Abstract
Objective
As the use of minimally invasive surgery in cardiothoracic surgery increases, so does the need for simulation and training. We developed a heart model for simulation and training of minimally invasive cardiac surgery, particularly minimally invasive mitral valve repair using our new three-dimensional printing system.
Methods
Digital imaging and communication in medicine data from patient computed tomography, three-dimensional computer-aided design, and three-dimensional printing helped create replicas of the heart and thoracic cavity. A polyvinyl alcohol model material with a texture and physical properties similar to those of heart tissue was initially used in mitral valve replicas to simulate surgical procedures. To develop this material, we mechanically investigated the composition of each part of the porcine heart.
Results
We investigated the elastic modulus and breaking strength of the porcine heart. Based on investigation results, the cardiac model was set at rupture strength 20 MPa, elastic modulus 0.17 MPa, and moisture content 85%. This provided a biotexture and feeling exactly like a patient heart. Computed tomography scans confirmed that the model shape was nearly the same as that of a human heart. We simulated minimally invasive mitral valve repair, including ring annuloplasty, chordal reconstruction, resection and suture, and edge-to-edge repair. Full surgery simulations using this model used minimally invasive cardiac surgery tools including a robot.
Conclusions
This life-like model can be used as a standard simulator to train younger, less experienced surgeons to practice minimally invasive cardiac surgery procedures and may help develop new operative tools.
Key Words: Surgical education, Minimally invasive cardiac surgery, 3D printing, Minimally invasive mitral valve repair
The use of minimally invasive cardiac surgery (MICS) began in the mid 1990s1,2 and has been gaining importance in cardiothoracic surgery. Although MICS has many advantages, including rapid postoperative recovery, reduced postoperative pain, and improved cosmesis,3 opportunities for simulation and training remain limited. Several recent publications have reported the benefits of training simulators,4 but no available simulators satisfactorily mimic both the anatomy and texture of human organs. The use of porcine hearts does not comply with ethical principles of animal welfare. We have used our existing three-dimensional (3D) printing technology, Biotexture (Fasotec Co, Ltd, Chiba, Japan), to create realistic replicas of human organs in simulated MICS, particularly for minimally invasive mitral valve repair (MIMVR).
MATERIALS AND METHODS
This project was a collaboration of medicine and engineering, and the initial development of biotexture models has been previously described.5,6 Realistic replicas of organs such as the kidney and liver have already been used to simulate surgical procedures and provide training (Fig. 1). We have also used this technology for cardiac surgery, specifically MICS.
FIGURE 1.
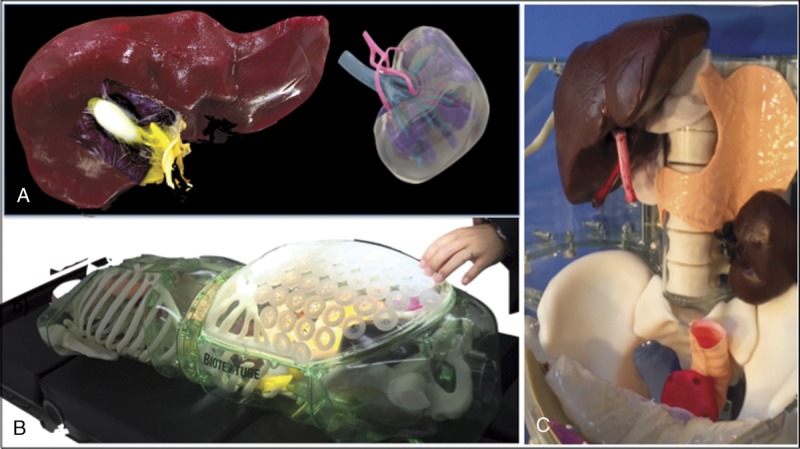
Biotexture, using CT and 3D printing systems, enabled us to replicate patient-specific 3D organs. Liver and kidney model (A), thoracic and abdominal cavity model (B), and simulator for gastrointestinal surgery and urology (C).
