Abstract
Background
Meningioma is the most common primary intracranial tumor and recurrence is one of the important challenges in patient management. Prognostic factors for tumor recurrences in these patients especially before surgical resection are not fully characterized. Several studies have indicated an association between changes in hematologic laboratory parameters with patient outcomes in solid malignancies. We aimed to assess the association between hematologic parameters and tumor recurrence in patients with meningioma.
Methods
Preoperative complete blood count (CBC) data were analyzed in patients with newly diagnosed meningioma (n = 222). Clinical data, including history of corticosteroid therapy, tumor characteristics, and follow-up, were obtained. Recurrence-free survival (RFS) was evaluated using Cox proportional hazards models and log-rank tests.
Results
Using preoperative CBC data from patients prior to any steroid therapy, 51 (23%) patients had neutrophilia. In univariate analysis, neutrophilia was significantly associated with meningioma recurrence (hazard ratio [HR] 2.73; P < 0.01). Neither leukocytosis nor lymphocytosis was associated with RFS. In multivariate analysis, after adjusting for tumor grade, tumor size, and extent of resection, neutrophilia remained an independent prognostic factor for RFS (HR 2.23, P = 0.01). Forty-six (21%) patients had low hemoglobin levels indicative of anemia, and the presence of anemia showed a trend toward high risk for recurrence (HR 1.83; P = 0.06).
Conclusions
The presence of neutrophilia was associated with higher rate of tumor recurrence in patients with meningioma. Validation of these results and the biologic role of neutrophilic inflammatory/immune reaction in meningioma requires further investigation.
Keywords: meningioma, preoperative neutrophilia, tumor recurrence
Importance of the study
A subset of patients with meningioma experience tumor recurrence, which is a major cause of morbidity in these patients. Clinical and pathological factors, including tumor grade, extent of resection, and tumor size, only partially account for recurrence rate. We assessed routinely obtained preoperative, pre-corticosteroid–therapy CBC data from 222 patients with meningioma (World Health Organization [WHO] grades I, II, and III). We observed neutrophilia in 23% of our cases distributed in all grades of meningioma. Using multivariate analysis to adjust for the WHO grade, extent of resection and tumor size showed that neutrophilia was an independent predictor for tumor recurrence in these patients. In addition, we identified anemia in 21% of our cases and univariate analysis indicated that it was marginally significant for worse RFS. If validated, the findings may be useful in the assessment of the biologic role of neutrophils in meningioma.
Meningioma is a neoplasm that originates from meningothelial cells. It is the most common primary brain tumor and accounts for 35%–40% of CNS tumors.1 The World Health Organization (WHO) grading of meningioma is based on histologic subtypes or utilizes histologic features, including mitotic activity, brain invasion, cellularity, growth pattern, and presence of nucleoli.2,3 Although most meningiomas are WHO grade I (85%–95%) and are considered benign solid tumors, these tumors can recur even after complete resection, and the recurrence rate in these patients has been reported as 7.5% at 10 years and 9.3% at 20 years.4,5 Furthermore, a subset of meningiomas are well known to have an aggressive clinical course in a manner that correlates with malignancy grade.2
Estimating recurrence risk is an important clinical challenge in therapeutic management of meningioma patients. Previous studies have established that WHO grade, extent of resection, and tumor size are important prognostic factors for tumor recurrence, of which only tumor size can be used to predict tumor recurrence before neurosurgical resection.2,6–9
Several studies showed that systemic inflammatory/immunologic reaction before treatment might influence and predict clinical course in cancer patients.10,11 The prognostic significance of preoperative hematologic parameters has been reported in several solid tumors, including melanoma and cancers of the breast, gastrointestinal tract, lung, bladder, ovary, head and neck, prostate, kidney, and other sites.12–22
Work from other brain tumor types, including gliomas,2,23 documents changes in the inflammatory landscape in both low grade glioma and glioblastoma.24–29 Among these factors, increased neutrophil/lymphocyte ratio (NLR) was associated with poor patient outcome.24,25 Similarly, neutrophilia at the time of initial diagnosis has been reported in meningioma and vestibular schwannoma.29 Inflammatory/immunologic reactions in meningioma indicated as peritumoral edema and stromal infiltration of inflammatory cells are also well described.30–32
Here, we examined the association of hematologic parameters in routine preoperative complete blood cell count (CBC) with tumor recurrence in patients with meningioma. Given the well-known association of steroid therapy and elevation of white blood cell count, and that a subset of patients with meningioma may receive corticosteroids preoperatively, we limited the cohort to only those patients who had CBC data prior to documented corticosteroid therapy.
