Abstract
Background:
The neuroinflammatory response to morphine exposure modulates its antinociceptive effects, tolerance, and dependence. Positron emission tomography radioligands for translocator protein-18kDa such as [18F]DPA-714 are noninvasive biomarkers of glial activation, a hallmark of neuroinflammation.
Methods:
[18F]DPA-714 positron emission tomography imaging was performed in 5 baboons at baseline and 2 hours after i.m. morphine injection (1 mg/kg). Brain kinetics and metabolite-corrected input function were measured to estimate [18F]DPA-714 brain distribution.
Results:
Morphine significantly increased [18F]DPA-714 brain distribution by a 1.3 factor (P<.05; paired t test). The effect was not restricted to opioid receptor-rich regions. Differences in baseline [18F]DPA-714 binding were observed among baboons. The response to morphine predominated in animals with the highest baseline uptake.
Conclusions:
[18F]DPA-714 positron emission tomography imaging may be useful to noninvasively investigate the brain immune component of morphine pharmacology. Correlation between baseline brain distribution and subsequent response to morphine exposure suggest a role for priming parameters in controlling the neuroinflammatory properties of opioids.
Keywords: neuroinflammation, TSPO, translocator protein 18 kDa, neuroimmunopharmacology, opioid.
Introduction
Morphine is the opioid most prescribed to treat acute and chronic pain. The effects of morphine exposure on CNS have mainly been investigated through the lens of neuronal pathways and the opioid systems in particular, with special emphasis on the development of tolerance and addiction (Henriksen and Willoch, 2008; Koob and Volkow, 2010; Williams et al., 2013). Imaging studies have considerably improved our knowledge regarding the function and regulation of the opioid system in the human brain (Henriksen and Willoch, 2008). However, these studies have not fully elucidated the parameters that control the great variability in the response to opioid drugs, in terms of their analgesic and adverse effects, observed in clinical practice (Nielsen and Kreek, 2012; Williams et al., 2013).
Many preclinical studies have highlighted the interaction of morphine and other opioids with the innate immune system of the brain, especially microglial cells (Watkins et al., 2009). Morphine and its metabolites have been shown to promote microglial activation and trigger inflammatory pathways such as that of Toll-like receptor 4, with the release of proinflammatory cytokines in the brain, in a similar fashion to the classic Toll-like receptor 4 ligand, lipopolysaccharide (Due et al., 2012; Wang et al., 2012). Interestingly, microglial activation may also modulate the pharmacological actions of morphine (Hutchinson et al., 2007), including their pain-relieving properties (Watkins et al., 2009) as well as the potency of their reward and dependence effects (Coller and Hutchinson, 2012; Hutchinson et al., 2012). Indeed, modulators of glial activation such as minocycline or ibudilast were shown to enhance acute morphine analgesia (Hutchinson et al., 2007). Blockade of the actions of proinflammatory cytokines such as IL-1β, IL-6, and TNFα were shown to prevent or at least attenuate the development of tolerance induced by chronic morphine exposure. Interestingly, modulators of glial activation may also impact morphine-induced behavioral reward, withdrawal symptoms, and dopamine release in the nucleus accumbens (for review, see Coller and Hutchinson, 2012). This suggests that glial modulation may be an underexplored parameter of the variability of response to opioids in patients. Suitable tools are therefore needed to highlight and measure the neuroimmune component of opioid pharmacology in humans and evaluate its contribution to pharmacodynamics (Hutchinson and Watkins, 2014).
The most advanced approach currently available for the noninvasive investigation of glial cell activation is positron emission tomography (PET) imaging using radiolabeled ligands of the translocator protein-18kDa (TSPO). Baseline TSPO expression in the brain differs from the measurable TSPO overexpression detected in many neuroinflammatory diseases (Jacobs et al., 2012). The neuroimmune response to lipopolysaccharide-induced systemic inflammation has recently been shown using TSPO PET imaging in baboons (Hannestad et al., 2012) and humans (Sandiego et al., 2015). [18F]DPA-714, a TSPO PET radioligand, is used to study the neuroinflammatory component of CNS diseases and has reached clinical status, allowing for the clinical translation of preclinical findings (Arlicot et al., 2012; Lavisse et al., 2015).
