Abstract
Background
Studies in child and adolescent offspring of patients with schizophrenia or bipolar disorders may help understand the influence of neurodevelopmental factors on the premorbid phenotype of these disorders.
Aims
To assess whether a combination of neurodevelopmental factors discriminates between young offspring of patients with schizophrenia (SzO) or bipolar disorder (BpO) and community controls (CcO). To assess the association between these factors and rates of psychiatric diagnoses in high risk (HR) youth.
Methods
One hundred thirty-three HR offspring (47 SzO and 86 BpO) and 84 CcO, aged 6–17, underwent cross-sectional clinical, neurocognitive, and structural neuroimaging assessment. Information on perinatal events and early childhood development was also obtained. General linear mixed models were performed to assess group discrimination and association with lifetime axis I psychiatric disorders.
Results
Multivariate analyses revealed that greater neurological soft signs (NSS), less total grey matter volume (GMV) and a higher frequency of obstetric complications discriminated HR offspring from CcO. When comparing each group individually, greater NSS and a higher frequency of obstetric complications discriminated SzO from CcO, and BpO from CcO, while lower intelligence also discriminated SzO from CcO and from BpO. Within HR offspring, lower intelligence and less total GMV were associated with lifetime incidence of psychiatric disorders.
Conclusions
Both SzO and BpO showed evidence of neurodevelopmental insult, although this may have a greater impact in SzO. Lower intelligence and less total GMV hold potential as biomarkers of risk for psychiatric disorders in HR youth.
Keywords: schizophrenia, bipolar disorder, relatives, neurodevelopmental disorders, neuroimaging
Introduction
Evidence of both a phenomenological and etiological overlap between schizophrenia and bipolar disorder1 has challenged the traditional diagnostic categorization of the 2 conditions.2 However, one of the remaining areas of controversy concerns the developmental trajectories preceding clinical onset of these disorders.3,4 The neurodevelopmental hypothesis of schizophrenia was put forward in the late 20th century, and suggested that a combination of genetic and environmental factors led to neurobiological changes in the pre- and perinatal period, increasing risk for the disorder later in life.5–7 Since then, a large evidence base has been accumulated in support of this model in schizophrenia, from epidemiological, cognitive, imaging, and molecular studies.8 With the turn of the 21st century a number of authors sought to investigate whether neurodevelopmental abnormalities also played a role in the pathophysiology of bipolar disorders,9,10 however, this line of enquiry has yielded equivocal findings. Substantial uncertainty remains as to the impact and timing of developmental disruption in bipolar disorder,11 and its similarities and differences with schizophrenia.
The study of child and adolescent offspring of probands with schizophrenia or bipolar disorder has the potential to help increase understanding on the premorbid phenotype of these disorders. Familial high-risk (HR) studies are underpinned by the fact that offspring of probands with these disorders carry a 10%–15% risk of developing the disease,12 and therefore constitute enriched samples for identifying inherited gene and gene–environment effects which may impact on risk for the disorders. An additional advantage of this kind of sample is that it provides the opportunity to assess changes in brain and behavior during what is considered a “critical” neurodevelopmental window.13
Studies ranging back to the 1950s have yielded evidence of developmental risk factors and antecedents in offspring of patients with schizophrenia (SzO), including obstetric and perinatal complications, delayed psychomotor milestones or greater neurological soft signs (NSS).14–17 In offspring of patients with bipolar disorder (BpO), 3 studies so far have provided mixed evidence of an association between adverse perinatal events and later affective disorders, however they failed to employ a control group.18–20 We are aware of no other studies reporting on early childhood development or neurological abnormalities in young BpO. A reduction in intelligence has emerged consistently across SzO samples,21 while in BpO, despite reports of specific deficits in terms of executive function, memory, and attention,22,23 evidence regarding general intelligence is equivocal.23,24 With the advent of magnetic resonance imaging, convergent findings have pointed to global and localized grey matter volume (GMV) reduction in young SzO25–28 but not in BpO,29,30 although there have been reports on regional volumetric increases in healthy BpO,31 and decreases in BpO with mood disorders.32
Birth cohort- and population-based studies have added to the evidence obtained from familial HR designs, and have confirmed associations between the presence of perinatal events, delayed development and reduced intelligence with later schizophrenia outcomes.33–37 Such studies have provided more limited evidence of associations between perinatal events, motor function and cognition and later bipolar disorders, with overall weaker effects.33–35,38,39 Advanced paternal age, which has been associated with delayed development40 and an increased risk of autism,41 has also been associated with higher rates of schizophrenia,42 and in some,43,44 yet not all45 studies, of bipolar disorder. More recently, advanced maternal age has also been associated with risk of schizophrenia,46 while there is limited evidence for this association in bipolar disorder.43–45 Although the data so far suggests some degree of specificity of developmental factors for schizophrenia,33–35 the paucity of studies assessing both conditions prospectively limits capacity for drawing conclusions.4
In this context, we set out to examine whether a range of “neurodevelopmental factors” discriminated between child and adolescent SzO or BpO and offspring of community controls (CcO). We focused on a combination of risk factors and antecedents considered to index neurodevelopmental disruption, some of which were reported by caregivers (parental age, obstetric complications, birth weight, difficulties in acquisition of language, motor, reading/writing skills, and elimination disorders) and others measured in offspring (NSS, general intelligence and global and lobar measures of GMV). We also set out to assess the potential clinical significance of these factors: although child and adolescent SzO and BpO have yet to reach the peak age of onset of the bipolar disorders and schizophrenia, they present with a range of mental health conditions at rates above those of the general population.47 It has been demonstrated that the presence of a nonspecific mental health disorder in youth is a risk factor in itself for later major psychiatric outcomes.48–50 Therefore, we sought to investigate whether the presence of neurodevelopmental factors were associated with psychiatric diagnoses during childhood and adolescence.
Methods
Sample
The study was conducted in the Child and Adolescent Psychiatry Department of the Hospital Clinic of Barcelona, Spain and Hospital General Gregorio Marañón, Spain. The Department of Psychiatry and Psychology of the Hospital Clinic and the Adult Psychiatry Unit of Hospital General Universitario Gregorio Marañón provided support in terms of recruitment of proband parents. The protocol was approved by the ethics review board at each site. All participants provided written informed consent/assent, parents provided informed consent and offspring provided assent. Further details on this sample can be found in Sanchez et al.51
Patients with a diagnosis of schizophrenia or bipolar disorder with offspring aged 6–17 years were identified and offered to participate in the study. All parents were outpatients at the time of recruitment with the exception of 2 mothers with schizophrenia who were admitted to chronic inpatient units. The exclusion criteria for proband parents were: intellectual disability and drug or medically induced psychosis or mania. Exclusion criteria for offspring included intellectual disability, head injury with loss of consciousness, or severe neurological conditions. Community control parents were recruited through advertisements posted in primary health care centers and other community locations within the same geographical area as the patients. The exclusion criteria were intellectual disability, severe neurological illness, and personal or first-degree family history of schizophrenia or bipolar spectrum disorders. All offspring of community control parents aged 6–17 years were invited to participate in the study; exclusion criteria were the same as for HR offspring.
