Abstract
Laser-generated electron beams are distinguished from conventional accelerated particles by ultrashort beam pulses in the femtoseconds to picoseconds duration range, and their application may elucidate primary radiobiological effects. The aim of the present study was to determine the dose-rate effect of laser-generated ultrashort pulses of 4 MeV electron beam radiation on DNA damage and repair in human cells. The dose rate was increased via changing the pulse repetition frequency, without increasing the electron energy. The human chronic myeloid leukemia K-562 cell line was used to estimate the DNA damage and repair after irradiation, via the comet assay. A distribution analysis of the DNA damage was performed. The same mean level of initial DNA damages was observed at low (3.6 Gy/min) and high (36 Gy/min) dose-rate irradiation. In the case of low-dose-rate irradiation, the detected DNA damages were completely repairable, whereas the high-dose-rate irradiation demonstrated a lower level of reparability. The distribution analysis of initial DNA damages after high-dose-rate irradiation revealed a shift towards higher amounts of damage and a broadening in distribution. Thus, increasing the dose rate via changing the pulse frequency of ultrafast electrons leads to an increase in the complexity of DNA damages, with a consequent decrease in their reparability. Since the application of an ultrashort pulsed electron beam permits us to describe the primary radiobiological effects, it can be assumed that the observed dose-rate effect on DNA damage/repair is mainly caused by primary lesions appearing at the moment of irradiation.
Keywords: ultrashort electron beam, DNA damage and repair; comet assay; dose-rate effect
INTRODUCTION
New laser-generated electron acceleration technology has been developed over the last decade. Laser-generated electron beams are distinguished from conventional, quasi-continuously accelerated particles by ultrashort beam pulses in the femtoseconds to picoseconds duration range [1]. Since the outcomes of most radiation-induced chemical reactions are determined by very fast processes, the application of ultrashort beam pulses can elucidate primary events triggered by the radiation. The identification of those primary events is essential in radiobiological studies for complete understanding of the effects of irradiation [2].
Recently, several in vitro and in vivo studies of the radiobiological effects of pulsed laser–generated electron and proton beams in the megaelectron volt energy domain have been undertaken using various cells, biological end points, and beam parameters [3–9]. For the majority of these studies, the impact of ultra-high-pulse dose rates on the radiobiological outcome was not reported [10]. According to the Beyreuther et al., increasing the electron energy to achieve a high dose rate with low-frequency pulses has no influence on the radiobiological effectiveness in vitro [11]. The aim of the present study was to determine (by varying the frequency of the pulses) the dose-rate effect of ultrashort pulses of 4 MeV electron beam radiation on DNA damage and repair in human cells.
MATERIALS AND METHODS
Cell culture and irradiation
The K562 (human chronic myeloid leukemia) cell line was maintained in RPMI-1640 (Sigma Aldrich, Germany), supplemented with 10% fetal bovine serum (HyClone, UK), 2 mM L-glutamine (Sigma Aldrich, Germany), 100 IU/ml penicillin (Sigma Aldrich, Germany) and 100 μg/ml streptomycin (Sigma Aldrich, Germany) at 37°C.
Radiation treatment was carried out using an electron beam generated by a laser-driven radiofrequency gun-based linear AREAL accelerator. The characteristics of the AREAL accelerator have previously been described [12]. The parameters of the AREAL laser-generated electron beam are presented in Table 1.
Table 1.
Characteristics of the AREAL laser-generated electron beam
| AREAL beam parameters | UV laser parameters | ||
|---|---|---|---|
| Beam charge (pС) | 30 | Wavelength (nm) | 258 |
| Electron energy (MeV) | 4 | Pulse energy | 200 |
| Pulse duration (fs) | 400 | Repetition rate (Hz) | 2/20 |
| Pulse repetition rate (Hz) | 2/20 | Energy stability | <2% |
| Beam spot (mm) | 15 | Beam divergence (mrad) | <0.3 |
| Norm. emittance (mm-mrad) | <0.5 | Beam diameter (mm) | 4.0 |
| RMS (root-mean-square) energy spread | <1.5% | ||
| Online dose information | Faraday cup | ||
Prior to irradiation, 2 ml of cell suspension was seeded in Eppendorf tubes (Sigma Aldrich, Germany) at a density of 0.5 × 105 cells/ml. For cell irradiation, each sample was placed in a sample holder facing towards the horizontal beam coming from the direction of the vacuum window, thus minimizing the material in front of the cell suspension. Cell samples were placed horizontally at the center of a 1 cm × 1 cm area at 1 cm from the beam exit point of the accelerator. Cells were irradiated on ice [13], with doses of 2, 4 and 8 Gy, and at the ‘low’ dose rate (LDR) of 3.6 (repetition rate 2 Hz) and the ‘high’ dose rate (HDR) of 36 Gy/min (repetition rate 20 Hz). Non-irradiated cell cultures were used as a control.