Previous studies have shown some relevance of electrolyte imbalance with clinical outcome in cancer patients.33,34 Hence, we also assessed the prognostic significance of preoperative serum electrolyte levels in patients with meningioma.
Materials and Methods
Patients and Clinical Data
We included patients with newly diagnosed, histologically confirmed meningioma (WHO grades I, II, and III) who underwent craniotomy at Toronto Western Hospital between 2001 and 2015. Appropriate ethics review was approved for this study. We obtained the following clinical information from electronic patient records including demographic information, history of preoperative corticosteroid therapy, and clinical follow-up. Regarding extent of resection (EOR), we reviewed both the operative/clinical notes and postoperative MRI reports from each patient. EOR was categorized as gross total resection (GTR) for complete resection/no residual and as subtotal resection (STR) for incomplete resection/presence of residual tumor, confirmed by postoperative MRIs. Where postoperative imaging was not available in the medical record, we relied on the surgeon’s observation based on the operative report. Patients with tumor recurrence/progression and patients with no tumor recurrence with follow-up time >2 years were included in our cohort. Tumor recurrence was defined as appearance of a new enhancing lesion on serial postoperative MRIs that required therapeutic intervention, either second surgery or radiotherapy. Pathology reports and original diagnostic histologic slides were reviewed to confirm the diagnosis and WHO grade. Tumor size was defined as the largest single dimension recorded in the radiology report and/or from the measurement of the MRI/CT preoperative imaging (when the radiology report was not available).
Laboratory Data
The CBC data utilized in our cohort represented the blood draw taken immediately before surgery, unless the patient had documented corticosteroid use, in which case we used CBC data from the last blood draw prior to steroid therapy. The following CBC data were collected: hemoglobin level, packed cell volume, erythrocyte cell count, mean corpuscular volume, total white blood cell count (WBC), and absolute neutrophil, monocyte, lymphocyte, and platelet counts. Normal ranges were obtained based on the clinical laboratory medicine program at the University Health Network, Toronto, Ontario (Supplementary Table S1). We defined leukocytosis as WBC >11.0 billion (bil)/L, neutrophilia as absolute neutrophil count >7.5 bil/L, lymphocytosis as absolute count >4 bil/L, lymphopenia as absolute count <1.5 bil/L, monocytosis as absolute count >0.8 bil/L, and anemia as hemoglobin level <120 g/L for females and <140 g/L for males. We also collected the data on preoperative serum levels of sodium (normal range, 135–145 mmol/L), potassium (normal range, 3.2–5 mmol/L), and chloride (normal range, 100–110 mmol/L).
Statistical Analyses
The primary outcome of interest was recurrence-free survival (RFS), defined as the time from the date of surgery to the date of recurrence or the last known follow-up. Kaplan–Meier curves were performed to evaluate the impact of the following parameters on RFS: malignancy grade (I–III), tumor location (skull base tumor vs non–skull base tumor), maximum tumor size (maximum tumor dimension, dichotomized by median value), extent of resection (GTR vs STR), and hematologic parameters. Associations between clinical and pathological characteristics of interest with time to recurrence were evaluated using multivariate Cox proportional hazards analyses, including the dependent variables that were significant in the univariate analyses.
Significance was determined as P < 0.05 on all univariate and multivariate analyses. Hazard ratios (HRs) were reported with 95% CIs. All statistical analyses were performed using Med Calc. To assess the robustness of the association between RFS and hematologic variables, we used these factors as continuous variables in both univariate and multivariate Cox proportional hazards models.
