngeal samples remains a significant barrier. Regular STI screening constitutes an important component of medical care for people with HIV infection such that the infections can be treated to reduce morbidity and secondary transmission of the both HIV and STIs to others.
Supplementary Material
APPENDIX
TABLE A.
CPT Codes Used to Identify STI Tests
Footnotes
This research was supported by grants from the California HIV/AIDS Research Program of the University of California (Grant RP11-LA-020). This work was also supported by the Center for HIV Identification, Prevention, and Treatment (NIMH Grant P30 MH058107-21), the UCLA Center for AIDS Research (Grant 5P30AI028697), and the National Institutes of Health/National Center for Advancing Translational Science (UCLA CTSI Grant No. UL1TR000124). Research was approved by UCLA Institutional Review Board (No. 10-000823).
Conflicts of Interest and Sources of Funding: None declared.
The funders had no role in data collection; management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript for publication.
REFERENCES
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2015. Atlanta: U.S. Department of Health and Human Services, 2016. Available at: https://www.cdc.gov.std.stats. Accessed Dec. 1, 2016.
- 2.Centers for Disease Control and Prevention. HIV Surveillance Report, 2015, Vol. 27. November 2016. Available at: http://www.cec.gov/hiv/library/reports/hiv-surveillance.html. Accessed June 20, 2017.
- 3.Centers for Disease Control and Prevention. HIV infection risk, prevention, and testing behaviors among men who have sex with men—National HIV Behavioral Surveillance, 20 US Cities, 2014. HIV Surveillance Report. Special Report #15. January 2016. Available at: http://www.cdc.gov/hiv/library'reports/surveillance/#panel2. Accessed October 15, 2016.
- 4.Peterman TA, Su J, Bernstein KT, et al. Syphilis in the United States: On the rise? Expert Rev Anti Infect Ther 2015; 13:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abara WE, Hess KL, Neblett Fanfair R, et al. Syphilis trends among men who have sex with men in the United States and Western Europe: A systematic review of trend studies published between 2004 and 2015. PLoS One 2016; 11:e0159309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchacz K, Patel P, Taylor M, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS 2004; 18:2075–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarzebowski W, Caumes E, Dupin N, et al. Effect of early syphilis infection on plasma viral load and CD4 cell count in human immunodeficiency virus–infected men: Results from the FHDH-ANRS CO4 cohort. Arch Intern Med 2012; 172:1237–1243. [DOI] [PubMed] [Google Scholar]
- 8.Barbee LA, Khosropour CM, Dombrowksi JC, et al. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis 2017; 44:385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesson HW, Pinkerton SD. Sexually transmitted diseases and the increased risk for HIV transmission: Implications for cost-effectiveness analyses of sexually transmitted disease prevention interventions. J Acquir Immune Defic Syndr 2000; 24:48–56. [DOI] [PubMed] [Google Scholar]
- 10.Kelley CF, Vaughan AS, Luisi N, et al. The effect of high rates of bacterial sexually transmitted infections on HIV incidence in a cohort of black and white men who have sex with men in Atlanta, Georgia. AIDS Res Hum Retroviruses 2015; 31:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workowski KA, Bolan GA. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR-03):1–137. Erratum in MMWR Recomm Rep. 2015; 64:924. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6403a1.htm. [PMC free article] [PubMed] [Google Scholar]
- 12.Flagg EW, Weinstock HS, Frazier EL, et al. Bacterial sexually transmitted infections among HIV-infected patients in the United States: Estimates from the Medical Monitoring Project. Sex Transm Dis 2015; 42:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Behavioral and Clinical Characteristics of Persons Receiving Medical Care for HIV Infection. Medical Monitoring Project, United States 2013 Cycle (June 2013–May 2014). HIV Surveillance Report Special Report #16. January 2016. Available at: http://wwwcdcgov/hiv/library'reports/surveillance/#panel2. Accessed October 15, 2016. [PubMed]
- 14.Pearson WS, Davis AD, Hoover KW, et al. Demographic and health services characteristics associated with testing for sexually transmitted infections among a commercially insured population of HIV-positive patients. J Acquir Immune Defic Syndr 2015; 70:269–274. [DOI] [PubMed] [Google Scholar]
- 15.Hoover KW, Butler M, Workowski K, et al. STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis 2010; 37:771–776. [DOI] [PubMed] [Google Scholar]
- 16.Leibowitz AA, Desmond K. Identifying a sample of HIV-positive beneficiaries from Medicaid claims data and estimating their treatment costs. Am J Public Health 2015; 105:567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landon BE, Wilson IB, McInnes K, et al. Physician specialization and the quality of care for human immunodeficiency virus infection. Arch Intern Med 2005; 165:1133–1139. [DOI] [PubMed] [Google Scholar]
- 18.Handford CD, Rackal JM, Tynan AM, et al. The association of hospital, clinic and provider volume with HIV/AIDS care and mortality: Systematic review and meta-analysis. AIDS Care 2012; 24:267–82. [DOI] [PubMed] [Google Scholar]
- 19.California State Office of AIDS. HIV/AIDS Surveillance in California. Available at: http://www.cdph.ca.gov/data/statistics/Documents/HIVSurveillanceReport2013dxBy2015yrenddata.pdf. Accessed March 24, 2017.
