Abstract
OBJECT
Decompression surgery followed by adjuvant radiotherapy is an effective therapy for preservation or recovery of neurologic function and achieving durable local disease control in patients suffering from metastatic epidural spinal cord compression. The authors examine the outcomes of postoperative image-guided intensity-modulated radiation therapy (IG-IMRT) delivered as single-fraction or hypofractionated stereotactic radiosurgery (SRS) for achieving long-term local tumor control.
METHODS
A retrospective chart review identified 186 patients with epidural spinal cord compression from spinal metastases who were treated with surgical decompression, instrumentation, and postoperative radiation delivered as either single-fraction SRS (24 Gy) in 40 patients (22%), high-dose hypofractionated SRS (24-30 Gy in 3 fractions) in 37 patients (20%), or low-dose hypofractionated SRS (18-36 Gy in 5 or 6 fractions) in 109 patients (58%). The relationships between postoperative adjuvant SRS dosing and fractionation, patient characteristics, tumor histology-specific radiosensitivity, grade of epidural spinal cord compression, extent of surgical decompression, response to preoperative radiotherapy, and local tumor control were evaluated by competing risks analysis.
RESULTS
The total cumulative incidence of local progression was 16.4% one year after SRS. Multivariate Gray’s competing risks analysis revealed a significant improvement in local control with high-dose hypofractionated SRS (4.1% cumulative incidence of local progression at 1 year; hazard ratio = 0.12, p = 0.04) as compared to low-dose hypofractionated SRS (22.6% local progression at 1 year; HR = 1). Although univariate analysis demonstrated a trend towards greater risk of local progression for patients that failed preoperative cEBRT (22.2% local progression at 1 year, HR = 1.96, p = 0.07) compared to patients who did not receive any preoperative radiotherapy (11.2% local progression at 1 year, HR = 1), this association was not confirmed with multivariate analysis. No other variable significantly correlated with progression-free survival, including radiation sensitivity of tumor histology, grade of epidural spinal cord compression, extent of surgical decompression, or gender.
CONCLUSIONS
Postoperative adjuvant SRS following epidural spinal cord decompression and instrumentation is a safe and effective strategy for establishing durable local tumor control regardless of tumor histology-specific radiosensitivity. Patients who received high-dose hypofractionated SRS demonstrated one-year local progression rates less than 5% (95% CI: 0-12.2%), which were superior to the results of low-dose hypofractionated SRS. The local progression rate after single-fraction SRS was less than 10% (95% CI: 0-19.0%).
Keywords: Spinal Metastases, Radiotherapy, Radiosurgery, Spinal Cord Compression, Surgery
Introduction
The aim of therapy in the treatment of metastatic spine tumors is palliative with the goals of improving or maintaining neurologic function, achieving spine stability, relieving pain, and providing durable tumor control. In our institution, the primary indications for surgery are relief of high-grade epidural spinal cord compression (ESCC) resulting from tumors radioresistant to conventional external beam radiation (cEBRT) or gross spinal instability.1,6 Multiple series have demonstrated that surgery is effective for addressing neurologic, mechanical stability, and pain issues, but very few have examined the ability to achieve durable control. With improvements in systemic therapy, patients are living longer, thus placing a greater emphasis on the need to prevent local tumor recurrence and spinal cord compression. Conventional EBRT, such as 30 Gy delivered in 10 fractions, has traditionally been used in the postoperative setting, but high local recurrence rates have been observed in up to 70% of patients at one year.18 Modern image-guided radiation methods allow precise delivery of high radiation doses administered as stereotactic radiosurgery, also known as stereotactic body radiation therapy (SBRT). SRS typically refers to high-dose, conformal radiation delivered in 1-5 fractions with daily image guidance. SRS as definitive therapy in patients with minimal or no ESCC has demonstrated response rates of 85-95% even in tumors considered radioresistant to cEBRT, such as renal cell carcinoma, melanoma, and sarcoma.9–12,29 Based on these improved response rates in the upfront setting, a number of centers have begun to explore the use of adjuvant SRS following surgical decompression of high-grade ESCC in order to achieve better tumor control than that seen with cEBRT and potentially to reduce the aggressiveness of tumor resection due to the expectation that residual tumor can be controlled with cytotoxic doses of radiation that spare the spinal cord.23,25–27
Moulding et al. previously reported a pilot study from our institution of 21 patients who underwent “separation surgery” in which the thecal sac was decompressed by limited posterolateral tumor resection and posterior segmental instrumentation.23 This limited tumor resection was followed by postoperative single-fraction SRS, with doses ranging from 18 to 24 Gy. The overall estimated one-year local progression risk was 9.5% and patients receiving high-dose single-fraction SRS (24 Gy) had a lower progression risk compared to those receiving low-dose single-fraction SRS (18 to 21 Gy), 6.3% and 20%, respectively. Given the improved outcomes of high-dose, the present series examines only those single-fraction SRS patients treated with 24 Gy. Additionally, in our institution, re-irradiated or large volume tumors are treated with hypofractionated SRS delivered in 3 to 5 fractions. Over the course of this study, hypofractionated SRS radiation schedules were escalated from low-dose 20-30 Gy in 5 fractions prior to 2008 to high-dose 24-30 Gy in 3 fractions. This study reports the local tumor control and toxicity for patients who underwent “separation surgery” followed by 24 Gy single-fraction SRS, high-dose hypofractionated SRS, or low-dose hypofractionated SRS.
