Abstract
The primary inhibitory neurotransitter γ-aminobutyric acid (GABA) and the major antioxidant glutathione (GSH) are compounds of high importance for the function and integrity of the human brain. In this study, a method for simultaneous J-difference spectral-edited MR spectroscopy of GSH and GABA with suppression of macromolecular (MM) signals at 3 T is proposed. MM-suppressed Hadamard encoding and reconstruction of MEGA-edited spectroscopy (HERMES) consists of four sub-experiments (TE = 80 ms), with 20-ms editing pulses applied at: (A) 4.56 ppm & 1.9 ppm; (B) 4.56 ppm & 1.5 ppm; (C) 1.9 ppm; and (D) 1.5 ppm. One Hadamard combination (A+B−C−D) yields GSH-edited, and another one (A−B+C−D) yields GABA-edited spectra with symmetric suppression of the co-edited MM signal.
MM-suppressed HERMES, conventional HERMES, and separate MEGA-PRESS data were successfully acquired from a (33 mm)3 voxel in the parietal lobe in 10 healthy subjects. GSH- and GABA-edited MM-suppressed HERMES spectra were in close agreement with the respective MEGA-PRESS spectra. Mean GABA (and GSH) estimates were 1.10 ± 0.15 i.u. (0.59 ± 0.12 i.u.) for MM-suppressed HERMES, and 1.13 ± 0.09 i.u. (0.66 ± 0.09 i.u.) for MEGA-PRESS. Mean GABA (and GSH) differences between MM-suppressed HERMES and MEGA-PRESS were −0.03 ± 0.11 i.u. (−0.07 ± 0.11 i.u.). Mean signal-to-noise ratio (SNR) improvement of MM-suppressed HERMES over MEGA-PRESS was 1.45 ± 0.25 for GABA, and 1.32 ± 0.24 for GSH. These results indicate that symmetric suppression of MM signal can be accommodated into the Hadamard editing framework. Compared to sequential single-metabolite MEGA-PRESS experiments, MM-suppressed HERMES allows for simultaneous edited measurements of GSH and GABA without MM contamination in only half the scan time, while SNR is maintained.
Keywords: GABA, Glutathione, Macromolecules, Editing, Hadamard, HERMES
Graphical abstract
Introduction
Magnetic resonance spectroscopy (MRS) of the main inhibitory neurotransmitter γ-aminobutyric acid (GABA) and the main antioxidant glutathione (GSH) can probe inhibitory and/or redox dysfunction in the brain, which are common features of psychiatric and neurological disease1,2. Both compounds have low in vivo concentration and coupled resonances, so are usually detected at 3T with J-difference-edited techniques such as MEGA-PRESS2–4. J-difference editing acquires two sub-experiments that differ in their treatment of the particular spin system of interest. Subtracting the two sub-experiments removes overlying signals from more concentrated compounds and reveals the target signals.
In the case of MEGA-PRESS editing of GABA and GSH, the two acquisitions are: 1) the ‘ON’ experiment, in which RF editing pulses are applied to the target resonance (either 1.9 ppm to edit GABA or 4.56 ppm to edit GSH); and 2) the ‘OFF’ experiment with the editing pulses turned off or applied at a different frequency. However, specificity of editing is limited by the selectivity of editing pulses. For example, conventional GABA editing co-edits unwanted macromolecular (MM) signals originating at 1.7 ppm with coupling to a resonance at 3 ppm (where GABA is measured), so that the combined GABA+MM peak (usually reported as ‘GABA+’) includes ~50% MM signal5, adding inter-subject variance to GABA measurements and limiting the conclusions that can be drawn from studies. MM contamination can be avoided by arranging the editing pulses symmetrically about the 1.7 ppm MM resonance, that is, at 1.9 ppm (for the GABA-ON experiment) and 1.5 ppm (for the GABA-OFF experiment). Thus, the MM resonances are inverted equally in the two experiments and the MM contribution is eliminated upon subtraction6,7.
Due to the low abundance of GABA and GSH in the brain (1–2 mmol/kg), edited MRS is time-consuming (~10 minutes per edited metabolite per region). This typically limits the number of measurements a research protocol can accommodate, and places restrictions on the degree of sophistication of MRS research study design. Although experiment durations are set by the need for signal averaging, recent developments have demonstrated the potential of multiplexed acquisition. Edited MRS data can be simultaneously acquired from more than one voxel8,9 or for more than one target compound.