Forty-seven SzO, 86 BpO (60 parents with bipolar I and 26 with bipolar II disorders; 39 BpO [45.3%] had a parent with a history of psychotic symptoms), and 84 CcO were included.
Clinical and Neurocognitive Assessment
The assessment of the participating family was carried out at the outpatient service of the Child and Adolescent Psychiatry Department of each hospital. The families received compensation for their time and travel expenses. Parental and offspring interviews were conducted by different team members, each blind to the others’ assessment. Clinical diagnoses in both parents were assessed by adult psychiatrists with the Structured Interview for DSM-IV disorders,52 Spanish version. Offspring were assessed by child and adolescent psychiatrists using The Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime,53 Spanish version. Socioeconomic status was assessed with the Hollingshead scale,54 where highest parental educational and employment status were registered. Obstetric complications were assessed with the Lewis Scale55 which assesses events occurring both during pregnancy (such as infections—syphilis or rubella, Rh incompatibility, pre-eclampsia or bleeding) and during labor (premature rupture of membranes, twin births, mechanical complications of umbilical cord, instrumentalization, or cesarean) and need for an incubator or postpartum reanimation. Presence of any reported complication was categorized dichotomously. Parents’ age at birth was also registered. Information on difficulties/delay in attainment of developmental milestones was obtained from parents/caregivers, including psychomotor, language, and reading/writing skills; elimination disorders were evaluated using the Kiddie-SADS-PL. These were categorized jointly in a dichotomous variable as “childhood developmental difficulties.” Pubertal development was assessed using a self-report pictorial questionnaire according to Tanner criteria.56
Intelligence was assessed using the Spanish version of the Wechsler Intelligence Scale for Children-Fourth Edition (WISCIV).57 This intelligence battery is designed to evaluate intellectual abilities in children and adolescents aged between 6 and 16 years old. WISC-IV provides 4 composite scores: verbal comprehension, perceptual reasoning, working memory, and processing speed. Previous studies have demonstrated that the working memory and processing speed indices may be impaired in HR offspring,58,59 therefore, in order to avoid the influence of each of these domains on the full scale intelligence quotient, the General Ability Index, derived from the Verbal Comprehension and Perceptual Reasoning indices was used as an index of intelligence level.60 This index will be referred to as “general intelligence.”
The presence and severity of NSS were assessed using the Neurological Evaluation Scale.61 This scale consists of 26 items (14 of which are scored separately right/left body) covering “Sensory integration,” “Motor coordination,” and “Sequencing of complex motor acts.” Higher total scores reflect greater neurological impairment. Neurocognitive assessments were performed by neuropsychologists with experience in child and adolescent population, blinded to parental status.
Neuroimaging Processing and Analysis
Details of image acquisition are provided in Supplement 1. Analyses were performed employing Statistical Parametric Mapping version 8, in a Matlab R2010a environment. Images were reoriented according to the anterior–posterior commissure line. Given the age range of the sample, customised tissue probability maps were created from the control group (n = 83), and were employed for tissue segmentation. The same maps were applied to the whole sample, as performed in a previous study.62 An inter-site compatibility study62 confirmed a high intraclass correlation coefficient for total GMV (r = .98) among healthy participants scanned at the 2 sites. We also set out to analyze GMV for brain structures most commonly implicated in offspring of probands with schizophrenia or bipolar disorders.26–28,31 Parcellations defined by the Automated Anatomical Labeling atlas63 corresponding to frontal, temporal, parietal lobes, and hippocampal–parahippocampal complex were corregistered to the anatomical space of each patient using their grey matter probability map. Corregistration was performed using the inverse nonlinear deformations obtained with Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL).64 All images were inspected for anatomical accuracy by an experienced technician (JP). GMV for each brain region of interest was extracted and retained for analyses.
Statistical Analysis
First, we examined the effect of group (SzO, BpO, CcO) on sociodemographic and clinical variables employing general linear mixed models and binary logistic regression, with family membership and site as random variables. For dichotomous dependent outcomes, the odds ratio and 95% confidence intervals (CIs) were also computed. The effect of group on factors considered to be associated with neurodevelopment—parental age, obstetric complications, birth weight, childhood developmental difficulties, NSS, general intelligence, and measures of GMV—were also assessed. Where significant, age, sex, and total intracranial volume (for GMV), were included. Given its association with group (decreased status in schizophrenia patients65) the effect of socioeconomic status was also examined: analyses of clinical and neurodevelopmental variables are presented with and without including this variable in the models.
Neurodevelopmental factors showing an effect of group (P-value < .10) were then included as explanatory variables in a multivariate binary logistic regression model, aimed at discriminating groups at a pairwise level (all HR offspring vs CcO, SzO vs CcO, BpO vs CcO, SzO vs BpO). We used a backward stepwise selection procedure, whereby at each step the least significant variable from the model was discarded, until all variables in the model reached P-values <.10. In order to avoid overfitting, the number of variables that could enter the multivariate model was limited using the P < m/10 rule.66
We also set out to assess whether neurodevelopmental factors were associated with a lifetime history of axis I psychopathology in HR offspring: the same variables were included in a multivariate binary logistic regression model following a comparable procedure to the one above, with lifetime axis I psychopathology categorized dichotomously as outcome variable. For this analysis, given the low incidence of cases, the 2 HR groups were considered jointly.
In order to evaluate the performance of the models we used received operational curve (ROC) methodology, computed using the predicted probability values of each model. The area under the curve (AUC) is reported for each model.
A 2-sided type I error of 5% was used for all tests. Statistical analyses were performed with PASW Statistics20 and R v.3.3.1.
Results
Sociodemographic and clinical information are depicted in table 1. Groups were comparable in terms of sex, age, and percentage of pre-pubertal children. SzO had lower socioeconomic status than CcO and than BpO. SzO had higher rates of lifetime axis I disorders than CcO and than BpO, and BpO had higher rates than CcO. Specifically, SzO exhibited higher rates of attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder than CcO and than BpO. When controlling for socioeconomic status, differences in rates of lifetime axis I disorders and ADHD between SzO and CcO, and in ADHD in SzO relative to both BpO and CcO, remained unchanged, while the effects of oppositional defiant disorder achieved trend-level, and differences in lifetime axis I disorders between SzO and BpO became nonsignificant. No participant met criteria for psychosis or a bipolar spectrum disorder. Rates of psychopharmacological treatment in SzO were higher than in CcO and BpO (medications consisted of stimulants and selective serotonin reuptake inhibitors, with the exception of one BpO with a history of treatment with risperidone 0.25 mg/day).
Table 1.