In this study, we used [18F]DPA-714 PET imaging to investigate neuroinflammatory changes in the brain following morphine exposure in a nonhuman primate model.
Methods
Study Design
The brain distribution of [18F]DPA-714 was measured in 5 baboons at baseline and after i.m. injection of a single dose of morphine (1 mg/kg) in awake animals 2 hours before [18F]DPA-714 PET imaging.
Animals
Healthy Male Papio anubis baboons (25–30 kg, 8–10 years old) were obtained from the primatology station at Rousset-sur-Arc (France). PET studies were carried out using 5 different animals for a total of 10 experiments. Animal use procedures were in strict accordance with the recommendations of the European Community (86/609/CEE) and the French National Committee (Decret 87/848) for the care and use of laboratory animals. A resting period of 35±13 days was observed between experiments. The experimental protocol was evaluated by a local ethics committee (CETA/APAFIS#892).
Drugs, Chemicals, and Radiochemicals
Sterile morphine sulfate solution was purchased from Aguettant (Lyon, France). Propofol (200 mg/20 mL) was purchased from Fresenius Kabi (Sèvres, France). DPA-714 was labeled with fluorine-18 at its 2-fluoroethyl moiety using a tosyloxy-for-fluorine nucleophilic aliphatic substitution, following slight modifications to procedures already reported and using a commercially available GE TRACERLab FXFN synthesizer (GE Medical Systems, Buc, France) (Kuhnast et al., 2012). Typically, 10 GBq [18F]DPA-714 was obtained within 60 minutes, including high-performance liquid chromatography purification and formulation. Ready-to-inject, >99% radiochemically pure [18F]DPA-714 (formulated in physiological saline containing <10% ethanol) was obtained with 15% to 20% nondecay corrected yields and specific radioactivity at the end of radiosynthesis ranging from 37 to 148 GBq/µmol (Kuhnast et al., 2012).
PET Imaging
[18F]DPA-714 brain PET imaging was performed using an HR+ Exact positron tomograph (Siemens Healthcare, Knoxville, TN) as previously described (Saba et al., 2015). Anesthesia was maintained using an i.v. bolus of propofol (20–30 mg) followed by 10 mg/kg/h i.v. infusion under oxygen ventilation. The head of the baboon was positioned in the tomograph in such a way as to have the brain and parotid glands in the same field of view. Animals were i.v. injected with [18F]DPA-714 (226 ± 21 MBq) followed by 120 minutes dynamic PET acquisition. Physiological monitoring, including heart rate, oxygen saturation (SpO2), respiratory rate, and end-tidal CO2, was performed throughout the duration of the PET scan.
Metabolite-Corrected Input Function
During PET scanning, arterial blood samples were drawn at designated times (from 0 to 120 min). Additional arterial plasma samples obtained at 5, 15, 30, 60, 90, and 120 minutes were analyzed using a validated solid-phase extraction method to determine the fraction of unmetabolized parent [18F]DPA-714 in plasma (Peyronneau et al., 2013). For each animal, unmetabolized parent [18F]DPA-714 in plasma was expressed as the standardized uptake value (SUV=[radioactivity per mL plasma/injected radioactivity] x body weight).
PET Data Processing
Before the study, each baboon underwent an anatomical 3D T1-weighted MRI. MR images were co-registered onto PET images summed over the 120-minute acquisition. The individual co-registered MR images were warped onto a baboon T1 MR template (Black et al., 2001). Matrices were then applied to the dynamic PET images, using SPM (statistical parametric mapping, Wellcome Department of Cognitive Neurology, London, UK), MATLAB (Math Works, Natick, MA), and PMOD (PFUSION tool, version 3.7, PMOD Technologies Ltd., Zurich, Switzerland). Twelve brain structures obtained from the template MR were analyzed as volume of interest (VOI). In addition, the parotid glands were manually drawn on PET images. [18F]DPA-714 kinetics was measured in each VOI for both conditions (baseline and acute morphine treatment). Regional time-activity curves, expressed in SUV units vs time, were generated by calculating the mean radioactivity in the 12 VOIs, whole brain, and parotid glands. Pharmacokinetic modeling was performed to describe the PET data and compare [18F]DPA-714 brain distributions. [18F]DPA-714 volume of distribution (VT; mL/cm3) was estimated in all VOIs using the Logan graphical method, considering arterial plasma corrected for metabolites as the input function (PMOD software, Zurich, Switzerland). Differences between groups were assessed using a 1-tailed paired t test (GraphPad Prism software). The threshold for statistical significance was set at P<.05.