Hadamard encoding and reconstruction of MEGA-edited spectroscopy (HERMES) has been presented for simultaneous spectral editing of NAA/NAAG10 and GABA+/GSH11. HERMES, edited GABA+ and GSH spectra can be acquired in a single experiment, halving the effective total scan time while preserving the signal-to-noise ratio (SNR), and allowing a greater number of edited MRS acquisitions to be considered in a research protocol.
However, like conventional MEGA-PRESS of GABA+, HERMES of GABA+/GSH suffers from co-editing of MM. Therefore, this manuscript proposes MM-suppressed HERMES for simultaneous edited measurement of GSH and GABA without MM contamination. By incorporating editing pulses at 1.5 ppm during the GABA-OFF sub-experiments, MM suppression can be implemented. We predict that MM-suppressed HERMES will be comparable with sequential MM-suppressed MEGA-PRESS of GABA and GSH, while acquiring the same amount of data in half the scan time.
Experimental
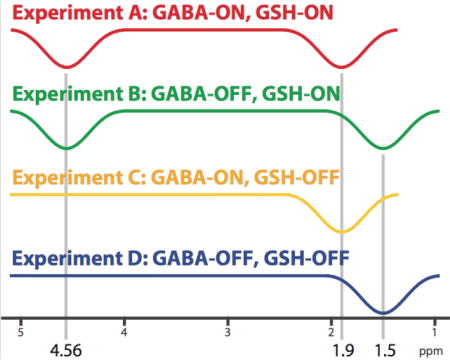
Simultaneous HERMES editing of two compounds can be thought of as two separate MEGA-PRESS experiments with different editing targets performed at the same time. Instead of two separate ON and OFF experiments tailored for each molecule, four different experiments are designed. For each compound, two of these sub-experiments are edit-ON, while two are edit-OFF, with the editing encoding of each of the two target molecules mutually orthogonal (Fig. 1a). Hadamard sum/difference combinations of the experiments yield separate edited spectra for each target without cross-contamination.
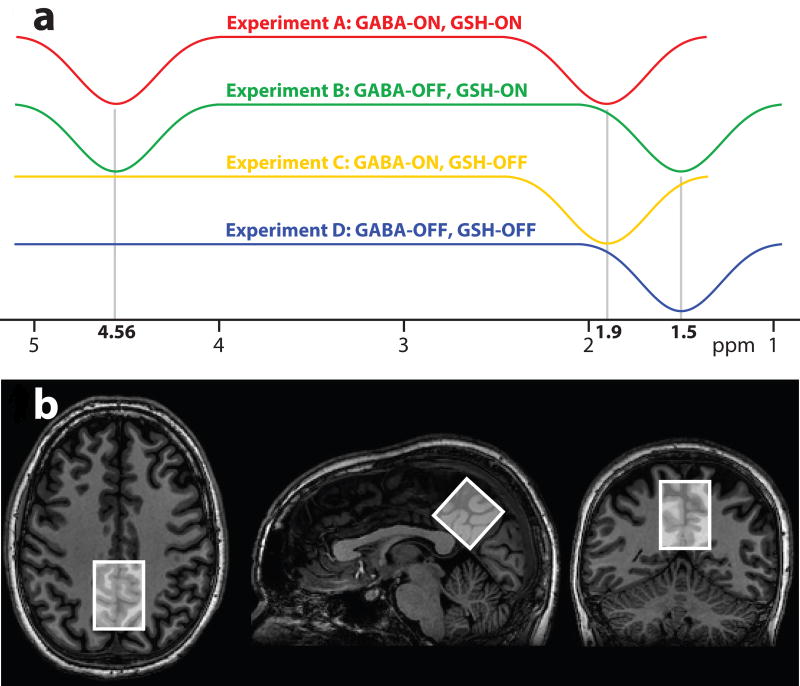
Figure 1.
(A) The MM-suppressed HERMES experiment incorporates six editing lobes, encoded in a Hadamard fashion to edit GSH and GABA with macromolecular suppression. (B) Exemplary in vivo voxel placement in the midline parietal area of the brain.
In the case of HERMES for GABA and GSH, four experiments are required: (A) GABA-ON/GSH-ON; (B) GABA-OFF/GSH-ON; (C) GABA-ON/GSH-OFF; and (D) GABA-OFF/GSH-OFF11. The Hadamard combination A+B−C−D yields a GSH-edited difference spectrum, while the combination A−B+C−D results in a GABA-edited difference spectrum. Within this framework, symmetric suppression of the GABA-co-edited MM signals can be achieved by applying editing pulses at 1.5 ppm in the GABA-OFF sub-experiments (as previously demonstrated for MEGA-PRESS).
