Abstract
Background and Aims Among the various floral traits involved in pollinator attraction and potentially under selection mediated by pollinators, floral scent/fragrance has been less investigated than other components of floral phenotype. Whether or not pollinator-mediated selection impacts floral scents depends on the heritability of scent/fragrance and the occurrence of some variation within species. Although most studies have investigated how scent varies among species, growing amounts of data are available on variation at the intraspecific level.
Methods The results of 81 studies investigating intraspecific variation of floral scents in 132 taxa were reviewed. For each study, whether variation was found in either identity, proportion or absolute quantities of volatile organic compounds (VOCs) was recorded, as well as information with the potential to explain variation, such as methodology, plant origin or pollination biology.
Key Results Variation was found for almost all investigated species, both among individuals (among and sometimes within populations) and within individuals across different temporal scales. Cases in which such variation is a possible result of pollinator-mediated selection were analysed, by discussing separately selection related to variation in pollinator identity/behaviour among populations or across time, deceit pollination and sex-specific selection. Not surprisingly, in many cases, pollinator-mediated selection alone does not explain the observed variation in floral scent. This led us to review current knowledge on less investigated factors, such as selection mediated by natural enemies, genetic drift and gene flow, environmental constraints, phylogenetic inertia, or biochemical constraints that could be invoked to explain scent variation.
Conclusions This review highlights the great potential of analysing floral scent variation and including it in integrated studies of floral phenotypes. We also have identified the current gaps in our understanding of this complex signal and we propose several methodological and conceptual future directions in this research area.
Keywords: Pollination, chemical ecology, intraspecific variation, evolution, reproductive strategies, odour
INTRODUCTION
The role of pollinator-mediated selection in floral design has been embraced for >250 years by dozens of biologists spanning Kolreuter (1761), Sprengl (1793), Darwin (1862) and Stebbins (1970), to name a few. However, the intraspecific variation of floral traits is a widespread characteristic of angiosperms, and its emergence and maintenance have been widely questioned by some biologists (Galen, 1999; Warren and Mackenzie, 2001; Schemske and Bierzychudek, 2007). While floral scent/fragrance is considered a crucial pollinator attractant, it remains relatively poorly understood compared with visual cues such as flower colour or morphology (Fenster et al., 2004; Dicke, 2006; Raguso, 2008a, b; Whitehead and Peakall, 2009) that together make up a suite of traits that underlie the idea of ‘pollination syndrome’ in nature (Fenster et al., 2004). Floral scent is not easy to quantify, thus data have been slow to accumulate in the literature. This may be attributable to the nature of floral scent itself, which is less obvious than visual cues and requires more specialized equipment and interdisciplinary skills to be characterized (Dicke, 2006). However, during the past decade, the development of highly efficient tools in plant chemical analyses and insect sensory biology has led to a growing body of literature on floral scents and their variation (e.g. Raguso, 2008b). It is now possible to investigate the ecological and evolutionary processes that influence floral scent.
Most of the studies that have investigated the variation in floral scents and its evolutionary implications have focused on interspecific comparisons. Such studies usually investigate which volatile organic compounds (VOCs) may be involved in reproductive isolation between two taxa (by usually attracting distinct pollinators) thereby reducing gene flow and contributing substantially to speciation (Byers et al., 2014; Schiestl, 2015). However, as pointed out by Knudsen (2002) and Raguso (2008a), a prerequisite to this scenario is the occurrence and the heritability of some variation at the intraspecific level. Thus, the description of patterns in intraspecific variation of floral scents, as well as the understanding of the evolutionary and ecological processes involved in the appearance and maintenance of such variation, are crucial points to be investigated.
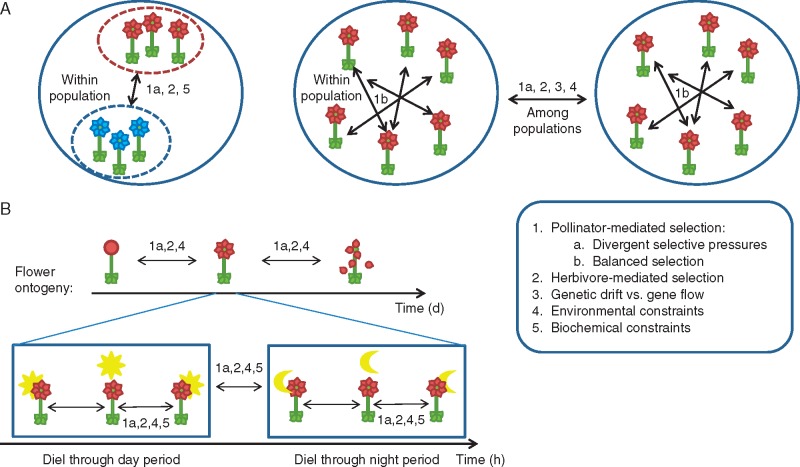
Studying floral scent separately from the other floral traits is somewhat artificial, as it precludes investigation of the interactive value of floral traits (see Fenster et al., 2004). By focusing on floral scent/fragrance, this review constitutes a first step in the incorporation of scent within evolutionary studies of floral phenotype. Specifically, we review the main patterns of variation in floral scent within species at two scales: among individuals at various geographical scales and within individuals across time (Fig. 1). Although these two categories of variation are usually investigated separately, they can be explained by common ecological and/or evolutionary processes. Herein, we consider the different factors that are expected to generate and maintain variation in floral scents. This includes pollinator-mediated selection which is generally the most often discussed cause of scent variation, but we also consider selection mediated by other non-pollinating insects, genetic drift, migration and various forms of constraints. We merge the current knowledge about spatial and temporal variation in order to discuss whether observed variation may actually reflect the impacts of these various factors. This allows us to underline the current gaps and to propose several stimulating perspectives in this research area.
Fig. 1.
Schematic representation of the various scales of floral scent variation and the main ecological and evolutionary processes associated. (A) Variation in floral scents among individuals can occur both within and among populations (solid circles). Within populations, variation can occur between groups of plants (e.g. sexual types, colour phenotypes, dashed circles) or among equivalent individuals. (B) Variation in floral scents within individuals occurs at several time scales: across flower ontogeny, between day and night and along a diel period. In the box are listed the main processes involved in variation of floral scents: (1) Pollinator-mediated selection can generate variation in floral scents (1a) if divergent selection occurs, either because the cost–benefit balance of the interaction with the same pollinator species varies (between plant genders ot between plant phenological stages) or because they interact with different pollinator species (that can vary both among populations and along with time); (1b) if pollination is achieved by deceit, since pollinators can cause variation through balanced selection. (2) If the identity, occurrence or effect of herbivores varies among populations or across time, herbivore-mediated selection can generate variation in floral scent. (3) The balance between the effects of genetic drift and gene flow impact variation among populations, particularly for biologically inactive volatile compounds. (4) The variation in some environmental components that affect the functioning of metabolic pathways or compound release (e.g. temperature, humidity or soil) should cause some variation in floral scents. (5) Finally, biochemical processes may explain some of the observed variations both within individuals (depending on the metabolic rhythms) and between individuals (of different colour).
Literature search and data extraction
We built our bibliographic database in Web of Sciences (until October 2016) by searching published articles containing the words ‘floral/flower’, ‘scent/odo(u)r//fragrance’ and ‘variation’. We retained all original studies that showed a statistical or graphical comparison of floral scents at the intraspecific level, among and/or within individuals, either in natural populations or in controlled conditions (greenhouse or experimental garden), leading to a list of 81 studies carried out on 132 species or subspecies, from 28 Angiosperm families and one Gymnosperm (Table 1). We chose not to include variation in floral scents due to pathogens, wounding or other external treatments. Studies reporting interspecific variation in floral scents were retained only in cases where they also investigated variation within at least one of their studied taxa. We excluded studies that presented variation among individuals for which the geographical origin was unclear. Some of the studies found with our literature search were reviews based on previously published results (Ashman, 2009; Juillet and Scopece, 2010; Ackerman et al., 2011). We cite and discuss these studies but did not include them in Table 1.
Table 1.
Recapitulative table of information for all species
| General |
Type of variation |
Methods |
References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Family/species | Repro | Poll | With Pop | Category | Btw Pop | With Indiv | Behav | N | Extr | |
| Angiosperms | ||||||||||
| Agavaceae | ||||||||||
| Yucca elata | H | S | 2 | qS | 34 | P | Svensson et al. (2006) | |||
| Y. filamentosa | H | S | 1 | Qs | 87 | P | Svensson et al. (2005) | |||
| Amaryllidaceae | ||||||||||
| Narcissus assoanus ssp. assoanus | H | G | 2 | S | 2 | M | Dobson et al. (1997) | |||
| N. a. ssp. praelongus | H | G | 2 | S | 3 | M | Dobson et al. (1997) | |||
| N. bugei | H | G | 2 | 3 | M | Dobson et al. (1997) | ||||
| N. bulbocodium ssp. bulbocodium | H | G | 2 | 3 | M | Dobson et al. (1997) | ||||
| N. gaditanus | H | G | 2 | 3 | M | Dobson et al. (1997) | ||||
| N. jonquilla | H | G | 2 | 2 | M | Dobson et al. (1997) | ||||
| N. papyraceus | H | G | 2 | 5 | M | Dobson et al. (1997) | ||||
| N. serotinus | H | G | 2 | 2 | M | Dobson et al. (1997) | ||||
| N. triandrus ssp. pallidulus | H | G | 2 | 3 | M | Dobson et al. (1997) | ||||
| Pancratium maritimum | H | S | 0 | S | 130 | P | Sanaa et al. (2012) | |||
| Apocynaceae | ||||||||||
| Xysmalobium parviflorum | H | S | 1 | S | 8 | P | Shuttleworth (2016) | |||
| Araceae | ||||||||||
| Homalomena propinqua | H | S | 0 | Sex: QS | DP: QS | T | 10 | P | Kumano-Nomura and Yamaoka (2009) | |
| Philodendron bipinnatifidum | H | S | 1 | Sex: qs | 12 | P | Gottsberger et al. (2013) | |||
| Philodendron form selloum | H | S | 1 | Sex: QS | 8 | P | Gottsberger et al. (2013) | |||
| Anthurium antioquiense | H | – | 0 | DP: S* | 1 | C | Kuanprasert et al. (1998) | |||
| Arecaceae | ||||||||||
| Chamaerops humilis | D | S, N, D | 2 | Sex: Qs | O: QS | 12 | C | Dufa et al. (2004) | ||
| Geonoma macrostachys var. macrostachys | M | G, D | 2 | QS | 54 | P | Knudsen (2002) | |||
| Geonoma macrostachys | M | G, D | 2 | Morphotypes: S* | S* | 22 | P | Borschsenius et al. (2016) | ||
| Asclepediaceae | ||||||||||
| Stephanotis floribunda | H | G | 0 | DP: QS | C | Pott et al. (2002) | ||||
| Ceropegia dolichophylla | H | G, D | 0 | DP: q*, IF: QS | B | 2 | C | Heiduk et al. (2010) | ||
| Asteraceae | ||||||||||
| Cirsium arvense | H | G | 0 | Sex: QS | DN: QS, DP: QS, O: QS | P | Theis et al. (2007) | |||
| C. repandum | H | G | 0 | DN: QS, DP: QS, O: QS | P | Theis et al. (2007) | ||||
| Brassicaceae | ||||||||||
| Arabidopsis thaliana | 0 | Q | 70 | M | Tholl et al. (2005) | |||||
| Hesperis matronalis | H | G | 0 | Colour: qs | QS white M, Qs purple M | DN: QS both M | 31 | M | Majetic et al. (2007) | |
| Hesperis matronalis | H | G | 2 | Colour: qs | QS | 99 | P | Majetic et al. (2008) | ||
| Cactaceae | ||||||||||
| Echinopsis ancistrophora ssp. ancistrophora | H | G | 2 | QS | DN: qs | B | 37 | P | Schlumpberger and Raguso (2008) | |
| Echinopsis chiloensis ssp. chiloensis | H | G | 0 | DN: S | Excl | P | Lemaitre et al. (2014) | |||