Methods
Study Design
A retrospective analysis was undertaken of all patients treated at Memorial Sloan-Kettering Cancer Center (MSKCC) between 2002 and 2011 who harbored spinal metastases and underwent surgery followed by SRS. This study was approved by MSKCC’s institutional review board under approval number WA0274-11. All patients were reviewed by the Memorial Sloan-Kettering Cancer Center spine tumor service during the weekly multidisciplinary clinic or tumor board. Exclusion criteria included patients with a post-RT follow-up less than 1 month (n = 15) or adjuvant low-dose single-fraction radiation (n = 4). The decision to operate was made using the NOMS decision framework with the primary operative indications being high-grade ESCC from tumors radioresistant to cEBRT or gross spinal instability that was not amenable to percutaneous cement augmentation.3 Patients with high-grade ESCC underwent surgical decompression in order to provide a separation between the tumor and the spinal cord, thereby enabling the safe delivery of a cytotoxic radiation dose to the tumor while avoiding spinal cord toxicity and radiation-induced myelopathy (Figure 1).
Figure 1.
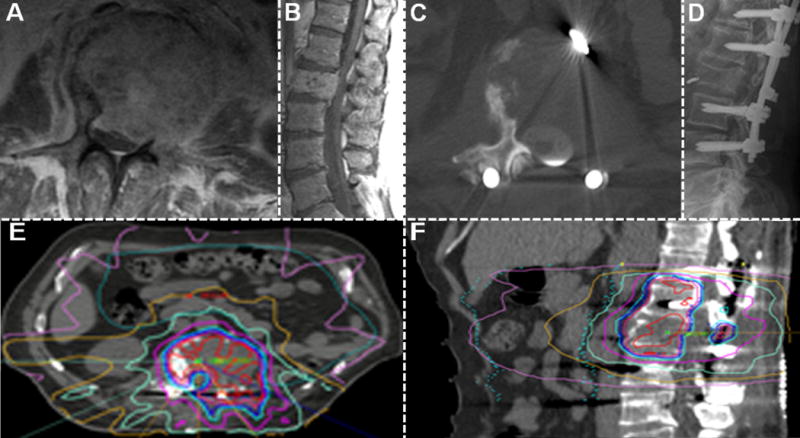
Treatment of a 66 year-old man with metastatic L2 renal cell carcinoma. Initial T1 post-contrast MRI demonstrated Grade 3 epidural spinal cord compression (A axial, B sagittal). The patient was neurologically intact. The patient then underwent “separation surgery” to decompress the spinal cord and CSF space, followed by instrumentation (C axial postoperative CT myelogram, D postoperative x-ray). Subsequently, the postoperative CT myelogram was used to guide planning for adjuvant high-dose hypofractionated SRS (E axial, F sagittal).
Charts and imaging were reviewed in order to evaluate the association of progression-free (PFS) and overall survival (OS) with tumor histology-specific radiosensitivity, preoperative radiotherapy, postoperative radiation dose and fractionation, degree of pre- and postoperative epidural tumor extension. The beginning of the OS and PFS time intervals was defined as the completion date of RT.
Surgery
All patients underwent “separation surgery” accomplished via a posterolateral laminectomy including a unilateral or bilateral facetectomy using a high-speed 3 mm matchstick bur, as previously described.28 Epidural tumor was resected circumferentially starting from normal dural planes. The posterior longitudinal ligament was resected in order to achieve a margin on the anterior dura and to ensure spinal cord decompression. Typically, a partial vertebral body resection was accomplished, but no attempt was made to resect aggressively either gross vertebral body or paraspinal tumor. Given the limited vertebral body resection, anterior reconstruction was rarely required. When greater than 50% of the vertebral body was resected, the discs were removed and vertebral body replacement was accomplished from a posterior only approach using either polymethylmethacrylate (PMMA) with Steinman pin or through placement of a titanium or PEEK carbon fiber cage.
SRS
Within two to four weeks of separation surgery either single-fraction (24 Gy) or hypofractionated SRS was administered. Hypofractionated SRS was further classified as low-dose (median total dose of 30 Gy in 5 or 6 fractions, total dose range 18-36 Gy) or high-dose (median total dose of 27 Gy in 3 fractions, total dose range 24-30 Gy). The preoperative MRI was used to delineate the gross tumor volume (GTV), which included the intraosseous, epidural, and paraspinal components. The GTV coverage was contoured to the preoperative tumor volume rather than the postoperative residual tumor. A postoperative CT myelogram was used to plan the tumor volume for radiation treatment and to define the dural margin allowing a clear delineation of the cerebrospinal fluid (CSF) space and the spinal cord in the setting of spinal instrumentation. The clinical tumor volume (CTV) was an expansion of the GTV contoured in order to account for microscopic tumor. For example, the assumption is made that the entire vertebral body is at risk for tumor infiltration even though MR imaging shows a discrete lesion.23 Thus, the CTV includes the entire volume of the vertebral body even in the setting of partial radiographic involvement. The planning target volume (PTV) typically represents a 2 mm expansion of the CTV that accounts for uncertainties in radiation set-up and delivery. All of the treatment volumes were contoured so they did not transgress the dural margins, as defined on the postoperative CT myelogram.