Comet assay
To estimate the wide range of DNA damages and repair, the alkaline comet assay was carried out [14] at 0 h and 24 h time points after irradiation of cell samples. Since the comet assay allows observation of differences in the patterns of DNA damage immediately after irradiation, the 0 h time point was selected to estimate the primary DNA-damage distribution. The 24 h incubation period after irradiation was selected in order to observe the recovery of the majority of the DNA damage [15]. To quantify the level of DNA damage, the extent of DNA migration was defined using the ‘Olive Tail Moment’ (OTM), which is the relative amount of DNA in the tail of the comet multiplied by the median migration distance. The comets were observed with a fluorescence microscope Zeiss III RS (Germany) equipped with a 560 nm excitation filter, a 590 nm barrier filter and a charged coupled device (CCD) PCO video camera (Germany). At least 150 cells were examined for each experimental point. Images were analysed using Comet Assay IV software (Perceptive Instruments, UK).
Statistical analysis
All experiments were repeated at least three times; triplicate cultures were scored for an experimental point. Experimental values were expressed as mean ± SEM. The non-parametric Mann–Whitney test was used for statistical analysis, and P < 0.05 was considered to indicate a statistically significant value. The R statistical software was used for distribution analysis.
RESULTS AND DISCUSSION
The effect of ultrashort electron beam irradiation on DNA damage and repair
The dose and dose-rate effects of ultrashort pulses of electron beam irradiation on DNA damage and repair, obtained by comet assay of cancer K562 cells, are presented in Table 2.
Table 2.
The level of DNA damage in K-562 cells after 0 and 24 h of irradiation with ultrashort pulses of the electron beam
| Dose, Gy | Mean (OTM) ± SEM | |||
|---|---|---|---|---|
| 3.6 Gy/min | 36 Gy/min | |||
| 0 h | 24 h | 0 h | 24 h | |
| 0 | 5.08 ± 1.91 | 7.67 ± 2.73 | 6.05 ± 2.73 | 4.26 ± 0.40 |
| 2 | 20.39 ± 6.24* | 12.24 ± 5.61# | 19.09 ± 2.11* | 9.17 ± 2.43*# |
| 4 | 35.04 ± 8.82* | 8.06 ± 4.63# | 28.01 ± 1.84* | 10.05 ± 3.24*# |
| 8 | 29.35 ± 8.22* | 5.39 ± 2.02# | 31.18 ± 3.08* | 13.39 ± 1.58*# |
*P < 0.05 in comparison with the corresponding control; #P < 0.05 in comparison with the corresponding data at the 0 h time point.
The dose-dependent increases in DNA damage, expressed as OTM values, are shown immediately after irradiation of up to 8 Gy at the LDR (3.6 Gy/min) and HDR (36 Gy/min). The range of OTM values at 0 h corresponded with the data reported in the literature for the similar doses of irradiation [16]. After 24 h of LDR irradiation, the levels of DNA breaks in K562 cells were significantly lower than immediately after exposure and did not differ from the corresponding control. Thus, the detected radiation-induced DNA damages can be regarded as having been completely reparable. The gradual decrease in the OTM values as the dose increased at 24 h in the LDR data can be explained by the elimination of non-viable cells with highly damaged DNA. Despite the significant decrease in the DNA breaks 24 h after HDR irradiation up to the dose of 8 Gy, the OTM values remained significantly higher compared with that of the corresponding control. Thus, DNA damages from HDR irradiation can be considered as partially reparable.
The probability distributions of OTM values
The distribution of the OTM (which is considered to be a sensitive indicator of DNA breakage) was analysed. The Weibull distribution was applied to fit the comet data.