Consequently, HERMES with MM suppression consists of the following four sub-experiments (as depicted in Fig. 1a): (A) GABA-ON/GSH-ON dual-band editing pulse applied at 1.9 ppm & 4.56 ppm; (B) GABA-OFF/GSH-ON dual-band editing pulse applied at 1.5 ppm & 4.56 ppm; (C) GABA-ON/GSH-OFF single-band editing pulse at 1.9 ppm; (D) GABA-OFF/GSH-OFF single-band editing pulse at 1.5 ppm. The GABA-edited difference combination A−B+C−D reconstructs the GABA-edited signal, but no signal from MM as these are treated the same in all four experiments and no signal from GSH as these are balanced (i.e. ON-ON+OFF-OFF). The GSH-edited difference combination A+B−C−D contains the reconstructed GSH-edited signal, but does not contain signal from MM or GABA.
Simulations
In order to confirm that the strict frequency stability requirements of symmetrical editing are met by the MM-suppressed HERMES editing scheme (i.e., that the dual-band editing pulses are congruent with their single-band equivalents), Bloch simulations were performed with the MATLAB-based toolbox FID-A12. The inversion profiles of the single- and dual-lobed editing pulses were separately determined for each of the four sub-experiments.
In vivo
Ten healthy subjects (6 males; aged 31.3 ± 8.3 y) were recruited with local IRB approval and written informed consent. MRS was performed on a Philips ‘Achieva’ scanner at 3 T field strength, using the body coil for transmit, and a 32-channel phased-array volume head coil (Invivo, Gainesville, Florida, USA) for receive. MRS data were acquired from a (33 mm)3 midline voxel in the parietal lobe of the brain (Fig. 1b), with the following common parameters: TR/TE = 2000/80 ms; 20-ms editing pulses (sinc-Gaussian for the single-band pulses, cosine-modulated sinc-Gaussian for the dual-band pulses; FWHM = 61.9 Hz for each inversion lobe); 2 kHz spectral width; 2048 data points; slice-selective excitation/refocusing pulse bandwidth 2.2/1.3 kHz. VAPOR water suppression was used for all experiments. Prospective frequency correction13 with one water-unsuppressed acquisition per eight water-suppressed transients was used in all experiments to maintain a high degree of B0 field stability. Dual-band pulses were calculated by multiplying the single-band sinc-Gaussian waveform by 2cos(π ΔΩ t), with ΔΩ being the frequency difference between the editing targets, that is, 340 Hz for the 4.56/1.9 ppm pulse, and 391 Hz for the 4.56/1.5 ppm pulse (assuming t = 0 occurs at the center of the pulse).
To demonstrate successful implementation of the MM-suppressed HERMES scheme, the following five MRS scans (total scan time: ~50 minutes) were acquired for each individual:
Experiment 1) MM-suppressed HERMES for GABA/GSH editing (GABA-ON/GSH-ON = 1.9/4.56 ppm, 320/40 water-suppressed/water-unsuppressed averages, 12 minutes) Experiment 2) HERMES for GABA+/GSH editing (GABA-ON/GSH-ON = 1.9/4.56 ppm, 320/40 water-suppressed/water-unsuppressed averages, 12 minutes)
Experiment 3) MEGA-PRESS for GABA+ editing (ON/OFF = 1.9/7.46 ppm, 160/20 water-suppressed/water-unsuppressed averages, 6 minutes)
Experiment 4) MEGA-PRESS for MM-suppressed GABA editing (ON/OFF = 1.9/1.5 ppm, 160/20 water-suppressed/water-unsuppressed averages, 6 minutes)
Experiment 5) MEGA-PRESS for GSH editing (ON/OFF = 4.56/8.00 ppm, 160/20 water-suppressed/water-unsuppressed averages, 6 minutes).
MEGA-PRESS experiments were performed with half the number of averages of the HERMES acquisitions, so that a single HERMES acquisition had the same duration as the two corresponding MEGA-PRESS experiments. GABA+ experiments 2 and 3 did not apply GABA-OFF editing at 1.5 ppm.
All data were processed using Gannet14, including frequency-and-phase correction (spectral registration15 for GABA data, choline-based alignment for GSH data). A post-processing water filter (HLSVD, Hankel-Lanczos Singular Value Decomposition) was applied to GSH spectra.