The MICS simulator was developed from cardiac and thoracic cavity models. The thoracic cavity model has already been developed by us. The cardiac model was created by Biotexture5,6 as follows. Cardiac computed tomography (CT) was used to obtain standard digital imaging and communications in medicine (DICOM) data of the heart. After the converting from DICOM data to stereolithography data, the 3D computer-aided design application was used to design the cardiac model (Fig. 2), and an anatomically accurate mold was created from the design using a 3D printer. The model material, which included polyvinyl alcohol (PVA), was injected into the mold (Fig. 3). A representative human heart model that was produced is shown in Figure 4. Note that PVA plays an important role in replicating the texture of the heart. To express the texture of each part of the heart by engineering parameters, the elastic modulus and breaking strength were used. The breaking strength indicates the strength of the material, and the elastic modulus indicates the hardness of the material. The elastic modulus is calculated from the amount of strain in the direction of compressive stress and corresponds to the inclination of the straight portion of the stress-strain curve, with stress on the vertical axis and strain on the horizontal axis. The analysis of breaking strength allows the determination of how much deformation or breakage occurs with compression at a constant speed. The elastic modulus and breaking strength of the model material were determined with a dedicated measuring device (Sunarrow Limited, Tokyo, Japan, original device; tip diameter = 0.5 mm, speed = 1.0 mm/s, load cell = NMB BCL - 300 GM - A, resolution = 1/1000 N, Fig. 5). We measured the elastic modulus and breaking strength of the left ventricle, papillary muscle, mitral annulus, fibrous trigone, mitral leaflet, and left atrium of the each three parts of three porcine hearts. In addition to these two parameters, to better simulate the texture of the heart, we measured the moisture content of the tissues by comparing the weight of the completely dried three porcine hearts with the weight before drying. These data were considered when developing the PVA heart model material. The cardiac mitral complex model (MCM) that was constructed included the left atrium, mitral valve, left ventricle, and other surrounding structures. We evaluated the shape of the replica by comparison to CT scans and the feeling of the material by performing real surgical procedures on the replica.
FIGURE 2.
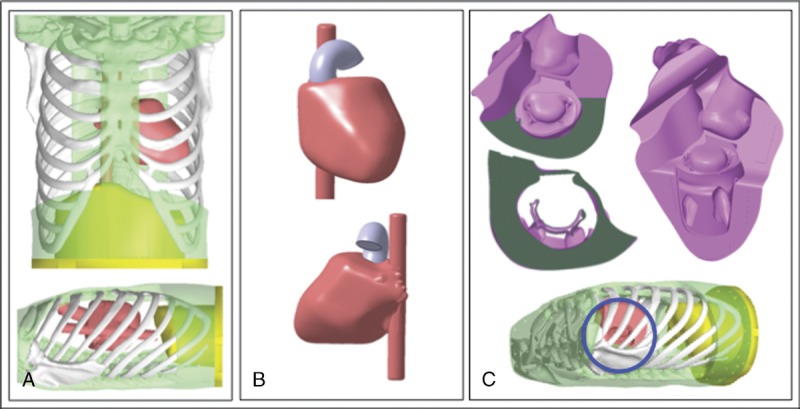
Cardiac CT was used to obtain standard DICOM data of the heart, which were used to design the cardiac model with a 3D CAD. The design of 3D CAD of thoracic cavity and cardiac model (A), cardiac model (B), and MCM (C).
FIGURE 3.
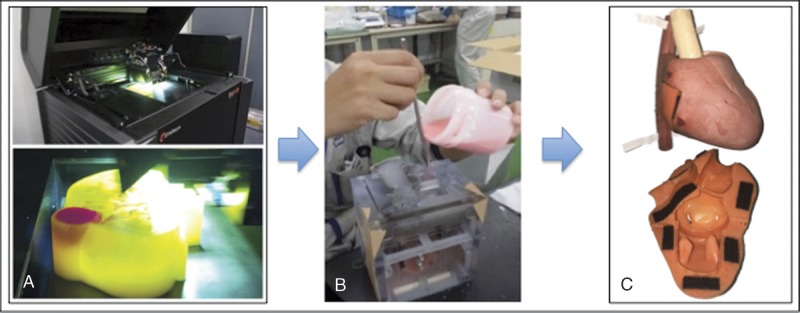
A, Accurate mold was created from the design using a 3D printer. Materials such as PVA were inserted into the mold (B), and the actual model was developed (C).
