Abstract
Apicomplexans are successful parasites responsible for severe human diseases including malaria, toxoplasmosis, and cryptosporidiosis. For many years, it has been discussed whether these parasites are in possession of peroxisomes, highly variable eukaryotic organelles usually involved in fatty acid degradation and cellular detoxification. Conflicting experimental data has been published. With the age of genomics, ever more high quality apicomplexan genomes have become available, that now allow a new assessment of the dispute. Here, we provide bioinformatic evidence for the presence of peroxisomes in Toxoplasma gondii and other coccidians. For these organisms, we have identified a complete set of peroxins, probably responsible for peroxisome biogenesis, division, and protein import. Moreover, via a global screening for peroxisomal targeting signals, we were able to show that a complete set of fatty acid β-oxidation enzymes is equipped with either PTS1 or PTS2 sequences, most likely mediating transport of these factors to putative peroxisomes in all investigated Coccidia. Our results further imply a life cycle stage-specific presence of peroxisomes in T. gondii and suggest several independent losses of peroxisomes during the evolution of apicomplexan parasites.
Keywords: apicomplexa, peroxisome, β-oxidation, fatty acid degradation, catalase
Introduction
Apicomplexans are a group of parasitic protists including severe pathogens that befall humans and cattle. One example is Toxoplasma gondii the causative agent of toxoplasmosis, which, when infecting immunosuppressed individuals or infants, can cause fatal disease. Toxoplasma gondii is a member of the Coccidia, a subclass of apicomplexans known to form environmentally resistant oocysts, which, if sporulated and ingested via food or water, may provoke host infection (Fritz et al. 2012; Lindsay et al. 1997). Besides T. gondii, Coccidia comprise other single-celled obligate parasites such as Eimeria, Isospora, Neospora, Cyclospora, Sarcocystis, and Hammondia, which usually infect vertebrates (Blazejewski 2015; Liu et al. 2016; Palmieri et al. 2017). Similar to other Coccidia, T. gondii has a complex life cycle involving three different infectious stages: Tachyzoites, bradyzoites, and sporozoites (Dubey et al. 1998). Whereas the latter develop outside the definitive host (felids) after release with feces as oocysts, tachyzoites, and bradyzoites are intracellular stages found inside a host (intermediate or definitive; Lyons et al. 2002; Tenter et al. 2000). Coccidia and most other apicomplexans, such as, for example, the malaria causing parasite Plasmodium falciparum, contain a residual, nonphotosynthetic complex plastid called apicoplast that goes back to a former free-living red alga (McFadden 2014). From an evolutionary perspective these organisms are thus the product of a eukaryote–eukaryote endosymbiosis with chromerids being their most closely related photosynthetic relatives known so far (Woo et al. 2015).
In contrast to plastids, peroxisomes have most likely a nonendosymbiotic origin. These organelles usually proliferate by division, but can also form de novo (Agrawal and Subramani 2016; Sugiura et al. 2017). Their biogenesis and maintenance is conducted by a conserved set of proteins known as peroxins (Pex) (Smith and Aitchison 2013). Peroxisomes are metabolically highly versatile, single membrane-bound organelles present in most eukaryotes (Deb and Nagotu 2017). Although peroxisomes can vary drastically in enzymatic content and number between different species and among different cell types, their most conserved metabolic functions are degradation of fatty acids (β-oxidation) and detoxification of reactive oxygen species (ROS; Gabaldon 2010). These two metabolic processes are usually strictly connected because the first reaction in peroxisomal fatty acid β-oxidation performed by acyl-CoA oxidase (ACOX) generates H2O2, which is subsequently converted to oxygen and H2O via the detoxifying action of the enzyme catalase (2 H2O2 → O2 + 2 H2O) to protect the cell from oxidative damage (Schrader and Fahimi 2006). The remaining steps of the peroxisomal β-oxidation produce acetyl-CoA, which can serve as a carbon or energy source for growth, for example, as a substrate for the mitochondrial TCA-cycle (Poirier et al. 2006; Schrader et al. 2015).
Peroxisomes can import their luminal (matrix) proteins via two specific import signals: Peroxisomal targeting signal type 1 (PTS1), a C-terminal tripeptide with the consensus sequence [SAC]-[KRH]-[LM] (Ast et al. 2013; Kim and Hettema 2015; Lametschwandtner et al. 1998; Rucktäschel et al. 2011), and type 2 (PTS2), an N-terminal nonapeptide comprised of the amino acids [RK]-[LVIQ]-X-X-[LVIHQ]-[LSGAK]-X-[HQ]-[LAF] (all known possibilities; Kunze et al. 2015). Whereas the PTS1 is recognized by the receptor peroxin 5 or Pex5, PTS2-mediated import requires Pex7 and an additional coreceptor (Pex5/Pex5L in plants and mammals; Freitag et al. 2012; Meinecke et al. 2016). A special feature of the peroxisomal matrix protein import is that even folded and oligomerized proteins can by transported into the organelle via a so-called piggy-back mechanism (Saryi et al. 2017). Besides the PTS1 and PTS2 receptors, peroxisomes rely on several other Pex for protein import as well as peroxisomal biogenesis and maintenance, which are generally conserved among eukaryotes with peroxisomes (Gabaldon et al. 2006; Schlüter et al. 2006). The core components comprise Pex13 and Pex14 (docking complex), the E3 ubiquitin ligases Pex2, Pex10 and Pex12, Pex4 (ubiquitin conjugation) and Pex22 (membrane anchor for Pex4), as well as the cytosolic AAA-ATPases Pex1 and Pex6 for receptor recycling—all part of the import machinery for soluble matrix proteins (Gould and Valle 2000). Moreover, Pex3, Pex16, and Pex19 are involved in the import of membrane proteins into the peroxisomal membrane and Pex11 plays an important role in the division of peroxisomes (e.g., Kim and Hettema 2015; Smith and Aitchison 2013).
Although the majority of eukaryotes are known to contain peroxisomes to compartmentalize particular (often “noxious”) metabolic pathways within a “special reaction chamber,” certain organisms—mostly parasitic protists containing mitochondria-related organelles instead of canonical aerobic mitochondria, but also some metazoans—seem to have lost these organelles (Gabaldon et al. 2016; Schlüter et al. 2006; Zarsky and Tachezy 2015). Among the parasites are Trichomonas and Giardia (Excavata), microsporidians (Fungi), and Entamoeba (Amoebozoa); for peroxisomes in apicomplexans conflicting data exists.
One of the first reports presenting experimental data on potential peroxisomes in apicomplexans came from Kaasch and Joiner in 2000. Here, T. gondii catalase which contains a putative PTS1 (AKM) was found to localize within defined punctuate and vesicular structures anterior to the parasite nucleus in intra- and extracellular tachyzoites (Kaasch and Joiner 2000). Yet in the same year, Ding and colleagues produced controversial results and found catalase to be mainly localized in the cytosol of T. gondii tachyzoite cell stages (Ding et al. 2000). Moreover, Ding et al. later on confirmed a cytosolic localization of catalase in T. gondii and reported the complete absence of Pex genes in the parasite (Ding et al. 2004). Work by Schlüter et al. (2006), finally described apicomplexans as “the first group of organisms devoid of peroxisomes, in the presence of mitochondria”. However, this view is in question because sequencing of several additional apicomplexan genomes has occurred from which new evidence emerged that might speak for the presence of peroxisomes or peroxisome-like structures in at least a subset of apicomplexan species (Gabaldon et al. 2016): Firstly, there is in silico data reporting the presence of classical peroxisomal enzymes such as Pex1, Pex5, and Pex6 as well as enzymes involved in typical peroxisomal fatty acid metabolism (β-oxidation) in T. gondii and Neospora caninum (Gabaldon et al. 2016; Kienle et al. 2016); secondly, some of the β-oxidation factors contain putative PTS1 sequences (Possenti et al. 2013); and thirdly, there is experimental evidence that one of the latter enzymes (TgHAD-2SCP-2; TGME49_234570) localizes at least in part to distinct vesicular structures in T. gondii tachyzoites (Lige et al. 2009). Taken together, the presence of peroxisomes in apicomplexans is still uncertain and although several leads in support for such organelles (at least in particular species) exist, the line of evidence is still scarce, especially with respect to experimental verification.
Here we provide strong bioinformatic evidence for the presence of peroxisomes in T. gondii and other coccidians by identification of a complete set of Pex that might be capable of producing and maintaining fully functional peroxisomes in these organisms. Moreover, we found the fatty acid β-oxidation pathway in coccidians to be equipped with classical PTSs. In T. gondii these factors are subjected to a life cycle stage-specific protein expression profile, which suggests that a putative peroxisomal β-oxidation takes place mainly during the oocyst/sporozoite stage.
