Abstract
Gray’s Reinforcement Sensitivity Theory (RST) asserts three core personality systems: the behavioral approach system (BAS), the fight-flight-freeze system (FFFS) and the revised behavioral inhibition system (r-BIS). Past models of frontal activity link greater relative left frontal activity with Carver and White’s (1994) BAS scale and trait impulsivity and greater relative right frontal activity with Carver and White’s (1994) BIS scale. However, the original BIS scale assesses both FFFS and r-BIS. Past work linking the BIS scale and right frontal activity does not indicate which system is related to right frontal activity. The current study (n = 182) examined frontal asymmetric activity with personality traits associated with approach (BAS), withdrawal (FFFS-Fear), behavioral inhibition (BIS-Anxiety) and impulsivity (UPPS-P). Resting frontal cortical activity was recorded using electroencephalography (EEG), and the traditional alpha band was examined. Greater BIS-Anxiety related to greater relative right frontal activity. Impulsivity related to less relative right frontal activity. BAS and FFFS-Fear (approach and withdrawal motivation) did not relate to asymmetric frontal activity. Regulatory control processes associated with r-BIS and impulsivity, rather than withdrawal motivation associated with FFFS, may be more closely related to right frontal activity.
Keywords: frontal asymmetry, EEG, personality, behavioral inhibition system, impulsivity
Gray’s influential Reward Sensitivity Theory (RST) has guided research in the realm of motivation and personality since its conception in 1970. The original theory emphasized two general motivational systems underlying human behavior: the behavioral activation system (BAS) and behavioral inhibition system (BIS). BAS was theorized to encompass approach and reward-related responses to appetitive stimuli (Gray, 1970). BIS comprised responses to aversive stimuli, and was activated by signals of punishment. BIS was thought to inhibit goal-directed behavior in order to respond to the aversive stimulus, and was accompanied by feelings of anxiety, frustration, and sadness.
In his later revision of the theory, Gray adjusted the function of BIS based on decades of research (Gray and McNaughton, 2000). While his original conception of RST mentioned a less defined fight-flight system (FFS), the revised RST developed this system further into a fight-flight-freeze system (FFFS). The original BIS dealt with responses to aversive stimuli, but in the revised RST, the FFFS was primarily responsible for this role. FFFS encompasses functional behavioral responses to threat, including fighting the threat, fleeing in active avoidance, or freezing to avoid attracting the attention of the predator. In the revised theory, revised-BIS (r-BIS) serves primarily to detect and resolve conflict between BAS and FFFS. Thus, r-BIS is responsible for regulatory responses to motivational urges from BAS and FFFS. Accordingly, higher levels of impulsivity relate to diminished functioning of the r-BIS system (Neal and Gable, 2016).
In line with Gray’s original RST, Carver and White (1994) developed a personality questionnaire that tapped the sensitivity of BAS and BIS on a dispositional level. These BIS/BAS scales have been extensively used in motivational and physiological research to relate approach- and withdrawal-motivated personality traits to behavior, affect and neurophysiological responses (for a review, Harmon-Jones et al., 2013). Specifically, much work on asymmetric activity of the frontal cortex as measured by electroencephalography (EEG) began to associate Carver and White’s (1994) BIS scale with relative right frontal activation. Since the BIS scale was formulated based on the original conception of BIS as an aversive-based avoidance system, this led to the model of frontal asymmetry associating approach motivation and left frontal activity and withdrawal motivation with right frontal activity.
BIS and asymmetric frontal activity
For the past few decades, researchers have linked the left and right hemispheres to approach and withdrawal motivation using EEG alpha asymmetry (Harmon-Jones and Gable, in press). The alpha band frequency has been theorized to reflect the inverse of cortical activity (Cook et al., 1998; Allen et al., 2004). This finding has been confirmed through use of source localization techniques (Pizzagalli et al., 2005). It is suggested that alpha frequency desynchronizes in response to activation, while other slower frequencies (e.g. delta and theta) synchronize (Knyazev and Slobodskaya, 2003). Thus, cortical activation should result in desynchronized oscillations in the alpha band (lower alpha power). Researchers typically measure frontal cortical activation in homologous areas of the left and right frontal hemispheres, then calculate a difference score between the two hemispheres to examine relative activation of the left vs right hemisphere.
Early research linking Carver and White’s (1994) BIS/BAS scales to resting EEG alpha activity found inconsistent results. This work has been predicated on the hypothesis that approach motivation is related to greater left frontal activity and withdrawal motivation is related to greater right frontal activity. Initially, two studies linked higher BAS sensitivity to greater relative left frontal activity (Harmon-Jones and Allen, 1997; Sutton and Davidson, 1997). Sutton and Davidson (1997) also found a correlation between BIS sensitivity and relative right frontal activity, while Harmon-Jones and Allen (1997) did not. Further research on resting frontal activity over the last 20 years has continued to produce inconsistent findings with the BIS scale and right frontal activity. Some studies have found a link between resting right frontal activity and BIS (Sutton and Davidson, 1997; Shackman et al., 2009; Quadflieg et al., 2015), while many others have not (Henriques and Davidson, 2000; Kline et al., 2000; Coan et al., 2001; Coan and Allen, 2003; Jackson et al., 2003; Hewig et al., 2004, 2006; Pizzagalli et al., 2005; Amodio et al., 2008; Wacker et al., 2008; Berkman and Lieberman, 2010; Wacker et al., 2010; Keune et al., 2012; De Pascalis et al., 2013; Quirin et al., 2013). Recently, there have also been some failures to replicate the link between BAS and left frontal activity (Shackman et al., 2009; Wacker et al., 2010; Gable et al., 2015; Neal and Gable, 2016). However, all but one of these studies (Shackman et al., 2009) also failed to replicate the link between BIS and right frontal activity.