- 20.Centers for Disease Control and Prevention. Tracking the Hidden Epidemics 2000. Trends in STDs in the United States. Available at: www.cdc.gov/std/trends2000/trends2000.pdf. Accessed September 1, 2016.
- 21.van Liere GA, Hoebe CJ, Niekamp AM, et al. Standard symptom– and sexual history–based testing misses anorectal Chlamydia trachomatis and Neisseria gonorrhoeae infections in swingers and men who have sex with men. Sex Transm Dis 2013; 40:285–289. [DOI] [PubMed] [Google Scholar]
- 22.Baffi CW, Aban I, Willig JH, et al. New syphilis cases and concurrent STI screening in a southeastern U.S. HIV clinic: A call to action. AIDS Patient Care STDS 2010; 24:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landovitz RJ, Desmond KA, Leibowitz AA. Antiretroviral therapy: racial disparities among publicly insured Californians with HIV. J Health Care Poor Underserved 2017; 28:406–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: A systemic review and meta-analysis. PLoS One 2015; 10:e0132776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patton ME, Kidd S, Llata E, et al. Extragenital gonorrhea and chlamydia testing and infection among men who have sex with men—STD Surveillance Network, United States, 2010–2012. Clin Infect Dis 2014; 58:1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry SA, Ghanem KG, Page KR, et al. Gonorrhoea and chlamydia testing rates of HIV-infected men: Low despite guidelines. Sex Transm Infect 2010; 86:481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van der Helm JJ, Hoebe CJ, van Rooijen MS, et al. High performance and acceptability of self-collected rectal swabs for diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men who have sex with men and women. Sex Transm Dis 2009; 36:493–497. [DOI] [PubMed] [Google Scholar]
- 28.Scarborough AP, Slome S, Hurley LB, et al. Improvement of sexually transmitted disease screening among HIV-infected men who have sex with men through implementation of a standardized sexual risk assessment tool. Sex Transm Dis 2015; 42:595–598. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Preventive Services Task Force. Final Update Summary: Chlamydia and Gonorrhea: Screening. Available at: https://www.uspreventiveser vicestaskforce.org/Page/Document/Update SummaryFinal/chlamydia-and-gonorrhea. Accessed June 23, 2017.
- 30.Centers for Medicare & Medicaid Services (CMS). Screening for Sexually Transmitted Infections (STIs) and High Intensity Behavioral Counseling (HIBC) to Prevent STIs. MLN Matters Number MM7610. May 23, 2012. Available at: http://www.cms.gov/MLNMattersArticles/downloads/MM7610.pdf. Accessed February 23, 2017.
- 31. CDC STD trends in the United States: 2010 National Data for Gonorrhea, Chlamydia, and Syphilis. Available at: www.cdc.gov/std/stats10/trends.htm. Accessed August 29, 2016.
- 32.Owusu-Edusei K, Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013; 40:197–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


