Abstract
Background
The intracerebroventricular injection of ouabain, a specific inhibitor of the Na+/K+-adenosine-triphosphatase (Na+/K+-ATPase) enzyme, induces hyperactivity in rats in a putative animal model of mania. Several evidences have suggested that the protein kinase C signaling pathway is involved in bipolar disorder. In addition, it is known that protein kinase C inhibitors, such as lithium and tamoxifen, are effective in treating acute mania.
Methods
In the present study, we investigated the effects of lithium and tamoxifen on the protein kinase C signaling pathway in the frontal cortex and hippocampus of rats submitted to the animal model of mania induced by ouabain. We showed that ouabain induced hyperlocomotion in the rats.
Results
Ouabain increased the protein kinase C activity and the protein kinase C and MARCKS phosphorylation in frontal cortex and hippocampus of rats. Lithium and tamoxifen reversed the behavioral and protein kinase C pathway changes induced by ouabain. These findings indicate that the Na+/K+-ATPase inhibition can lead to protein kinase C alteration.
Conclusions
The present study showed that lithium and tamoxifen modulate changes in the behavior and protein kinase C signalling pathway alterations induced by ouabain, underlining the need for more studies of protein kinase C as a possible target for treatment of bipolar disorder.
Keywords: bipolar disorder, animal model of mania, ouabain, Na+/K+-ATPase, protein kinase C
Significance Statement
The bipolar disorder (BD) is a chronic and recurrent psychiatric disorder. Although little is known about the pathophysiology of BD, some studies have strongly suggested that protein kinase C (PKC) alterations are an important component of this disorder The present study evaluated the action of lithium (a mood stabilizer) and tamoxifen (a PKC inhibitor) on behavior and PKC activity in an animal model of mania induced by ouabain. It was demonstrated that tamoxifen could act on PKC activity and reversed the manic-like behaviors in animals submitted to the administration of ouabain. This research can contribute with information about a new therapeutic target for bipolar disorder, as well as elucidating mechanisms involved in pathophysiology of this disorder.
Introduction
Bipolar disorder (BD) is the third most impactful mood disorder, with a lifetime prevalence of approximately 5% (Proudfoot et al., 2009). BD is characterized by mood changes that alternate between mania, depression, mixed states, and euthymia, all of which cause psychosocial impairments for the patient. Despite its importance, the pathophysiology of BD remains unknown (American Psychiatric Association, 2014).
A number of clinical and preclinical studies have suggested that the protein kinase C (PKC) plays an important role in the pathophysiology of BD (Zaratte and Manji, 2009; Cechinel-Recco et al., 2012; Steckert et al., 2012). In this context, initial interest in the role of PKC arose from the discovery that the mood stabilizer drugs lithium (Li) and valproate directly inhibited this enzyme (Zaratte and Manji, 2006). Additionally, clinical and preclinical studies have showed that tamoxifen (TMX), a PKC inhibitor, is effective in treating manic-like behavior (Amrollahi et al., 2011; Moretti et al., 2011; Fallah et al., 2016). Furthermore, Birnbaum and colleagues (2004) have demonstrated that excessive activation of PKC in rodents impairs cognitive functions related to frontal cortex and mood stabilizers protected cognitive functions. Even though cognitive impairment is not a clinical mark of mania, it is important to emphasize that bipolar patients show cognitive damage (Rolstad et al., 2016) and prefrontal alterations (Blumberg et al., 1999) compared with healthy control groups. Amodeo and colleagues (2017) demonstrated that ouabain induces cognitive flexibility impairment and alters plasticity in frontal cortex in rats submitted to the model of mania.
Indeed, PKC was implicated in the regulation of systems involved in the modulation of moods, including neuronal excitability, neurotransmitter release, and long-term alterations in gene expression and plasticity (DiazGranados and Zarate, 2008). A previous preclinical study has suggested that myristoylated alanine-rich C kinase substrate (MARCKS) plays an important role in the manic-like behaviors (Szabo et al., 2009). MARCKS is a substrate of PKC, which has been implicated in the neuronal membrane trafficking. The phosphorylation of MARCKS by PKC results in the translocation of MARCKS from the membrane to the cytosol (Aderem, 1992). MARCKS is an important protein in the transduction of calcium- and PKC-mediated signaling events (Ramakers et al. 1999).
Recent advances in genetic, neurobiological, and pharmacological methodologies have enabled the development of animal models, which are important tools in investigating new intracellular systems that may be involved in the pathophysiology of BD (Einat et al., 2003; Valvassori et al., 2013). The animal model of mania induced by ouabain is regarded as suitable to study bipolar mania (El-Mallakh et al., 2003; Valvassori et al., 2013). Ouabain is a cardiac glycoside that inhibits the Na+/K+-adenosine-triphosphatase (Na+/K+-ATPase) enzyme. The intracerebroventricular (ICV) injection of ouabain in rats mimics certain manic-like symptoms, which can then be reverted by the administration of classical mood stabilizers, including Li and valproate (Li et al., 1997; El-Mallakh et al., 2003; Jornada et al., 2010, 2011).
Therefore, the present study aimed to evaluate the effects of Li or TMX on the PKC activity and phosphorylation, as well as the MARCKS phosphorylation in the frontal cortex and hippocampus of rats submitted to an animal model of mania induced by ouabain.