To summarize, most past studies have either replicated the link between BAS and left frontal activity but failed to find a relationship between BIS and right frontal activity, or failed to replicate both relationships with frontal asymmetric activity. The relationship between BAS and left frontal activity seems to be more replicable than the relationship between BIS and right frontal activity. This reduced replicability may be due to a smaller effect size for the relationship between BIS and right frontal activity than the relationship between BAS and left frontal activity.
One possible explanation for the inconsistent relationship between BIS and frontal asymmetry could be that Carver and White’s (1994) BIS/BAS scales were created based on original RST, but no changes were made in light of revised RST theory. One of the major criticisms of the BIS/BAS scales is its failure to separate the r-BIS and FFFS systems (Corr, 2016). Thus, the BIS scale may be capturing processes of conflict detection associated with r-BIS, as well as withdrawal-motivated processes of the FFFS (Smillie et al., 2006; Corr and McNaughton, 2008). Heym et al. (2008) asserted that some items of the BIS subscale (e.g. 1, 4 and 6) address fear responses to threatening stimuli, and comprise a subscale termed ‘FFFS-Fear’. The remaining items of the BIS subscale (e.g. 2, 3, 5 and 7) capture anxiety over conflict, or BIS-Anxiety. For an overview of these items, please see Supplementary Figure S1. This BIS-Anxiety scale best captures the conflict detection and resolution function of r-BIS defined in revised RST. Confirmatory factor analysis on the BIS scale found a two-factor model was the best fit, splitting the scale into BIS-Anxiety and FFFS-Fear (Heym et al., 2008). Past studies examining the BIS subscale with right frontal activity did not consider that Carver and White’s (1994) BIS scale assesses both FFFS and r-BIS. It may be that only one of these systems is related to right frontal activity.
r-BIS and right frontal activity
Much work has linked right frontal activity to r-BIS related processes of regulatory control, response inhibition, motivational control and risk appraisal (for a review, see Gable et al., in press). Recent attempts to examine the r-BIS and FFFS have suggested that r-BIS rather than withdrawal motivation may be responsible for right frontal activity. For example, Wacker et al. (2008) found that during an emotional imagery task participants exhibited greater right frontal activation in situations evoking behavioral inhibition (r-BIS) than situations evoking withdrawal motivation.
Other EEG work has linked diminished r-BIS activation, assessed by trait impulsivity, with right frontal EEG activity. Santesso et al. (2008) linked greater relative left frontal activity (less relative right frontal activity) to higher trait sensation seeking as measured by the Zuckerman (1994) Sensation Seeking Scale. Positive urgency, or impulsive behaviors in a positive emotional context, has been related to reduced relative right frontal activity (Gable et al., 2015). Multiple facets of impulsivity as measured by the UPPS-P Behavioral Impulsivity Scale (Whiteside et al., 2005) have been related to reduced relative right frontal activity (Neal and Gable, 2016). These past findings suggest that trait related to diminished r-BIS functioning relates to reduced right frontal activity, but no past work has linked greater trait r-BIS functioning with greater right frontal asymmetry.
Due to the large volume of studies that have failed to link Carver and White’s BIS scale with right frontal activity, it seems likely that one of the two systems comprising the BIS scale (r-BIS and FFFS) may not relate to right frontal activity. According to other models of frontal asymmetry, FFFS could be related to greater right frontal activation. However, based on much past work linking personality traits of regulatory control to right frontal activity, we predict that r-BIS is related to right frontal activity. Control of motivational processes may be more closely tied to right frontal activity than withdrawal motivation. Specifically, we predicted that greater relative right frontal activity would be related to higher levels of r-BIS as measured by greater BIS-Anxiety. We predicted that greater trait impulsivity would be related to reduced right frontal activity because greater impulsivity should reflect diminished functioning of the r-BIS.
Methods
Participants
One hundred and eighty two undergraduate introductory psychology students (109 female) participated for course credit. For inclusion in the study, participant must have been at least 18 years old and right handed. Handedness of participant was assessed using a 13-item checklist (Gable and Poole, 2014). Participants indicated which hand (right, left, or both) they used to perform a variety of common tasks. All participants were verified as right-handed before participation by reporting performing no more than one item with their left hand.
Materials
Behavioral activation sensitivity/behavioral inhibition sensitivity
Participants completed Carver and White’s (1994) 20-item BIS/BAS scales. Items were presented one at a time and the participant was asked to respond to a 4 point scale ranging from 1 Strongly Disagree to 4 Strongly Agree.