The 3 ppm GABA resonance and 3.75 ppm Glx resonance in the GABA-edited spectra were fit using Gannet with a combined model using a linear baseline estimation, two Gaussian peaks for Glx, and one Gaussian peak for GABA. The 2.95 ppm GSH resonance in the GSH-edited spectra was fit with a single Gaussian model using an adaptive baseline estimation implemented in the MATLAB-based Peakfit tool (Dr. Tom O’Haver, University of Maryland). GABA and GSH levels were subsequently quantified with respect to the unsuppressed water reference, using the default Gannet relaxation time parameters: T1,GABA / T2,GABA = 1310/88 ms14,16; T1,GSH / T2,GSH = 397/120 ms17,18; and T1,water / T2,water = 1100/95 ms (averaged from the gray and white matter values in Wansapura et al.19). Fit errors were estimated by dividing the standard deviation of the residual over the peak fit range (GABA: 2.79–3.55 ppm, GSH: 2.85–3.05 ppm) by the height of the respective fitted peak. The GABA peak integrals from the MM-suppressed experiments were divided by the GABA+ peak integrals from the conventional experiments to determine the fraction of the GABA+MM integral that can be attributed to ‘pure’ GABA. GABA signal-to-noise ratio (SNR) was calculated for the MM-suppressed HERMES and MEGA-PRESS spectra by dividing the height of the modelled Gaussian GABA peak by the standard deviation of the signal between 0 ppm and the right end of the spectrum, where a flat baseline can be expected.
Results
Simulation results of the editing pulse inversion profiles indicated correct implementation of the MM-suppressed HERMES editing scheme with the intended editing pulse offsets of 4.56 ppm, 1.9 ppm and 1.5 ppm. Specifically, the offsets of the dual-lobe inversion profiles with respect to their single-lobe equivalents were small (<1 Hz, see Fig. S1 in Supplementary Material). These results establish that the proposed pulses meet the requirements for GSH editing and GABA editing with MM suppression to be performed independently and simultaneously.
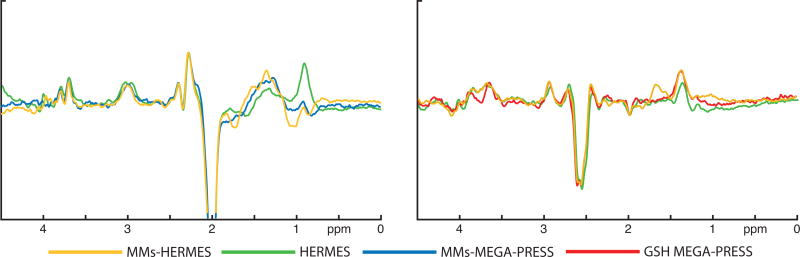
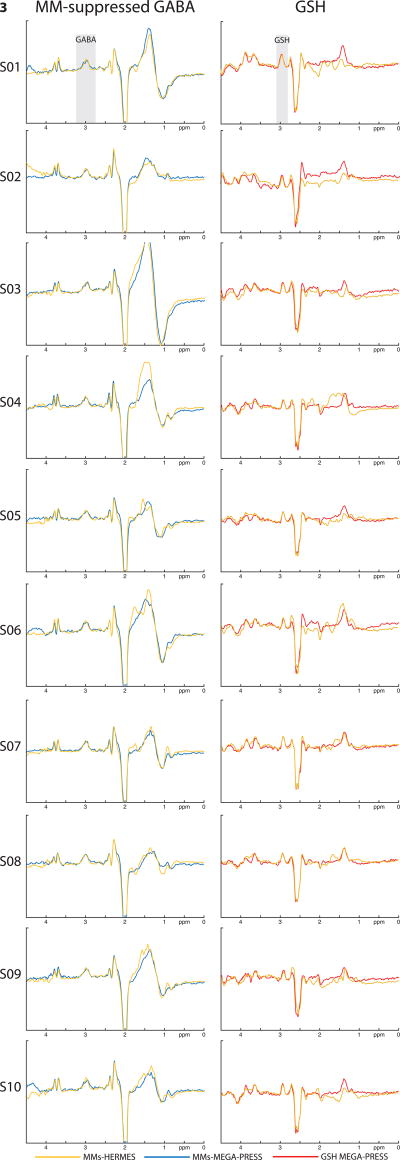
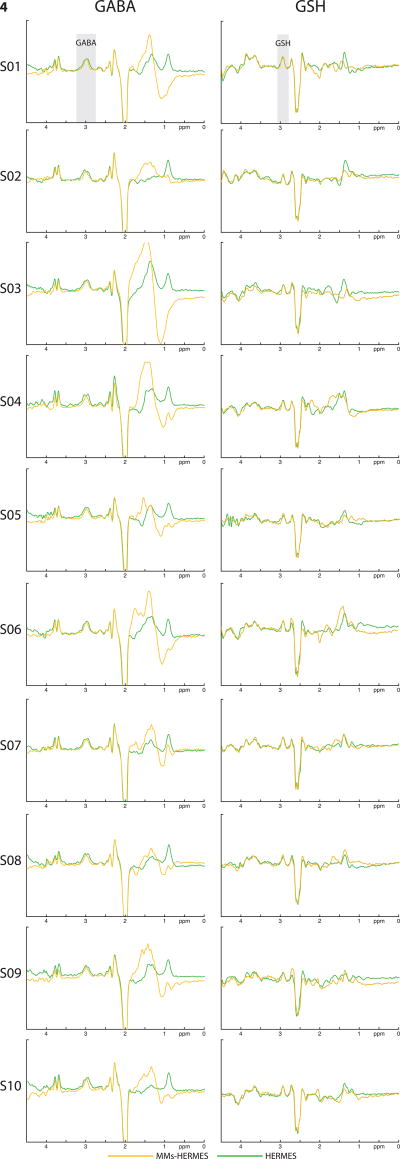
