Abstract
Background and Aims Temporal flooding is a common environmental stress for terrestrial plants. Aquatic adventitious roots (aquatic roots) are commonly formed in flooding-tolerant plant species and are generally assumed to be beneficial for plant growth by supporting water and nutrient uptake during partial flooding. However, the actual contribution of these roots to plant performance under flooding has hardly been quantified. As the investment into aquatic root development in terms of carbohydrates may be costly, these costs may – depending on the specific environmental conditions – offset the beneficial effects of aquatic roots. This study tested the hypothesis that the balance between potential costs and benefits depends on the duration of flooding, as the benefits are expected to outweigh the costs in long-term but not in short-term flooding.
Methods The contribution of aquatic roots to plant performance was tested in Solanum dulcamara during 1–4 weeks of partial submergence and by experimentally manipulating root production. Nutrient uptake by aquatic roots, transpiration and photosynthesis were measured in plants differing in aquatic root development to assess the specific function of these roots.
Key Results As predicted, flooded plants benefited from the presence of aquatic roots. The results showed that this was probably due to the contribution of roots to resource uptake. However, these beneficial effects were only present in long-term but not in short-term flooding. This relationship could be explained by the correlation between nutrient uptake and the flooding duration-dependent size of the aquatic root system.
Conclusions The results indicate that aquatic root formation is likely to be selected for in habitats characterized by long-term flooding. This study also revealed only limited costs associated with adventitious root formation, which may explain the maintenance of the ability to produce aquatic roots in habitats characterized by very rare or short flooding events.
Keywords: Adventitious root removal, benefit, cost, flooding duration, nutrient uptake, partial submergence, plasticity, root function, Solanum dulcamara, water uptake
Introduction
Temporal flooding of soils and total or partial submergence of above-ground parts can present a major environmental stress for terrestrial plants (Voesenek et al., 2006; Colmer and Voesenek, 2009). Upon flooding, O2 is rapidly depleted in the rhizosphere due to respiration of roots and aerobic micro-organisms, which subsequently causes loss of function of the root system as the oxygen-requiring metabolic processes are strongly hampered (Armstrong, 1979; Sauter, 2013). However, many plant species can produce adventitious roots on their submerged stems during flooding events (Jackson and Drew, 1984; Rich et al., 2008; Steffens et al., 2012; Steffens and Rasmussen, 2016). These roots contain aerenchyma, a highly porous tissue type that facilitates O2 diffusion from the shoot into the root (Takahashi et al., 2014; Armstrong and Armstrong, 2014). This improves the internal aeration of the plant, particularly when parts of the shoot are still extending above the floodwater surface, and allows energy-dependent root functions such as water and nutrient uptake to continue (Colmer, 2003). Therefore, adventitious root formation is considered an important adaptation of plants to flooding. Remarkably, empirical studies quantifying these assumed benefits of adventitious roots for plant performance are still scarce. This study aims to provide evidence for a positive contribution of stem-borne aquatic adventitious roots, i.e. adventitious roots that are suspended in the water column, to plant performance, and investigates if this relationship depends on flooding duration.
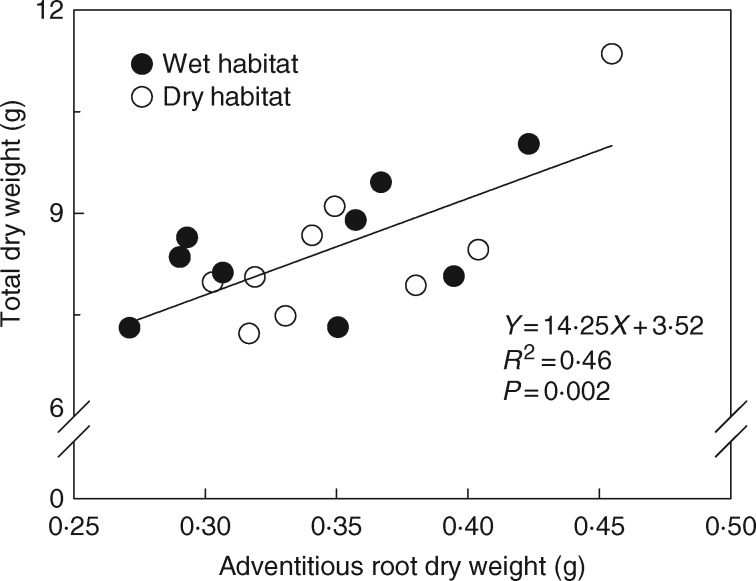
Previous studies particularly linked the capacity of adventitious root formation to plant performance by quantifying variation in adventitious rooting among closely related species growing along a flooding gradient. These studies revealed that species from habitats characterized by different hydrological regimes differed significantly in the morphology and size of their adventitious root system (Laan et al., 1989; Blom et al., 1994; Visser et al., 1996). The flooding tolerance of species seems to be closely linked to their ability to form adventitious roots. For example, Whiteman et al. (1984) investigated the flooding tolerance of 17 tropical pasture legumes, and found that species with a capacity to produce medium to large adventitious root systems have superior flooding tolerance. The number and porosity of adventitious roots also positively correlated with the flooding tolerance of woody Hakea species from contrasting hydrological environments (Poot and Lambers, 2003). However, even individuals of a single species can be subjected to distinctively different flooding regimes if the species occurs along a flooding gradient (Lenssen et al., 2004; Chen et al., 2009; Huber et al., 2014; Zhang et al., 2016). Covering such a wide range of flooding conditions potentially selects for within-species difference in adaptive traits that are related to flooding tolerance, such as adventitious root development, and is thus expected to lead to genetic differentiation along the flooding gradient in terms of flooding resistance. A previous study testing this hypothesis revealed considerable among-population variation in aquatic adventitious root formation of Solanum dulcamara (bittersweet), a plant species occurring in contrasting hydrological environments and producing large numbers of aquatic adventitious roots when partially submerged (Zhang et al., 2015, 2016). However, surprisingly, this variation in root production could not be linked to flooding frequency in the local habitat types (Zhang et al., 2016). We re-analysed data from that study, which revealed a positive correlation between aquatic adventitious root biomass and the performance of the partially submerged plants (Fig. 1). These results indicate that while hydrological regimes under natural conditions apparently did not select for differences in aquatic adventitious root formation between habitats, overall natural variation in aquatic adventitious root formation might result in fitness differences in terms of biomass production in S. dulcamara. To unambiguously show the fitness benefits of aquatic adventitious roots, experiments manipulating the formation of aquatic adventitious roots coupled to measurements of fitness parameters such as plant biomass production are needed.
Fig. 1.
Relationship between the dry weight of aquatic adventitious roots and the total dry weight (adventitious root dry weight excluded) of the entire plant after 3 weeks of experimental partial flooding in plants originating from dry (open symbols) and wet (shaded symbols) habitats along the Dutch coast (original data from Zhang et al., 2016). To test the relationship between adventitious root dry weight with total plant dry weight excluding adventitious root dry weight across 18 populations of S. dulcamara (Zhang et al., 2016), a linear regression model was fitted with adventitious root dry weight and the total plant dry weight as independent and dependent variables, respectively.