Results
Study Population
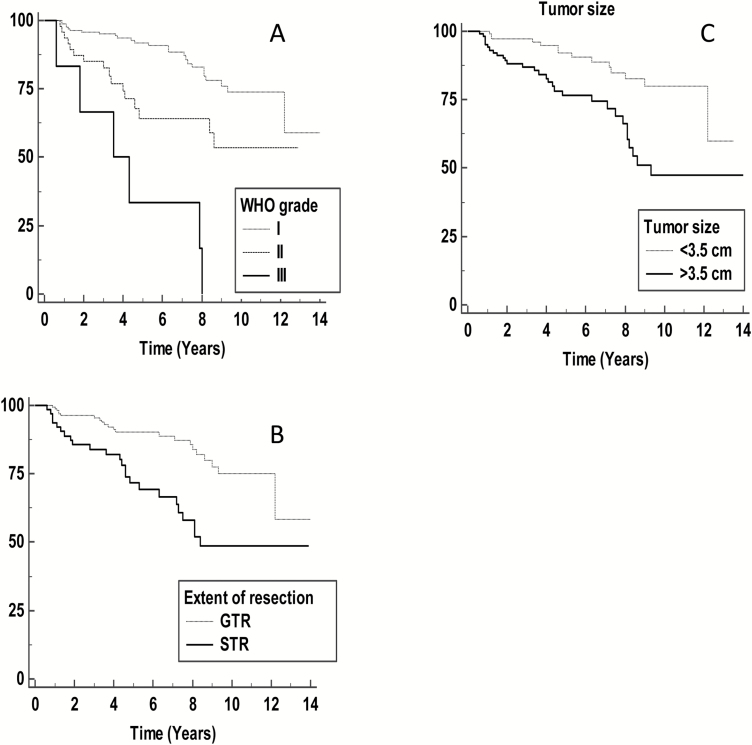
Our initial cohort included 226 patients with newly diagnosed meningioma who underwent surgical resection (WHO grades I, II, and III). We excluded 4 patients due to lack of CBC data prior to the administration of corticosteroid therapy. Of the 222 patients, the final cohort included 169 grade I, 47 grade II, and 6 grade III tumors. Demographics, tumor characteristics, and the results of univariate analyses are included in Table 1, showing an expected preponderance of female patients (F/M ratio = 2.5), with a median age of 58 years (range, 20–88 y). The maximum tumor diameters were available for 205 patients, and the median tumor diameter was 3.5 cm (range, 0.5–8.4). Twenty-eight (13%) patients had a history of corticosteroid use prior to the surgery. Regarding EOR, 158 patients had GTR, 63 patients had STR, and data for EOR was not available for 1 patient. Of the cohort of 222 patients, EOR data were obtained by analysis of the presence of residual tumor in all patients for whom postoperative imaging was available, comprising 215 patients (97%), and by the surgeon’s observation (from the operative note) in 6 patients (3%), with the remaining patient having unavailable EOR data. The patient median follow-up time was 6.0 years (range, 0.6–14 y). In total, 48 (22%) patients had a documented tumor recurrence. As expected, Kaplan–Meier survival analysis showed that both WHO grade and EOR correlated with poor RFS (P < 0.01 for each, log-rank test) (Fig. 1A, B). Also, tumor size, as a continuous parameter, was predictive for tumor recurrence (P < 0.01), and when we dichotomized this factor at the 3.5 cm median value, it remained prognostic for poor RFS (Fig. 1C). Tumor location was not prognostic for tumor recurrence in our cohort (P = 0.3).
Table 1.
Patient characteristics and univariate analyses
| Characteristic | Total Number (%) | P-value | HR | 95% CI |
|---|---|---|---|---|
| WHO grade | <0.01 | 2.98 | 1.95–4.56 | |
| I | 169 (76) | |||
| II | 47 (21) | |||
| III | 6 (3) | |||
| Gender | 0.10 | 0.61 | 0.34–1.10 | |
| Female | 158 (71) | |||
| Male | 64 (29) | |||
| Tumor location | 0.59 | 1.17 | 0.65–2.11 | |
| Skull base | 70 (32) | |||
| Non–skull base | 152 (68) | |||
| Max. tumor size, cm | <0.01 | 1.49 | 1.24–1.78 | |
| >3.5 | 123 (55) | |||
| <3.5 | 99 (45) | |||
| Extent of resection | <0.01 | 2.63 | 1.50 to 4.65 | |
| GTR | 158(71) | |||
| STR | 63(29) | |||
| Anemia | 0.06 | 1.83 | 0.97–3.48 | |
| Present | 46 (21) | |||
| Absent | 176 (79) | |||
| Leukocytosis | 0.10 | 1.74 | 0.90–3.36 | |
| Present | 42 (19) | |||
| Absent | 180 (81) | |||
| Neutrophilia | <0.01 | 2.73 | 1.52–4.88 | |
| Present | 51 (23) | |||
| Absent | 171 (77) | |||
| Lymphocytosis | 0.31 | 2.82 | 0.38–20.82 | |
| Present | 4 (2) | |||
| Absent | 218 (98) | |||
| Lymphopenia | 0.26 | 1.4 | 0.78–2.46 | |
| Present | 83 (37) | |||
| Absent | 139 (63) | |||
| Monocytosis | 0.25 | 1.6 | 0.72–3.56 | |
| Present | 24 (11) | |||
| Absent | 198 (89) |
Fig. 1.