Results
Arterial Input Function
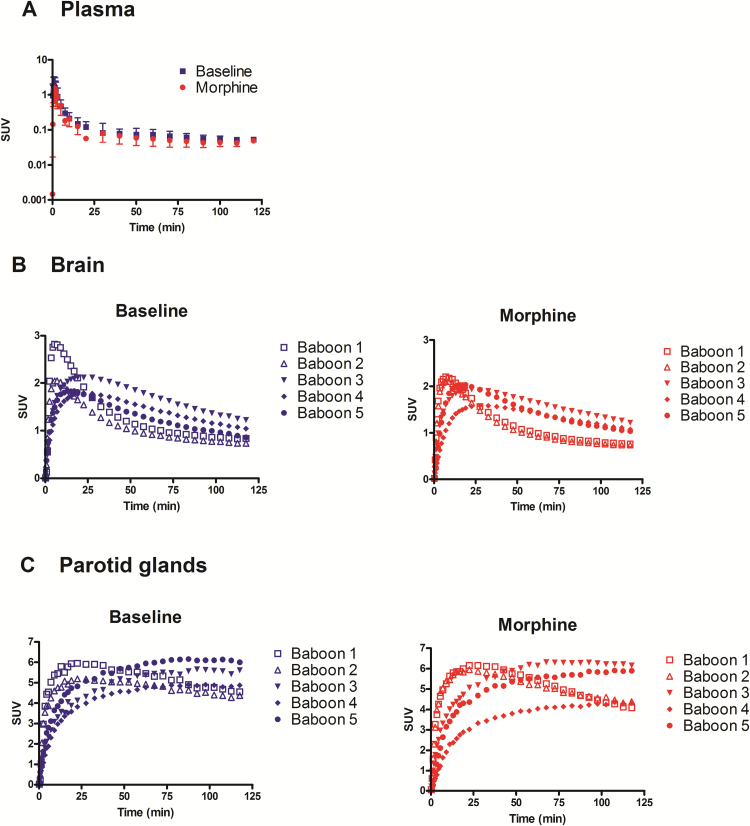
The plasma kinetics of parent [18F]DPA-714 was described by a distribution phase followed by an elimination phase that started 25 minutes after injection (Figure 1a). Mean SUVs measured from 40 to 120 minutes (elimination phase) were not different between baseline (0.06 ± 0.01) and acute morphine conditions (0.05 ± 0.01).
Figure 1.
[18F]DPA-714 positron emission tomography (PET) kinetics in the plasma, brain, and parotid glands. Animals were injected with 226 ± 21 MBq [18F]DPA-714 i.v. followed by 120 minutes PET scanning. The plasma kinetics of unmetabolized parent [18F]DPA-714 in the presence or absence of morphine (1 mg/kg: i.m.) is shown in A (mean ± SD; n=5). Individual PET kinetics of radioactivity in the brain and parotid glands are shown in B and C, respectively. Data are shown as standardized uptake values (SUV).