Under anaerobic conditions, aquatic adventitious roots have been shown to play an important role in nutrient uptake (Trought and Drew, 1980; Khan et al., 1982). As uptake of several mineral nutrients depends strongly on the total root surface area (Comas et al., 2013), the beneficial effects of adventitious roots may be more apparent in long-term flooding events where the adventitious roots have grown substantially larger than in short-term flooding. Furthermore, many flooding-induced adventitious roots in wetland species typically grow in the floodwater column rather than in the sediment, and most of these roots may die after flooding subsides (Rich et al., 2012; Zhang et al., 2015). The loss of aquatic adventitious roots after short-term flooding may negatively affect plant performance, as the initial investment into cell division and elongation during aquatic adventitious root development might have not been returned in terms of increased water and nutrient uptake (Takahashi et al., 2003). Consequently, the net benefits of aquatic adventitious roots are expected to depend on flooding duration.
In the present study, we used the species S. dulcamara to (1) quantify the benefits of aquatic adventitious roots during prolonged partial flooding by experimentally manipulating the number of aquatic adventitious roots formed, (2) show the contribution of these aquatic adventitious roots to nutrient and water uptake under partially submerged conditions and (3) elucidate whether the effect of aquatic adventitious root formation on plant performance depends on flooding duration.
Materials and Methods
Species and plant material
Solanum dulcamara L. (bittersweet) is a perennial, potentially climbing species (D’Agostino et al., 2013), native to Europe and occurring in contrasting ecological habitats, such as permanently flooded wetlands and dry sand dunes (Visser et al., 2015; Zhang et al., 2015). For a larger research programme, seeds were collected from S. dulcamara populations originating from either wet or dry habitats at nine locations along the coastline of the Netherlands (Zhang et al., 2016) in autumn 2012 and 2013. Detailed information about these contrasting habitats has been described in Zhang et al. (2016). The seeds of these 18 populations were cleaned and dried at room temperature, and then stored separately at 4 °C. For the present experiments, seeds collected along a dune lake on the island of Texel, one of the nine locations, were used. This population was chosen for its representative position in terms of aquatic adventitious root production among the 18 populations.
Prior to sowing, seeds were surface sterilized with 15 % (w/v) tri-sodium phosphate (Sigma Aldrich Chemie GmbH, Steinheim, Germany) solution in water for 20 min and rinsed with abundant tap water. After sterilization, seeds were either directly sown in seed trays with cells of 3·5×3·5×4 cm (length ×width×depth) filled with commercial sowing compost (Horticoop substrate, Lentse potgrond, Cuijk, the Netherlands) or first placed on wet filter paper in Petri dishes and then transplanted to the seed trays after germination. To induce germination, seeds were stratified to break dormancy by placing the wet seeds at 4 °C for 2–3 weeks. After stratification, seeds were transferred to 20 °C and, upon germination, plants were defined to be 0 weeks old. After approximately 3 weeks, when plants had developed four to five leaves, homogenously grown seedlings were selected and transplanted to individual pots of 1 L (experiments 1 and 4) or 1·35 L (experiments 2 and 3), filled with a mixture of sandy soil (70 % sand, 30 % clay) and 4 g L−1 slow-release coated fertilizer (Osmocote Exact Standard, 3–4 months; Everris International, Geldermalsen, the Netherlands). Plants were grown in a greenhouse and watered regularly with tap water. Within 2 weeks after repotting, plants were fertilized twice with an additional 50 mL of nutrient solution (2 g L−1 of Kristalon, Yara International ASA, Vlaardingen, the Netherlands). The average temperature in the greenhouse was 20.1°C and the average air humidity was 58 %.
Experimental designs
Experiment 1: effects of decreased aquatic adventitious root formation on plant performance.
We aimed to evaluate the importance of aquatic adventitious roots by experimentally manipulating the formation of these roots. In a pilot experiment, we first investigated the optimal methods to prevent the formation of aquatic adventitious roots (Supplementary Data Fig. S1). Of seven adventitious root manipulation treatments, two treatments resulted in the lowest and second lowest number of adventitious roots, respectively: a removal treatment where all the visible adventitious root primordia on the stem were removed before partial submergence, and a Vaseline® treatment where a thick layer (∼ 0·5 cm) of Vaseline covered the stem and prevented the stem from being in contact with the surrounding floodwater. These two treatments were chosen for the current experiment based on the absence of negative side effects of the manipulations per se and the inhibiting effects on root expansion in the pilot experiment, which were compared to a control without manipulation of root formation and a control treatment where wounds similar to those in the primordium removal treatment were made to the stem tissue (leaving the primordia intact). The latter treatment tested for potential side effects of the inevitable wounding caused by the removal treatment. All four treatments were then subjected to either partially submerged conditions, or irrigated but drained conditions, respectively, for 3 weeks. The latter conditions served as a control to examine the effects of flooding on plant growth.
Eighty-eight plants 64 ± 12 (mean ± 1 s.e.) cm high were selected for homogeneity and subsequently assigned to the four treatments under both drained and partially submerged conditions for 3 weeks. Plants in drained conditions were well watered and plants in partially submerged conditions remained flooded to 10 cm above the soil surface throughout the experiment. During partial submergence, plants developed occasionally aquatic adventitious roots from root primordia covered by Vaseline (on average seven roots per plant, and mainly only after the second week of flooding), or, more frequently, from newly formed primordia in the removal treatment (37 roots per plant). These new roots were removed every day, and therefore never reached a length exceeding 1 cm. In the untreated partially submerged plants, on average 69 ± 10·7 (mean ± 1 s.e.) roots per plant were formed throughout the experiment.
After 3 weeks of treatment, all plants were harvested (for measurements, see below).
Experiment 2: nutrient uptake by aquatic adventitious roots during flooding.
In this experiment, we evaluated the effect of the size of the aquatic adventitious root system on the capacity for uptake of mineral nutrients. Here, we used the uptake of phosphate (P), an essential macronutrient for plant growth, as an illustration. Plants displaying a range of adventitious root number and length were created by subjecting 12 8-week-old plants to different flooding treatments. Half of the plants were partially submerged to 15 cm above the soil surface for 2 weeks. The remaining plants were first soil flooded (1 cm above the soil surface) for 1 week, and then partially submerged at 15 cm depth for another week to have fewer and shorter aquatic adventitious roots while keeping the sediment roots flooded for the same period of time.
To be able to supply nutrient solution to the aquatic adventitious roots on the stem only, and not to the original root system in the soil, a glass cylindrical cuvette (diameter = 10 cm, height = 8 cm) with detachable top and bottom lids was constructed (Supplementary Data Fig. S2). After attaching the cuvette to the stem with a clamp around the top lid, the bottom lid was sealed to the cuvette with a mixture (v/v, 1 : 1) of silicon sealant (Silicone Glass Sealant, Bison International B.V., Goes, the Netherlands) and corn starch (Maizena, Koopmans, Amersfoort, the Netherlands). This mixture shortens the hardening of silicon sealant to about 45 min. During this time, the floodwater (tap water; P concentration 0·316 µmol L−1) was lowered to facilitate access to the aquatic adventitious roots on the stem, and the adventitious roots were temporarily loosely wrapped in plastic foil after spraying with water to prevent dehydration. The cuvette was then filled with a known volume of nutrient solution (ranging between 540 and 570 mL; composition given below), the plastic foil around the roots was removed, the cuvette was closed and the floodwater level was increased again to the level of the bottom of the cuvette.
