Abstract
Emotional intelligence (EI) is defined as an individual’s capacity to accurately perceive, understand, reason about, and regulate emotions, and to apply that information to facilitate thought and achieve goals. Although EI plays an important role in mental health and success in academic, professional and social realms, the neurocircuitry underlying this capacity remains poorly characterized, and no study to date has yet examined the relationship between EI and intrinsic neural network function. Here, in a sample of 54 healthy individuals (28 women, 26 men), we apply independent components analysis (ICA) with dual regression to functional magnetic resonance imaging (fMRI) data acquired while subjects were resting in the scanner to investigate brain circuits (intrinsic resting state networks) whose activity is associated with greater self-reported (i.e. Trait) and objectively measured (i.e. Ability) EI. We show that higher Ability EI, but not Trait EI, is associated with stronger negatively correlated spontaneous fMRI signals between the basal ganglia/limbic network (BGN) and posterior default mode network (DMN), and regions involved in emotional processing and regulation. Importantly, these findings suggest that the functional connectivity within and between intrinsic networks associated with mentation, affective regulation, emotion processing, and reward are strongly related to ability EI.
Keywords: emotional intelligence, resting state functional connectivity, FSL, fMRI, neuroimaging
Introduction
Individuals differ widely in their ability to accurately perceive, understand, and reason about emotional information, and to effectively apply that knowledge to enhance cognitive and behavioral performance—a capacity known as emotional intelligence (EI) (Salovey and Mayer, 1990). People who possess high EI are skilled at reasoning about emotional issues and are effective at using emotional information to solve problems and achieve goals (Mayer et al., 2008a). Because EI capacities are often highly predictive of important aspects of social/interpersonal functioning and professional success (Brackett and Mayer, 2003; Brackett et al., 2006; Cherniss et al., 2006; Talarico et al., 2013; Libbrecht et al., 2014; Moslehi et al., 2015), it is of critical importance to understand the neurobiological underpinnings of these capacities and the extent to which they can be improved through training. The goal of the present study is to address the existing knowledge gap regarding the neurocircuitry that underlies EI.
Since EI was first proposed in the early 1990s (Mayer et al., 1990; Salovey and Mayer, 1990), two different approaches to conceptualizing and assessing this construct have emerged—the “trait” and the “ability” models (Webb et al., 2013). The Trait model considers EI as a set of emotional self-perceptions and dispositions that are best assessed via introspection and self-report, similar to personality traits (Petrides et al., 2007; Siegling et al., 2015), while the Ability model considers EI as a set of cognitive capacities that permit an individual to accurately perceive, reason about, and use emotional information in adaptive ways, akin to other models of intelligence that focus on demonstrated knowledge and performance ability (Mayer et al., 2001; Mayer et al., 2008a). Although both conceptual models lay claim to the term “emotional intelligence” and often appear to be addressing similar theoretical domains (Petrides and Furnham, 2003), these two approaches are often only modestly correlated, if at all (Brannick et al., 2009; Webb et al., 2013). The lack of statistical association between these two approaches has led to some confusion in the field and may be due to differences in measurement approaches or to core differences in the processes being measured (Petrides and Furnham, 2003). Each approach accounts for unique aspects of emotional, personality and intellectual functioning (Mayer et al., 2008b), with Trait indices accounting for relatively stable self-perceived competencies in handling emotional and social situations, similar to stable self-rated personality traits (Bar-On, 2006; Mikolajczak et al., 2007; Nozaki and Koyasu, 2013), while the Ability approach emphasizes objective scoring and measured performance capacities with meaningful benchmarks or comparison metrics, and shares considerable variance with other indices of cognitive intelligence (Webb et al., 2013). Interestingly, a recent meta-analysis showed that Ability EI was significantly associated with better performance on a variety of emotional (i.e. “hot” cognition) tasks whereas Trait EI measures were not associated with such outcomes (Gutierrez-Cobo et al., 2016). On the other hand, Trait EI has been shown to predict career success and satisfaction over many years (Amdurer et al., 2014). Because emotional processes are multifaceted and complex, and their adaptive use to achieve goals requires the integration of many capacities, including self-awareness, subjective perceptions, reasoning, and skilled behavioral responses, it is likely that any comprehensive approach to EI will require an understanding of both Trait and Ability aspects of EI. Therefore, in the present study, we include well-validated metrics of both the Trait and Ability models and assess their association with the functional connectivity of large-scale brain networks.
An influential neurocircuitry model of EI posited that these capacities emerge from the interaction of several key brain regions involved in emotional perception, experience, and decision-making (Bar-On et al., 2003), including (but not limited to) the amygdala, insular cortex, ventromedial prefrontal cortex (vmPFC), and anterior cingulate cortex (ACC), which are key nodes of functional brain networks that are consistently linked with emotional experience, perception (Kober et al., 2008; Lindquist et al., 2012; Guillory and Bujarski, 2014), and affectively guided decision making (Bartra et al., 2013; Clithero and Rangel, 2014). The first task-based neuroimaging study of EI found that higher scores on a standard measure of Ability EI were negatively associated with activation of nodes of this neurocircuitry, particularly the anterior medial prefrontal cortex (PFC), during a social reasoning task (Reis et al., 2007), suggesting that EI may be directly related to measureable brain activation patterns. Our group reported similar findings for a measure of Trait EI in adolescents during a simple emotional face perception task (Killgore and Yurgelun-Todd, 2007), suggesting a common neurocircuitry might be involved in both models of EI. On the other hand, subsequent task-based functional magnetic resonance imaging (fMRI) work from our lab found that only higher Ability (but not Trait) EI was associated with greater activation of the vmPFC, amygdala, and insula in response to facial cues reflecting changing levels of trustworthiness (Killgore et al., 2013), findings that were essentially supported in other recent work (Quarto et al., 2016). The role of these same brain regions in EI has also been supported by volumetric studies of brain structure, with Ability EI showing positive correlations with the volume of the insula and vmPFC (Killgore et al., 2012), while various components of Trait EI showed positive correlations with regions of the medial frontal cortex and anterior cingulate cortex (Koven et al., 2011; Takeuchi et al., 2011; Killgore et al., 2012). Thus, task-related and structural volumetric studies have begun to map out a consistent set of brain regions that appear to be important for EI, though the specific regions appear to differ slightly depending on whether the Trait or Ability model is the focus.
As evident in the foregoing paragraphs, the Trait and Ability models both emphasize complex and highly inter-related capacities for emotion perception, comprehension and regulation, but each show different associations with behavior and brain function, suggesting that it is critical to study the neurocircuitry underlying both models. Because of the multifaceted nature of EI, it is unlikely to be easily reducible to simple patterns of activation in specific brain regions or discrete organization of brain volume and neurocircuitry. Rather, sophisticated capacities such as EI are more likely to be reflected in the way that emotion-related perceptual, interoceptive and experiential systems interact with higher-order integrative and problem solving systems of the brain. A first step in characterizing this complexity is to map the patterns of intrinsic connectivity within and among these systems. Even during spontaneous resting cognition, these brain regions are self-organized into intrinsic networks that interact with one another in meaningful ways that clarify the circuitry that underlies task-based functioning (Smith et al., 2009).
Takeuchi et al. (2013) recently conducted the first investigation of the association between Trait EI and resting state functional connectivity and found that higher EI scores were associated with greater connectivity within the medial regions of the default mode network (DMN). Moreover, they showed that higher Trait EI was also associated with greater anti-correlation between certain regions of the DMN and a task positive network, or TPN (i.e. dorsolateral PFC). This is critical, as recent evidence suggests that normal brain development and the acquisition of superior cognitive performance is related to the magnitude of negatively correlated or “push–pull”, activity among major cerebral networks. Notably, superior cognitive performance is associated with greater negative correlation (i.e. anti-correlation) between cortical TPNs that are typically involved in attention demanding cognition and the “task-negative” or DMN, which is typically suppressed during attentionally demanding or externally focused cognition (Kelly et al., 2008; Hampson et al., 2010; Barber et al., 2013). Individuals with greater cognitive abilities tend to show stronger anti-correlations between these networks. In a parallel fashion, it has recently been shown that the strength of anti-correlation between TPNs and DMN increases over the developmental span from childhood into early adulthood (Chai et al., 2014). On the other hand, lack of anti-correlation or even positive correlation between these normally anti-correlated networks has been associated with developmental immaturity (Chai et al., 2014), affective disorders (Marchetti et al., 2012), schizophrenia (Whitfield-Gabrieli et al., 2009), and risk for psychosis (Wotruba et al., 2013). Thus, greater ability to suppress the DMN during important task-relevant cognitive processing while increasing activity in relevant TPNs appears to be related to better performance in several domains.