Materials and Methods
Animals
The subjects were adult male Wistar rats (Rattus norvegicus; body weight ranging from 250 to 350 g) obtained from our breeding colony. Animals were housed 5 to a cage with ad libitum food and water and were maintained on a 12-h-light/-dark cycle (lights on at 7:00 am) at 22 ± 1°C. All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Brazilian Society for Neuroscience and Behavior. This study was approved by the local ethics committee, Comissão de Etica no Uso de Animais da Universidade do Extremo Sul Catarinense. All experiments were performed at the same time during the day to avoid circadian variations.
Surgical Procedure
Animals were anesthetized via i.p. injection of ketamine (80 mg/kg) plus xylazine (10 mg/kg) and were kept in a stereotaxic apparatus. The skin covering the rat skull was excised and a 27-gauge, 9-mm guide cannula was put on the surface of cranial bone according to the following coordinates: 0.9 mm posterior to bregma, 1.5 mm right from the midline, and 1.0 mm above the lateral cerebral ventricle. Thereafter, a cannula was ventrally implanted 2.6 mm to the superior surface of the skull through a 2-mm-diameter orifice and fixed with acrylic cement. Immediately after the surgical procedure, the animals received a single intramuscular injection of tramadol hydrochloride (10 mk/kg). Within 3 days, the rats presented complete recovery from the procedure.
Treatment
The model included in the present study was designed to reproduce the management of an acute manic episode. Animals (n = 10/group) received a single ICV injection of ouabain 10−3 M (5 μL) dissolved in artificial cerebrospinal fluid (ACSF) or 5 μL of ACSF alone on the fourth day following surgery (El-Mallakh et al., 2003; Riegel et al., 2009). A 30-gauge cannula was placed inside the guide cannula and connected to a microsyringe through a polyethylene tube. The tip of the cannula infusion protruded 1.0 mm beyond the guide cannula to target the right lateral cerebral ventricle. From the day following the injection of ouabain or ACSF, the rats were treated for 7 days with i.p. injections of saline, Li, or TMX in 6 experimental groups (n = 10 animals/group): (1) ACSF ICV + Saline i.p.; (2) ACSF ICV + Li i.p.; (3) ACSF ICV + TMX i.p.; (4) Ouabain ICV + Saline i.p.; (5) Ouabain ICV + Li i.p., (6) Ouabain ICV + TMX i.p. Animals received twice a day i.p. injections of Li (47.5 mg/kg) and TMX (1 mg/kg) (Cechinel-Recco et al., 2012). The rats were euthanized 24 hours after the last injection of Li, TMX, or saline.
Immunoblotting
The hippocampus and frontal cortex were dissected (n=8/group). The tissues were promptly homogenized in extraction buffer (1% Triton-X 100, 100 mM Tris, pH 7.4, containing 100 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM EDTA, 10 mM sodium vanadate, 2 mM phenylmethylsulfonyl fluoride (PMSF), and 0.1 mg of aprotinin/mL) at 4°C with a Polytron PTA 20S generator (Brinkmann Instruments model PT 10/35) operating at maximum speed for 30 seconds. Centrifugation of the extracts at 11000 rpm and 4°C in a Beckman 70.1 Ti rotor for 40 minutes was performed to remove insoluble debris. The resulting supernatants were used for protein quantification using the assay described by Bradford (1976). Denaturation of proteins was carried out by boiling in Laemmli (Laemmli, 1970) sample buffer supplemented with 100 mM 2-mercaptoethanol (DTT) (De Souza et al., 2003). Thereafter, 0.2 mg of protein extracts collected from each tissue was fractioned by SDS-PAGE, transferred to nitrocellulose membranes, and blotted with anti-total PKC, anti-total MARCKS, anti-phospho-PKC, and anti-phospho-MARCKS (Ser96). The antibodies (1:1000) were diluted in TBS-Tween supplemented with azide. Antibodies were purchased from Millipore. Chemiluminescent detection was performed with horseradish secondary antibodies conjugated to peroxidase. Membranes were exposed to RX-films to support the visualization of protein bands. Ponceau solution was used to stain the membrane after transfer. Bands were visualized, photographed, and quantified prior to the primary antibody to control the transfer. Quantification of the band intensities was carried out by optical densitometry (Scion Image Software, ScionCorp) of the developed autoradiographs.
PKC Activity Analysis by ELISA
The PKC activity was measured in frontal cortex and hippocampus of rats using the PKC Kinase Activity Assay Kit (AbCam) following the instructions of the manufacturer. Assays were performed in triplicate with the mean ± SEM shown.
Protein Determination
All biochemical measures were normalized to the protein content with bovine albumin as standard (Lowry et al., 1951).
Open-Field Task
The locomotor activity (crossings and rearings) were assessed 7 days after ICV injection of ouabain or aCSF using the open-field task. This task was carried out in a 40- x 60-cm open field surrounded by 50-cm-high walls made of brown plywood with a frontal glass wall. The floor of the open field was divided into 9 equal rectangles by black lines. The animals were gently placed to explore the arena for 5 minutes. The following behavioral parameters were assessed in the open field test (crossings: total number of square crossings during the entire test period [Ericson et al., 1991]; rearings: total number of erect postures during the entire test period [Ericson et al., 1991]). It is important to note that a 5-minute test is a short test and represents one aspect of motor activity, the initial phase of novelty exploration (Platel and Porsolt, 1982; Thiel et al., 1999).