(A) Kaplan–Meier analysis of the association of WHO grade (P < 0.01); (B) extent of resection—gross total resection (GTR) versus subtotal resection (STR) (P < 0.01); and (C) tumor size (maximum tumor dimension) dichotomized at 3.5 cm (P < 0.01) with recurrence-free survival.
WBC Count and Differential in CBC: Neutrophilia Is a Prognostic Factor for High Rate of Tumor Recurrence in Patients with All Grades of Meningioma
Leukocytosis
We identified leukocytosis in 42 (19%) patients. Univariate analysis of WBC as a continuous parameter was prognostically significant for tumor recurrence (P = 0.01) (Supplementary Table S1); however, univariate analysis showed that leukocytosis was not associated with worse RFS in meningioma patients (P = 0.1) (Table 1).
Neutrophilia
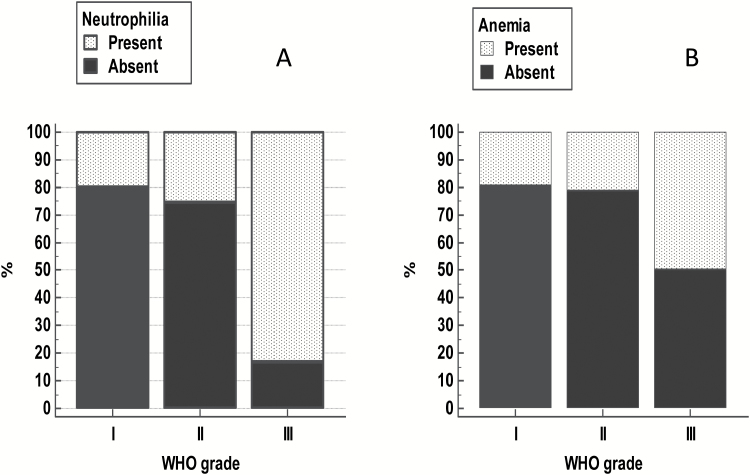
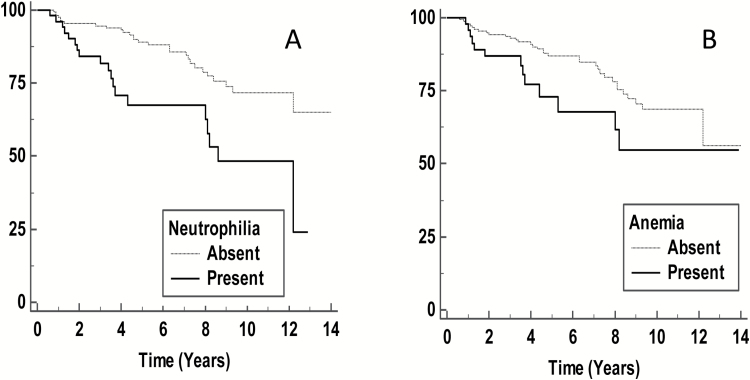
The median of neutrophil count in the cohort was 4.55 (bil/L) (range, 0.6–21.5 bil/L). Interestingly, absolute neutrophilia (defined in our clinical laboratory as a neutrophil count >7.5 bil/L) was observed in 51 (23%) and was distributed among all grades of meningioma, including grade I: 34 (15%), grade II: 12 (5%), and grade III: 5 (2%) tumors (Fig. 2A). Kaplan–Meier analysis indicated that the presence of neutrophilia was a significant predictor of poor RFS (P < 0.01) (Fig. 3A). In addition, when neutrophil count was used as a continuous variable, we found that it remained a statistically significant parameter for tumor recurrence (HR 1.10, P < 0.01, 95% CI: 1.03–1.17) (Supplementary Table S1). This finding suggested that a unit increase in neutrophil count was associated with a 10% increase in the rate of tumor recurrence. Using the Cox proportional hazards model, when adjusting for WHO grade, maximum tumor size, and EOR, the presence of neutrophilia remained as an independent factor for predicting tumor recurrence (P = 0.01) (Table 2). To examine the effect of neutrophilia in grade I meningioma, we used the Cox proportional hazards model, which showed that in this subset alone, neutrophilia lacked prognostic significance for prediction of tumor recurrence (Supplementary Table S3). We observed that the number and proportion of events (tumor recurrence) were lower in patients with grade I meningiomas (26/169, 15%) compared with grades II–III meningiomas combined (21/53, 40%), which may account for this finding.