Materials and Methods
Genomic Screening for Peroxins in Chromerids and Apicomplexans
Pex in chromerids were identified via two different strategies: 1) using KEGG (Kyoto Encyclopedia of Genes and Genomes; http://www.genome.jp/kegg/; last accessed November 10, 2017) classification of the 31,799 Chromera velia CCMP2878 annotated proteins (CryptoDB-31_CveliaCCMP2878_AnnotatedProteins.fasta) downloaded from the Cryptosporidium Genomics Resource, CryptoDB (http://cryptodb.org/cryptodb/home.do; last accessed November 10, 2017) screening the category “peroxisome”; and 2) by performing a blastP search against the C. velia genome database ((http://cryptodb.org/cryptodb/showQuestion.do?questionFullName=UniversalQuestions.UnifiedBlast; last accessed November 10, 2017, e-value cutoff: e-4) using known sequences of Pex from yeast, plants, and animals (NCBI) followed by analysis of the hits using reciprocal blastP versus the NCBI nr protein database (https://blast.ncbi.nlm.nih.gov/blast/Blast.cgi; last accessed November 10, 2017) to check the annotation of similar sequences, areas of sequence conservation and potential functional domains. The same strategy was chosen to screen for Pex in T. gondii ME49 (KEGG “peroxisome”: http://www.genome.jp/kegg-bin/show_pathway?tgo04146; blastP: http://toxodb.org/toxo/; last accessed November 10, 2017, e-value cutoff: e-4), except that this time the identified C. velia Pex were used as additional queries for the database search.
For the following blastP analyses in other apicomplexans including further coccidians such as N. caninum Liverpool, Eimeria tenella Houghton, Cyclospora cayetanensis CHN_HEN01, Hammondia hammondi H.H.34, and Sarcocystis neurona SO SN1 (http://toxodb.org/toxo/; last accessed November 10, 2017) as well as P. falciparum 3D7 (http://plasmodb.org/plasmo/; last accessed November 10, 2017), Cryptosporidium parvum Iowa II, and Gregarina niphandrodes (http://cryptodb.org/cryptodb/; last accessed November 10, 2017), putative Pex identified in C. velia and T. gondii have been used as queries for genome searches.
Peroxisomal Proteome Prediction in T. gondii
To predict potential PTS1-containing proteins in T. gondii, we used an in-house Perl script specifically identifying those sequences in the 8,322 T. gondii proteins (ToxoDB-32_TgondiiME49_AnnotatedProteins) that possess a combination of the amino acid sequence [SAC]-[KRH]-[LM] within the last three positions towards their C-terminus. The hit sequences were then functionally categorized via performing a blastP analysis against the NCBI nr database (https://blast.ncbi.nlm.nih.gov/blast/Blast.cgi; last accessed November 10, 2017). Furthermore, all of them were subjected to a stringent targeting prediction pipeline as already described in Moog et al. (2015). Briefly, sequences were analyzed with PTS1 predictor (http://mendel.imp.ac.at/mendeljsp/sat/pts1/PTS1predictor.jsp; last accessed November 10, 2017; Neuberger et al. 2003) and Target Signal Predictor (http://216.92.14.62/Target_signal.php; last accessed November 10, 2017, Schlüter et al. 2007) for potential peroxisomal targeting sequences, SignalP3.0 (http://www.cbs.dtu.dk/services/SignalP-3.0/; last accessed November 10, 2017, Nielsen et al. 1997), SignalP4.1 (http://www.cbs.dtu.dk/services/SignalP/; last accessed November 10, 2017, Petersen et al. 2011), TargetP1.1 (http://www.cbs.dtu.dk/services/TargetP/; last accessed November 10, 2017, Emanuelsson et al. 2000), Predotar (https://urgi.versailles.inra.fr/predotar/; last accessed November 10, 2017; Small et al. 2004), and PredSL (http://aias.biol.uoa.gr/PredSL/input.html; last accessed November 10, 2017, Petsalaki et al. 2006) were used to predict N-terminal targeting signals such as signal peptides and mitochondrial targeting peptides, whereas potential transmembrane domains were predicted with TMHMM (http://www.cbs.dtu.dk/services/TMHMM/; last accessed November 10, 2017, Krogh et al. 2001), and TOPCONS (http://topcons.cbr.su.se/; last accessed November 10, 2017, Bernsel et al. 2009). Proteins were classified as “most likely peroxisomal,” if not more than one of the individual targeting predictions contradicted a peroxisomal localization (see supplementary table S2, Supplementary Material online, red letters) and the proteins were not predicted to contain transmembrane domains.
PTS1-independent searches for proteins potentially involved in β-oxidation of fatty acids, as it was the case for T. gondii 3-ketoacyl-CoA thiolase (3KCT), were performed via blastP using queries of the diatom Phaeodactylum tricornutum (Gonzalez et al. 2011). Sequences were subjected to the targeting prediction pipeline as described above. Additionally, sequences were manually inspected for the presence of a putative PTS2 using all known motif possibilities ([RK]-[LVIQ]-X-X-[LVIHQ]-[LSGAK]-X-[HQ]-[LAF]) as reported by Kunze et al. (2015).
Identification of T. gondii Proteins in Proteomics Data
To check whether proteins identified during our searches are permanently present during the life cycle of T. gondii or are part of the proteome of a specific life cycle stage in the parasite we used all existing mass spectrometry data incorporated into the Toxoplasma Genomics Resource (http://toxodb.org/toxo/; last accessed November 10, 2017) and oocyst/sporozoite proteomic data published by Possenti et al. (2013). The presence of at least one unique peptide sequence mapping to the protein of interest was considered evidence of expression during a specific life cycle stage (T: tachyzoite; O: oocyst/sporozoite) of T. gondii. Existing proteomics data for N. caninum and E. tenella were also analyzed via the Toxoplasma Genomics Resource.
Screening for β-Oxidation Enzymes in Apicomplexans
Enzymes for β-oxidation in Coccidia and other apicomplexans were identified using T. gondii candidate sequences obtained in the course of the peroxisomal proteome prediction in reciprocal blastP searches (see above). All hit sequences were checked for the presence of a potential PTS1 using PTS1 predictor and/or PTS2 (see above).
Results
Peroxins in Chromerids and Apicomplexans
We first wanted to investigate whether the closest photosynthetic relative of apicomplexans contains the molecular equipment for peroxisome biogenesis, maintenance and protein import factors—peroxins (Pex). To this end we conducted a genomic screen in C. velia, as described in the Materials and Methods section, that led to the identification of a full set of factors potentially sufficient to maintain functional peroxisomes in the chromerid (table 1). Chromera velia contains the two import receptors required for PTS1- and PTS2-mediated matrix protein import—Pex5 and Pex7—as well as one central component of the peroxisomal docking complex, Pex14, although Pex13 seems to be absent. In addition, all components for receptor recycling including the E3 ligases Pex2, Pex10, and Pex12 (RING-finger complex), the ubiquitin-conjugating enzyme Pex4 its putative membrane anchor Pex22 and the AAA ATPases Pex1 and Pex6 were identified (table 1). With respect to components for peroxisomal membrane protein (PMP) import and de novo formation Pex3 and Pex16 were detected in C. velia, whereas Pex19, the receptor for PMPs, has not been identified in the Chromera genome (table 1). However, by screening the genome of a closely related chromerid species, Vitrella brassicaformis CCMP3155, a Pex19 candidate containing a conserved Pex19 superfamily domain showing similarity to fungal Pex19 sequences was identified (NCBI accession number: CEL92557.1). Besides Pex necessary for protein import and biogenesis, C. velia also contains a Pex11 homolog, which is known to be a crucial factor for peroxisomal division and proliferation.
Table 1.