Sociodemographic and Clinical Information
| SzO | BpO | CcO | F | P | SzO Vs CcO | BpO Vs CcO | SzO Vs BpO | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 47 | N = 86 | N = 84 | ||||||||||||
| Least Square Means (SE) | OR/Beta [95% CI] | t | P | OR/Beta [95% CI] | t | P | OR/Beta [95% CI] | t | P | |||||
| Age (years) | 11.3 (0.49) | 12.4 (0.37) | 12.2 (0.44) | 2.0 | .13 | |||||||||
| Tanner stage (% prepubertal) | 53.7% (7.6) | 37.0% (5.5%) | 46.2% (6.5%) | 1.8 | .16 | |||||||||
| Sex (%♀) | 43.6% (7.9) | 50.5% (6.1) | 61.3% (6.9) | 1.9 | .14 | |||||||||
| Socioeconomic status | 33.0 (2.0) | 50.6 (1.4) | 51.1 (1.5) | 32.7 | <.001 | Beta = −18.1 [−22.9 to −13.3] | −7.4 | <.001 | Beta = −0.42 [−4.5 to 3.6] | −.37 | .71 | Beta = −17.7 [−22.5 to −12.9] | −7.2 | <.001 |
| Lifetime axis I diagnosisa | 50.1% (12.2) | 31.7% (9.7) | 16.1% (6.5) | 8.1 | <.001 | OR = 5.4 [2.4 to 12.2] | 4.1 | <.001 | OR = 2.5 [1.2 to 5.2] | 2.5 | .014 | OR = 2.2 [1.0 to 4.5] | 2.1 | .041 |
| Attention deficit hyperactivity disorderb | 44.3% (8.9) | 16.9% (5.0) | 3.4% (2.5) | 10.4 | <.001 | OR = 22.8 [4.9 to 106.1] | 4.0 | <.001 | OR = 5.8 [1.2 to 27.4] | 2.2 | .025 | OR = 3.9 [1.7 to 9.2] | 3.1 | .002 |
| Mood disorders1 | 6.2% (4.6) | 8.4% (5.3) | 4.9% (3.5) | 0.50 | .60 | |||||||||
| Anxiety disorders2 | 18.1% (7.9) | 11.3% (5.1) | 6.4% (3.4) | 2.2 | .12 | |||||||||
| Oppositional defiant disorderc | 14.9% (5.2) | 4.2% (2.2) | 3.5% (2.0) | 3.3 | .037 | OR = 4.8 [1.2 to 19.9] | 2.2 | .030 | OR = 1.2 [0.25 to 5.8] | 0.23 | .82 | OR = 4.0 [1.1 to 15.2] | 2.05 | .041 |
| Other axis I disorders3 | 7.8% (4.6) | 2.8 % (1.8) | 4.8% (2.4) | 0.64 | .53 | |||||||||
| Psychotropic treatment | 27.7% (6.5) | 7.6% (2.9) | 2.9% (1.8) | 8.3 | <.001 | OR = 12.96 [3.1 to 54.6] | 3.5 | .001 | OR = 2.77 [0.61 to 12.6] | 1.3 | .19 | OR = 4.7 [1.7 to 13.1] | 3.0 | .003 |
Note: SzO, offspring of probands with schizophrenia; BpO, offspring of probands with bipolar disorder; CcO, offspring of community controls; OR, odds ratio; CI, confidence interval. Generalized linear mixed model analyses including family and site as random variables. Diagnoses were elicited using DSM-IV criteria (Kiddie-SADS).
1Includes major depressive disorder, adjustment disorders, and dysthimia.
2Includes generalized anxiety disorder, simple phobia, panic disorders, separation anxiety disorder, and obsessive compulsive disorder.
3Includes tic disorders and eating disorders. Given group differences in socioeconomic status, rates of lifetime axis I diagnoses were re-analyzed controlling for socioeconomic status:
a F = 5.0, P = .008. SzO vs CcO: OR = 3.6 [1.4 to 9.2], t = 2.6, P = .010. BpO vs CcO: OR = 2.5 [1.2 to 5.1], t = 2.4, P = .016. SzO vs BpO: ns.
b F = 8.1, P < .001. SzO vs CcO: OR = 22.5 [4.5 to 113.2], t = 3.8, P < .001. BpO vs CcO: OR = 5.8 [1.2 to 27.4], t = 2.5, P = .026. SzO vs BpO: OR = 3.8 [1.4 to 10.3], t = 2.7, P = .008.
c F = 2.1, P = .13. SzO vs CcO: OR = 4.3 [0.90 to 21.4], t = 1.8, P = .070. BpO vs CcO: ns. SzO vs BpO: OR 3.6 [0.80 to 16.3], t = 1.7, P = .092.
Group differences in neurodevelopmental factors are displayed in table 2. There were group effects in obstetric complications and NSS (SzO more than CcO and BpO more than CcO), general intelligence (SzO less than CcO and than BpO) and total GMV (SzO less than CcO). There were also group effects in childhood developmental difficulties (SzO more than CcO and than BpO); assessment of this measure as a continuous variable yielded similar group effects (F = 8.8, P < .001). There were no significant effects in either parental age or birth weight. There was an effect of socioeconomic status on rates of obstetric complications and general intelligence, however, inclusion of this variable in the model did not change significant group effects.
Table 2.
Neurodevelopmental Factors by Group
| SzO | BpO | CcO | F | P | SzO Vs CcO | BpO Vs CcO | BpO Vs SzO | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Least Square Means (SE) | OR/Beta [95% CI] | t | P | OR/Beta [95% CI] | t | P | OR/Beta [95% CI] | t | P | |||||
| Paternal age at birth | 35.6 (0.74) | 35.1 (0.54) | 34.0 (0.55) | 1.8 | .17 | |||||||||
| Maternal age at birth | 31.1 (0.62) | 30.7 (0.46) | 31.8 (0.46) | 1.6 | .21 | |||||||||
| Obstetric complicationsa | 65.9% (6.9) | 56.8% (5.3) | 38.1% (5.3) | 5.6 | .004 | OR = 3.1 [1.5 to 6.6] | 3.0 | .003 | OR = 2.2 [1.2 to 4.2] | 2.6 | .010 | OR = 1.4 [0.70 to 2.9] | 0.88 | .38 |
| Birth weight | 3.23 (0.12) | 3.17 (0.10) | 3.29 (0.11) | 0.76 | .47 | |||||||||
| Childhood developmental difficulties* | 44.6% (7.7) | 23.5% (4.7) | 18.6% (4.4) | 4.9 | .008 | OR = 3.5 [1.5 to 8.0] | 3.0 | .003 | OR = 1.3 [0.6 to 2.8] | 0.78 | .44 | OR = 2.6 [1.2 to 5.7] | 2.4 | .017 |
| Neurological soft signs | 14.7 (3.3) | 13.2 (3.3) | 8.8 (3.5) | 12.4 | <.001 | Beta = 5.8 [3.4 to 8.3] | 4.7 | <.001 | Beta = 4.3 [2.0 to 6.6] | 3.7 | <.001 | Beta = 1.7 [−1.1 to 4.44] | 1.2 | .23 |
| General intelligenceb | 92.3 (2.0) | 104.8 (1.5) | 106.9 (1.5) | 22.6 | <.001 | Beta = −14.7 [−19.1 to −10.2] | −6.5 | <.001 | Beta = −2.1 [−5.8 to 1.6] | −1.1 | .27 | Beta = −12.6 [8.1 to 17.0] | −5.6 | .<001 |
| Grey matter volume | ||||||||||||||
| Total | 759.1 (6.4) | 762.9 (6.2) | 765.8 (6.4) | 4.4 | .014 | Beta = −6.7 [−11.3 to −2.2] | −2.95 | .003 | Beta = −2.9 [−6.9 to 1.0] | −1.5 | .14 | Beta = −3.8 [−8.3 to 0.67] | −1.7 | .095 |
| Frontal lobe | 143.0 (4.4) | 143.3 (4.4) | 144.3 (4.4) | 0.91 | .40 | |||||||||
| Temporal lobe | 123.5 (2.2) | 123.2 (2.1) | 123.8 (2.2) | 0.30 | .74 | |||||||||
| Parietal lobe | 91.9 (1.1) | 92.8 (1.0) | 93.5 (1.0) | 1.5 | .23 | |||||||||
| Hippocampus/ parahippocampal gyrus | 13.4 (0.22) | 13.5 (0.20) | 13.5 (0.22) | 0.24 | .78 | |||||||||
Note: SzO, offspring of probands with schizophrenia; BpO, offspring of probands with bipolar disorder; CcO, offspring of community controls; OR, odds ratio; CI, confidence interval. Generalized linear mixed model analyses including family and site as random variables adjusted by age, gender, and total intracranial volume (for grey matter volume) where significant. 1Includes difficulties reported in acquisition of language, motor, reading/writing skills, and elimination disorders. Socioeconomic status had an effect on obstetric complications and general intelligence. When controlling for socioeconomic status in group analyses:
a F = 4.6, P = .011. SzO vs CcO: OR = 2.8 [1.2 to 6.5], t = 2.4, P = .016. BpO vs CcO: OR = 2.25 [1.2 to 4.2], t = 2.6, P = .010. SzO vs BpO: ns.
