Abstract
Background:
Major depressive disorder has been associated with dysfunctional astrocytic networks. The underlying causes, extent, and consequences of such dysfunctions remain to be characterized. Astrocyte-astrocyte communication occurs principally through gap junction channels primarily formed by connexin 30 and 43 (CX30 and CX43). We previously reported decreased connexin expression in the prefrontal cortex of depressed suicides. In the present study, we investigated whether these changes are mediated by epigenetic regulation, and expanded gene expression quantifications to other cortical and subcortical regions to assess the regional distribution of connexion disruptions in depressed suicides.
Methods:
The expression of CX30 and CX43 was measured by real-time PCR in samples of neocortex (Brodmann areas 4 and 17), cerebellar cortex, mediodorsal thalamus, and caudate nucleus of 22 depressed suicides and 22 matched sudden-death controls. Chromatin immunoprecipitation was used to measure enrichment levels of the repressive chromatin mark H3K9me3 in the prefrontal cortex.
Results:
We found a consistent downregulation of connexin genes in all regions examined, except in the cerebellum where an increase in the expression of CX30 was measured and using chromatin immunoprecipitation we observed an enrichment of H3K9me3 for both Cx30 and Cx43 in the prefrontal cortex.
Conclusions:
Our study shows widespread astrocytic CX gene repression in depressed suicides that is mediated, at least in part, through epigenetic mechanisms. Taken together, these findings support the notion of widespread cerebral astrocytic dysfunction in major depressive disorder.
Keywords: chromatin modification, astrocytes, connexions, depression, suicide
Significance Statement
The research presented here shows a widely distributed dysregulation of the astrocytic connexin genes CX30 and CX43 across the brain of depressed suicides. Disruption to connexins may have important consequences for astrocytes given their numerous and significant roles in normal astrocytic function. We have also shown that the decrease in CX30 and CX43 expression is likely mediated by an epigenetic mechanism in the form of chromatin conformational changes, as we found significant increases in the repressive H3K9me3 histone mark. These findings are in line with the growing body of literature that implicates aberrant astrocytic function in the pathophysiology of major depression.
Introduction
Glial cells have been implicated in major depression for nearly 20 years (Ongür et al., 1998), with much of the data pointing toward astrocytes as the main glial contributor (Rajkowska and Stockmeier, 2013). Of all cells in the brain, astrocytes exhibit the highest level of connexin (Cx) expression (Giaume and Naus, 2013). Cxs form the base unit of gap junction channels (GJC), and it is estimated that each cell will form up to 30000 GJCs (Rohlmann and Wolff, 1996). These specialized channels facilitate intercellular communication through direct transfer of molecules between adjacent cells (Rash et al., 2012), allowing for the regulation of metabolic and homeostatic functions (Pannasch and Rouach, 2013) and propagating Ca2+ waves (Verkhratsky and Kettenmann, 1996). GJCs are comprised of 2 hemichannels (connexins) that can be composed of 6 identical connexins (homomeric) or of multiple connexin isotypes (heteromeric). Each of the 2 participating cells contribute one hemichannel, and each Cx combination (heter- or homo- meric or type) endows unique physiological properties to the channel (Kumar and Gilula, 1996). Though mRNA for Cx26, Cx30, Cx32, Cx40, and Cx43 have been detected by single-cell RT-PCR using astrocytes from brain slices (Blomstrand et al., 2004), the major Cxs have been identified as Cx30 and Cx43 (Giaume and McCarthy, 1996; Rouach and Giaume, 2001), which form either homotypic or heterotypic gap junctions (Nagy et al., 1999). The high expression levels of Cx30 and 43 throughout the brain (Nagy et al., 2004) and the fact that Cx30/Cx43 double-knockout mice lack GJCs (Wallraff et al., 2006) both support these as the main 2 Cxs isotypes of astrocytes. Astrocytic connexins have previously been implicated in depression by our group (Ernst et al., 2011; Nagy et al., 2015) and others (Bernard et al., 2011; Miguel-Hidalgo et al., 2014). Similarly, studies using animal models of depression have shown decreased expression and function of Cx43, with recovery using antidepressants such as fluoxetine, duloxetine, or the tricyclic amitriptyline (Li et al., 2010; Sun et al., 2012b; Morioka et al., 2014; Mostafavi et al., 2014; Quesseveur et al., 2015). Given their abundance, role in communication, and association with antidepressant response, these proteins represent markers that may provide insight into the functional status of astrocytes in depression.
Astrocytes are known to be both sensitive and reactive to various microenvironmental cues, such as those arising from traumatic brain injury or autoimmune disorders (Sofroniew, 2009). Likewise, epigenetic modifications, such as histone modifications, constitute molecular mechanisms that respond to environmental changes. Histone modifications have previously been identified as a form of gene regulation in mood disorders, including major depression (for review, see Sun et al., 2013). Chromatin conformation can be altered so that DNA becomes more or less accessible to transcription factors or other DNA binding proteins. Modifications to the histone tail, often at a lysine, are known to create either an open/active state or a closed/repressive state. Histone 3 lysine 9 trimethylation (H3K9me3), for example, is almost exclusively associated with repressed chromatin states and is often found near the promoter region of a gene (Bannister and Kouzarides, 2011). H3K9me3 in particular has been associated with stress regulation (Hunter et al., 2009) and antidepressant response (Jiang et al., 2010), making it an interesting target in the context of depression.