FIGURE 4.
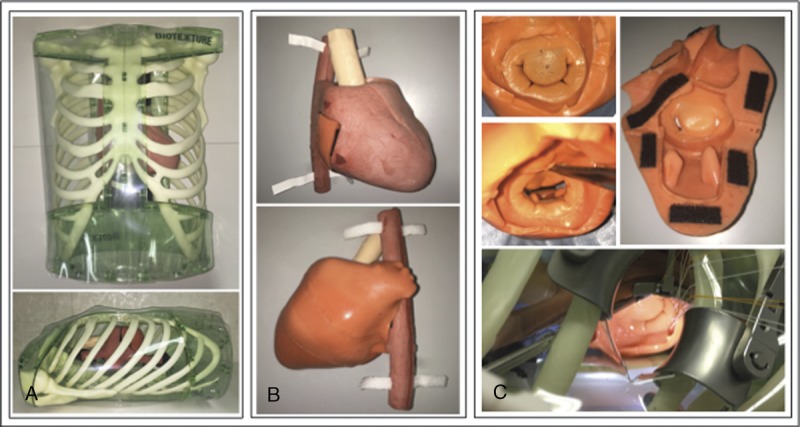
Actual thoracic cavity and cardiac model (A), cardiac model (B), and MCM (C), with similar shapes.
FIGURE 5.

Systems of measurement of breaking strength and elastic modulus (Sunarrow Limited original device). The breaking strength is obtained by dividing the fracture Newton value by the tip cross-sectional area. The elastic modulus is obtained by dividing the rupture strength gradient of unit displacement by the cross-sectional area.
RESULTS
Figure 6 shows the elastic modulus and breaking strength of the porcine heart. The breaking strength, with tissue location and texture, was the strongest in the fibrous trigone, and the papillary muscle was brittle and delicate. These differences in physical properties were similar to those in the feeling experienced in an actual operation. Based on these data, we developed a PVA model material with a breaking strength of 20 MPa and an elastic modulus of 0.17 MPa, which realistically simulated the heart tissues. In addition, the moisture content of the porcine heart was 76%, which we used in the model. At this percentage, the feeling of needle insertion was most similar to the feeling experienced during an actual surgery. Computed tomography scans confirmed the accurate structure and position of each valve compared with an actual human heart (Fig. 7).
FIGURE 6.
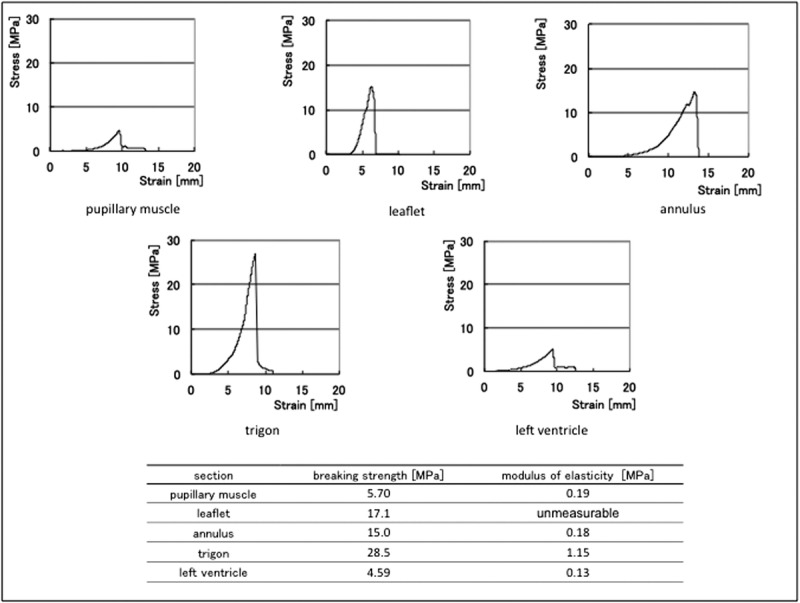
This graph shows characteristic data of elastic modulus and breaking strength obtained from three porcine hearts. The breaking strength data represent the difference in the texture of each part; for example, the trigon is the strongest and the papillary muscle is brittle and delicate, and it is similar to the feeling experienced in an actual operation. Based on these data, we developed an original PVA with a breaking strength of 20.3 MPa and an elastic modulus of 0.17 MPa. Only the leaflet could not be measured for the elastic strength because the tissue was very thin.