Statistical Analysis
Data are presented as mean ± SEM. The variables were analyzed according to their distribution. The Shapiro–Wilk’s test for normality was used to this purpose. Among experimental groups, the homogeneity of variances was evaluated by the Levene’s test, whereas the differences were determined by 2-way ANOVA followed by Tukey’s posthoc test when ANOVA was significant. Correlations were assessed using the Pearson’s correlation test. P < .05 was rated as statistically significant.
Results
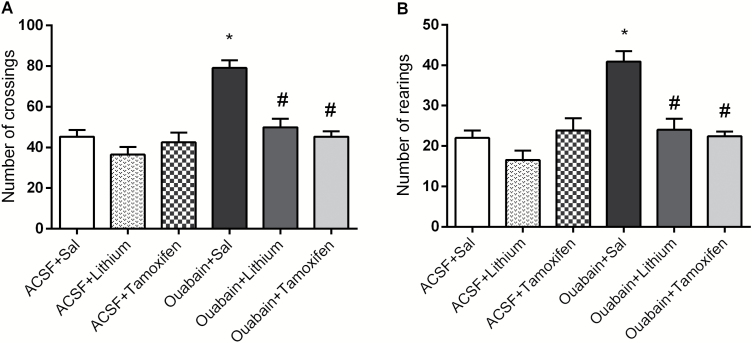
In Figure 1, ouabain increased crossings (A) and rearings (B) in rats, and both Li and TMX reversed ouabain-related hyperactive behavior. The administration of Li or TMX in ACSF-treated animals did not change behavioral measures, indicating that the effects of the drugs in ouabain-treated rats were not associated with sedation. Two-way ANOVA revealed significant effects of ouabain administration [crossings: F(1.38) = 28.19, P<.001; rearings: F(1.38) = 18.06, P<.001] and treatment [crossings: F(2.38) = 16.38, P<.001; rearings: F(2.38) = 12.22, P<.001] and a significant ouabain administration × treatment interaction [crossings: F(2.38) = 8.74, P<.001; rearings: F(2.38) = 9.33, P<.001].
Figure 1.
Effects of the ouabain administration on the number of crossings (A) and rearings (B) in animals submitted to ouabain-induced animal model (n = 10/group). Data were analyzed by 2-way ANOVA followed by the Tukey test when F was significant. Values are expressed as mean ± SEM. *P < .05 compared with ACSF group. #P < .05 compared with ouabain group.
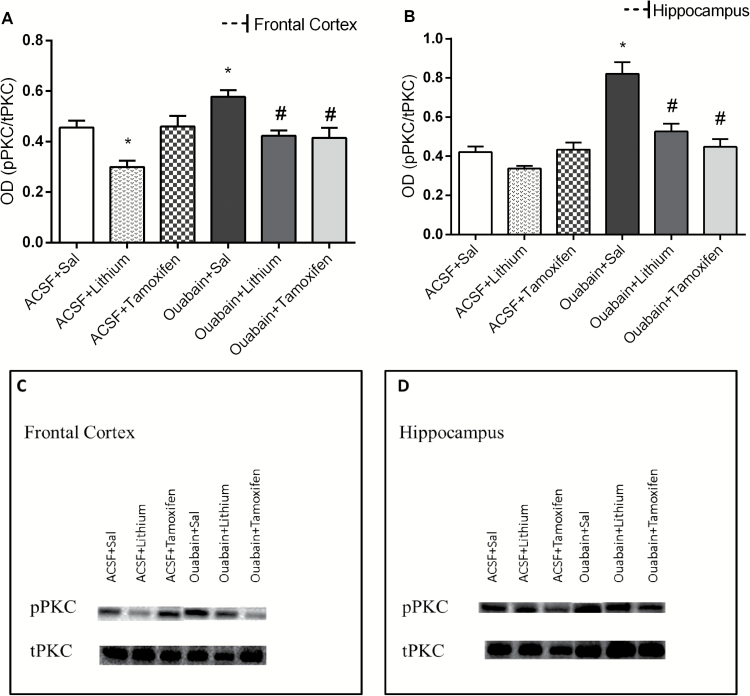
It can be observed in Figure 2 that ouabain administration increased PKC phosphorylation in rat frontal cortex (A) and hippocampus (B); however, Li and TMX reversed this enzyme alteration. The treatment with Li per se decreased the PKC phosphorylation. Data from the 2-way ANOVA revealed significant effects of ICV ouabain administration [frontal cortex: F(1.38) = 7.07, P=.011; hippocampus: F(1.38) = 38.87, P<.001] and treatment [frontal cortex: F(2.38) = 13.00, P<.001; hippocampus: F(2.38) = 15.31, P<.001] and a significant ouabain administration × treatment interaction [frontal cortex: F(2.38) = 4.87, P=.013; hippocampus: F(2.38) = 12.27, P<.001].
Figure 2.