All items contributing to BAS were calculated into a single BAS factor (BAS Total) comprised of 13 items. All scores were within 2.01 standard deviations of the mean. Responses to original BIS items on Carver and White’s (1994) scale were calculated into a total score reflecting original BIS. This score included 7 items and all scores were within 2.9 standard deviations of the mean.
BIS-Anxiety and FFFS sub-scales were calculated according to Heym et al. (2008) revised RST factor analysis. The breakdown of which items from the original BIS scale make up each subscale is presented in Supplementary Table S1. BIS items were calculated into two subscales: FFFS-Fear and BIS-Anxiety. FFFS-Fear consisted of 3 items relating to avoidance motivation. BIS-Anxiety consisted of 4 items reflecting r-BIS. All scores were within 2.55 (FFFS-Fear) and 2.72 (BIS-Anxiety) standard deviations of the mean.
UPPS-P behavioral impulsivity scale
The UPPS-P Impulsive Behavior Scale (Whiteside et al., 2005; Cyders and Smith, 2007) was used to measure trait impulsivity. The UPPS-P consists of 59 items assessing multiple facets of impulsivity including negative urgency, lack of premeditation, lack of perseverance, sensation seeking and positive urgency. Items were averaged into a single Impulsivity composite score. Impulsivity is thought to index the inverse of control over motivational impulses (r-BIS). All scores were within 2.37 standard deviations of the mean.
Procedure
Participants were brought into the lab and consented to participate. They completed demographic and personality questionnaires. Then, EEG electrodes were applied. Participants then completed 8 min of baseline resting EEG recording, half with eyes open and half with eyes closed and counterbalanced across participants.
EEG recording and processing
Participants were fitted with a 64-channel tin electrode stretch lycra cap (Electro-Caps, Eaton, OH). Sensor placement was based on the 10-20 system with a ground electrode mounted between FPZ and FZ. Electrode impedances were kept under 5 kΩ, with homologous sites kept within 1 kΩ of one another. EEG activity was referenced online to the left earlobe. Data were collected using a Neuroscan SynAmps RT amplifier unit (El Paso, TX). Data were filtered online with a low pass filter at 100 Hz, high pass filter at 0.05 Hz, notch filter at 60 Hz and were digitized at 500 Hz. The filter slope was set at 12 dB per octave. In order to eliminate artifacts, all data were visually inspected. Visible artifacts of muscle movement and horizontal eye movement were removed by hand. Then, a regression-based eyeblink correction was applied (Semlitsch et al., 1986). Finally, data were visually inspected a second time to ensure proper removal and correction of artifacts.
All epochs 1.024 s in duration from all 8 min of baseline activity were extracted through a Hamming window. Consistent with much past research connecting frontal activity and individual differences in BIS and BAS (Sutton and Davidson, 1997; Amodio et al., 2008) data were rereferenced using an average ears reference. Each consecutive epoch overlapped by 50%. Power spectra were calculated using a fast Fourier transform. The band inspected was the traditional alpha band (8–13 Hz), and power values were averaged across epochs. An asymmetry score was calculated at medial frontal sites by subtracting natural logarithm log (base e) transformed alpha power for left (F3) from right (F4) sites (Harmon-Jones and Allen, 1997; Sutton and Davidson, 1997; Pauls et al., 2005). Asymmetry scores were also calculated for posterior sites (P4–P3) and central sites (C4–C3) to examine whether the effect was specific to frontal regions. Because alpha power inversely relates to cortical activity, lower asymmetry scores indicated greater relative right frontal activity. An average of 831.67 (s.d. = 153.81) usable epochs were analyzed for each participant.
