Abstract
HIV-1 clades (subtypes) differentially contribute to the neuropathogenesis of HIV-associated dementia (HAD) in neuroAIDS. HIV-1 envelop protein, gp120, plays a major role in neuronal function. It is not well understood how these HIV-1 clades exert these neuropathogenic differences. The N-methyl-D-aspartate (NMDA) receptor-reduced glutamine synthesis could lead to secretion of neurotoxins such as arachidonic acid (AA) which plays a significant role in the neuropathogenic mechanisms in neuroAIDS. We hypothesize that clade B and C gp120 proteins exert differential effects on human primary astrocytes by production of the neurotoxin arachidonic acid. Our results indicate that clade B gp120 significantly downregulated NMDA receptor gene and protein expression, and level of glutamine while increasing expression of prostaglandin E2 (PGE2) and thromboxane A2 receptor (TBXA2 R) compared to HIV-1 clade C gp120 protein. Thus, our studies for the first time demonstrate that HIV-1 clade B-gp120 protein appears to induce higher levels of expression of the neuropathogenic molecule cyclooxygenase-2 (COX-2)-mediated arachidonic acid by-products, PGE2, and TBXA2 R compared to HIV-1 clade C gp120 protein. These studies suggest that HIV-1 clade B and C gp120 proteins may play a differential role in the neuropathogenesis of HAD in neuroAIDS.
Keywords: HIV-1 gp120, NMDA receptor, COX-2, PGE2, TBXA2 R
Introduction
HIV-1 infection is known to cause neurodegeneration in the neuronal cells which is a major risk factor in the neuropathogenesis of brain disease (Ensoli et al. 1993). HIV-1 directly and indirectly affects the central nervous system causing neurological impairments such as HIV/AIDS dementia, cognitive, behavioral, and motor abnormalities caused by a massive death of neurons in all the brain regions (Nath 2002; Gonzalez-Scarano and Baltuch 1999). This can be initiated following activation of brain cells such as microglia and astrocytes. There is evidence association with the secretory neurotoxin metabolites, glutamate (Dreyer and Lipton 1995), arachidonic acid (Gendelman al. 1994), quinolinic acid (Nottet et al. 1996; Sei et al. 1995), nitric oxide (NO), reactive oxygen species (ROS), and inflammatory cytokines and chemokines (Gray et al. 2001).
The HIV-1 gp120 protein is required for viral entry into the host cells. It facilitates the viral replication and enhances neurotoxicity through inducible nitric oxide synthesis (Raber et al. 1996; Dawson et al. 1993), and causes cellular oxidative stress which progressively affects the central nervous system (CNS; Brenneman et al. 1994; Galicia et al. 2002). Gp120 binds to glutamate (Scorziello et al. 1998) and increases intracellular calcium levels (Dreyer et al. 1990), which eventually leads to lipid peroxidation (Corasaniti et al. 1998) causing “excitotoxic,” N-methyl-D-aspartate (NMDA) receptor-mediated neuronal damage (Corasaniti et al. 1996; Lipton et al. 1991; Holden et al. 1999). Previous studies have shown that gp120 induces overstimulation of glutamate which could lead to the generation of reactive nitrogen species in neurons and astrocytes (Kaul and Lipton 1999). Furthermore, these reactive nitrogen species cause damage to the mitochondrial function which in turn may lead to an AIDS dementia complex (ADC), neurobehavioral abnormalities, and eventually death.
Previous studies suggest that genetic variations in HIV strains play critical roles in influencing and modifying disease progression in gp120 protein (Kaleebu et al. 2001; Satishchandra et al. 2000). HIV-1 clade B- and C-infected subjects show a differential degree of neurological problems (Satishchandra et al. 2000). It has been shown that different sequences and genetic polymorphisms in the viral protein and variations in the viral gene enzymes ultimately lead to differential expressions of ADC. Recent studies reported that gp120 induced the activation of NMDA receptor; dependent on HIV-1 clade variations (Li et al. 2008). Previous studies have shown that HIV-1 clade C Tat gene sequence dicysteine is changed to C30C31 motif and alters the functional property compared to HIV-1 clade B Tat (Mishra et al. 2008). We have recently reported that there are clade-specific differences in the regulation of inflammatory cytokine, chemokines, and the enzyme indoleamine 2, 3-dioxygenase (IDO) activity (Gandhi et al. 2009; Samikkannu et al. 2009). However, the underlying mechanisms are not yet clearly understood. Therefore, we sought to investigate if HIV-1 clade B and C gp120 proteins differentially modulate NMDA receptor functions through a similar or distinct mechanism(s) in human primary astrocytes.
In the present study, we show that clade B gp120 is involved in the activation of NMDA receptor gene and protein expression compared to HIV-1 gp120 clade C protein in primary human astrocytes.