| Gymnocalicium bruchii | H | G | 0 | IF: Q* | C | Schlumpberger et al. (2004) | ||||
| G. andreae | H | G | 2 | S | DN: Qs, DP: Qs, IF: Q* | 121 | C | Schlumpberger et al. (2004) | ||
| G. bodenbenderianum spp. intertextum | H | G | 0 | DN: Qs, DP: Qs, IF: QS* | C | Schlumpberger et al. (2004) | ||||
| Rebutia arenacea | H | G | 0 | IF: Q* | C | Schlumpberger et al. (2004) | ||||
| R. fabrisii | H | G | 0 | DN: Qs, DP: Qs, IF: Q* | C | Schlumpberger et al. (2004) | ||||
| R. heliosa | H | G | 0 | IF: Q* | C | Schlumpberger et al. (2004) | ||||
| R. marsoneri | H | G | 0 | DN: Qs, DP: Qs, IF: Q* | C | Schlumpberger et al. (2004) | ||||
| Caryophyllaceae | ||||||||||
| Silene ciliata | H | G, N | 1 | DN=QS* | EAG, Excl | P | Gimenez-Benavides et al. (2007) | |||
| S. dioica | D | G (N) | 0 | DN: qS | Supp | 52 | C | Waelti et al. (2008) | ||
| S. latifolia | D | G, N | 2 | Sex: q | qS | 98 | C | Dötterl et al. (2007) | ||
| S. latifolia | D | G, N | 0 | DN: QS | Supp | 55 | C | Waelti et al. (2008) | ||
| S. latifolia | D | G, N | 0 | Sex: q | qs | DN: Q*, DP: Q*, O: Q*, P: Q*, S: Q* | 98 | C | Dötterl et al. (2005) | |
| S. stellata | H | G,N | 0 | DN: QS* | 5 | P | Castillo et al. (2014) | |||
| S. otites | D | G | 0 | DN: QN, DP: QS | T | 4 | C | Dötterl et al. (2012) | ||
| S. otites | D | G | 1 | Sex: Q | S | B | 63 | C | Salma Juhmur et al. (2008) | |
| S. sennenii | H | G | 0 | DN=S* | Excl | P | Martinell et al. (2010) | |||
| Ericaceae | ||||||||||
| Pyrola grandiflora | H | G | 2 | 4 | P | Knudsen (1994) | ||||
| P. rotundifolia ssp. maritima | H | G | 2 | 9 | P | Knudsen (1994) | ||||
| P. rotundifolia ssp. rotundifolia | H | G | 2 | S | 14 | P | Knudsen (1994) | |||
| Vaccinium corrymbosum | H | G | 0 | DN: Q, DP: QS, P: QS | T, Excl | C | Rodriguez-Saona et al. (2011) | |||
| Fumariaceae | ||||||||||
| Corydalis cava | H | G | 2 | Colour: qs* | S | 35 | P | Olesen and Knudsen (1994) | ||
| Magnoliaceae | ||||||||||
| Magnolia kobus | H | G | 2 | QS | 52 | P | Azuma et al. (2001) | |||
| Magnolia sprengeri | H | G | 1 | O and Sex: S | 1 | P | Wang et al. (2014) | |||
| Mimosoideae | ||||||||||
| Parkia biglobosa | H | G | 2 | QS* | DP: QS | 15 | P | Petterson and Knudsen (2001) | ||
| Moraceae | ||||||||||
| Ficus auriculata | D | S, D, N | 1 | Sex: qS | 8 | P | Hossaert-McKey et al. (2016) | |||
| Ficus carica | D | S, D, N | 0 | Sex: qS, spring ♂/♀; qs, summer ♂/♀ | O: qS | EAG | 23 | M | Soler et al. (2012) | |
| Ficus exasperata | D | S, D, N | 1 | Sex: qS | 23 | P | Hossaert-McKey et al. (2016) | |||
| Ficus fistulosa | D | S, D, N | 1 | Sex: Qs | 11 | P | Hossaert-McKey et al. (2016) | |||
| Ficus fulva | D | S, D, N | 1 | Sex: qs | 7 | P | Hossaert-McKey et al. (2016) | |||
| Ficus hispida | D | S, D, N | 1 | Sex: qs | 18 | P | Hossaert-McKey et al. (2016) | |||
| F. hispida | D | S, D, N | 1 | S | 19 | P | Soler et al. (2011) | |||
| F. hispida | D | S, D, N | 0 | Sex: QS* | O: QS, P: QS | 4 | M | Proffit et al. (2008) | ||
| F. racemosa | M | S, N | 1 | S | 22 | P | Soler et al. (2011) | |||
| F. racemosa | M | S, N | 1 | DN: QN, O: QS | 10 | P | Borges et al. (2013) | |||
| F. semicordata | D | S, D, N | 1 | Sex: qS | 10 | P | Hossaert-McKey et al. (2016) | |||
| F. septica | D | S, D, N | 1 | Sex: qS | 12 | P | Hossaert-McKey et al. (2016) | |||
| Nyctagenaceae | ||||||||||
| Abronia umbellate | H | G | 0 | QS† | 129 | PC | Doubleday et al. (2013) | |||
| Orchidaceae | ||||||||||
| Anacamptis coriophora | H | G | 2 | EAG | 43 | P | Salzman et al. (2007a) | |||
| A. morio | H | G, D | 2 | EAG, Supp | 59 | P | Salzman et al. (2007a) | |||
| Calanthe sylvatica | H | G, D | 2 | Colour: S | S | 58 | P | Delle-Vedove et al. (2011) | ||
| Cypripedium calceolus var. parviflorum | H | D | 2 | 0‡ | 15 | P | Barkman et al. (1997) | |||
| Dactylhoriza romana | H | G, D | 2 | Colour: Q | 37 | P | Salzmann and Schiestl (2007) | |||
| Epidendrum ciliare | H | G, D | 2 | S | O: S | >29 | C | Moya and Ackermann (1993) | ||
| Gongora pleiochroma | H | G | 2 | Colour: s | s | 16 | C | Hetherington-Rauth and Ramirez (2016) | ||
| Gymnadenia conopsea | H | G | 0 | DN: QS | EAG, T | 94 | P | Huber et al. (2005) | ||
| G. odoratissima | H | G | 0 | DN: QS | EAG, T | 118 | P | Huber et al. (2005) | ||
| G. odoratissima | H | G | 1 | qS | >103 | P | Gross et al. (2016) | |||
| Op. arachnitiformis | H | S, D | 2 | Colour: qs | 0§ | B | 45 | M | Vereecken and Schiestl (2009) | |
| Op. exaltata | H | S, D | 1 | s | Trans | >60 | M | Breitkopf et al. (2013) | ||
| Op. exaltata | H | S, D | 2 | T | 161 | M | Mant et al. (2005) | |||
| Op. exaltata | H | S, D | 1 | S | 103 | P | Vereecken and Schiestl (2008) | |||
| Op. garganica | H | S, D | 2 | T | 22 | M | Mant et al. (2005) | |||
| Op. monte-gargano | H | D | 2 | 22 | M | Mant et al. (2005) | ||||
| Op. sphegodes | H | S, D | 1 | S | Trans | >60 | M | Breitkopf et al. (2013) | ||
| Op. sphegodes | H | D | 2 | T | 142 | M | Mant et al. (2005) | |||
| Op. sphegodes | H | S, D | 0 | DN: Q, P: QS | B | 63 | M | Schiestl et al. (1997) | ||
| Orchis mascula | H | G, D | 2 | Colour: s | 36 | P | Dormont et al. (2010) | |||
| Or. mascula | H | G, D | 2 | S | 76 | P | Salzman et al. (2007b) | |||
| Or. pauciflora | H | G, D | 2 | S | 76 | P | Salzman et al. (2007b) | |||
| Platanthera bifolia | H | G | 2 | QS* | 34 | P | Tollsten and Bergstrom (1993) | |||
| P. chlorantha | H | G | 2 | QS* | 25 | P | Tollsten and Bergstrom (1993) | |||
| Schizochilus angustifolius | H | G | 0 | 5 | P | Van der Niet et al. (2010) | ||||
| S. bulbinella | H | G | 0 | QS | 10 | P | Van der Niet et al. (2010) | |||
| S. flexuosus | H | G | 0 | QS | 13 | P | Van der Niet et al. (2010) | |||
| S. zeyheri | H | G | 0 | QS | 5 | P | Van der Niet et al. (2010) | |||
| Phyllanthaceae | ||||||||||
| Antidesma japonicum | D | G | 1 | Sex: s | 21 | M | Okamoto et al. (2013) | |||
| Breynia vitis-idaea | M | S, N | 1 | Sex: S | 34 | M | Okamoto et al. (2013) | |||
| Breynia vitis-idaea | M | S, N | 0 | Sex: QS | EAG, B | 37 | P | Svensson et al. (2010) | ||
| Flueggea suffruticosa | M | G | 1 | Sex: s | 5 | M | Okamoto et al. (2013) | |||
| Glochidion acuminatum | M | S, N | 1 | Sex: S | 18 | M | Okamoto et al. (2013) | |||
| G. daltonii | M | S,N | 1 | Sex:QS | M | Huang et al. (2015) | ||||
| G. eriocarpum | M | S,N | 1 | Sex:QS | M | Huang et al. (2015) | ||||
| G. hirsutum | M | S,N | 1 | Sex: QS | M | Huang et al. (2015) | ||||
| G. lanceolatum | M | S, N | 1 | Sex: S | 14 | M | Okamoto et al. (2013) | |||
| G. obovatum | M | S, N | 1 | Sex: S | 36 | M | Okamoto et al. (2013) | |||
| G. rubrum | M | S, N | 1 | Sex: S | 12 | M | Okamoto et al. (2013) | |||
| G. sphaerogynum | M | S,N | 1 | Sex: QS | M | Huang et al. (2015) | ||||
| G. zeylanicum | M | S,N | 1 | Sex: QS | M | Huang et al. (2015) | ||||
| G. zeylanicum | M | S, N | 1 | Sex: S | B | 12 | M | Okamoto et al. (2013) | ||
| Phyllanthus flexuosus | M | G | 1 | Sex: s | 14 | M | Okamoto et al. (2013) | |||
| P. reticulatus | M | S, N | 1 | Sex: S | 32 | M | Okamoto et al. (2013) | |||
| P. roseus | M | G | 1 | Sex: s | 10 | M | Okamoto et al. (2013) | |||
| Plantaginaceae | ||||||||||
| Penstemon digitalis | H | G | 0 | Colour: S | S | 42 | C | Parachnowitsch et al. (2012) | ||
| Penstemon digitalis | H | G | 0 | DN–DP: QS | 12 | P | Burdon et al. (2015) | |||
| Polemoniaceae | ||||||||||
| Linanthus dichotomus subsp. dichotomus | H | S | 2 | Q | 38 | C | Chess et al. (2008) | |||
| L. dichotomus sbsp. meridianus | H | G | 2 | Q | DN: QS* | 41 | C | Chess et al. (2008) | ||
| Phlox divaricata | H | G | 1 | DP: QS* | 5 | C | Majetic et al. (2015) | |||
| Polemonium viscosum | H | G | 2 | B, Supp | 16 | P | Galen et al. (2011) | |||
| Primulaceae | ||||||||||
| Primula elatior | H | G | 0 | Sex: s | 30 | P | Gaskett et al. (2005) | |||
| P. farinosa | H | G | 0 | Sex: s | 39 | P | Gaskett et al. (2005) | |||
| Proteaceae | ||||||||||
| Protea caffra | H | G | 0 | O: QS | 5 | L | Steenhuisen et al. (2010) | |||
| P. dracomontana | H | G | 0 | O: QS | 5 | L | Steenhuisen et al. (2010) | |||
| P. simplex | H | G | 0 | O: QS | 5 | L | Steenhuisen et al. (2010) | |||
| P. welwitschii | H | G | 0 | O: QS | 5 | L | Steenhuisen et al. (2010) | |||
| Ranunculaceae | ||||||||||
| Trollius europeaus | H | S, N | 2 | qS | IF: S | Supp | 47 | P | Ibanez et al. (2010) | |
| Saxifragaceae | ||||||||||
| Lithophragma affine | H | S | 1 | O: Qs | 19 | C | Friberg et al. (2013) | |||
| L. cymbalaria | H | S | 1 | O: Qs | 14 | C | Friberg et al. (2013) | |||
| L. heterophyllum | H | S | 1 | 15 | C | Friberg et al. (2013) | ||||
| L. parviflorum | H | S | 1 | 23 | C | Friberg et al. (2013) | ||||
| Scrophulariaceae | ||||||||||
| Antirrhinum majus | H | G | 0 | QS† | EAG, B | 70 | C | Suchet et al. (2011) | ||
| Scrophulariaceae | ||||||||||
| Buddleja fallowiana | 2 | S | 9 | P | Gong et al. (2014) | |||||
| B. officinalis | 2 | s | 14 | P | Gong et al. (2014) | |||||
| Solanaceae | ||||||||||
| ‘Rastroensis’ | H | G | 0 | DN: QS* | 4 | C | Raguso et al. (2006) | |||
| Nicotiana africana | H | G | 0 | DN: qs* | 5 | C | Raguso et al. (2006) | |||
| N. alata | H | G | 0 | DN: QS* | 3 | C | Raguso et al. (2003) | |||
| N. alata | H | G | 0 | QS* | cf2003 | 4 | C | Raguso et al. (2006) | ||
| N. bonariensis | H | G | 0 | DN: QS* | 3 | C | Raguso et al. (2003) | |||
| N. bonariensis | H | G | 0 | QS* | cf2003 | 4 | C | Raguso et al. (2006) | ||
| N. cavicola | H | G | 0 | 1 | C | Raguso et al. (2006) | ||||
| N. forgetiana | H | G | 0 | DN: QS* | 4 | C | Raguso et al. (2003) | |||
| N. ingulba | H | G | 0 | 1 | C | Raguso et al. (2006) | ||||
| N. langsdorffii | H | G | 0 | DN: QS* | 4 | C | Raguso et al. (2003) | |||
| N. langsdorffii | H | G | 0 | QS* | DN: QS* | 6 | C | Raguso et al. (2006) | ||
| N. longiflora | H | G | 0 | DN: QS* | 3 | C | Raguso et al. (2003) | |||
| N. mutabilis | H | G | 0 | DN: QS* | 4 | C | Raguso et al. (2006) | |||
| N. rustica | H | G | 0 | DN: QS* | 4 | C | Raguso et al. (2003) | |||
| N. suaveolens | H | G | 0 | DN: QS* | 4 | C | Raguso et al. (2003) | |||
| N. sylvestris | H | G | 0 | DN: QS* | 3 | C | Raguso et al. (2003) | |||
| Petunia axillaris | H | G | 0 | QS | 13 | C | Kondo et al. (2006 | |||
| Vitaceae | ||||||||||
| Vitis vinifera ssp. sylvestris | D | G | 1 | Sex=qS | 16 | C | Zito et al. (2016) | |||
| Zamiaceae | ||||||||||
| Encephalartos villosus | H | G | 0 | S | 73 | P | Suinyuy et al. (2012) | |||
| Gymnosperms | ||||||||||
| Macrozamia lucida | D | S | 0 | DP: S* | EAG | P | Terry et al. (2007) | |||
General features include: (1) reproductive system: Repro (H, hermaphroditism; M, monoecy, D, dioecy); (2) pollination system: Poll (S, specialized; G, generalized; D, deceit pollination; N, nursery pollination). Type of variation informs about variation (3) within populations: With Pop (0, no information; 1, only a few metrics of variation, e.g. s.d., coefficients of variation; 2, several metrics and/or graphics, variation mentioned and discussed), ‘Category’ referring to comparisons between sex or colour categories of individuals; (4) among populations: Btw Pop; (5) within individuals across time: With Indiv (DN, day vs. night; DP, diel period; O, with ontogeny; P, before vs. after pollination; IF, between different floral parts; Sex, male vs. female phase). Quantitative (Q) and semi-quantitative (S) variation are coded in lower case letters when no variation was found and in upper case letters otherwise. Methods include: (6) behavourial tests on pollinators: Behav (T, test of odour attraction though insect traps in the field; B, behavioural test with wind tunnel or Y tube, Supp: test of the effect of odour supplementation on insect visitations; Trans, study of pollinator attraction on transplanted plant individuals; EAG. electro-antennography; Excl. pollinator exclusion experiments); (7) number of plant individuals used in the study, N; and (8) condition of odour extraction: Extr (P, natural populations, C, controlled conditions, M, cases implying manipulation such as flower cutting).