We previously reported a robust downregulation of Cx30 and Cx43 in the prefrontal cortex (PFC) (BA8/9 and BA10) of people died by suicide (Nagy et al., 2015). In the present study, we set out to determine if disrupted CX gene expression in depression and suicide is confined to these cortical areas, or if it is a more widespread phenomenon in the brain. As the motor, visual, and cerebellar cortex are not typically implicated in mood disorders, they were selected to test if altered connexin expression is a general phenomenon in the brain, or if it is localized to regions implicated in mood disorders. Additionally, to determine the subcortical involvement of astrocytic connexin dysregulation, the mediodorsal thalamus and caudate nucleus were selected given their known connectivity with neocortical regions involved in mood regulation. Furthermore, we sought to determine in PFC samples whether decreased astrocytic CX expression is associated with an altered chromatin state. We found both CX30 and CX43 were downregulated across a number of brain regions and that PFC tissue showed an enrichment of the repressive histone mark H3K9me3 in cases compared with controls.
Materials and Methods
Subjects
This study was approved by the Douglas Hospital Research Ethics Board, and written informed consent from next-of-kin was obtained for each subject. Postmortem brain samples were provided by the suicide section of the Douglas-Bell Canada Brain Bank (www.douglasbrainbank.ca). Frozen gray matter samples were dissected from the left hemisphere of 22 male depressed suicides and 22 matched sudden-death controls for the mediodorsal thalamus (mdThal), caudate nucleus (CN), cerebellar cortex and cerebral cortex (Brodmann areas [BAs] 4 and 17). Suicides were selected from a recently characterized large cohort of subjects based on extreme low expression of astrocytic genes GFAP, ALDH1L1, SOX9, GLUL, SCL1A3, GJA1, and GJB6 in 2 prefrontal cortical areas (BA8/9, BA10), as previously described (Nagy et al., 2015). Overall, a significant decrease in the expression of all these genes was found in suicides within a subgroup displaying extremely low expression defined by those found in the bottom quartile of at least 5/7 genes (Nagy et al., 2015). For each individual, the cause of death was ascertained by the Quebec Coroner’s office, and psychological autopsies were performed by proxy-based interviews, as described previously (Dumais et al., 2005). Cases met criteria for major depressive disorder, Depressive Disorder Not Otherwise Specified, or had evidence of depressive symptoms at the moment of death. Controls were individuals who died suddenly and did not have evidence of any axis I disorders (Table 1). Postmortem interval (PMI) represents the delay between a subject’s death and collection and processing of the brain. There were no statistical differences found for age (t=0.40 P = .70), tissue pH (t=1.64, P = .11), or PMI (U=198.0, P =.31) between groups.
Table 1:
Subject Information
| Age (years) | Sex M/F | Tissue pH | PMI (hours) | Cause of death | Clinical information | ||
|---|---|---|---|---|---|---|---|
| ETOH | AD p | ||||||
| Controls (n=22) | 41.64±4.35 | 22/0 | 6.58±0.04 | 21.53±2.76 | 10 natural deatha, 7 road accident, 5 other accident | 1 | - |
| Cases (n=22) | 39.73±2.45 | 22/0 | 6.71±0.04 | 17.50±3.93 | 18 hanging, 4 intoxication | 4 | 11b |
aNatural death includes 2 myocardial infarctions, 3 acute myocardial insufficiencies, 2 cardiorespiratory arrest, 1 cardiac arrhythmia, 1 stroke, and 1 unknown medical cause. b For the 11 subjects with histories of antidepressant use, antidepressant levels were only detected in 5 individuals by toxicological analysis. All values are Mean ± SEM. Abbreviations: ADp; Antidepressants prescribed, ETOH; Alcohol dependence, PMI; Post mortem interval
Absolute Quantification of Gene Expression
Total RNA was extracted from 40mg of frozen tissues using the RNeasy Lipid Tissue Mini Kit (Qiagen Inc., Mississauga, Canada) with DNase digestion following the manufacturer’s instructions. Total RNA content was quantified using Nanodrop spectrophotometer (NanoDrop technologies, Rockland, DE) and RNA integrity was determined with an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). The average RNA Integrity Number for all brain regions was 6.99±0.11 for all subjects. cDNA was synthesized from 500ng of total RNA using 400U M-MLV reverse transcriptase (Gibco BRL Life Technologies, Burlington, ON, Canada) and oligo-deoxythymidine-16 according to the instructions of M-MLV manufacturer. To reduce variability in cDNA synthesis, 2 independent plates of cDNA were simultaneously synthesized, and the end product was combined and used for Real-Time PCR. The GJB6 (CX30) Taqman probesets (TaqMan assay id: Hs00917676_m1) and GJA1(CX43) (TaqMan assay id: Hs00748445_s1) labelled with FAM targets all transcripts for each gene. Endogenous controls β-actin (TaqMan assay id: Hs99999903_m1) and GAPDH (Life Technologies: 4310884E) were labelled with the fluorogenic reporter VIC. Real-time PCR reactions were run with the endogenous controls in 3 replicates with 2 µL of cDNA, 0.5 µL of each 20x Taqman assays specific to each quantified gene, 6 µL Mastermix (Applied Biosystems, Foster City, CA), and water. A standard curve was made of pooled cDNA from all subjects and all regions. Samples were analyzed with ABI PRISM 7900HT Sequence Detection System or QuantStudio 6 Flex Real-Time PCR System Sequence Detection System (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s protocol. Standard curve quantification of expression was assessed using QuantStudio Real-Time PCR Software version 1.2 (ThermoFisher Scientific) or SDS software version 2.4 (Applied Biosystems, Foster City, CA). The quantity values for all replicates were averaged to get the mean quantity per subject and then normalized to the geometric mean of GAPDH and β-actin. Samples with Cycle threshold SDs >0.3 were excluded from analysis to avoid excessive variability among replicates.