PET Imaging
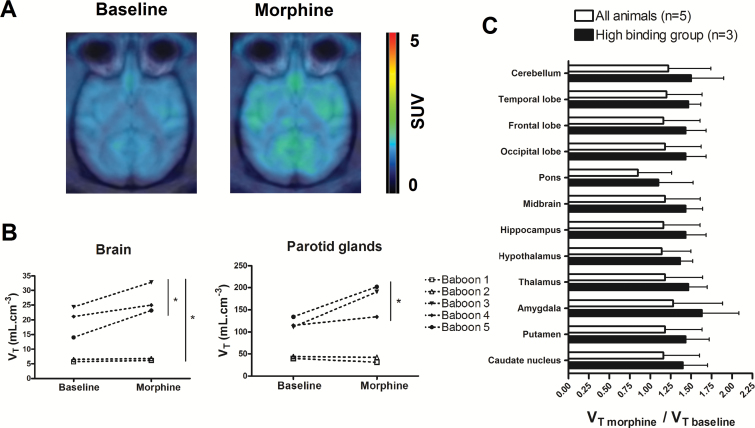
Figure 1b displays the individual brain kinetics of [18F]DPA-714 under the conditions tested. Two distinct baseline time-activity curves profiles could be distinguished among the 5 baboons. In baboons 1 and 2, baseline brain radioactivity increased rapidly (Tmax ~ 7 min), followed by fast elimination from the brain. In the 3 other animals, radioactivity peaked later (Tmax ~ 20 min) with a much slower decrease in the brain. The estimation of brain [18F]DPA-714 VT confirmed this observation with VT baseline=5.7 and 6.5 mL.cm-3 in the low-binding group (n=2) and 19.8 ± 5.3 mL.cm-3 in the high-binding group (n=3). In morphine-treated animals, [18F]DPA-714 VT (VT morphine=18.8 ± 11.8 mL.cm-3) was significantly higher than VT baseline (14.3±8.4 mL.cm-3; P<.05, n=5). Regional analysis and ANOVA (P>.05) showed that the response to morphine, estimated by VT morphine/VT baseline, was not different between the different brain regions (Figure 2).
Figure 2.
[18F]DPA-714 brain distribution obtained before and after morphine administration in baboons. Representative [18F]DPA-714 positron emission tomography (PET) images obtained at baseline and 2 hours after morphine administration (1 mg/kg; i.m.) are shown in A. These are summed (0 to 120 minutes) and standardized uptake value (SUV)-normalized PET images obtained in an animal of the high-binding group (baboon 5). Estimated distribution volume (VT) of [18F]DPA-714 in the whole brain and parotid glands of each animal are shown in B. The ratio of VT morphine to VT baseline (means ± SD) in different brain regions is shown in C for all animals (n = 5) and for animals of the high-binding group only (n = 3).
Interestingly, the increase in VT in the low-binding group was modest compared with the high-binding group. Representative PET images obtained in the high-binding group are shown in Figure 2. The increase in VT was significant even when only animals from the high-binding group were considered (P<.05, n=3), with no regional difference between brain regions (P>.05, n=3) (Figure 2).
In the parotid glands, a TSPO-rich region, differences in radioactivity kinetics between the low- and high-binding groups were also observed under both baseline and acute morphine conditions (Figure 1c). Compared with baseline (VT baseline=88.8±43.7 mL.cm-3), morphine exposure resulted in higher VT (VT morphine=121.6±78 mL.cm-3), although this difference was not statistically significant when including all 5 animals (P>.05). There was no increase in VT in the low-binding group, but the increase was statistically significant when only animals from the high-binding group were included (n=3, P<.05) (Figure 2). There was a significant correlation between VTs measured in the brain and VTs estimated in the parotid glands for the 10 [18F]DPA-714 PET experiments (r = 0.88, P<.001, linear regression).
Discussion
TSPO expression in the brain, estimated by [18F]DPA-714 VT in nonhuman primates, was significantly increased after acute morphine exposure. The effect was observed globally across all brain regions and was not restricted to opioid receptor-rich regions (Koob and Volkow, 2010).
Several ex vivo studies, mainly performed in rodents, have shown the morphine-induced upregulation of microglial and/or astrocytic activation markers as well as the morphine-induced upregulation and/or release of proinflammatory cytokines and chemokines. In vitro studies have also reported the direct actions of opioids on glia (Watkins et al., 2009). Our results suggest that the neuroimmune response to acute morphine exposure can be detected and quantified in vivo by PET imaging using the TSPO ligand [18F]DPA-714.
In animals from the high-binding group, the increase in [18F]DPA-714 binding was also significant in the parotid glands, a peripheral region rich in TSPO. This suggests that the immune response to morphine is not restricted to the brain but may involve peripheral processes.