Studies for which statistical tests were not available.
Variation occurred between two groups of populations that correspond to different subspecies.
No significant differences were found between individuals of different populations but this was expressed in presence/absence.
No significant differences found between individuals of two populations on a subset of the emitted VOCs corresponding to active compounds.
For all included studies, we extracted the following information: the method used for scent extraction, conditions in which scent extraction was performed (in natural populations vs. controlled conditions), total number of sampled individuals, the scale of variation investigated (geographic vs. temporal), characteristics of scent variation investigated (quantitative and/or semi-quantitative, see below), reproductive system of the plant, and any information provided on the pollination ecology and their pollinators, as well as behavioural tests on pollinators if included in the study. All information is synthesized in Table 1, except detailed information about pollinators that is presented in the Supplementary Data Table S1. For each investigated scale of variation, we aimed at reporting whether some variation effectively occurred. In some cases, it was possible to use the results of statistical tests to assess whether a given scent characteristic significantly varied or not, along with geography or time. When variation was presented graphically without statistical tests, we based our report mainly on the interpretation made by the authors. Regarding the variation among individuals within populations, because there is no way to test statistically for such variation, and also because of the large diversity in the estimates of such variation, we could only report in Table 1 whether any basic data on variation within populations were available from the study (coefficient of variation, variation in occurrence, similarity indexes and graphical representations) and whether this variation was explicitly mentioned and discussed.
Below, we briefly report and describe the basic data extracted from studies (Table 1). We then review the main processes involved in floral scent variation in these 132 taxa: the various forms of selection mediated by pollinators, selection mediated by natural enemies, and non-selective forces. Finally, we briefly present several methodological and conceptual future directions that should improve our understanding of the evolutionary ecology of floral scent.
CHARACTERISTICS OF THE STUDIES INCLUDED IN THE REVIEW
Scales of scent variation
Two main scales of variation have been investigated. Studies that looked at variation among individuals often investigated and found geographical variation by comparing two or more populations at various spatial scales, from nearby populations, a few kilometres away (e.g. Parachnowitsch et al., 2012), to populations located on different continents (e.g. Dötterl et al., 2005; Soler et al., 2011). In only a few studies, basic metrics such as coefficient of variation or graphical representation of individual blends allow evaluation of how floral scents vary among plants within populations, although such a scale of variation is clearly not the primary interest of the authors in most cases.
It is noteworthy that our current knowledge of scent variation among individuals is thus not only incomplete but also probably biased in some cases. Indeed, some studies are based on the sampling of only a small number of individuals per population and provide a graphical representation of the average bouquet per population. In cases of large variation within populations, because of sampling effects, this approach may artificially create a data set with a strong variation among populations, giving the wrong impression that ‘population’ is the main source of scent variation. Regarding the temporal scale, changes in floral scent between day and night were the most investigated and almost always found to be significant. Other types of temporal variation have been studied, such as variation during a diel period (different time periods across 12 h or 24 h), along with the flower ontogeny, and variation associated with post-pollination. Similarly, many of these studies do not discuss and often do not even present the variance among individuals for a given time period.
Methods used for scent extraction and analysis
In only a few studies, the method used involved solid phase micro extraction (SPME; Pott et al., 2002; Raguso et al., 2003, 2006; Dormont et al., 2010; Delle-Vedove et al., 2011; Friberg et al., 2013) or solvent extracts of flowers (Mant et al., 2005; Vereecken and Schiestl, 2008, 2009; Sanaa et al., 2012; Breitkopf et al., 2013). In the majority of cases, dynamic headspace was used to sample floral scents, sometimes combined with other methods (SPME or solvent extracts) to confirm compound identifications or identify particular sites of scent production. Traps used for headspace sampling are composed of a blend of different adsorbents, generally Tenax and Carbogentrap. VOCs are then generally analysed through gas chromatography and mass spectrometry (GC-MS) using an elution of the glass cartridge in a solvent (hexane, dichloromethane or pentane) or thermodesorbtion of the trap. According to the study, scent extraction was performed either in natural populations or in controlled conditions (greenhouse or common garden), or more rarely on flowers harvested for further analyses in the laboratory (generally with a control of the changes in scent emissions induced by the damage).
Investigated characteristics of floral scent
Three different characteristics of floral scents are presented and analysed by various studies. Semi-quantitative (relative proportions of the different VOCs) and qualitative variation (identity of the VOCs) are the most commonly investigated traits. In contrast, although quantification of the emission rate is made possible by the dynamic headspace through the use of internal standards, the absolute quantitative variation (emission rate, generally expressed in ng h−1) has been investigated less often. While both semi-quantitative and quantitative variation were generally analysed with various statistical tools, qualitative variation is presented in tables that in general report the occurrence of VOCs in different blends. The magnitude of such variation is therefore difficult to rank among studies, as it ranges from strikingly different chemotypes (e.g. Suinyuy et al., 2012) through blends that vary only through the occurrence of one or a few minor compounds (e.g. Dormont et al., 2010) to cases in which all investigated individuals produce exactly the same compounds (e.g. Delle-Vedove et al., 2011). For this reason, we could not synthesize the information on qualitative variation in Table 1.
Information and data on pollination ecology
For most of the studied species, the authors provided some information about the pollinators or floral visitors. Although some studies included data on the identity of insect visitors (observations, insect net catches or trapping experiments), the source of information was not always clear and could be based on previous reports from the literature or on assumptions made from the classic idea of ‘pollination syndrome’ (Faegri and Van Der Pijl, 1979), which may lead to some misinterpretation (see below). In Table 1, we only report some basic information on pollination, indicating whether plants are pollinated by one or very few pollinator species (referred to here as ‘specialized’ pollination) or by several species/groups of insects (‘generalized’ pollination). All available information on insect visitors/pollinators is provided in Table S1.
Although the effects of floral scents on pollinators (and in return, the effect of natural selection mediated by pollinators on floral scents) are extensively discussed in most of the studies, very few attempt to investigate directly the role of floral scents on pollinator attraction. This can be done through different approaches, including traps baited with floral compounds or floral extracts, counts of insect visits on odour-supplemented plants or behavioural observations in wind-tunnel or Y-tube tests. The use of electro-antennography (EAG) alone or in combination with gas chromatography (GC-EAD) is a promising approach, since it allows one to identify, among a blend, the VOCs detected by the insects. However, the use of such techniques in combination with the study of the intraspecific variation remains scarce (seven studies).
WHY DOES FLORAL SCENT VARY?
The most invoked explanation for variation in floral traits in general, and for floral scents in particular, is pollinator-mediated selection (reviewed in Fenster et al., 2004). Because floral traits impact both pollinator visitation and pollination efficiency (Young and Stanton, 1990; Jones and Reithel, 2001; Reynolds et al., 2009), they directly affect individual reproductive output and should be under strong selective pressures when the observed variation has a genetic basis (Gigord et al., 2001; Streisfeld and Kohn, 2007; Majetic et al., 2009a). In the three following parts, we review the different scenarios under which pollinator-mediated selection can lead to intraspecific variation in floral traits at geographical and/or temporal scales. First, pollinator identity can vary across populations or with time, thereby leading to perhaps divergent selective pressures at these two scales. Secondly, interactions with pollinators can sometimes generate balanced selection, particularly in deceit pollination, leading to some intrapopulation variation in floral scents. Thirdly, in species in which several sexual morphs exist, selective pressures mediated by pollinators can differ between morphs, thus leading to intersexual morph variation (or between male and female flowers/phases in monoecious and hermaphroditic species). Finally, we review the current knowledge on factors others than pollinator-mediated selection, which have been less investigated so far: selection mediated by natural enemies and non-selective factors.
Does scent variation reflect divergence in pollinator-mediated selection?
Because pollinators are expected to mediate selective pressures on floral scents, any variation in their occurrence and/or their impact on plant reproduction should also influence the force and the direction of selection. Here, we first investigate whether differences in floral scents among populations or groups of populations necessarily reflect differences in pollination biology, as expected under the hypothesis of local adaptation to pollinators. Then, we investigate whether patterns of temporal variation in floral scents can be explained by some temporal variation in pollinator occurrence or behaviour that would lead to shifts in the strength or the direction of selection.
Scent variation among populations.
Among studies that investigated geographical variation in floral scents, a sub-sample also provided some information on pollinator assemblage, making it possible to investigate whether scent and pollinator variation show some consistency. This has been done at different taxonomic scales, by comparing several populations within a given plant species (Dötterl et al., 2005, 2007; Mant et al., 2005; Svensson et al., 2005; Jhumur et al., 2008; Schlumberger and Raguso, 2008; Vereecken and Schiestl, 2008, 2009; Ibanez et al., 2010; Soler et al., 2011; Parachnowitsch et al., 2012; Suinyuy et al., 2012; Gross et al., 2016), different geographical ecotypes or subspecies (Chess et al., 2008; Schlumberger and Raguso, 2008; Suchet et al., 2011; Breitkopf et al., 2013; Doubleday et al., 2013) or closely related allopatric species (Svensson et al., 2006). Among the studies included in the present review, we found that variation in floral scents only rarely matches (only in five studies) with variation in pollinator identities. For example, Ophrys sphegodes exhibited two distinct scent profiles between ecotypes that were found to attract different pollinators, suggesting adaptation to locally available pollinators (Breitkopf et al., 2013). Between Linanthus dichotomus ssp. meridianus and L. dichotomus ssp. dichotomus (more specialized on noctuid pollinators), significant differences were found, with higher quantities of VOCs attracting noctuid moths (e.g. lilac aldehydes) emitted by the dichotomus subspecies (Chess et al., 2008). Results found in Echinopsis ancistrophora ssp. ancistrophora were less clear: this subspecies contains two groups of populations differing in their pollinators (bees vs. moths), which also show some small differences in terms of scent composition. However, compounds usually associated with moth pollination were not found in the moth-pollinated plants and moths were equally attracted by the two groups of plants (Schlumpberger and Raguso, 2008). Doubleday et al. (2013) found both quantitative and semi-quantitative variation between two subspecies of Abronia umbellata that differ in their mating systems, with smaller quantities of scents detected in selfing populations compared with outcrossing populations (probably from relaxed selection). Finally, the most convincing evidence for pollinator-mediated selection has been provided by Gross et al. (2016) on Gymnadenia odoratissima, in which not only the semi-quantitative variation in floral scent between lowland and mountain populations seems consistent with the variation in pollinator assemblage, but the measurement of selective gradients directly shows that selection favours different blends between the two habitats.
However, pollinator-mediated selection does not appear to be the only explanation for geographical variation in floral scents. Indeed, three studies showed no significant difference in floral scents among populations/taxa that were associated with different pollinators. In Silene latifolia, Dötterl et al. (2005) found an extraordinary (and unexplained) variation among populations, but no significant difference between European and North American populations, despite a difference in pollinator taxa between the two continents. This study, however, analysed the full bouquet composition, with VOCs probably not involved in pollinator attraction. The wide variation in floral scents could hide a possible difference in the VOCs effectively involved in the attraction of European vs. North-American pollinators (see below). In the very specialized Yucca genus, two studies found very low variation among populations, even when comparing groups of plants associated with different insect species: between Y. filamentosa and Y. elata (Svensson et al., 2006), and between two groups of populations of Y. filamentosa (Svensson et al., 2005). The strong homogeneity within Y. filamentosa is possibly due either to a recent pollinator shift or to the obligatory nature of this interaction which could constrain the evolution of chemical communication (Svensson et al., 2006).
Finally, in most cases, studies found the opposite pattern, with some variation in floral scents but no documented variation in pollinators among the sampled populations (Dötterl et al., 2005; Jhumur et al., 2008; Suchet et al., 2011; Parachnowitsch et al., 2012; Suinyuy et al., 2012), including five cases of highly specific pollination systems [Ophrys exaltata/Colletes cunicularius (Mant et al., 2015); O. arachnitiformis/C. cunicularius (Vereecken and Schiestl, 2008, 2009); Trollius europeaus/Chiastocheta sp. (Ibanez et al., 2010); Ficus hispida/Ceratosolen solmsi ssp. marchali and F. racemosa/C. fusciceps (Soler et al., 2011)]. Of course, since only a part of the VOCs available in a given scent is usually involved in pollinator attraction (e.g. Huber et al., 2005; Salzmann et al., 2007a; Svensson et al., 2010), a part of the observed variation may be neutral from the pollinator’s perspective (Raguso et al., 2003; Huber et al., 2005; Dötterl et al., 2007). In a few cases, additional information about which VOCs in the blend can be actively detected by pollinator(s) was available and provided contrasting results. In S. latifolia, whose floral scent shows a spectacular variation among populations in both Europe and North America (Dötterl et al., 2005), it appears that only lilac aldehyde is responsible for most of the attraction of the main pollinator (Hadena moth) in European populations (Dötterl et al., 2006), in particular the ratio of the various isomers of lilac aldehyde, which is particularly stable in these locations (Dötterl et al. 2007; but see Schneider et al., 2013). This might suggest that all other VOCs are more or less neutral for pollinator attraction, and that the spectacular variation did not result from pollinator-mediated selection but from other processes. In contrast, in several Ophrys species (Mant et al., 2005) and in Trollius europaeus (Ibanez et al., 2010), some important variation was found among populations associated with the same pollinator, even when focusing on active VOCs. Alternative explanations (e.g. genetic drift or hybridization) were sometimes proposed by the authors and will be discussed below. These different results also suggest that a given interaction can vary in some important traits from one locality to another. This is consistent with the predictions from the theory of a geographic mosaic of coevolution (Thompson, 1996), which postulates that one given interaction between two species often exhibits some differences among populations, in terms of genetics, demography or communities of associated species. Such differences can lead to some variation in the selective pressures involved in the coevolutionary processes and generate a geographic mosaic rather than a homogeneous interaction (Thompson, 1996). For example, although Ophrys exaltata is pollinated by Colletes cunicularius in every studied locality, significant inter-region differences were found in active VOCs (Mant et al., 2005). As floral scents mimic the pheromone of the pollinator in this system (but see Vereecken and Schiestl, 2008), any geographical difference in the composition of the pheromone could lead to some divergence in the floral scents, through adaptation to the local characteristics of the pollinator species (Mant et al., 2005).