Fig. 2.
(A) Distribution of neutrophilia. (B) Anemia in preoperative, pre-steroid complete blood cell count among WHO grades I, II, and III meningiomas.
Fig. 3.
Kaplan–Meier analysis of the association of (A) preoperative neutrophilia (P < 0.01) and (B) anemia (P = 0.06) with recurrence-free survival.
Table 2.
Multivariate analysis of clinical and pathological characteristics with tumor recurrence in meningioma patients
| Covariate | P-value | Hazard Ratio | 95% CI |
|---|---|---|---|
| Neutrophilia | 0.01 | 2.23 | 1.20–4.20 |
| WHO grade | 0.04 | 1.68 | 1.01–2.78 |
| Extent of resection | 0.002 | 2.55 | 1.40–4.70 |
| Tumor size | 0.01 | 1.3 | 1.10–1.60 |
Additional clinical laboratory data
While lymphocytosis was detected in only 4 patients (2%), lymphopenia was noted in 83 (37%). Univariate analysis showed that lymphopenia was not a predictor for poor RFS (P = 0.26). Twenty-four patients (11%) showed absolute monocytosis, distributed among all grades of meningioma, including 16 (7%) grade I, 5 (2%) grade II, and 3 (1%) grade III; however, the univariate analysis suggested that it was not associated with higher risk for tumor recurrence (P = 0.25). Preoperative serum levels of sodium, potassium, and chloride were not prognostically significant for tumor recurrence in our cohort (data not shown).
Preoperative anemia and recurrence-free survival
Preoperative anemia was present in 46/222 (21%) patients (Table 1). Thirty-three of 46 anemic patients (71%) had grade I meningioma, while the remainder had atypical meningioma (10; 5%) and malignant meningioma (3; 1%) (Fig. 2B). Regarding the type of anemia, 37/46 patients (80%) of the anemic cases had normocytic anemia, with the remaining 9 (20%) having macrocytic (n = 5) and microcytic anemia (n = 4). Overall, for all types of anemia, Kaplan–Meier analysis showed that the presence of preoperative anemia was marginally significant (P = 0.06) for predicting unfavorable RFS in meningioma patients (Fig. 3B).
Discussion
Estimating recurrence risk is an important clinical problem in meningioma. A subset of patients will experience tumor recurrence, and efforts to estimate this risk include assessment of WHO grade, along with EOR and other metrics, such as tumor size. With these parameters, there remains uncertainty for individual patients as to their individual recurrence risk, which is of importance when considering different treatment options such as neurosurgical resection, adjuvant radiation therapy, and clinical follow-up. In an effort to identify pretreatment clinical parameters that predict recurrence of meningioma, we examined routinely collected laboratory data from patients assessed preoperatively. Our study identifies an elevated neutrophil count as correlated with recurrence. We identified the presence of preoperative neutrophilia in 23% of the patients in our cohort, distributed among all grades of meningioma. Multivariate analyses, adjusting for WHO grade, maximum tumor size, and EOR, indicated that this parameter was an independent prognostic factor for worse RFS in our cohort. We also accounted for 2 important confounding factors for neutrophilia in these patients, including surgical resection and corticosteroid therapy. Preoperative hematologic testing for CBC is routine in all patients and inexpensive and can be widely used to predict risk of tumor recurrence in meningioma before surgical resection.
Our results align with previous findings regarding the prognostic role of inflammatory changes in other tumor types.29,35 The prognostic role of tumor-infiltrating neutrophils, neutrophilia, and elevated blood NLR in predicting poor clinical outcome has been shown in several cancers.15,21,22,36 The definition for cutoffs for neutrophilia across these studies varied between 3.5–7.5 109/L. The cutoff of 7.5 bil/L, defined by the laboratory at our hospital, is set at the high end of cutoffs relative to other studies, and it has been used in at least 2 previous studies that examined neutrophilia and prognosis in renal cell carcinoma and malignant melanoma.37,38 Among brain tumors, previous studies showed that elevated NLR and overall neutrophilic activation are associated with worse outcome in glioblastoma.24,25,27 In our cohort of meningiomas, we observed lymphopenia in 83 (37%) of our cases, but this finding was not associated with worse RFS. Therefore it appears that neutrophil count alone might be a more relevant parameter in meningioma rather than NLR. Future studies may prove useful for further elucidation.