While the study of Trait EI by Takeuchi et al. (2013) discussed above represents an important first step in addressing the role of these large-scale networks in EI, it did not examine Ability EI. That study also only examined functional connectivity between specific network regions of the DMN and TPN; it did not examine interactions within and between intrinsic networks themselves. Further, as it used a Japanese self-report instrument in a college student cohort, it remains unclear whether such anti-correlated networks would extend to the capacities measured by Ability EI assessments, and whether their findings would replicate for Trait EI among English speaking populations with a broader age range. To address these issues, we chose to examine intrinsic networks in relation to both Trait and Ability EI. We tested the hypothesis that a “push–pull” relationship between relevant resting state networks (RSNs) may also exist with regard to the networks involved in Ability EI. We further hypothesized that both higher Trait and Ability EI capacities would be directly related to the strength of the anti-correlation between the DMN and the primary networks involved in emotional processing (rather than traditional cognitive TPNs involved in attention and working memory), but that this pattern would be most strongly observed for the Ability model, given that it involves actual performance and is, therefore, most analogous to other cognitive ability studies. To provide an additional within-network test of this “push–pull” hypothesis, we also tested the prediction that, with higher Trait and Ability EI, these networks would also individually show stronger negative connectivity with a range of emotion-related brain regions—as might be expected in association with greater cognitive control of emotion. Based on prior findings from our structural and task-based functional studies of EI from this same sample (Killgore et al., 2012; Killgore et al., 2013; Weber et al., 2013; Alkozei and Killgore, 2015; Smith et al., 2016), we focused on networks comprised of emotion processing regions that are typically activated in response to emotional stimuli and which we have previously shown to be involved in EI (e.g. insular and orbitofrontal cortex, ventral striatum, amygdala); we also focused on the DMN, which is engaged during self-introspection and Theory of Mind (Abu-Akel and Shamay-Tsoory, 2011) processes that are critical to EI. To test these associations, we applied independent components analysis (ICA) with dual regression to functional magnetic resonance imaging (fMRI) data acquired while subjects were resting in the scanner. We then tested the relationships between network connectivity and total scores on well-established metrics of Trait and Ability EI.
Materials and methods
Participants
Seventy right-handed adults (32 women, 38 men), ranging in age from 18–45 years (mean age = 30.9 years; SD = 8.4 years) from the Boston metropolitan area provided written informed consent to participate in a neuroimaging study. Participants underwent initial telephone screening to rule out any significant history of medical, neurological, psychiatric, or substance use disorders. Following consent, eight participants withdrew voluntarily or were dropped from the study due to failure to comply with study requirements. Data from an additional eight participants were excluded due to excess motion or other scan-related artifacts. The final sample consisted of 54 healthy right-handed adults (28 women, 26 men), with an average age of 30.1 (SD = 7.5) years. Participants were well educated, with an average of 15.2 years of formal education (SD = 2.2 years), and all were native English speakers. Some prior task-based fMRI and behavioral data from subsets of these same participants have been published elsewhere (Killgore et al., 2012; Killgore et al., 2013; Weber et al., 2013; Alkozei and Killgore, 2015; Smith et al., 2016), but the current EI-resting-state network correlational findings are novel and have not been presented previously. Participants were provided nominal financial compensation for their time. This research protocol was reviewed and approved by the McLean Hospital Institutional Review Board and the U.S. Army Human Research Protection Office.
EI instruments
Prior to scanning, participants completed two computer-administered measures of EI. As a measure of Trait EI, participants completed the Bar-On Emotional Quotient Inventory (EQ-i) (Bar-On, 2002), a 125-item self-report measure that provides a global measure of EI (Total EQ). Total EQ was the primary variable of interest from this scale, but five composite subscales were also available, measuring the ability to understand emotions in others (Interpersonal), emotional self-awareness and self-confidence (Intrapersonal), problem solving and emotional flexibility (Adaptability), coping capacity under stress (Stress Management), and general optimism and positive outlook (General Mood). Raw scores were transformed into standard scaled scores based on the general population norms available in the computerized scoring program (Bar-On, 2002). Subscale scores were only included in ancillary analyses if the Total EQ was found to be significantly related to brain function (which it was not in this study). While other measures of Trait EI were available, we selected the EQ-i given that it is one of the most commonly used self-report measures of EI, it is commercially available, and its model has generated considerable research interest (Daus, 2006). In addition, Ability EI was assessed with the Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT) (Mayer et al., 2002), which measures emotional reasoning and problem solving. The primary outcome variable was the MSCEIT Total score. Additionally, four branch scores known as Perceiving Emotions, Facilitating Emotions, Understanding Emotions, and Managing Emotions were also calculated, but only included in ancillary analyses if the MSCEIT Total was found to be significantly related to brain function. Scaled scores were calculated and normalized based on the general consensus scoring method described in the test manual (Mayer et al., 2002). The MSCEIT was selected for this study, as it is the preeminent and most widely used Ability measure of EI (Daus, 2006).
Functional neuroimaging
All MRI data were acquired on a Siemens Tim Trio 3 Tesla scanner (Erlangen, Germany) with a 12-channel head coil. High-resolution structural images were acquired using a magnetization-prepared rapid acquisition with gradient echo (MPRAGE) pulse sequence with the following parameters: TR/TE = 2.1 s/2.25 ms, slices = 128, matrix = 256 × 256, flip angle = 12°, resolution = 1.0 × 1.0 × 1.33 mm. Gradient echo echo-planar images sensitive to BOLD contrast were acquired using the following parameters: TR/TE = 2.0 s/30 ms, flip angle = 90°, slices = 34, voxel size = 3.5 mm isotropic. During the 6:04 minute resting state fMRI scan participants were asked to remain awake with their eyes open.
Data pre-processing
Most data processing was done using the FMRIB Software Library (Smith et al., 2004; Woolrich et al., 2009; Jenkinson et al., 2012), FSL release 4.1 (FMRIB Analysis Group; Oxford University, UK; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) and MATLAB R2012a (The Mathworks, Inc, Natick, MA).
First, the raw data underwent quality assurance (QA) to assess for scanner artifacts and subject motion. A de-spiking algorithm (using an in-house program) was used to correct any datasets that had spikes (e.g. slice dropouts or slices/volumes with spikes in intensity). Motion correction was accomplished with MCFLIRT (Jenkinson et al., 2002) to do the initial QA. Data were excluded from further analysis if there was greater than one voxel motion in any direction or if the raw FMRI data showed variations in image intensity across the axial planes (e.g. intensity rolling due to interactions of motion with RF pulses), no matter how small the movement as these effects are not correctable with any widely available motion correction scheme. Based on the QA, data from eight participants were removed from further analysis, with one dataset showing excessive non-stationary ghosting, one large male subject with excess pulsations (e.g. physiological noise due to respiration), two with excessive scanner electronic noise and four with excessive motion. Pre-processing included: motion correction, slice timing correction, brain extraction using BET (Smith, 2002), spatial smoothing with a Gaussian kernel of full-width half-maximum 7 mm and high-pass temporal filtering with Gaussian-weighted least-squares straight-line fitting with σ = 150 s. Functional MRI data were aligned to the high-resolution MPRAGE scan with 12 degrees of freedom. The MPRAGE scan was aligned with the MNI152 standard brain using non-linear registration. Transformation of the functional results into MNI space was done following concatenation of the two alignments into a single matrix. All spatially normalized fMRI data were re-sampled to 2 mm3 resolution.
Statistical modeling
Independent component analysis (ICA)
FSL MELODIC [Multivariate Exploratory Linear Decomposition into Independent Components (Beckmann and Smith, 2004)] was used to perform an independent component analysis of the fMRI data from the sample of subjects. This method decomposes the spatially normalized fMRI data from all subjects (e.g. concatenated into a single data matrix) into a set of “independent components” (ICs), with each IC being a distributed set of brain regions with a temporal trace that describes the evolution of that particular spatial pattern over time. The number of spatio-temporal patterns estimated by the group ICA was set to 30, run eight times, followed by a meta-level ICA fed by all of the spatial maps from the eight decompositions following the procedures used in Smith et al. (2009), which identified 26 stable independent components. In this case, each independent component is comprised of a spatial map and a corresponding timecourse and represents a spatio-temporal pattern of activity that is common to the entire set of participants.