Imaging
Patients were imaged with serial gadolinium-enhanced MR imaging at 4 to 6 month intervals following radiation in order to monitor for tumor recurrence or sooner if symptomatic recurrence was suspected. Tumor recurrence was determined radiographically with MRI or CT myelogram, as indicated. Additional images reviewed include chest, abdomen and PET CTs. Actual imaging and reports of all patients were reviewed by a neuroradiologist (EL) and a neurosurgical member of the spine team, who were blinded to the treatment that the patients received. All measurements were made by consensus and in consultation with the original imaging reports, using the validated 6-point ESCC scale.2
Statistical Analysis
Univariate analysis of OS was performed using a proportional hazards model; while univariate and multivariate analyses of PFS were performed using Gray’s competing risks method.15 P-values < 0.05 were considered statistically significant and p-values < 0.10 were considered to show a trend toward association. For the purpose of variable selection for multivariate analysis, p < 0.10 was used as a threshold due to the small number of local progression events. The statements regarding the difference in PFS are based on the statistical comparison of the entire Gray’s competitive risks functions, rather than individual time points. With a median follow-up for survivors of 11 months (range 1.5 – 63.2), 1-year cumulative local progression rates were calculated for the entire study population. R package cmprsk version 2.9.2 and SAS version 9.2 (Cary, NC) software were used for statistical analysis.
Results
Patient and Tumor Characteristics
A total of 203 patients were identified that fit the inclusion criteria, of which 19 were excluded for inadequate follow-up (n = 15) or low-dose single-fraction radiation (n = 4). Univariate analysis failed to reveal significant associations between overall survival and either gender, tumor radiation sensitivity, preoperative radiation type, postoperative radiation fractionation or dose, degree of pre- or postoperative ESCC, or tumor resection extent. Among the 186 evaluated patients, radiographic high-grade ESCC was present preoperatively in 136 patients (73.1%; Table 1). Tumors were categorized as either radiosensitive or resistant to cEBRT. The most prevalent primary radiosensitive tumors were breast and prostate, whereas radioresistant tumors included colorectal, non-small cell lung, renal cell carcinoma, and sarcoma (Table 2). Patients were followed for an overall median of 7.6 months (range 1.0 – 66.4) following SRS. Among the patients who were alive at the conclusion of the analysis, the median follow-up time was 11.0 months (range 1.5 – 63.2, n = 54). The median survival among patients who died was 6.1 months (range 1.0 – 66.4, n = 132).
TABLE 1.
Tumor characteristics and local progression*
| Characteristic | Local Progression†
|
||
|---|---|---|---|
| Total | Yes | No | |
| tumor location | |||
| cervical | 15 | 4 (26.7) | 10 (66.7) |
| cervicothoracic | 7 | 3 (42.9) | 3 (42.9) |
| thoracic | 107 | 18 (16.8) | 78 (72.9) |
| thoracolumbar | 10 | 1 (10.0) | 6 (60.0) |
| lumbar | 47 | 8 (17.0) | 34 (72.3) |
| preop ESCC grade‡ | |||
| no compression (0, 1a) | 6 | 1 (16.7) | 4 (66.7) |
| dural compression (1b, 1c) | 40 | 9 (22.5) | 31 (77.5) |
| cord compression (2, 3) | 136 | 23 (16.9) | 95 (69.9) |
Values in data cells represent numbers of cases (%). The values in parentheses under preop ESCC represent ESCC grades.
In 21 cases, the patients died without imaging follow-up sufficient to determine presence or absence of local progression.
Preoperative imaging for analysis was unavailable in 4 cases.
TABLE 2.
| Local Progression | |||||
|---|---|---|---|---|---|
| Total | Yes | No | |||
|
| |||||
| n | n | (%) | n | (%) | |
| Radiation Sensitive | 42 | 9 | (21.4) | 26 | (61.9) |
|
| |||||
| Breast | 11 | 1 | (9.1) | 9 | (81.8) |
|
| |||||
| Prostate | 24 | 7 | (29.2) | 12 | (50.0) |
|
| |||||
| Other | 7 | 1 | (16.7) | 5 | (83.3) |
|
| |||||
| Radiation Resistant | 144 | 25 | (17.4) | 103 | (71.5) |
|
| |||||
| Colorectal | 15 | 1 | (6.7) | 10 | (66.7) |
|
| |||||
| Hepatocellular | 6 | 1 | (16.7) | 4 | (66.7) |
|
| |||||
| Lung, non-small cell | 15 | 3 | (20.0) | 10 | (66.7) |
|
| |||||
| Melanoma | 9 | 0 | (0) | 9 | (100) |
|
| |||||
| Renal cell | 41 | 8 | (19.5) | 31 | (75.6) |
|
| |||||
| Sarcoma | 33 | 7 | (21.2) | 25 | (75.8) |
|
| |||||
| Squamous cell | 3 | 1 | (33.3) | 2 | (66.7) |
|
| |||||
| Thyroid | 5 | 2 | (40.0) | 3 | (60.0) |
|
| |||||
| Other | 17 | 2 | (11.8) | 11 | (64.7) |
Surgery and SRS
A median of 2 spinal levels (range 1-8 levels) were decompressed and then treated with either low-dose hypofractionated SRS (n = 109, 58.6%), high-dose hypofractionated SRS (n = 37, 19.9%), or single-fraction SRS (n = 40, 21.5%). (Table 3) The RT regimen was completed within a median of 1.6 months from the date of surgery. There were no neurological complications due to radiotherapy and four patients underwent re-operation due to hardware failure, one of whom had local progression.