The local inflammatory/immune response in meningioma has been described, including peritumoral edema, which is a predictor of tumor recurrence in meningioma.31 Interestingly, one of the important contributing mediators for peritumoral edema in meningioma is interleukin-6,39 which has also been implicated as an activated mediator for neutrophils in glioblastoma.27 Stromal infiltration of lymphocytes and macrophages has been largely studied in meningioma.30,40–42
While this requires further investigation, several large-scale studies indicated that neutrophils are the most important prognostic factors in the biology of cancer,43,44 with possible roles of angiogenesis, migration, invasion, metastasis, mutagenesis, or immunosuppression.36 Prior studies on meningioma point to roles of an intratumoral inflammatory response, including biological roles of T lymphocytes, macrophages, and microglia, as well as programmed cell death ligand 1 expression in meningioma.41,45,46 As these studies did not specifically investigate changes in circulating neutrophils or intratumoral neutrophils, the biologic role of these inflammatory cells in meningioma remains unclear.
We also identified anemia in 21% of the patients in our cohort, the majority of whom were normocytic, with smaller proportions showing microcytic and macrocytic anemia. Univariate analysis showed borderline significance of anemia for predicting tumor recurrence (P = 0.06). While this exploratory analysis precludes a definitive conclusion regarding the role of anemia as a robust predictor for tumor recurrence, this finding warrants further study for cause and possible correlation of anemia with worst outcome in meningioma. Pre-radiotherapy anemia has been studied in brain tumors and is reported as a poor prognostic factor in patients with malignant gliomas.47 A prior study noted pretreatment low hemoglobin levels in patients with meningioma and vestibular schwannoma compared with a normal control group.29 Interestingly, anemia has been implicated in chordoid meningioma and lymphoplasmacytic meningioma48,49 (perhaps in relation to associated comorbidities), and our findings suggest that further investigations may be warranted for all meningioma subtypes.
Limitations of our study design include its retrospective nature in a single center, its exploratory nature, and presence of possible confounding factors for neutrophilia. We could not collect CBC data in a defined time period before surgery because the time of starting steroid therapy varied in our patients. Our sample size was limited by the availability of preoperative and pre-steroid CBC in the medical records. In addition, we required patients with clinical follow-up, and excluded meningioma patients with less than 2 years follow-up (assuming no evidence of recurrence). While we addressed steroid use and surgical resection as possible confounders for neutrophilia in our patients, we could not exclude other possible causes of neutrophilia in them, including infectious, inflammatory, immunologic, smoking, seizure, and stress induced adrenalin release before surgery. Future studies will attempt to monitor the changes in neutrophil count after resection; in the current cohort we were unable to follow up these changes due to missing data: absence of systematic CBC collection at follow-up and possible confounding factors after surgery including steroid therapy.
In conclusion, our study indicated a prognostic association of preoperative neutrophilia in meningioma patients. If our results are validated in an independent cohort, they could potentially be used as one of the preoperative predictors for risk of tumor recurrence in these patients. This exploratory study may serve to stimulate additional prospective studies to investigate whether routinely assessed hematologic parameters might help predict tumor recurrence in meningioma patients. In addition, there is a need for future research studies to better understand the biologic role of tumor-associated inflammation in meningioma. New perspectives learned from immunologic characteristics of meningioma may help us to tailor new therapeutic approaches for these patients.
Supplementary material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by the Canadian Institutes of Health Research.
Conflict of interest statement. None.