Results
Effect of HIV-1 clade B and C gp120 on NMDA-Receptor, PGE2, and TBXA2 R gene expression
Since HIV-1B infection has been reported to induce more neuropathogenesis than HIV-1C infection (Mishra et al. 2008), we investigated whether HIV-1B- and C clade-derived gp120 differentially modulate the expression of the neurotoxin arachidonic acid and its by-products PGE2 and TBXA2 in primary human astrocytes. Data presented in Fig. 1a show that astrocytes treated with clade B (Bal) gp120 at 50 ng (p<0.03) and 100 ng (p<0.01) significantly downregulated N-methyl-D-aspartate receptors gene expression compared to clade C (CN54) gp120-treated cultures or untreated control at 24 h. Figure 1b shows the kinetics results which demonstrate significant decrease in NMDA receptor gene expression at 24 h (p<0.03) treatment compared to clade C gp120. We examined whether clade B gp120 proteins Bal, MN and JRCSF and clade C gp120 CN54 differ in their expression. Figure 2 results indicate that in gp120 clade B, Bal (0.01), MN (p<0.02), and JRCSF (p<0.02) significantly downregulated the NMDA receptor gene expression, compared to clade C gp120 CN54 at 50 ng concentration. Since maximum response was found at 50 ng/ml, we used this particular concentration in subsequent experiments. Furthermore, the downstream effects of the activation of PGE2 and TBXA2 R were also examined. The PGE2 (p<0.006) and TBXA2 R (p<0.02) expressions were upregulated in HIV-1 clade B gp120 Bal-treated cells, compared to the clade C gp120 CN54 (Fig. 3a, b).
Fig. 1.
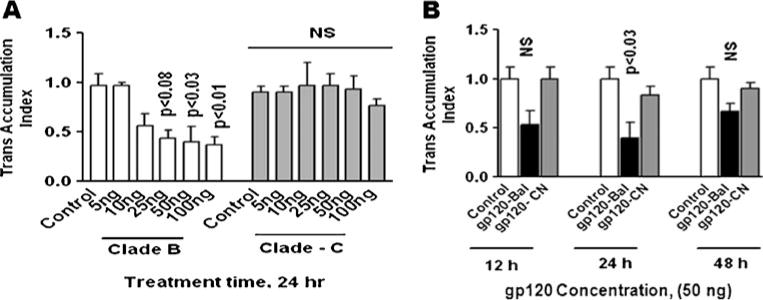
Effect of HIV-1 clade B and C gp120 protein on NMDA receptor gene expression by dose-response (a) and kinetics (b) in human astrocytes. Astrocytes (1 × 106 cells/ml) were separately treated with HIV-1 clade B (Bal) and C (CN54) gp120 at 5–100 ng/ml for 24 h and 25 ng/ml for 12, 24, and 48 h. RNA was extracted and reverse transcribed followed by quantitative real-time PCR for NMDA receptor and housekeeping gene β-actin-specific primers. Data are expressed as mean±SD of TAI values of three independent experiments
Fig. 2.
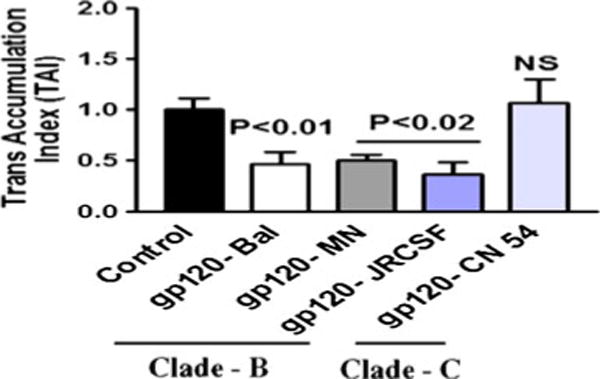
Effect of HIV-1 clade B and C gp120 protein on NMDA Receptor gene expression in human astrocytes. Astrocytes (1×106 cells/ml) were separately treated with HIV-1 clade B (Bal, MN, and JRCSF) and C (CN54) gp120 at 25 ng/ml for 24 h, and the NMDA receptor expression was quantified by quantitative real-time PCR. Data are expressed as mean±SD of TAI values of three independent experiments
Fig. 3.
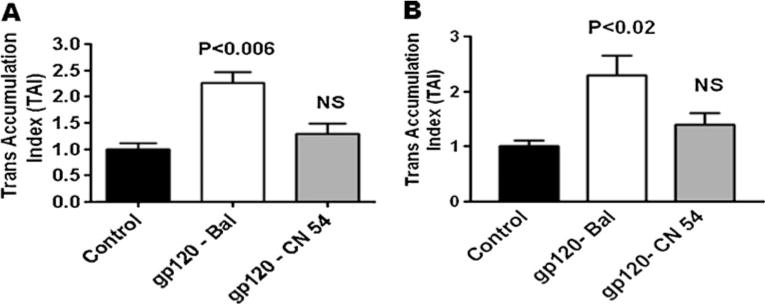
Effect of HIV-1 clade B and C gp120 protein on PGE2 (a) and TBXA2 R (b) gene expression in human astrocytes. Astrocytes (1 × 106 cells/ml) were separately treated with HIV-1 clade B (Bal) and C gp120 (CN54) at 25 ng/ml for 24 h, and the PGE2 and TBXA2 R expression were quantified by quantitative real-time PCR. Data are expressed as mean±SD of TAI values of three independent experiments
Glutamine levels induced by HIV-1 B and C gp120 proteins
Glutamine synthetase (GS) is a marker of astrocytes, and it plays a significant role in the enzymatic conversion of the excitatory amino acid glutamate to glutamine (Souba 1992). Previous studies demonstrate that this enzyme significantly modulates neuronal disorders of the central nervous system. We investigated the role of clade B and C gp120 induction of glutamate formation and enzyme glutamine level in astrocytes. Data presented in Fig. 4a show that astrocytes treated with 50 ng of clade B gp120 Bal have reduced the level of glutamine at 24 h (p<0.04) compared to clade C gp120 CN54 or the untreated control group in which glutamine levels were lower. Furthermore, there is an increase in glutamate (p<0.02) for the astrocytes treated with clade B gp120 Bal compared to clade C gp120 CN54 treatment (Fig. 4b).