Results
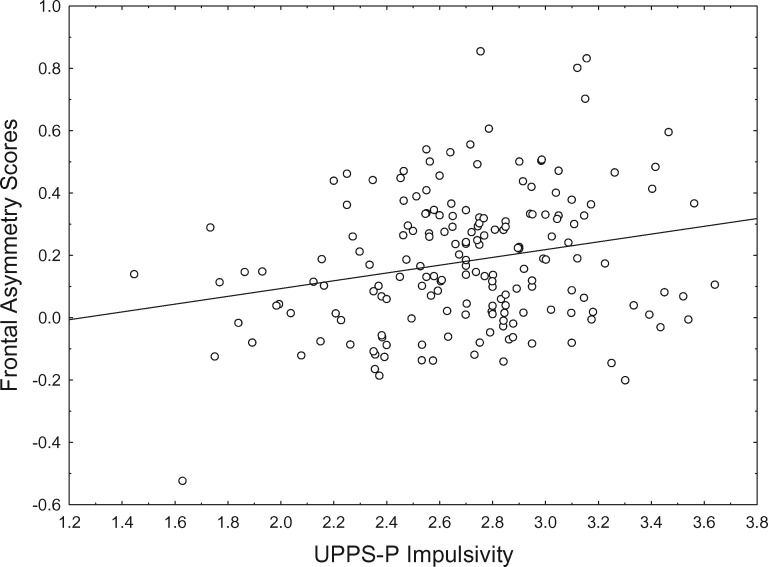
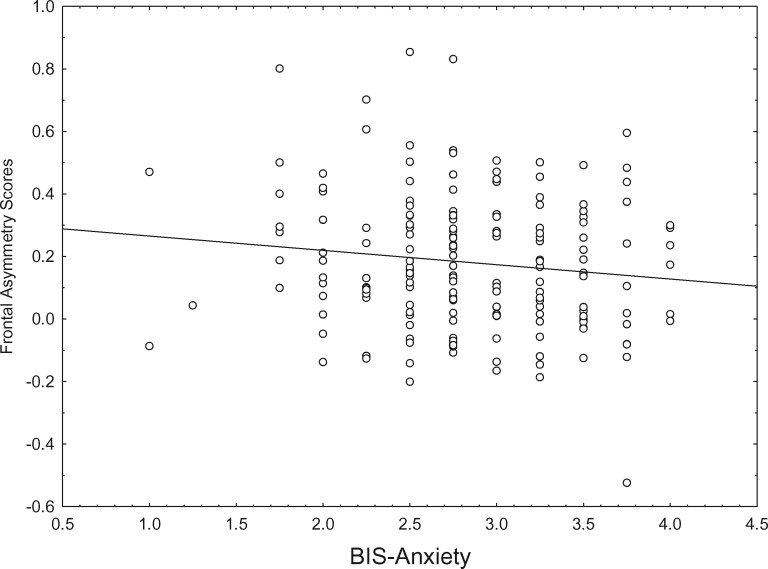
Descriptive statistics and reliability for all key variables are presented in Table 1. Correlations among personality variables and frontal asymmetry scores are presented in Table 2. Resting frontal asymmetry scores were regressed on each of the personality variables individually. UPPSP Impulsivity predicted less relative right frontal activity, ß = 0.23, p < 0.002 (Figure 1). BIS-Anxiety predicted greater relative right frontal activity, ß = –0.15, p < 0.05 (Figure 2).1 BAS ( ß = 0.01, p = 0.92) and FFFS ( ß = –0.09, p = 0.23) were unrelated to frontal activity.
Table 1.
Descriptive statistics and reliability for key variables
| Mean (s.d.) | Male mean (s.d.) | Female mean (s.d.) | Reliability (Cronbach’s α) | |
|---|---|---|---|---|
| UPPS-P | 2.70 (0.39) | 2.64 (0.32)a | 2.73 (0.43)a | 0.92 |
| BIS-anxiety | 2.84 (0.60) | 2.61 (0.57)a | 2.98 (0.54)b | 0.73 |
| FFFS-fear | 2.99 (0.57) | 2.74 (0.50)a | 3.14 (0.55)b | 0.56 |
| BAS | 3.02 (0.35) | 3.00 (0.32)a | 3.04 (0.48)a | 0.81 |
| Original BIS | 2.91 (0.52) | 2.67 (0.48)a | 3.04 (0.48)b | 0.74 |
| Frontal asymmetry | 0.18 (0.22) | 0.18 (0.20)a | 0.17 (0.22)a | 0.97 |
Notes and Sources: UPPS-P and BAS scales are comprised of all items in each of the subscales of these measures. Frontal Asymmetry is the natural logarithm log (base e) difference scores at sites F4 and F3 for each minute of resting activity. Between-column differences between men and women are indicated by different superscripts (P < 0.05).
Table 2.
Correlations among personality variables
| Variables | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. UPPS-P impulsivity | –– | |||||
| 2. BIS-anxiety | 0.14 | –– | ||||
| 3. FFFS-fear | 0.00 | 0.62* | –– | |||
| 4. BAS | –0.26* | –0.04 | 0.08 | –– | ||
| 5. Original BIS | 0.09 | 0.93* | 0.87* | 0.01 | –– | |
| 6. Frontal asymmetry | 0.23* | –0.15* | –0.09 | 0.01 | –0.14 | –– |
P < 0.05.
Fig. 1.
UPPSP impulsivity and frontal asymmetry scores (F4–F3). Smaller Frontal Asymmetry Scores indicate greater relative right frontal activity.
Fig. 2.
BIS-anxiety scores and frontal asymmetry scores (F4–F3). Smaller Frontal Asymmetry Scores indicate greater relative right frontal activity.
In order to determine whether the effects were specific to frontal regions, the bivariate relationships were examined between the personality variables and asymmetry scores for central and parietal sites. No relationships were found between personality and central or parietal asymmetry scores (p’s > 0.18). Results of these analyses are presented in Table 3.
Table 3.
Correlations between personality measures and central and parietal asymmetry scores
| Frontal asymmetry | Central asymmetry | Parietal asymmetry | |
|---|---|---|---|
| UPPS-P impulsivity | 0.23* | 0.09 | 0.08 |
| BIS-anxiety | –0.15* | –0.08 | 0.10 |
| FFFS-fear | –0.09 | –0.09 | 0.00 |
| BAS | 0.01 | –0.09 | –0.02 |
p < 0.05.