Supplementary Material
References
- 1. Ostrom QT, Gittleman H, Fulop J et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bi WL, Zhang M, Wu WW, Mei Y, Dunn IF. Meningioma genomics: diagnostic, prognostic, and therapeutic applications. Front Surg. 2016;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Reifenberger G et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 4. McNeill KA. Epidemiology of brain tumors. Neurol Clin. 2016;34(4):981–998. [DOI] [PubMed] [Google Scholar]
- 5. Nakasu S, Fukami T, Jito J, Nozaki K. Recurrence and regrowth of benign meningiomas. Brain Tumor Pathol. 2009;26(2):69–72. [DOI] [PubMed] [Google Scholar]
- 6. Champeaux C, Dunn L. World Health Organization grade II meningioma. A 10-year retrospective study for recurrence and prognostic factor assessment. World Neurosurg. 2016;89:180–186. [DOI] [PubMed] [Google Scholar]
- 7. Durand A, Labrousse F, Jouvet A et al. WHO grade II and III meningiomas: a study of prognostic factors. J Neurooncol. 2009;95(3):367–375. [DOI] [PubMed] [Google Scholar]
- 8. Gallagher MJ, Jenkinson MD, Brodbelt AR, Mills SJ, Chavredakis E. WHO grade 1 meningioma recurrence: Are location and Simpson grade still relevant? Clin Neurol Neurosurg. 2016;141:117–121. [DOI] [PubMed] [Google Scholar]
- 9. Rogers L, Barani I, Chamberlain M et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Detti B, Scoccianti S, Di Cataldo V et al. Atypical and malignant meningioma: outcome and prognostic factors in 68 irradiated patients. J Neurooncol. 2013;115(3):421–427. [DOI] [PubMed] [Google Scholar]
- 11. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. [DOI] [PubMed] [Google Scholar]
- 12. Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33(Suppl 1):S79–S84. [DOI] [PubMed] [Google Scholar]
- 13. Templeton AJ, McNamara MG, Šeruga B et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. [DOI] [PubMed] [Google Scholar]
- 14. Varlotto J, Stevenson MA. Anemia, tumor hypoxemia, and the cancer patient. Int J Radiat Oncol Biol Phys. 2005;63(1):25–36. [DOI] [PubMed] [Google Scholar]
- 15. Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol. 2013;23(3):200–207. [DOI] [PubMed] [Google Scholar]
- 16. Berardi R, Braconi C, Mantello G et al. Anemia may influence the outcome of patients undergoing neo-adjuvant treatment of rectal cancer. Ann Oncol. 2006;17(11):1661–1664. [DOI] [PubMed] [Google Scholar]
- 17. Chen XF, Qian J, Pei D et al. Prognostic value of perioperative leukocyte count in resectable gastric cancer. World J Gastroenterol. 2016;22(9):2818–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoff CM. Importance of hemoglobin concentration and its modification for the outcome of head and neck cancer patients treated with radiotherapy. Acta Oncol. 2012;51(4):419–432. [DOI] [PubMed] [Google Scholar]
- 19. Kozak MM, von Eyben R, Pai JS et al. The prognostic significance of pretreatment hematologic parameters in patients undergoing resection for colorectal cancer. Am J Clin Oncol. 2015. [DOI] [PubMed] [Google Scholar]
- 20. Lutterbach J, Guttenberger R. Anemia is associated with decreased local control of surgically treated squamous cell carcinomas of the glottic larynx. Int J Radiat Oncol Biol Phys. 2000;48(5):1345–1350. [DOI] [PubMed] [Google Scholar]
- 21. Ozcan C, Telli O, Ozturk E et al. The prognostic significance of preoperative leukocytosis and neutrophil-to-lymphocyte ratio in patients who underwent radical cystectomy for bladder cancer. Can Urol Assoc J. 2015;9(11–12):E789–E794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Su Z, Mao YP, OuYang PY, Tang J, Xie FY. Initial hyperleukocytosis and neutrophilia in nasopharyngeal carcinoma: incidence and prognostic impact. PLoS One. 2015;10(9):e0136752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bi WL, Wu WW, Santagata S, Reardon DA, Dunn IF. Checkpoint inhibition in meningiomas. Immunotherapy. 2016;8(6):721–731. [DOI] [PubMed] [Google Scholar]
- 24. Bambury RM, Teo MY, Power DG et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol. 2013;114(1):149–154. [DOI] [PubMed] [Google Scholar]
- 25. Alexiou GA, Vartholomatos E, Voulgaris S. Prognostic value of neutrophil-to-lymphocyte ratio in patients with glioblastoma. J Neurooncol. 2013;115(3):521–522. [DOI] [PubMed] [Google Scholar]
- 26. Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999;98(4):349–354. [DOI] [PubMed] [Google Scholar]