Effects of the lithium (Li) and tamoxifen (TMX) administration on the phosphorylation of protein kinase C (PKC) in frontal cortex (A) and hippocampus (B) in animals submitted to ouabain-induced animal model (n = 8/group). Data were analyzed by 2-way ANOVA followed by the Tukey test when F was significant. Values are expressed as mean ± SEM. *P < .05 compared with ACSF group. #P < .05 compared with ouabain group.
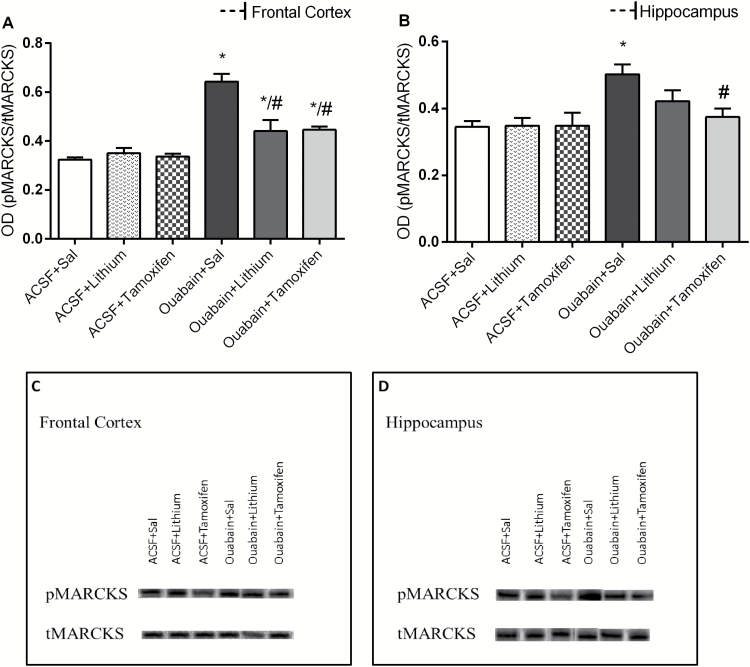
As shown in Figure 3, ouabain increased MARCKS phosphorylation, and the treatment with Li and TMX decreased this enzyme alteration in frontal cortex (A). In the hippocampus (B), only TMX reversed the increase in MARCKS phosphorylation induced by ouabain. Data from the 2-way ANOVA for ICV ouabain administration [frontal cortex: F(1.38) = 68.61, P<.001; hippocampus: F(1.38) = 13.68, P<.001] and treatment [frontal cortex: F(2.38) = 8.61, P<.001; hippocampus: F(2.38) = 2.53, P=.093] and a significant ouabain administration × treatment interaction [frontal cortex: F(2.38) = 12.78, P<.001; hippocampus: F(2.38) = 2.83, P=.072].
Figure 3.
Effects of lithium (Li) and tamoxifen (TMX) administration on the activity of protein kinase C (PKC) in frontal cortex (A) and hippocampus (B) of animals submitted to ouabain-induced animal model (n = 8/group). Data were analyzed by 2-way ANOVA followed by the Tukey test when F was significant. Values are expressed as mean ± SEM. *P < .05 compared with the ACSF group. #P < .05 compared with the ouabain group.
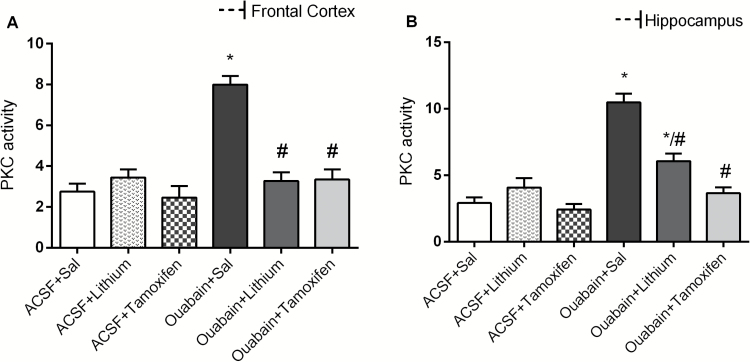
In Figure 4, ouabain increased PKC activity in frontal cortex (A) and hippocampus (B). TMX reversed the PKC activity alteration induced by ouabain in all structures evaluated. Li treatment reversed this enzyme alteration in frontal cortex and partially reversed in hippocampus. Data from the 2-way ANOVA revealed significant effects of ICV ouabain administration [frontal cortex: F(1.38) = 29.19, P<.001; hippocampus: F(1.38) = 63.37, P<.001] and treatment [frontal cortex: F(2.38) = 17.78, P<.001; hippocampus: F(2.38) = 22.47, P<.001] and a significant ouabain administration × treatment interaction [frontal cortex: F(2.38) = 21.31, P<.001; hippocampus: F(2.38) = 20.44, P<.001].
Figure 4.
Effects of lithium (Li) and tamoxifen (TMX) administration on the phosphorylation of myristoylated alanine-rich C kinase substrate (MARCKS) in frontal cortex (A) and hippocampus (B) of animals submitted to ouabain-induced animal model (n = 8/group). Data were analyzed by 2-way ANOVA followed by the Tukey test when F was significant. Values are expressed as mean ± SEM. *P < .05 compared with the ACSF group. #P < .05 compared with the ouabain group.