Fig. 4.
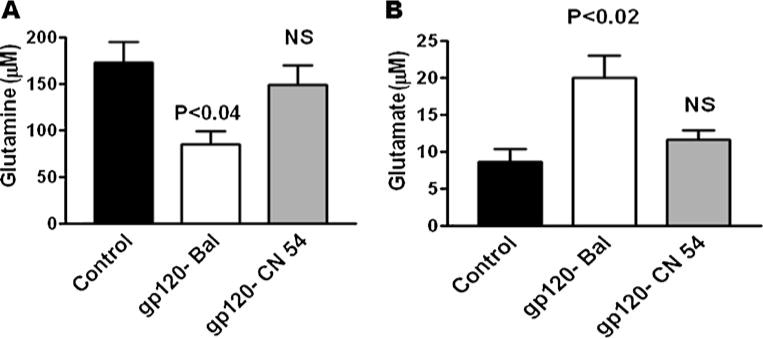
Effect of HIV-1 clade B and C gp120 protein on glutamine and glutamate levels. Astrocytes (1 × 106 cells/ml) were separately treated with HIV-1 clade B (Bal) and C (CN54) gp120 at 25 ng/ml for 24 h, and the cell lysates were examined for glutamine (a) and glutamate level (b). Data are expressed as mean±SD of three independent experiments
Effect of HIV-1 clade B and C gp120 on NMDA-Receptor, GS, PGE2, and TBXA2 R protein expression
We also examined whether clade B and C gp120 differentially modulate the NMDA receptor (NR2A) protein expression in astrocytes. Data presented in Fig. 5a show that clade B gp120 Bal-treated cells significantly decreased the NR2A protein expression compared to clade C gp120 CN54. In addition, clades B gp120 (Bal, MN, and JRCSF) show similar significant decreases in NR2A protein expression compared to clade C gp120 CN54 or untreated control (Fig. 5b). Figure 5c shows the densitometric scanning of NR2A protein levels in clade B gp120 (Bal, MN, and JRCSF) compared with clade C gp120 (CN54). Results indicate significantly reduced levels for all clade B gp120 proteins when compared to the clade C gp120 protein. In order to understand whether reduced glutamine level will affect the downstream effect of GS protein modification by clade B and C gp120, we observed the results (Fig. 5d, e) which demonstrate that GS protein is significantly downregulated by clade B gp120 compared to clade C gp120 consistent with NMDA receptor activation. Furthermore, the downstream effect of neurotoxin arachidonic acid cascade induction through COX-2-mediated byproducts of PGE2 and TBXA2 R is shown in Fig. 6a, c. Data presented in Fig. 6b, d show the densitometry evaluation respectively PGE2 (p<0.004) and TBXA2 R (p<0.009). These results confirm the decreased in NMDA receptor levels of glutamine synthesis and increased glutamate formation in clade B gp120 compared to clade C gp120.
Fig. 5.
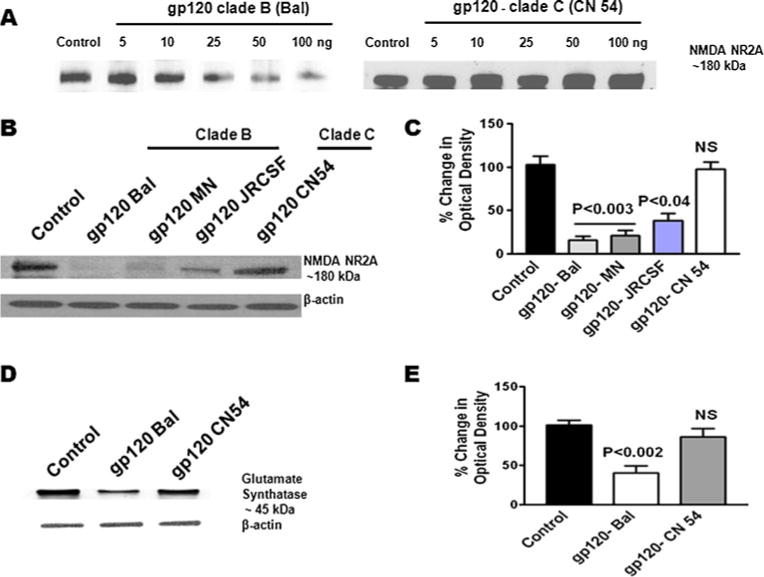
Effect of HIV-1 clade B and C gp120 protein on NMDA Receptor (NR2A) and glutamine synthetase (GS) protein expression. Astrocytes (1×106 cells/ml) were separately treated with HIV-1 clade B and C gp120 at 25 ng/ml for 24 h and analyzed by Western blot. a The dose-response effect of NR2A protein in gp120 clade B (Bal) and clade C (CN54). b A representative experiment: lane 1 control, lane 2 gp120 Bal, lane 3 MN, lane 4 JRCSF, lane 5 CN54 on NR2A protein and densitometric evaluation (c). d A representative experiment: lane 1 control, lane 2 gp120 Bal, lane 3 CN54 on glutamate synthetase protein and densitometric evaluation (e). Data are representative of three independent experiments
Fig. 6.