Exemplary MM-suppressed GABA-edited and GSH-edited HERMES spectra (orange), conventional GABA-edited and GSH-edited HERMES spectra (green), and MM-suppressed GABA-edited (blue) and GSH-edited MEGA-PRESS spectra (red) are overlaid for one subject in Fig. 2. MM-suppressed GABA-edited and GSH-edited HERMES spectra (orange) and MM-suppressed GABA-edited (blue) and GSH-edited MEGA-PRESS spectra (red) are further shown for each of the ten subjects in Fig. 3. Likewise, Fig. 4 shows an overlay of the GABA-edited and GSH-edited MM-suppressed HERMES spectra (orange) with the GABA+-edited and GSH-edited conventional HERMES (green) for all ten subjects. For better visibility of the agreement between similar editing modalities, the MM-suppressed and conventional experiments are presented separately in Figs. 3 and 4.
Figure 2.
Exemplary MM-suppressed HERMES, conventional HERMES, and conventional MEGA-PRESS spectra from one subject. Left: MM-suppressed GABA-edited HERMES (orange, Experiment 1), GABA+ conventional HERMES (green, Experiment 2), and MM-suppressed GABA-edited MEGA-PRESS (blue, Experiment 4) spectra. Right: GSH-edited MM-suppressed HERMES (orange, Experiment 1), conventional HERMES (green, Experiment 2), and MEGA-PRESS (red, Experiment 5) spectra.
Figure 3.
MM-suppressed HERMES and conventional MEGA-PRESS spectra of all 10 healthy subjects. Left column: MM-suppressed GABA-edited HERMES (orange, Experiment 1) and MEGA-PRESS (blue, Experiment 4) spectra; right column: GSH-edited MM-suppressed HERMES (orange, Experiment 1) and MEGA-PRESS (red, Experiment 5) spectra.
Figure 4.
MM-suppressed HERMES and conventional HERMES spectra of all 10 healthy subjects. Left column: MM-suppressed GABA-edited HERMES (orange, Experiment 1) and GABA+ conventional HERMES (green, Experiment 2) spectra; right column: GSH-edited MM-suppressed HERMES (orange, Experiment 1) and conventional HERMES (green, Experiment 2) spectra.
Results of the quantitative analysis are summarized in Table 1. Mean GABA estimates were 1.10 ± 0.15 i.u. for MM-suppressed HERMES, and 1.13 ± 0.09 i.u. for MEGA-PRESS (mean difference −0.03 ± 0.11 i.u., paired t-test: p = 0.42). The mean GABA/GABA+ ratio was 0.60 ± 0.06 for the HERMES experiments, and 0.67 ± 0.08 for the MEGA-PRESS experiments. Mean GSH estimates were 0.59 ± 0.12 i.u. for MM-suppressed HERMES, and 0.66 ± 0.09 i.u. for MEGA-PRESS (mean difference −0.07 ± 0.11 i.u., paired t-test: p = 0.07). Mean SNR improvement of MM-suppressed HERMES over MEGA-PRESS was 1.45 ± 0.25 for GABA and 1.32 ± 0.24 for GSH, that is, close to the expected SNR improvement of √2 resulting from the doubled number of acquired averages.
Table 1.