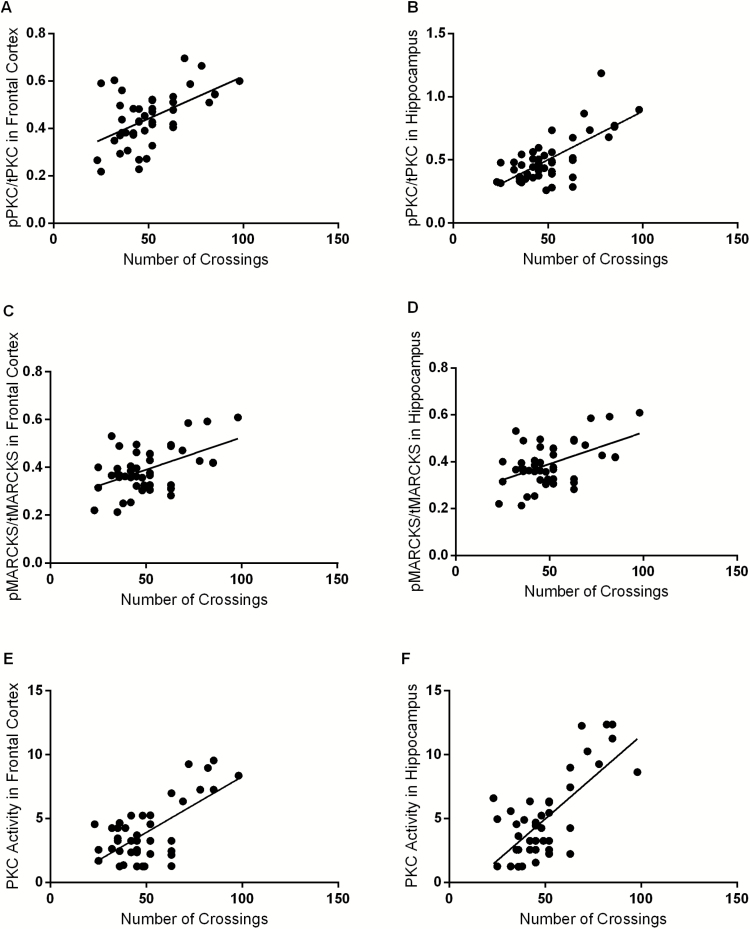
In Figure 5, it can be observed the correlation between locomotor activity and PKC phosphorylation in frontal cortex (A) and hippocampus (B), MARCKS phosphorylation in frontal cortex (C) and hippocampus (D), as also PKC activity in frontal cortex (E) and hippocampus (F) of rats. Locomotor activity was positively correlated with PKC activity in all brain structures evaluated. Data from Pearson correlation to PKC phosphorylation x Crossings [frontal cortex (n = 44; r2 = 0.29; P < .001), hippocampus (n = 44; r2 = 0.48; P < .001)], MARCKS phosphorylation x Crossings [frontal cortex (n = 44; r2 = 0.25; P < .001), hippocampus (n = 44; r2 = 0.25; P < .001)] and PKC activity x Crossings [frontal cortex (n = 44; r2 = 0.44; P < .001), hippocampus (n = 44; r2 = 0.5; P < .001)].
Figure 5.
Correlations between locomotor activity (number of crossings) and protein kinase C (PKC) phosphorylation in frontal cortex (A). Correlations between locomotor activity (number of crossings) and PKC phosphorylation in hippocampus (B). Myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation in frontal cortex (C). Correlations between locomotor activity (number of crossings) and MARCKS phosphorylation in hippocampus (D); and PKC activity in frontal cortex (E). Correlations between locomotor activity (number of crossings) and PKC activity in hippocampus (F) of animals submitted to ouabain-induced animal model. Results were assessed using the Pearson correlation test. PKC phosphorylation x crossings [frontal cortex (n=44; r2=0.29; P<.001), hippocampus (n = 44; r2 = 0.48; P<.001)]. MARCKS phosphorylation x crossings [frontal cortex (n = 44; r2 = 0.25; P<.001), hippocampus (n = 44; r2 = 0.25; P<.001)]. PKC activity x crossings [frontal cortex (n = 44; r2 = 0.44; P<.001), hippocampus (n = 44; r2 = 0.5; P<.001)].
Discussion
The ICV administration of ouabain in rats induces hyperlocomotion, mimicking the manic-like behavior (Machado-Vieira et al., 2004; Valvassori et al., 2013). In the present study, the administration of Li and TMX reverses the hyperlocomotion induced by ouabain. Previous studies also showed that Li and TMX reversed the hyperlocomotion induced by amphetamine (Einat et al., 2007; Sabioni et al., 2008) and by sleep deprivation (Armani et al., 2012; Abrial et al., 2014). TMX, which is also an estrogenic inhibitor, triggers antimanic effect assigned to inhibition of PKC (Sabioni et al., 2008). Furthermore, Sabioni and colleagues (2008) have demonstrated that the administration of Li, TMX, or chelerythrine (a specific PKC inhibitor) completely blocked the amphetamine-induced manic-like behavior. However, while an intermediate dose of medroxyprogesterone (an estrogenic inhibitor) partially reduced the amphetamine-induced hyperlocomotion, lower and higher doses produced no effect. These findings suggest a pivotal role for PKC inhibition in the antimanic-like effect of TMX, although its antiestrogenic properties may also have contributed to this effect.