Temporal scent variation.
Because pollinators are not always needed or not always active, the benefit of attracting them varies across time, which should lead to a decrease or even an absence of scent production when energetic costs of scent emission are not counterbalanced by benefits.
At a large temporal scale, one should thus expect differences between receptive flowers and other phenological stages in the emission rate of VOCs, in particular those involved in pollinator attraction, leading to quantitative, semi-quantitative and possibly qualitative variation across time. At this scale, temporal variation in floral scents includes the effect of the stage of the flower (e.g. floral bud, receptive flowers or pollinated flowers) or the effect of flower age from anthesis to senescence. In most documented cases, some scent emission has been detected in non-receptive phases, but with a significantly lower quantity compared with the peak associated with anthesis (Dufa et al., 2004; Dötterl et al., 2005; Theis et al., 2007; Steenhuisen et al., 2010), including post-pollination changes (Schiestl et al., 1997; Dötterl et al., 2005; Proffit et al., 2008; Rodriguez-Saona et al., 2011). A decrease in attractiveness thus seems more often to be due to a decrease in VOC quantity rather than a shut off. Moreover, all studies that analysed scent composition across different floral stages found a significant variation in the proportion of VOCs (Table 1), although stage-specific compounds (i.e. released only during floral receptivity) have been rarely documented (found in Ficus racemosa: Borges et al., 2013). Finally, an increase in the proportion of some compounds after pollination has been recorded in some cases, and could function as a repellent (e.g. germacrene A and indole in Ficus hispida; Proffit et al., 2008) that would either prevent additional pollinator visits or deter enemies (Raguso, 2003), and/or allow pollinators to be guided towards unpollinated flowers (Schiestl and Ayasse, 2001). It is noteworthy that a decrease in pollinator attraction can also be beneficial if pollinators ensure a high cost, such as in nursery pollination systems (Dötterl et al., 2005). The impact of the cost induced by natural enemies, and sometimes by pollinators, will be discussed in a later section.
At a more detailed temporal scale, studies often compare scent emission between day and night and sometimes between different time periods across 12 h or 24 h (diel period). When pollination is temporally specialized (diurnal or nocturnal pollination), both (semi-)quantitative and qualitative variation between day and night are expected. For species with a mixed pollination system (pollinated by both diurnal and nocturnal insects), qualitative differences between day and night should occur, reflecting ‘temporally local’ adaptation to different pollinator species if they present different olfactory preference. In terms of scent quantity, one may expect higher amounts at night, as visual cues are sometimes less efficient. Finally, regarding variation along a diel period, one expects scent quantity (in particular active VOCs) to correlate with pollinator activity. In addition to pollinator-mediated selection, environmental constraints such as temperature can impact temporal variation in floral scents and will be discussed in a later section.
Only some of the species with temporally specialized pollination systems show patterns that match these expectations. In Penstemon digitalis, Linanthus dichotomus subsp. meridianus, Silene latifolia, S. stellata, Parkia biglobosa, Vaccinium corrymbosum, Gymnocalycium andreae, Cirsium repandum and C. arvense, the maximal production of odour was observed during the period of pollinator activity (day or night), with almost no or very few emissions outside of this phase (Petterson and Knudsen, 2001; Schlumpberger et al., 2004; Dötterl et al., 2005; Theis et al., 2007; Chess et al., 2008; Rodriguez-Saona et al., 2011; Castillo et al., 2014; Burdon et al., 2015). The same pattern has been found at finer temporal scales in Cirsium arvense that shows a peak of scent emission when pollinators are most active (Theis et al., 2007).
In other species, the patterns of variation do not match with temporal variation in pollinator activity. A first type of incongruence concerns species [Cirsium repandum (Theis et al., 2007) and Phlox divaricata (Majetic et al., 2015)] that show a decrease in scent emission at a phase when (some) pollinators are active, which could be due to a low sensitivity of these pollinators to floral scents. The reverse pattern has also been found, as in four (nocturnal) hawkmoth-pollinated Nicotiana species, which release the maximal quantity of scent at night but also substantial VOC emission during the day (Raguso et al., 2003). Similarly, in Ficus racemosa strictly pollinated by a diurnal wasp, almost no quantitative variation of scent emission was observed between day and night (although there was a reduction in the number of emitted VOCs in the night volatile bouquet) (Borges et al., 2013). Finally, striking incongruences have been found in a few other species: in the two hummingbird- (diurnal) pollinated Nicotiana species (N. langsdorffi and N. forgetiana), the total scent emission reaches its maximal intensity at night (Raguso et al., 2003, 2006). In the primarily day-pollinated Silene dioica, the flowers emit not only similar quantities of scent during night and day, but also a higher relative amount of moth attractants at night (Waelti et al., 2008). In such cases, the question of the factors that maintain floral scent production at times other than the pollinator activity period remains open. It may be related to either phylogenetic constraints, biochemical constraints and/or adaptation to other interacting species [either herbivores or pathogens active outside of the phase of pollinator activity (Raguso et al., 2003, 2006) or nocturnal pollinators that have not been described so far (Waelti et al., 2008)]. Moreover, measurement of costs linked to the scent emission remain technically difficult, reducing our understanding of this process.
The second group comprises species with a mixed pollination system. Each time this was investigated, a quantitative difference between day and night scents was found, with a higher quantity in the evening or night in Hesperis matronalis, Silene otites and S. ciliata (Majetic et al., 2007; Gimenez-Benavides et al., 2007; Dötterl et al., 2012), and a higher quantity during the day in Gymnadenia odoratissima and G. conopsea (Huber et al., 2005). Semi-quantitative differences were found in all studied species, and were often consistent with pollination biology. For instance, Echinopis chiloensis ssp. chiloensis, pollinated by nocturnal hawkmoths and various diurnal bees, releases nocturnal scents dominated by terpenes (usual attractants of hawkmoths) and diurnal scents composed of a larger number of compounds as expected in flowers with different pollinators (Lemaitre et al., 2014). Moreover, in both S. otites and Gymnadenia species, temporal variation was particularly marked for compounds known or suspected to be involved in the attraction of some of the pollinators (Huber et al., 2005; Dötterl et al., 2012). Interestingly, several species with mixed pollination systems (such as as notably many Sileneae species) were at first considered with a nocturnal (or diurnal) pollination syndrome but were found to release high quantities of floral scents and receive efficient insect visits during the day (or night) (Gimenez-Benavides et al., 2007; Lemaitre et al., 2014; Prieto-Benitez et al., 2015). This could reflect past ecological context, ongoing transition in pollination biology or some bet-hedging strategy (that would allow plants to be pollinated even when the preferred pollinator is absent) and clearly highlights the potential bias of studies that consider a priori pollination syndrome.
Determining the role of pollinator-mediated selection on day vs. night variation in floral scent is often precluded by the lack of direct experimental tests. To date, only the study of Majetic et al. (2009a), on mixed-pollinated Hesperis matronalis, has experimentally addressed the adaptive value of temporal variation in floral scents and shown a positive correlation of both emission rates and scent composition (notably for terpenoids) with plant fitness. Nevertheless, the effect of night vs. day scent was found to depend on the context (experimental array vs. wild population), suggesting that the adaptive value of odour variation is probably more complex than usually hypothesized. Correlative studies of scent characteristics and plant fitness are thus needed to understand how selection impacts scent variation.
Within population variation in floral scents: the impact of deceit pollination
Earlier we discussed how directional selection in space or time can promote scent variation. Other forms of selection can generate diversification: pollinator-mediated selection can also promote scent polymorphism within populations by providing a selective advantage to rare floral scents, which is expected when flowers are deceptive (Smithson and McNair, 1997; Gigord et al., 2001). Because pollinators can learn to associate a given signal with the absence of reward, and then subsequently avoid this signal, plants that emit rare signals should be more often visited and thus benefit through higher reproductive success. Pollination by deceit is thus a common explanation when some variation is known within natural populations (e.g. Moya and Ackerman, 1993; Azuma et al., 2001) and many of the studies that investigated variation at this geographical scale are focused on deceptive plants (see Table 1).
A few studies have measured and compared variation in floral scents among orchid species in order to test whether deceptive species exhibit more variation than non-deceptive species. By comparing one rewarding and one deceptive species from the same genus, Salzmann et al. (2007a) found a significantly lower variation in the rewarding species, particularly among biologically active compounds. Similarly, Dormont et al. (2014) found that their two deceptive orchid species emitted a more variable blend than a third, co-flowering and rewarding species. In their large study focusing on 20 deceptive and 41 rewarding orchid species, Ackerman et al. (2011) found the same tendency, but it was not significant. Unfortunately, available data from the studies included in the current review did not allow us to rank species according to their level of scent variation. Indeed, there is a wide variety of ways for describing such variation, including ranges of VOC proportions in the floral bouquet, coefficients of variation for VOC proportions, similarity indexes and graphical representations of individual scents, not to mention a large variation in the sampling size, which impacts the estimates of variation.
We found, however, that the level of variation in scent profiles within populations seems to vary a lot among species but with no obvious trend in regard to deceit pollination. Whereas some rewarding species show remarkable stability in blend composition [e.g. very stable proportions of the main compounds in Gymnocalcium andreae (Schlumpberger et al., 2004) and within-population similarity indexes of around 70 % in four Lithophragma species (Friberg et al., 2013)], dramatic variation was found in both deceptive and rewarding species. In food-deceptive species, such as Epidendrum ciliare or Orchis mascula, some VOCs emitted in high proportion in some individuals were absent from others (Moya and Ackerman, 1993; Dormont et al., 2010), and in food-deceptive Tolumnia variegata, both fragrant and non-fragrant individuals co-occur within populations (Ackerman et al., 1997). Similar variations in scent profiles were found in some rewarding species. The total quantity of scents emitted by flower or inflorescence varied by a factor of 10 in Parkia biglobosa (Petterson and Knudsen, 2001) and 100 in Trollius europeaus (Ibanez et al., 2010). In T. europeaus and Platanthera chlorantha, the proportion of some VOCs varied from 0 to 80 % (Tollsten and Bergström, 1993; Ibanez et al., 2010), while it varied up to two orders of magnitude among individuals of Corydalis cava (Olesen and Knudsen, 1994), Polemonium viscosum (Galen et al., 2011) and Parkia biglobosa (Petterson and Knudsen, 2001).
Although potentially important, negative frequency-dependent selection is thus unlikely to be the only factor involved in floral scent polymorphism within populations. Several studies have investigated this question in deceptive species, either by manipulating local variation of floral scent phenotypes (Ackerman et al., 1997; Salzmann et al., 2007a) or by measuring plant reproductive success as a function of the ratio of floral phenotypes (Pellegrino et al., 2005; Jersáková et al., 2006), but none of them found results consistent with expectations under frequency-dependent selection. Both these studies and the fact that some rewarding species exhibit spectacular levels of variation within population suggest that other factors play a role in promoting variation in floral scent. Finally, it is noteworthy that in some species, the level of variation differs strikingly from one population to another [e.g. Platanthera bifolia (Tollsten and Bergström, 1993) and Echinopsis ancistrophora ssp. ancistrophora (Schlumpberger and Raguso, 2008)]. The comparison of variable vs. non-variable populations constitutes an interesting perspective to better understand the factors involved in floral scent variation.
The role of sex-specific selection mediated by pollinators
The last scenario under which pollinator-mediated selection is expected to generate scent variation is when male and female plants (dioecy) or flowers within plants (monoecy) co-occur, or also in the case of functional shifts in hermaphroditic flowers. On one hand, because the relationship between pollinator attraction and reproductive success can differ between males and females (sexual selection), males should be under stronger selection to attract pollinators, compared with females (Bateman, 1948). Thus, males should produce higher amounts of floral scents. On the other hand, pollinator-mediated selection may also maintain odour similarity between males and females as it ensures pollinator constancy and movements between conspecifics (Ashman, 2009). In a review of 33 dioecious and gynodioecious species, Ashman (2009) found that males (or hermaphrodites) often produced higher amounts of volatiles than females, as expected under sexual selection. Moreover, males and females seem to exhibit similar scent composition more often when females are pollinated by deceit (as expected if the rewardless sex is particularly selected to resemble the rewarding sex).
The few studies included in the current work that were published after Ashman’s review (2009) bring new interesting elements to these conclusions. First, the hypothesis of intersexual mimicry has been reinforced by several studies conducted on dioecious Ficus species, in which males produce rewarding figs while females are pollinated by deceit. Proffit et al. (2008) showed that male and female figs of F. hispida produced distinct floral bouquets except during the phase of receptivity. Hossaert-McKey et al. (2016) showed that in fig species in which males and females flower synchronously, i.e. in systems in which dissimilarity should be counter-selected through pollinator choice, male and female scents are similar, while they differ in species with asynchronous flowering of male and female plants. Finally, Soler et al. (2012) found in F. carica that intersex similarity was higher between female and male figs that are produced synchronously during summer than between female figs (produced during summer) and male figs produced during spring. These different results highlight that when no selective pressures for mimicry occurs, genders tend to produce different scents. Moreover, selection for intersex mimicry seems to occur in both male and female plants.