Chromatin Immunoprecipitation (ChIP)
Chromatin was prepared by douncing 80mg of human brain tissue in 400 μL of cold PBS using 25 controlled strokes. Then 7 μL of micrococcal nuclease (Sigma, Oakville, ON, Canada, N3755) was added, and samples were incubated for 12 minutes at 37°C to digest DNA between nucleosomes. Then 8 μL of 0.5% EDTA was used to stop the enzymatic reaction, and 1x protease inhibitor cocktail was added to the solution to prevent protein degradation caused by sample processing. The prepared chromatin was then immunoprecipitated using the Millipore Magna ChIP A kit (Millipore Etobicoke, ON, Canada). Briefly, 50 µL of cut chromatin solution was mixed with 450 µL of dilution buffer and 2.25 µL of protease inhibitor cocktail, 20 µL of protein A magnetic beads, and 4 µg of target antibody or IgG control. The Rabbit polyclonal anti-histone H3 (tri methyl K9) antibody-ChIP grade (Abcam ab8898, Toronto, ON, Canada) was used to enrich for the histone modification. The solution was incubated for 16 hours, magnets were used to separate the bound fractions, and the solution was washed with various immune complex buffers. Proteinase K was used to elute at 62°C, and samples were purified by phenol/chloroform extraction. Primers specific to H3K9me3 ENCODE peak sites were designed to detect enrichment of chromatin modification. Enrichment was quantified by normalizing immunoprecipitated sample over an input sample from the same dounced, sheared chromatin not subjected to antibodies. It was further normalized to a negative control region of the gene GAPDH. The results are presented as a fold change of cases to controls.
Statistical Analyses
Statistical analyses were performed using SPSS 18 (Statistical Product and Service Solutions, Chicago, IL) and Prism 6 (GraphPad Software, Inc., La Jolla, CA). All measurements were expressed as mean ± SEM, and P ≤ 0.05 was considered significant in all statistical tests. Normality was assessed using Shapiro-Wilk tests. Two-tailed U-tests were used for nonnormally distributed data and Student’s t tests otherwise. ROUT test (Q=1.0%) was applied to each group and all significant outliers were removed. Grubb’s test was applied to ChIP data because of its strong non-Gaussian distribution. The number of subjects included in each statistical test after exclusion is reflected in the degrees of freedom of each result. All correlations were subjected to Bonferroni correction for multiple comparisons.
Results
Regional Expression of Astrocyte-Specific Connexin Genes
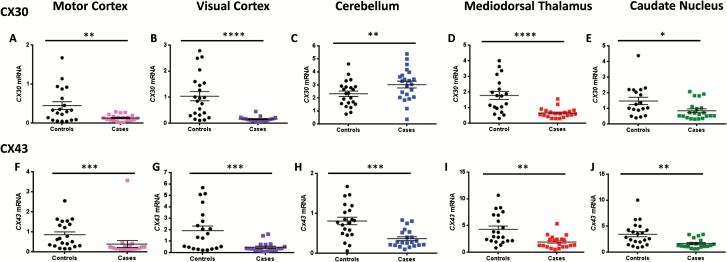
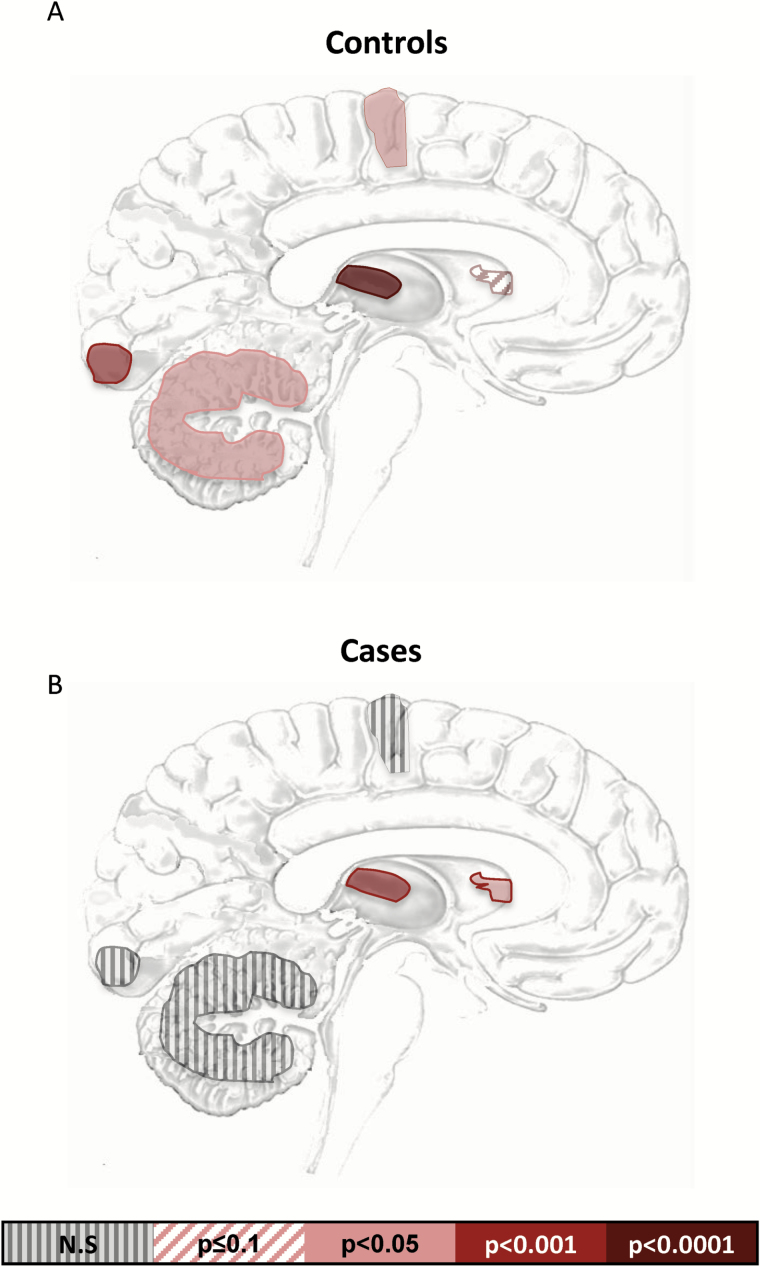
All brain regions investigated in this study showed differences between cases and controls (Figure 1a-j) (P values ranging between .05 and .0001). The cerebellum was the only region to display an increase of either connexin, with CX30 being increased in cases compared with controls [F(2,41)= 2.51, P =.0082] (Figure 1c). Given that Cx30 and Cx43 colocalize, as confirmed by coimmunolabelings observed with electron and fluorescence microscopy (Nagy and Rash, 2000), we expected their expression patterns to correlate with each other. Interestingly, in controls, correlations were found in all brain regions [BA4: rs(19)=0.70 P =.005 BA17: rs(21)=0.73, P =.0009; cerebellum: rs(21)=-0.54 P =.05; mdThal: rs(18)=0.92, P <.0001] but the CN [rs(18)=0.54, P =.10] (Figure 2a), whereas in cases, we observed a correlation in only the subcortical regions, mdThal [rs(18) =0.75, P =.0007], and caudate [rs(18) =0.74, P =.002] (Figure 2b).