A Multiple Regression analysis was conducted to investigate which personality variables predicted frontal activity controlling for the other scales. UPPS-P Impulsivity, BIS-Anxiety, FFFS-Fear and BAS were entered into a multiple regression model with frontal activity as the dependent variable. A significant regression equation was found F(3, 173) = 4.02, p < 0.009, R2 = 0.07. In this model, only UPPS-P Impulsivity and BIS-Anxiety were significant predictors of frontal activity (Table 4).
Table 4.
Multiple regression of frontal asymmetry on personality
| ß | t | p | |
|---|---|---|---|
| UPPS-P impulsivity | 0.28 | 3.65 | 0.0003 |
| BIS-anxiety | –0.21 | –2.24 | 0.02 |
| FFFS-fear | 0.04 | 0.37 | 0.71 |
| BAS | 0.07 | 0.90 | 0.37 |
Frontal asymmetry scores did not differ based on sex, t(170) = –0.21, p = 0.84. Sex was entered as a predictor in multiple regression analyses with each personality variable predicting frontal asymmetry score, and then in the full model with all personality variables predicting frontal asymmetry. When examining the relationships between personality variables and frontal asymmetry controlling for sex, sex was not a significant predictor (p’s > 0.59).
Discussion
Greater trait BIS-Anxiety was related to greater relative right frontal activation. Importantly, these results are the first to link individual difference measures of greater regulatory control with greater resting right frontal activity. Greater trait impulsivity was related to less relative right frontal activation. These findings support previous work establishing the connection between reduced regulatory control and diminished right frontal activity (Gable et al., 2015; Mechin et al., 2016; Neal and Gable, 2016). Additionally, this study is the first to investigate the relative contributions of traits relating to r-BIS and FFFS in Carver and White’s (1994) BIS scale to resting frontal activity. The results suggest that the regulatory r-BIS system may be more closely tied to right frontal activity than the avoidance-motivating FFFS system. Our study supports recent advances in RST theory aimed at differentiated the functions and neural correlates of the r-BIS and FFFS (De Pascalis et al., 2017).
Past research has been inconsistent when relating the BIS scale to resting frontal activity. This study suggests that these mixed results may be due to items in the BIS scale assessing both FFFS and r-BIS. The BIS/BAS scales were created prior to a substantial revision of RST, and have not been updated to reflect the nuances between the functioning of the BIS and FFFS systems. Individual differences in r-BIS assessed by items in the BIS scale may account for some past research finding a relationship between the BIS scale and right frontal activity. It may be that conflict arising between approach and avoidance motivations in these past studies activated r-BIS and resulted in greater right frontal activity. In contrast, in studies where no relationship was found between frontal activity and the BIS scale, combining individual differences in trait FFFS-Fear may have masked the relationship between BIS scale and resting frontal activity. By separately accounting for the variance in BIS-Anxiety and FFFS-Fear, rather than combining them into a single BIS scale, the current results help clarify the relationship between greater right frontal activity and the BIS scale may be driven by individual difference in regulatory control measured by BIS-Anxiety. Our findings that the UPPS-P Impulsive Behavior Scale relates to greater right frontal activity further support the idea that regulatory control relates to right frontal activity.
The current findings relating BIS-Anxiety to right frontal activation are consistent with past work linking traits associated with r-BIS to frontal asymmetry (Wacker et al., 2003, 2008, 2010; Gable et al., 2015; Neal and Gable, 2016). However, this study relates right frontal activity to regulatory traits beyond those investigated in past research. Past work has focused on diminished functioning of r-BIS. The current findings extend these past results to relate right frontal activity to enhanced functioning of regulatory control. In addition, impulsivity measures a lack of ability to regulate approach urges in the absence of an approach-avoidance conflict. The use of both BIS-Anxiety and UPPS-P Impulsivity scales encompasses both control of conflicting urges and ability to regulate motivational urges in the absence of conflict.
The current findings that r-BIS rather than withdrawal motivation is related to right frontal activity is supported by other neurophysiological work. Diminished activity in the right prefrontal cortex has been related to greater risk-taking behavior (Gianotti et al., 2009). Lesions of the right prefrontal cortex lead to poor inhibition in a stop signal task (Aron et al., 2003) and risky decisions in a gambling task (Tranel et al., 2002; Clark et al., 2003). Enhancing activity in the right inferior frontal gyrus using transcranial direct current stimulation (tDCS) leads to better response inhibition in a stop signal task (Jacobson et al., 2011; Stramaccia et al., 2015). Additionally, tDCS stimulation of the right dorsolateral prefrontal cortex led to less risky decision making in a gambling task (Fecteau et al., 2007). Kelley and Schmeichel (2016) demonstrated that the right frontal cortex is involved in the inhibition of both approach and avoidance behavior, a key function of the r-BIS. Participants who received right frontal tDCS excitatory stimulation were faster to respond to motivationally-incongruent trials in an Approach-Avoidance task for both approach-motivated and avoidance-motivated stimuli.