Dual regression
To assess inter-subject variability in the independent components, the group ICA maps are “back-projected” into each subject’s fMRI data to identify their unique spatial and temporal pattern for each ICA map. These “subject-specific spatial maps” (SSSMs) and “subject-specific time courses” (SSTCs) corresponding to each IC from the group ICA were estimated using the dual regression procedure with timecourse normalization (Beckmann et al., 2009; Filippini et al., 2009). The SSSMs were used to conduct a “within-network” functional connectivity analysis to identify how the shape (e.g. spatial pattern) of a network might relate to EI covariates of interest. This analysis proceeded similarly to higher level group analyses of task-based fMRI, only instead of activation contrast maps for the subjects being passed to the group level analysis that would be done in a task fMRI study, in our resting state study, the SSSMs for the subjects were collected together (into a 4D data file, with subject being the fourth dimension) and submitted to a higher level general linear model of the group effects.
Additionally, the SSTCs were used to conduct a “between-network” functional connectivity analysis. In this case, for the RSN pairs of interest, the timecourses of the networks in each subject were used to calculate the within-subject partial correlation between the networks (e.g. taking all other components into account) to give one correlation value between each network pair in each individual. These between-network correlation values were collected together across all the subjects and tested to see if the strength of the correlation between a pair of networks was related to Trait and Ability EI (i.e. EQ-i and MSCEIT scores, respectively).
Estimation and inference
To test for statistically significant linear relationships between within-network functional connectivity and EI, the 4D collection of SSSMs for each RSN of interest was submitted to a group general linear model (GLM) with the design matrix setup recommended on the FSL website (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/GLM#Single-Group_Average_with_Additional_Covariate), using two-sided tests. The model included the two covariates as measures of EI: EQ-i Total score and MSCEIT Total score (which were not correlated in this sample, r = 0.05, P = 0.71). As the between-subjects effects may be non-Gaussian and/or non-stationary, which can greatly decrease the performance of standard parametric inference methods (Hayasaka and Nichols, 2003; Hayasaka et al., 2004), we employed non-parametric permutation testing for estimation and inference (Filippini et al., 2012) using FSL Randomise. A novel permutation-based method to correct for the number of tests across both number of voxels and number of components was implemented (Licata et al., 2013) to control the FWE across voxels and components at P < 0.05 for the within-network functional connectivity analysis (which was assessed for four different networks). The procedure was implemented as follows: (i) for each component, the same seed was set in the call to Randomise to ensure that the permutations would be identical for all 5000 permutations for every component, with cluster-mass based thresholding (with cluster-forming threshold of 2.3, e.g. -C 2.3 options), and –N and −P options set to output the null distribution text file for each component (ii) the output null distribution text files for all of the components that were tested (e.g. a subset of the full set of independent components) were loaded into Matlab (The Mathworks, Natick, MA). Each text file contained a 5000-element long vector of the maximum cluster-mass statistic over all voxels obtained for each permutation (which are the values to construct the null distribution that is used to control the family-wise error over all voxels), (iii) a maximum of the maximums data matrix (a vector containing 5000 values) was created by recording for each permutation, the max (cluster-mass) value obtained across components, with (iv) the resulting max of the max values were used to construct the null distribution for the maximum cluster-mass statistic across voxels and components. This information then was used to control the FWE across voxels and components at P < 0.05 by identifying the threshold corresponding to the 95th percentile.
The primary hypotheses were restricted to Total EI scores for the MSCEIT and EQ-i, not the individual sub-scales of each measure. However, following a hierarchical analysis strategy, only RSNs that showed significant relationships between within-network functional connectivity and Total EI scores were further probed to identify which subscales of each test (e.g. four MSCEIT branch subscales; five EQ-i facet subscales) were driving the results. These ancillary analyses were included only if the parent scale was significant and are provided merely for purposes of clarification, but no a priori hypotheses were posed for the subscales. As described below, since only the MSCEIT Total score was significant, only the four branch sub-scales of that test were subsequently analyzed. For each of these sub-scales, we show plots of the regression weights averaged over the regions showing a statistically significant linear relationship with Total EI (e.g. “Functional Connectivity”) versus the sub-scale value.
Relationships between covariates of interest and between-network functional connectivity were assessed using the FSLNets toolbox (Smith et al., 2011) (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLNets). Briefly, for each subject, the partial correlation coefficient (controlling for the contributions of other effects represented in other SSTCs) between the SSTCs for each pair of RSNs of interest was calculated. For each pair of networks, the relationship between covariates of interest (EQ-i and MSCEIT Total scores) and the between-network partial correlation coefficients was assessed using FSL Randomise (with a design matrix setup the same as for the spatial map group GLM), accounting for FWE by correcting for multiple tests (e.g. network pairs and contrasts using a Bonferroni correction) with the significance set at P < 0.05.
Results
Association between trait and ability measures
The EQ-i (Trait EI) and MSCEIT (Ability EI) were first examined with a zero-order correlation to determine their association in this sample. As expected from prior work (Brannick et al., 2009; Webb et al., 2013), the correlation between EI measures was non-significant (r = 0.052, P = 0.71).
ICA
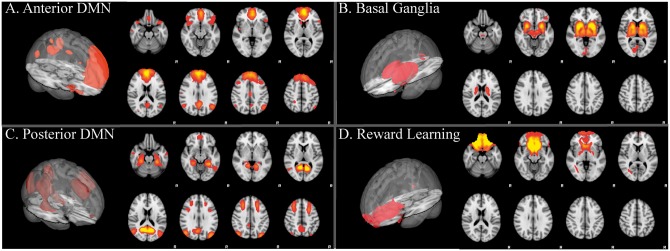
RSNs of interest in the current study were those that contained ventral PFC and limbic/paralimbic regions, including basal ganglia, amygdala, anterior cingulate, hippocampus, anterior thalamus, orbitofrontal cortex (OFC) and nucleus accumbens (NAcc), which are involved in emotion processing (Meeks and Jeste, 2009; Killgore et al., 2012; Catani et al., 2013; Killgore et al., 2013; Takeuchi et al., 2013; Weber et al., 2013; Alkozei and Killgore, 2015; Smith et al., 2016), as well as two sub-networks of the DMN that have been reported to be involved with episodic memory retrieval, mentalizing, Theory of Mind, and autobiographical thought (Andrews-Hanna et al., 2014). Prior to assessing for statistically significant relationships between network connectivity and EI, four networks, shown in Figure 1, were selected that contained these sub-regions:
Fig. 1.
3D views and several axial slices are shown for the: (A) Anterior default mode network (A-DMN): ventral and dorsal medial prefrontal cortex (PFC), anterior and posterior cingulate (A/PCC), precuneus, caudate and posterior parietal regions, (B) Basal ganglia/limbic network: limbic-paralimbic-striatal regions including inferior insula, basal ganglia (striatum, pallidum, NAcc, subthalamic nucleus, and substantia nigra), amygdala, and thalamus, (C) Posterior default mode network (P-DMN): precuneus/PCC, posterior parietal, and frontal cortex (including Brodmann area 8), (D) Reward learning network: ventromedial and orbital PFC, caudate, and nucleus accumbens. RSNs shown in red-yellow and overlaid onto the MNI 152 2 mm standard brain (Z > 2.6).
Anterior default mode network (Damoiseaux et al., 2006; Uddin et al., 2009): recent findings show the existence of several “default mode” sub-networks that can be parcellated from resting state data (Buckner et al., 2008; Andrews-Hanna et al., 2010; Smith et al., 2012). Consistent with prior observations (Andrews-Hanna et al., 2010), here we find a sub-network comprising ventral and dorsal medial PFC, anterior and posterior cingulate (A/PCC), precuneus, caudate, and posterior parietal regions that is engaged during autobiographical thought (Andrews-Hanna et al., 2010).
Basal ganglia/limbic network (Marchand, 2010; Laird et al., 2011; Janes et al., 2012; Licata et al., 2013; Beliveau et al., 2015): a limbic-paralimbic-striatal network comprising basal ganglia (striatum, pallidum, NAcc, subthalamic nucleus and substantia nigra), inferior insula, amygdala, and thalamus (Seeley et al., 2007; Touroutoglou et al., 2012) that has been associated with activation during reward and emotional tasks (Laird et al., 2011).