Reproductive systems other than dioecy can also inform us about sex-specific selection. In monoecious species, the evolutionary processes leading to scent differences between male and female flowers within the same plant may be quite similar to the mechanisms occurring in dioecious species. In Breynia vitis-idaea, male flowers produce a quantity of scent (total bouquet or active compounds only) that is ten times higher than produced by female flowers, illustrating a possible effect of sexual selection at the flower level (Svensson et al., 2010). Moreover, the composition of scent significantly differs between male and female flowers in several species of this genus, in which specialized pollinators seek different rewards in male and female flowers (Svensson et al., 2010; Okamoto et al., 2013; Huang et al., 2015).
Reproductive systems with unisexual flowers or individuals are valuable models for the study of chemical mediation and the role of pollinator-mediated selection through male and female fitness. Thus, the comparison of scents between male and female flowers/plants should be systematically integrated in studies on dioecious or monoecious species. We also suggest that the study of sex-specific selection should be simultaneously examined with temporal variation of floral scent. For example, the comparison of floral scents emitted by hermaphroditic plants during their distinct male vs. female phases should allow one to investigate patterns linked with sexual selection and intersexual mimicry. In Magnolia sprengeri, flowers are deceptive during their female phase, but scent profiles are similar in these two phases (Wang et al., 2014). On the other hand, Gottsberger et al. (2013) found semi-quantitative variation between male and female phases in Philodendron form selloum but not in P. bipinnatifidum, and higher scent quantity during the female phase in both species. It is noteworthy that in most Philodendron species, specialized pollinators are attracted during the female phase, trapped within inflorescences and released after the staminate phase. Thus, the benefits of pollinator attraction appear variable during female and male phases, which leads to very different patterns in terms of sex-specific selection.
The underestimated role of natural enemies as selective agents
When floral scents are also used by natural enemies (e.g. florivores, larcenists or herbivores) to locate food or egg-laying sites, they can be associated with a fitness cost. However, the way in which interactions with enemies can shape emission and characteristics of floral scents has to date been poorly documented (but see Baldwin et al. 1997; Kessler et al., 2013). Several hypotheses can nevertheless be formulated and are supported by some studies. One expects a decrease in total emission rates when the cost of damage is high (e.g. in populations associated with a high prevalence of natural enemies) and/or when the cost is not counter-balanced by the benefit of attracting pollinators (e.g. outside phases of pollinator activity or flower receptivity). In the same way, both the decrease of antagonist-attracting VOCs and the increase of repellent compounds can drive some (semi-) quantitative variation.
Several studies, mentioned earlier, report some temporal variation that could also be linked to the repellent function of some compounds: increases in the proportion of germacrene A and indole after pollination in Ficus hispida (Proffit et al., 2008) and of methyl salicylate at night in day-pollinated Penstemon digitalis (Burdon et al., 2015). Methyl salicylate was also found in F. racemosa, but specifically in scents of non-receptive figs, and could repel parasites of pollinator larvae (Borges et al., 2013). These interpretations were based on the known potential repellent function of some VOCs that increase during phases at which pollinator attraction is not beneficial, but with no direct link between scent variation and prevalence of natural enemies. Only one study, in Cirsium arvense, specifically correlated variation in floral scents with activity of both pollinators and florivores, and strongly suggested that temporal variation in both scent quantity and composition has been selected to increase pollinator attraction during their maximal activity (mid-day) and decrease florivore attraction during early morning and late afternoon (Theis et al., 2007).
For studies that reported scent variation among populations, a possible selective role for herbivory in addition to pollinators may help explain observed scent variation. For instance, the fact that two subspecies of Antirrhinum majus differ in floral scent (notably with high quantity vs. absence of acetophenone) while sharing the same pollinator may be linked with the role of acetophenone in deterring herbivores (Suchet et al., 2011). Herbivore-mediated selection may also explain some variation within population. In Polemonium viscosum, the major VOC, 2-phenylethanol (2PE), reduces the cost of herbivory but also deters pollinators when emitted in high quantity (Galen et al., 2011). The strong within-population variation in the relative amount of 2PE in floral scents could be explained by the possible promotion of several strategies (highly attractive vs. poorly attractive for both pollinators and herbivores) with equivalent reproductive success. Moreover, any annual variation in the abundance of pollinators vs. herbivores within the same population (Galen et al., 2011) may also partially maintain different floral scents through balancing selection. However, the impact of such herbivore-mediated selection on the variation in floral scents remains speculative and constitutes an exciting perspective.
Interestingly, such contrasting selective pressures may also occur within plant–pollinator interactions. Pollination possibly involves a fitness cost, with insects consuming nectar and pollen, damaging the flowers or laying eggs within inflorescences in the case of nursery pollination systems (Pyke, 1991; Castellanos et al., 2002; Dufay and Anstett, 2003; Kephart et al., 2006) and plants may be under selection to reduce the ‘antagonistic side’ of their interaction with pollinators. For example, the strong cost in nursery pollination of some Silene species with nocturnal Hadena moths is likely to favour pollination by diurnal insects and lead selection to promote scent emission during the day (Prieto-Benitez et al., 2015) (although this does not seem to occur in all Silene species, e.g. Castillo et al., 2014).
Finally, in some instances, selection seems to have favoured strategies that attract pollinators and repel them afterwards. In the above-mentioned P. viscosum, the major VOC (2PE) is also contained in nectar and may play a role in decreasing nectar consumption and even increasing pollen transfer by reducing the time spent by pollinators on the flower (Galen et al., 2011). In cycad Macrozamia lucida, the increase in quantity of β-myrcene between morning and mid-day seems to explain why specialized pollinators leave male cones in large numbers, while the decrease of this compounds later in the day should re-increase attractiveness to pollinators (Terry et al., 2007). This fascinating push–pull strategy illustrates the contradictory selective pressures that occur on floral traits that must not attract enemies but must attract attract pollinators, but not for too long.
How do non-selective factors affect scent variation?
Variation in floral scents is not always explained by natural selection alone, and many authors have pointed out the potential effect of other evolutionary forces (genetic drift and gene migration, e.g. Mant et al., 2005; Suinyuy et al., 2012), environmental (abiotic) factors (Knudsen, 1994; Dötterl et al., 2005; Majetic et al., 2009b), biochemical (Raguso et al., 2003; Dötterl et al., 2007; Delle-Vedove et al., 2011; Suchet et al. 2011) or phylogenetic constraints (Raguso et al., 2006; Waelti et al., 2008; Delle-Vedove et al., 2011), although only a few studies have specifically tried to examine these other forces.
The role of genetic drift and gene migration on geographical variation.
Studies that report unexplained variation in floral scents (e.g. strong variation within populations in non-deceptive species or divergence among populations with the same pollinators) sometimes invoke genetic drift. For instance, to explain the high diversity in floral scents found among individuals in Magnolia kobus, Azuma et al. (2001) discuss the possible minor role of volatile signals compared with visual cues, which should lower the force of selection on odour relative to genetic drift. The strong variation in scents observed in North American populations of Silene latifolia can be explained by the recent introduction of several small, isolated populations (Dötterl et al., 2005). In contrast, studies that report no difference in floral odours among populations or taxa when some level of variation was expected (e.g. differences in the identity of the main pollinator) sometimes propose that gene flow may hamper chemical differentiation (e.g. Svensson et al., 2005). As expected for any genetically determined trait, the relative weight of genetic drift and gene flow should impact scent differentiation among populations. However, very few studies have attempted to quantify the relative magnitude of these two evolutionary forces on floral scent variation, as compared with pollinator-mediated selection.
In their synthesis paper, Whitehead and Peakall (2009) advocate that the study of floral scent variation should integrate population genetics, since the pollinators attracted by floral scents impact pollen flow between plants and populations and, thus, patterns of population genetic structure. In return, we suggest that population genetics studies can help in understanding some patterns of floral scent variation. One promising approach is the comparison of the spatial patterns of variation for active compounds, non-active compounds and neutral genetic markers, because they are differently impacted by genetic drift, gene flow and selection. For example, in Ophrys exaltata, active compounds show globally lower variation than non-active compounds; this illustrates the role of genetic drift in the variation of one part of the chemical blend, with only the active VOCs being under stabilizing selection mediated by the specific pollinator (Mant et al., 2005). However, in the same species at a different scale, active compounds show stronger differentiation among two regions than non-active compounds. This result, and the fact that both types of VOC show a stronger differentiation than neutral genetic markers, suggest (1) a globally weak role for genetic drift in the geographical variation of floral VOCs and (2) some divergent pollinator-mediated selection between regions possibly driven by a divergence in pollinator behaviour (Mant et al., 2005).
When genetic data and knowledge on the active/inactive role of the different VOCs are not available, other indirect approaches are possible, such as a characterization of the covariance between odour variation and geographical distance. For example, no correlation was found between Euclidian distances calculated on floral scents and geographical distance between populations either in Yucca filamentosa or in Silene otites, indicating no isolation by distance and suggesting a low impact of genetic drift (Svensson et al., 2005; Jhumur et al., 2008). In contrast, Suinyuy et al. (2012) found some isolation by distance in volatiles emitted by cones of the cycad Encephalartos villosus, and concluded that restricted gene flow among populations could explain the spatial patterns of variation in floral scent. It is difficult, however, to interpret such a correlative approach, because isolation by distance in floral scents is not necessarily an indicator of drift and could also reflect the impact of factors that follow a geographical gradient. As highlighted by Whitehead and Peakall (2009), the joint use of population genetics and chemical ecology constitutes an interesting and necessary perspective, and we trust that many future studies will integrate these two methodological approaches. However, some formalization of how the effects of selection, drift, and gene flow should translate into patterns of active and non-active VOCs in the blends, and how to interpret the results of such multidisciplinary studies is clearly needed.
At another taxonomic level, hybridization between species might be detected through blended scent profiles indicative of gene flow (Raguso, 2008b). For example, in Encephalartos villosus, cone volatiles across their range show a marked discontinuity that could be explained by some hybridization (Suinyuy et al., 2012). Similarly, the emission of intermediate scents in hybrid individuals between several Orchis species could account for the high variability observed within species (Nilsson, 1983; Salzmann et al., 2007b; Stökl et al., 2008, 2009; Schatz et al., 2010). The impact of hybridization on patterns of floral scents also suggests that data in chemical ecology can be valuable when examining taxonomic issues, as in the subgenus Hieracium (Feulner et al., 2011). Similarly, the screening of several chemotypes may help in detecting cryptic species (e.g. Hetherington and Ramirez, 2016).
The effect of environmental factors.
The variation observed for in situ extracted floral scents among populations can also be explained by phenotypic plasticity related to variation in edaphic conditions, light, temperature or any environmental factor that varies among study sites. Similarly, temporal variation can be at least partly attributed to the same factors varying between the investigated time phases. A small number of studies (not listed in Table 1) have investigated changes in scent phenotypes due to various abiotic factors (moisture, temperature and irradiance; reviewed by Majetic et al., 2009b; see also Falara et al., 2013; Farre-Armengol et al., 2014). The studies included in the current review have little complementary information on the topic. Some suggest a potential correlation between environment and scent emission, generally not supported by any statistical analysis, and the direction can vary among species. For example, in Silene latifolia, seasonal changes in the total scent emission were found to be potentially attributable to weather conditions, with lower quantities emitted during hot and dry summer months (Dötterl et al., 2005), while in Pyrola grandiflora, scent emissions were lower in a population when temperatures were also low (Knudsen, 1994).
Importantly, the fact that a part of the variation in scent emission is due to some variation in environmental factors does not mean that such variation is not adaptive. Farre-Armengol et al. (2015) showed that although temperature impacted terpene emission in six Mediterranean species, the temperature that optimizes terpene emission differs among them and globally matches with temperature during their flowering season. This suggests that plasticity of scent emission to the environment can evolve and be optimized by natural selection.
One useful and common experimental approach is to control for the environment when comparing scent over time, floral phases or populations. For instance, by surveying plant individuals within a growth chamber, Majetic et al. (2015) was able to dismiss the possibility that diel variation of scent in Phlox divaricata (with peaks of scent emission that globally correspond to pollinator activity) was explained by variation in temperature during the day. Studies controlling for environmental constraints by measuring scent emission on individuals from different origins in a common garden experiment (e.g. Dötterl et al. 2005, 2012; Waelti et al., 2008; Majetic et al., 2009b; Parachnowitsch et al., 2012) provide a better snapshot of the genetic basis of floral scent variation but are instead not fully representative of ecological situations. Surprisingly, this aspect is generally not discussed by the studies. Depending on the question, the two approaches (natural population vs. controlled conditions) provide different information, but only the comparison of floral scent between these two conditions would allow understanding of the plastic vs. genetic component of floral scent variation.
To our knowledge, only the study of Majetic et al. (2009b) has addressed the question of phenotypic plasticity as a function of all potential environmental factors in Hesperis matronalis, by comparing scent composition of potted plants originating from different populations but reared in a common garden with those of the same population grown in their natural habitat. Their results clearly showed environmentally induced variation of both qualitative and quantitative components of floral scents. Differences in floral scent composition (aromatic vs. terpenoid compounds) in individuals from different natural populations were not conserved in plants grown in a common garden, but the changes were mostly attributable to one population, indicating that the plastic response may be population (or genotype) specific. Although this study did not investigate environmental parameters linked to scent variation, nor control for genetic differentiation among individuals of both populations, it nevertheless demonstrates the potential role of phenotypic plasticity in interpopulation floral scent variation.
Biochemical and phylogenetic constraints.