Figure 1.
Astrocytic connexin gene expression is strongly repressed in both cortical and subcortical regions in cases compared to controls. CX30 (a-e) is significantly downregulated in the (a) motor cortex (U(38)=40, P=.024), (b) visual cortex (U(38)=40, P<.0001), (d) mdThAl (U(39)=57, P<.0001), and (e) caudate nucleus (U(39)=103, P=.015), and CX43 (f-j) showed decreases in the (f) motor cortex (U(40)=67, P=.0002), (g) visual cortex (U(40)=81.5, P=.001), (h) cerebellum (U(42)=84, P=.0004), and (j) caudate nucleus (U(40)=83, P=.0012). Cases showed a significant increase of CX30 in (c) cerebellum (t(41)= 2.78, P=.008).
Figure 2.
Correlations of connexin 30 and 43 across brain regions. P values for Spearman correlations between connexins for each region in (a) controls and (b) cases. P values defined by color scale, and all values are Bonferroni corrected for multiple testing.
Evidence in animals shows that antidepressant treatment can reverse the effects of downregulated Cx43 (Sun et al., 2012a). Here, we found no significant correlation between antidepressant use and CX43 expression, though all the r values suggest an inverse relationship (data not shown).
Enrichment of Histone Methylation in the PFC
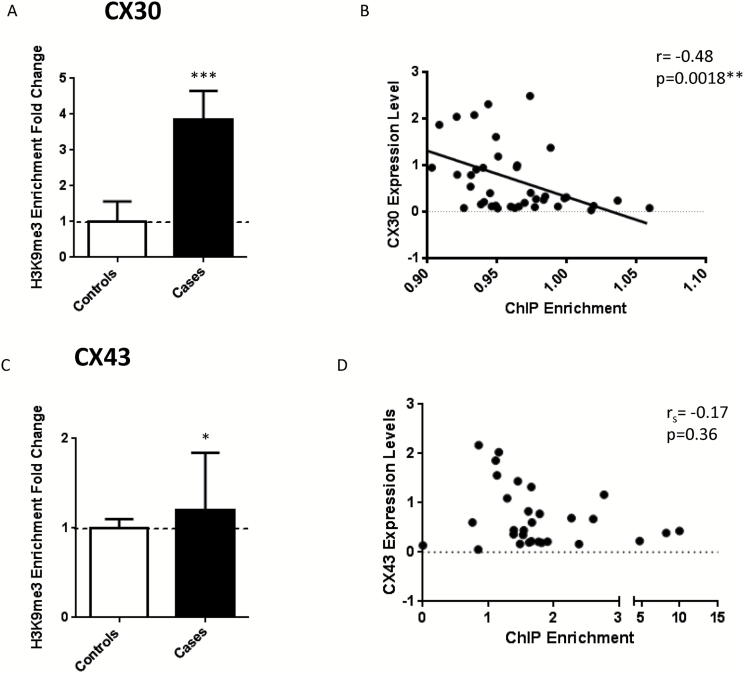
Since cases demonstrated a strong depletion in the expression of astrocytic connexins, we sought to investigate the underlying molecular mechanism. Epigenetic mechanisms explain, at least in part, altered gene expression and have previously been reported to play an important role in the depressed brain (Fiori et al., 2011; Fiori and Turecki, 2011; Turecki, 2014). We previously looked at DNA methylation and found no site-specific methylation changes associated with either CX30 or CX43 (Nagy et al., 2015). We therefore hypothesized that chromatin rearrangement could be responsible for this gene repression. As patterns of expression were similar across regions with the exception of the cerebellum, we opted to conduct chromatin immunoprecipitation studies in the PFC (BA8/9), a representative region with pronounced changes to the astrocytic connexin genes. ChIP was performed to enrich for H3K9me3 modifications representative of hetero- or inaccessible chromatin. We found that for both CX genes, the case group showed enrichment in H3K9me3 (Cx30 [t(40)=4.036, P =.0002] and CX43 [U(35)=93, P =.048]) (Figure 3a-c). BA8/9 CX gene expression levels inversely correlated with the level of histone modification, but only the correlation between Cx30 and H3K9me3 was significant [rs(40)=-0.48; P =.0018] (Figure 3b-d). There was no relationship found between age, tissue pH or PMI, and H3K9me3 enrichment for either Cx30 or 43.