One limitation of the current study is that the original BIS scale items separate into two subscales (e.g. BIS-Anxiety and FFFS-Fear) with only three and four items each. Because so few items make up each subscale, it can be difficult to obtain high internal consistency. In our results, the BIS Anxiety scale obtained adequate internal consistency. However, the FFFS Fear scale had poor internal consistency. A revised version of the BIS/BAS scales may more effectively measure sensitivity of the three personality systems. Newer scales assessing revised RST may be better able to capture sensitivity of the FFFS and BIS systems (Corr, 2016), and may more strongly relate to neural correlates such as frontal asymmetry.
The effect sizes observed between personality traits and resting EEG activity were small. These small effects may be due in part to the influence of state variance in resting EEG activity (Hagemann et al., 2002). However, even true effects do not always replicate in null-hypothesis testing (Cumming, 2014). For example, many studies have found that higher trait BAS is related to left frontal activity, but the current results did not find a relationship between left frontal activity and BAS. The relationship between approach motivation and frontal asymmetric activity is related to individual differences in approach motivation to stimuli and may be largely driven by situational context, such as emotional/motivation states (Coan et al., 2006; Gable and Poole, 2014).
While resting frontal activity has been extensively studied as a stable trait measure, it is worth noting that state factors may be influencing EEG during a resting state recording in the laboratory. For instance, Kline et al. (2002) found that the sex of the experimenter interacted with the trait defensiveness of the participant to affect frontal asymmetrical activity. Peterson and Harmon-Jones (2009) found that time of day and time of year also affect frontal EEG activity. Body posture of the participant during EEG recording can also influence frontal activity (Price and Harmon-Jones, 2011). Much of the variance in resting frontal activity may stem from state influences (Hagemann, 2004). However, Hagemann et al. (2002) have found that 60% of the variance in resting frontal activity is a result of trait influences. Results of the current study suggest that trait regulatory control appears to be related to greater right frontal activity, even while state influences cause fluctuations in resting alpha power.
Decades of research have related basic biological systems to lateralized frontal activity. The current study suggests that control of motivational impulses of approach and avoidance may be strongly related to frontal activity. Gaining a better understanding of what traits contribute to frontal activity at rest may help us better understand the relationship between neural correlates and behavior in regards to functional and dysfunctional behavior.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Supplementary Material
Footnotes
The participant with the lowest asymmetry score was not considered an outlier based on Standard Deviations. When removed from the analyses, results from the multiple regression analyses remain similar when excluding this participant. In the multiple regression model, UPPS-P Impulsivity and BIS-Anxiety remain significant predictors (ß = 0.24 and ß = –0.20, respectively) while BAS and FFFS-Fear remain non-significant (P’s > 0.29).
References
- Allen J.J., Coan J.A., Nazarian M. (2004). Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology, 67(1), 183–218. [DOI] [PubMed] [Google Scholar]
- Amodio D.M., Master S.L., Yee C.M., Taylor S.E. (2008). Neurocognitive components of the behavioral inhibition and activation systems: implications for theories of self‐regulation. Psychophysiology, 45(1), 11–9. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Fletcher P.C., Bullmore E.T., Sahakian B.J., Robbins T.W. (2003). Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience, 6(2), 115–6. [DOI] [PubMed] [Google Scholar]
- Berkman E.T., Lieberman M.D. (2010). Approaching the bad and avoiding the good: lateral prefrontal cortical asymmetry distinguishes between action and valence. Journal of Cognitive Neuroscience, 22(9), 1970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver C.S., White T.L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67(2), 319–33. [Google Scholar]
- Clark L., Manes F., Antoun N., Sahakian B.J., Robbins T.W. (2003). The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia, 41(11), 1474–83. [DOI] [PubMed] [Google Scholar]
- Coan J.A., Allen J.J. (2003). Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology, 40(1), 106–14. [DOI] [PubMed] [Google Scholar]
- Coan J.A., Allen J.J., Harmon‐Jones E. (2001). Voluntary facial expression and hemispheric asymmetry over the frontal cortex. Psychophysiology, 38(6), 912–25. [DOI] [PubMed] [Google Scholar]
- Coan J.A., Allen J.J., McKnight P.E. (2006). A capability model of individual differences in frontal EEG asymmetry. Biological Psychology, 72(2), 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook I.A., O'Hara R., Uijtdehaage S.H., Mandelkern M., Leuchter A.F. (1998). Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalography and Clinical Neurophysiology, 107(6), 408–14. [DOI] [PubMed] [Google Scholar]