We used H2 to estimate P net uptake. A concentration of 50 µm H2 in a 1/40 strength modified Hoagland solution (further containing in µm: 440, K+ 340, Ca2+ 200, Mg2+ 50, 175, Na+ 6·5, Fe3+ 9·0, Cl− 5·0, 2·5, Mn2+ 0·2, Zn2+ 0·2, Cu2+ 0·05, 0·05; pH 5·6) was supplied to the aquatic adventitious roots. Before and after an uptake period of 6 h, a sample of 10 mL was taken from the solution in the cuvette to determine P depletion. The cuvette was then removed from the plant. The concentration of total phosphorus in the nutrient solution was measured by spectrometry (ICP-OES ICap 6000, Thermo Fischer Scientific, Waltham MA, USA). The aquatic adventitious roots were counted, and scanned using an A3-sized flatbed scanner (Epson Expression 11000XL, Japan), after which the total length, surface area and volume of these roots were calculated in WinRHIZO (Regent Instruments Inc., Québec, Canada). The fresh weight of these adventitious roots was obtained after gently removing the extra water from the root surface.
Experiment 3: effects of the number of aquatic adventitious roots on leaf transpiration and photosynthesis.
Eight-week-old plants were subjected to drained conditions, or to partial submergence with two adventitious root manipulation treatments (stems covered with Vaseline and stems without Vaseline, respectively), with six replicates per treatment. Plants were well watered in the drained treatment and flooded to 10 cm above the soil surface in both partial submergence treatments. Plants were allowed to form aquatic adventitious roots freely in partial submergence without Vaseline, whereas in partial submergence with Vaseline formation of aquatic adventitious roots was prevented by covering the basal 12-cm stem with a 0·5-cm-thick layer of Vaseline (Daro Vaseline, the Netherlands) prior to flooding. After 3 weeks, transpiration and photosynthesis were measured on a young mature leaf from each plant with a Li-Cor 6400 portable photosynthesis system (Li-Cor, Inc., Lincoln, NE, USA). The measurements were taken at a leaf temperature of 23 °C, with saturating light at 1000 μmol photons m−2 s−1 and 400 μmol mol−1 CO2. The temperature and relative air humidity of the climate room were kept at 20 °C and 60 %, respectively, throughout the experiment.
Experiment 4: benefits of aquatic adventitious root formation under different flooding duration.
Nine weeks after germination, plants were subjected to a combination of treatments modulating the number of aquatic adventitious roots and duration of partial submergence. Plants (in six to eight replicates) were partially submerged to 5 cm above the soil surface for either 1, 2 or 4 weeks, and subjected to root removal or control treatments. In the root removal treatment, newly formed aquatic adventitious roots (≥2 mm) of each plant were removed every other day. In the control treatment, aquatic adventitious roots were left intact. Plants were harvested after 1, 2 or 4 weeks of partial submergence.
Measurements of stem height, leaf size and biomass in all experiments
Initial stem height was measured for each plant in expt 1 before subjecting plants to the treatments. In both expts 1 and 4, the youngest fully expanded leaf on the main shoot was marked at the onset of the experiments to be able to distinguish leaves that were still developing at the onset of treatments from leaves that had finished expansion prior to the flooding treatment. The leaf above the marked leaf was harvested 1 day before the final harvest to determine leaf size (LI-3100 Area Meter, Li-Cor, Inc., Lincoln, NE, USA). During harvest, stem height was measured and roots were carefully washed free of substrate. Plants were divided into leaves, stems, sediment roots and aquatic adventitious roots, of which dry weights were determined after drying the plants to constant weight at 70°C in all experiments.
Data analysis
All statistical analyses were conducted in R (R Development Core Team, 2015). In expt 1, to test the effects of damage (control and Vaseline vs. wounding and removal treatments) and aquatic adventitious root removal (Vaseline and removal vs. control and wounding treatments) on total plant dry weight, stem height and leaf size, two-way ANCOVAs (type III) and ANOVA (type III) with damage and aquatic adventitious root removal as main effects were conducted using the car package. Initial stem height was treated as a covariate in the ANCOVAs for total plant dry weight and stem height. Separate one-way ANOVAs were then conducted to test the effects of the four different treatments (control, wounding, Vaseline and removal) on total plant dry weight, stem height and leaf size under both partially submerged and drained conditions. Total plant dry weight and initial stem height were ln-transformed to improve normality. For expt 2, linear regression analysis was performed to test the relationship between phosphate uptake rate and the surface area of the adventitious roots. For expt 3, the effect of duration of partial submergence on the aquatic adventitious root growth (adventitious root number and biomass) of plants with intact adventitious roots was analysed by one-way ANOVA (type III) and pairwise comparisons were conducted using pairwise t tests with Bonferroni corrections for the significance of the P values to compensate for the increased likelihood of a Type I error due to the multiple comparisons. Separate one-way ANOVAs (type III) were performed on plants in partial submergence without Vaseline and partial submergence with Vaseline treatments to test the effect of presence of aquatic adventitious roots on leaf transpiration, stomatal conductance and photosynthesis. For expt 4, the main effects of adventitious roots (with adventitious roots vs. without adventitious roots) and duration of partial submergence and their interaction were analysed in a two-way ANOVA (type III). Since the interaction was significant, pairwise comparisons were conducted using Student’s t tests to examine the effects of adventitious root treatment at different durations of partial submergence. P values were adjusted by means of Bonferroni correction.