Figure 3.
Enrichment of the repressive chromatin mark H3K9me3 is increased in cases compared to controls. (a) Enrichment of H3K9me3 in CX30 is increased in cases (t(40)=4.036, P=.0002) and inversely correlate with gene expression (b) Pearson correlation (r(40)=-0.48, P=.0018). (c) CX43 also shows an increase in H3K9me3 enrichment (U(35)=93, P=.048) though the fold change was not as strong and d) the correlation though inverse, was not significant (Spearman Rho(30)=-0.17, P=.36).
Discussion
We recently reported that the astrocyte-specific marker glial-fibrillary acidic protein (GFAP) is downregulated in subcortical (Torres-Platas et al., 2016) and prefrontal cortical (Nagy et al., 2015), areas implicated in mood disorders, but not in the other neocortical regions examined (BA4, BA17) nor in cerebellar cortex. Here we sought to determine whether the astrocyte-specific CX30 and CX43 would display a similar pattern of expression. In samples from depressed suicides relative to psychiatrically healthy controls, we found that the expression of both CX30 and CX43 was strongly repressed in all regions examined, except for CX30 in the cerebellum, where it was significantly increased in cases. It thus appears that astrocytic connexin dysregulation in the depressed brain is not limited to regions known to be implicated in mood regulation and is thus more globally affected than GFAP.
To determine a possible mechanism for the strong repression of connexin genes, we examined the PFC, a region canonically implicated in depression (Koenigs and Grafman, 2009) that is strongly associated with the subcortical regions (Mega and Cummings, 1994) examined in this study. We found that the repressive histone mark H3K9me3 is enriched in cases compared to controls for both astrocytic CX genes and that this increase is inversely correlated with expression of CX30. CX43 histone methylation did not correlate with expression, but this could be due to a lack of power, as the enrichment was more subtle within this gene. Though there was an inverse relationship between enrichment and expression, it was not significant. It is likely that increasing the sample size would provide the required power to establish a significant relationship.
As expected from the colocalization of these 2 connexins using electron microscopy (Nagy and Rash, 2000), these genes correlate well with each other across most regions, showing proportional expression in the control group. However, this relationship was found to be highly altered when examining cases alone, where the only correlation observed was subcortical and in a different pattern than controls. This indicates that in the normally functioning neocortex, astrocyte connexins are proportionally expressed, and dysregulation in depressed suicides interferes with this relationship.
In the animal literature, there is evidence that decreases of Cx43 in animals subjected to chronic unpredictable stress can be reversed by antidepressant drug treatment (Sun et al., 2012b). Additionally, treating primary mouse astrocytes with the antidepressant amitriptyline increases levels of mRNA, protein, and gap junction intercellular communication (Morioka et al., 2014). This was shown to affect only Cx43 but not Cx30 expression (Morioka et al., 2014), indicating that restoring levels of this Cx may be critical to antidepressant effects. Though we found no significant relationship between antidepressant use and level of CX43 gene expression, it is possible that we do not have enough power to detect such a change, as the presence of antidepressants by toxicological reports was found in only 5 cases at the time of death.
Previous human postmortem brain studies have described a significant decrease in the expression of both CX43 and CX30 in brain samples from depressed individuals relative to controls (Bernard et al., 2011; Ernst et al., 2011; Nagy et al., 2015). In fact, Ernst and colleagues (2011) described a robust decrease of both astrocytic connexion genes in a widely encompassing prefrontal cortical region including BAs 9,10,11, 44a, 45,46, and 47, and further demonstrated a strong protein decrease in the PFC. Here, we found a strongly significant decrease in CX30 expression in BA4, BA17 mdThal, and a milder but still significant decrease in the CN. Somewhat surprisingly, we found an increase in CX30 expression in the cerebellum of cases compared to controls. Certainly, this still represents a decoupling of CX30 with CX43, as the latter displays a strong downregulation in this same brain region in the same samples. This is not the first report of inverse expression of astrogial connexins in the cerebellum. Mice infected with Borna Disease Virus show increased Cx43 together with decreased Cx30 expression which, in turn, resulted in changes to the functional coupling of these proteins (Koster-Patzlaff et al., 2007).
A number of studies have described a reduction of glial density and/or glial number (Ongür et al., 1998; Rajkowska et al., 1999; Rajkowska, 2000; Cotter et al., 2001, 2002; Rajkowska and Stockmeier, 2013) and molecular markers of glia (Choudary et al., 2005; Chandley et al., 2013), commonly concluding that reduced GFAP expression or immunoreactivity reflects a loss of astrocytic cells in particular (Webster et al., 2005). However, GFAP is not always a reliable indicator of astrocyte numbers, as not all astrocytes express GFAP and not all GFAP expression is from astrocytes (Khakh and Sofroniew, 2015). Animal studies using models of depression report a reduction of GFAP-IR cells but no change to other markers (Gosselin et al., 2009) or cell counts (Leventopoulos et al., 2007; Tynan et al., 2013). The growing research in animal models of depression has shown a certain malleability of GFAP as many have shown a drug effect on GFAP-IR (Czeh et al., 2006; Banasr and Duman, 2008; Banasr et al., 2010; Ye et al., 2011; Sun et al., 2012a). These data open the door to the possibility of molecular alterations or even cellular atrophy rather than a loss in astrocytic cell numbers per se. In line with the idea of malleable GFAP, connexins are known to interact with the cytoskeleton, for example astrocytes with altered Cx43 expression have been shown to differ in their morphology (Olk et al., 2009). In an in situ study using hippocampal brain slices from Cx43 KO mice, astrocytes were found to have increased somal size, though no difference in GFAP immunolabelling astrocytes was reported (Chever et al., 2014). Taken together, this suggests a link between connexins and cell shape through cytoskeletal reorganization.