Posterior default mode network: We identified another subnetwork of the default mode comprising precuneus, PCC, posterior parietal, medial frontal cortex (including Brodmann area 8), retrosplenial cortex, and mesial temporal lobe, including amygdala. This network is a memory-related network associated with self-relevant recall (Shirer et al., 2012; Andrews-Hanna et al., 2014) and evaluation of emotional valence and arousal (Torta and Cauda, 2011)
Reward Learning (Laird et al., 2011): A network comprising ventromedial and orbitofrontal cortex, caudate, and nucleus accumbens that is also activated during reward and emotional tasks (Laird et al., 2011; Janes et al., 2014).
Relationships between functional connectivity, EQ-I, and MSCEIT
Within-network functional connectivity. There were no significant effects associated with EQ-i (reflecting Trait EI) for any of the primary networks of interest. On the other hand, the functional connectivity of each network was related to MSCEIT Total (reflecting Ability EI) and its branch sub-scales as follows:
Anterior default mode network (A-DMN). Total scores on the MSCEIT were unrelated to functional connectivity of the A-DMN.
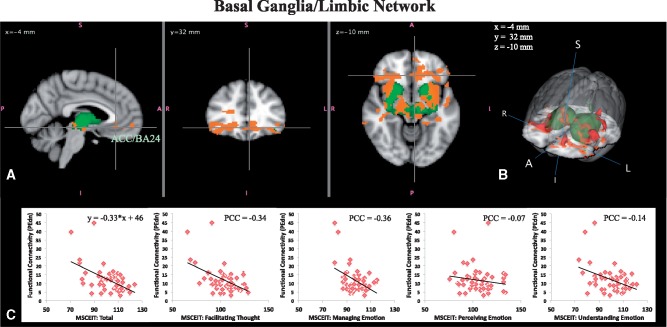
Basal ganglia/limbic network (BGN). A negative linear relationship between within-network functional connectivity and MSCEIT Total was found for the BGN. Figure 2A and B shows brain regions in which lower MSCEIT Total scores were related to the strength of functional connectivity with the BGN, including hypothesized emotion processing regions in vmPFC, AI, amygdala, lateral OFC and other widespread regions including pallidum, thalamus, midbrain, subcallosal cortex, paracingulate, ventral ACC, and white matter. Table 1 shows information regarding the clusters showing a statistically significant negative linear relationship with MSCEIT Total in Figure 2A and B. The plots in Figure 2C show the average of the parameter estimate values from the dual regression for each subject versus MSCEIT Total scores. From the plots, it is evident that increased connectivity of the BGN with these brain regions in individuals with lower Ability EI was driven primarily by scores on the MSCEIT Managing Emotions and Facilitating Thought branches. These analyses were repeated with the two data points removed that appear to be outliers in the plots. The results held without these two subjects.
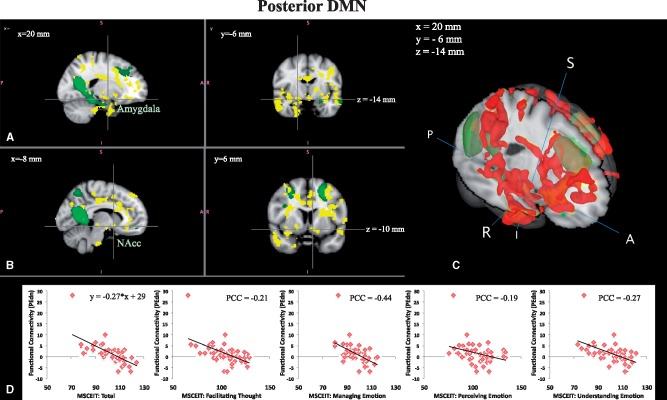
Posterior default mode network (P-DMN). Within-network connectivity of the P-DMN was also related to MSCEIT Total scores in that the functional connectivity between P-DMN and a spatially distributed pattern of regions, including hypothesized emotion processing structures such as the amygdala, vmPFC, AI, PI, OFC, and other non-hypothesized regions such as the temporal pole, middle temporal gyrus, hippocampus, nucleus accumbens, and the ACC, precuneus/posterior cingulate cortex, and superior parietal regions, was negatively linearly related to MSCEIT Total. Interestingly, higher MSCEIT Total scores were associated with stronger anti-correlations between the P-DMN and these regions (e.g. with negative average regression weights), while lower MSCEIT Total was associated with positive correlations between the P-DMN and these various regions (see Figure 3). The correlations with MSCEIT Total were primarily driven by the Managing Emotions branch. All analyses were repeated with removal of the single data point that appears to be an outlier in the plots; all results held without this subject. Table 2 shows information regarding the clusters showing a statistically significant negative linear relationship with MSCEIT Total in Figure 3A and B.
Reward Learning (RL). Total scores on the MSCEIT were unrelated to functional connectivity with this network.
Fig. 2.
Basal ganglia/limbic network. (A) Three orthogonal slices showing regions whose functional connectivity with the basal ganglia network (green) is negatively linearly associated with MSCEIT scores (i.e. reflecting Ability EI; red–yellow, P < 0.05 corrected). The crosshairs show one region in the medial PFC. (B) 3D view to better illustrate the spatial extent/pattern. (C) Functional connectivity values (regression coefficients averaged over voxels in red–yellow) plotted against MSCEIT: Total and MSCEIT subscales for Facilitating, Managing, Perceiving, and Understanding. Partial correlation coefficients (PCC) between functional connectivity values and MSCEIT scores are shown for each subscale.
Table 1.
Clusters with cluster size 10 or larger found in regions whose functional connectivity with the basal ganglia network (BGN) were negatively correlated with MSCEIT total scores
| Cluster name | Voxels | MAX | MAX X (mm) | MAX Y (mm) | MAX Z (mm) |
|---|---|---|---|---|---|
| R. Brainstem/hippocampus | 2727 | 5.13 | 12 | −10 | −12 |
| R. Inferior orbitofrontal gyrus | 955 | 4.93 | 38 | 28 | −16 |
| L. Precentral gyrus | 311 | 4.09 | −40 | 8 | 34 |
| R. Caudate | 162 | 3.94 | 22 | 28 | 14 |
| L. Hippocampus | 137 | 3.97 | −40 | −34 | −8 |
| R. Hippocampus | 65 | 3.22 | 40 | −28 | −10 |
| L. Pallidum | 43 | 2.94 | −16 | 0 | 2 |
| R. Heschl’s gyrus | 40 | 3.32 | 38 | −24 | 18 |
| L. Postcentral gyrus | 37 | 3.26 | −46 | −8 | 34 |
| L. Medial orbiofrontal gyrus | 30 | 2.98 | −4 | 56 | −8 |
| L. Amygdala | 30 | 3.08 | −16 | 0 | −12 |
| L. Superior temporal gyrus | 30 | 3.32 | −52 | −2 | −12 |
| R. Inferior temporal gyrus/hippocampus | 22 | 3.47 | 48 | −14 | −20 |
| L. Middle temporal gyrus | 16 | 3.4 | −52 | −16 | −2 |
| R. Middle temporal gyrus | 11 | 3.67 | 48 | −20 | −10 |
Cluster labeling was based on the nearest gray matter label to the cluster maximum according to the Automated Anatomical Labeling Atlas. R = right; L = left.
Fig. 3.
Posterior DMN network. Two orthogonal slices for two different x locations showing regions whose functional connectivity with the DMN is negatively linearly related to MSCEIT scores (i.e. reflecting Ability EI; red–yellow, P < 0.05, corrected). Crosshairs in (A) bisect the amygdala and crosshairs in (B), the nucleus accumbens. (C) 3D view to better illustrate the spatial extent/pattern. (D) Functional connectivity values plotted against MSCEIT scores (PCCs are shown for each subscale).
Table 2.