FIGURE 7.
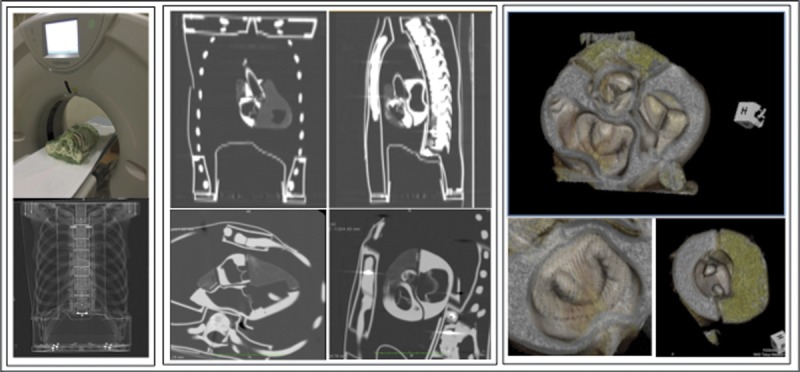
Computed tomography scan proved that the structure and position of each valve are equivalent to those of the actual human body.
We simulated MIMVR including ring annuloplasty (Carpentier-Edwards Physio II ring; Edwards Lifesciences Corp, Irvine, CA USA), artificial chordal reconstruction, resection and suture, and edge-to-edge repair, with actual MICS tools (Delacroix-Chevalier surgical instruments, Paris, France), camera systems (KARL STORZ GmbH & Co KG, Tuttlingen, Germany), and robotic surgery (da Vinci Surgical System; Intuitive Surgical, Sunnyvale, CA USA) (Fig. 8). The feel of the replica was remarkably similar to that of the actual biotexture of a patient heart. The difficulty of the simulated procedures, particularly the limited surgical working space caused by the ribs, was the same as that during an actual surgery. The positions of the heart, incision, and surgical ports were interchangeable; this way, we could simulate various MICS procedures.
FIGURE 8.
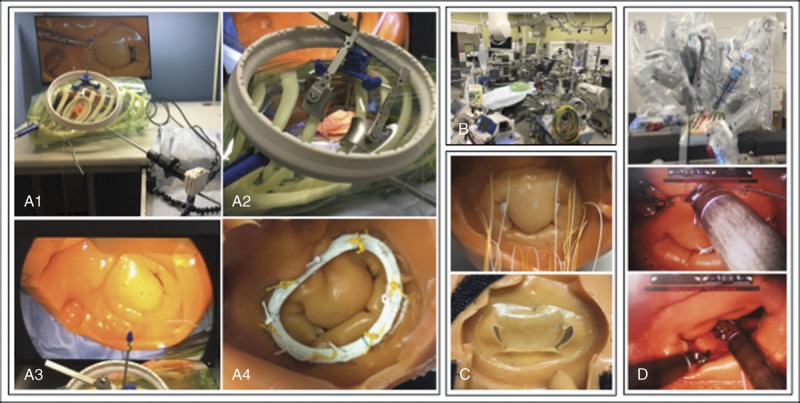
Thoracic and cardiac simulator was used for simulation of MIMVR including ring annuloplasty, artificial chordal reconstruction, resection and suture, and edge-to-edge, with actual MICS tools with or without a robot.