In addition to their role in the formation of GJCs and cytoskeletal interactions, connexins have an important role in the maintenance of ion homeostatsis (Wallraff et al., 2006), minimizing damage in the CNS (Lin et al., 2008), extracellular exchanges through hemichannels, as well as channel-independent functions involving cell adhesion /signalling (Theis et al., 2005; Elias et al., 2007; Olk et al., 2009), cell proliferation (Cheng et al., 2004), migration (Scemes et al., 2003), and differentiation (Duval et al., 2002). In the context of adult hippocampal neurogenesis, a double knockout model of Cx30/Cx43 seemed to suggest Cx- 30 and 43 affect neurogenic processes in an opposing manner, where Cx43 promotes survival of newborn neurons and Cx30 restricts their survival (Liebmann et al., 2013). This is interesting in the context of major depression, since there have been links made between adult hippocampal neurogenesis and the etiopathogenesis of depression (Lee et al., 2013; Mahar et al., 2014).
Astrocytic connexins have also been shown to altered oligodendrocytic functions (Lutz et al., 2009). Cx30 and Cx43 are known to form heterotypic GJCs with the somata and lamellae of oligodendrocytes (Kamasawa et al., 2005), double knockout of these Cxs resulted in widespread edema and vacuolation in white matter resulting in oligodendrocyte and myelin pathology, while astrocytes were found to have structural changes. In the gray matter, astrocytes were found to be swollen, though even a single allele of either Cx was sufficient to protect against glial edema, suggesting a partial redundancy in these connexins (Lutz et al., 2009). Hypertrophic astrocytes have previously been described in the white matter of brains from depressed suicides, but no such changes were found in gray matter (Torres-Platas et al., 2011). As white matter astrocytes are devoid of Cx30 (Nagy et al., 1999), the potential for connexin compensation is lost, suggesting a possible link between the globally strong decreases of CX43 found here and the morphological changes to white matter astrocytes previously described. This is, however, an extrapolation of our results as only gray matter expression was examined in the present work. More studies are therefore required.
One of the mechanisms shown to regulate Cx expression is inflammatory responses through microglial activation. It was previously demonstrated that microglial activation decreases Cx43 expression resulting in a loss of functional coupling between astrocytic cells (Faustmann et al., 2003; Hinkerohe et al., 2005; Meme et al., 2006). A recent study examining cerebral inflammatory markers in depressed suicides revealed increased microglial/macrophage activity in the anterior cingulate cortex of cases relative to controls (Torres-Platas et al., 2014). Interestingly, these changes were found in the same region as the morphological changes mentioned above (Torres-Platas et al., 2011). Macrophage metabolic status has been linked to the activity of chromatin-modifying enzymes (Baardman et al., 2015), and a number of studies have implicated epigenetic modifications as a regulatory mechanism in depression (for review, see Sun et al., 2013). Based on our current findings, whatever may be triggering a downregulation of gene expression seems to be mediated through altered chromatin state, an epigenetic regulatory factor.
Our study is not without limitations. In particular, epigenetic modifications are cell and tissue specific; therefore, the altered histone marks in the prefrontal cortex may not apply to all brain regions studied here. Additionally, given the strong evidence that antidepressants can alter the expression of connexins, it would have been interesting to have access to more samples from individuals using antidepressants at the time of death.
In conclusion, our study shows that CX genes specific to astrocytes are widely dysregulated in depression and suicide. The findings in this study implicate the involvement of an epigenetic molecular mechanism in the regulation of CX gene expression. Our data are correlational and do not discount the possibility of cell loss but are suggestive of something beyond cell loss as being involved in the astrocytic abnormalities seen in major depressive disorder.
Statement of Interest
None.
Acknowledgments
We thank Quebec’s Coroner office as well as the next-of-kin of the deceased for their support. We also thank the expert staff of the Douglas-Bell Canada Brain Bank.
This work has been supported by operating grants to G.T. from Canadian Institutes of Health Research (grant no. MOP-93775) and to N.M. from ERANET-NEURON (FRQ-S). N.M. is a FRQ-S chercheur-boursier and CIHR New Investigator. C.N. is the recipient of FRQ-S doctoral scholarship, and S.G.T.-P. is the recipient of a CONACyT doctoral scholarship.