b F = 6.8, P = .001. SzO vs CcO: Beta = −8.7 [−13.5 to −4.1], t = 3.7, P < .001 BpO vs CcO: Beta = −2.0 [−5.5 to 1.5], t = −1.1, P = .27 SzO vs BpO: Beta = −6.8 [−11.5 to −2.1], t = −2.9, P = .005.
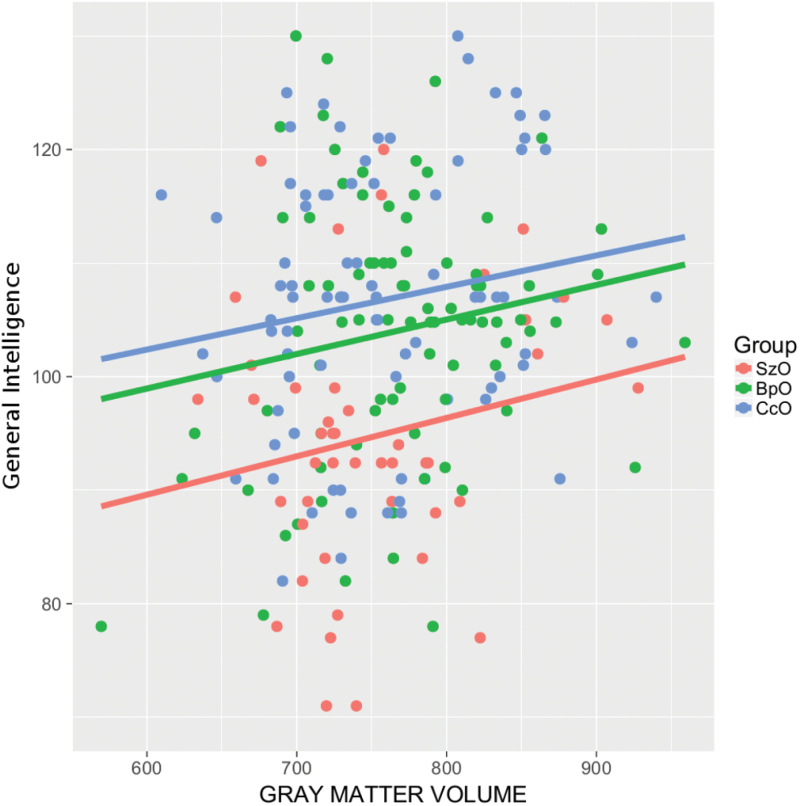
Multivariate analysis (table 3) revealed that, when considering HR offspring jointly, these were discriminated from CcO by obstetric complications, NSS and total GMV (AUC = 0.85; 95% CI = [0.80–0.90], P < .001). Obstetric complications, NSS and general intelligence distinguished SzO from CcO (0.88 [0.82–0.94], P < .001), obstetric complications and NSS discriminated BpO from CcO (0.85 [0.80–0.90], P < .001), and general intelligence distinguished SzO from BpO (0.82 [0.75–0.89], P < .001). Childhood developmental difficulties did not contribute significantly to any of the above models. For the sake of illustrating the results, general intelligence and GMV are plotted for each of the 3 groups in figure 1.
Table 3.
Multivariate Group Discrimination
| All High Risk Offspring Vs CcO | SzO Vs CcO | BpO Vs CcO | SzO Vs BpO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR [95% CI] | t | P | OR [95% CI] | t | P | OR [95% CI] | t | P | OR [95% CI] | t | P | |
| Obstetric complications | 2.8 [1.4–5.5] | 2.9 | .004 | 3.2 [1.3–8.5] | 2.4 | .017 | 2.4 [1.1–5.1] | 2.2 | .028 | |||
| Neurological soft signs | 1.2 [1.1–1.3] | 4.6 | <.001 | 1.2 [1.1–1.3] | 3.9 | <.001 | 1.2 [1.1–1.3] | 3.6 | <.001 | |||
| General intelligence | 0.92 [0.88–0.96] | −3.6 | <.001 | 0.92 [0.89–0.96] | −4.4 | <.001 | ||||||
| Total grey matter volume | 0.98 [0.95–0.99] | −2.0 | .043 | |||||||||
Note: SzO, offspring of probands with schizophrenia; BpO, offspring of probands with bipolar disorder; CcO, offspring of community controls; OR, odds ratio; CI, confidence interval. Binary logistic regression models including family and site as random variables adjusted for age, sex, and total intracraneal volume (for grey matter volume) where significant.
Fig. 1.
Scatterplot representing the relationship between general intelligence and total grey matter volume between groups. SzO, offspring of probands with schizophrenia; BpO, offspring of probands with bipolar disorder; CcO, offspring of community controls.
Within HR offspring, lifetime psychopathology was associated with total GMV and general intelligence (AUC = 0.75, 95% CI: 0.67–0.84, P < .001; table 4).
Table 4.
Prediction of Lifetime History of Axis I Disorders
| All High Risk Offspring | |||
|---|---|---|---|
| OR [95% CI] | t | P | |
| General intelligence | 0.96 [0.93–0.99] | −2.6 | .009 |
| Total grey matter volume | 0.99 [0.98–0.99] | −2.2 | .026 |
Note: OR, odds ratio; CI: confidence interval. Binary logistic regression model including family and site as random variables, adjusted for sex.