Peroxins in Chromerids and Apicomplexans
| Peroxin | Cv | Gn | Cp | Tg | Nc | Et | Cc | Hh | Sn | Pf |
|---|---|---|---|---|---|---|---|---|---|---|
| Pex1 | + | nd | nd | + | + | + | + | + | nd | nd |
| Pex2 | + | nd | nd | +T | + | + | + | + | nd | nd |
| Pex3 | + | nd | nd | +O, T | + | ? | nd | + | + | nd |
| Pex4 | + | + | + | +O, T | + | nd | + | + | nd | + |
| Pex5 | + | nd | nd | + | + | + | + | + | + | nd |
| Pex6 | + | nd | nd | +T | + | + | + | + | nd | nd |
| Pex7 | + | nd | nd | + | + | + | + | + | + | nd |
| Pex10 | + | nd | nd | +T | + | + | nd | + | nd | ? |
| Pex11 | + | nd | nd | +O | + | + | + | + | + | nd |
| Pex12 | + | nd | nd | + | + | + | + | + | + | nd |
| Pex13 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Pex14 | + | nd | nd | + | + | nd | nd | + | nd | nd |
| Pex16 | + | nd | nd | + | nd | + | + | + | nd | nd |
| Pex19 | nda | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Pex22 | + | + | + | +T | + | + | ? | + | + | + |
Note.—Organism name (and strain) abbreviations: Cv: Chromera velia CCMP2878; Gn: Gregarina niphandrodes; Cp: Cryptosporidium parvum Iowa II; Tg: Toxoplasma gondii ME49; Nc: Neospora caninum Liverpool; Et: Eimeria tenella Houghton; Cc: Cyclospora cayetanensis CHN_HEN01; Hh: Hammondia hammondi H.H.34; Sn: Sarcocystis neurona SO SN1; Pf: Plasmodium falciparum 3D7. Other abreviations: +: Ortholog present; ?: orthology status unclear; nd: not detected. See supplementary table S1, Supplementary Material online, for protein identification numbers. O, T: protein detected in oocyst and/or tachyzoite proteome. Proteomics data from Toxoplasma gondii genomics resource ToxoDB (http://toxodb.org/toxo/; last accessed November 10, 2017).
A Pex19 candidate was detected in a related chromerid species, Vitrella brassicaformis CCMP3155 (NCBI accession number: CEL92557.1).
In the apicomplexan T. gondii ME49 the situation is identical to C. velia (table 1): All candidates for the basic set of Pex are encoded in the genome of the parasite except a homolog for Pex19, which either might be truly absent or due to sequence divergence, could not be identified in the genome of the coccidian. Other factors required for peroxisome biogenesis and membrane protein import (Pex3 and Pex16), organelle division (Pex11) and import of soluble proteins and receptor recycling are present (table 1).
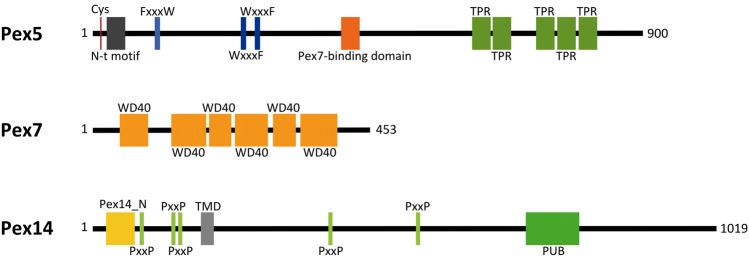
From an in silico perspective all “central” components of the PTS1- and PTS2-dependant matrix protein import in T. gondii (Pex5, Pex7, and Pex14) contain the conserved motifs known to be required for potential interaction of these factors (fig. 1). Toxoplasma gondii Pex5, for example, possesses a cysteine at position 16—the putative mono-ubiquitination site (receptor recycling)—followed by a conserved N-terminal motif of ∼30 residues around the amino acids NPL (position 34–36; Schliebs and Kunau 2006). Moreover, the protein contains a FxxxW and two WxxxF/Y motifs located between amino acid position 102 and 271 known to play a role in interaction of Pex5 with the docking complex proteins Pex13 and Pex14 (Effelsberg et al. 2016; Galland and Michels 2010), of which the first one seems to be absent from T. gondii (table 1). These motifs are followed by a Pex7-binding domain between position 414 and 437 (Dodt et al. 2001) and, finally, several TPR (tetratricopeptide repeat) domains (starting from amino acid 601), which are very common for Pex5 proteins (fig. 1; Galland and Michels 2010).
Fig. 1.
—Putative central peroxin factors for PTS1- and PTS2-mediated peroxisomal matrix protein import in Toxoplasma gondii. Pex5, Pex7, and Pex14 possess all the required motifs for proper function/interaction during peroxisomal matrix protein import. Numbers indicate amino acid positions of the individual primary sequences. Cys: cysteine; N-t motif: conserved N-terminal motif of Pex5; FxxxW and WxxxF/Y: motifs for interaction of Pex5 with Pex13 and Pex14; Pex7-binding domain: motif known to be crucial for interaction of the co-receptor Pex5 with the PTS2 receptor Pex7; TPR: tetratricopeptide repeat, protein–protein interaction motif; WD40: protein–protein interaction motif; Pex14_N: N-terminal domain of Pex14 known to be required for interaction with the PTS1 receptor Pex5; PxxP: potential Pex13 binding motif of Pex14; PUB: PNGase/UBA or UBX domain.
The T. gondii Pex7 candidate is a highly conserved protein with six predicted WD-40 domains (fig. 1) similar in domain organization to other Pex7 factors known from yeast, plants and humans (Galland and Michels 2010). This protein usually interacts with PTS2-containing peroxisomal cargo proteins and is led to the docking complex with the aid of a coreceptor (Pex5 in plants, Pex5L in mammals, and Pex18/21 or Pex21 in yeasts; Schliebs and Kunau 2006). The docking complex in T. gondii, according to our results, either consists of a single protein, Pex14, or the primary sequence of the putative T. gondii Pex13 is divergent to a degree that is preventing identification in the genome of the parasite. Similar to mammalian, yeast, plant and trypanosome Pex14 proteins, the homolog of T. gondii contains a conserved N-terminal domain (amino acids 22–62) known to be required for interaction with the PTS1 receptor Pex5 (Galland and Michels 2010; Schliebs et al. 1999). This domain is followed by a predicted transmembrane domain (amino acids 176–196) and potential Pex13 binding motifs (PxxP) were also detected in the T. gondii Pex14 primary sequence. The C-terminal part of the protein is less conserved, but similar to the homolog of C. velia (table 1), the T. gondii Pex14 possess a PUB (PNGase/UBA or UBX) domain (fig. 1) known to function as a Cdc48 interaction module (Madsen et al. 2009), in this case representing a potential site for interaction with the Cdc48-related ATPases Pex1 and/or Pex6.
Besides the conserved components, a subset of putative Pex identified in T. gondii is showing an increased sequence divergence. Unlike other RING-finger complex E3 ligases such as those from C. velia, Saccharomyces cerevisiae, Arabidopsis thaliana, or Homo sapiens, the T. gondii homologs seem to lack N-terminal sequence conservation and thus are devoid of a predicted Pex10 or Pex2/Pex12 superfamily domain. However, they possess the conserved C-terminal RING-finger domain (E3 ubiquitin ligase activity), which still exhibits a high degree of sequence conservation and is therefore sufficient for identification of the potential molecular function of these components. Additionally, the T. gondii Pex3 lacks significant sequence conservation, when it is used as a query against the NCBI nr database. However, it has been identified by using the C. velia Pex3 as a query for which a conserved Pex3 domain was detected. The T. gondii Pex22 primary sequence also seems to be highly derived. However, besides two predicted transmembrane domains in the relatively nonconserved N-terminal half, the protein shares sequence similarity to other proteins annotated as “Pex22” or “Pex22-like” in its C-terminal portion. Thus, although in parts less conserved as in other organisms, it seems that T. gondii possess the molecular tools to form and maintain functional peroxisomes.
Next, we wanted to analyze whether the Pex identified in T. gondii are permanently expressed, or whether they might be present during a specific stage in the parasite’s life cycle only. To this end we analyzed existing proteomics data integrated into the Toxoplasma Genomics Resource (see Materials and Methods). Whereas there was no proteomic evidence for a fraction of the putative Pex (Pex1, Pex5, Pex7, Pex12, Pex14, Pex16) in general, several of these proteins were identified to be present in the proteome during tachyzoite (Pex2, Pex6, Pex10, Pex22), tachyzoite and oocyst (Pex3 and Pex4), and oocyst (Pex11) life cycle stages (see table 1 and supplementary table S2, Supplementary Material online).
In addition to T. gondii, we searched the genomes of other coccidians including N. caninum, E. tenella, C. cayetanensis, H. hammondi, and S. neurona for the presence of genes encoding sequences homologous to the Pex identified in C. velia and T. gondii. As shown in table 1 this screening resulted in the identification of several Pex candidates in these organisms. Although in some cases this ended up in a fragmentary list only (table 1), with S. neurona showing the most incomplete set of putative Pex probably due to the quality of the current genomic data, these results generally support the presence of peroxisomes in coccidian parasites. Screenings of the N. caninum and E. tenella proteomics data concerning a potential stage-specific presence of Pex did not provide any results.