Summary of quantitative metabolite concentration estimation. Water-scaled metabolite levels are provided in institutional units (i.u.).
| GABA | GABA+ | GSH | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MMs-HERMES | Fit error (%) | MEGA-PRESS | Fit error (%) | HERMES | Fit error (%) | MEGA-PRESS | Fit error (%) | MMs-HERMES | Fit error (%) | HERMES | Fit error (%) | MEGA-PRESS | Fit error (%) | |
| 01 | 1.15 | 11.0 | 1.17 | 7.0 | 1.89 | 10.4 | 1.72 | 4.4 | 0.69 | 1.4 | 0.71 | 5.7 | 0.75 | 4.1 |
| 02 | 0.89 | 6.7 | 0.93 | 7.2 | 1.75 | 10.6 | 1.46 | 5.1 | 0.45 | 2.8 | 0.43 | 4.3 | 0.56 | 5.9 |
| 03 | 1.10 | 8.1 | 1.23 | 7.2 | 1.69 | 10.6 | 1.62 | 5.1 | 0.57 | 2.8 | 0.51 | 5.1 | 0.65 | 5.9 |
| 04 | 1.15 | 12.5 | 1.09 | 10.5 | 2.10 | 9.9 | 1.99 | 6.6 | 0.59 | 3.3 | 0.66 | 5.9 | 0.60 | 2.4 |
| 05 | 1.03 | 15.1 | 1.12 | 10.8 | 1.80 | 10.7 | 1.57 | 8.5 | 0.58 | 2.7 | 0.51 | 10.9 | 0.54 | 8.0 |
| 06 | 1.00 | 8.1 | 1.10 | 11.5 | 1.85 | 14.1 | 1.77 | 6.0 | 0.61 | 5.2 | 0.66 | 8.4 | 0.63 | 2.7 |
| 07 | 1.44 | 6.3 | 1.22 | 6.4 | 2.13 | 7.2 | 1.86 | 7.2 | 0.84 | 2.3 | 0.81 | 3.9 | 0.79 | 4.1 |
| 08 | 1.08 | 14.7 | 1.12 | 10.3 | 1.71 | 13.8 | 1.62 | 7.3 | 0.59 | 3.5 | 0.57 | 7.5 | 0.67 | 2.7 |
| 09 | 1.21 | 9.0 | 1.19 | 12.5 | 1.80 | 13.7 | 2.02 | 8.4 | 0.44 | 5.5 | 0.71 | 6.2 | 0.78 | 6.4 |
| 10 | 0.98 | 11.7 | 1.14 | 9.0 | 1.77 | 6.3 | 1.41 | 7.0 | 0.50 | 4.4 | 0.53 | 4.9 | 0.60 | 3.8 |
Discussion
Hadamard encoding of J-difference editing with HERMES allows for the simultaneous, separable detection of multiple target metabolites. Here, we have demonstrated the successful implementation of MM-suppressed GABA editing and GSH editing in the same four-step HERMES framework. As expected, signal-to-noise is improved by approximately √2 for the multiplexed HERMES experiment compared to two sequential single-metabolite experiments of the same total duration. Good agreement is seen, qualitatively between the spectra of simultaneously acquired MM-suppressed HERMES and their MEGA-edited equivalents, and between quantitative measurements from automated processing. These results indicate successful incorporation of MM suppression into multiplexed editing, removing one of the major shortcomings of conventional HERMES of GSH and GABA+.
Comparing the MM-suppressed and GABA+ data acquired from a midline parietal region, the MM-suppressed GABA integral is 60–67% of the GABA+MM peak, measured with a conventional editing scheme and 20-ms editing pulses. This is broadly consistent with a previous study that reported MM fractions of 50 ± 14 %5, comparing MM-suppressed measurements to GABA+ with 14-ms editing pulses – shorter editing pulses co-edit more MM signal. In a similar study, Mikkelsen et al. observed MM fractions between 57 % (anterior cingulate) and 52 % (occipital cortex), again using shorter 16-ms editing pulses20. The aforementioned studies also observed no consistent relationship between GABA and GABA+ levels, indicating strong variability of the MM contribution across brain regions and subjects.
Like simultaneous NAA/NAAG10 and GABA+/GSH11 editing, the MM-suppressed HERMES scheme is compatible with simultaneous data acquisition from two different brain regions. The Parallel Reconstruction In Accelerated Multivoxel (PRIAM) approach is based on dual-band excitation and separation of the spectra using phased-array coil sensitivity profiles8,9. While multi-voxel encoding with PRIAM is performed within a single TR, multi-metabolite encoding with HERMES happens between consecutive TR. Encoding for multiple compounds and multiple regions is therefore orthogonal, leading to a potential fourfold increase in data acquisition rates, as a single 11-min MM-suppressed HERMES-PRIAM scan acquires MM-suppressed GABA- and GSH-edited spectra from two brain regions. To achieve the same amount of information without SNR penalty, four consecutive conventional 11-min MEGA-PRESS experiments would be required.