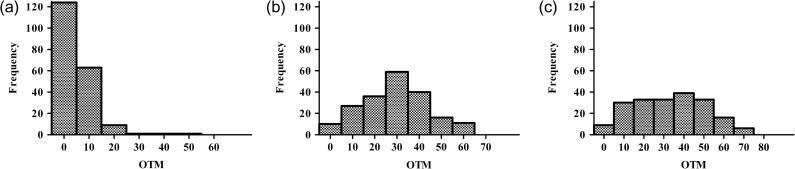
The probability distribution of the OTM values for K562 cells irradiated with a dose of 8 Gy compared with that of the control is presented in Fig. 1. Immediately after irradiation at the HDR (Fig. 1c), the shift to a higher OTM and a broadening in the comet distribution was observed (compared with the LDR) (Fig. 1b). Although, the HDR and LDR irradiation with doses up to 8 Gy leads to approximately the same mean level of DNA damage, the distributions of this damage are different. Thus, the differences in the distribution of the DNA damage can be the reason for the above-mentioned varying levels of repair in cells irradiated with HDR versus LDR irradiation. The shift to the higher OTM values in this case suggests the formation of highly damaged DNA, leading to the accumulation of unreparable DNA damages.
Fig. 1.
Probability distribution of the OTM value for unirradiated K-562 cells (a) and those exposed to 8 Gy with the ultrashort electron beam at low (3.6 Gy/min) (b) and high (36 Gy/min) (c) dose rates at the 0 h time point after irradiation.
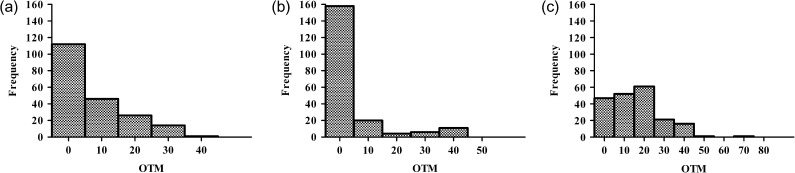
After 24 h of irradiation, the shift to lower OTM values was evident due to DNA repair (Fig. 2b and c). In the case of the LDR irradiation, the distribution of the DNA damages was similar to that of the control (Fig. 2a and b). However, some cells with residual DNA damage were still present. In the case of HDR irradiation, the OTM was shifted towards higher values, demonstrating the presence of cells with highly damaged DNA (Fig. 2c). Thus, the lower level of repair in the cells irradiated with HDR irradiation, compared with LDR, can be explained by an accumulation of cells with higher levels of DNA damage.
Fig. 2.
Probability distribution of the OTM value for unirradiated K-562 cells (a) and those exposed to 8 Gy with the ultrashort electron beam at low (3.6 Gy/min) (b) and high (36 Gy/min) (c) dose rates at the 24 h time point after irradiation.
So, our results indicated that increasing the dose rate via changing the frequency of the pulses led to an increase in the complexity of the DNA damages, with a consequent decrease in their reparability. Previously, the dose-rate effect in the range of 1–5 Gy/min was described in radiobiological studies using conventional accelerators [17]. The irradiation time in the range of minutes to hours was comparable with that of the radiation response, since the biological processes (repair, repopulation or reoxygenation), starting after 10–15 min of irradiation [18], can modify the radiation effect. So, longer exposure with LDR irradiation leads to a lower radiation effect, and the shorter exposure with HDR irradiation leads to a higher radiation effect. We demonstrated the dose-rate effect of an ultrashort pulsed electron beam at 3.6 Gy/min (the duration was <6 min) and at 36 Gy/min (the duration was <40 s) at a low temperature, when the DNA repair was minimized, and repopulation and reoxygenation factors were excluded. Thus, it can be assumed that the observed dose-rate effect on DNA damage and repair is mainly caused by primary lesions appearing at the moment of irradiation. The reduced reparability of DNA damages in HDR-irradiated cells compared with in LDR-irradiated cells may be associated with the formation of more complex DNA clustered damages.
The latest in vivo data has demonstrated that pulsed, ultrahigh-dose-rate electron irradiation increases the differential response between normal and tumor tissue, although the molecular mechanisms underlying those differential responses are still unknown [6]. Thus, our preliminary findings can enhance future radiobiological studies on the elucidation of dose-rate effect mechanisms. Our results may also be useful in the development of effective parameters for the clinical application of pulsed ultrashort electron beam radiation.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
This work was supported by the RA MES State Committee of Science of Armenia [grant number 14AR-1f06].