Results
Decreased numbers of aquatic adventitious roots negatively affected plant growth during partial submergence
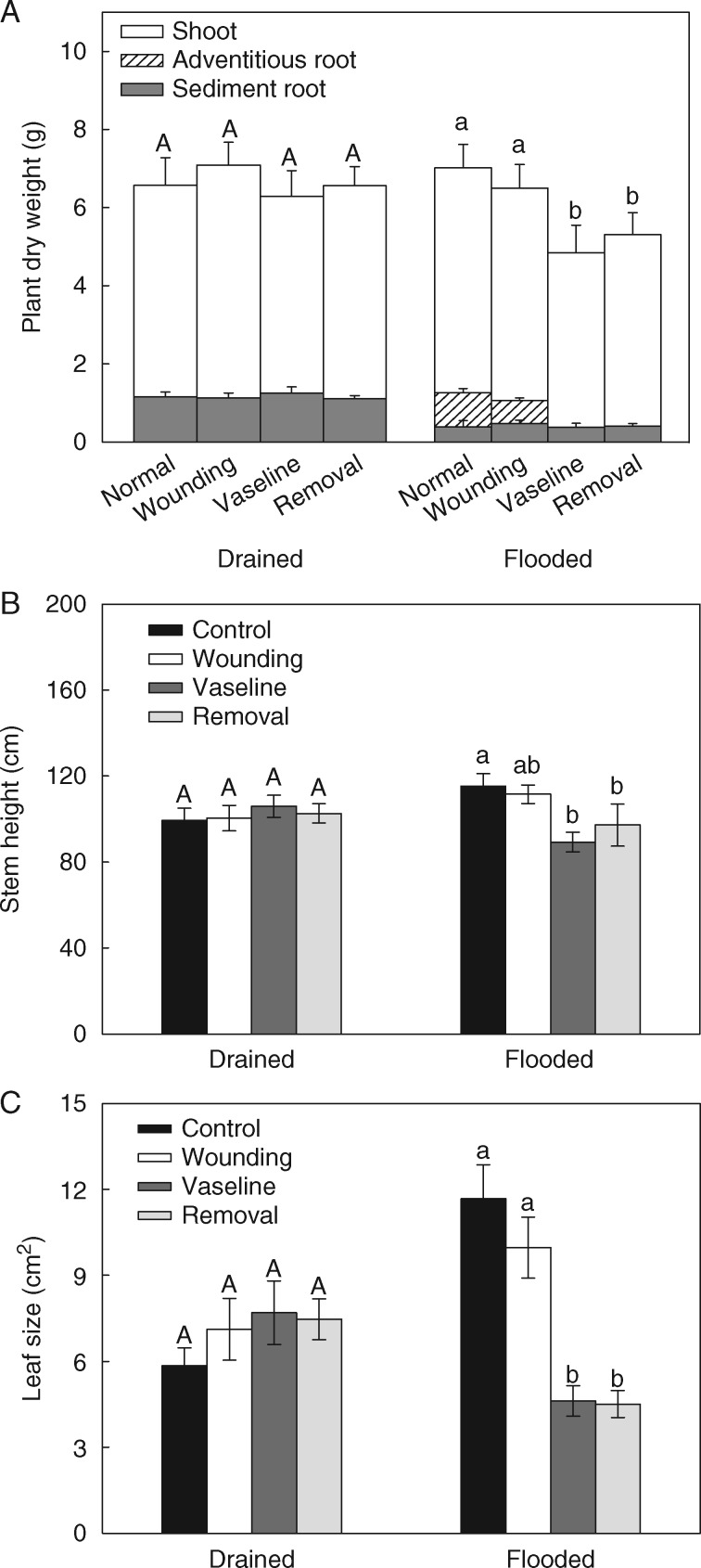
To be able to comprehensively understand the importance of aquatic adventitious roots, preventing their formation provides an opportunity to evaluate whether negative effects occur when plants are not able to develop these roots during partial submergence. Plant performance in terms of biomass gain, plant size and leaf expansion was significantly decreased (by 25, 18 and 58 %, respectively) after removal of the aquatic adventitious root primordia or Vaseline application on the stem prior to flooded conditions (Fig. 2, Table 1). Damaging the stem by removing small parts of the epidermis and upper cortical layers, but leaving the primorida and developing roots intact (wounding treatment) had no effect on the final total biomass, stem height and leaf size under partially submerged conditions (Fig. 2, Table 1), indicating that the negative effects on plant growth caused by aquatic adventitious root removal were not due to damage but due to the lack of adventitious roots. None of the three treatments (Vaseline, removal and wounding) affected total biomass, stem height and leaf size of plants under drained conditions (Fig. 2), suggesting that these treatments did not limit plant growth per se.
Fig. 2.
Mean (± 1 s.e.) shoot and root (aquatic adventitious roots and non-adventitious sediment roots) dry weight (A), stem height (B) and leaf size (C) after 3 weeks of drained or partially flooded conditions, and control treatment (no primordia removal), Vaseline treatment (where the lower stem parts including the root primordia were covered with Vaseline), removal of adventitious root primordia, or wounding treatment (where similar wounds as with primordia removal were made on the stem without damaging the primordia), respectively. Significant differences among treatments within drained or flooded conditions are indicated with different letters (P < 0·05, n = 11).
Table 1.
Results of two-way ANCOVA on total dry weight and stem height, and results of two-way ANOVA on leaf size with aquatic adventitious root (AR) removal (Vaseline and removal treatments vs. intact and wounding treatments) and damage (Vaseline and intact treatments vs. removal and wounding treatments) as main effects; for the analysis of total dry weight and stem height, initial stem height was included as a covariate
|
F-values |
||||
|---|---|---|---|---|
| d.f. | Total dry weight | Stem height | Leaf size | |
| AR removal | 1 | 18·153*** | 15·387*** | 64·075*** |
| Damage | 2 | 0·705 | 0·157 | 0·695 |
| AR removal × Damage | 1 | 1·397 | 1·014 | 0·570 |
| Initial stem height | 1 | 243·972*** | 26·944*** | — |
Total dry weight was ln-transformed to increase normality. Significance is indicated by asterisks, ***P < 0·001. Values in bold type indicate significant effects.
Aquatic adventitious roots contributed to nutrient and water uptake
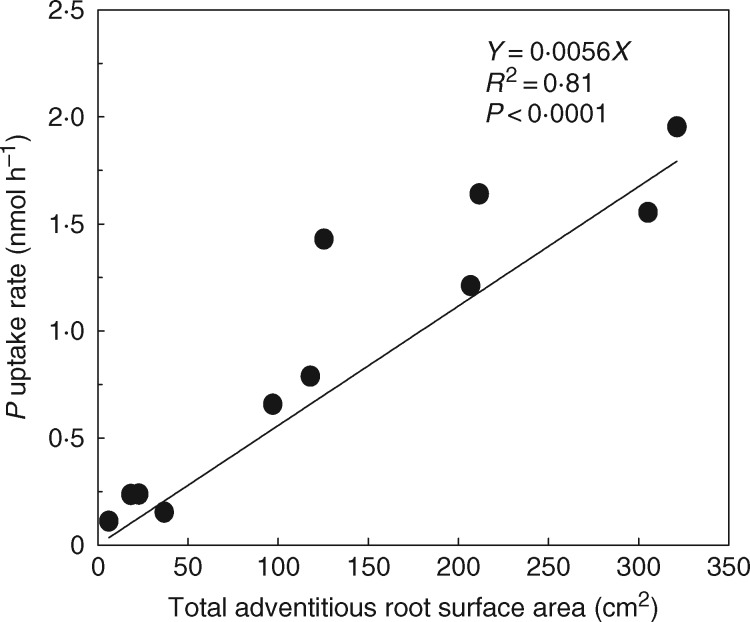
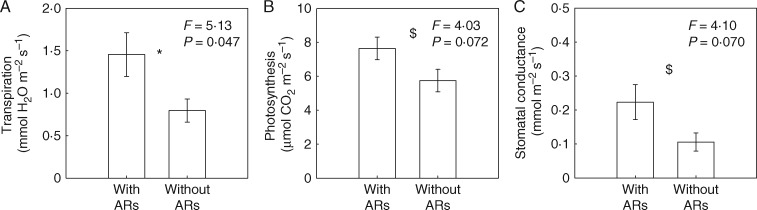
To reveal potential mechanisms contributing to the beneficial role of adventitious roots during partial submergence, we investigated the main function of these roots by evaluating nutrient uptake, and by estimating water status of the plant via transpiration measurements. After 6 h of incubation, P was shown to have been taken up by the aquatic adventitious roots at rates that ranged from 0·11 to 1·95 µmol h−1, depending on root size (on average 0·52 ± 0·076 µmol h−1 per g of fresh roots) (Fig. 3). The uptake rate of P strongly correlated with the total surface area of the aquatic adventitious roots (Fig. 3), suggesting that this surface determines ion uptake during partial submergence. Transpiration, stomatal conductance and photosynthesis were (marginally) significantly influenced by not being able to produce aquatic adventitious roots (Fig. 4). On average, transpiration, stomatal conductance and photosynthesis rates were respectively 45, 53 and 25 % lower in plants without aquatic adventitious roots, compared to plants with aquatic adventitious roots, while the latter group had similar photosynthesis but higher stomatal conductance and transpiration rates than the control plants (Fig. 4).