Interestingly, we show that the response to morphine exposure in the brain and parotid glands can be predicted from the baseline VT of [18F]DPA-714. Animals in the group with low binding at baseline showed a negligible response to acute morphine compared with animals in the high-binding group, in which the increase in VT was significant. This inter-individual difference in baseline [18F]DPA-714 binding appears to be an indicator of the previous priming of the immune response to morphine. From a methodological point of view, the absence of response in the low-binding group indicates that the effect observed in the high-binding group is not due to any pharmacokinetic or TSPO-independent interaction between morphine and [18F]DPA-714 kinetics in the brain. To the best of our knowledge, such variability in the baseline PET kinetics of [18F]DPA-714 has not been reported when considering animals scanned in the same PET center but can be observed when comparing for baboons from different centers (James et al., 2008; Saba et al., 2015). Differences between baboons in the baseline brain kinetics of the TSPO radiotracer [11C]PBR28 have been reported (Hannestad et al., 2012). Similar variability in baseline [18F]DPA-714 brain distribution (VT=6.58±4.13 mL.cm-3) could be observed in healthy male cynomolgus macaques (Lavisse et al., 2014).
Further investigations are needed to assess whether the differences in baseline [18F]DPA-714 binding and response to morphine exposure correspond to the known genetic variability observed in humans (Guo et al., 2013) or to environmental factors linked to an increased sensitivity of microglial function (Williamson et al., 2016). We did not identify any parameter (origin, age, nature/number of previous anesthesia, or treatments) that could explain the disparity between the low- and high-binding groups, but our results suggest that they may be of prime importance for the interpretation of TSPO PET data imaging in humans and nonhuman primates. Moreover, long-term changes in microglial function have been associated with an increased risk of drug-induced reinstatement of addiction behavior in adulthood (Schwarz and Bilbo, 2013). One could thus hypothesize that baseline [18F]DPA-714 kinetics and/or response to morphine-induced proinflammatory stimuli would predict the variability in pharmacodynamics and tolerance.
Acute morphine exposure increases the binding of [18F]DPA-714 to the brain in nonhuman primates. The effect predominated in animals with the highest baseline uptake, suggesting a role for priming parameters that remain to be identified in controlling the neuroimmune response to morphine.
Statement of Interest
None.
Acknowledgments
We thank Vincent Brulon and Thierry Lekieffre for helpful technical assistance.
This work was supported by a public grant overseen by the French National research Agency (ANR) as part of the Investissement d’Avenir program through the Lidex-PIM project funded by the IDEX Paris-Saclay, ANR-11-IDEX-0003-02.
References
- Arlicot N, Vercouillie J, Ribeiro M-J, Tauber C, Venel Y, Baulieu J-L, Maia S, Corcia P, Stabin MG, Reynolds A, Kassiou M, Guilloteau D. (2012) Initial evaluation in healthy humans of [18F]DPA-714, a potential PET biomarker for neuroinflammation. Nucl Med Biol 39:570–578. [DOI] [PubMed] [Google Scholar]
- Black KJ, Snyder AZ, Koller JM, Gado MH, Perlmutter JS. (2001) Template images for nonhuman primate neuroimaging: 1. Baboon. NeuroImage 14:736–743. [DOI] [PubMed] [Google Scholar]
- Coller JK, Hutchinson MR. (2012) Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther 134:219–245. [DOI] [PubMed] [Google Scholar]
- Due MR, Piekarz AD, Wilson N, Feldman P, Ripsch MS, Chavez S, Yin H, Khanna R, White FA. (2012) Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling. J Neuroinflammation 9:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Colasanti A, Owen DR, Onega M, Kamalakaran A, Bennacef I, Matthews PM, Rabiner EA, Turkheimer FE, Gunn RN. (2013) Quantification of the specific translocator protein signal of 18F-PBR111 in healthy humans: a genetic polymorphism effect on in vivo binding. J Nucl Med 54:1915–1923. [DOI] [PubMed] [Google Scholar]