TABLE 3.
Treatment characteristics and local progression*
| Characteristic | Total | Local Progression
|
|
|---|---|---|---|
| Yes | No | ||
| preop RT failure† | 91 | 21 (23.1) | 57 (62.6) |
| cEBRT | 58 | 15 (25.9) | 32(55.2) |
| hypofractionated SRS | 18 | 4 (22.2) | 14 (77.8) |
| single-fraction SRS | 7 | 1 (14.3) | 6 (85.7) |
| surgical decompression | |||
| age at surgery (yrs) | |||
| median | 58.9 | 61.5 | 57.9 |
| range | 14.8–81.4 | 16.2–79.4 | 14.8–81.4 |
| no. of spinal levels | |||
| median | 2 | 3 | 2 |
| range | 1–8 | 1–8 | 1–6 |
| time to RT (mos) | |||
| median | 1.6 | 1.5 | 1.8 |
| range | 0.4–46.1 | 0.6–20.3 | 0.4–46.1 |
| postop ESCC | |||
| no compression (0, 1a) | 67 | 10 (14.9) | 50 (74.6) |
| dural compression (1b, 1c) | 98 | 19 (19.4) | 71 (72.4) |
| cord compression (2, 3) | 21 | 5 (23.8) | 10 (47.6) |
| postop adjuvant SRS | |||
| low-dose hypo | 109 | 28 (23.5) | 65 (54.6) |
| high-dose hypo | 37 | 1 (2–7) | 34(91.9) |
| single-fraction | 40 | 5(12.5) | 32 (80.0) |
| no. of spinal levels | |||
| median | 2 | 2 | 3 |
| range | 1–11 | 1–8 | 1–11 |
Values in data cells represent numbers of cases (%) unless otherwise indicated Abbreviation: hypo = hypofractionated.
The fractionation scheme of preoperative radiation was unknown in 8 patients.
Local Tumor Control
Local progression was observed in 34 (18.3%) patients at a median of 4.8 months (range 0.2 – 38.3) following SRS, while 103 (55.4%) died without local progression (median survival 5.6 months, range 1.0 – 66.4). (Table 4) The remaining 49 patients (26.3%) were alive and free of local progression at last follow-up (median 7.1 months, range 1.3 – 55.6). The cumulative incidence of local progression was 16.4% at 1 year (95% CI: 10.7 – 22.2%). Univariate analysis by Gray’s competing risks method revealed a significant association between PFS and postoperative radiation delivery scheme (hazard ratios: low-dose hypofractionated SRS = 1; high-dose hypofractionated SRS = 0.12, p = 0.04; single-fraction SRS = 0.45, p = 0.09) and a trend toward significant association between PFS and preoperative cBERT (HR: no pre-op radiation = 1; failed pre-op cEBRT = 1.96, p = 0.07; Table 5). Of the 91 patients who progressed following attempted definitive radiation therapy that ultimately required separation surgery, two (2.2%) were treated with postoperative SRS, 14 (15.4%) with high-dose hypofractionated SRS, and 75 (82.4%) with low-dose hypofractionated SRS. All 48 patients treated with postoperative low-dose hypofractionated SRS prior to 2008 had previously failed preoperative radiotherapy. The 95 patients without preoperative radiotherapy were treated with either postoperative SRS (n = 38, 40.0%), high-dose hypofractionated SRS (n = 24, 25.3%), or low-dose hypofractionated SRS (n = 36, 37.9%). When stratified according to the postoperative radiation therapy received, the 1-year cumulative local progression rates were 22.6% for low-dose hypofractionated SRS, 4.1% for high-dose hypofractionated SRS, and 9.0% for single-fraction SRS (95% CI: 14.3 – 30.8%, 0 – 12.2%, and 0 – 19.0%, respectively; Figure 2). Stratification by preoperative fractionation scheme revealed a 1-year cumulative local progression rate of 22.2% for preoperative cBERT (95% CI: 10.9 – 33.6%), compared to 11.2% for patients that did not receive preoperative radiotherapy (95% CI: 4.6 – 17.9%; Figure 3).
TABLE 4.
| Total | Local Progression | ||||
|---|---|---|---|---|---|
| Yes | No | ||||
| Patient Characteristics | n | n | (%) | n | (%) |
| Total Patients | 186 | 34 | (18.3) | 131 | (70.4) |
| Median f/u (mo; range) | 7.6 (1.0–66.4) | 4.8 | (1.0–38.3) | 6.8 | (1.0–66.4) |
| Alive at last f/u | 54 | 5 | (9.3) | 49 | (90.7) |
f/u: follow-up
TABLE 5.