Suppression of MM co-editing, while desirable, is hampered by the extreme vulnerability of symmetrical editing to frequency instabilities, as previously demonstrated for MEGA-PRESS21. Very stable magnetic field conditions are therefore obligatory, either from stable hardware and compliant participants or active frequency adjustments (e.g., by prospective frequency correction using interleaved water reference scans13, as applied in this paper).
While our work shows that it is possible to perform J-difference editing of two species in a single experiment, this does require a more complex experiment, that is, a four-step as opposed to a two-step editing scheme. However, the more complex experiment can be thought of as consisting of two independent MEGA-PRESS experiments (for GABA and GSH) occurring at the same time, and the impact of subject motion on the data will be the same as for a MEGA-PRESS experiment with the same timing. However, post-processing frequency-and-phase correction is always performed on the individual transients to improve linewidth and to reduce subtraction artefacts resulting from frequency drift and signal phasing errors. Compared to MEGA-PRESS, such correction is both more necessary (as it is a four-step rather than two-step editing scheme) and more challenging (the four sub-experiments differentially saturate key spectral features). Several sophisticated methods for the alignment of MEGA-edited GABA MRS data have been introduced in the past, most notably using the creatine signal as a frequency and phase reference, and spectral registration, an approach based on frequency-and-phase correction of the full time-domain data15,22. Alignment for GSH-edited spectra has also received some attention23. While spectral registration yielded convincing GABA-edited HERMES and MEGA-PRESS spectra, the best results for GSH-edited MM-suppressed HERMES and MEGA-PRESS spectra were achieved using frequency-and-phase correction based on modeling the choline peak. Despite these efforts, several spectra continue to exhibit subtraction artefacts, which also remained in a number of GABA-edited spectra, as indicated by considerable fit errors in some cases, resulting from calculating the residual over a range including the potential choline subtraction artefact at 3.2 ppm. While differences between the single- and dual-metabolite experiments in the estimates of GSH did not reach statistical significance (p = 0.07), the MEGA-PRESS estimates showed a tendency to be slightly higher than their HERMES equivalents, further indicating that the various frequency-and-phase correction approaches may introduce a certain bias into metabolite estimation. Advanced alignment routines for multiplexed experiments (e.g., two-step approaches using two different reference resonances, or spectral registration within each sub-experiment followed by additional alignment) are expected to improve their quantitative results, and agreement with single-metabolite MEGA-PRESS experiments.
Conclusion
Symmetrical suppression of co-edited MM signal can be performed within a HERMES experiment that simultaneously edits GABA and GSH. This method improves the efficiency and specificity of edited MRS for studies of pathophysiological dysfunction of inhibitory neurotransmission and redox balance. While studying metabolite levels of both GABA and GSH in multiple brain regions with conventional edited MRS would rapidly exceed the time frame of typical research study protocols, MM-suppressed HERMES opens up new possibilities to measure both GABA and GSH, mitigating the confound of MM contamination of the GABA+ resonance.
Supplementary Material
Supplementary Figure S1: (A) Bloch simulations of the four sub-experiments demonstrate correct implementation of the MM-suppressed HERMES editing scheme. (B) Magnified portions of the inversion profiles shown in Fig. 2a. Offsets between the dual-lobed editing pulses and their single-lobed equivalents are small (< 1 Hz deviation).
Acknowledgments
The authors are grateful to Robin A. de Graaf for his permission to use his MATLAB implementation of the HLSVD filter. This work was supported by NIH grants R01 EB016089, R01 MH106564, and P41 EB015909.