We performed the same bioinformatic screening for Pex in related, noncoccidian apicomplexans: The malaria causing agent P. falciparum, the parasite responsible for cryptosporidiosis—C. parvum—as well as the gregarine G. niphandrodes. Our analysis revealed that all of the three representatives seem to lack essential Pex necessary to form and maintain functional peroxisomes (table 1). Interestingly though, in each of the three organisms, a homolog for the peroxisomal ubiquitin-conjugating enzyme Pex4 and its potential membrane anchoring interaction partner Pex22 was identified via blastP searches (table 1). Similar to T. gondii, the primary sequence of the Pex4 candidates is highly conserved, which is not the case for the putative Pex22 proteins (orthology unclear). Whereas proteomic evidence for the Pex4 and Pex22 candidates in C. parvum and G. niphandrodes is absent, in P. falciparum, these two proteins are most abundant in gametocytes, but have also been detected in schizont, trophozoite, merozoite, and sporozoite proteomics data (supplementary table S2, Supplementary Material online). Moreover, in P. falciparum a protein containing a “rudimentary” Pex10 domain at the N-terminus overlapping with a RING-finger domain has been detected (table 1). Although representing an unusual domain organization for Pex10 (RING domain usually C-terminally located) the protein might still constitute a functional E3 ligase.
Prediction of PTS1-Based Peroxisomal Proteome in T. gondii
Based on our results showing the presence of a nearly complete set of Pex in coccidians, and thus suggesting that peroxisomes may exist in these organisms, we wanted to analyze whether there is any evidence for proteins that are potentially targeted to such organelles. To this end we screened the genome data of T. gondii for encoded proteins containing a putative PTS1 at the C-terminus (see Materials and Methods). This signal was chosen because the vast majority of characterized peroxisomal proteins in organisms studied so far is targeted to peroxisomes via a PTS1 (Brocard and Hartig 2006). The signal had to be composed of the amino acid motif [SAC]-[KRH]-[LM], the classical PTS1 consensus sequence (Kim and Hettema 2015), and had to be located within the last three positions of an individual protein primary sequence. Interestingly, our search resulted in the identification of a total of 22 protein sequences only (supplementary table S2, Supplementary Material online). These sequences were further subjected to a very stringent targeting prediction pipeline, verifying the predicted PTS1 with other PTS1 prediction tools and screening for potential N-terminal targeting sequences and transmembrane domains, to exclude false positive predictions. Only those sequences with a clearly detectable PTS1 not conflicted by more than one diverging prediction were classified as potentially PTS1-targeted proteins (supplementary table S2, Supplementary Material online). In total this set contained eight sequences, which were subsequently investigated concerning their potential molecular function via blastP analysis against the NCBI protein database. Interestingly, six of the eight proteins were identified as typical peroxisomal functions including catalase and enzymes of the fatty acid β-oxidation pathway (supplementary table S2, Supplementary Material online). These six out of 22 proteins were finally defined as the “high confidence” PTS1-targeted peroxisomal proteome for T. gondii (table 2). The potential β-oxidation enzymes comprise: Long chain fatty acid-CoA ligase (FACS) for activation of fatty acids prior oxidation, ACOX, catalyzing the initial step of peroxisomal β-oxidation, enoyl-CoA hydratase (ECH) and (3R)-hydroxyacyl-CoA dehydrogenase, which catalyze the middle part of the β-oxidation reaction, as well as 2, 4-dienoyl-CoA reductase (DECR), an additional enzyme required for degradation of polyunsaturated fatty acids (table 2).
Table 2.
Predicted High Confidence Toxoplasma gondii Peroxisomal Proteome
| IDa | Pred. PTS1b | Function (BlastP)c | Proteomicsd |
|---|---|---|---|
| TGME49_297220 | AKL | Long chain fatty acid-CoA ligase | O, T |
| TGME49_229140 | AKL | Enoyl-CoA hydratase | O, T |
| TGME49_232250 | AKM | Catalase | O, T |
| TGME49_226300 | SRL | 2, 4 dienoyl-CoA reductase | O |
| TGME49_234570 | SRL | (3R)-hydroxyacyl-CoA dehydrogenase | O |
| TGME49_247500 | SKL | Acyl-CoA oxidase | O |
| TGME49_242390 | PKLe | Enoyl-Coa hydratase/isomerase | O |
| Pred. PTS2e, f | |||
| TGME49_273740g | RLTTLSGQF | 3-Ketoacyl-CoA thiolase | O, T |
Note.—See supplementary table S2, Supplementary Material online, for detailed information on detected protein sequences including an in-depth targeting prediction.
Identification number according to the Toxoplasma gondii genomics resource ToxoDB (http://toxodb.org/toxo/; last accessed November 10, 2017).
Predicted PTS1 using a Perl script searching for sequences with terminal amino acids [SAC]-[KRH]-[LM].
Functional annotation based on NCBI protein–protein BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi; last accessed November 10, 2017).
Detection in proteomics data from Toxoplasma gondii genomics resource ToxoDB (http://toxodb.org/toxo/; last accessed November 10, 2017). O: Oocyst proteome; T: Tachyzoite proteome; nd: not detected.
Manually detected PTS, not predicted via automated search.
Predicted PTS2 instead of PTS1.
Manually identified protein that might catalyze the last step of the fatty acid beta oxidation in T. gondii; possibility of dual targeting to peroxisome and mitochondria (see supplementary table S2, Supplementary Material online).
In addition to these enzymes, another candidate for degradation of unsaturated fatty acids might be targeted to putative T. gondii peroxisomes: enoyl-CoA isomerase (ECI). This accessory factor already identified by Possenti et al. (2013) contains a predicted PTS1 composed of the amino acids PKL and was thus not detected by our screening. However, the prediction of a PTS1 using other tools (see Materials and Methods) was positive (supplementary table S2, Supplementary Material online), qualifying the protein as a further candidate of the “high confidence” PTS1-targeted peroxisomal proteome of T. gondii (table 2).
PTS1-prediction allowed us to identify an almost complete fatty acid β-oxidation pathway, which is probably targeted to peroxisomes in T. gondii, with the exception of one enzyme: 3KCT. This enzyme catalyzes the last step of the β-oxidation, generating acetyl-CoA and fatty acyl-CoA shortened by two carbon units (Poirier et al. 2006). We manually screened the T. gondii genome data for genes encoding 3KCT via blastP using a query from P. tricornutum (protein ID JGI: 41969) and obtained two hits. One was annotated as an acetyl-CoA acyltransferase the other as acetyl-CoA acetyltransferase. A detailed analysis of the primary sequences of both proteins revealed that the first one is indeed a 3KCT and that both proteins, based on the detection of N-terminal targeting signals and due to a missing C-terminal PTS1, seem to be targeted to the mitochondrion (supplementary table S2, Supplementary Material online). However, when inspecting the putative T. gondii 3KCT for the presence of a potential PTS2 we detected a motif very close to the N-terminus (RLTTLSGQF) that, according to Kunze et al. (2015), might resemble a functional peroxisomal targeting sequence of type 2 (supplementary table S2, Supplementary Material online). Although it cannot be ruled out at this point that the T. gondii 3KCT is targeted to the mitochondrion (or possibly dually targeted to mitochondrion and peroxisome), because of the very clear manual identification of a putative PTS2 motif, the enzyme is a further candidate for the “high confidence” peroxisomal proteome of T. gondii, resulting in a list of eight proteins in total (table 2).
We have also detected a putative delta(3, 5)-delta(2, 4)-dienoyl-CoA isomerase (TGME49_310830) via blastP with the P. tricornutum bifunctional enzyme sequence (55069), which might be involved in peroxisomal β-oxidation in T. gondii. However, PTS1 prediction was vague (AAL; supplementary table S2, Supplementary Material online), which is why the enzyme was not added to the predicted “high confidence” peroxisomal proteome. In addition to the putative peroxisomal fatty acid β-oxidation pathway, a complete set of enzymes containing predicted mitochondrial targeting signals was identified in T. gondii (see supplementary table S2, Supplementary Material online).
As for the detected Pex, we wanted to analyze if there is a tendency towards the presence of the predicted “high confidence” T. gondii peroxisomal proteome components in certain life cycle stages of the parasite. As shown in table 2 we found a clear abundance of all eight proteins within the oocyst proteome, with some of them being additionally found in the tachyzoite proteomics data (FACS, ECH, CAT, 3KCT). As already observed by Possenti et al. (2013), this points to an elevated oocyst-specific presence of the β-oxidation enzymes in T. gondii.