Biochemical processes have been also suggested partly to explain floral scent variation in some species. For example, in Silene otites, the general increased production of lilac aldehyde at night is synchronized with a decrease in the emission of its precursor, linalool, leading to variation in the relative proportion of each VOC in the floral blend (Dötterl et al., 2012). Another example is where substrate competition between different branches of a biosynthetic pathway may lead to variation in scent among individuals of a species. This has been shown in Phlox subulata where different cultivars have been found to vary in the quantity of some benzenoid and phenylpropanoid emissions produced by the same pathway (Majetic and Sinka, 2013).
The question of biochemical constraints has been particularly explored in the case of specific colour–scent associations. Pigments and VOC production often share biochemical pathways, potentially leading to specific combination of both traits. Two main biosynthetic connections are known (1) the shikimate pathway, which links the synthesis of anthocyanin pigments and benzenoid/phenylpropanoid VOCs, and (2) the MEP (methyl d-erythritol 4-phosphate) biosynthetic pathway, leading to the production of carotenoid pigments and some terpenoid compounds. The impact of the pigment/scent relationship on variation in floral scent is linked not only to the constraints imposed by enzymatic machinery, but also to the selective pressures acting on flower colour that may generate a pleiotropic impact on floral scents. For example, anthocyanins are known to play a role in heat or UV plant protection. The anthocyanin/benzenoid link has been a particular focus, especially since the work of Zuker et al. (2002) showed that laboratory-manipulated white null-mutants of Dianthus caryophyllus emitted more methyl benzoate than anthocyanin-pigmented individuals. Nevertheless, studies performed in natural populations of other species presenting white vs. purple variants (Olesen and Knudsen, 1994; Majetic et al., 2007, 2008; Dormont et al., 2010, 2014; Delle-Vedove et al. 2011) or other types of colour variation (e.g. Hetherington-Rauth and Ramirez, 2016) found no such generalizable pattern of floral scent variation. As pointed out by Majetic et al. (2008), white-flowered phenotypes can possibly be produced by several independent mutations, acting at different steps of the biosynthetic pathway. Consequently, several chemotypes (including individuals that produce no odour at all) may be associated with the same colour phenotype.
Although phylogenetic constraints are unlikely to explain intraspecific variation in floral scents directly, we briefly discuss this factor because it may help explain some patterns of floral scents emissions. For example, in Silene dioica (and possibly in other Sileneae species; see Prieto-Benitez et al., 2015), the production of lilac aldehyde on a nocturnal rhythm while the main pollinators are thought to be diurnal may be explained by the fact that the ancestor species was moth pollinated (Waelti et al., 2008). Understanding which characteristics of floral scents are constrained by phylogeny requires studies at the interspecific level, and has been tested in a few taxa. For instance, Steiner et al. (2011) showed a stronger influence of historical constraints compared with pollinator-mediated selection to explain scent composition in oil-secreting orchids. Similar results were obtained by the studies of lineages of the tobacco genus (Nicotiana), including both hummingbird- and hawkmoth-pollinated species (Raguso et al., 2003, 2006). In the section Alatae, all species were found to emit the so-called ‘cineole cassette’ during the night, which consisted of the joint emission of 1,8-cineole with small amounts of related monoterpenes (α-pinene, β-pinene, sabinene, β-myrcene, limonene and α-terpineol). The relatively well conserved ratios of these compounds are similar to those produced by 1–8 cineole synthase activity which may reveal the biosynthetic origin of this blend rather than any ecological constraint (Raguso et al., 2003). This implies that lineage history can explain some patterns of scent composition, and in this case it may also explain observed temporal variation in Nicotiana species that are putatively hummingbird pollinated and for which nocturnal emission of scent does not result from current pollinator-mediated selection.
PROSPECTS
To date, several exciting and challenging issues have already been highlighted to integrate chemical ecology in the fields of community ecology (Dicke, 2006; see also Larue et al., 2016; Junker, 2016; Kuppler et al., 2016 for recent investigations of the role of floral scent on the plant-insect community), pollination ecology (Raguso, 2008a, b), and population genetics (Whitehead and Peakall, 2009). They have underlined the necessity for better knowledge of floral scent variation, especially in terms of their role on pollinator behaviour. This review identifies some additional gaps in current knowledge on the role of floral scent variation in plant–pollinator interaction, as well as in methods of data analyses that may be easily addressed. Hereafter, we detail several major future issues that should allow for a better understanding of the factors leading to this variation and facilitate future comparative analyses.
Standardization of the variation metrics
We found that many studies do not present data on variation among individuals and sometimes not even among populations. Moreover, the high heterogeneity of metrics and graphic representation makes any further quantitative meta-analysis very difficult. Some classical metrics should be provided systematically for each identified VOC: the mean and range (or s.d.) of proportion and/or quantities, the coefficient of variation (or of similarity) and the occurrence of each identified VOC among individuals of a given population. We also argue that future studies should (1) sample a reasonable number of individuals per population (minimum of ten individuals) and (2) analyse variance in the floral bouquet within vs. between populations (e.g. Mant et al., 2005).
Identify the signal and its behavioural implication in complex floral blends
Thanks to electrophysiological technics (EAG/GC-EAD), it is now possible to identify active compounds in complex floral blends. This is a first step to understanding which part of a scent variation is likely to be under pollinator-mediated selection vs. neutral evolution (e.g. Huber et al., 2005; Dötterl et al., 2007). Secondly, behavioural tests are the sole way to provide information both on the behavioural function (repellent, neutral or attractive) of each VOC for the insect and on the potential synergies/inhibitions among VOCs of the same blend, which largely influence the level of floral visitation and plant fitness (Kessler and Halitschke, 2009; Junker et al., 2010). Both techniques are complementary in investigating the role of specific compounds and must be considered in combination to understand the signals involved in plant–pollinator interactions.
Disentangling the role of environmental factors in observed floral scent variation
Phenotypic plasticity due to abiotic factors may potentially explain floral scent variation at both spatial and temporal scales (Majetic et al., 2009b), and several studies have shown the role of environmental factors on floral scent variation (Hansted et al., 1994; Jakobsen et al., 1994; Nielsen et al., 1995). In this regard, both transplantation experiments and surveys of scent variation in common gardens are useful approaches and should be more often used.
Disentangling the role of non-pollinators species
Non-pollinator agents such as herbivores, florivores or larcenists may influence plant scent variation in two different ways: (1) by being selective agents acting directly on plant fitness as discussed earlier and (ii) by directly inducing changes in floral scent emissions (Kessler and Baldwin, 2001). While the role of plant chemicals in the mediation of plant–herbivore interaction has been extensively studied, relatively few studies have investigated their role in floral scent variation. Further studies should link non-pollinator floral visitors with floral scent variation and estimate their impact on plant fitness in natural populations.
Establish the link between odour variation and fitness
Very few studies have directly investigated the impact of floral scents on pollinator visitation, and this often precludes discussing the consequences of floral scent variation on plant fitness. Moreover, one should keep in mind that pollination visitation is not always a good a proxy of plant fitness (see Reynolds and Fenster, 2008, for a standardized approach to estimate pollinator importance and to place error bars when comparing potential pollinators). Because pollinator visitation sometimes incurs some cost to the plant, and because female and male fitness are expected to respond differently to pollinator visitation, there is a need for studies linking more directly realized plant fitness (through fruit set, seed set and/or pollen removal or deposition) and the major characteristics that vary in floral scents. A first step should be to collect such components of plant fitness while extracting floral scents in a natural population, as in Majetic et al. (2009a) and Parachnowitsch et al. (2012). Ultimately, measurements of selection gradients, as done by Gross et al. (2016), are probably the best approaches for understanding the evolutionary ecology of floral scent. Finally, understanding how pollinator-mediated selection should impact floral scent characteristics obviously requires the study of heritability of such traits. To this end, marker-based approaches already prove to be fruitful to assess heritability of plant traits and should be developed in further studies (Bessega et al., 2009; Castellanos et al., 2011). Selection experiments such as that of Zu et al. (2015) on Brassica rapa and that of Byers et al. (2014) on genetic mapping/transgenic manipulation on Mimulus will allow one to consider pleiotropic effects between different VOCs or between VOCs and other plant traits, and more realistically understand how they can respond to selection.
Some recent studies included in this review have employed approaches of evolutionary biology (selection gradients, simultaneous study of trait variation and population genetics, selection experiments, genetic manipulation, phylogenetics studies), allowing one to study the effects of selection, gene flow, stochasticity and historical/genetic constraints on the evolution of floral scents. Hopefully, these interdisciplinary approaches will continue in the future, allowing us to better understand evolution of floral scents, and to predict their potential ecological impact on ecosystem functioning in our fast-changing world.
SUPPLEMENTARY INFORMATION
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: recapitulative table of the knowledge on the pollination system for all plant species listed in Table 1. Glossary.
Supplementary Material
ACKNOWLEDGEMENTS
We are extremely grateful to Isabelle De Cauwer, Jeffrey D. Karron, Michele R. Dudash and four anonymous reviewers for their helpful comments on previous versions of this manuscript. This work was funded by the University of Lille 1 which supported R.D.V. as a temporary lecturer in 2012–2014.
LITTERATURE CITED
- Ackerman J, Melendez-Ackerman E, Salguero-Faria J.. 1997. Variation in pollinator abundance and selection on fragrance phenotypes in an epiphytic orchid. American Journal of Botany 84: 1383–1390. [PubMed] [Google Scholar]
- Ackerman JD, Cuevas AA, Hof D.. 2011. Are deception-pollinated species more variable than those offering a reward? Plant Systematics and Evolution 293: 91–99. [Google Scholar]
- Ashman TL. 2009. Sniffing out patterns of sexual dimorphism in floral scent. Functional Ecology 23: 852–862. [Google Scholar]
- Azuma H, Toyota M, Asakawa Y.. 2001. Intraspecific variation of floral scent chemistry in Magnolia kobus DC. (Magnoliaceae). Journal of Plant Research 114: 411–422. [Google Scholar]
- Baldwin IT, Preston C, Euler M, Gohram D.. 1997. Patterns and consequences of benzyl acetone floral emissions from Nicotiana attenuata plants. Journal of Chemical Ecology 23: 2327–2343. [Google Scholar]
- Barkman TJ, Beaman JH, Gage DA.. 1997. Floral fragrance variation in Cypripedium: implications for evolutionary and ecological studies. Phytochemistry 44: 875–882. [Google Scholar]
- Bateman AJ. 1948. Intra-sexual selection in Drosophila. Heredity 2: 349–368. [DOI] [PubMed] [Google Scholar]
- Bessega C, Saidman BO, Darquier MR, et al. 2009. Consistency between marker- and genealogy-based heritability estimates in an experimental stand of Prosopis alba (Leguminosae). American Journal of Botany 96: 458–465. [DOI] [PubMed] [Google Scholar]
- Borschsenius F, Lozada T, Knudsen JT.. 2016. Reproductive isolation of sympatric forms of the understorey palm Geonoma macrostachys in western Amazonia. Botanical Journal of the Linnean Society 182: 398.–. [Google Scholar]
- Borges RM, Bessière JM, Ranganathan Y.. 2013. Diel variation in fig volatiles across syconium development: making sense of scents. Journal of Chemical Ecology 39: 630–642. [DOI] [PubMed] [Google Scholar]
- Breitkopf H, Schlüter PM, Xu S, Schiestl FP, Cozzolino S, Scopece G.. 2013. Pollinator shifts between Ophrys sphegodes populations: might adaptation to different pollinators drive population divergence? Journal of Evolutionary Biology 26: 2197–2208. [DOI] [PubMed] [Google Scholar]
- Burdon RCF, Raguso RA, Kessler A, Parachnowitsch AL.. 2015. Spatiotemporal floral scent variation of Penstemon digitalis. Journal of Chemical Ecology 41: 641–650. [DOI] [PubMed] [Google Scholar]