Univariate competing risks analysis*
| Univariate
|
Est Cumulative 1-Yr Incidence (%)
|
|||
|---|---|---|---|---|
| Factor | HR | p Value | Value | 95% CI |
| postop adjuvant SRS | ||||
| low-dose hypo | reference | 22.6 | 14.3–30.8 | |
| high-dose hypo | 0.12 | 0.04 | 4.1 | 0–12.2 |
| single-fraction | 0.45 | 0.09 | 9.0 | 0–19.0 |
| preop RT failure | ||||
| no preop RT | reference | 11.2 | 4.6–17.9 | |
| cEBRT | 1.96 | 0.07 | 22.2 | 10.9–33.6 |
| hypo SRS | 1.84 | 0.29 | 23.8 | 2.4–45.2 |
| single-fraction SRS | 0.98 | 0.99 | 17.1 | 0–51.2 |
| radiation sensitivity | 1.23 | 0.60 | — | — |
| male sex | 0.72 | 0.34 | — | — |
| total incidence | NA | NA | 16.4 | 10.7–22.2 |
Est = Estimated; NA = not applicable.
Figure 2.
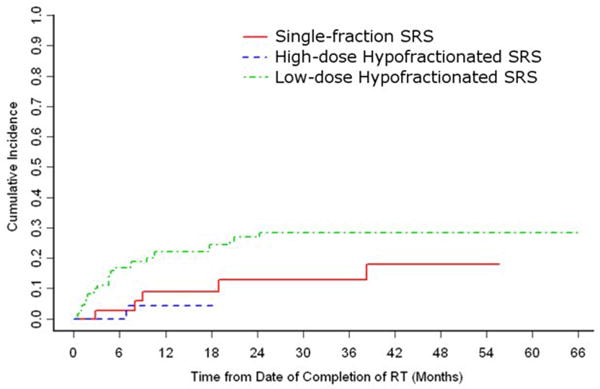
The cumulative incidence of local progression by postoperative adjuvant SRS fractionation regimen.
Figure 3.
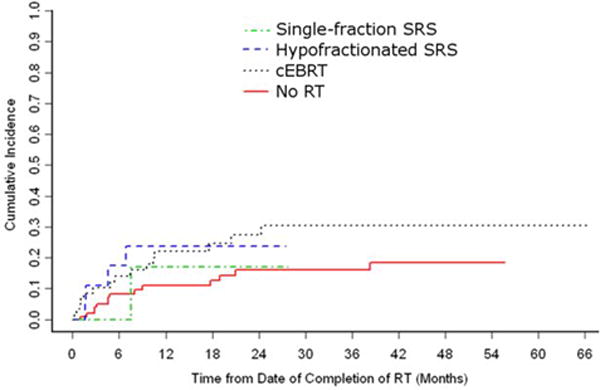
The cumulative incidence of local progression by preoperative RT fractionation regimen.
Multivariate Analysis
Preoperative and postoperative radiation variables met the selection criteria for multivariate analysis. Multivariate analysis that included the preoperative and postoperative radiation variables confirmed the significant improvement in local control after high-dose hypofractionated SRS compared to low-dose hypofractionated SRS (low-dose hypofractionated SRS HR = 1, high-dose hypofractionated SRS HR = 0.12, p = 0.04), but displayed no statistically significant difference between single-fraction SRS (HR = 0.57, p = 0.30) and low-dose hypofractionated SRS. Controlling for postoperative radiation during multivariate analysis eliminated the trend towards significance difference between the PFS in patients who did not receive preoperative radiation and patients who received preoperative cEBRT.
Discussion
Treatment paradigms for metastatic spinal tumors must incorporate a wide range of radiation, surgical, and medical options currently available. Advances in systemic therapy have significantly extended the expected survival for patients with various tumor histologies. With improved survival comes an increasing emphasis on the maintenance of quality of life and durable local tumor control. Spinal cord decompression in the setting of high-grade ESCC and restoration of mechanical stability represent the main surgical indications.1,5 In the absence of mechanical instability, tumor histology serves as a primary determinant in multi-modality treatment decisions. The main distinction among tumor histologies lies in their sensitivity to cEBRT, for example 30 Gy in 10 treatments.12,13 Tumors such as lymphoma myeloma, seminoma, breast, and prostate carcinomas are markedly radiosensitive to cEBRT.14,21,22 The remaining solid tumor histologies fall into the spectrum of moderately to highly radioresistant. In the setting of radiosensitive metastases, durable local tumor control can be reliably obtained with cEBRT regardless of the degree of epidural spinal cord compression; however, radioresistant tumors demonstrate poor response rates on the order of 30% with progression seen within 3 months of radiation.21,22 Recently, a number of studies have demonstrated improved control of radioresistant tumors with the delivery of high radiation doses, administered as single-fraction or hypofractionated SRS using image-guided, intensity modulated radiation therapy (IG-IMRT).10,29,31 We have previously reported a 90% one-year tumor control rate, regardless of tumor histology with higher single-fraction doses (24 Gy) providing control rates of 95%.23,29
The recommendation for surgical decompression as the initial treatment in the setting of high-grade ESCC and myelopathy caused by solid tumor metastases is based principally on a single prospective randomized trial and to a lesser extent on lower quality evidence provided by retrospective reviews.1,24 A systematic review of publications reporting outcomes after cEBRT in the setting of metastatic ESCC reported post-radiation ambulation rates of 31-76% and ambulation recovery rates of 16-51%.1 The rates of ambulation after surgical decompression and stabilization in patients with metastatic ESCC were significantly better, ranging between 74% and 100%, with ambulation recovery rates ranging between 57% and 82%. Patchell et al. conducted a prospective randomized trial that, to date, provides the most convincing evidence for the superiority of surgical decompression over cEBRT in patients with high-grade ESCC and myelopathy secondary to metastatic solid tumors.24 They reported significantly superior rates of overall ambulation (84 vs. 57%), maintenance of ambulation (94 vs. 74%), recovery of ambulation (62 vs. 19%), bowel and bladder continence, narcotic requirements, and survival in patients who underwent surgical decompression followed by cEBRT compared to cEBRT alone. Based on these data and expert opinion, the Spine Oncology Study Group published recommendations that patients with high-grade spinal cord compression resulting from solid tumor malignancies undergo surgical decompression followed by radiation therapy.1