- 27. Rahbar A, Cederarv M, Wolmer-Solberg N et al. Enhanced neutrophil activity is associated with shorter time to tumor progression in glioblastoma patients. Oncoimmunology. 2016;5(2):e1075693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang PF, Song HW, Cai HQ et al. Preoperative inflammation markers and IDH mutation status predict glioblastoma patient survival. Oncotarget. 2017;doi: 10.18632/oncotarget.15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Subeikshanan V, Dutt A, Basu D, Tejus MN, Maurya VP, Madhugiri VS. A prospective comparative clinical study of peripheral blood counts and indices in patients with primary brain tumors. J Postgrad Med. 2016;62(2):86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Domingues P, González-Tablas M, Otero Á et al. Tumor infiltrating immune cells in gliomas and meningiomas. Brain Behav Immun. 2016;53:1–15. [DOI] [PubMed] [Google Scholar]
- 31. Nowak A, Dziedzic T, Krych P, Czernicki T, Kunert P, Marchel A. Benign versus atypical meningiomas: risk factors predicting recurrence. Neurol Neurochir Pol. 2015;49(1):1–10. [DOI] [PubMed] [Google Scholar]
- 32. Osawa T, Tosaka M, Nagaishi M, Yoshimoto Y. Factors affecting peritumoral brain edema in meningioma: special histological subtypes with prominently extensive edema. J Neurooncol. 2013;111(1): 49–57. [DOI] [PubMed] [Google Scholar]
- 33. Furukawa J, Miyake H, Kusuda Y, Fujisawa M. Hyponatremia as a powerful prognostic predictor for Japanese patients with clear cell renal cell carcinoma treated with a tyrosine kinase inhibitor. Int J Clin Oncol. 2015;20(2):351–357. [DOI] [PubMed] [Google Scholar]
- 34. Yoon J, Ahn SH, Lee YJ, Kim CM. Hyponatremia as an independent prognostic factor in patients with terminal cancer. Support Care Cancer. 2015;23(6):1735–1740. [DOI] [PubMed] [Google Scholar]
- 35. Kataki A, Skandami V, Memos N et al. Similar immunity profiles in patients with meningioma and glioma tumors despite differences in the apoptosis and necrosis of circulating lymphocyte and monocyte populations. J Neurosurg Sci. 2014;58(1):9–15. [PubMed] [Google Scholar]
- 36. Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23(3):141–148. [DOI] [PubMed] [Google Scholar]
- 37. Négrier S, Escudier B, Gomez F et al. Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Français d’Immunothérapie. Ann Oncol. 2002;13(9):1460–1468. [DOI] [PubMed] [Google Scholar]
- 38. Schmidt H, Suciu S, Punt CJ et al. ; American Joint Committee on Cancer Stage IV Melanoma; EORTC 18951. Pretreatment levels of peripheral neutrophils and leukocytes as independent predictors of overall survival in patients with American Joint Committee on Cancer Stage IV Melanoma: results of the EORTC 18951 Biochemotherapy Trial. J Clin Oncol. 2007;25(12):1562–1569. [DOI] [PubMed] [Google Scholar]
- 39. Park KJ, Kang SH, Chae YS et al. Influence of interleukin-6 on the development of peritumoral brain edema in meningiomas. J Neurosurg. 2010;112(1):73–80. [DOI] [PubMed] [Google Scholar]
- 40. Han SJ, Reis G, Kohanbash G et al. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J Neurooncol. 2016;130(3):543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Domingues PH, Teodósio C, Otero Á et al. Association between inflammatory infiltrates and isolated monosomy 22/del(22q) in meningiomas. PLoS One. 2013;8(10):e74798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Domingues PH, Teodósio C, Ortiz J et al. Immunophenotypic identification and characterization of tumor cells and infiltrating cell populations in meningiomas. Am J Pathol. 2012;181(5):1749–1761. [DOI] [PubMed] [Google Scholar]
- 43. Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One. 2014;9(6):e98259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moses K, Brandau S. Human neutrophils: their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 2016;28(2):187–196. [DOI] [PubMed] [Google Scholar]
- 45. Fang L, Lowther DE, Meizlish ML et al. The immune cell infiltrate populating meningiomas is composed of mature, antigen-experienced T and B cells. Neuro Oncol. 2013;15(11):1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Du Z, Abedalthagafi M, Aizer AA et al. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget. 2015;6(7):4704–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Céfaro GA, Genovesi D, Vinciguerra A et al. Prognostic impact of hemoglobin level and other factors in patients with high-grade gliomas treated with postoperative radiochemotherapy and sequential chemotherapy based on temozolomide: a 10-year experience at a single institution. Strahlenther Onkol. 2011;187(12):778–783. [DOI] [PubMed] [Google Scholar]
- 48. Kepes JJ, Chen WY, Connors MH, Vogel FS. “Chordoid” meningeal tumors in young individuals with peritumoral lymphoplasmacellular infiltrates causing systemic manifestations of the Castleman syndrome. A report of seven cases. Cancer. 1988;62(2):391–406. [DOI] [PubMed] [Google Scholar]
- 49. Zhu HD, Xie Q, Gong Y et al. Lymphoplasmacyte-rich meningioma: our experience with 19 cases and a systematic literature review. Int J Clin Exp Med. 2013;6(7):504–515. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.