Indeed, the present study demonstrated that ouabain increases PKC activity and phosphorylation. This study also showed that there is a positive correlation between manic-like behavior and PKC activity and phosphorylation in the frontal cortex and hippocampus of rats. In addition, the antimanic effects of Li and TMX was accompanied by decreases in the PKC activity and phosphorylation. Accordingly, Abrial and colleagues (2013) showed that PKC activation induces manic-like behaviors, whereas PKC inhibition produces antimanic-like effects. Several evidences indicate that inhibition of the Na+/K+-ATPase by ouabain induces depolarization and subsequent neuronal excitation, which leads to increased intracellular Ca2+ levels via the Na+-Ca2+ exchange system and neurotransmitter release (Scheiner-Bobis, 2002; Albers and Siegel, 2012). These molecular events have been associated with hyperactivity in rats and manic episodes in BD patients (Jornada et al., 2010; Banerjee et al., 2012). An in vitro study performed by Wu and coworkers (2015) using ventricular myocytes showed that ouabain markedly increased late sodium current and reversed the Na+-Ca2+ exchange. Interestingly, these effects induced by ouabain were suppressed by bisindolylmaleimide, a PKC inhibitor. The authors also showed that ouabain increases PKC activation, leading to phosphorylation of membrane Na+ channels (Wu et al., 2015). Therefore, these studies and our data suggest that PKC activity can be mediated and modulated by effects of ouabain.
To evaluate the PKC signaling pathway involved in the effects of ouabain, we evaluated MARCKS phosphorylation in the brain of ouabain-administered rats. As previously described, MARCKS is a substrate of PKC implicated in the transduction of calcium- and PKC-mediated signaling events (Ramakers et al. 1999). The present study demonstrated that ouabain induces increase in MARCKS phosphorylation in the frontal cortex and hippocampus of rats. In addition, a positive correlation was demonstrated between manic-like behavior and MARCKS phosphorylation. According to our results, Szabo and colleagues (2009) detected an increase in MARCKS phosphorylation in the frontal cortex of rats submitted to the animal model of mania induced by amphetamine and sleep deprivation. A clinical study demonstrated an increase of MARCKS expression in the platelets collected from BD patients (Pandey et al., 2002). Additionally, MARCKS mRNA expression was found to have increased in the dorsolateral prefrontal cortex of BD subjects (Konopaske et al., 2015). Together with our data, these findings indicate an important role of MARCKS in both manic-like behaviors and BD pathophysiology.
In the present study, treatments with Li and TMX reversed the increase of MARCKS phosphorylation induced by ouabain in the frontal cortex of rats. In the hippocampus, only TMX significantly reversed this enzyme alteration. In accordance with our data, Szabo and coworkers (2009) showed a decrease of MARCKS phosphorylation after the treatment with Li. Furthermore, a previous study demonstrated that chronic treatment with Li decreased the hippocampal levels of the MARCKS protein (Lenox et al., 1992). Watson and coworkers (1998) showed that valproate, at therapeutic concentrations, induces a concentration- and time-dependent reduction of MARCKS in immortalized hippocampal cells. Collectively, these findings suggest that the regulation of MARCKS is a target for the action of mood stabilizers whose effect is mediated by PKC and may be specific to a class of drugs effective in the treatment of BD.
In conclusion, we showed an important relationship between the manic-like behavior and alterations in the PKC signaling pathway induced by ouabain in rats. Moreover, treatments with Li or TMX prevented the manic-like behavior while protecting the brain against alterations in the PKC signaling pathway. Therefore, we can suggest that the Na+/K+-ATPase inhibition observed in BD patients may be associated with alterations in the PKC signaling pathway. Moreover, we can provide additional evidence for the involvement of the PKC signaling pathway in the therapeutic effects of Li and TMX.
The brain structures used in this study were dissected entirely, not taking into account the specific subareas of frontal cortex and hippocampus.
Statement of Interest
None.
Acknowledgments
The Translational Psychiatry Program (USA) is funded by the Department of Psychiatry and Behavioral Sciences, McGovern Medical School, The University of Texas Health Science Center at Houston (UTHealth). Laboratory of Neurosciences (Brazil) is one of the centers of the National Institute for Molecular Medicine (INCT-MM) and one of the members of the Center of Excellence in Applied Neurosciences of Santa Catarina (NENASC). Its research is supported by grants from CNPq, FAPESC; Instituto Cérebro e Mente and UNESC. J.Q. is a 1A CNPq Research Fellow.