Fig. 3.
Correlation between total surface area of the aquatic adventitious roots of S. dulcamara and the uptake rates of phosphate under partially flooded conditions. The uptake rate was calculated after 6 h of incubation of the adventitious roots in a closed cuvette that was fixed around the stem and contained nutrient solution. Slopes, correlation coefficients and statistical significance of the linear regression line are indicated.
Fig. 4.
Mean (± 1 s.e.) rates and ANOVA results of leaf transpiration (A), photosynthesis (B) and stomatal conductance (C) of plants in partial submergence treatments (With ARs: plants were able to maintain aquatic adventitious roots during flooding; Without ARs: plants were not able to produce aquatic adventitious roots due to full coverage of Vaseline on the flooded stem). The mean (± 1 s.e.) rates of leaf transpiration, stomatal conductance and photosynthesis of plants under drained conditions were 1·10 (± 0·22), 0·13 (± 0·026) and 7·86 (± 1·05) (all units as given in the body of the figure), respectively. The significance levels were *0·01 < P < 0·05, $0·05 < P < 0·1.
The effect of removing aquatic adventitious roots on plant growth was dependent on partial submergence duration
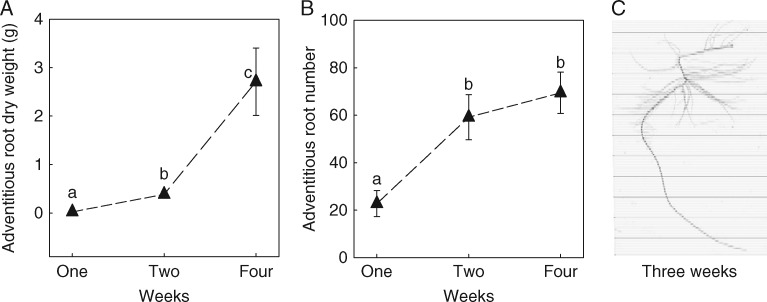
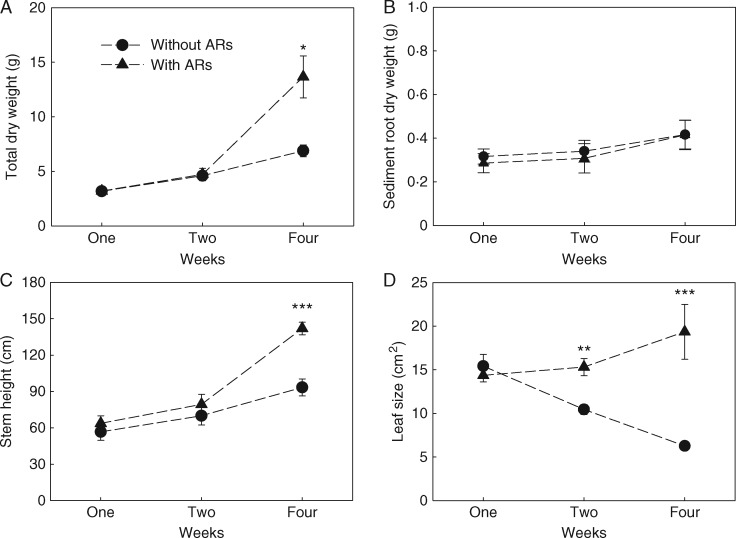
The size of the aquatic adventitious root system was significantly determined by the duration of partial submergence (Fig. 5), because elongation and branching of the aquatic adventitious roots, rather than the fast initiation of primordia outgrowth, were primarily determining root system size. Since nutrient uptake significantly correlated with the size of the aquatic adventitious root system (Fig. 3) the potential benefits of developing aquatic adventitious roots were also expected to depend on the duration of partial submergence. To test this hypothesis, we conducted an experiment investigating the effects of aquatic adventitious root removal in plants subjected to different durations of partial submergence. Removing the aquatic adventitious roots significantly decreased biomass accumulation, leaf size and the size of the plants subjected to 4 weeks of partial submergence, but not of plants subjected to 1 or 2 weeks of partial submergence (Fig. 6, Table 2). In contrast to the significant changes observed in the above-ground parts, absolute sediment root dry weight was not influenced by aquatic root removal (Fig. 6B, Table 2).
Fig. 5.
Mean (± 1 s.e.) aquatic adventitious root dry weight (A) and number (B) of S. dulcamara plants after 1, 2 and 4 weeks of partial submergence, respectively, and scanning image of an individual main adventitious root including lateral roots (C) after 3 weeks of partial submergence. Significant differences are indicated with different letters (P < 0·05 after Bonferroni correction for pairwise t test in R, n = 6–8).
Fig. 6.
Mean (± 1 s.e.) total dry weight (A), sediment root dry weight (B), stem height (C) and leaf size (D) for plants with aquatic adventitious roots (ARs) and without ARs after 1, 2 and 4 weeks of partial submergence, respectively. Significant effects of aquatic adventitious root removal within each submergence duration are indicated by asterisks, ***P < 0·0003, **0·0003 < P < 0·003, *0·003 < P < 0·002 (P values were adjusted by Bonferroni correction, n = 6–8).
Table 2.
The results of two-way ANOVA on total dry weight, sediment root (SR) dry weight, stem height and leaf size with aquatic adventitious root treatment (with ARs vs. without ARs) and flooding duration (1, 2 and 4 weeks) as main effects
|
F-values |
|||||
|---|---|---|---|---|---|
| d.f. | Total dry mass | SR dry mass | Stem height | Leaf size | |
| AR treatment (A) | 1 | 31·980*** | 0·208 | 14·816*** | 42·655*** |
| Duration (D) | 2 | 8·779** | 2·307 | 38·918*** | 7·488** |
| A × D | 2 | 8·621*** | 0·048 | 5·943** | 22·084*** |
Leaf size was ln-transformed to meet the assumption of ANOVA. Significance is indicated by asterisks, ***P < 0·001, **P < 0·01. Values in bold type indicate significant results.