DISCUSSION
The surgical procedures, tools, and techniques of MICS have continued to advance since 1998.7–9 It is important for cardiac surgeons to perform MICS easily and safely. Consequently, effective simulation and training are needed. Virtual simulation using equipment such as “flight simulators” is useful for operating an endoscope,10 and traditional “black-box” training is useful for surgeons.11 However, MICS has a steep learning curve.12
We began developing MICS simulators approximately 3 years ago. Several have been developed for simulation training in cardiothoracic surgery and have been previously described.13 Simulators that are both realistic and accurate are not currently available. Three-dimensional printing is very useful for making life-like heart replicas.14 We took advantage of this technique to develop not only an anatomically accurate heart model but also a surgical simulator using a material with a biotexture that mimics the feel of human tissue.
To reproduce the actual shape and texture of the heart, we designed the model using data obtained from CT scans and developed the material based on engineering data from porcine heart tissue. Note that CT scans of the cardiac model resulted in images equivalent to those normally obtained for hearts. In addition, breaking strength and elastic modulus measurements of the porcine heart were numerically similar to values obtained during an actual surgery. For example, the fibrous trigone is the strongest and the papillary muscle is the most delicate. However, accurate reproduction of each region would be expensive. Because printing cast molds is a standard technique of 3D printing, we used it to produce a cardiac model comprising several parts.
At first, we focused on MIMVR because it is the most frequently performed MICS procedure. We simulated ring annuloplasty, artificial chordal reconstruction, resection and suture, and edge-to-edge repair via left atriotomy. We therefore developed an MCM that included the left atrium, mitral valve, and left ventricle. This cardiac model can be used with all surgical instruments used for actual MICS including thoracotomy, such as left atrium retractors, endoscope cameras, dedicated needle holders, liner, and ports (Fig. 8). Use with the da Vinci Surgical System is also shown in Figure 8. This makes it possible to closely simulate surgery as practiced in an operating room. It is also useful as an educational tool for surgical residents, and from the perspective of the heart team, it can help make actual surgery proceed more smoothly.
We are certain that this simulator can help in training younger and less experienced surgeons to learn and practice MICS procedures with life-like models rather than virtual simulators and animal organs. Hopefully, it will become a standard simulator and trainer for MICS procedures. Finally, we developed this heart model with the aim of replacing wet lab animal models. The porcine heart model has been reported to be more useful than a simple dry box,15 but in recent years, the use of animal models has been restricted because of animal welfare concerns. In addition, the human and porcine hearts do not have exactly the same anatomy and texture. Needless to say, model human organs and not animals will become increasingly used in surgical education in the future. Furthermore, the cardiac model can be used without an accompanying thoracic cavity model for simulation of and training for conventional heart procedures.
We have already begun the next steps by not only developing valves such as the aortic valve and the tricuspid valve but also developing models of coronary artery and internal thoracic arteries for minimally invasive off-pump coronary artery bypass grafting. To develop these models, detailed CT data are required to show the details of the heart, and it is important to construct a mechanism for beating heart for off-pump coronary artery bypass grafting. A CT scan can clearly demonstrate the aortic valve but not the mitral valve and tricuspid valve, particularly the chordae and leaflets. Therefore, we have started to consider how to obtain the clearest image of the mitral valve by a CT scan. If we succeed, we would be able to develop a cardiac model that is specific to each patient, which would allow optimal preoperative simulation and training and help educate patients about their disease and treatment. In addition to allowing less experienced operators to plan for repair of more complicated diseases such as Barlow’s disease, they can teach patients and co-medical staffs about diseases and treatments. This is an important advance in the era when the learning curve no longer becomes acceptable to patients, facilities, or the public. In the future, this simulator will help increase the safety and effectiveness of MICS procedures and the development of new surgical procedures and tools.