Glossary
- ACSF
artificial cerebrospinal fluid
- MARCKS
myristoylated alanine-rich C kinase substrate
References
- Abrial E, Etievant A, Bétry C, Scarna H, Lucas G, Haddjeri N, Lambás-Señas L (2013) Protein kinase C regulates mood-related behaviors and adult hippocampal cell proliferation in rats. Prog Neuropsychopharmacol Biol Psychiatry 43:40–48. [DOI] [PubMed] [Google Scholar]
- Abrial E, Bétourné A, Etiévant A, Lucas G, Scarna H, Lambás-Señas L, Haddjeri N (2014) Protein kinase C inhibition rescues manic-like behaviors and hippocampal cell proliferation deficits in the sleep deprivation model of mania. Int J Neuropsychopharmacol 18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A. (1992) The MARCKS brothers: a family of protein kinase C substrates. Cell 71:713–716. [DOI] [PubMed] [Google Scholar]
- Albers RW, Siegel GJ (2012) Membrane transport. In: Basic neurochemistry: principles of molecular, cellular and medical neurobiology, 8th ed ( ST, Brady GJ, Siegel RW, Albers D, Price, eds), pp 41–62. Cambridge, MA: Elsevier Academic Press. [Google Scholar]
- American Psychiatric Association (2014) DSM-5: manual diagnóstico e estatístico de transtornos mentais, 5th ed Porto Alegre: Artmed. [Google Scholar]
- Amrollahi Z, Rezaei F, Salehi B, Modabbernia AH, Maroufi A, Esfandiari GR, Naderi M, Ghebleh F, Ahmadi-Abhari SA, Sadeghi M, Tabrizi M, Akhondzadeh S (2011) Double-blind, randomized, placebo-controlled 6-week study on the efficacy and safety of the tamoxifen adjunctive to lithium in acute bipolar mania. J Affect Disord 129:327–331. [DOI] [PubMed] [Google Scholar]
- Armani F, Andersen ML, Andreatini R, Frussa-Filho R, Tufik S, Galduróz JC (2012) Successful combined therapy with tamoxifen and lithium in a paradoxical sleep deprivation-induced mania model. CNS Neurosci Ther 18:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee U, Dasgupta A, Rout JK, Singh OP (2012) Effects of lithium therapy on Na+–K+-ATPase activity and lipid peroxidation in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 37:56–61. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, Gobeske KT, Sweatt JD, Manji HK, Arnsten AF (2004) Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science 306:882–884. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Stern E, Ricketts S, Martinez D, de Asis J, White T, Epstein J, Isenberg N, McBride PA, Kemperman I, Emmerich S, Dhawan V, Eidelberg D, Kocsis JH, Silbersweig DA (1999) Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 156:1986–1988. [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. [DOI] [PubMed] [Google Scholar]
- Cechinel-Recco K, Valvassori SS, Varela RB, Resende WR, Arent CO, Vitto MF, Luz G, de Souza CT, Quevedo J (2012) Lithium and tamoxifen modulate cellular plasticity cascades in animal model of mania. J Psychopharmacol 26:1594–1604. [DOI] [PubMed] [Google Scholar]
- De Souza CT, Gasparetti AL, Pereira-da-Silva M, Araújo EP, Carvalheira JB, Saad MJ, Boschero AC, Carneiro EM, Velloso LA (2003) Peroxisome proliferator-activated receptor gamma coactivator-1-dependent uncoupling protein-2 expression in pancreatic islets of rats: a novel pathway for neural control of insulin secretion. Diabetologia 46:1522–1531. [DOI] [PubMed] [Google Scholar]
- DiazGranados N, Zarate CA Jr (2008) A review of the preclinical and clinical evidence for protein kinase C as a target for drug development for bipolar disorder. Curr Psychiatry Rep 10:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, Manji HK, Chen G (2003) The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci 23:7311–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H, Yuan P, Szabo ST, Dogra S, Manji HK (2007) Protein kinase C inhibition by tamoxifen antagonizes manic-like behavior in rats: implications for the development of novel therapeutics for bipolar disorder. Neuropsychobiology 55:123–131. [DOI] [PubMed] [Google Scholar]
- El-Mallakh RS, El-Masri MA, Uhf MO, Li XP, Decker S, Levy RS (2003) Intracerebroventricular administration of ouabain as a model of mania in rats. Bipolar Disord 5:362–365. [DOI] [PubMed] [Google Scholar]
- Ericson E, Samuelsson J, Ahlenius S (1991) Photocell measurements of rat motor activity. A contribution to sensitivity and variation in behavioral observations. J Pharmacol Methods 25:111–122. [DOI] [PubMed] [Google Scholar]
- Fallah E, Arman S, Najafi M, Shayegh B (2016) Effect of tamoxifen and lithium on treatment of acute mania symptoms in children and adolescents. Iran J Child Neurol 10:16–25. [PMC free article] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF (2009) Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci U S A 106:17957–17962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jornada LK, Moretti M, Valvassori SS, Ferreira CL, Padilha PT, Arent CO, Fries GR, Kapczinski F, Quevedo J (2010) Effects of mood stabilizers on hippocampus and amygdala BDNF levels in an animal model of mania induced by ouabain. J Psychiatr Res 44:506–510. [DOI] [PubMed] [Google Scholar]