The above results were confirmed in the subsample that had not received psychopharmacological treatment (Supplement 2), with the exception of the effect of GMV toward discrimination of HR offspring, and the association between general intelligence and psychopathology. Results of between group comparisons employing only the healthy control group are presented in Supplement 3.
Discussion
To the best of our knowledge, this is the first study to perform a comparison of child and adolescent offspring of patients with schizophrenia, offspring of patients with bipolar disorder and community controls integrating data from different modalities. We have demonstrated that a combination of neurodevelopmental factors—antecedents of obstetric complications, NSS, and total GMV—was able to distinguish between HR offspring and controls with what has been considered good discrimination performance.67 When assessed independently, SzO and BpO were each distinguished from CcO by obstetric complications and NSS, although in SzO, general intelligence also contributed to group discrimination.
Our findings concerning higher rates of obstetric complications in HR offspring are unsurprising, given the reported increase in perinatal adverse outcomes in parents with major psychiatric disorders, widely supported by studies in the field of obstetrics and pediatrics.68 However, the fact that NSS had an independent contribution to group discrimination of both SzO and BpO relative to CcO, regardless of the increased rates of obstetric complications and the effects of psychotropic medications, is noteworthy. There is longstanding evidence of minor neurologic alterations in patients with schizophrenia,69 while findings in samples of patients with bipolar disorder are more recent.69–71 Comparative studies in clinical samples so far have reported on higher rates of NSS in both patient groups relative to controls,72–74 although one sample suggested that these may be more salient in schizophrenia than in bipolar disorder.74 Adult relatives of probands with bipolar disorder have been associated with greater NSS than in controls,70,75 although we are unaware of any study so far reporting on NSS in child and adolescent BpO. A recent meta-analysis76 has confirmed evidence of greater NSS in adult relatives of probands with schizophenia, although results were weaker in younger samples.16,17,77 Our findings are the first to report on comparative rates of NSS in child and adolescent SzO and BpO: although NSS were numerically higher in SzO, NSS were significantly higher than CcO in both HR groups. NSS are thought to reflect disruption in cortico-basal ganglia-cerebellar connections.78 In a subgroup of the current sample we have recently identified differences in resting state connectivity in a left cortical-basal ganglia network, which was significantly reduced in SzO relative to CcO, but not in relation to BpO, and was associated with GMV of the left caudate nucleus in SzO.79 This was independent of ADHD diagnosis or the effects of psychotropic medication. It is therefore likely that our clinical observations reflect gene–environment effects common to both SzO and BpO, which may have a stronger biological signature in SzO.
SzO showed a GMV decrease relative to CcO and BpO at a univariate level. Although GMV is a dynamic measure, approximately 75% of brain volumes are determined by early childhood, and are thought to reflect early developmental processes.80 We have previously demonstrated that GMV decrease is present in SzO independently of age,62 and that reduction of global cortical surface area, which has been closely associated with GMV, is present in childhood and adolescence over 2 points in time.81 At a multivariate level, GMV decrease was detected when assessing both groups jointly, and may not have emerged in the contrasts for SzO versus CcO or BpO due to a comparatively stronger effect of intelligence in the model. Higher rates of childhood developmental difficulties were also detected in SzO relative to CcO and BpO, however this did not distinguish between groups in multivariate analyses, also likely related to a stronger effect of intelligence, obstetric complications and NSS.
Intelligence clearly distinguished between SzO and BpO. The single study so far to directly compare young SzO and BpO23 failed to identify group differences in full scale intelligence, which may have been related to a lack of power, although it did identify working memory and attentional deficits in SzO and BpO relative to controls, respectively. Our findings are in keeping with a number of studies pointing to lower intelligence in first-degree relatives of patients with schizophrenia in relation to controls,21 in contrast to the mixed evidence from studies in bipolar relatives.22–24 A more recent meta-analysis of comparative studies between schizophrenia and bipolar disorder concluded that reductions in premorbid intelligence in bipolar disorder were only detected in retrospective studies, and thus concurs with our own prospective observations.82
Taken together, our evidence points to a potential continuum of neurodevelopmental disruption between SzO and BpO, both from a qualitative (number of domains affected) and quantitative (effect sizes, comparative accuracy of discriminatory models) perspective. Although not a primary outcome measure of the study, the higher rates of ADHD, considered to be a disorder of neurodevelopment,83 in SzO relative to CcO, with intermediate values for BpO, also concurs with this notion. Our observations are in keeping with results from molecular and genetic studies in patient samples, which have demonstrated that although the impact is greater in schizophrenia, bipolar disorders are also associated with a degree of alteration in neuromaturational processes.84–89
Our second objective aimed to assess the clinical relevance of these findings: GMV and intelligence were independently associated with lifetime axis I psychopathology in HR youth, which may index greater risk of later schizophrenia or bipolar disorder in these youth.48–50 Kendler et al90 in a recent conscript study, reported an inverse relationship between scholastic achievement at age 16 and risk for schizophrenia; this association was weaker in individuals who later developed bipolar disorder. In an earlier cohort study assessing neuropsychological performance at age 7,91 both children who later developed schizophrenia or bipolar disorder performed worse than controls, although impairment was more severe in those who went on to develop schizophrenia. Interestingly, the presence of a positive family history in first-degree relatives significantly increased the severity of childhood neuropsychological impairment for schizophrenia but not for bipolar disorder, which resonates with our own findings. Neuroimaging studies in familial HR youth have revealed an association between a longitudinal decrease in total and fronto-temporal volume and schizophrenia outcomes in young adult SzO,92 while regional GMV predicted prodromal symptomatology at follow-up in an adolescent sample of SzO.27 Although GMV reduction does not appear to characterize young BpO as a group,29,30 it has been shown to identify those who go on to develop mood symptoms,32 which is in keeping with (a) our lack of case–control neuroimaging differences in BpO, and (b) the fact that, when considered together with SzO, total GMV was associated with axis I psychopathology, and therefore may serve in future to distinguish those HR offspring who will develop a major psychiatric outcome.48
Limitations
The above data is limited by a number of shortcomings. First, data on perinatal events and childhood development are dependent on recall capacity of the parents; hence our decision to focus on a small number of objective items. We also lacked information on maternal substance misuse, smoking or psychopharmacological treatment during pregnancy, or on minor gestational events which have later been associated with psychiatric outcomes such as influenza or urinary tract infections,93 which will ideally need to be assessed in studies including newborn offspring. Parents of children with a history of developmental problems may have been more motivated to participate in the study, however control families were screened for this and excluded if this was their main reason for participating in the study. In proband families we also consider it unlikely that this could have driven our findings: families were approached following a systematic procedure, and over 85% of families who were contacted went on to participate in the study.51 Our experience is that parents with schizophrenia or bipolar disorder are eager for their offspring to participate in such studies regardless of whether they have presented with previous developmental difficulties. We also prioritized sample size at the expense of having to accommodate our neuroimaging analyses to a multicenter design, hence our focus on a measures of total and regional brain volumes rather than more refined measures of brain morphology. Patients with schizophrenia, especially, are known to have low fertility rates,94 which is why we decided to recruit from 2 sites. Furthermore, we decided against comparing between subtypes of bipolar disorder in order to minimize multiple comparisons. There was a proportion of offspring receiving psychopharmacological medication; however, results in the unmedicated subsample were very similar, and risk of exclusion of the more severely affected offspring and subsequently those carrying greatest illness-related effects must also be taken into account. Finally, although psychopathology in HR youth has been associated with adult outcomes, only long-term follow-up will allow to elucidate which of these neurodevelopmental factors are predictive of schizophrenia or bipolar disorder.