The pain, functional and neurologic results of posterolateral decompression and stabilization were previously analyzed and reported by our institution, therefore we did not repeat this analysis for the current patient series and concentrated instead on the analysis of local tumor control.28 While multiple series have demonstrated that surgery for metastatic ESCC provides excellent rates of neurologic recovery and stability, very few have examined the durability of tumor control. Klekamp and Samii examined tumor control in 101 patients who underwent surgical decompression, of which 60% received cEBRT.18 Overall recurrence was 57.9% at six months, 69.3% at one year and 96% at four years, with favorable histologies to cEBRT showing more durable control. It is a vexing proposition to subject a patient to major spine operation for palliation only to have the tumor return within a few months. The deleterious effects of tumor recurrence have been adeptly documented previously.8,16,20 The failure of surgery to control metastatic disease reflects the inability to achieve negative margins based on anatomic constraints and aggressive tumor biology. With the integration of effective spinal radiation methods even for radiation resistant histologies, “separation surgery” provides effective spinal cord decompression and stabilization reducing the need for complex approaches and attempted gross total resection of spinal metastases. In order to safely administer the tumoricidal radiation doses afforded by SRS and SBRT, a small margin of 2-3 mm created by separation surgery between the tumor and the spinal cord allows a full radiation dose to the entire tumor volume while minimizing the radiation exposure to the spinal cord. Thus, in patients with radioresistant tumors causing high-grade ESCC, separation surgery is undertaken with the primary purpose of providing a small separation between the tumor and the spinal cord, but avoiding the risks associated with extensive or gross total tumor resection.
The goals of the current analysis were to determine the long-term tumor control rates after “separation surgery” for metastatic spinal tumors and to delineate the oncologic and surgical factors associated with tumor control. The overall progression rate after radiation was 16% at one year. The only factor significantly associated with local tumor progression was the postoperative radiation dose, with high-dose hypofractionated SRS resulting in a 4% local progression rate after one year, compared to the significantly higher 22% one-year local progression rate for low-dose hypofractionated SRS. Single-fraction SRS one-year local progression rate was 9%, which did not differ significantly from low-dose hypofractionated SRS. The lack of statistical significance may be due to the low number of local progression events in the SRS group, since the study is likely underpowered to adequately evaluate this difference. This finding echoes the results of previous publications where the radiation dose was observed to be inversely proportionate to recurrence rate.29
There was no association between histology-specific sensitivity to radiation, previous radiation, and the degree of pre- and postoperative epidural spinal cord compression, confirming that there is no evidence to suggest that certain tumor characteristics may portend a poor response to high-dose radiation. The tumoricidal mechanisms activated by high-dose radiation require further elucidation; however, mounting evidence indicates that these mechanisms differ from those employed by conventional radiotherapy using multiple fractions of low-dose radiation. The linear-quadratic model describes the effect of conventionally fractionated radiation, but fails to accurately predict tumor response to radiosurgery.17 Tumor xenograft experiments have shown that high radiation doses activate microvascular endothelial apoptosis, which is associated with tumor growth arrest, whereas low-dose radiation does not.7 The difference in tumor arrest mechanisms may account for the finding that SRS provides improved local tumor control irrespective of histology and size, which differs from the results observed with conventional fractionation.4,19,28,30 Because SRS provides local tumor control irrespective of the tumor volume, the extent of tumor resection loses importance as long as there is adequate separation of the tumor from vital structures to allow for optimal radiation dosimetry.
Several publications describe the results of postoperative SRS. Rock et al. administered radiosurgical treatment to 18 patients who underwent surgery for spinal metastatic tumors, with a median follow-up of 7 months.26 One patient suffered neurological deterioration secondary to rapid tumor progression, while 30% remained neurologically stable and 62% improved. The MSKCC experience with postoperative SRS in radioresistant tumors was initially described in 21 patients, in which the overall estimated incidence of local progression at one year was 9.5%, with patients who received 24 Gy doses having a one year recurrence risk of 6.3%.23 Finally, Garg et al. prospectively evaluated tumor control rates after spinal re-irradiation using hypofractionated SRS (6 Gy × 5 or 9 Gy × 3) for 59 patients with mostly radioresistant histologies that failed prior cEBRT, with more than half of the patients having undergone prior surgical intervention.8 They reported an actuarial one-year local tumor control of 76% irrespective of tumor histology. An identical one-year tumor control rate (76%) was reported by Damast et al. for patients who underwent re-irradiation with the 6 Gy × 5 paradigm at MSKCC, which was independent of histology.5 The results presented in the current analysis may demonstrate better tumor control, however, one limitation of this small sample size may be insufficient power to discern a statistical difference.