Clusters with cluster size 10 or larger found in regions whose functional connectivity with the posterior default mode network (P-DMN) were negatively correlated with MSCEIT Total scores
| Cluster name | Voxels | MAX | MAX X (mm) | MAX Y (mm) | MAX Z (mm) |
|---|---|---|---|---|---|
| R. Hippocampus | 5871 | 6.16 | 26 | −18 | −8 |
| L. Middle temporal gyrus | 1390 | 5.16 | −38 | −50 | 2 |
| L. Insula | 1033 | 4.84 | −34 | 2 | 16 |
| R. Middle occipital gyrus | 814 | 4.85 | 30 | −66 | 36 |
| L. Cuneus | 543 | 3.99 | −20 | −54 | 28 |
| L. Precentral gyrus | 422 | 4.61 | −36 | 10 | 42 |
| L. Middle cingulate gyrus | 256 | 4.24 | −8 | −18 | 42 |
| L. Inferior occipital gyrus | 256 | 4.53 | −22 | −104 | −6 |
| L. Cerebellum crus 1 | 203 | 3.76 | −36 | −84 | −18 |
| L. Supramarginal gyrus | 187 | 4.75 | −44 | −34 | 30 |
| R. Paracentral lobule | 182 | 4.3 | 4 | −42 | 72 |
| R. Parahippocampal gyrus | 163 | 3.73 | 24 | −40 | −4 |
| L. Middle occipital gyrus | 160 | 4.01 | −38 | −76 | 18 |
| L. Hemisphere white matter | 143 | 3.96 | −24 | −2 | 32 |
| R. Cerebellum area 6 | 121 | 4.69 | 36 | −58 | −20 |
| L. Cerebellum area 6 | 117 | 4.14 | −34 | −62 | −22 |
| R. Middle cingulate gyrus | 115 | 4.23 | 12 | −28 | 40 |
| L. Middle occipital gyrus | 114 | 3.55 | −30 | −70 | 30 |
| L. Hemisphere white matter | 87 | 3.48 | −22 | −40 | 42 |
| L. Superior occipital gyrus | 78 | 3.44 | −18 | −98 | 20 |
| R. Gyrus rectus | 76 | 3.44 | 10 | 20 | −12 |
| L. Hippocampus | 72 | 4.73 | −36 | −8 | −14 |
| R. Postcentral gyrus | 71 | 4.03 | 28 | −36 | 68 |
| R. Middle frontal gyrus | 64 | 3.91 | 34 | 44 | 12 |
| L. Cerebellum Area 10 | 62 | 3.52 | −22 | −32 | −42 |
| R. Middle frontal gyrus | 58 | 4.26 | 24 | 46 | 2 |
| R. Posterior cingulate gyrus | 53 | 3.61 | 8 | −40 | 14 |
| L. Thalamus | 52 | 4.39 | −16 | −6 | 4 |
| R. Superior frontal gyrus | 50 | 3.49 | 18 | 50 | 18 |
| L. Brainstem | 43 | 3.36 | −16 | −12 | −8 |
| R. Middle temporal gyrus | 27 | 3.19 | 52 | −24 | −4 |
| L. Middle frontal gyrus | 25 | 3.12 | −24 | 40 | 32 |
| R. Caudate | 25 | 3.3 | 12 | 24 | 2 |
| R. Superior temporal gyrus | 24 | 3.64 | 50 | −4 | −2 |
| L. Hemisphere white matter | 22 | 2.96 | −18 | −16 | 34 |
| L. Rolandic operculum | 20 | 3.19 | −62 | 6 | 6 |
| R. Precuneus | 17 | 3.45 | 6 | −50 | 14 |
| L. Cerebellum area 6 | 16 | 3.19 | −26 | −52 | −20 |
| L. Middle occipital gyrus | 15 | 3.74 | −28 | −78 | 42 |
| L. Precentral gyrus | 15 | 3.2 | −56 | 4 | 38 |
| R. Caudate | 15 | 3.18 | 6 | 16 | 0 |
| L. Superior temporal gyrus | 14 | 3.26 | −54 | −6 | 6 |
| L. Middle frontal gyrus | 13 | 3.31 | −34 | 20 | 58 |
| R. Precuneus | 12 | 3.11 | 10 | −56 | 70 |
| R. Calcarine cortex | 12 | 2.89 | 30 | −60 | 12 |
| R. Superior parietal cortex | 11 | 3.1 | 20 | −64 | 70 |
| L. Calcarine cortex | 11 | 3.05 | −14 | −50 | 12 |
| L. Precuneus | 11 | 3.07 | −4 | −44 | 10 |
| L. Pallidum | 11 | 3.36 | −26 | −6 | −4 |
| R. Insula | 11 | 3.33 | 38 | 14 | −16 |
Cluster labeling was based on the nearest gray matter label to the cluster maximum according to the Automated Anatomical Labeling Atlas. L, left; R, right.
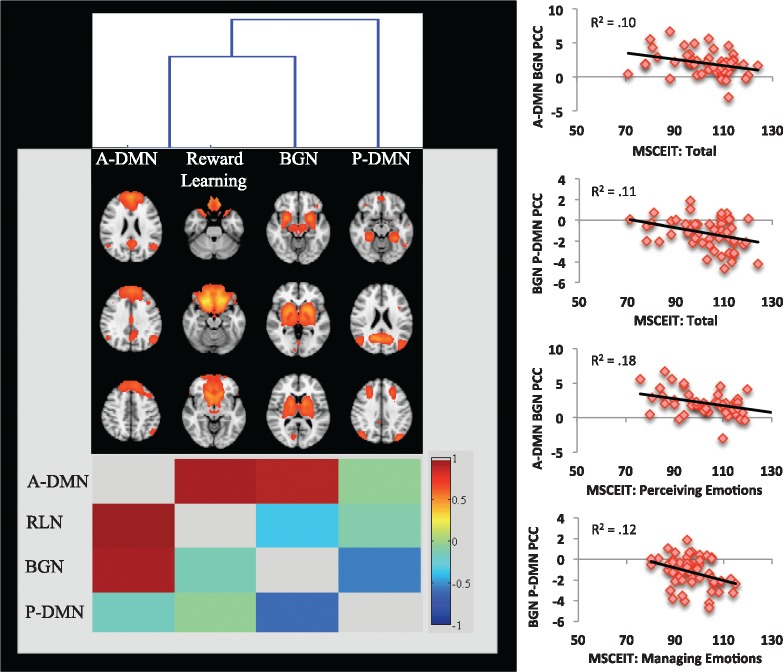
Between-network functional connectivity. For this sample of healthy adults, the A-DMN was strongly significantly correlated (P < 0.05 corrected) with both the BGN (partial correlation coefficient or PCC = 0.93) and the Reward Learning network (PCC = 0.95). In addition, the P-DMN was significantly anti-correlated with the BGN network (PCC = −0.75). These between network relationships are illustrated in Figure 4. Furthermore, the correlations among these networks showed statistically significant relationships with MSCEIT subscales as follows:
A-DMN with BGN: partial correlations between these two networks were negatively linearly related to MSCEIT Total (P = 0.05, corrected) and MSCEIT Perceiving (P = 0.004, corrected), such that the greater the positive correlation strength between the two networks, the lower the MSCEIT Perceiving Emotions score, whereas near-zero correlations between the two networks were associated with the highest MSCEIT scores.
BGN and P-DMN: negative partial correlation (e.g. anti-correlation) between these two networks was linearly related to MSCEIT Total (P = 0.03, corrected) and MSCEIT Managing Emotions, (P = 0.03, corrected), such that the greater the strength of the anti-correlation, the higher the MSCEIT score. The lowest MSCEIT scores were associated with partial correlations near zero.
Fig. 4.
(Left) Hierarchical clustering shows between-network correlations. The dendrogram is shown in the top of the figure, the RSNs in the middle, and the correlation matrix at the bottom (correlation coefficients shown in the bottom triangular portion and partial correlations shown in the top triangular portion). (Right) Scatterplots showing the significant association between Ability EI (MSCEIT) scales and the major network connectivity (represented as z-transformed partial correlation coefficients in the y-axis). A-DMN, anterior default mode network; BGN, basal ganglia/limbic network; P-DMN, posterior default mode network; PCC, partial correlation coefficient; RLN, reward learning network.