Conclusions
We provide evidence of a potential continuum of neurodevelopmental insult between SzO and BpO. Clinicians should be alerted to the potential association between lower intelligence and total GMV and risk for psychopathology, which may index increased risk for adverse clinical outcomes in HR youth.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
This work was supported by the Spanish Ministry of Economy and Competitiveness / Instituto de Salud Carlos III (FIS PI 07/0066, PI 11/00683, PI12/00912, PI 15/00467), European Regional Development Fund, European Union Seventh Framework Program under grant agreement FP7- HEALTH-2013-2.2.1-2-602478 (Project METSY), La Marato de TV3 Foundation (091630), CERCA Programme / Generalitat de Catalunya, Catalonia Government (2009SGR1119, 2014SGR441, 2014SGR489, 2014_ SGR_398, 2014_ SGR_398), and Madrid Regional Government (S2010/ BMD-2422 AGES).
Supplementary Material
Acknowledgments
The authors would like to thank Miss Mireia Rosa and Miss Marina Redondo for their help with data collection. The authors also thank the support of the Spanish Ministry of Economy and Competitiveness/ISCIII, co-financed by ERDF Funds from the European Commission (“A way of making Europe”), CIBERSAM, Alicia Koplowitz Foundation, Fundación Mutua Madrileña, European Union Structural Funds and European Union Seventh Framework Program. Dr Moreno has served as consultant for Janssen, Servier, and Lundbeck. Dr Vieta has received grants and served as consultant, advisor or CME speaker for the following entities: AB-Biotics, Actavis, Aequus, Adamed, Alexza, Almirall, AstraZeneca, Bial, Bristol-Myers Squibb, Elan, Eli Lilly, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen-Cilag, Jazz, Johnson & Johnson, Lundbeck, Merck, Novartis, Organon, Otsuka, Pfizer, Pierre-Fabre, Qualigen, Roche, Sanofi-Aventis, Servier, Shering-Plough, Shire, Solvay, Sumitomo Dainippon, Sunovion, Takeda, Telefónica, Teva, the Spanish Ministry of Science and Innovation (CIBERSAM), the Seventh European Framework Programme (ENBREC), the Stanley Medical Research Institute, United Biosource Corporation, and Wyeth. The remaining authors declare no conflicts of interest in relation to the subject of this study.
References
- 1. Ivleva E, Thaker G, Tamminga CA. Comparing genes and phenomenology in the major psychoses: schizophrenia and bipolar 1 disorder. Schizophr Bull. 2008;34:734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kraepelin E. Psychiatrie: ein Lehrbuch für Studirende und Aertze [Psychiatry: A Textbook for Students and Doctors]. Lepizig, Germany: JA Barth; 1899. [Google Scholar]
- 3. Demjaha A, MacCabe JH, Murray RM. How genes and environmental factors determine the different neurodevelopmental trajectories of schizophrenia and bipolar disorder. Schizophr Bull. 2012;38:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laurens KR, Luo L, Matheson SL, et al. Common or distinct pathways to psychosis? A systematic review of evidence from prospective studies for developmental risk factors and antecedents of the schizophrenia spectrum disorders and affective psychoses. BMC Psychiatry. 2015;15:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. [DOI] [PubMed] [Google Scholar]
- 6. Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed). 1987;295:681–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. [DOI] [PubMed] [Google Scholar]
- 8. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nasrallah HA. Neurodevelopmental aspects of bipolar affective disorder. Biol Psychiatry. 1991;29:1–2. [DOI] [PubMed] [Google Scholar]
- 10. Sanches M, Keshavan MS, Brambilla P, Soares JC. Neurodevelopmental basis of bipolar disorder: a critical appraisal. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1617–1627. [DOI] [PubMed] [Google Scholar]
- 11. Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. 2016;387:1561–1572. [DOI] [PubMed] [Google Scholar]
- 12. Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moran ME, Hulshoff Pol H, Gogtay N. A family affair: brain abnormalities in siblings of patients with schizophrenia. Brain. 2013;136:3215–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hameed MA, Lewis AJ. Offspring of parents with schizophrenia: a systematic review of developmental features across childhood. Harv Rev Psychiatry. 2016;24:104–117. [DOI] [PubMed] [Google Scholar]
- 15. Forsyth JK, Ellman LM, Tanskanen A, et al. Genetic risk for schizophrenia, obstetric complications, and adolescent school outcome: evidence for gene–environment interaction. Schizophr Bull. 2013;39:1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rieder RO, Nichols PL. Offspring of schizophrenics. III. Hyperactivity and neurological soft signs. Arch Gen Psychiatry. 1979;36:665–674. [DOI] [PubMed] [Google Scholar]
- 17. Onal O, Demir C, Ceylan ME. Soft neurological signs in children of schizophrenic parents: a controlled study [Turkish]. Klinik Psikofarmakoloji Bulteni. 2002;12:78–85. [Google Scholar]
- 18. Waters B, Marchenko I, Smiley D. Affective disorder, paranatal and educational factors in the offspring of bipolar manic-depressives. Can J Psychiatry. 1983;28:527–531. [DOI] [PubMed] [Google Scholar]
- 19. Wals M, Reichart CG, Hillegers MH, et al. Impact of birth weight and genetic liability on psychopathology in children of bipolar parents. J Am Acad Child Adolesc Psychiatry. 2003;42:1116–1121. [DOI] [PubMed] [Google Scholar]
- 20. Freed RD, Tompson MC, Otto MW, et al. Early risk factors for psychopathology in offspring of parents with bipolar disorder: the role of obstetric complications and maternal comorbid anxiety. Depress Anxiety. 2014;31:583–590. [DOI] [PubMed] [Google Scholar]
- 21. Agnew-Blais J, Seidman LJ. Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: a quantitative and qualitative review. Cogn Neuropsychiatry. 2013;18:44–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klimes-Dougan B, Ronsaville D, Wiggs EA, Martinez PE. Neuropsychological functioning in adolescent children of mothers with a history of bipolar or major depressive disorders. Biol Psychiatry. 2006;60:957–965. [DOI] [PubMed] [Google Scholar]
- 23. Diwadkar VA, Goradia D, Hosanagar A, et al. Working memory and attention deficits in adolescent offspring of schizophrenia or bipolar patients: comparing vulnerability markers. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDonough-Ryan P, DelBello M, Shear PK, Ris DM, Soutullo C, Strakowski SM. Academic and cognitive abilities in children of parents with bipolar disorder: a test of the nonverbal learning disability model. J Clin Exp Neuropsychol. 2002;24:280–285. [DOI] [PubMed] [Google Scholar]
- 25. Rajarethinam R, Upadhyaya A, Tsou P, Keshavan MS. Caudate volume in offspring of patients with schizophrenia. Br J Psychiatry. 2007;191, 258–259. [DOI] [PubMed] [Google Scholar]