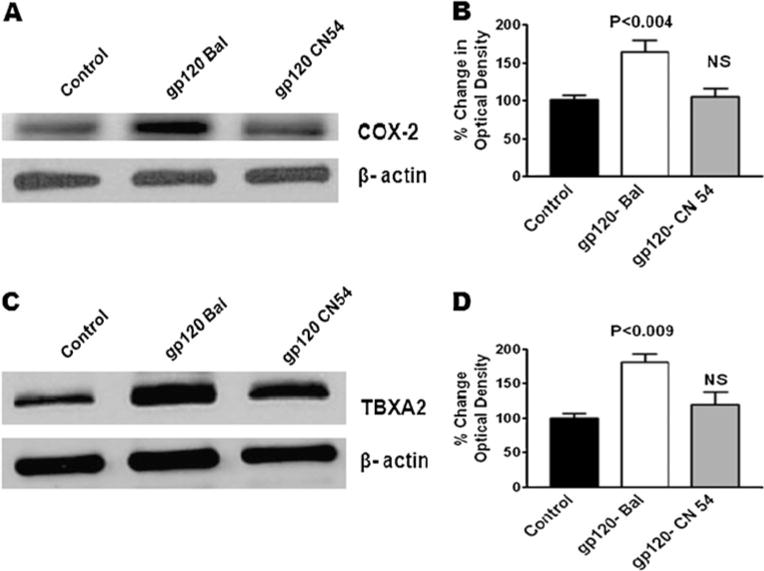
Effect of HIV-1 clade B- and C-gp120 protein on COX-2 (PGE2) (a), densitometry evaluation (b), TBXA2 (c), and densitometry evaluation (d). Astrocytes (1×106 cells/ml) were separately treated with HIV-1 clade B (Bal) and C (CN54) gp120 at 25 ng/ml for 24 h and analyzed by Western blot. Data are representative of three independent experiments
Discussion
The present study provides new insights into the functional role of the NMDA receptor in primary astrocytes following clade B and C gp120 exposure, and how the clade-specific gp120 proteins modulate NMDA receptor expression. We have demonstrated for the first time the differential role of clade B and clade C gp120 in the modulation of the NMDA receptor mRNA expression. These findings are of considerable interest because it indicates that the NMDA receptor and the arachidonic acid by-products of PGE2 and TBXA2 R are effective in inducing the production of the neurotoxin, arachidonic acid. In addition, our results suggest that the NMDA receptor-mediated downregulation of protein modifications in clade B gp120 is more potent than clade C gp120.
Previous studies have shown that HIV-1-induced neurotoxin, arachidonic acid, plays a major role in the NMDA receptor-mediated inhibition of excitatory amino acid uptake in neuronal dysfunction (Ushijima et al. 1995). It has been reported that the HIV-1 gp120 protein induces a complex glutamate receptor and calcium-dependent cascade alteration that leads to brain dementia and neuronal cell death (Corasaniti et al. 1995; Brooke et al. 1997; Vesce et al. 1997). HIV-1 infection is associated with increased levels of the neurotoxin, arachidonic acid, in cerebrospinal fluid (CSF; Genis et al. 1992). Furthermore, an increase in nitric oxide (NO) and PGE2 synthesis in HIV-associated dementia (HAD) patients has also been reported (Sardar and Reynolds 1995), suggesting an association of NMDA receptor with neuronal dysfunction.
Studies have shown that HIV-1 geographical variations and genetic polymorphisms may lead to a differential expression of HAD. HIV-1 clade B infections occur predominantly in North America, Western Europe, and Australia. Conversely, HIV-1 clade C is found in Africa, Latin America, and Asia (Robertson et al. 2001). Current estimates place the prevalence of HAD in the USA and Western Europe between 10% and 20% (Grant et al. 2005), compared to only 1–8% in India (Shankar et al. 2005; Wadia et al. 2001). HAD appears to be the most common with HIV-1 clade B infections, whereas much lower level of HAD occurs with HIV-1 clade C infection. This suggests that the prevalence of HAD may be correlated with the difference in subtypes of HIV-1.
Our recent studies corroborate other reports in which HIV-1 clade B Tat was shown to be more potent in inducing neuropathogesis compared to clade C Tat (Samikkannu et al. 2009, 2010). However, there are no reports on the molecular mechanisms of NMDA receptor-mediated expression of COX-2 by HIV-1 clade C gp120 protein. In the present study, we have shown, for the first time, differences in HIV-1 clades by studying modulation of the NMDA receptor pathway and COX-2 activation in primary human astrocytes. We demonstrate that clade B gp120 significantly decreased the NMDA receptor mRNA expression compared with clade C gp120 (Fig. 1a, b). In order to understand the various clades B gp120 proteins such as HIV-1 clade B gp120 MN and JRCSF, we also tested the functional effect and similarity of the gene expression and protein modification (Figs. 2 and 5b). The NMDA receptor activation data confirm the downstream signals of PGE2 and TBXA2 R (Fig. 3a, b). Furthermore, our results also demonstrate that the induction of NMDA receptor expression reduced the level of glutamine (Fig. 4a), and significantly increased glutamate formation (Fig. 4b) with HIV-1 clade B gp120 protein. This result suggests that HIV-1 clade C gp120 showed less effect and lack of functional activity in NMDA receptor-mediated induction.