Discussion
Consistent with our “push–pull” hypothesis with respect to Ability EI, we found that higher MSCEIT Total scores were associated with more negative intrinsic connectivity values between specific “task negative” and “task positive” networks, and between those networks and many brain regions implicated in emotion processing. However, contrary to our expectations, the Bar-On Total EQ-i (Trait EI) was unrelated to resting state connectivity within or between the networks we investigated. Specifically, based on findings from our prior functional and structural studies in EI (Killgore et al., 2012; Killgore et al., 2013; Weber et al., 2013; Alkozei and Killgore, 2015; Smith et al., 2016), we selected four brain networks associated with key emotional and integrative processes from the results of a group ICA of resting state fMRI data that included: an (i) Anterior Default Mode network, (ii) Basal Ganglia/Limbic network, (iii) Posterior Default Mode network, and (iv) a Reward Learning network. The strength of resting state functional connectivity within each of these four networks (within-network connectivity) and with each other (between-network connectivity) was then statistically evaluated against Trait and Ability measures of EI using a dual regression procedure. Whereas Trait EI (EQ-i) was unrelated to brain connectivity in these four networks, Ability EI (MSCEIT) was significantly negatively associated with the functional connectivity of the Basal Ganglia/Limbic network and the Posterior DMN network with other brain regions involved in a wide range of emotion-related processes, including primary threat/salience assessment (e.g. amygdala), visceral sensations (e.g. insula), autobiographical memory retrieval (e.g. posterior cingulate), behavioral control (e.g. lateral orbitofrontal cortex), conflict monitoring (e.g. anterior cingulate cortex), reward processing (e.g. ventral striatum), and many others. In contrast, neither the Anterior DMN nor Reward Learning network connectivity patterns were associated with Ability EI. Given that the Trait and Ability models of EI were uncorrelated here, these findings suggest that the ability to reason about emotional information (Ability EI), but not necessarily perceived self-efficacy with regard to emotional competencies (Trait EI), is associated with greater negative functional connectivity between diverse brain regions and networks involved in the rapid assessment and interoceptive aspects of emotional experience, autobiographical memory, and regulatory control, but not significantly with intrinsic connectivity of areas involved in reward learning or self-referential processing.
Interestingly, in the P-DMN, a network often associated with autobiographical memory retrieval (Whitfield-Gabrieli et al., 2011; Qin et al., 2012), we found that higher Ability EI (MSCEIT) was associated with synchronous, but negative, correlations between the DMN, and other key affective brain regions, while lower Ability EI appeared to involve positively correlated signals between this network and the same brain regions. In other words, individuals with high MSCEIT Total scores showed anti-correlations between the P-DMN and distributed brain regions involved in the perception, experience and regulation of emotion, while individuals with low MSCEIT Total scores showed positive correlations between the P-DMN and these regions, a finding generally consistent with another recent brain imaging study of Trait EI (Takeuchi et al., 2013). Although the interpretation of negative correlations in resting state data continues to be a subject of debate, when data are processed with the group ICA with dual regression, negative correlations in spontaneous fluctuations across spatially distributed regions may indeed reflect valid neurobiological phenomena (Fox et al., 2009; Chai et al., 2012) owing to the fact that this approach does not bring to bear the issue of global signal regression. As such, our findings suggest that, similar to observations of superior task performance in individuals with strongest anti-correlations between the P-DMN and Task Positive networks (Chai et al., 2014), superior Ability EI is associated with strong anti-correlations between the P-DMN and key affective brain regions, while conversely, individuals with poor Ability EI show positive correlations between P-DMN and widespread cortico-limbic and striatal regions involved in emotional processing.
The present findings are consistent with numerous other studies that show that superior cognitive performance is often associated with greater suppression of the DMN, or a kind of “push–pull” association between DMN and TPN regions, while sub-optimal, developmentally immature, or clinically aberrant patterns of performance tend to be associated with reduced anti-correlation or even positive correlations between these networks (Barber et al., 2013; Chai et al., 2014). For example, among healthy adults, greater anti-correlation between DMN and TPN is associated with better attentional performance (Kelly et al., 2008). Similarly, healthy adults show greater anti-correlation between DMN and TPN than children or adolescents, a pattern that correlates with greater inhibitory control, but the strength of these anti-correlations declines again in older age (Spreng et al., 2016). By extension, even more severe versions of this pattern may be seen among individuals with various forms of psychopathology, including affective disorders (Marchetti et al., 2012; McEvoy et al., 2013; Wilkinson et al., 2013) or schizophrenia (Whitfield-Gabrieli et al., 2009). This is not to say that decreased anti-correlations are necessarily directly associated with psychopathology, but they tend to be associated with lower EI capacities, which in itself may serve as a potential risk factor (Paradiso et al., 2016). This possibility remains an open question for further research.
Consistent with these prior studies, we propose that Ability EI may also involve a similar relationship to the patterns of connectivity between the P-DMN and the emotion-related systems described above. Specifically, our data suggest that a person with high Ability EI would be expected to show largely anticorrelated activation between the emotion-related and self-referential P-DMN regions. Such suppression of self-reflective systems when emotion-processing systems are active may facilitate clear boundaries between emotional responses and self-relevant cognitions. On the other hand, our data suggest that a person with low Ability EI would be expected to show positive coupling between these systems, perhaps contributing to difficulties separating emotional responses from self-reflective processing. We posit that this neurobiological obscuring of the boundaries between internal self-referential cognitions and emotional responses may be associated ultimately with a difficulty reasoning objectively about emotions and managing them in accord with external constraints (i.e. difficulty maintaining “emotional perspective”). Our findings are consistent with such a conceptualization, but replication in other domains of emotional behavior will be necessary to determine the extent to which these patterns of connectivity contribute to the ability to deal effectively with real-world social and emotional phenomena.
For the BGN, individuals with the lowest Ability EI (MSCEIT) had the strongest positive connectivity within the network itself and between the network and regions in vmPFC, anterior insula (AI), amygdala and lateral OFC. Prior research suggests that these regions are important in the regulation of emotion. Specifically, the vmPFC and contiguous regions of the rACC/sgACC have been proposed to modulate activation within key affective regions such as the amygdala (Kim et al., 2004; Urry et al., 2006; Diekhof et al., 2011; Etkin et al., 2011; Seo et al., 2013; Wagner and Heatherton, 2013; Zotev et al., 2013), whereas the lateral OFC appears to be involved in regulating emotion through reappraisal processes (Golkar et al., 2012; Buhle et al., 2013; Vijayakumar et al., 2014). The less functionally connected these regions were with the BGN, the higher an individual’s Ability EI. Furthermore, the BGN was more strongly functionally connected to the A-DMN in individuals with the lowest Ability EI. Thus, similar to the P-DMN findings above, strong positive coupling between the BGN and affective regions was associated with lower Ability EI.
The positive connectivity between emotional regulation regions and the BGN may suggest that low EI individuals may have difficulty regulating their emotional experiences and interoceptive sensations via prefrontal control systems. This is consistent with research showing that activation of the vmPFC is negatively related to activation in the amygdala in healthy individuals but is positively coupled in depressed patients (Johnstone et al., 2007) as well as among individuals who have not obtained adequate sleep (Killgore, 2013). Notably, the association between Total EI and the connectivity among these regions was driven most strongly by the Managing Emotions branch of the MSCEIT, consistent with the hypothesis that poorer emotion regulation is associated with greater in-phase correlation between the aforementioned regulatory regions and the BGN (i.e. when prefrontal activity is high, activity within emotional and interoceptive regions also remains high, rather than being suppressed or effectively modulated). While causal direction cannot be inferred because these data are strictly correlational, they are nevertheless in accord with the growing consensus that the vmPFC plays a modulatory role over primary emotionally responsive regions within the limbic system (e.g. amygdala) (Kim et al., 2004; Urry et al., 2006; Johnstone et al., 2007). Thus, we speculate that one of the neurobiological mechanisms underlying lower EI capacities may involve ineffective modulation of the BGN by prefrontal control regions.
It is also worthwhile to note that while we found significant associations between Ability EI (MSCEIT) and functional connectivity of the BGN and P-DMN, Trait EI (EQ-i) was not significantly related to connectivity patterns among the four the networks we studied. This stands in contrast to a recently published seed-based functional connectivity study that found significant correlations between a Japanese measure of Trait EI [i.e. Emotional Intelligence Scale (Fukunishi et al., 2001)] and functional connectivity between several regions involved in social and emotional processing (Takeuchi et al., 2013). However, that study differed significantly from the present investigation in several important respects, including their use of a seed-based analysis to investigate the specific connectivity of the medial PFC and the left anterior insula as opposed to our use of ICA with dual regression to investigate the connectivity of the networks that contain these regions. In addition, they used a different Trait EI measure with a different factor structure, presented in a different language to a more homogeneous group of younger and more highly educated university students. Further research will be necessary to determine the role of these factors in the observed differences between studies. Nonetheless, the present results strongly suggest that higher Ability EI capacities appear to be associated with stronger anti-correlations between key networks involved in affective control versus emotional responses, and between emotionally responsive networks and those involved in self-reflective cognition.