The retrospective nature of the analysis engenders several limitations. The study includes a heterogeneous population of patients with numerous tumor histologies undergoing a wide range of systemic therapies. Although the majority of chemotherapies has little effect on bone metastases, several agents may have contributed to the local control provided by SRS. However, due to the myriad of systemic treatments available, we could not effectively control for this factor. The study was not designed to objectively compare the efficacy of postoperative SRS to other available treatments such as cEBRT or more aggressive tumor excision. The results reported in this manuscript can only be compared to the results of previously published studies. Finally, our data indicate that imaging failed to confirm complete spinal cord decompression in 21 patients (11%). Whether this finding is due to the limitations of the postoperative imaging used or to the true failure to achieve spinal cord decompression is difficult to determine. Although the degree of posterior and lateral epidural decompression was always confirmed intraoperatively using direct visualization, the ventral epidural space cannot be directly visualized using the posterior approach and instead we had to rely on probing of the ventral epidural space using surgical instruments or intraoperative ultrasound. Postoperatively our patients routinely underwent CT myelography, rather than MR imaging, in order to evaluate the degree of epidural decompression. MR evaluation of the epidural space is generally limited due to instrumentation artifact, while myelography provides clear CSF definition. However, CT myelography cannot reliably differentiate residual tumor in the epidural space from postoperative blood products that may account for a portion of the patients in whom spinal cord decompression was not documented. It is interesting to note that the presence of spinal cord compression on the postoperative myelogram was not associated with local tumor progression. Further investigations are needed to address these limitations and to fully delineate their roles in establishing durable local tumor control in the spine.
Conclusions
Spinal cord decompression, spinal stabilization, and durable tumor control represent the goals of treatment of spinal metastatic tumors. While spinal cord compression secondary to radiosensitive histologies may be effectively treated with cEBRT, patients with radioresistant tumors causing high-grade spinal cord compression benefit from surgical decompression and postoperative single-fraction or hypofractionated SRS. The long-term tumor control provided by high dose-per-fraction postoperative SRS schema, which is irrespective of tumor histology-specific radiosensitivity, obviates the need for extensive tumor resection in favor of a limited spinal cord decompression and reconstitution of the CSF space around the spinal cord. Furthermore, SRS provides durable postoperative tumor control regardless of the previous radiation treatment or the degree of epidural tumor extension.
Acknowledgments
We would like to thank Cynthia Correa from the Spine Tumor Center for her invaluable clinical support, and Shahiba Ogilvie from the Spine Tumor Center for her generous administrative support.
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Bilsky MH, Laufer I, Burch S. Shifting paradigms in the treatment of metastatic spine disease. Spine. 2009;34(22 Suppl):S101–107. doi: 10.1097/BRS.0b013e3181bac4b2. [DOI] [PubMed] [Google Scholar]
- 2.Bilsky MH, Laufer I, Fourney DR, Groff M, Schmidt MH, Varga PP, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13(3):324–328. doi: 10.3171/2010.3.SPINE09459. [DOI] [PubMed] [Google Scholar]
- 3.Bilsky M, Smith M. Surgical approach to epidural spinal cord compression. Hematol Oncol Clin North Am. 2006;20(6):1307–1317. doi: 10.1016/j.hoc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Chang UK, Cho WI, Lee DH, Kim MS, Cho CK, Lee SY, et al. Stereotactic radiosurgery for primary and metastatic sarcomas involving the spine. J Neurooncol. 2012;107(3):551–557. doi: 10.1007/s11060-011-0777-0. [DOI] [PubMed] [Google Scholar]
- 5.Damast S, Wright J, Bilsky M, Hsu M, Zhang Z, Lovelock M, et al. Impact of dose on local failure rates after image-guided reirradiation of recurrent paraspinal metastases. Int J Radiat Oncol Biol Phys. 2011;81(3):819–826. doi: 10.1016/j.ijrobp.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Fisher CG, DiPaola CP, Ryken TC, Bilsky MH, Shaffrey CI, Berven SH, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine. 2010;35(22):E1221–1229. doi: 10.1097/BRS.0b013e3181e16ae2. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 8.Garg AK, Wang XS, Shiu AS, Allen P, Yang J, McAleer MF, et al. Prospective evaluation of spinal reirradiation by using stereotactic body radiation therapy: The University of Texas MD Anderson Cancer Center experience. Cancer. 2011;117(15):3509–3516. doi: 10.1002/cncr.25918. [DOI] [PubMed] [Google Scholar]