- Jornada LK, Valvassori SS, Steckert AV, Moretti M, Mina F, Ferreira CL, Arent CO, Dal-Pizzol F, Quevedo J (2011) Lithium and valproate modulate antioxidant enzymes and prevent ouabain-induced oxidative damage in an animal model of mania. J Psychiatr Res 45:162–168. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Subburaju S, Coyle JT, Benes FM (2015) Altered prefrontal cortical MARCKS and PPP1R9A mRNA expression in schizophrenia and bipolar disorder. Schizophr Res 164:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. [DOI] [PubMed] [Google Scholar]
- Lenox RH, Watson DG, Patel J, Ellis J (1992) Chronic lithium administration alters a prominent PKC substrate in rat hippocampus. Brain Res 570:333–340. [DOI] [PubMed] [Google Scholar]
- Li R, el-Mallakh RS, Harrison L, Changaris DG, Levy RS (1997) Lithium prevents ouabain-induced behavioral changes. Toward an animal model for manic depression. Mol Chem Neuropathol 31:65–72. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. [PubMed] [Google Scholar]
- Machado-Vieira R, Kapczinski F, Soares JC (2004) Perspectives for the development of animal models of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 28:209–224. [DOI] [PubMed] [Google Scholar]
- Moretti M, Valvassori SS, Steckert AV, Rochi N, Benedet J, Scaini G, Kapczinski F, Streck EL, Zugno AI, Quevedo J (2011) Tamoxifen effects on respiratory chain complexes and creatine kinase activities in an animal model of mania. Pharmacol Biochem Behav 98:304–310. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, SridharaRao J, Ren X, Janicak PG, Sharma R (2002) Protein kinase C and phospholipase C activity and expression of their specific isozymes is decreased and expression of MARCKS is increased in platelets of bipolar but not in unipolar patients. Neuropsychopharmacology 26:216–228. [DOI] [PubMed] [Google Scholar]
- Platel A, Porsolt RD (1982) Habituation of exploratory activity in mice: a screening test for memory enhancing drugs. Psychopharmacology 78:346–352. [DOI] [PubMed] [Google Scholar]
- Proudfoot JG, Parker GB, Benoit M, Manicavasagar V, Smith M, Gayed A (2009) What happens after diagnosis? Understanding the experiences of patients with newly-diagnosed bipolar disorder. Health Expect 12:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers GMJ, McNamara RK, Lenox RH, De Graan PNE (1999) Differential changes in the phosphorylation of the protein kinase C substrates myristoylated alanine-rich C kinase substrate and growth-associated protein-43/B-50 following schaffer collateral long-term potentiation and long-term depression. J Neurochem 73:2175–2183. [PubMed] [Google Scholar]
- Riegel RE, Valvassori SS, Elias G, Reus GZ, Steckert AV, de Souza B, Petronilho F, Gavioli EC, Dal-Pizzol F, Quevedo J (2009) Animal model of mania induced by ouabain: evidence of oxidative stress in submitochondrial particles of the rat brain. Neurochem Int 55:491–495. [DOI] [PubMed] [Google Scholar]
- Rolstad S, Abé C, Olsson E, Eckerström C, Landén M (2016) Cognitive reserve lessens the burden of white matter lesions on executive functions in bipolar disorder. Psychol Med 46:3095–3104. [DOI] [PubMed] [Google Scholar]
- Sabioni P, Baretta IP, Ninomiya EM, Gustafson L, Rodrigues AL, Andreatini R (2008) The antimanic-like effect of tamoxifen: behavioural comparison with other PKC-inhibiting and antiestrogenic drugs. Prog Neuropsychopharmacol Biol Psychiatry 32:1927–1931. [DOI] [PubMed] [Google Scholar]
- Scheiner-Bobis G. (2002) The sodium pump. Its molecular properties and mechanics of ion transport. Eur J Biochem 269:2424–2433. [DOI] [PubMed] [Google Scholar]
- Steckert AV, Valvassori SS, Mina F, Lopes-Borges J, Varela RB, Kapczinski F, Dal-Pizzol F, Quevedo J (2012) Protein kinase C and oxidative stress in an animal model of mania. Curr Neurovasc Res 9:47–57. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Machado-Vieira R, Yuan P, Wang Y, Wei Y, Falke C, Cirelli C, Tononi G, Manji HK, Du J (2009) Glutamate receptors as targets of protein kinase C in the pathophysiology and treatment of animal models of mania. Neuropharmacology 56:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CM, Muller CP, Huston JP, Schwarting RKW (1999) High versus low reactivity to a novel environment: behavioural, pharmacological and neurochemical assessments. Neuroscience 93:243–251. [DOI] [PubMed] [Google Scholar]
- Valvassori SS, Budni J, Varela RB, Quevedo J (2013) Contributions of animal models to the study of mood disorders. Rev Bras Psiquiatr 35:S121–131. [DOI] [PubMed] [Google Scholar]
- Watson DG, Watterson JM, Lenox RH (1998) Sodium valproate down-regulates the myristoylated alanine-rich C kinase substrate (MARCKS) in immortalized hippocampal cells: a property of protein kinase C-mediated mood stabilizers. J Pharmacol Exp Ther 285:307–316. [PubMed] [Google Scholar]
- Wu Y, Wang L, Ma J, Song Y, Zhang P, Luo A, Fu C, Cao Z, Wang X, Shryock JC, Belardinelli L (2015) Protein kinase C and Ca2+-calmodulin-dependent protein kinase II mediate the enlarged reverse /NCX induced by ouabain-increased late sodium current in rabbit ventricular myocytes. Exp Physiol 100:399–409. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Manji HK (2006) Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biol Psychiatry 59:1006–1020. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Manji HK (2009) Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs 23:569–582. [DOI] [PMC free article] [PubMed] [Google Scholar]