Funding
This study was supported by a grant from the U.S. Army Medical Research Acquisition Activity (USAMRAA) to WDSK (W81XWH-09-1-0730) and R01 DA037265 to LDN.
Conflict of interest. None declared.
References
- Abu-Akel A., Shamay-Tsoory S. (2011). Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia, 49, 2971–84. [DOI] [PubMed] [Google Scholar]
- Alkozei A., Killgore W.D. (2015). Emotional intelligence is associated with reduced insula responses to masked angry faces. Neuroreport, 26, 567–71. [DOI] [PubMed] [Google Scholar]
- Amdurer E., Boyatzis R.E., Saatcioglu A., Smith M.L., Taylor S.N. (2014). Long term impact of emotional, social and cognitive intelligence competencies and GMAT on career and life satisfaction and career success. Frontiers in Psychology, 5, 1447.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. (2010). Functional-anatomic fractionation of the brain's default network. Neuron, 65, 550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Saxe R., Yarkoni T. (2014). Contributions of episodic retrieval and mentalizing to autobiographical thought: evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. Neuroimage, 91, 324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On R. (2002) BarOn Emotional Quotient Inventory: A Measure of Emotional Intelligence–Technical Manual. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Bar-On R. (2006). The Bar-On model of emotional-social intelligence (ESI). Psicothema, 18, 13–25. [PubMed] [Google Scholar]
- Bar-On R., Tranel D., Denburg N.L., Bechara A. (2003). Exploring the neurological substrate of emotional and social intelligence. Brain, 126, 1790–800. [DOI] [PubMed] [Google Scholar]
- Barber A.D., Caffo B.S., Pekar J.J., Mostofsky S.H. (2013). Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia, 51, 156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O., McGuire J.T., Kable J.W. (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage, 76, 412–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Mackay C.E., Filippini N., Smith S.M. (2009). Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Neuroimage, 47, S39–41. [Google Scholar]
- Beckmann C.F., Smith S.M. (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging, 23, 137–52. [DOI] [PubMed] [Google Scholar]
- Beliveau V., Svarer C., Frokjaer V.G., Knudsen G.M., Greve D.N., Fisher P.M. (2015). Functional connectivity of the dorsal and median raphe nuclei at rest. Neuroimage, 116, 187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackett M.A., Mayer J.D. (2003). Convergent, discriminant, and incremental validity of competing measures of emotional intelligence. Personality and Social Psychology Bulletin, 29, 1147–58. [DOI] [PubMed] [Google Scholar]
- Brackett M.A., Rivers S.E., Shiffman S., Lerner N., Salovey P. (2006). 'Relating emotional abilities to social functioning: a comparison of self-report and performance measures of emotional intelligence. Journal of Personality and Social Psychology, 91, 780–95. [DOI] [PubMed] [Google Scholar]
- Brannick M.T., Wahi M.M., Arce M., Johnson H.A., Nazian S., Goldin S.B. (2009). Comparison of trait and ability measures of emotional intelligence in medical students. Medical Education, 43, 1062–8. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. (2008). The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2013) Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Dell'acqua F., Thiebaut de Schotten M. (2013). A revised limbic system model for memory, emotion and behaviour. Neuroscience and Biobehavioral Reviews, 37, 1724–37. [DOI] [PubMed] [Google Scholar]
- Chai X.J., Castanon A.N., Ongur D., Whitfield-Gabrieli S. (2012). Anticorrelations in resting state networks without global signal regression. Neuroimage, 59, 1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Ofen N., Gabrieli J.D., Whitfield-Gabrieli S. (2014). Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. Journal of Cognitive Neuroscience, 26, 501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniss C., Extein M., Goleman D., Weissberg R.P. (2006). Emotional Intelligence: What does the research really indicate?. Educational Psychologist, 41, 239–45. [Google Scholar]
- Clithero J.A., Rangel A. (2014). Informatic parcellation of the network involved in the computation of subjective value. Social Cognitive and Affective Neuroscience, 9, 1289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F., et al. (2006). Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America, 103, 13848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daus C.S. (2006). The case for an ability-based model of emotional intelligence In: Murphy K. R., editor. A Critique of Emotional Intelligence: What Are the Problems and How Can They Be Fixed? New York: Lawrence Earlbaum. [Google Scholar]
- Diekhof E.K., Geier K., Falkai P., Gruber O. (2011). Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage, 58, 275–85. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., et al. (2009). Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proceedings of the National Academy of Sciences of the United States of America, 106, 7209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N., Nickerson L.D., Beckmann C.F., et al. (2012). Age-related adaptations of brain function during a memory task are also present at rest. Neuroimage, 59, 3821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. (2009). The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology, 101, 3270–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunishi I., Wise T.N., Sheridan M., et al. (2001). Validity and reliability of the Japanese version of the Emotional Intelligence Scale among college students and psychiatric outpatients. Psychological Reports, 89, 625–32. [DOI] [PubMed] [Google Scholar]
- Golkar A., Lonsdorf T.B., Olsson A., et al. (2012). Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One, 7, e48107.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillory S.A., Bujarski K.A. (2014). Exploring emotions using invasive methods: review of 60 years of human intracranial electrophysiology. Social Cognitive and Affective Neuroscience, 9, 1880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Cobo M.J., Cabello R., Fernandez-Berrocal P. (2016). The relationship between emotional intelligence and cool and hot cognitive processes: a systematic review. Frontiers in Behavioral Neuroscience, 10, 101.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M., Driesen N., Roth J.K., Gore J.C., Constable R.T. (2010). Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magnetic Resonance Imaging, 28, 1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S., Nichols T.E. (2003). Validating cluster size inference: random field and permutation methods. Neuroimage, 20, 2343–56. [DOI] [PubMed] [Google Scholar]
- Hayasaka S., Phan K.L., Liberzon I., Worsley K.J., Nichols T.E. (2004). Nonstationary cluster-size inference with random field and permutation methods. Neuroimage, 22, 676–87. [DOI] [PubMed] [Google Scholar]
- Janes A.C., Farmer S., Frederick B., Nickerson L.D., Lukas S.E. (2014). An increase in tobacco craving is associated with enhanced medial prefrontal cortex network coupling. PLoS One, 9, e88228.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes A.C., Nickerson L.D., Frederick Bde B., Kaufman M.J. (2012). Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug and Alcohol Dependence, 125, 252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17, 825–41. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. (2012). Fsl. Neuroimage, 62, 782–90. [DOI] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. (2007). Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27, 8877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.M., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. (2008). Competition between functional brain networks mediates behavioral variability. Neuroimage, 39, 527–37. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S. (2013). Self-reported sleep correlates with prefrontal-amygdala functional connectivity and emotional functioning. Sleep, 36, 1597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W.D.S., Schwab Z.J., Tkachenko O., et al. (2013). Emotional intelligence correlates with functional responses to dynamic changes in facial trustworthiness. Social Neuroscience, 8, 334–46. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S., Weber M., Schwab Z.J., et al. (2012). Gray matter correlates of Trait and Ability models of emotional intelligence. Neuroreport, 23, 551–5. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S., Yurgelun-Todd D.A. (2007). Neural correlates of emotional intelligence in adolescent children. Cognitive, Affective and Behavioral Neuroscience, 7, 140–51. [DOI] [PubMed] [Google Scholar]
- Kim H., Somerville L.H., Johnstone T., et al. (2004). Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience, 16, 1730–45. [DOI] [PubMed] [Google Scholar]
- Kober H., Barrett L.F., Joseph J., Bliss-Moreau E., Lindquist K., Wager T.D. (2008). Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage, 42, 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koven N.S., Roth R.M., Garlinghouse M.A., Flashman L.A., Saykin A.J. (2011). Regional gray matter correlates of perceived emotional intelligence. Social Cognitive and Affective Neuroscience, 6, 582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A.R., Fox P.M., Eickhoff S.B., et al. (2011). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23, 4022–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbrecht N., Lievens F., Carette B., Cote S. (2014). Emotional intelligence predicts success in medical school. Emotion, 14, 64–73. [DOI] [PubMed] [Google Scholar]
- Licata S.C., Nickerson L.D., Lowen S.B., Trksak G.H., Maclean R.R., Lukas S.E. (2013). The hypnotic zolpidem increases the synchrony of BOLD signal fluctuations in widespread brain networks during a resting paradigm. Neuroimage, 70, 211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A., Wager T.D., Kober H., Bliss-Moreau E., Barrett L.F. (2012). The brain basis of emotion: a meta-analytic review. Behavioral and Brain Sciences, 35, 121–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand W.R. (2010). Cortico-basal ganglia circuitry: a review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Structure and Function, 215, 73–96. [DOI] [PubMed] [Google Scholar]
- Marchetti I., Koster E.H., Sonuga-Barke E.J., De Raedt R. (2012). The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychology Review, 22, 229–51. [DOI] [PubMed] [Google Scholar]
- Mayer J.D., DiPaolo M., Salovey P. (1990). Perceiving affective content in ambiguous visual stimuli: a component of emotional intelligence. Journal of Personality Assessment, 54, 772–81. [DOI] [PubMed] [Google Scholar]
- Mayer J.D., Roberts R.D., Barsade S.G. (2008a). Human abilities: emotional intelligence. Annual Review of Psychology, 59, 507–36. [DOI] [PubMed] [Google Scholar]
- Mayer J.D., Salovey P., Caruso D.R. (2002) Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) User's Manual. North Tonawanda, NY, MHS.