- 9.Gerszten PC, Monaco EA., 3rd Complete percutaneous treatment of vertebral body tumors causing spinal canal compromise using a transpedicular cavitation, cement augmentation, and radiosurgical technique. Neurosurg Focus. 2009;27(6):E9. doi: 10.3171/2009.9.FOCUS09184. [DOI] [PubMed] [Google Scholar]
- 10.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32(2):193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 11.Gerszten PC, Burton SA, Quinn AE, Agarwala SS, Kirkwood JM. Radiosurgery for the treatment of spinal melanoma metastases. Stereotact Funct Neurosurg. 2005;83(5-6):213–221. doi: 10.1159/000091952. [DOI] [PubMed] [Google Scholar]
- 12.Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine. 2009;34(22 Suppl):S78–92. doi: 10.1097/BRS.0b013e3181b8b6f5. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3(1):40–51. doi: 10.1002/ana.410030107. [DOI] [PubMed] [Google Scholar]
- 14.Katagiri H, Takahashi M, Inagaki J, Kobayashi H, Sugiura H, Yamamura S, et al. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys. 1998;42(5):1127–1132. doi: 10.1016/s0360-3016(98)00288-0. [DOI] [PubMed] [Google Scholar]
- 15.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13(2 Pt 1):559–565. doi: 10.1158/1078-0432.CCR-06-1210. [DOI] [PubMed] [Google Scholar]
- 16.Kim KT, Lee SH, Suk KS, Bae SC. The quantitative analysis of tissue injury markers after mini-open lumbar fusion. Spine. 2006;31(6):712–716. doi: 10.1097/01.brs.0000202533.05906.ea. [DOI] [PubMed] [Google Scholar]
- 17.Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18(4):240–243. doi: 10.1016/j.semradonc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neurochir (Wien) 1998;140(9):957–967. doi: 10.1007/s007010050199. [DOI] [PubMed] [Google Scholar]
- 19.Lovelock DM, Zhang Z, Jackson A, Keam J, Bekelman J, Bilsky M, et al. Correlation of local failure with measures of dose insufficiency in the high-dose single-fraction treatment of bony metastases. Int J Radiat Oncol Biol Phys. 2010;77(4):1282–1287. doi: 10.1016/j.ijrobp.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahadevan A, Floyd S, Wong E, Jeyapalan S, Groff M, Kasper E. Stereotactic body radiotherapy reirradiation for recurrent epidural spinal metastases. Int J Radiat Oncol Biol Phys. 2011;81(5):1500–1505. doi: 10.1016/j.ijrobp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Maranzano E, Bellavita R, Rossi R, De Angelis V, Frattegiani A, Bagnoli R, et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol. 2005;23(15):3358–3365. doi: 10.1200/JCO.2005.08.193. [DOI] [PubMed] [Google Scholar]
- 22.Maranzano E, Latini P, Perrucci E, Beneventi S, Lupattelli M, Corgna E. Short-course radiotherapy (8 Gy × 2) in metastatic spinal cord compression: an effective and feasible treatment. Int J Radiat Oncol Biol Phys. 1997;38(5):1037–1044. doi: 10.1016/s0360-3016(97)00128-4. [DOI] [PubMed] [Google Scholar]
- 23.Moulding HD, Elder JB, Lis E, Lovelock DM, Zhang Z, Yamada Y, et al. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J Neurosurg Spine. 2010;13(1):87–93. doi: 10.3171/2010.3.SPINE09639. [DOI] [PubMed] [Google Scholar]
- 24.Patchell RA, Posner JB. Neurologic complications of systemic cancer. Neurol Clin. 1985;3(4):729–750. [PubMed] [Google Scholar]
- 25.Rades D, Huttenlocher S, Bajrovic A, Karstens JH, Adamietz IA, Kazic N, et al. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int J Radiat Oncol Biol Phys. 2011;81(5):e861–868. doi: 10.1016/j.ijrobp.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 26.Rock JP, Ryu S, Shukairy MS, Yin FF, Sharif A, Schreiber F, et al. Postoperative radiosurgery for malignant spinal tumors. Neurosurgery. 2006;58(5):891–898. doi: 10.1227/01.NEU.0000209913.72761.4F. [DOI] [PubMed] [Google Scholar]
- 27.Sahgal A, Bilsky M, Chang EL, Ma L, Yamada Y, Rhines LD, et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine. 2011;14(2):151–166. doi: 10.3171/2010.9.SPINE091005. [DOI] [PubMed] [Google Scholar]
- 28.Wang JC, Boland P, Mitra N, Yamada Y, Lis E, Stubblefield M, et al. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(3):287–298. doi: 10.3171/spi.2004.1.3.0287. [DOI] [PubMed] [Google Scholar]
- 29.Yamada Y, Bilsky MH, Lovelock DM, Venkatraman ES, Toner S, Johnson J, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71(2):484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 30.Yamada Y, Cox BW, Zelefsky MJ, Lovelock DM, Kollmeier MA, Tam M, et al. An analysis of prognostic factors for local control of malignant spine tumors treated with spine radiosurgery. Int J Radiat Oncol Biol Phys. 2011;81(2):S132–S133. (Abstract) [Google Scholar]
- 31.Zelefsky MJ, Greco C, Motzer R, Magsanoc JM, Pei X, Lovelock M, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82(5):1744–1748. doi: 10.1016/j.ijrobp.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