- Byers KJRP, Vela JP, Peng F, Riffel JA, Bradshaw HD Jr.. 2014. Floral volatile alleles can contribute to pollinator-mediated reproductive isolation in monkeyflowers (Mimulus). The Plant Journal 80: 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos MC, Wilson P, Thomson JD.. 2002. Dynamic nectar replenishment in flowers of Penstemon (Scrophulariaceae). American Journal of Botany 89: 111–118. [DOI] [PubMed] [Google Scholar]
- Castellanos MC, Alcantara JM, Rey PJ, Bastida JM.. 2011. Intra-population comparison of vegetative and floral traits heritabilities estimated from molecular markers in wild Aquilegia populations. Molecular Ecology 20: 3513–3524. [DOI] [PubMed] [Google Scholar]
- Castillo DM, Kula AAR, Dötterl S, Dudash MR, Fenster CB.. 2014. Invasive Silene latifolia may benefit from a native pollinating seed predator Hadena ectypa, in North America. International Journal of Plant Sciences 175: 80–91 [Google Scholar]
- Chess SKR, Raguso RA, LeBuhn G.. 2008. Geographic divergence in floral morphology and scent in Linanthus dichotomus (Polemoniaceae). American Journal of Botany 95: 1652–1659. [DOI] [PubMed] [Google Scholar]
- Darwin C. 1862. On the various contrivances by which British and foreign orchids are fertilized. London: Murray. [PMC free article] [PubMed] [Google Scholar]
- Delle-Vedove R, Juillet N, Bessière JM, et al. B. 2011. Color–scent associations in a tropical orchid: three colors but two odors. Phytochemistry 72: 735–742. [DOI] [PubMed] [Google Scholar]
- Dicke M. 2006. Chemical ecology: from genes to communities In: Dicke M, Taken W, eds. Chemical ecology: from genes to ecosystem. Berlin: Springer, 175–189. [Google Scholar]
- Dobson HEM, Arroyo J, Bergström G, Groth I.. 1997. Interspecific variation in floral fragrances within the genus Narcissus (Amaryllidaceae). Biochemical Systematics and Ecology 25: 685–706. [Google Scholar]
- Dormont L, Delle‐Vedove R, Bessière JM, Hossaert-McKey M, Schatz B.. 2010. Rare white‐flowered morphs increase the reproductive success of common purple morphs in a food‐deceptive orchid. New Phytologist 185: 300–310. [DOI] [PubMed] [Google Scholar]
- Dormont L, Delle-Vedove R, Bessière JM, Schatz B.. 2014. Floral scent emitted by white and colored morphs in orchids. Phytochemistry 100: 51–59. [DOI] [PubMed] [Google Scholar]
- Dötterl S, Wolfe LM, Jürgens A.. 2005. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry 66: 203–213. [DOI] [PubMed] [Google Scholar]
- Dötterl S, Jürgens A, Seifert K, Laube T, Weissbecker B, Schutz S.. 2006. Nursery pollination by a moth in Silene latifolia: the role of odours in eliciting antennal and behavioural responses. New Phytologist 169: 707–718. [DOI] [PubMed] [Google Scholar]
- Dötterl S, Burkhardt D, Jürgens A, Mosandl A.. 2007. Stereoisomeric pattern of lilac aldehyde in Silene latifolia, a plant involved in a nursery pollination system. Phytochemistry 68: 499–504. [DOI] [PubMed] [Google Scholar]
- Dötterl S, Jareiß K, Salma Jhumur U, Jürgens A.. 2012. Temporal variation of flower scent in Silene otitis (Caryophyllaceae): a species with a mixed pollination system. Botanical Journal of the Linnean Society 169: 447–460. [Google Scholar]
- Doubleday LAD, Raguso RA, Eckert CG.. 2013. Dramatic vestigialization of floral fragrance across a transition from outcrossing to selfing in Abronia umbellata (Nyctaginaceae). American Journal of Botany 100: 2280–2292. [DOI] [PubMed] [Google Scholar]
- Dufa M, Hossaert-McKey M, Anstett MC.. 2004. Temporal and sexual variation of leaf-produced pollinator-attracting odours in the dwarf palm. Oecologia 139: 392–398. [DOI] [PubMed] [Google Scholar]
- Dufaÿ M, Anstett MC.. 2003. Conflicts between plants and pollinators that reproduce within inflorescences: evolutionary variations on a theme. Oikos 100: 3–14. [Google Scholar]
- Fægri K, van der Pijl L.. 1979. The principles of pollination ecology, 3rd edn.Oxford: Pergamon. [Google Scholar]
- Falara V, Amarasinghe R, Poldy J, Pichersky E, Barrow RA, Peakall R.. 2013. The production of a key floral volatile is dependent on UV light in a sexually deceptive orchid. Annals of Botany 111: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farre-Armengol G, Filella I, Llusia J, Niinemets U, Penuelas J.. 2014. Changes in floral bouquets from compound-specific responses to increasing temperatures. Global Change Biology 20: 3660–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farre-Armengol G, Filella I, Llusia J, Niinemets U, Penuelas J.. 2015. Optimum temperature for floral terpene emissions tracks the mean temperature of the flowering season. Functional Plant Biology 42: 851–857. [DOI] [PubMed] [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD.. 2004. Pollination syndromes and floral specialization. Annals of Botany 35: 375–403. [Google Scholar]
- Feulner M, Schuhwerk F, Dötterl S.. 2011. Taxonomical value of inflorescence scent in Hieracium s. str. Biochemical Systematics and Ecology 39: 732–743. [Google Scholar]
- Friberg M, Schwind C, Raguso RA, Thompson JN.. 2013. Extreme divergence in floral scent among woodland star species (Lithophragma spp.) pollinated by floral parasites. Annals of Botany 111: 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen C. 1999. Why do flowers vary? The functional ecology of variation in flower size and form within natural plant populations. Bioscience 49: 631–640. [Google Scholar]
- Galen C, Kaczorowski R, Todd SL, Geib J, Raguso RA.. 2011. Dosage-dependent impacts of a floral volatile compound on pollinators, larcenists, and the potential for floral evolution in the alpine skypilot Polemonium viscosum. American Naturalist 177: 258–272. [DOI] [PubMed] [Google Scholar]
- Gaskett AC, Conti E, Schiestl FP.. 2005. Floral odor variation in two heterostylous species of Primula. Journal of Chemical Ecology 31: 1223–1228. [DOI] [PubMed] [Google Scholar]
- Gigord LDB, Macnair MR, Smithson A.. 2001. Negative frequency-dependent selection maintains a dramatic flower color polymorphism in the rewardless orchid Dactylorhiza sambucina (L.). Proceedings of the National Academy of Sciences, USA 98: 6253–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Benavides L, Dötterl S, Jürgens A, Escudero A, Iriondo JM.. 2007. Generalist diurnal pollination provides greater fitness in a plant with nocturnal pollination syndrome: assesing the effects of a Silene–Hadena interaction. Oikos 116: 1461–1472. [Google Scholar]
- Gong WC, Chen G, Liu CQ, Dunn BL, Sun WB. 2014. Comparison of floral scent between and within Buddleja fallowiana and Buddleja officinalis (Scrophulariaceae). Biochem Syst Ecol 55: 322–328. [Google Scholar]
- Gottsberger G, Silberbauer-Gottsberger I, Dötterl S.. 2013. Pollination and floral scent differentiation in species of the Philodendron bipinnatifidum complex (Araceae). Plant Systematics and Evolution 299: 793–809. [Google Scholar]
- Gross K, Sun M, Schiestl FP.. 2016. Why do floral perfumes become different? Region-specific selection on floral scent in a terrestrial orchid. PLoS One 11: e0147975 doi:10.1371/journal.pone.0147975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansted L, Jakobsen HB, Olsen CE.. 1994. Influence of temperature on the rhythmic emission of volatiles from Ribes nigrum flowers in situ. Plant, Cell & Environment 17: 1069–1072. [Google Scholar]
- Heiduk A, Brake I, Tolasch T, et al. 2010. Scent chemistry and pollinator attraction in the deceptive trap flowers of Ceropegia dolichophylla. South African Journal of Botany 76: 762–769. [Google Scholar]
- Hetherington-Rauth MD, Ramirez SR.. 2016. Evolution and diversity of floral scent chemistry in the euglossine bee-pollinated orchid genus Gongora. Annals of Botany 118: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossaert-McKey M, Proffit M, Soler CCL, et al. 2016. How to be a dioecious fig: chemical mimicry between sexes matters only when both sexes flower synchronously. Scientific Reports 6: 21236. doi:10.1038/srep21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Fuchen S, Minwei C, Li R, Houhun L.. 2015. Interspecific and intersexual differences in the chemical composition of floral scent in Glochidion species (Phyllanthaceae) in South China. Journal of Chemistry Article ID 865694. doi:10.1155/2015/865694. [Google Scholar]
- Huber FK, Kaiser R, Sauter W, Schiestl FP.. 2005. Floral scent emission and pollinator attraction in two species of Gymnadenia (Orchidaceae). Oecologia 142: 564–575. [DOI] [PubMed] [Google Scholar]
- Ibanez S, Dötterl S, Anstett MC, et al. 2010. The role of volatile organic compounds, morphology and pigments of globeflowers in the attraction of their specific pollinating flies. New Phytologist 188: 451–463. [DOI] [PubMed] [Google Scholar]
- Jakobsen HB, Olsen CE.. 1994. Influence of climatic factors on emission of flower volatiles in situ. Planta 192: 365–371. [Google Scholar]
- Jersáková J, Kindlmann P, Renner SS.. 2006. Is the colour dimorphism in Dactylorhiza sambucina maintained by differential seed viability instead of frequency-dependent selection? Folia Geobotanica 41: 61–76. [Google Scholar]
- Jhumur US, Dötterl S, Jürgens A.. 2008. Floral odors of Silene otites: their variability and attractiveness to mosquitoes. Journal of Chemical Ecology 34: 14–25. [DOI] [PubMed] [Google Scholar]
- Jones KN, Reithel JS.. 2001. Pollinator-mediated selection on a flower color polymorphism in experimental populations of Antirrhinum (Scrophulariaceae). American Journal of Botany 88: 447–454. [PubMed] [Google Scholar]
- Juillet N, Scopece G.. 2010. Does floral trait variability enhance reproductive success in deceptive orchids? Perspectives in Plant Ecology, Evolution and Systematics 12: 317–322. [Google Scholar]
- Junker RR. 2016. Multifunctional and diverse floral scents mediate biotic interactions embedded in communities In: Blande JD, Glinwood RT, eds. Deciphering chemical language of plant communication. Signaling and communication in plants. Heidelberg: Springer, 257–282. [Google Scholar]
- Junker RR, Höcherl N, Blüthgen N.. 2010. Responses to olfactory signals reflect network structure of flower–visitor interactions. Journal of Animal Ecology 79: 818–823. [DOI] [PubMed] [Google Scholar]
- Kephart S, Reynolds RJ, Rutter MT, Fenster CB, Dudash MR.. 2006. Pollination and seed predation by moths on Silene and allied Caryophyllaceae: evaluating a model system to study the evolution of mutualisms. New Phytologist 169: 637–651. [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT.. 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141. [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R.. 2009. Testing the potential for conflicting selection on floral chemical traits by pollinators and herbivores: predictions and case study. Functional. Ecology 23: 901–912. [Google Scholar]
- Kessler D, Diezel C, Clark DG, Colquhoun TA, Baldwin IT.. 2013. Petunia flowers solve the defence/apparency dilemma of pollinator attraction by deploying complex floral blends. Ecology Letters 16: 299–306. [DOI] [PubMed] [Google Scholar]
- Kolreuter JG. 1761. Vorlaufige Nachrichten von einigen das Geschlecht der Pflanzen betreffenden Versuchen und Beobachtungen. Leipzig: Gleditschischen Handlung. [Google Scholar]
- Knudsen JT. 1994. Floral scent variation in the Pyrola rotundifolia complex in Scandinavia and western Greenland. Nordic Journal of Botany 14: 277–282. [Google Scholar]
- Knudsen JT. 2002. Variation in floral scent composition within and between populations of Geonoma macrostachys (Arecaceae) in the western Amazon. American Journal of Botany 89: 1772–1778. [DOI] [PubMed] [Google Scholar]
- Knudsen JT, Eriksson R, Gershenzon J, Ståhl B.. 2006. Diversity and distribution of floral scent. Botanical Review 72: 1–120. [Google Scholar]
- Kondo M, Oyama-Okubo N, Ando T, Marchesi E, Nakayama M.. 2006. Floral scent diversity is differently expressed in emitted and endogenous components in Petunia axillaris lines. Annals of Botany 98: 1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuanprasert N, Kuehnle AR, Tang CS.. 1998. Floral fragrance compounds of some Anthurium (Araceae) species and hybrids. Phytochemistry 49: 521–528. [Google Scholar]
- Kumano-Nomura Y, Yamaoka R.. 2009. Beetle visitations, and associations with quantitative variation of attractants in floral odors of Homalomena propinqua (Araceae). Journal of Plant Research 122: 183–192. [DOI] [PubMed] [Google Scholar]
- Kuppler J, Höfers MK, Wiesmann L, Junker RR.. 2016. Time-invariant differences between plant individuals in interactions with arthropods correlate with intraspecific variation in plant phenology, morphology and floral scent. New Phytologist 210: 1357–1368. [DOI] [PubMed] [Google Scholar]
- Larue AAC, Raguso RA, Junker RR.. 2016. Experimental manipulation of floral scent bouquets restructures flower–visitor interactions in the field. Journal of Animal Ecology 85: 396–408. [DOI] [PubMed] [Google Scholar]
- Lemaitre AB, Pinto CF, Niemeyer HM.. 2014. Generalized pollination system: are floral traits adapted to different pollinators? Arthropod-plant interactions 8: 261–272. [Google Scholar]
- Majetic CJ, Sinka BN.. 2013. Diverging pathways: differential benzenoid and phenylpropanoid volatile production in Phlox subulata L. cultivars. Biochemical Systematics and Ecology 50: 75–81. [Google Scholar]
- Majetic CJ, Raguso RA, Tonsor SJ, Ashman TL.. 2007. Flower color–flower scent associations in polymorphic Hesperis matronalis (Brassicaceae). Phytochemistry 68: 865–874. [DOI] [PubMed] [Google Scholar]
- Majetic CJ, Raguso RA, Ashman TL.. 2008. The impact of biochemistry vs. population membership on floral scent profiles in colour polymorphic Hesperis matronalis. Annals of Botany 102: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majetic CJ, Raguso RA, Ashman TL.. 2009a. The sweet smell of success: floral scent affects pollinator attraction and seed fitness in Hesperis matronalis. Functional Ecology 23: 480–487. [Google Scholar]