Previous studies have shown that HIV-1 genetic variations play a critical role in influencing HIV-1 infection and differently modify disease progression in the clade B and C (Satishchandra et al. 2000). There are differences in sequential and structure of HIV-1 clade B gp120 compared to HIV-1 clade C gp120, especially in V3 and C3 regions of gp120 (Gnanakaran et al. 2007; Rizzuto et al. 1998; Choe et al. 1998). These differences might account for the observed differences in the activities of the clade B and C proteins. Recent studies have shown that the hypervariable V3 domain of gp120 is an important determinant of neuropathogenesis (Pattarini et al. 1998). Regardless of the HIV clade from which it is derived, distinct HIV envelope sequences have been associated with the clinical expression of dementia (Zhang et al. 2001). Previous studies showing analysis of differences in sequence of the V3 region of various HIV-1 isolates have provided evidence that genetically unique sequence of the infecting virus exists in different brain regions (Chang et al. 1998). The influence of brain-specific, actively replicating viral reservoirs may be important for the development of dementia (Mattson et al. 2005) therefore, raising the possibility that these neuropathogenic factors may be involved in the observed effects.
There are several reports stating that NMDA receptor expression alters ion channels with increased level of Ca2+ which activate several pathways (Lipton et al. 1991; Muller et al. 1992; Meucci and Miller 1996). We have shown here that the gp120 protein alters NMDA receptor expression and may have an impact on the glutamate enzyme activation. It has been shown that activating a number of Ca2+ ion-dependent enzymes and phopholipase A2 (PLA2) increases secretion of arachidonic acid and various by-products in COX and 5-LOX by NMDA receptor expression (Tapia-Arancibia et al. 1992). Arachidonic acid induction is also known to rapidly activate PGE2 and TBXA2 R signaling pathways that promote neuropathogenesis preferentially via COX-2 (Maccarrone et al. 1998; Corasaniti et al. 2000, 2003; Griffin et al. 1994). These studies suggest a possible role for the neuropathogenic molecules regulated by NMDA activation in HAD (Griffin et al. 1994). Further studies are needed to fully understand the mechanism of HIV-1 clade Band C gp120-induced release of the neurotoxin arachidonic acid.
Observed protein modification demonstrates that clade B gp120-induced activation of the NMDA receptor (Fig. 5a, b) correlates with the observed NMDA receptor mRNA expression (Figs. 1 and 2). The decrease in glutamine may be due to suppression of the GS protein expression (Fig. 5d, e). We consistently found that clade B gp120 significantly increased arachidonic acid cascade of COX-2 (PGE2) and TBXA2 R, as compared with HIV-1 clade C gp120 (Fig. 6a–d). Observed effects are more pronounced for clade B gp120 and, therefore, raised the possibility that these factors may be involved in the observed effects. However, the mechanisms underlying these alterations and their protein modifications are yet to be elucidated. The main observation in this report is that gp120 clade B is significantly higher than clade C gp120 and that these two different clades may have distinct in vitro mechanisms. Overall, these data provide evidence of a connection between NMDA receptor gene expression, glutamate activation, and enzyme uptake and secretion of arachidonic acid by-products PGE2 and TBXA2 R in gp120 clade B-exposed cells.
In conclusion, HIV-1 gp120 protein treatment down-regulates NMDA receptor mRNA expression as well as protein modification in cultured primary human astrocytes. Furthermore, HIV-1 clade B gp120 could induce more arachidonic acid by-products compared to HIV-1 clade C gp120 in human primary astrocytes. Based on these results, it appears that HIV-1 clade B gp120 is more potent than HIV-1 clade C gp120, and this might play a critical role in the neuropathogenesis of HAD in HIV-1 B-infected patients.
Materials and methods
Reagents
Cell culture reagents were purchased from Sciencell (Carlsbad, CA), NMDA receptor (NR2A) antibody were purchased from Invitrogen (Camrillo, CA), antiglutamine synthetase antibody were purchased from BD Transduction Laboratories (San Jose, CA), COX-2 antibody were purchased from (Abcam, Cambridge, MA), TBXA2 R antibody were purchased from Cayman Chemical (Ann Arbor, MI), goat antirabbit IgG and goat antimouse IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), electrophoresis reagents were purchased from Bio-Rad (Richmond, CA), nitrocellulose membrane were purchased from Amersham, and glutamine and glutamate assay kit as well as all reagents were purchased from Sigma-Aldrich, St. Louis, MO, USA Scientific.
HIV-1 clade gp120 recombinant proteins
HIV-1 clade B gp210 Bal and gp120 clade C CN54 proteins were obtained from NIH AIDS Research and Reference Reagent; HIV-1 clade B MN and JRCSF were obtained from Immune Technology Corp (New York). These were highly purified recombinant gp120 proteins, clade B (>95%) and clade C (>90%) purities, respectively.
Primary human astrocyte culture
Cells were maintained in basal medium containing 10% fetal bovine serum, 50 units/ml penicillin, astrocyte growth supplement, and 100 μg/ml streptomycin. Cells and medium were obtained from Sciencell (Carlsbad, CA), and the cells were grown to 80–90% confluence.