β-Oxidation Enzymes and Catalase in Coccidia Contain Putative PTSs
We further aimed to inspect the presence and localization of putative orthologs of the T. gondii “high confidence” PTS1-targeted peroxisomal proteome in other coccidians. To this end, we performed an in silico search using the eight proteins of T. gondii (Tab. 2) as queries for blastP analyses against the genome data of N. caninum, E. tenella, C. cayetanensis, H. hammondi, and S. neurona. The screening revealed that in each investigated species orthologs to all of the eight T. gondii proteins are present (table 3 and supplementary table S2, Supplementary Material online). For the majority of the identified sequences unambiguous PTS1 (FACS, ACOX, ECH, 3HCDH, ECI, DECR, CAT) or PTS2 motifs (3KCT) were detected (see Materials and Methods). In the rare cases in which no potential peroxisomal targeting sequence for one of the eight proteins was identified, this was predominantly due to an incomplete protein sequence in the genome database (indicated by blastP). An analysis of the amino acid frequencies within the last 12 C-terminal residues of all 37 coccidian proteins with predicted PTS1 revealed that the classical SKL is the dominant motif, but there seem to be further, not so common residues ([SAPC]-[KRNS]-[LMF]) which might be important elements of PTS1 motifs in coccidians (supplementary table S2, Supplementary Material online).
Table 3.
Toxoplasma gondii Fatty Acid Beta Oxidation Enzymes, Catalase, Orthologs from Other Apicomplexans and Predicted Peroxisomal Targeting Signals
| Enzyme | Tg | Nc | Et | Cc | Hh | Sn | Pf | Cp | Gn |
|---|---|---|---|---|---|---|---|---|---|
| FACS | +/PTS1 (AKL) | +/PTS1 (AKL) | +/PTS1 (CKL) | +/PTS1 (AKL) | +/PTS1 (AKL) | +/PTS1 (SKL) | +/no PTSa | +/SP, PTS1a | +/no PTSa |
| ACOX | +/PTS1 (SKL) | +/PTS1 (CKL) | +/PTS1 (PKL) | +/no PTS | +/PTS1 (SKL) | +/PTS1 (AKL) | nd | nd | nd |
| ECH | +/PTS1 (AKL) | +/PTS1 (SKL) | +/no PTS | +/PTS1 (AKL) | +/PTS1 (AKL) | +/PTS1 (ARM) | nd | nd | nd |
| 3HCDH | +/PTS1 (SRL) | +/PTS1 (SRL) | +/PTS1 (SKL) | +/PTS1 (SKL) | +/PTS1 (SRL) | +/no PTSb | nd | nd | nd |
| ECI | +/PTS1 (PKL) | +/PTS1 (PKL) | +/PTS1 (SKM) | +/PTS1 (SRF) | +/PTS1 (PKL) | +/PTS1 (SNL) | nd | nd | nd |
| DECR | +/PTS1 (SRL) | +/PTS1 (SRL) | +/no PTSb | +/PTS1 (SSL) | +/PTS1 (SRL) | +/PTS1 (AKL) | nd | nd | nd |
| 3KCT | +/PTS2 (RLTTLSGQF) | +/PTS2 (RLTTLSGQF) | +/PTS2? (RLVLGVVAL) | +/PTS2? (RLQAVTRQV) | +/PTS2 (RLTTLSGQF) | +/no PTSb | nd | nd | nd |
| CAT | +/PTS1 (AKM) | +/PTS1 (AKM) | +/no PTSb | +/PTS1 (ARM) | +/PTS1 (AKM) | +/PTS1 (SKM) | nd | nd | +/no PTS |
Note.—Enzyme abbreviations: FACS: fatty acyl-CoA synthetase; ACOX: acyl-CoA oxidase; ECH: enoyl-CoA hydratase; 3HCDH: (3R)-hydroxyacyl-CoA dehydrogenase; ECI: enoyl-CoA isomerase; DECR: 2, 4-dienoyl-CoA reductase; 3KCT: 3-ketoacyl-CoA thiolase; CAT: catalase. See supplementary table S2, Supplementary Material online, for protein identification numbers. Organism name (and strain) abbreviations: Tg: Toxoplasma gondii ME49; Nc: Neospora caninum Liverpool; Et: Eimeria tenella Houghton; Cc: Cyclospora cayetanensis CHN_HEN01; Hh: Hammondia hammondi H.H.34; Sn: Sarcocystis neurona SO SN1; Pf: Plasmodium falciparum 3D7; Cp: Cryptosporidium parvum Iowa II; Gn: Gregarina niphandrodes. Other abbreviations: +: Ortholog present; nd: not detected; PTS: peroxisomal targeting signal type 1 or 2; SP: signal peptide; no PTS: no PTS predicted.
Best hit (blastP).
Protein sequence probably incomplete. PTS prediction not possible.
We also screened the genomes of the related apicomplexans P. falciparum and C. parvum. With exception of homologous sequences to FACS, none of the eight queries from T. gondii produced any hits when performing blastP analyses. The potential FACS homolog detected in P. falciparum and C. parvum is probably involved in fatty acid metabolism apart from the β-oxidation pathway, which seems to be completely absent in both organisms. Whereas the P. falciparum FACS candidate showed no indication of a PTS, the putative FACS of C. parvum possesses a C-terminal NKL motif recognized as a PTS1 by in silico prediction tools. However, the fact that there is no evidence for peroxisomes in C. parvum in addition to a predicted signal peptide at the N-terminus of the protein cast doubt on a functionality of the putative FACS PTS1 in C. parvum.
Further Factors Connected to β-Oxidation That Might Play a Role in Putative Peroxisomes of T. gondii and Other Coccidia
Fatty acids must reach the peroxisomal lumen to be channeled into the peroxisomal β-oxidation pathway and so far there is no evidence for membrane transporters that might take over this function in coccidians. By screening the T. gondii genome data using the protein sequence of an A. thaliana peroxisomal fatty acid translocator—an ABC class D transporter—(NCBI accession number BAB84551.1) as a query, a putative homolog could be detected (TGME49_314330). By analyzing the T. gondii Genomics Resource for existing proteomic data, we found that this putative transporter, which is also present in other coccidians (data not shown), is indeed an oocyst specific protein. A screening for the presence of putative targeting sequences within the protein neither resulted in the detection of a PTS nor any other targeting sequence, except that five transmembrane domains were predicted via TMHMM (see supplementary table S2, Supplementary Material online).
In addition of the requirement for fatty acids to enter the peroxisome to fuel the β-oxidation pathway, it is equally important for the end products to leave the organelle for supplying other parts of cellular metabolism. One of the most important products of the β-oxidation of fatty acids is acetyl-CoA. Besides a potential direct transport via putative translocators, this compound, as known from mitochondria, can be converted into citrate by citrate synthase and cross membranes, such as the mitochondrial inner membrane, via the citrate-shuttle (Eckardt 2005). Interestingly, T. gondii contains at least two putative citrate synthase isoenzymes of which one (TGME49_203110) is an oocyst/sporozoite-specific protein (Possenti et al. 2013). By analyzing the protein primary sequence for the presence of potential targeting signals, we identified a predicted PTS2 (RLSVINAHL) in the very N-terminal part of the protein (see supplementary table S2, Supplementary Material online), which might direct the enzyme into the peroxisome of T. gondii.
Discussion
It is still a matter of debate whether peroxisomes exist in apicomplexan parasites. Whereas classical peroxisomal marker proteins seem to be absent from Plasmodium spp., Theileria annulata, Babesia bovis, and C. parvum, the results for T. gondii are somewhat controversial, but imply that at least a subset of molecular components necessary for peroxisome biosynthesis as well as some classical peroxisomal metabolic enzymes are encoded in the genome of the coccidian parasite (e.g., Gabaldon et al. 2016).
In this study, we conducted a thorough in silico screening to identify peroxins (Pex) in coccidians and other apicomplexans as well as their photosynthetic relatives, the chromerids. We were able to show that an almost complete set of Pex, probably sufficient for biogenesis, maintenance and proliferation of peroxisomes, is present in the chromerid C. velia, but also in T. gondii and related coccidians (table 1). Strikingly, orthologs for the PMP receptor Pex19 or the docking complex component Pex13 were neither identifiable in C. velia, nor in T. gondii and other coccidians. It is possible that these components are too divergent to be identified via the methods here applied or that they are truly absent from the genomes of these organisms. In the latter case their functions might have become redundant or have been taken over by so far unknown factors, although a candidate for Pex19 could at least be identified in the chromerid V. brassicaformis, indicating that Pex19 function is at least important in some of the photosynthetic relatives of apicomplexans (see table 1).