Discussion
Flooding regimes in natural habitats often are unpredictable, as the occurrence of flooding may vary both in time and in space (van der Sman et al., 1993; Bailey-Serres et al., 2012). This has been argued to affect the selection on the adaptive traits that are essential to buffer the potentially negative effects of flooding on plant growth (Setter and Laureles, 1996; Chen et al., 2011). If such traits are constitutively present in a flood-prone plant population, plants may incur costs if flooding does not occur permanently. Moreover, expression of flooding-induced traits may not be beneficial if the duration of flooding is too short. In this study, we quantified the contribution of flooding-induced adventitious root formation to plant growth and subsequently investigated whether this contribution depends on duration of the partial submergence. Our results revealed a positive effect of the presence of aquatic adventitious roots on plant performance, with respect to transpiration, photosynthesis and biomass accumulation, in long-term partial submergence but not in short-term flooding. Regarding the function of adventitious roots, we found a clear positive correlation between nutrient uptake and the size of the aquatic adventitious root system. This relationship, possibly also valid for water uptake, may be the underlying reason why aquatic adventitious roots were only advantageous under a long duration of partial submergence. This may be due to the aquatic adventitious roots only gradually taking over the function of the sediment roots, as their biomass relative to the sediment roots increased exponentially over time, from less than 10 % after 1 week of partial submergence to 600–700 % after 4 weeks. This implies that there is a time-lag between the onset of flooding and sustained root function. In addition, aquatic adventitious roots that grow in the floodwater mostly dry out and die upon subsidence of the flooding. Our data show that aquatic adventitious roots convey benefits under long-term but not under short-term partial submergence, corroborating our hypothesis that aquatic adventitious roots can contribute to increased plant growth by stimulating resource uptake during partial submergence. However, in contrast to our expectation, no clear costs in terms of energy investment into adventitious root production and maintenance were detected under short-term partial submergence, which may explain why the ability to produce aquatic adventitous roots is maintained over a wide range of habitats, ranging from permanent wetlands to drought-prone coastal sand dunes.
Aquatic adventitious roots confer fitness advantages in long-term but not in short-term flooding
In accordance with our hypothesis that the balance between potential costs and benefits associated with adventitious root development depends on the duration of flooding, our experiment revealed a net benefit mediated by adventitious roots in terms of increased biomass accumulation only for long-term partial submergence (3 and 4 weeks of flooding in the present study), but not for short-term partial submergence (<2 weeks of flooding in the present study). This observation is probably explained by the increased size of the adventitious root system with longer duration of partial submergence. The formation of the first adventitious roots upon partial submergence of S. dulcamara commences very quickly, usually within 2 days (Dawood et al., 2014; Zhang et al., 2015). However, a relatively large adventitious root system characterized by the presence of lateral roots and a large surface area needs significantly longer time to develop (Q. Zhang, pers. observ.). Within the first 2 weeks of partial submergence, the aquatic adventitious roots are unbranched and only constitute approx. 0·5 % (1 week) to 1·2 % (2 weeks) of the total plant dry weight, which is more than 10-fold smaller than the biomass of adventitous roots produced during longer flooding duration (11 % of total plant weight after partial submergence lasting for 3 weeks and 20 % after 4 weeks). Roots of this size substantially contribute to resource acquisition, and consequently also to plant growth. The beneficial effects of aquatic adventitious roots have also been shown in previous studies in several other species during relatively long-lasting flooding events, varying from 15 to 110 d. Tsukahara and Kozlowski (1985) found that removing the aquatic adventitious roots of seedlings of the tree species Platanus occidentalis significantly reduced leaf initiation and expansion, growth rates and dry weight. Aquatic adventitious root removal also resulted in significantly lower dry weight of the legumes Macroptilium lathyroides (16 % reduction) and Vigna luteola (26 % reduction) (Javier, 1987). However, as in these studies the effects of aquatic adventitious roots on plant growth were only measured at one time point, they did not provide information about the production and potential costs and benefits of adventitous roots during the course of flooding events.
As plastic responses are usually not only associated with benefits but also with costs in terms of energy investment into, for example, production and maintenance of the plastic response (DeWitt et al., 1998; Dorn et al., 2000; Huber et al., 2004; Weijschedé et al., 2006; Murren et al., 2015), it can be expected that plants may incur net costs from aquatic adventitious root formation, especially when partial submergence lasts only for a few days. However, in contrast to our hypothesis, plants that invested into aquatic adventitous root development attained the same biomass as plants whose aquatic adventitious roots were removed immediately after emergence from the stem during short-term partial submergence. This implies that the costs of investing into the growth and maintenance of these, still small, aquatic adventitious roots are relatively low. Moreover, the highly porous aerenchyma formed in the aquatic adventitious roots is known to reduce respiration, making aquatic adventitious roots potentially cheaper to maintain than regular root tissues (Fan et al., 2003; Postma and Lynch, 2011). Such presumably low costs in aquatic adventitious root growth and maintenance may contribute to the lack of habitat effect on aquatic adventitious root formation in S. dulcamara, such as found in a previous study (Zhang et al., 2016), where the potential to produce aquatic adventitious roots is even maintained in plants originating from dry populations that never experienced flooding (Zhang et al., 2016).
Aquatic adventitious roots maintain nutrient and water uptake during flooding
Aquatic adventitious roots have been found to be beneficial under flooding that lasted for long periods. In order to unravel the mechanism underlying this benefical role, further studies into the function of these roots were needed. Soil rapidly becomes anoxic upon flooding, causing nutrient availability in the soil to decrease (Kozlowski and Pallardy, 1984; Ponnaperuma, 1984). In addition, the lack of oxygen may cause decay of the primary root system (Visser et al., 2015). Such lower nutrient availability together with impeded functioning of the primary roots may lead to nutrient deficiency in the entire plant (Drew and Sisworo, 1979; Trought and Drew, 1980). It has been shown that the aquatic adventitious roots may take up nutrients and water from the floodwater, thereby partly buffering the reduced functionality of the root system produced prior to flooding and not adapted to flooded conditions (Jackson, 1955; Javier, 1987). In the present study, we quantified the uptake capacity of phosphate by the adventitious roots of S. dulcamara during flooding. The aquatic adventitious roots showed considerable P acquisition, as 4–73 % of P in the cuvette was taken up within 6 h, depending on the surface area of these adventitious roots. Potentially, uptake rates could decrease when low P concentrations were reached in the cuvette, and this may lead to an underestimation of uptake rates, but given the linear dependence on root surface area, it is unlikely that this played a major role. Other species such as deep-water rice have also been shown to take up considerable amounts of nutrients through the nodal adventitious roots (roots generated from the stem nodes) generated during flooding (Khan et al., 1982). In wheat, the nodal adventitious roots contributed equally to nitrate and potassium uptake in O2-deficient media compared to the sediment root system (Kuiper et al., 1994). Although natural floodwater may not contain such high concentrations of P as in our nutrient solution (e.g. P concentration varied between 0·3 and 2 µmol L−1 in the floodwater of rice fields; Setter et al., 1987), the continuous exposure of the roots to these concentrations may still lead to substantial uptake. Therefore, being able to take up nutrients through the aquatic adventitious roots from the floodwater may substantially improve plant survival and performance during partial submergence and play a major role in increased plant performance upon aquatic adventitious root formation.