- 26. Sişmanlar SG, Anik Y, Coşkun A, Ağaoğlu B, Karakaya I, Yavuz CI. The volumetric differences of the fronto-temporal region in young offspring of schizophrenic patients. Eur Child Adolesc Psychiatry. 2010;19:151–157. [DOI] [PubMed] [Google Scholar]
- 27. Bhojraj TS, Sweeney JA, Prasad KM, et al. Gray matter loss in young relatives at risk for schizophrenia: relation with prodromal psychopathology. Neuroimage. 2011;54(suppl 1):S272–S279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dougherty MK, Gu H, Bizzell J, Ramsey S, Gerig G, Perkins DO, Belger A. Differences in subcortical structures in young adolescents at familial risk for schizophrenia: a preliminary study. Psychiatry Res. 2012;204:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh MK, Delbello MP, Adler CM, Stanford KE, Strakowski SM. Neuroanatomical characterization of child offspring of bipolar parents. J Am Acad Child Adolesc Psychiatry. 2008;47:526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karchemsky A, Garrett A, Howe M, et al. Amygdalar, hippocampal, and thalamic volumes in youth at high risk for development of bipolar disorder. Psychiatry Res Neuroimaging. 2011;194:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ladouceur CD, Almeida JR, Birmaher B, et al. Subcortical gray matter volume abnormalities in healthy bipolar offspring: potential neuroanatomical risk marker for bipolar disorder? J Am Acad Child Adolesc Psychiatry. 2008;47:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nickson T, Chan SW, Papmeyer M, et al. Prospective longitudinal voxel-based morphometry study of major depressive disorder in young individuals at high familial risk. Psychol Med. 2016;46:2351–2361. [DOI] [PubMed] [Google Scholar]
- 33. Leask SJ, Done DJ, Crow TJ. Adult psychosis, common childhood infections and neurological soft signs in a national birth cohort. Br J Psychiatry. 2002;181:387–392. [DOI] [PubMed] [Google Scholar]
- 34. Cannon M, Caspi A, Moffitt TE, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–456. [DOI] [PubMed] [Google Scholar]
- 35. Osler M, Lawlor DA, Nordentoft M. Cognitive function in childhood and early adulthood and hospital admission for schizophrenia and bipolar disorders in Danish men born in 1953. Schizophr Res. 2007;92:132–141. [DOI] [PubMed] [Google Scholar]
- 36. Keskinen E, Miettunen J, Koivumaa-Honkanen H, Mäki P, Isohanni M, Jääskeläinen E. Interaction between parental psychosis and risk factors during pregnancy and birth for schizophrenia—the Northern Finland 1966 Birth Cohort study. Schizophr Res. 2013;145:56–62. [DOI] [PubMed] [Google Scholar]
- 37. O’Neill SM, Curran EA, Dalman C, et al. Birth by caesarean section and the risk of adult psychosis: a population-based cohort study. Schizophr Bull. 2016;42:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meyer SE, Carlson GA, Wiggs EA, et al. A prospective study of the association among impaired executive functioning, childhood attentional problems, and the development of bipolar disorder. Dev Psychopathol. 2004;16:461–476. [DOI] [PubMed] [Google Scholar]
- 39. Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS. Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry. 2013;70:677–685. [DOI] [PubMed] [Google Scholar]
- 40. Nishimura T, Takei N, Tsuchiya KJ, Asano R, Mori N. Identification of neurodevelopmental trajectories in infancy and of risk factors affecting deviant development: a longitudinal birth cohort study. Int J Epidemiol. 2016;45:543–553. [DOI] [PubMed] [Google Scholar]
- 41. Sandin S, Schendel D, Magnusson P, et al. Autism risk associated with parental age and with increasing difference in age between the parents. Mol Psychiatry. 2016;21:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ek M, Wicks S, Svensson AC, Idring S, Dalman C. Advancing paternal age and schizophrenia: the impact of delayed fatherhood. Schizophr Bull. 2015;41:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chudal R, Gissler M, Sucksdorff D, et al. Parental age and the risk of bipolar disorders. Bipolar Disord. 2014;16:624–632. [DOI] [PubMed] [Google Scholar]
- 44. Lehrer DS, Pato MT, Nahhas RW, et al. Paternal age effect: replication in schizophrenia with intriguing dissociation between bipolar with and without psychosis. Am J Med Genet B Neuropsychiatr Genet. 2016;171:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brown A, Bao Y, McKeague I, Shen L, Schaefer C. Parental age and risk of bipolar disorder in offspring. Psychiatry Res. 2013;208:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mehta D, Tropf FC, Gratten J, et al. Evidence for genetic overlap between schizophrenia and age at first birth in women. JAMA Psychiatry. 2016;73:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rasic D, Hajek T, Alda M, Uher R. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta-analysis of family high-risk studies. Schizophr Bull. 2014;40:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry. 2003;60:709–717. [DOI] [PubMed] [Google Scholar]
- 49. Duffy A, Alda M, Crawford L, Milin R, Grof P. The early manifestations of bipolar disorder: a longitudinal prospective study of the offspring of bipolar parents. Bipolar Disord. 2007;9:828–838. [DOI] [PubMed] [Google Scholar]
- 50. Welham J, Scott J, Williams G, et al. Emotional and behavioural antecedents of young adults who screen positive for non-affective psychosis: a 21-year birth cohort study. Psychol Med. 2009;39:625–634. [DOI] [PubMed] [Google Scholar]
- 51. Sanchez-Gistau V, Romero S, Moreno D, et al. Psychiatric disorders in child and adolescent offspring of patients with schizophrenia and bipolar disorder: a controlled study. Schizophr Res. 2015;168:197–203. [DOI] [PubMed] [Google Scholar]
- 52. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 53. Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 54. Hollingshead AB, Redlich FC. Social class and mental illness: a community study. 1958. Am J Public Health. 2007;97:1756–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lewis S, Owen M, Murray R. Obstetric complications and schizophrenia: methodology and mechanisms. In: Schulz S, Tamminga C, eds. Schizophrenia: Scientific Progress. New York, NY: Oxford University Press; 1989. [Google Scholar]
- 56. Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wechsler D. WISC-IV Escala de Inteligencia Wechsler para Niños. Madrid: TEA Ediciones; 2003. [Google Scholar]
- 58. O’Connor M, Harris JM, McIntosh AM, Owens DG, Lawrie SM, Johnstone EC. Specific cognitive deficits in a group at genetic high risk of schizophrenia. Psychol Med. 2009;39:1649–1655. [DOI] [PubMed] [Google Scholar]
- 59. Duffy A, Hajek T, Alda M, Grof P, Milin R, MacQueen G. Neurocognitive functioning in the early stages of bipolar disorder: visual backward masking performance in high risk subjects. Eur Arch Psychiatry Clin Neurosci. 2009;259:263–269. [DOI] [PubMed] [Google Scholar]