As already suggested by other studies, we were not able to detect the molecular tool set necessary to generate and sustain peroxisomes in all other noncoccidian apicomplexans investigated (table 1). Remarkably, though, P. falciparum, C. parvum, and G. niphandrodes seem to maintain remnants of this set, namely homologs of the peroxins Pex4 (E2 enzyme) and perhaps Pex22 (unclear; sequence highly derived), which are usually involved in conjugation of ubiquitin to the peroxisomal import receptor Pex5 at the peroxisomal membrane in the course of receptor recycling (El Magraoui et al. 2014; Williams et al. 2012; Zolman et al. 2005). As a peroxisomal membrane is most likely absent from these organisms, as is the PTS1 receptor Pex5 (table 1), it is unclear what function these factors fulfill (see discussion later), but their presence indicates that they seem to be of high importance to the parasites.
In P. falciparum there is another protein that might trace back to a former peroxisomal biogenesis factor: A protein containing a residual Pex10 and RING-finger domain. Pex10 in organisms with peroxisomes acts in concert with Pex4 and is normally involved in ligation of (mono-) ubiquitin to the PTS1 receptor Pex5 (Platta et al. 2014). Because of the unusual domain organization of this protein compared with other Pex10 sequences (RING-finger domain at the N- instead of the C-terminus, see Results section) in addition to the rather high sequence divergence, it is unknown whether this protein maintains any functional relatedness to Pex10. Thus, although traces of former peroxisomal proteins are noticeable in P. falciparum, C. parvum, and G. niphandrodes there is no full set of factors necessary for the biogenesis and maintenance of peroxisomes, strongly suggesting that such organelles are absent in these parasites. Furthermore, according to Gabaldon and colleagues, piroplasms (Theileria and Babesia) are devoid of Pex (Gabaldon et al. 2016). This is in total contrast to T. gondii and other coccidians, for which clear evidence for the genomic presence of Pex exists (table 1). But the line of evidence is not only based on genomics. As depicted in table 1, we also found that Pex in T. gondii can be detected in proteomics data obtained from tachyzoite and oocyst stages, suggesting that many of these factors are indeed expressed in the coccidian (see below). Moreover, and in part already known from earlier work (Kaasch and Joiner 2000; Lige et al. 2009; Possenti et al. 2013), we have identified catalase and a complete fatty acid β-oxidation pathway to contain putative signals for peroxisomal import (PTS1/PTS2) in coccidia, which we have defined as “high confidence” peroxisomal proteome in case of T. gondii (see tables 2 and 3).
If the molecular components necessary to form peroxisomes are present in the genomes of coccidians, and classical peroxisomal components such as catalase and enzymes for fatty acid β-oxidation enzymes have been detected with putative peroxisomal targeting sequences, why have these compartments never been clearly observed at the ultrastructural level before? There are at least two studies—one focusing on T. gondii catalase, the other on the D-bifunctional protein (TgHAD-2SCP-2; TGME49_234570; named 3HCDH here, see tables 2 and 3) of the parasite—in which typical peroxisomal proteins containing classical peroxisomal targeting sequences (PTS1) were shown to be targeted at least partially to vesicular structures in T. gondii tachyzoites and to peroxisomes when expressed in mammalian cells (Kaasch and Joiner 2000; Lige et al. 2009). In the case of both enzymes, catalase and TgHAD-2SCP-2, controversial results challenged a solely peroxisomal localization of the proteins as they have been frequently observed to localize to the cytosol of T. gondii tachyzoite cells as well (Ding et al. 2000; Lige et al. 2009). However, it has been shown that the C-terminal PTS1 of TgHAD-2SCP-2 has an important influence as a targeting signal mediating vesicular localization because blocking of the putative signal led to an exclusively cytosolic localization of the protein (Lige et al. 2009). Thus, although the results from earlier studies are controversial, together with the findings of our study, there is now an explicit lead towards the presence of peroxisomes in T. gondii (and other coccidians).
Maybe the reason why peroxisomes have so far not been clearly observed in T. gondii is more straightforward than one might think. It is well-known that peroxisomes are highly dynamic organelles—not only with respect to their enzymatic composition, but also in terms of their abundance within cells and different cell types depending on factors such as nutrition, environmental influences and developmental stages (Lodhi and Semenkovich 2014; Smith and Aitchison 2013). Kinetoplastids, which are mostly parasitic organisms, but are not related to apicomplexans, contain peroxisomal derivatives—so-called glycosomes—so named because a part of the glycolytic pathway is taking place inside these compartments (Haanstra et al. 2016). In trypanosomatids, obligate parasitic kinetoplastids, for example, the abundance of glycosomes is tightly regulated via autophagy (pexophagy, peroxisome degradation) and the biogenesis of new ones, to rapidly adapt to metabolic changes during the different parasite life cycle stages (Szöör et al. 2014). Toxoplasma gondii and other coccidians might be confronted with similar problems, as they all undergo a complex life cycle including sporozoite, tachyzoite, and bradyzoite stages with variable nutritional availabilities (Dubey et al. 1998).
We have investigated the putative Pex detected in our screen with respect to their presence during specific life cycle stages of T. gondii using existing proteomic data integrated into the T. gondii genomics resource (see table 1 and supplementary table S2, Supplementary Material online) and found that several of the key factors can be detected within oocyst and tachyzoite proteomic data. Pex11, a PMP promoting peroxisome division (Li and Gould 2002), and thus a potential regulator of peroxisome proliferation, was even specific to the oocyst/sporozoite proteome data set (table 1 and supplementary table S2, Supplementary Material online; see also Possenti et al. 2013). The machinery for peroxisome biogenesis, division and matrix protein import is, according to our predicted high confidence PTS1-specific T. gondii peroxisomal proteome (see table 2 and supplementary table S2, Supplementary Material online), primarily present to generate a compartment in which the β-oxidation of fatty acids can take place (fig. 2). Fatty acid β-oxidation is known to generate ROS (H2O2) as a side product (Poirier et al. 2006), which is probably directly neutralized by a peroxisome-targeted catalase (see table 2 and supplementary table S2, Supplementary Material online). Interestingly, the entire predicted high confidence T. gondii peroxisomal proteome (including a putative PTS2-targeted 3KCT) is, according to existing proteomics data, expressed during oocyst/sporozoite stages, some of them also during tachyzoite stages (see table 2). Moreover, when consulting additional transcriptomic data it is clearly noticeable that expression of, for example, those genes encoding Pex11 and the majority of the β-oxidation enzymes are extremely upregulated during the early oocyst stage (not shown). Thus, based on the current data, we hypothesize that if peroxisomes are present in T. gondii, they might be specific to the oocyst/sporozoite and (early) tachyzoite stages and probably completely absent in bradyzoites. This might also provide an explanation for the differently observed catalase localizations either to the cytosol and/or punctuate structures within T. gondii (Ding et al. 2000; Kaasch and Joiner 2000).
Fig. 2.