In addition to nutrient uptake, aquatic adventitious roots may also contribute to water uptake during flooding (Calvo-Polanco et al., 2012). Unlike nutrient uptake, which is via active transport and thus depends on the energy status of the flooded plants, water uptake is maintained by passive mechanisms (transpiration). Flooding causes stomatal closure, following a reduced water uptake of the flooded plant (Kozlowski and Pallardy, 1984; Else et al., 2009). In our study, both leaf transpiration rates and leaf size were significantly reduced by aquatic root removal, suggesting that transpiration rates at plant level were higher in plants that were allowed to produce adventitious roots during partial submergence. Similar results were found in the woody species Fraxinus pennsylvanica, which maintained higher transpiration rates in the presence of adventitious roots (Gomes and Kozlowski, 1980). Interestingly, in the present study, while aquatic adventitious roots gradually took over the function of the original sediment roots, in flooded plants the biomass of non-adventitious sediment roots still remained constant [0·29 ± 0·04, 0·31± 0·06 and 0·42 ± 0·07 g (mean ± 1 s.e.) after 1, 2 and 4 weeks of partial submergence, respectively]. The survival and, in the upper parts of the pots, even growth of these sediment roots may suggest that in addition to their importance for anchoring, the sediment roots still have other functions. Potentially, they are also important for the continuation of plant growth after the water level decreases again. However, further studies focusing on the contribution of adventitious roots and sediment roots to resource acquisition are required to assess the relative importance of each of the two root systems during flooding. The mechanism by which these sediment roots survive in hypoxic conditions may also deserve more attention.
Conclusions
We have comprehensively demonstrated in this study the importance of aquatic adventitious roots during partial submergence, the underlying mechanisms of the benefits associated with the aquatic adventitious roots, and the benefits and/or costs of these roots under different flooding regimes. Aquatic adventitious roots of S. dulcamara plants were capable of taking up nutrients and water from the floodwater, thus conferring benefits to plant performance. However, as the nutrient uptake was strongly affected by the size of the aquatic adventitious root system, the fitness advantages were only pronounced in long-term flooding, after a sizeable aquatic adventitious root system had developed. This fitness advantage associated with aquatic adventitious root production may provide a potential for selection on adventitious root formation in environments with predictable long-lasting flooding events. In contrast to our expectation, the aquatic adventitious roots did not confer apparent costs when flooding lasted for a short period of time, suggesting that aquatic adventitious root formation is not a very costly process. This makes it likely that species can maintain this plasticity in unpredictable or dry environments. This notion was confirmed in earlier work on this species, where populations from dry habitats that had never experienced flooding retained the same potential for aquatic adventitious root development as populations originating from wet habitats (Zhang et al., 2016). Such constitutive plasticity may enable this species to occur in the contrasting hydrological environments without evolving specific adaptation to the local environment (Zhang et al., 2016).
Supplementary Data
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1 illustration of the effect of different methods to prevent the aquatic adventitious root formation on root outgrowth during partial submergence. Figure S2: the cuvette used for nutrient uptake (P) by aquatic adventitious roots of S. dulcamara in experiment 2.
Supplementary Material
Acknowledgements
This work is part of the B’sweet collaborative research initiative of the Departments of Experimental Plant Ecology and Plant Science of the Radboud University Nijmegen. Collection of seeds was supported by a grant from Stichting Schure-Beijerinck-Popping Fonds, and permission from Natuurmonumenten, Zuidhollands Landschap, Staatsbosbeheer and Waternet to sample populations at their properties. Q.Z. was supported by China Scholarship Council grant number 201206510011.
Literature Cited
- Armstrong W. 1979. Aeration in higher plants In: Woolhouse HW, ed. Advances in botanical research. London, UK: Academic Press, 236–332. [Google Scholar]
- Armstrong W, Armstrong J.. 2014. Plant internal oxygen transport (diffusion and convection) and measuring and modelling oxygen gradients In: van Dongen JT, Licausi F, eds. Low-oxygen stress in plants: sensing and adaptive responses to hypoxia. Plant Cell Monographs, 21. Berlin: Springer, 267–298. [Google Scholar]
- Bailey-Serres J, Lee SC, Brinton E.. 2012. Waterproofing crops: effective flooding survival strategies. Plant Physiology 160: 1698–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom CWPM, Voesenek LACJ, Banga M, et al. 1994. Physiological ecology of riverside species: adaptive responses of plants to submergence. Annals of Botany 74: 253–263. [Google Scholar]
- Calvo-Polanco M, Senorans J, Zwiazek JJ.. 2012. Role of adventitious roots in water relations of tamarack (Larix laricina) seedlings exposed to flooding. BMC Plant Biology 12: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Huber H, de Kroon H, et al. 2009. Intraspecific variation in the magnitude and pattern of flooding-induced shoot elongation in Rumex palustris. Annals of Botany 104: 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Visser EJW, de Kroon H, Pierik R, Voesenek LACJ, Huber H.. 2011. Fitness consequences of natural variation in flooding-induced shoot elongation in Rumex palustris. New Phytologist 190: 409–420. [DOI] [PubMed] [Google Scholar]
- Colmer TD. 2003. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment 26: 17–36. [Google Scholar]
- Colmer TD, Voesenek LACJ.. 2009. Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology 36: 665–681. [DOI] [PubMed] [Google Scholar]
- Comas LH, Becker SR, Cruz VMV, Byrne PF, Dierig DA.. 2013. Root traits contributing to plant productivity under drought. Frontiers in Plant Science 4: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino N, Golas T, van de Geest H, et al. 2013. Genomic analysis of the native European Solanum species, S. dulcamara. BMC Genomics 14: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood T, Rieu I, Wolters-Arts M, Derksen EB, Mariani C, Visser EJW.. 2014. Rapid flooding-induced adventitious root development from preformed primordia in Solanum dulcamara. AoB PLANTS 6: plt058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt TJ, Sih A, Wilson DS.. 1998. Costs and limits of phenotypic plasticity. Trends in Ecology & Evolution 13: 77–81. [DOI] [PubMed] [Google Scholar]
- Dorn LA, Pyle EH, Schmitt J.. 2000. Plasticity to light cues and resources in Arabidopsis thaliana: testing for adaptive value and costs. Evolution 54: 1982–1994. [DOI] [PubMed] [Google Scholar]
- Drew MC, Sisworo EJ.. 1979. The development of waterlogging damage in young barley plants in relation to plant nutrient status and changes in soil properties. New Phytologist 82: 301–314. [Google Scholar]