Abbreviations
- GSH
Glutathione
- GABA
γ-aminobutyric acid
- MM
macromolecules
- MEGA-PRESS
Mescher-Garwood Point Resolved Spectroscopy
- HERMES
Hadamard encoding and reconstruction of MEGA-edited Spectroscopy
- SNR
signal-to-noise ratio
- NAA
n-acetylaspartate
- NAAG
n-acetylaspartylglutamate
References
- 1.Puts NAJ, Edden RAE. In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog Nucl Magn Reson Spectrosc. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terpstra M, Marjanska M, Henry P-G, Tkáč I, Gruetter R. Detection of an antioxidant profile in the human brain in vivo via double editing with MEGA-PRESS. Magn Reson Med. 2006;56(6):1192–1199. doi: 10.1002/mrm.21086. [DOI] [PubMed] [Google Scholar]
- 3.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90(12):5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266–272. doi: 10.1002/(SICI)1099-1492(199810)11:6<266::AID-NBM530>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Harris AD, Puts NAJ, Barker PB, Edden RAE. Spectral-editing measurements of GABA in the human brain with and without macromolecule suppression. Magn Reson Med. 2014;74(6):1523–1529. doi: 10.1002/mrm.25549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry P-G, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn Reson Med. 2001;45(3):517–520. doi: 10.1002/1522-2594(200103)45:3<517::AID-MRM1068>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Edden RAE, Puts NAJ, Barker PB. Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magn Reson Med. 2012;68(3):657–661. doi: 10.1002/mrm.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boer VO, Klomp DWJ, Laterra J, Barker PB. Parallel reconstruction in accelerated multivoxel MR spectroscopy. Magn Reson Med. 2015;74(3):599–606. doi: 10.1002/mrm.25718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oeltzschner G, Puts NAJ, Chan KL, Boer VO, Barker PB, Edden RAE. Dual-volume excitation and parallel reconstruction for J-difference-edited MR spectroscopy. Magn Reson Med. 2017;77(1):16–22. doi: 10.1002/mrm.26536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan KL, Puts NAJ, Schär M, Barker PB, Edden RAE. HERMES: Hadamard encoding and reconstruction of MEGA-edited spectroscopy: HERMES. Magn Reson Med. 2016;76(1):11–19. doi: 10.1002/mrm.26233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saleh MG, Oeltzschner G, Chan KL, et al. Simultaneous edited MRS of GABA and glutathione. NeuroImage. doi: 10.1016/j.neuroimage.2016.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)—An open source, MATLAB-based toolkit. Magn Reson Med. 2015 Dec; doi: 10.1002/mrm.26091. [DOI] [PubMed] [Google Scholar]
- 13.Edden RAE, Oeltzschner G, Harris AD, et al. Prospective frequency correction for macromolecule-suppressed GABA editing at 3T. J Magn Reson Imaging. 2016 May; doi: 10.1002/jmri.25304. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40(6):1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2015;73(1):44–50. doi: 10.1002/mrm.25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puts NAJ, Barker PB, Edden RAE. Measuring the longitudinal relaxation time of GABA in vivo at 3 tesla. J Magn Reson Imaging. 2013;37(4):999–1003. doi: 10.1002/jmri.23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi I-Y, Lee P. Doubly selective multiple quantum chemical shift imaging and T1 relaxation time measurement of glutathione (GSH) in the human brain in vivo. NMR Biomed. 2013;26(1):28–34. doi: 10.1002/nbm.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheidegger M, Hock A, Fuchs A, Henning A. Proc. Intl. Soc. Magn. Reson. Med. Vol. 23. Milan: 2014. T2 relaxation times of 18 brain metabolites determined in 83 healthy volunteers in vivo; p. 2947. [Google Scholar]
- 19.Wansapura JP, Holland SK, Dunn RS, Ball WS. NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging. 1999;9(4):531–538. doi: 10.1002/(SICI)1522-2586(199904)9:4<531::AID-JMRI4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 20.Mikkelsen M, Singh KD, Sumner P, Evans CJ. Comparison of the repeatability of GABA-edited magnetic resonance spectroscopy with and without macromolecule suppression. Magn Reson Med. 2016;75(3):946–953. doi: 10.1002/mrm.25699. [DOI] [PubMed] [Google Scholar]
- 21.Harris AD, Glaubitz B, Near J, et al. Impact of frequency drift on gamma-aminobutyric acid-edited MR spectroscopy. Magn Reson Med. 2014;72(4):941–948. doi: 10.1002/mrm.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans CJ, Puts NAJ, Robson SE, et al. Subtraction artifacts and frequency (Mis-)alignment in J-difference GABA editing. J Magn Reson Imaging. 2013;38(4):970–975. doi: 10.1002/jmri.23923. [DOI] [PubMed] [Google Scholar]
- 23.An L, Zhang Y, Thomasson DM, et al. Measurement of glutathione in normal volunteers and stroke patients at 3T using J-difference spectroscopy with minimized subtraction errors. J Magn Reson Imaging. 2009;30(2):263–270. doi: 10.1002/jmri.21832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: (A) Bloch simulations of the four sub-experiments demonstrate correct implementation of the MM-suppressed HERMES editing scheme. (B) Magnified portions of the inversion profiles shown in Fig. 2a. Offsets between the dual-lobed editing pulses and their single-lobed equivalents are small (< 1 Hz deviation).