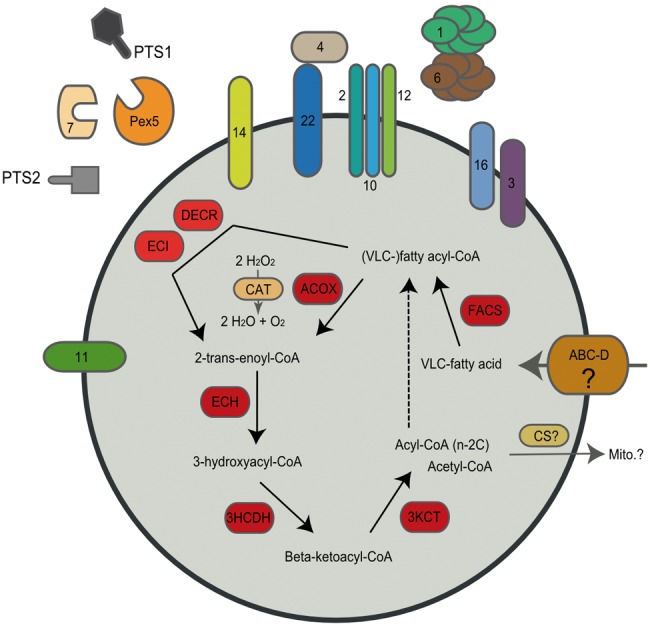
—Model of the Toxoplasma gondii peroxisome. The hypothetical peroxisome of T. gondii consists of a biogenesis/division/maintenance system that is also responsible for matrix protein import (peroxins, Pex, see numbers). The β-oxidation of fatty acids takes place inside the peroxisomal lumen (matrix), mainly during the oocyst life cycle stage of the parasite. Fatty acids might be imported into the peroxisome via an ABC class D transporter (ABC-D) and activated by FACS. Unsaturated fatty acids are converted by the accessory enzymes DECR and ECI to suitable substrates to be channeled into the β-oxidation cycle, which is catalyzed by ACOX, ECH, 3HCDH, and 3KCT. Hydrogen peroxide (H2O2) generated as a consequence of the ACOX reaction, is neutralized by the peroxisomal CAT. Acetyl-CoA, the end product of the fatty acid β-oxidation, finally may be transported to the mitochondria for energy production via the TCA-cycle, maybe after conversion by a putative PTS2-targeted peroxisomal CS into citrate, whereas acyl-CoA shortened by 2 C-atoms (n-2C) can be further oxidized in another round of β-oxidation in the peroxisome (or mitochondrion). VLC: very long chain; FACS: fatty acyl-CoA synthetase; ACOX: acyl-CoA oxidase; ECH: enoyl-CoA hydratase; 3HCDH: (3R)-hydroxyacyl-CoA dehydrogenase; ECI: enoyl-CoA isomerase; DECR: 2, 4-dienoyl-CoA reductase; 3KCT: 3-ketoacyl-CoA thiolase; CAT: catalase; CS: citrate synthase; PTS1/2: peroxisomal targeting signal of type 1 or 2; Mito.: mitochondrion. Numbers in the figure correspond to the individual peroxins (Pex). See supplementary table S2, Supplementary Material online, for protein identification numbers.
With respect to the observation of a stage-specific expression of β-oxidation enzymes in T. gondii the present study is not the first report on this topic. As already noted by Possenti and colleagues several years ago, enzymes for fatty acid β-oxidation are encoded in the genome of T. gondii and the products of six of them are part of the T. gondii oocyst/sporozoite-specific proteome data set—with four of them containing predicted PTSs (Possenti et al. 2013). In the present study we have provided further evidence that, besides the presence of a potential mitochondrial-targeted fatty acid β-oxidation pathway (supplementary table S2, Supplementary Material online), a complete set of β-oxidation enzymes (seven enzymes in total) is equipped with classical PTSs of either type 1 (FACS, ACOX, ECH, 3HCDH, ECI, DECR) or type 2 (3KCT; see tables 2,3 and supplementary table S2, Supplementary Material online) in T. gondii (fig. 2). Moreover, we have shown that the same is true for related coccidian species including N. caninum, E. tenella, C. cayetanensis, H. hammondi, and S. neurona, with the exception of a few scattered enzymes without positive PTS prediction, which is in most cases probably due to incomplete gene models present in the genome databases (see table 3 and supplementary table S2, Supplementary Material online). Thus, the detection of classical PTS1 and PTS2 sequences together with the presence of a conserved set of Pex in T. gondii and other coccidians strongly suggest that peroxisomes exist in these organisms and, as evident from T. gondii, are probably present only during specific life cycle stages.
The presence of FACS, DECR and ECI with PTS1 further suggests that coccidians have to metabolize long and very-long chain fatty acids (FACS), which have been reported to be synthesized during tachyzoite stages (Ramakrishnan et al. 2012), as well as unsaturated (DECR and ECI) fatty acids within their putative peroxisomes (fig. 2). This is most likely taking place mainly during the oocyst/sporozoite stage, when the cells are outside their hosts and have a high demand of energy production via the mitochondrial TCA cycle, which is probably fueled with acetyl-CoA produced by the peroxisomal β-oxidation (Possenti et al. 2013). Peroxisomal acetyl-CoA might be converted to citrate via a putative citrate synthase (TGME49_203110), which according to our results contains a putative PTS2, prior export into the cytoplasm and further transport to the mitochondrion (fig. 2). Interestingly, this enzyme is an oocyst/sporozoite-specific protein (Possenti et al. 2013), further supporting the idea of an oocyst-specific presence of peroxisomes and peroxisomal β-oxidation.
Is there anything else that might support our hypothesis for life cycle stage-specific peroxisomes in T. gondii and other coccidians? At least in T. gondii differences at the morphological level between the three cell types/life cycle stages have been observed that might be indicative. One such example is lipid bodies, which are abundant in sporozoites, but less numerous in tachyzoites, whereas they are reported to be absent in bradyzoites (Dubey et al. 1998). This electron microscopical observation might also speak for an elevated rate of lipid metabolism (catabolism and anabolism) especially during the sporozoite stage, supporting the life cycle stage-specific presence of β-oxidation enzymes, which might be compartmentalized within mitochondria and peroxisomes in T. gondii (and other coccidians). Additionally, we have detected a putative ABC class D transporter in T. gondii being homologous to plant peroxisomal fatty acid translocators. This putative transporter seems to be conserved in coccidians and, according to the T. gondii Genomics Resource, is an oocyst specific protein. It thus might participate in the import process of fatty acids into peroxisomes of T. gondii (fig. 2) and other coccidians during the oocyst stage, and thus represents another supportive element for our hypothesis.
By taking all of the present data into account, a potential model for peroxisome regulation in coccidians emerges: Once oocysts are released by the host into the environment, Pex11 might be activated via elevated expression initiating an increased proliferation of peroxisomes in early oocyst/sporozoite stages (most likely during the developmental period of sporulation), while other Pex are moderately expressed. The emerging number of peroxisomes is then mainly filled with enzymes of the β-oxidation pathway and catalase to cover the cellular needs for energy production in “subcellular reaction chambers” preventing the parasites cytoplasm from oxidative damage through side products of the pathway. As soon as the parasites have become intracellular again (tachyzoite stage) they probably turn off or decrease expression of Pex as well as fatty acid degradation genes to a basic level and probably degrade peroxisomes via pexophagy, a specific form of autophagy. The latter has been reported to take place in apicomplexans, although the classical factors for pexophagy known from yeast seem to be absent (Besteiro 2012). It is thus unknown whether autophagy plays a role in a hypothesized life cycle stage-specific degradation of peroxisomes in T. gondii, but it is also possible that some as yet unknown factors participate in this process. From a metabolic standpoint the described model seems to be reasonable, because during the intracellular life cycle stages (tachyzoites, bradyzoites) the parasites are supplied with abundant metabolites and energy by the host, whereas they are pretty much left to their own resources once shed as oocysts by their hosts and released to the environment (Fritz et al. 2012) where it might be crucial for survival to be able to rapidly and efficiently mobilize stored energy required for sporulation (Ginger 2006).
In other apicomplexans this might be totally different. Plasmodium sp. for example seems to lack a β-oxidation pathway (e.g., Olszewski and Llinas 2011; Oppenheim et al. 2014) and does not have a life cycle stage taking place outside of the host (Doerig et al. 2015; Menard et al. 2013). Thus, the parasite probably has no need to increase energy production via metabolism of internal storage products, because a continuous supply by the host might be ensured. However, cryprosporidians and gregarines show host-external life cycle stages including oocyst formation (e.g., Barta and Thompson 2006; Bouzid et al. 2013; Clopton et al. 2016; Toso and Omoto 2007), but nevertheless seem to lack peroxisomes (table 1) as well as peroxisomal and mitochondrial fatty acid β-oxidation (table 3). Based on our findings peroxisomes of Coccidia are most likely primarily needed to degrade very-long and long-chain fatty acids (see above)—in addition to fatty acid degradation in mitochondria—and to protect the cytoplasm from the harmful side products of this reaction. The absence of β-oxidation in P. falciparum, C. parvum, and G. niphandrodes is most likely a result of reductive evolution coming along with a general reduction of lipid metabolism in many parasitic protists (Gabaldon et al. 2016) and suggests that the latter two organisms may have found a strategy to persist during environmental stages which is different to the one of coccidians. These noncoccidian apicomplexans probably survive via metabolic quiescence or mobilization of metabolites other than the products of fatty acid degradation (acetyl-CoA) to fuel their energetic requirements (Clopton et al. 2016). One possible explanation would be that the noncoccidian apicomplexans investigated here simply do not need the amount of energy, which is required by coccidians during the environmental life cycle stage. This might be due to the fact that in most coccidians (except Sarcocystis) sporulation occurs outside the host in environmental oocysts and probably relies on stored energy reservoirs whereas cryptosporidians, Plasmodium spp. as well as several gregarines sporulate within their hosts (Bouzid et al. 2013; Menard et al. 2013) where they are supplied with sufficient metabolites to fulfill their reproduction cycle and generate sporozoites. Thus, peroxisomes are probably metabolically redundant in those noncoccidian apicomplexans, due to their individually adapted life cycles in conjunction with their highly specialized parasitic life styles.