RNA extraction and real-time quantitative PCR
Total RNA from 1×106 cultured primary astrocytes cells were extracted using the Qiagen kit (Invitrogen Life Technologies, Carlsbad, CA, USA) following the manufacturer’s instructions. The total RNA (5 μg) was used for the synthesis of the first strand of cDNA. The amplification of cDNA was performed using primer (Applied Biosystems, Foster City, CA) specific for NMDA receptor (Assay ID Hs00609557_m1), COX-2 (Assay ID, Hs00153133_m1), TBXA2 R (Assay ID, Hs00169054_m1), and β-actin (Assay ID, Hs99999903_m1) that was used as housekeeping gene for quantifying real-time PCR. Relative abundance of each mRNA species was assessed using the Taqman master mix from Applied Biosystems to perform real-time quantitative PCR (Stratagene PCR machine), which detects and plots the increase in fluorescence versus PCR cycle number to produce a continuous measure of PCR amplification. All the results were expressed as the ratio of normalized expression of the target gene in treated cells to the normalized expression of the target gene in untreated control cells (Shively et al. 2003).
Glutamine and glutamate assay
Intracellular glutamine changes were determined by using a glutamine assay kit (Sigma) based on the reductive deamination of glutamine and glutamate by a proprietary enzyme. Briefly, 2×106 cells were disrupted, and an equal concentration of cell supernatants, glutamine standards, and cell culture medium were incubated with the reaction buffer, the diluent buffer, and the specific enzyme for 1 hr at 37°C. The color reagent was added to each sample and allowed to develop for 5 min at room temperature, and then measured at 550 nm using a spectrophotometer. To calculate the quantity of glutamine and glutamate, a linear regression analysis of the standard curve was performed.
Western blot analysis
To determine the NMDA Receptor (NR2A), glutamine synthetase (GS), COX-2 and TBXA2 R protein modification in astrocytes by HIV-1 clade B gp210 Bal, MN, JRCSF, and gp120 clade C CN54, at the end of the time period, cells were washed and lysed by lysis buffer (Pierce, IL) with 1× complete cocktail of protease inhibitors. Equal amount of total cellular protein was resolved on a gradient of 4–15% polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and incubated with their respective primary antibodies. Immunoreactive bands were visualized using a chemiluminescence western blotting system according to the manufacturers’ instructions (Amersham).
Statistics
Results presented in the study are triplicate values and representative of three or more independent experiments. Statistical significance was analyzed with the computer software GraphPad Prism using ANOVA or Student’s t test for unpaired observations. The values presented are means±SE, and p<0.05 was considered to be significant.
Acknowledgments
The present study was supported by grants from National Institute of Health (NIH): DA012366, DA 021537, DA 025576, and MH08529.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
References
- Brenneman DE, McCune SK, Mervis RF, Hill JM. gp120 as an etiologic agent for neuroAIDS: neurotoxicity and model systems. Adv Neuroimmunol. 1994;4:157–165. doi: 10.1016/s0960-5428(06)80252-4. [DOI] [PubMed] [Google Scholar]
- Brooke S, Chan R, Howard S, Sapolsky R. Endocrine modulation of the neurotoxicity of gp120: implications for AIDS related dementia complex. Proc Natl Acad Sci USA. 1997;94:9457–9462. doi: 10.1073/pnas.94.17.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Jozwiak R, Wang B, Ng T, Ge YC, Bolton W, Dwyer DE, Randle C, Osborn R, Cunningham AL, Saksena NK. Unique HIV type 1 V3 region sequences derived from six different regions of brain: region-specific evolution within host-determined quasispecies. AIDS Res Hum Retroviruses. 1998;14:25–30. doi: 10.1089/aid.1998.14.25. [DOI] [PubMed] [Google Scholar]
- Choe H, Martin KA, Farzan M, Sodroski J, Gerard NP, Gerard C. Structural interactions between chemokine receptors, gp120 Env and CD4. Semin Immunol. 1998;10:249–257. doi: 10.1006/smim.1998.0127. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Melino G, Navarra M, Garaci E, Finazzi-Agro A, Nistico G. Death of cultured neuroblastoma cells induced by HIV-1 gp120 is prevented by NMDA receptor antagonists and inhibitors of nitric oxide and cyclooxygenase. Neurodegeneration. 1995;4:315–321. doi: 10.1016/1055-8330(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Navarra M, Catani MV, Melino G, Nistico G, Finazzi-Agro A. NMDA and HIV-1 coat protein, GP120, produce necrotic but not apoptotic cell death in human CHP100 neuroblastoma cultures via a mechanism involving calpain. Biochem Biophys Res Commun. 1996;229:299–304. doi: 10.1006/bbrc.1996.1796. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Navarra M, Nistico S, Rotiroti D, Maccarrone M, Melino G, Finazzi Agro A. Requirement for membrane lipid peroxidation in HIV-1 gp120-induced neuroblastoma cell death. Biochem Biophys Res Commun. 1998;246:686–689. doi: 10.1006/bbrc.1998.8687. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Strongoli MC, Piccirilli S, Nistico R, Costa A, Bilotta A, Turano P, Finazzi-Agro A, Bagetta Apoptosis induced by gp120 in the neocortex of rat involves enhanced expression of cyclooxygenase type 2 (COX-2) and is prevented by NMDA receptor antagonists and by the 21-aminosteroid U-74389G. Biochem Biophys Res Commun. 2000;274:664–669. doi: 10.1006/bbrc.2000.3160. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Bellizzib C, Russo R, Colica C, Amantea D, Di Renzo G. Caspase-1 inhibitors abolish deleterious enhancement of COX-2 expression induced by HIV-1 gp120 in human neuroblastoma cells. Toxicol Lett. 2003;139:213–219. doi: 10.1016/s0378-4274(02)00436-8. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM, Uhl GR, Synder SH. Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical cultures. Proc Natl Acad Sci USA. 1993;90:3256–3259. doi: 10.1073/pnas.90.8.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer EB, Lipton SA. The coat protein gp120 of HIV-1 inhibits astrocyte uptake of exciTatory amino acids via macrophage arachidonic acid. Eur J Neurosci. 1995;7:2502–2507. doi: 10.1111/j.1460-9568.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- Dreyer EB, Kaiserr PK, Offermann JT, Lipton SA. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galicia O, Sanchez-Alavez M, Mendez Diaz M, Navarro L, Prospero-Garcia O. HIV glycoprotein 120: possible etiological agent of AIDS-associated dementia. Rev Invest Clin. 2002;54:437–452. [PubMed] [Google Scholar]
- Gandhi N, Saiyed Z, Samikkannu T, Rodriguez JW, Rao KVK, Nair MN. Differential effects of HIV-1 clade B and clade C Tat protein on expression of proand anti-inflammatory cytokines by primary monocytes. AIDS Res Hum Retroviruses. 2009;25:691–699. doi: 10.1089/aid.2008.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, Genis P, Jett M, Zhai QH, Nottet HS. An experimental model system for HIV-1-induced brain injury. Adv Neuroimmunol. 1994;4:189–193. doi: 10.1016/s0960-5428(06)80256-1. [DOI] [PubMed] [Google Scholar]
- Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ, et al. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992;176:1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanakaran S, Lang D, Daniels M, Bhattacharya T, Derdeyn CA, Korber B. Clade-specific differences between human immunodeficiency virus type 1 clades B and C: diversity and correlations in C3-V4 regions of gp120. J Virol. 2007;81:4886–4891. doi: 10.1128/JVI.01954-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- Grant I, Sacktor H, McArthur J. HIV neurocognitive disorders. In: Gendelman HE, Grant I, Everall I, Lipton SA, Swindells S, editors. The neurology of AIDS. 2nd. Oxford University Press; London, UK: 2005. pp. 357–373. [Google Scholar]
- Gray F, Adle-Biassette H, Chrétien F, Lorin de la Grandmaison G, Force G, Keohane C. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol. 2001;20:146–155. [PubMed] [Google Scholar]
- Griffin DE, Wesselingh SL, McArthur JC. Elevated central nervous system prostaglandins in human immunodeficiency virus-associated dementia. Ann Neurol. 1994;35:592–597. doi: 10.1002/ana.410350513. [DOI] [PubMed] [Google Scholar]
- Holden CP, Nath A, Haughey NJ, Geiger JD. Involvement of Na+/H+ exchangers, Ca2+ channels, and excitatory amino acid receptors in intracellular Ca2+ responses to HIV-1 gp120 in cultured human fetal brain cells. Neuroscience. 1999;91 doi: 10.1016/s0306-4522(98)00714-3. 13691378. [DOI] [PubMed] [Google Scholar]
- Kaleebu P, Ross A, Morgan D, Yirrell D, Oram J, Rutebemberwa A, Lyagoba F, Hamilton L, Biryahwaho B, Whitworth J. Relationship between HIV-1 Env subtypes A and D and disease progression in a rural Ugandan cohort. AIDS. 2001;15:293–299. doi: 10.1097/00002030-200102160-00001. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci USA. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Huang Y, Reid R, Steiner J, Malpica-Llanos T, Darden TA, Shankar SK, Mahadevan A, Satishchandra P, Nath A. NMDA receptor activation by HIV-Tat protein is clade dependent. J Neurosci. 2008;28:12190–12198. doi: 10.1523/JNEUROSCI.3019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Navarra M, Corasaniti MT, Nisticò G, Finazzi Agrò A. Cytotoxic effect of HIV-1 coat glycoprotein gp120 on human neuroblastoma CHP100 cells involves activation of the arachidonate cascade. Biochem J. 1998;333:45–49. doi: 10.1042/bj3330045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12:893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Meucci O, Miller RJ. gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-beta1. J Neurosci. 1996;335:639–642. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M, Vetrivel S, Siddappa NB, Ranga U, Seth P. Clade-specific differences in neurotoxicity of human immunodeficiency virus-1 B and C Tat of human neurons: significance of dicysteine C30C31 motif. Ann Neurol. 2008;63:366–376. doi: 10.1002/ana.21292. [DOI] [PubMed] [Google Scholar]