- Else MA, Janowiak F, Atkinson CJ, Jackson MB.. 2009. Root signals and stomatal closure in relation to photosynthesis, chlorophyll a fluorescence and adventitious rooting of flooded tomato plants. Annals of Botany 103: 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Zhu J, Richards C, Brown KM, Lynch JP.. 2003. Physiological roles for aerenchyma in phosphorus-stressed roots. Functional Plant Biology, 30: 493–506. [DOI] [PubMed] [Google Scholar]
- Gomes AS, Kozlowski T.. 1980. Growth responses and adaptations of Fraxinus pennsylvanica seedlings to flooding. Plant Physiology 66: 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H, Kane Nolan C, et al. 2004. Frequency and microenvironmental pattern of selection on plastic shade-avoidance traits in a natural population of Impatiens capensis. The American Naturalist 163: 548–563. [DOI] [PubMed] [Google Scholar]
- Huber H, Visser EJW, Clements G, Peters JL.. 2014. Flooding and fragment size interact to determine survival and regrowth after fragmentation in two stoloniferous Trifolium species. AoB PLANTS, 6: plu024. doi:010.1093/aobpla/plu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Drew MC.. 1984. Effects of flooding on growth and metabolism of herbaceous plants In: Kozlowski TT, ed. Flooding and plant growth. Orlando, FL: Academic Press, 47–128. [Google Scholar]
- Jackson WT. 1955. The role of adventitious roots in recovery of shoots following flooding of the original root systems. American Journal of Botany 42: 816–819. [Google Scholar]
- Javier RR. 1987. Effects of adventitious root removal on the growth of flooded tropical pasture legumes. Transactions of the National Academy of Science and Technology 9: 443–449. [Google Scholar]
- Khan MR, Ventura W, Vergara BS.. 1982. Uptake through aquatic roots and distribution of 15N-tagged ammonium in deepwater rice In Proceedings of the 1981 International Deepwater Rice Workshop. Los Baños, Philippines: International Rice Research Institute. [Google Scholar]
- Kozlowski T, Pallardy S.. 1984. Effect of flooding on water, carbohydrate, and mineral relations In: Kozlowski TT, ed. Flooding and plant growth. Orlando, FL: Academic Press, 165–193. [Google Scholar]
- Kuiper P, Walton C, Greenway H.. 1994. Effect of hypoxia on ion uptake by nodal and seminal wheat roots. Plant Physiology and Biochemistry 32: 267–276. [Google Scholar]
- Laan P, Berrevoets MJ, Lythe S, Armstrong W, Blom C.. 1989. Root morphology and aerenchyma formation as indicators of the flood-tolerance of Rumex species. Journal of Ecology 77: 693–703. [Google Scholar]
- Lenssen JPM, van Kleunen M, Fischer M, de Kroon H.. 2004. Local adaptation of the clonal plant Ranunculus reptans to flooding along a small-scale gradient. Journal of Ecology 92: 696–706. [Google Scholar]
- Murren CJ, Auld JR, Callahan H, et al. 2015. Constraints on the evolution of phenotypic plasticity: limits and costs of phenotype and plasticity. Heredity 115: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnaperuma F. 1984. Effects of flooding on soils In: Kozlowski TT, ed. Flooding and plant growth. Orlando, FL: Academic Press, 9–45. [Google Scholar]
- Poot P, Lambers H.. 2003. Growth responses to waterlogging and drainage of woody Hakea (Proteaceae) seedlings, originating from contrasting habitats in south-western Australia. Plant and Soil 253: 57–70. [Google Scholar]
- Postma JA, Lynch JP.. 2011. Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiology 156: 1190–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. 2015. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; http://www.R-project.org/. [Google Scholar]
- Rich SM, Ludwig M, Colmer TD.. 2008. Photosynthesis in aquatic adventitious roots of the halophytic stem-succulent Tecticornia pergranulata (formerly Halosarcia pergranulata). Plant, Cell & Environment 31: 1007–1016. [DOI] [PubMed] [Google Scholar]
- Rich SM, Ludwig M, Colmer TD.. 2012. Aquatic adventitious root development in partially and completely submerged wetland plants Cotula coronopifolia and Meionectes brownii. Annals of Botany 110: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter M. 2013. Root responses to flooding. Current Opinion in Plant Biology 16: 282–286. [DOI] [PubMed] [Google Scholar]
- Setter TL, Laureles EV.. 1996. The beneficial effect of reduced elongation growth on submergence tolerance of rice. Journal of Experimental Botany 47: 1551–1559. [Google Scholar]
- Setter TL, Kupkanchanakul T, Pakinnaka L, Aguru Y, Greenway H.. 1987. Mineral nutrients in floodwater and floating rice growing at water depths up to two metres. Plant and Soil 104: 147–150. [Google Scholar]
- Steffens B, Rasmussen A.. 2016. The physiology of adventitious roots. Plant Physiology 170: 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Kovalev A, Gorb SN, Sauter M.. 2012. Emerging roots alter epidermal cell fate through mechanical and reactive oxygen species signaling. The Plant Cell 24: 3296–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi F, Sato-Nara K, Kobayashi K, Suzuki M, Suzuki H.. 2003. Sugar-induced adventitious roots in Arabidopsis seedlings. Journal of Plant Research 116: 83–91. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yamauchi T, Colmer TD, Nakazono M.. 2014. Aerenchyma formation in plants In: van Dongen JT, Licausi F, eds. Low-oxygen stress in plants: sensing and adaptive responses to hypoxia. Plant Cell Monographs, 21. Berlin: Springer, 247–265. [Google Scholar]
- Trought MCT, Drew MC.. 1980. The development of waterlogging damage in young wheat plants in anaerobic solution cultures. Journal of Experimental Botany 31: 1573–1585. [Google Scholar]
- Tsukahara H, Kozlowski TT.. 1985. Importance of adventitious roots to growth of flooded Platanus occidentalis seedlings. Plant and Soil 88: 123–132. [Google Scholar]
- van der Sman AJM, Joosten NN, Blom CWPM.. 1993. Flooding regimes and life-history characteristics of short-lived species in river forelands. Journal of Ecology 81: 121–130. [Google Scholar]
- Visser EJW, Blom CWPM, Voesenek LACJ.. 1996. Flooding-induced adventitious rooting in Rumex: morphology and development in an ecological perspective. Acta Botanica Neerlandica 45: 17–28. [Google Scholar]
- Visser EJW, Zhang Q, De Gruyter F, Martens S, Huber H.. 2015. Shade affects responses to drought and flooding-acclimation to multiple stresses in bittersweet (Solanum dulcamara L.). Plant Biology 18: 112–119. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM.. 2006. How plants cope with complete submergence. New Phytologist 170: 213–226. [DOI] [PubMed] [Google Scholar]
- Weijschedé J, Martínková J, de Kroon H, Huber H.. 2006. Shade avoidance in Trifolium repens: costs and benefits of plasticity in petiole length and leaf size. New Phytologist 172: 655–666. [DOI] [PubMed] [Google Scholar]
- Whiteman PC, Seitlheko M, Siregar ME, Chudasama AK, Javier RR.. 1984. Short-term flooding tolerance of seventeen commercial tropical pasture legumes. Tropical Grasslands 18: 91. [Google Scholar]
- Zhang Q, Visser EJW, de Kroon H, Huber H.. 2015. Life cycle stage and water depth affect flooding-induced adventitious root formation in the terrestrial species Solanum dulcamara. Annals of Botany 116: 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Peters JL, Visser EJW, de Kroon H, Huber H.. 2016. Hydrologically contrasting environments induce genetic but not phenotypic differentiation in Solanum dulcamara. Journal of Ecology 104: 1649–1661. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.