- 60. Flanagan D, Kaufman A. Essentials of WISC-IV Assessment. Madrid: TEA Ediciones; 2008. [Google Scholar]
- 61. Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res. 1989;27:335–350. [DOI] [PubMed] [Google Scholar]
- 62. Sugranyes G, de la Serna E, Romero S, et al. Grey matter volume decrease distinguishes schizophrenia from bipolar offspring during childhood and adolescence. J Am Acad Child Adolesc Psychiatry. 2015;54:677.e2–684.e2. [DOI] [PubMed] [Google Scholar]
- 63. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 64. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 65. Muntaner C, Eaton WW, Miech R, O’Campo P. Socioeconomic position and major mental disorders. Epidemiol Rev. 2004;26:53–62. [DOI] [PubMed] [Google Scholar]
- 66. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. [DOI] [PubMed] [Google Scholar]
- 67. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York, NY: Wiley-Interscience; 2000. [Google Scholar]
- 68. Pereira PK, Lima LA, Legay LF, de Cintra Santos JF, Lovisi GM. Maternal mental disorders in pregnancy and the puerperium and risks to infant health. World J Clin Pediatr. 2012;1:20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Goswami U, Sharma A, Khastigir U, et al. Neuropsychological dysfunction, soft neurological signs and social disability in euthymic patients with bipolar disorder. Br J Psychiatry. 2006;188:366–373. [DOI] [PubMed] [Google Scholar]
- 70. Mrad A, Wassim Krir M, Ajmi I, Gaha L, Mechri A. Neurological soft signs in euthymic bipolar I patients: a comparative study with healthy siblings and controls. Psychiatry Res. 2016;236:173–178. [DOI] [PubMed] [Google Scholar]
- 71. Baş TÖ, Poyraz CA, Baş A, Poyraz BÇ, Tosun M. The impact of cognitive impairment, neurological soft signs and subdepressive symptoms on functional outcome in bipolar disorder. J Affect Disord. 2015;174:336–341. [DOI] [PubMed] [Google Scholar]
- 72. Whitty P, Clarke M, McTigue O, et al. Diagnostic specificity and predictors of neurological soft signs in schizophrenia, bipolar disorder and other psychoses over the first 4 years of illness. Schizophr Res. 2006;86:110–117. [DOI] [PubMed] [Google Scholar]
- 73. Zhao Q, Ma YT, Lui SS, et al. Neurological soft signs discriminate schizophrenia from major depression but not bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:72–78. [DOI] [PubMed] [Google Scholar]
- 74. Rigucci S, Dimitri-Valente G, Mandarelli G, et al. Neurological soft signs discriminate schizophrenia from bipolar disorder. J Psychiatr Pract. 2014;20:147–153. [DOI] [PubMed] [Google Scholar]
- 75. Sharma S, Bhatia T, Mazumdar S, Deshpande SN. Neurological soft signs and cognitive functions: amongst euthymic bipolar I disorder cases, non-affected first degree relatives and healthy controls. Asian J Psychiatr. 2016;22:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Burton BK, Hjorthøj C, Jepsen JR, Thorup A, Nordentoft M, Plessen KJ. Research Review: Do motor deficits during development represent an endophenotype for schizophrenia? A meta-analysis. J Child Psychol Psychiatry. 2016;57:446–456. [DOI] [PubMed] [Google Scholar]
- 77. Keshavan M, Montrose DM, Rajarethinam R, Diwadkar V, Prasad K, Sweeney JA. Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophr Res. 2008;103:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Whitty PF, Owoeye O, Waddington JL. Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr Bull. 2009;35:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Solé-Padullés C, Castro-Fornieles J, de la Serna E, et al. Altered cortico-striatal connectivity in offspring of schizophrenia patients relative to offspring of bipolar patients and controls. PLoS One. 2016;11:e0148045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. [DOI] [PubMed] [Google Scholar]
- 81. Sugranyes G, Solé-Padullés C, de la Serna E, et al. Cortical morphology characteristics of young offspring of schizophrenia or bipolar disorder patients. J Am Acad Child Adolesc Psychiatry. 2015. [DOI] [PubMed] [Google Scholar]
- 82. Trotta A, Murray RM, MacCabe JH. Do premorbid and post-onset cognitive functioning differ between schizophrenia and bipolar disorder? A systematic review and meta-analysis. Psychol Med. 2015;45:381–394. [DOI] [PubMed] [Google Scholar]
- 83. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Pub; 2013. [Google Scholar]
- 84. Sullivan PF, Magnusson C, Reichenberg A, et al. Family history of schizophrenia and bipolar disorder as risk factors for autism. Arch Gen Psychiatry. 2012;69:1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tang H, Shen N, Jin H, Liu D, Miao X, Zhu LQ. GSK-3β polymorphism discriminates bipolar disorder and schizophrenia: a systematic meta-analysis. Mol Neurobiol. 2013;48:404–411. [DOI] [PubMed] [Google Scholar]
- 86. Ruderfer DM, Fanous AH, Ripke S, et al. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol Psychiatry. 2014;19:1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Georgieva L, Rees E, Moran JL, et al. De novo CNVs in bipolar affective disorder and schizophrenia. Hum Mol Genet. 2014;23:6677–6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ziats MN, Rennert OM. Identification of differentially expressed microRNAs across the developing human brain. Mol Psychiatry. 2014;19:848–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Erhardt S, Schwieler L, Imbeault S, Engberg G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology. 2017;112:297–306. [DOI] [PubMed] [Google Scholar]
- 90. Kendler KS, Ohlsson H, Mezuk B, Sundquist K, Sundquist J. A Swedish national prospective and co-relative study of school achievement at age 16, and risk for schizophrenia, other nonaffective psychosis, and bipolar illness. Schizophr Bull. 2016;42:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Seidman LJ, Cherkerzian S, Goldstein JM, Agnew-Blais J, Tsuang MT, Buka SL. Neuropsychological performance and family history in children at age 7 who develop adult schizophrenia or bipolar psychosis in the New England Family Studies. Psychol Med. 2013;43:119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. McIntosh AM, Owens DC, Moorhead WJ, et al. Longitudinal volume reductions in people at high genetic risk of schizophrenia as they develop psychosis. Biol Psychiatry. 2011;69:953–958. [DOI] [PubMed] [Google Scholar]
- 93. Suvisaari JM, Taxell-Lassas V, Pankakoski M, Haukka JK, Lönnqvist JK, Häkkinen LT. Obstetric complications as risk factors for schizophrenia spectrum psychoses in offspring of mothers with psychotic disorder. Schizophr Bull. 2013;39:1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Power RA, Kyaga S, Uher R, et al. Fecundity of patients with schizophrenia, autism, bipolar disorder, depression, anorexia nervosa, or substance abuse vs their unaffected siblings. JAMA Psychiatry. 2013;70:22–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.