- Muller WEG, Schroder HC, Ushijima H, Dapper J, Bormann J. gp120 of HIV-1 induces apoptosis in rat cortical cell cultures: prevention by memantine. Eur J Pharmacol. 1992;226:209–214. doi: 10.1016/0922-4106(92)90063-2. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–S198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Nottet SLM, Jett M, Flanagan CR, Zhai QH, Persidsky Y, Rizzino A, Bernton EW, Genis P, Baldwin T, Schwartz J. A regulatory role for astrocytes in HIV-1 encephalitis: an overexpression of eicosanoids, platelet-activating factor, and tumor necrosis factor-by activated HIV-1-infected monocytes is attenuated by primary human astrocytes. J Immunol. 1996;154:3567–3581. [PubMed] [Google Scholar]
- Pattarini R, Pittaluga A, Raiteri M. The human immunodeficiency virus-1 envelope protein gp120 binds through its V3 sequence to the glycine site of N-methyl-D-aspartate receptors mediating noradrenaline release in the hippocampus. Neuroscience. 1998;87:147–157. doi: 10.1016/s0306-4522(98)00125-0. [DOI] [PubMed] [Google Scholar]
- Raber J, Toggas SM, Lee S, Bloom FE, Epstein CJ, Mucke L. Central nervous system expression of HIV-1 gp120 activates the hypothalamic-pituitary-adrenal axis: evidence for involvement of the NMDA receptors and nitric oxide synthase. Virology. 1996;226:362–373. doi: 10.1006/viro.1996.0664. [DOI] [PubMed] [Google Scholar]
- Rizzuto CD, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong PD, Hendrickson WA, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- Robertson D, Anderson J, Bradac J, Carr J, Foley B, Funkhouser R, Gao F, Hahn B, Kalish M, Kuiken C, Learn G, Leitner T, McCutchan F, Osmanov S, Peeters M, Pieniazek D, Salminen OM, Sharp P, Wolinsky SM, Korber B. A reference guide to HIV-1 classification. Los Alamos National Laboratory; Los Alamos, New Mexico: 2001. [Google Scholar]
- Samikkannu T, Saiyed Z, Rao KVK, Dakshayani KB, Rodriguez JW, Papuashvili MN, Nair MP. Differential regulation of indoleamine-2, 3-dioxygenase (IDO) by HIV-1 clade B and C Tat proteins. AIDS Res Hum Retroviruses. 2009;25:329–335. doi: 10.1089/aid.2008.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samikkannu T, Rao KV, Gandhi N, Saxena SK, Nair MP. Human immunodeficiency virus type 1 clade B and C Tat differentially induce indoleamine 2,3-dioxygenase and serotonin in immature dendritic cells: Implications for neuroAIDS. J Neurovirol. 2010;16:255–263. doi: 10.3109/13550284.2010.497809. [DOI] [PubMed] [Google Scholar]
- Sardar AM, Reynolds GP. Frontal cortex indoleamine-2,3-dioxygenase activity is increased in HIV-1-associated dementia. Neurosci Lett. 1995;187:9–12. doi: 10.1016/0304-3940(95)11324-p. [DOI] [PubMed] [Google Scholar]
- Satishchandra P, Nalini A, Gourie-Devi M, Khanna N, Santosh V, Ravi V, Desai A, Chandramuki A, Jayakumar PN, Shankar SK. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, South India (1989–96) Indian J Med Res. 2000;111:14–23. [PubMed] [Google Scholar]
- Scorziello A, Floriao T, Bajetto A, Schettini G. Intracellular signaling mediating HIV-1 gp120 neurotoxicity. Cell Signal. 1998;10:75–84. doi: 10.1016/s0898-6568(97)00093-4. [DOI] [PubMed] [Google Scholar]
- Sei S, Saito K, Stewart SK, Crowley JS, Brouwers P, Kleiner DE, Katz DA, Pizzo PA, Heyes MP. Increased human immunodeficiency virus (HIV) type 1 DNA content and quinolinic acid concentration in brain tissues from patients with HIV encephalopathy. J Infect Dis. 1995;172:638–647. [Google Scholar]
- Shankar AV, Sastry J, Erande A. Making the choice: the translation of global HIV and infant feeding policy to local practice among mothers in Pune, India. J Nutr. 2005;135:960–965. doi: 10.1093/jn/135.4.960. [DOI] [PubMed] [Google Scholar]
- Shively CA, Mirkes SJ, Lu NZ, Henderson JA, Bethea CL. Soy and social stress affect serotonin neurotransmission in primates. Pharmacogenomics J. 2003;3:114–121. doi: 10.1038/sj.tpj.6500166. [DOI] [PubMed] [Google Scholar]
- Souba WW. In: Glutamine—physiology, biochemistry and nutrition in critical illness. Landes RG, editor. R.G. Landes Co; Austin, TX: 1992. p. 5. [Google Scholar]
- Tapia-Arancibia L, Rage F, Récasens M, Pin JP. NMDA receptor activation stimulates phospholipase A2 and somatostatin release from rat cortical neurons in primary cultures. Eur J Pharmacol. 1992;225:253–262. doi: 10.1016/0922-4106(92)90027-s. [DOI] [PubMed] [Google Scholar]
- Ushijima H, Nishio O, Klöcking R, Perovic S, Müller WEG. Exposure to gp120 of HIV-1 induces an increased release of arachidonic acid in rat primary neuronal cell culture followed by NMDA receptor-mediated neurotoxicity. Eur J Neurosci. 1995;7:1353–1359. doi: 10.1111/j.1460-9568.1995.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Vesce S, Bezzi P, Rossi D, Meldolesi J, Volterra A. HIV-1 gp120 glycoprotein affects the astrocyte control of extracellular glutamate by both inhibiting the uptake and stimulating the release of the amino acid. FEBS Lett. 1997;411:107–109. doi: 10.1016/s0014-5793(97)00674-1. [DOI] [PubMed] [Google Scholar]
- Wadia RS, Pujari SN, Kothari S. Neurological manifestations of HIV disease. J Assoc Physicians India. 2001;49:343–348. [PubMed] [Google Scholar]
- Zhang K, Hawken M, Rana F, Welte FJ, Gartner S, Goldsmith MA, Power C. Human immunodeficiency virus type 1 clade A and D neurotropism: molecular evolution, recombination, and coreceptor use. Virology. 2001;283:19–30. doi: 10.1006/viro.2001.0876. [DOI] [PubMed] [Google Scholar]