- Corr P.J. (2016). Reinforcement sensitivity theory of personality questionnaires: structural survey with recommendations. Personality and Individual Differences, 89, 60–4. [Google Scholar]
- Corr P.J., McNaughton N. (2008). Reinforcement sensitivity theory and personality In: Corr P.J., editor. The Reinforcement Theory of Personality. Cambridge: Cambridge University Press. [Google Scholar]
- Cumming G. (2014). The new statistics: why and how. Psychological Science, 25(1), 7–29. [DOI] [PubMed] [Google Scholar]
- Cyders M.A., Smith G.T. (2007). Mood-based rash action and its components: positive and negative urgency. Personality and Individual Differences, 43(4), 839–50. [Google Scholar]
- De Pascalis V., Cozzuto G., Caprara G.V., Alessandri G. (2013). Relations among EEG-alpha asymmetry, BIS/BAS, and dispositional optimism. Biological Psychology, 94(1), 198–209. [DOI] [PubMed] [Google Scholar]
- De Pascalis V., Fracasso F., Corr P.J. (2017). Personality and Augmenting/Reducing (A/R) in auditory event-related potentials (ERPs) during emotional visual stimulation. Scientific Reports, 7, 41588.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S., Pascual-Leone A., Zald D.H., et al. (2007). Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. The Journal of Neuroscience, 27(23), 6212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable P.A., Mechin N.C., Hicks J.A., Adams D.L. (2015). Supervisory control system and frontal asymmetry: neurophysiological traits of emotion-based impulsivity. Social, Cognitive, and Affective Neuroscience, 10(10), 1310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable P.A., Neal L.B., Threadgill A.H. (in press). Regulatory behavior and frontal asymmetry: considering the role of revised-BIS in relative right frontal asymmetry. Psychophysiology, DOI: 10.1111/psyp.12910. [DOI] [PubMed] [Google Scholar]
- Gable P.A., Poole B.D. (2014). Influence of trait behavioral inhibition and behavioral approach motivation systems on the LPP and frontal asymmetry to anger pictures. Social Cognitive and Affective Neuroscience, 9(2), 182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianotti L.R., Knoch D., Faber P.L., et al. (2009). Tonic activity level in the right prefrontal cortex predicts individuals' risk taking. Psychological Science, 20(1), 33–8. [DOI] [PubMed] [Google Scholar]
- Gray J.A. (1970). The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy, 8(3), 249–66. [DOI] [PubMed] [Google Scholar]
- Gray J.A., McNaughton N. (2000). The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. Oxford: Oxford University Press. [Google Scholar]
- Hagemann D. (2004). Individual differences in anterior EEG asymmetry: methodological problems and solutions. Biological Psychology, 67(1), 157–82. [DOI] [PubMed] [Google Scholar]
- Hagemann D., Naumann E., Thayer J.F., Bartussek D. (2002). Does resting electroencephalograph asymmetry reflect a trait? An application of latent state-trait theory. Journal of Personality and Social Psychology, 82(4), 619.. [PubMed] [Google Scholar]
- Harmon-Jones E., Allen J.J. (1997). Behavioral activation sensitivity and resting frontal EEG asymmetry: covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology, 106(1), 159–63. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Gable P.A. (in press). On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: an updated review of the evidence. Psychophysiology, DOI: 10.1111/psyp.12879. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Price T.F., Peterson C.K., Gable P.A., Harmon-Jones C. (2013). The influence of behavioral approach and behavioral inhibition sensitivities on emotive cognitive processes In: Robinson M. D., Watkins E. R., Harmon-Jones E., editors. Handbook of Cognition and Emotion. New York: Guilford Publications. [Google Scholar]
- Henriques J.B., Davidson R.J. (2000). Decreased responsiveness to reward in depression. Cognition and Emotion, 14(5), 711–24. [Google Scholar]
- Hewig J., Hagemann D., Seifert J., Naumann E., Bartussek D. (2004). On the selective relation of frontal cortical asymmetry and anger-out versus anger-control. Journal of Personality and Social Psychology, 87(6), 926.. [DOI] [PubMed] [Google Scholar]
- Hewig J., Hagemann D., Seifert J., Naumann E., Bartussek D. (2006). The relation of cortical activity and BIS/BAS on the trait level. Biological Psychology, 71(1), 42–53. [DOI] [PubMed] [Google Scholar]
- Heym N., Ferguson E., Lawrence C. (2008). An evaluation of the relationship between Gray’s revised RST and Eysenck’s PEN: distinguishing BIS and FFFS in Carver and White’s BIS/BAS scales. Personality and Individual Differences, 45(8), 709–15. [Google Scholar]
- Jackson D.C., Mueller C.J., Dolski I., et al. (2003). Now you feel it, now you don't frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science, 14(6), 612–7. [DOI] [PubMed] [Google Scholar]
- Jacobson L., Javitt D.C., Lavidor M. (2011). Activation of inhibition: diminishing impulsive behavior by direct current stimulation over the inferior frontal gyrus. Journal of Cognitive Neuroscience, 23(11), 3380–7. [DOI] [PubMed] [Google Scholar]