References
- Baardman J, Licht I, de Winther MP, Van den Bossche J. (2015) Metabolic-epigenetic crosstalk in macrophage activation. Epigenomics 7:1155–1164. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman R. (2008) Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biological psychiatry 64:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. (2010) Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 15:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. (2011) Regulation of chromatin by histone modifications. Cell Res 21:381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ. (2011) Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry 16:634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstrand F, Venance L, Siren AL, Ezan P, Hanse E, Glowinski J, Ehrenreich H, Giaume C. (2004) Endothelins regulate astrocyte gap junctions in rat hippocampal slices. Eur J Neurosci 19:1005–1015. [DOI] [PubMed] [Google Scholar]
- Chandley MJ, Szebeni K, Szebeni A, Crawford J, Stockmeier CA, Turecki G, Miguel-Hidalgo JJ, Ordway GA. (2013) Gene expression deficits in pontine locus coeruleus astrocytes in men with major depressive disorder. J Psychiatry Neurosci 38:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Tang H, Cai J, Zhu M, Zhang X, Rao M, Mattson MP. (2004) Gap junctional communication is required to maintain mouse cortical neural progenitor cells in a proliferative state. Dev Biol 272:203–216. [DOI] [PubMed] [Google Scholar]
- Chever O, Pannasch U, Ezan P, Rouach N. (2014) Astroglial connexin 43 sustains glutamatergic synaptic efficacy. Philos Trans R Soc Lond B Biol Sci 369:20130596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudary P, Molnar M, Evans S, Tomita H, Li J, Vawter M, Myers R, Bunney W, Akil H, Watson S, Jones E. (2005) Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad U S A 102:15653–15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. (2001) Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 58:545–553. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall I. (2002) Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex 12:386–394. [DOI] [PubMed] [Google Scholar]
- Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. (2006) Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology 31:1616–1626. [DOI] [PubMed] [Google Scholar]
- Dumais A, Lesage AD, Alda M, Rouleau G, Dumont M, Chawky N, Roy M, Mann JJ, Benkelfat C, Turecki G. (2005) Risk factors for suicide completion in major depression: a case-control study of impulsive and aggressive behaviors in men. Am J Psychiatry 162:2116–2124. [DOI] [PubMed] [Google Scholar]
- Duval N, Gomes D, Calaora V, Calabrese A, Meda P, Bruzzone R. (2002) Cell coupling and Cx43 expression in embryonic mouse neural progenitor cells. J Cell Sci 115:3241–3251. [DOI] [PubMed] [Google Scholar]
- Elias LA, Wang DD, Kriegstein AR. (2007) Gap junction adhesion is necessary for radial migration in the neocortex. Nature 448:901–907. [DOI] [PubMed] [Google Scholar]
- Ernst C, Nagy C, Kim S, Yang J, Deng X, Hellstrom I, Choi K, Gershenfeld H, Meaney M, Turecki G. (2011) Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biol Psychiatry 70:312–319. [DOI] [PubMed] [Google Scholar]
- Faustmann PM, Haase CG, Romberg S, Hinkerohe D, Szlachta D, Smikalla D, Krause D, Dermietzel R. (2003) Microglia activation influences dye coupling and Cx43 expression of the astrocytic network. Glia 42:101–108. [DOI] [PubMed] [Google Scholar]
- Fiori L, Gross J, Turecki G. (2011) Effects of histone modifications on increased expression of polyamine biosynthetic genes in suicide. Int J Neuropsychop:1–7. [DOI] [PubMed] [Google Scholar]
- Fiori L, Turecki G. (2011) Epigenetic regulation of spermidine/spermine N1-acetyltransferase (SAT1) in suicide. J Psychiatr Res 45:1229–1264. [DOI] [PubMed] [Google Scholar]
- Giaume C, McCarthy KD. (1996) Control of gap-junctional communication in astrocytic networks. Trends Neurosci 19:319–325. [DOI] [PubMed] [Google Scholar]
- Giaume C, Naus CC. (2013) Connexins, gap junctions, and glia. WIREs membr transp signal 2:133–141. [Google Scholar]
- Gosselin RD, Gibney S, O’Malley D, Dinan TG, Cryan JF. (2009) Region specific decrease in glial fibrillary acidic protein immunoreactivity in the brain of a rat model of depression. Neuroscience 159:915–925. [DOI] [PubMed] [Google Scholar]
- Hinkerohe D, Smikalla D, Haghikia A, Heupel K, Haase CG, Dermietzel R, Faustmann PM. (2005) Effects of cytokines on microglial phenotypes and astroglial coupling in an inflammatory coculture model. Glia 52:85–97. [DOI] [PubMed] [Google Scholar]
- Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, McEwen BS. (2009) Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci U S A 106:20912–20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Jakovcevski M, Bharadwaj R, Connor C, Schroeder FA, Lin CL, Straubhaar J, Martin G, Akbarian S. (2010) Setdb1 histone methyltransferase regulates mood-related behaviors and expression of the NMDA receptor subunit NR2B. J Neurosci 30:7152–7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamasawa N, Sik A, Morita M, Yasumura T, Davidson KG, Nagy JI, Rash JE. (2005) Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: implications for ionic homeostasis and potassium siphoning. Neuroscience 136:65–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV. (2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. (2009) The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res 201:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster-Patzlaff C, Hosseini SM, Reuss B. (2007) Persistent Borna Disease Virus infection changes expression and function of astroglial gap junctions in vivo and in vitro. Brain Res 1184:316–332. [DOI] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. (1996) The gap junction communication channel. Cell 84:381–388. [DOI] [PubMed] [Google Scholar]
- Lee MM, Reif A, Schmitt AG. (2013) Major depression: a role for hippocampal neurogenesis? Curr Top Behav Neurosci 14:153–179. [DOI] [PubMed] [Google Scholar]
- Leventopoulos M, Ruedi-Bettschen D, Knuesel I, Feldon J, Pryce CR, Opacka-Juffry J. (2007) Long-term effects of early life deprivation on brain glia in Fischer rats. Brain Res 1142:119–126. [DOI] [PubMed] [Google Scholar]
- Li DQ, Li XJ, Duan JF, Cai W. (2010) Wuling Capsule promotes hippocampal neurogenesis by improving expression of connexin 43 in rats exposed to chronic unpredictable mild stress. Zhong Xi Yi Jie He Xue Bao 8:662–669. [DOI] [PubMed] [Google Scholar]
- Liebmann M, Stahr A, Guenther M, Witte OW, Frahm C. (2013) Astrocytic Cx43 and Cx30 differentially modulate adult neurogenesis in mice. Neurosci Lett 545:40–45. [DOI] [PubMed] [Google Scholar]
- Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J, Kang J, Nedergaard M. (2008) A central role of connexin 43 in hypoxic preconditioning. J Neurosci 28:681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz SE, Zhao Y, Gulinello M, Lee SC, Raine CS, Brosnan CF. (2009) Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J Neurosci 29:7743–7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar I, Bambico FR, Mechawar N, Nobrega JN. (2014) Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev 38:173–192. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL. (1994) Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci 6:358–370. [DOI] [PubMed] [Google Scholar]
- Meme W, Calvo CF, Froger N, Ezan P, Amigou E, Koulakoff A, Giaume C. (2006) Proinflammatory cytokines released from microglia inhibit gap junctions in astrocytes: potentiation by beta-amyloid. FASEB J 20:494–496. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wilson BA, Hussain S, Meshram A, Rajkowska G, Stockmeier CA. (2014) Reduced connexin 43 immunolabeling in the orbitofrontal cortex in alcohol dependence and depression. J Psychiatr Res 55:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka N, Suekama K, Zhang FF, Kajitani N, Hisaoka-Nakashima K, Takebayashi M, Nakata Y. (2014) Amitriptyline up-regulates connexin43-gap junction in rat cultured cortical astrocytes via activation of the p38 and c-Fos/AP-1 signalling pathway. Br J Pharmacol 171:2854–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi H, Khaksarian M, Joghataei MT, Hassanzadeh G, Soleimani M, Eftekhari S, Soleimani M, Mousavizadeh K, Hadjighassem MR. (2014) Fluoxetin upregulates connexin 43 expression in astrocyte. Basic Clin Neurosci 5:74–79. [PMC free article] [PubMed] [Google Scholar]
- Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, Turecki G. (2015) Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry 20:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Rash JE. (2000) Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Brain Res Rev 32:29–44. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Dudek FE, Rash JE. (2004) Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Brain Res Rev 47:191–215. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Patel D, Ochalski PA, Stelmack GL. (1999) Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience 88:447–468. [DOI] [PubMed] [Google Scholar]
- Olk S, Zoidl G, Dermietzel R. (2009) Connexins, cell motility, and the cytoskeleton. Cell Motil Cytoskeleton 66:1000–1016. [DOI] [PubMed] [Google Scholar]
- Ongür D, Drevets W, Price J. (1998) Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences of the United States of America 95:13290–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannasch U, Rouach N. (2013) Emerging role for astroglial networks in information processing: from synapse to behavior. Trends Neurosci 36:405–417. [DOI] [PubMed] [Google Scholar]
- Quesseveur G, Portal B, Basile JA, Ezan P, Mathou A, Halley H, Leloup C, Fioramonti X, Deglon N, Giaume C, Rampon C, Guiard BP. (2015) Attenuated Levels of hippocampal connexin 43 and its phosphorylation correlate with antidepressant- and anxiolytic-like activities in mice. Front Cell Neurosci 9:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G. (2000) Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biological psychiatry 48:766–777. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo J, Wei J, Dilley G, Pittman S, Meltzer H, Overholser J, Roth B, Stockmeier C. (1999) Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45:1085–1098. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Stockmeier CA. (2013) Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14:1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Kamasawa N, Davidson KG, Yasumura T, Pereda AE, Nagy JI. (2012) Connexin composition in apposed gap junction hemiplaques revealed by matched double-replica freeze-fracture replica immunogold labeling. J Membr Biol 245:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlmann A, Wolff JR. (1996) Gap Junctions in the Nervous System. Austin, TX: Springer Berlin Heidelberg. [Google Scholar]
- Rouach N, Giaume C. (2001) Connexins and gap junctional communication in astrocytes are targets for neuroglial interaction. Prog Brain Res 132:203–214. [DOI] [PubMed] [Google Scholar]
- Scemes E, Duval N, Meda P. (2003) Reduced expression of P2Y1 receptors in connexin43-null mice alters calcium signaling and migration of neural progenitor cells. J Neurosci 23:11444–11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Kennedy PJ, Nestler EJ. (2013) Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology 38:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J-D, Liu Y, Yuan Y-H, Li J, Chen N-H. (2012. a) Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology 37:1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JD, Liu Y, Yuan YH, Li J, Chen NH. (2012. b) Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology 37:1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis M, Sohl G, Eiberger J, Willecke K. (2005) Emerging complexities in identity and function of glial connexins. Trends Neurosci 28:188–195. [DOI] [PubMed] [Google Scholar]
- Torres-Platas S, Hercher C, Davoli M, Maussion G, Labonté B, Turecki G, Mechawar N. (2011) Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology 36:2650–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. (2014) Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 42:50–59. [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Nagy C, Wakid M, Turecki G, Mechawar N. (2016) Glial fibrillary acidic protein is differentially expressed across cortical and subcortical regions in healthy brains and downregulated in the thalamus and caudate nucleus of depressed suicides. Mol Psychiatry 21:509–515. [DOI] [PubMed] [Google Scholar]
- Turecki G. (2014) The molecular bases of the suicidal brain. Nat Rev Neurosci 15:802–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, Walker FR. (2013) Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol 126:75–91. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Kettenmann H. (1996) Calcium signalling in glial cells. Trends Neurosci 19:346–352. [DOI] [PubMed] [Google Scholar]
- Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. (2006) The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci 26:5438–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MJ, O’Grady J, Kleinman JE, Weickert CS. (2005) Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience 133:453–461. [DOI] [PubMed] [Google Scholar]
- Ye Y, Wang G, Wang H, Wang X. (2011) Brain-derived neurotrophic factor (BDNF) infusion restored astrocytic plasticity in the hippocampus of a rat model of depression. Neurosci Lett 503:15–19. [DOI] [PubMed] [Google Scholar]