- Mayer J.D., Salovey P., Caruso D.R. (2008b). Emotional intelligence: new ability or eclectic traits?. American Psychologist, 63, 503–17. [DOI] [PubMed] [Google Scholar]
- Mayer J.D., Salovey P., Caruso D.R., Sitarenios G. (2001). Emotional intelligence as a standard intelligence. Emotion, 1, 232–42. [PubMed] [Google Scholar]
- McEvoy P.M., Watson H., Watkins E.R., Nathan P. (2013). The relationship between worry, rumination, and comorbidity: evidence for repetitive negative thinking as a transdiagnostic construct. Journal of Affective Disorders, 151, 313–20. [DOI] [PubMed] [Google Scholar]
- Meeks T.W., Jeste D.V. (2009). Neurobiology of wisdom. A literature overview. Archives of General Psychiatry, 66, 355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczak M., Luminet O., Leroy C., Roy E. (2007). Psychometric properties of the Trait Emotional Intelligence Questionnaire: factor structure, reliability, construct, and incremental validity in a French-speaking population. Journal of Personality Assessment, 88, 338–53. [DOI] [PubMed] [Google Scholar]
- Moslehi M., Samouei R., Tayebani T., Kolahduz S. (2015). A study of the academic performance of medical students in the comprehensive examination of the basic sciences according to the indices of emotional intelligence and educational status. Journal of Education and Health Promotion, 4, 66.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki Y., Koyasu M. (2013). The relationship between trait emotional intelligence and interaction with ostracized others' retaliation. PLoS One, 8, e77579.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso S., Beadle J.N., Raymont V., Grafman J. (2016). Suicidal thoughts and emotion competence. Journal of Clinical and Experimental Neuropsychology, 38, 887–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides K.V., Furnham A. (2003). Trait emotional intelligence: behavioral validation in two studies of emotion recognition and reactivity to mood induction. European Journal of Personality, 17, 39–57. [Google Scholar]
- Petrides K.V., Pita R., Kokkinaki F. (2007). The location of trait emotional intelligence in personality factor space. British Journal of Psychology, 98, 273–89. [DOI] [PubMed] [Google Scholar]
- Qin P., Liu Y., Shi J., et al. (2012). Dissociation between anterior and posterior cortical regions during self-specificity and familiarity: a combined fMRI-meta-analytic study. Human Brain Mapping, 33, 154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarto T., Blasi G., Maddalena C., et al. (2016). Association between ability emotional intelligence and left insula during social judgment of facial emotions. PLoS One, 11, e0148621.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis D.L., Brackett M.A., Shamosh N.A., Kiehl K.A., Salovey P., Gray J.R. (2007). Emotional Intelligence predicts individual differences in social exchange reasoning. Neuroimage, 35, 1385–91. [DOI] [PubMed] [Google Scholar]
- Salovey P., Mayer J.D. (1990). Emotional intelligence. Imagination, Cognition, and Personality, 9, 185–211. [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27, 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D., Olman C.A., Haut K.M., Sinha R., Macdonald A.W. 3rd, Patrick C.J. (2014). Neural correlates of preparatory and regulatory control over positive and negative emotion. Social Cognitive and Affective Neuroscience, 9, 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W.R., Ryali S., Rykhlevskaia E., Menon V., Greicius M.D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex, 22, 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegling A.B., Furnham A., Petrides K.V. (2015). Trait emotional intelligence and personality: gender-invariant linkages across different measures of the big five. Journal of Psychoeducational Assessment, 33, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Alkozei A., Killgore W.D.S. (2017). Contributions of self-report and performance-based individual differences measures of social cognitive ability to large-scale neural network functioning. Brain Imaging Behav, 11, 685–97. [DOI] [PubMed] [Google Scholar]
- Smith S.M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., et al. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106, 13040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23, S208–19. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Miller K.L., Moeller S., et al. (2012). Temporally-independent functional modes of spontaneous brain activity. Proceedings of the National Academy of Sciences of the United States of America, 109, 3131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Miller K.L., Salimi-Khorshidi G., et al. (2011). Network modelling methods for FMRI. Neuroimage, 54, 875–91. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Stevens W.D., Viviano J.D., Schacter D.L. (2016). Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiology of Aging, 45, 149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Taki Y., Nouchi R., et al. (2013). Resting state functional connectivity associated with trait emotional intelligence. Neuroimage, 83, 318–28. [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Taki Y., Sassa Y., et al. (2011). Regional gray matter density associated with emotional intelligence: evidence from voxel-based morphometry. Human Brain Mapping, 32, 1497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico J.F., Varon A.J., Banks S.E., et al. (2013). Emotional intelligence and the relationship to resident performance: a multi-institutional study. Journal of Clinical Anesthesia, 25, 181–7. [DOI] [PubMed] [Google Scholar]
- Torta D.M., Cauda F. (2011). Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage, 56, 2157–72. [DOI] [PubMed] [Google Scholar]
- Touroutoglou A., Hollenbeck M., Dickerson B.C., Feldman Barrett L. (2012). Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage, 60, 1947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Kelly A.M., Biswal B.B., Castellanos F.X., Milham M.P. (2009). Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Human Brain Mapping, 30, 625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H.L., van Reekum C.M., Johnstone T., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26, 4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Whittle S., Yucel M., Dennison M., Simmons J., Allen N.B. (2014). Thinning of the lateral prefrontal cortex during adolescence predicts emotion regulation in females. Social Cognitive and Affective Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.D., Heatherton T.F. (2013). Self-regulatory depletion increases emotional reactivity in the amygdala. Social Cognitive and Affective Neuroscience, 8, 410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C.A., Schwab Z.J., Weber M., et al. (2013). Convergent and divergent validity of integrative versus mixed model measures of emotional intelligence. Intelligence, 41, 149–56. [Google Scholar]
- Weber M., Webb C.A., Deldonno S.R., et al. (2013). Habitual ‘sleep credit’ is associated with greater grey matter volume of the medial prefrontal cortex, higher emotional intelligence and better mental health. Journal of Sleep Research, 22, 527–34. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Moran J.M., Nieto-Castanon A., Triantafyllou C., Saxe R., Gabrieli J.D. (2011). Associations and dissociations between default and self-reference networks in the human brain. Neuroimage, 55, 225–32. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Thermenos H.W., Milanovic S., et al. (2009). Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 106, 1279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P.O., Croudace T.J., Goodyer I.M. (2013). Rumination, anxiety, depressive symptoms and subsequent depression in adolescents at risk for psychopathology: a longitudinal cohort study. BMC Psychiatry, 13, 250.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Jbabdi S., Patenaude B., et al. (2009). Bayesian analysis of neuroimaging data in FSL. Neuroimage, 45, S173–86. [DOI] [PubMed] [Google Scholar]
- Wotruba D., Michels L., Buechler R., et al. (2013). Aberrant coupling within and across the default mode, task-positive, and salience network in subjects at risk for psychosis. Schizophrenia Bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Phillips R., Young K.D., Drevets W.C., Bodurka J. (2013). Prefrontal control of the amygdala during real-time fMRI neurofeedback training of emotion regulation. PLoS One, 8, e79184.. [DOI] [PMC free article] [PubMed] [Google Scholar]