- Hannestad J, Gallezot J-D, Schafbauer T, Lim K, Kloczynski T, Morris ED, Carson RE, Ding Y-S, Cosgrove KP. (2012) Endotoxin-induced systemic inflammation activates microglia: [11C]PBR28 positron emission tomography in nonhuman primates. NeuroImage 63:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen G, Willoch F. (2008) Imaging of opioid receptors in the central nervous system. Brain 131:1171–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, et al. (2012) Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 32:11187–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. (2007) Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal 7:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Watkins LR. (2014) Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacology 76Pt B:218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AH, Tavitian B, INMiND consortium (2012) Noninvasive molecular imaging of neuroinflammation. J Cereb Blood Flow Metab 32:1393–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ML, Fulton RR, Vercoullie J, Henderson DJ, Garreau L, Chalon S, Dolle F, Costa B, Selleri S, Guilloteau D, Kassiou M. (2008) DPA-714, a new translocator protein-specific ligand: synthesis, radiofluorination, and pharmacologic characterization. J Nucl Med 49:814–822. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacol 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnast B, Damont A, Hinnen F, Catarina T, Demphel S, Le Helleix S, Coulon C, Goutal S, Gervais P, Dollé F. (2012) [18F]DPA-714, [18F]PBR111 and [18F]FEDAA1106-selective radioligands for imaging TSPO 18 kDa with PET: automated radiosynthesis on a TRACERLAb FX-FN synthesizer and quality controls. Appl Radiat Isot 70:489–497. [DOI] [PubMed] [Google Scholar]
- Lavisse S, Inoue K, Jan C, Peyronneau MA, Petit F, Goutal S, Dauguet J, Guillermier M, Dollé F, Rbah-Vidal L, Camp NV, Aron-Badin R, Remy P, Hantraye P. (2014) [18F]DPA-714 PET imaging of translocator protein TSPO (18 kDa) in the normal and excitotoxically-lesioned nonhuman primate brain. Eur J Nucl Med Mol Imaging 42:478–494. [DOI] [PubMed] [Google Scholar]
- Lavisse S, García-Lorenzo D, Peyronneau M-A, Bodini B, Thiriez C, Kuhnast B, Comtat C, Remy P, Stankoff B, Bottlaender M. (2015) Optimized Quantification of translocator protein radioligand 18F-DPA-714 uptake in the brain of genotyped healthy volunteers. J Nucl Med 56:1048–1054. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Kreek MJ. (2012) Common and specific liability to addiction: approaches to association studies of opioid addiction. Drug Alcohol Depend 123:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyronneau M-A, Saba W, Goutal S, Damont A, Dollé F, Kassiou M, Bottlaender M, Valette H. (2013) Metabolism and quantification of [(18)F]DPA-714, a new TSPO positron emission tomography radioligand. Drug Metab Dispos 41:122–131. [DOI] [PubMed] [Google Scholar]
- Saba W, Goutal S, Kuhnast B, Dollé F, Auvity S, Fontyn Y, Cayla J, Peyronneau M-A, Valette H, Tournier N. (2015) Differential influence of propofol and isoflurane anesthesia in a non-human primate on the brain kinetics and binding of [(18)F]DPA-714, a positron emission tomography imaging marker of glial activation. Eur J Neurosci 42:1738–1745. [DOI] [PubMed] [Google Scholar]
- Sandiego CM, Gallezot J-D, Pittman B, Nabulsi N, Lim K, Lin S-F, Matuskey D, Lee J-Y, O’Connor KC, Huang Y, Carson RE, Hannestad J, Cosgrove KP. (2015) Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci 112:12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. (2013) Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction. J Neurosci 33:961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H. (2012) Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci 109:6325–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF. (2009) The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci 30:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, Zastrow M von, Schulz S, Koch T, Evans CJ, Christie MJ. (2013) Regulation of µ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65:223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LL, McKenney EA, Holzknecht ZE, Belliveau C, Rawls JF, Poulton S, Parker W, Bilbo SD. (2016) Got worms? Perinatal exposure to helminths prevents persistent immune sensitization and cognitive dysfunction induced by early-life infection. Brain Behav Immun 51:14–28. [DOI] [PubMed] [Google Scholar]