- Kelley N.J., Schmeichel B.J. (2016). Noninvasive stimulation over the dorsolateral prefrontal cortex facilitates the inhibition of motivated responding. Journal of Experimental Psychology: General, 145(12), 1702–12. [DOI] [PubMed] [Google Scholar]
- Keune P.M., Bostanov V., Kotchoubey B., Hautzinger M. (2012). Mindfulness versus rumination and behavioral inhibition: a perspective from research on frontal brain asymmetry. Personality and Individual Differences, 53(3), 323–8. [Google Scholar]
- Kline J.P., Blackhart G.C., Joiner T.E. (2002). Sex, lie scales, and electrode caps: an interpersonal context for defensiveness and anterior electroencephalographic asymmetry. Personality and Individual Differences, 33(3), 459–78. [Google Scholar]
- Kline J.P., Blackhart G.C., Woodward K.M., Williams S.R., Schwartz G.E. (2000). Anterior electroencephalographic asymmetry changes in elderly women in response to a pleasant and an unpleasant odor. Biological Psychology, 52(3), 241–50. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G., Slobodskaya H.R. (2003). Personality trait of behavioral inhibition is associated with oscillatory systems reciprocal relationships. International Journal of Psychophysiology, 48(3), 247–61. [DOI] [PubMed] [Google Scholar]
- Mechin N., Gable P.A., Hicks J.A. (2016). Frontal asymmetry and alcohol cue reactivity: influence of core personality systems. Psychophysiology, 53(8), 1224–31. [DOI] [PubMed] [Google Scholar]
- Neal L.B., Gable P.A. (2016). Neurophysiological markers of multiple facets of impulsivity. Biological Psychology, 115, 64–8. [DOI] [PubMed] [Google Scholar]
- Pauls C.A., Wacker J., Crost N.W. (2005). The two components of social desirability and their relations to resting frontal brain asymmetry. Journal of Individual Differences, 26(1), 29–42. [Google Scholar]
- Peterson C.K., Harmon-Jones E. (2009). Circadian and seasonal variability of resting frontal EEG asymmetry. Biological Psychology, 80(3), 315–20. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A., Sherwood R.J., Henriques J.B., Davidson R.J. (2005). Frontal brain asymmetry and reward responsiveness a source-localization study. Psychological Science, 16(10), 805–13. [DOI] [PubMed] [Google Scholar]
- Price T.F., Harmon‐Jones E. (2011). Approach motivational body postures lean toward left frontal brain activity. Psychophysiology, 48(5), 718–22. [DOI] [PubMed] [Google Scholar]
- Quaedflieg C.W.E.M., Meyer T., Smulders F.T.Y., Smeets T. (2015). The functional role of individual-alpha based frontal asymmetry in stress responding. Biological Psychology, 104, 75–81. [DOI] [PubMed] [Google Scholar]
- Quirin M., Gruber T., Kuhl J., Düsing R. (2013). Is love right? Prefrontal resting brain asymmetry is related to the affiliation motive. Frontiers in Human Neuroscience, 7, 902.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J., Ashbaugh A.R., Antony M.M., McCabe R.E., Schmidt L.A. (2008). Frontal EEG asymmetry and sensation seeking in young adults. Biological Psychology, 78(2), 164–72. [DOI] [PubMed] [Google Scholar]
- Semlitsch H.V., Anderer P., Schuster P., Presslich O. (1986). A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology, 23(6), 695–703. [DOI] [PubMed] [Google Scholar]
- Shackman A.J., McMenamin B.W., Maxwell J.S., Greischar L.L., Davidson R.J. (2009). Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychological Science, 20(12), 1500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie L.D., Pickering A.D., Jackson C.J. (2006). The new reinforcement sensitivity theory: implications for personality measurement. Personality and Social Psychology Review, 10(4), 320–35. [DOI] [PubMed] [Google Scholar]
- Stramaccia D.F., Penolazzi B., Sartori G., Braga M., Mondini S., Galfano G. (2015). Assessing the effects of tDCS over a delayed response inhibition task by targeting the right inferior frontal gyrus and right dorsolateral prefrontal cortex. Experimental Brain Research, 233(8), 2283–90. [DOI] [PubMed] [Google Scholar]
- Sutton S.K., Davidson R.J. (1997). Prefrontal brain asymmetry: a biological substrate of the behavioral approach and inhibition systems. Psychological Science, 8(3), 204–10. [Google Scholar]
- Tranel D., Bechara A., Denburg N.L. (2002). Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex, 38(4), 589–612. [DOI] [PubMed] [Google Scholar]
- Wacker J., Chavanon M.L., Leue A., Stemmler G. (2008). Is running away right? The behavioral activation-behavioral inhibition model of anterior asymmetry. Emotion, 8(2), 232–49. [DOI] [PubMed] [Google Scholar]
- Wacker J., Chavanon M.L., Stemmler G. (2010). Resting EEG signatures of agentic extraversion: new results and meta-analytic integration. Journal of Research in Personality, 44(2), 167–79. [Google Scholar]
- Wacker J., Heldmann M., Stemmler G. (2003). Separating emotion and motivational direction in fear and anger: effects on frontal asymmetry. Emotion, 3(2), 167.. [DOI] [PubMed] [Google Scholar]
- Whiteside S.P., Lynam D.R., Miller J.D., Reynolds S.K. (2005). Validation of the UPPS impulsive behaviour scale: a four‐factor model of impulsivity. European Journal of Personality, 19(7), 559–74. [Google Scholar]
- Zuckerman M. (1994). Behavioral Expressions and Biosocial Bases of Sensation Seeking. New York: Cambridge university press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.