- Majetic CJ, Raguso RA, Ashman TL.. 2009b. Sources of floral scent variation: can environment define floral scent phenotype? Plant Signaling & Behavior 4: 129–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majetic CJ, Wiggam SD, Ferguson CJ, Raguso R.. 2015. Timing is everything: temporal variation in floral scent, and its connections to pollinator behavior and female reproductive success in Phlox divaricata. American Midland Naturalist 173: 191–207. [Google Scholar]
- Martinell MC, Dötterl S, Blanche C, Rovira A, Masso S, Bosch M.. 2010. Nocturnal pollination of the endemic Silene sennenii (Caryophyllaceae): an endangered mutualism?. Plant Ecology 211: 203–218. [Google Scholar]
- Mant J, Peakall R, Schiestl FP.. 2005. Does selection on floral odor promote differentiation among populations and species of the sexually deceptive orchid genus Ophrys? Evolution 59: 1449–1463. [PubMed] [Google Scholar]
- Moya S, Ackerman JD.. 1993. Variation in the floral fragrance of Epidendrum ciliare (Orchidaceae). Nordic Journal of Botany 13: 41–47. [Google Scholar]
- Nielsen JK, Jakobsen HB, Friis P, Hansen K, Moller J, Olsen CE.. 1995. Asynchronous rhythms in the emission of volatiles from Hesperis matronalis flowers. Phytochemistry 38: 847–851. [Google Scholar]
- Nilsson LA. 1983. Processes of isolation and introgressive interplay between Platanthera bifolia (L.) Rich and Platanthera chlorantha (Custer) Reichb. (Orchidaceae). Botanical Journal of the Linnean Society 87: 325–350. [Google Scholar]
- Okamoto T, Kawakita A, Goto R, Svensson GP, Kato M.. 2013. Active pollination favours sexual dimorphism in floral scent. Proceedings of the Royal Society B: Biological Sciences 280: 2013–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen JM, Knudsen JT.. 1994. Scent profiles of flower colour morphs of Corydalis cava (Fumariaceae) in relation to foraging behaviour of bumblebee queens (Bombus terrestris). Biochemical Systematics and Ecology 22: 231–237. [Google Scholar]
- Parachnowitsch AL, Raguso RA, Kessler A.. 2012. Phenotypic selection to increase floral scent emission, but not flower size or colour in bee‐pollinated Penstemon digitalis. New Phytologist 195: 667–675. [DOI] [PubMed] [Google Scholar]
- Pellegrino G, Caimi D, Noce ME, Musacchio A.. 2005. Effects of local density and flower colour polymorphism on pollination and reproduction in the rewardless orchid Dactylorhiza sambucina (L.) Soò. Plant Systematics and Evolution 251: 119–129. [Google Scholar]
- Pettersson S, Knudsen JT.. 2001. Floral scent and nectar production in Parkia biglobosa Jacq. (Leguminosae: Mimosoideae). Botanical Journal of the Linnean Society 135: 97–106. [Google Scholar]
- Pott MB, Pichersky E, Piechulla B.. 2002. Evening specific oscillations of scent emission, SAMT enzyme activity, and SAMT mRNA in flowers of Stephanotis floribunda. Journal of Plant Physiology 159: 925–934. [Google Scholar]
- Prieto-Benitez S, Doetterl S, Gimenez-Benavides L.. 2015. Diel variation in flower scent reveals poor consistency of diurnal and nocturnal pollination syndromes in Sileneae. Journal of Chemical Ecology 41: 1095–1104. [DOI] [PubMed] [Google Scholar]
- Proffit M, Schatz B, Bessiere JM, Chen C, Soler C, Hossaert-McKey M.. 2008. Signalling receptivity: comparison of the emission of volatile compounds by figs of Ficus hispida before, during and after the phase of receptivity to pollinators. Symbiosis 45: 15–24. [Google Scholar]
- Pyke GH. 1991. What does it cost a plant to produce floral nectar? Nature 350: 58–59. [Google Scholar]
- Raguso RA. 2008a. Wake up and smell the roses: the ecology and evolution of floral scent. Annual Review of Ecology, Evolution, and Systematics 39: 549–569. [Google Scholar]
- Raguso RA. 2008b. Start making scents: the challenge of integrating chemistry into pollination ecology. Entomologia Experimentalis et Applicata 128: 196–207. [Google Scholar]
- Raguso RA, Levin RA, Foose SE, Holmberg MW, Mcdade LA.. 2003. Fragrance chemistry, nocturnal rhythms and pollination ‘syndromes’ in Nicotiana. Phytochemistry 63: 265–284. [DOI] [PubMed] [Google Scholar]
- Raguso RA, Schlumpberger BO, Kaczorowski RL, Holtsford TP.. 2006. Phylogenetic fragrance patterns in Nicotiana sections alatae and suaveolentes. Phytochemistry 67: 1931–1942. [DOI] [PubMed] [Google Scholar]
- Reynolds RJ, Westbrook MJ, Rohde AS, Cridland JM, Fenster CB, Dudash MR.. 2009. Pollinator specialization and pollination syndromes of three related North American Silene. Ecology 90: 2077–2087. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Saona C, Parra L, Quiroz A, Isaacs R.. 2011. Variation in highbush blueberry floral volatile profiles as a function of pollination status, cultivar, time of day and flower part: implications for flower visitation by bees. Annals of Botany 107. doi:10.1093/aob/mcr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann CC, Schiestl FP.. 2007. Odour and colour polymorphism in the food-deceptive orchid Dactylorhiza romana. Plant Systematics and Evolution 267: 37–45. [Google Scholar]
- Salzmann CC, Nardella AM, Cozzolino S, Schiestl FP.. 2007a. Variability in floral scent in rewarding and deceptive orchids: the signature of pollinator-imposed selection? Annals of Botany 100. doi:10.1093/aob/mcm161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann CC, Cozzolino S, Schiestl FP.. 2007b. Floral scent in food‐deceptive orchids: species specificity and sources of variability. Plant Biology 9: 720–729. [DOI] [PubMed] [Google Scholar]
- Sanaa A, Boulila A, Bejaoui A, Boussaid M, Fadhel NB.. 2012. Variation of the chemical composition of floral volatiles in the endangered Tunisian Pancratium maritimum L. Populations (Amaryllidaceae). Industrial Crops and Products 40: 312–317. [Google Scholar]
- Schatz B, Geoffroy A, Dainat B, et al. 2010. A case study of modified interactions with symbionts in a hybrid Mediterranean orchid. American Journal of Botany 97: 1278–1288. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Bierzychudek P.. 2007. Spatial differentiation for flower color in the desert annual Linanthus parryae: was Wright right? Evolution 61: 2528–2543. [DOI] [PubMed] [Google Scholar]
- Schiestl FP. 2015. Ecology and evolution of floral volatile-mediated information transfer in plants. New Phytologist 206: 571–577. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M.. 2001. Post-pollination emission of a repellent compound in a sexually deceptive orchid: a new mechanism for maximizing reproductive success? Oecologia, 126: 531–534. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M, Paulus HF, Erdmann D, Francke W.. 1997. Variation of floral scent emission and postpollination changes in individual flowers of Ophrys sphegodes subsp. Sphegodes. Journal of Chemical Ecology 23: 2881–2895. [Google Scholar]
- Schlumpberger BO, Raguso RA.. 2008. Geographic variation in floral scent of Echinopsis ancistrophora (Cactaceae); evidence for constraints on hawkmoth attraction. Oikos 117: 801–814. [Google Scholar]
- Schlumpberger BO, Jux A, Kunert M, Boland W, Wittmann D.. 2004. Musty‐earthy scent in cactus flowers: characteristics of floral scent production in dehydrogeosmin‐producing cacti. International Journal of Plant Sciences 165: 1007–1015. [Google Scholar]
- Schneider MA, Dötterl S, Seifert K.. 2013. Diastereoselective synthesis of a lilac aldehyde isomer and its electrophysiological detection by a moth. Chemistry and Biodiversity 10: 1252–1259. [DOI] [PubMed] [Google Scholar]
- Shuttleworth A. 2016. Smells like debauchery: the chemical composition of semen-like, sweat-like and faintly foetid floral odours in Xysmalobium (Apocynaceae: Asclepiadoideae). Biochemical Systematics and Ecology 66: 63–75. [Google Scholar]
- Smithson A, Macnair MR.. 1997. Negative frequency-dependent selection by pollinators on artificial flowers without rewards. Evolution 51: 715–723. [DOI] [PubMed] [Google Scholar]
- Soler C, Hossaert-McKey M, Buatois B, Bessière JM, Schatz B, Proffit M.. 2011. Geographic variation of floral scent in a highly specialized pollination mutualism. Phytochemistry 72: 74–81. [DOI] [PubMed] [Google Scholar]
- Soler C, Proffit M, Bessière JM, Hossaert-McKey M, Schatz B.. 2012. Evidence for intersexual chemical mimicry in a dioecious plant. Ecology Letters 15: 978–985. [DOI] [PubMed] [Google Scholar]
- Sprengel CK. 1793. Das entdeckte Geheimniss der Natur im Bau und in der Befruchtung der Blumen. Berlin: Vieweg. [Google Scholar]
- Stebbins GL. 1970. Adaptive radiation of reproductive characteristics in angiosperms. I: pollination mechanisms. Annual Review in Ecology Systematics 1: 307–326. [Google Scholar]
- Steenhuisen SL, Raguso RA, Jürgens A, Johnson SD.. 2010. Variation in scent emission among floral parts and inflorescence developmental stages in beetle-pollinated Protea species (Proteaceae). South African Journal of Botany 76: 779–787. [Google Scholar]
- Steiner KE, Kaiser R, Doetterl S.. 2011. Phylogenetic effects on floral scent variation of oil-secreting orchids in South Africa. American Journal of Botany 98: 1663–1679. [DOI] [PubMed] [Google Scholar]
- Stökl J, Schlüter PM, Stuessy TF, Paulus HF, Assum G, Ayasse M.. 2008. Scent variation and hybridization cause the displacement of a sexually deceptive orchid species. American Journal of Botany 95: 472–481. [DOI] [PubMed] [Google Scholar]
- Stökl J, Schlüter PM, Stuessy TF, et al. 2009. Speciation in sexually deceptive orchids: pollinator‐driven selection maintains discrete odour phenotypes in hybridizing species. Biological Journal of the Linnean Society 98: 439–451. [Google Scholar]
- Streisfeld MA, Kohn JR.. 2007. Environment and pollinator-mediated selection on parapatric floral races of Mimulus aurantiacus. Journal of Evolutionary Biology 20: 122–132. [DOI] [PubMed] [Google Scholar]
- Suchet C, Dormont L, Schatz B, Giurfa M, Simon V, Raynaud C, Chave J.. 2011. Floral scent variation in two Antirrhinum majus subspecies influences the choice of naïve bumblebees. Behavioral Ecology and Sociobiology 65: 1015–1027. [Google Scholar]
- Suinyuy TN, Donaldson JS, Johnson SD.. 2012. Geographical variation in cone volatile composition among populations of the African cycad Encephalartos villosus. Biological Journal of the Linnean Society 106: 514–527. [Google Scholar]
- Svensson GP, Hickman MO Jr, Bartram S, Boland W, Pellmyr O, Raguso RA.. 2005. Chemistry and geographic variation of floral scent in Yucca filamentosa (Agavaceae). American Journal of Botany 92: 1624–1631. [DOI] [PubMed] [Google Scholar]
- Svensson GP, Pellmyr O, Raguso RA.. 2006. Strong conservation of floral scent composition in two allopatric yuccas. Journal of Chemical Ecology 32: 2657–2665. [DOI] [PubMed] [Google Scholar]
- Svensson GP, Okamoto T, Kawakita A, Goto R, Kato M.. 2010. Chemical ecology of obligate pollination mutualisms: testing the ‘private channel’ hypothesis in the Breynia–Epicephala association. New Phytologist 186: 995–1004. [DOI] [PubMed] [Google Scholar]
- Terry I, Walter GH, Moore C, Roemer R, Hull C.. 2007. Odor-mediated push–pull pollination in cycads. Science 318: 70. [DOI] [PubMed] [Google Scholar]
- Theis N, Lerdau M, Raguso RA.. 2007. The challenge of attracting pollinators while evading floral herbivores: patterns of fragrance emission in Cirsium arvense and Cirsium repandum (Asteraceae). International Journal of Plant Sciences 168: 587–601. [Google Scholar]
- Tholl D, Chen F, Petri J, Gershenzon J, Pichersky E.. 2005. Two sesquiterpene synthases are responsible for the complex mixture of sesquiterpenes emitted from Arabidopsis flowers. The Plant Journal 42: 757–771. [DOI] [PubMed] [Google Scholar]
- Thompson JN. 1996. Evolutionary ecology and the conservation of biodiversity. Trends in Ecology and Evolution 11: 300–303. [DOI] [PubMed] [Google Scholar]
- Tollsten L, Bergström LG.. 1993. Fragrance chemotypes of Platanthera (Orchidaceae) – the result of adaptation to pollinating moths? Nordic Journal of Botany 13: 607–613. [Google Scholar]
- Van der Niet T, Jürgens A, Johnson SD.. 2010. Pollinators, floral morphology and scent chemistry in the southern African orchid genus Schizochilus. South African Journal of Botany 76: 726–738. [Google Scholar]
- Vereckeen NJ, Schiestl FP.. 2008. The evolution of imperfect floral mimicry. Proceedings of National Academy of Sciences USA 105: 7484–7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecken NJ, Schiestl FP.. 2009. On the roles of colour and scent in a specialized floral mimicry system. Annals of Botany 104: 1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelti MO, Muhlemann JK, Widmer A, Schiestl FP.. 2008. Floral odour and reproductive isolation in two species of Silene. Journal of Evolutionary Biology 21: 111–121. [DOI] [PubMed] [Google Scholar]
- Wang R, Xu S, Xiangyu S, Zhang Y, Wang J, Zhang Z.. 2014. Thermogenesis, flowering and the association with variation in floral odour attractants in Magnolia sprengeri (Magnoliaceae). PLoS ONE 9: e99356. doi:10.1371/journal.pone.0099356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J, Mackenzie S.. 2001. Why are all colour combinations not equally represented as flower-colour polymorphisms? New Phytologist 151: 237–241. [DOI] [PubMed] [Google Scholar]
- Whitehead MR, Peakall R.. 2009. Integrating floral scent, pollination ecology and population genetics. Functional Ecology 23: 863–874. [Google Scholar]
- Young HJ, Stanton ML.. 1990. Influences of floral variation on pollen removal and seed production in wild radish. Ecology 35: 536–547. [Google Scholar]
- Zito P, Scrima A, Sajeva M, Carimi F, Dötterl S.. 2016. Dimorphism in inflorescence scent of dioecious wild grapevine. Biochemical Systematics and Ecology 66: 58–62. [Google Scholar]
- Zu P, Blanckenhom WU, Schiestl FP.. 2015. Heritability of floral volatiles and pleiotropic responses to artificial selection in Brassica rapa. New Phytologist 209: 1208–1219. [DOI] [PubMed] [Google Scholar]
- Zuker A, Tzfira T, Ben-Meir H, et al. 2002. Modification of flower color and fragrance by antisense suppression of the flavanone 3-hydroxylase gene. Molecular Breeding 9: 33–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.