REFERENCES
- 1. Gauduel Y. Laser-driven particle acceleration towards radiobiology and medicine In Giulietti A. (ed). Laser-Plasma Accelerators Based Ultrafast Radiation Biophysics. Switzerland: Springer, 2016, 19–41. [Google Scholar]
- 2. Oulianov DA, Li Y, Crowell RA et al. Femtosecond electron and x-ray source based on laser wakefield accelerator. In: Kleinfelder S, Paisley DL, Chang Z et al. (eds). Ultrafast X-Ray Detectors, High-Speed Imaging, and Applications. Proceedings of the SPIE, San Diego, CA, 31 July 2005.
- 3. Laschinsky L, Baumann M, Beyreuther E et al. Radiobiological effectiveness of laser accelerated electrons in comparison to electron beams from a conventional linear accelerator. J Radiat Res 2012;53:395–403. [DOI] [PubMed] [Google Scholar]
- 4. Labate L, Andreassi MG, Baffigi F et al. Small-scale laser based electron accelerators for biology and medicine: a comparative study of the biological effectiveness. In: Esarey E, Schroeder CB, Leemans WP et al. (eds). Laser Acceleration of Electrons, Protons, and Ions II; and Medical Applications of Laser-Generated Beams of Particles II; and Harnessing Relativistic Plasma Waves III. Proceedings of the SPIE, Prague, Czech Republic, 15 April 2013.
- 5. Rigaud O, Fortunel NO, Vaigot P et al. Exploring ultrashort high-energy electron-induced damage in human carcinoma cells. Cell Death Dis 2010;1:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Favaudon V, Caplier L, Monceau V et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 2014;6:245ra93. [DOI] [PubMed] [Google Scholar]
- 7. Wilson P, Jones B, Yokoi T et al. Revisiting the ultra-high dose rate effect: implications for charged particle radiotherapy using protons and light ions. Br J Radiol 2012;85:e933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zlobinskaya O, Dollinger G, Michalski D et al. Induction and repair of DNA double-strand breaks assessed by gamma-H2AX foci after irradiation with pulsed or continuous proton beams. Radiat Environ Biophys 2012;51:23–32. [DOI] [PubMed] [Google Scholar]
- 9. Zlobinskaya O, Siebenwirth C, Greubel C et al. The effects of ultra-high dose rate proton irradiation on growth delay in the treatment of human tumor xenografts in nude mice. Radiat Res 2014; 181:177–83. [DOI] [PubMed] [Google Scholar]
- 10. Laschinsky L, Karsch L, Leßmann E et al. Radiobiological influence of megavoltage electron pulses of ultra-high pulse dose rate on normal tissue cells. Radiat Environ Biophys 2016;55:381–91. [DOI] [PubMed] [Google Scholar]
- 11. Beyreuther E, Karsch L, Laschinsky L et al. Radiobiological response to ultra-short pulsed megavoltage electron beams of ultra-high pulse dose rate. Int J Radiat Biol 2015;91:643–52. [DOI] [PubMed] [Google Scholar]
- 12. Tsakanov VM, Aroutiounian RM, Amatuni GA et al. AREAL low energy electron beam applications in life and materials sciences. Nucl Instrum Methods Phys Res 2016;829:248–53. [Google Scholar]
- 13. Nakamura A, Sedelnikova OA, Redon C et al. Techniques for gamma-H2AX detection. Methods Enzymol 2006;409:236–50. [DOI] [PubMed] [Google Scholar]
- 14. Singh NP, McCoy MT, Tice RR et al. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 1988;175:184–91. [DOI] [PubMed] [Google Scholar]
- 15. Lomax ME, Folkes LK, O'Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol 2013;25:578–85. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Xu C, Du LQ et al. Evaluation of the comet assay for assessing the dose–response relationship of DNA damage induced by ionizing radiation. Int J Mol Sci 2013;14:22449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van der Kogel A. The dose-rate effect In: Joiner M, van der Kogel A (eds). Basic Clinical Radiobiology. London: CRC Press, 2009, 158–68. [Google Scholar]
- 18. Mazeron JJ, Scalliet P, Van Limbergen E et al. Radiobiology of brachytherapy and the dose-rate effect In: Gerbaulet A, Pötter R, Mazeron JJ et al. (eds). The GEC-ESTRO Handbook of Brachytherapy. Brussels: ESTRO, 2002, 95–121. [Google Scholar]