ACKNOWLEDGMENTS
The authors thank Fasotec Co, Ltd, and Sunarrow Limited for their continuing support and constant encouragement.
CLINICAL PERSPECTIVE
This report from Dr. Yamada and his colleagues examined the use of three-dimensional printing to create life-like models for simulation and training inminimally invasive cardiac surgery. Replicas of the heart and thoracic cavity were created fromcardiac computed tomography scans. They investigated the elastic modules and breaking strength of the porcine heart and then developed a cardiac model based on these parameters. They then used this model to simulate minimally invasive mitral valve repair.
The authors showed the feasibility of this approach, but it remains to be seen whether this technology will enhance training sufficiently to justify the cost. Further studies will be needed to evaluate the value of three-dimensional printing in training cardiothoracic surgeons on new procedures. Future studies from this group are keenly anticipated.
Footnotes
Disclosure: The authors declare no conflicts of interest.
REFERENCES
- 1.Navia JL, Cosgrove DM. Minimally invasive mitral valve operations. Ann Thorac Surg. 1996;62:1542–1544. [DOI] [PubMed] [Google Scholar]
- 2.Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg. 1997;226:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modi P, Hassan A, Chitwood WR., Jr Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2008;34:943–952. [DOI] [PubMed] [Google Scholar]
- 4.Joyce DL, Dhillon TS, Caffarelli AD, et al. Simulation and skills training in mitral valve surgery. J Thorac Cardiovasc Surg. 2011;141:107–112. [DOI] [PubMed] [Google Scholar]
- 5.Sugimoto M, Azuama T, Watanabe K, et al. Method for manufacturing three-dimensional molded model and support tool for medical treatment, medical training, research, and education. US patent 20140017651 A1. Accessed September 15, 2017. [Google Scholar]
- 6.Fasotec Co., Ltd. Available at: http://www.fasotec.co.jp/common/pdf/Comp_prof.pdf. Accessed September 15, 2017.
- 7.Yozu R, Okamoto K, Kudo M, Nonaka H, Adams DH. New innovative instruments facilitate both direct-vision and endoscopic-assisted mini-mitral valve surgery. J Thorac Cardiovasc Surg. 2012;143:S82–S85. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto K, Yozu R, Kudo M. Loop-in-loop technique in mitral valve repair via minithoracotomy. Ann Thorac Surg. 2012;93:1329–1330. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto K, Yozu R. Designing innovative retractors and devices to facilitate mitral valve repair surgery. Ann Cardiothorac Surg. 2015;4:364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Våpenstad C, Buzink SN. Procedural virtual reality simulation in minimally invasive surgery. Surg Endosc. 2013;27:364–377. [DOI] [PubMed] [Google Scholar]
- 11.Jensen K, Ringsted C, Hansen HJ, Petersen RH, Konge L. Simulation-based training for thoracoscopic lobectomy: a randomized controlled trial: virtual-reality versus black-box simulation. Surg Endosc. 2014;28:1821–1829. [DOI] [PubMed] [Google Scholar]
- 12.Holzhey DM, Seeburger J, Misfeld M, Dasseux JL. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation. 2013;30:483–491. [DOI] [PubMed] [Google Scholar]
- 13.Fann JI, Feins RH, Hicks GL, Jr, et al. Evaluation of simulation training in cardiothoracic surgery: the Senior Tour perspective. J Thorac Cardiovasc Surg. 2012;143:264–272. [DOI] [PubMed] [Google Scholar]
- 14.Schmauss D, Haeberle S, Hagl C, Sodian R. Three-dimensional printing in cardiac surgery and interventional cardiology: a single-centre experience. Eur J Cardiothorac Surg. 2015;47:1044–1052. [DOI] [PubMed] [Google Scholar]
- 15.Valdis M, Chu MW, Schlachta C, Kiaii B. Evaluation of robotic cardiac surgery simulation training: a randomized controlled trial. J Thorac Cardiovasc Surg. 2016;151:1498–1505. [DOI] [PubMed] [Google Scholar]