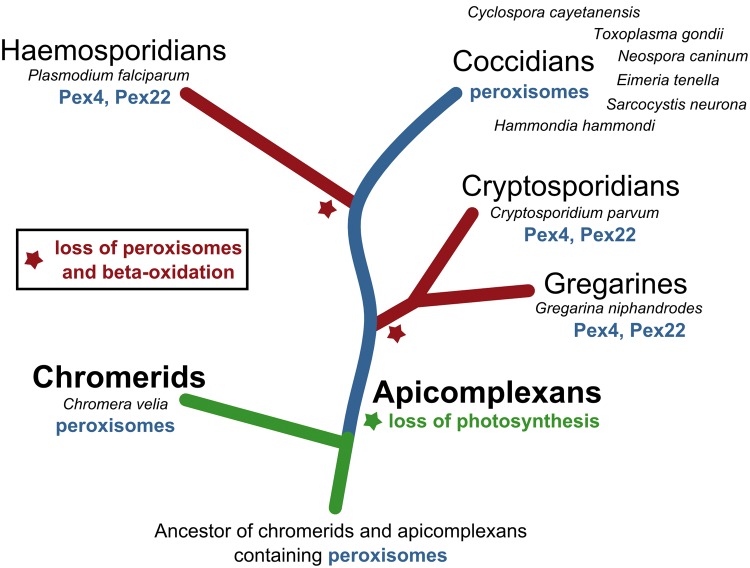
Taken together, the growing evidence from our and previous studies (Gabaldon et al. 2016; Kienle et al. 2016; Possenti et al. 2013) suggests that coccidians contain peroxisomes, whereas other apicomplexans such as P. falciparum, C. parvum, and G. niphandrodes as well as piroplasms (Babesia and Theileria), lack these organelles. Thus, according to the current notion of the general apicomplexan phylogeny (Arisue and Hashimoto 2015; Simdyanov et al. 2017), multiple independent losses (at least two) of peroxisomes have probably occurred during the evolution of these parasites (fig. 3; Gabaldon et al. 2016). The persistence of putative Pex4 and Pex22 homologs in P. falciparum, C. parvum, and G. niphandrodes, however, indicates that these proteins may have acquired a new essential function, possibly acting in ubiquitination (Pex4 homologs, E2) apart from peroxisomal biogenesis, or undergone some kind of neofunctionalization within the different parasite branches (fig. 3). This has probably happened already early in apicomplexan evolution, making these components essential to both, apicomplexans with or without peroxisomes.
Fig. 3.
—Multiple losses of putative peroxisomes in apicomplexans. The schematic tree depicts the potential evolutionary history of peroxisomes in chromerids and apicomplexans. Group names on branches specifically refer to the individual species listed besides/below, that is, those analyzed in this study. The presence of peroxisomes (indicated by blue letters) is hypothetical, that is, mainly based on bioinformatic results, and requires experimental verification. A green line/branch indicates that a photosynthetic plastid is present in these organisms, whereas a green asterisk denotes loss of photosynthesis, which is not necessarily accompanied by a loss of the plastid/apicoplast. Red lines/branches represent groups of apicomplexans probably devoid of peroxisomes, with a red asterisk indicating loss of putative peroxisomes in addition to a loss of β-oxidation enzymes in these organisms. Coccidians are the only apicomplexans for which bioinformatic and proteomic evidence for the presence of peroxisomes exists. All other non-coccidian apicomplexans seem to lack these organelles, but have retained two proteins possessing sequence similarity to the peroxisomal factors Pex4 and Pex22. Piroplasms, such as Theileria and Babesia, group together with the haemosporidians and were not included into the figure, but according to Gabaldon and colleagues, just as Plasmodium spp. these organisms are devoid of specific peroxisomal markers and thus peroxisomes (Gabaldon et al. 2016). The tree architecture is based on recently published phylogenies of apicomplexans and their sister groups (Arisue and Hashimoto 2015; Simdyanov et al. 2017).
Interestingly, those apicomplexans lacking evidence for peroxisomes seem to be devoid of the molecular toolset to degrade fatty acids via β-oxidation as well (see table 3). This is noteworthy because β-oxidation can take place in both, peroxisomes and mitochondria (Schrader et al. 2015). As all of the noncoccidian apicomplexans studied here are in possession of a mitochondrion or mitochondrion-related organelle (Cryptosporidium), occurrence of a general loss of the β-oxidation pathway in the course of streamlining the parasites metabolisms during evolution is likely.
Conclusions
Our data suggest a final solution to the question of whether apicomplexan parasites contain peroxisomes. By studying a haemospridian, crytosporidian, gregarine, and several coccidian genomes for encoded Pex and comparing our obtained results with already existing data, one should conclude that Plasmodium spp. (and piroplasms), Cryptosporidium spp. and gregarines have lost peroxisomes, whereas coccidians seem to generate these organelles. However, peroxisomes are probably mainly present during their life cycle stage that is located outside of the host (oocyst). The primary function of peroxisomes in coccidians is probably degradation of stored long-chain and very long-chain fatty acids via β-oxidation, thereby providing acetyl-CoA for energy mobilization (mitochondrial TCA cycle) required for sporulation under energy depleted conditions in an external environment. This is in contrast to Plasmodium spp. and Cryptosporidium, which fulfill sporulation during an intracellular life cycle stage, making a rapid mobilization of stored metabolites for sexual reproduction superfluous. Gregarines on the other hand might or might not sporulate inside the host. In any case these organisms seem to have found other strategies to overcome energy depleted conditions in case of environmental sporulation, probably by using other metabolites, making peroxisomes and peroxisomal β-oxidation redundant. Future studies should address the experimental verification of peroxisomes in coccidians by focusing specifically on oocyst/sporozoite stages.
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Supplementary Material
Acknowledgments
We would like to thank the members of our labs for helpful discussions on the topic. We are supported by the Deutsche Forschungsgemeinschaft (Ma 1232/15, Ma 1232/16, Ma 1232/17 and CRC 987 to U.G.M.) and the LOEWE Centre for Synthetic Microbiology (SynMikro).
Literature Cited
- Agrawal G, Subramani S.. 2016. De novo peroxisome biogenesis: evolving concepts and conundrums. Biochim Biophys Acta. 1863(5):892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arisue N, Hashimoto T.. 2015. Phylogeny and evolution of apicoplasts and apicomplexan parasites. Parasitol Int. 64(3):254–259.http://dx.doi.org/10.1016/j.parint.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Ast J, Stiebler AC, Freitag J, Bölker M.. 2013. Dual targeting of peroxisomal proteins. Front Physiol. 4:297.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta JR, Thompson RC.. 2006. What is Cryptosporidium? Reappraising its biology and phylogenetic affinities. Trends Parasitol. 22(10):463–468.http://dx.doi.org/10.1016/j.pt.2006.08.001 [DOI] [PubMed] [Google Scholar]
- Bernsel A, Viklund H, Hennerdal A, Elofsson A.. 2009. TOPCONS: consensus prediction of membrane protein topology. Nucleic Acids Res. 37(Web Server issue):W465–W468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besteiro S. 2012. Which roles for autophagy in Toxoplasma gondii and related apicomplexan parasites? Mol Biochem Parasitol. 184(1):1–8. [DOI] [PubMed] [Google Scholar]
- Blazejewski T. 2015. Systems-based analysis of the Sarcocystis neurona genome identifies pathways that contribute to a heteroxenous life cycle. MBio 6:e02445–e02414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzid M, Hunter PR, Chalmers RM, Tyler KM.. 2013. Cryptosporidium pathogenicity and virulence. Clin Microbiol Rev. 26(1):115–134.http://dx.doi.org/10.1128/CMR.00076-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard C, Hartig A.. 2006. Peroxisome targeting signal 1: is it really a simple tripeptide? Biochim Biophys Acta. 1763(12):1565–1573. [DOI] [PubMed] [Google Scholar]
- Clopton RE, Steele SM, Clopton DT.. 2016. Environmental persistence and infectivity of oocysts of two species of gregarines, Blabericola migrator and Blabericola cubensis (Apicomplexa: Eugregarinida: Blabericolidae), parasitizing blaberid cockroaches (Dictyoptera: Blaberidae). J Parasitol. 102(2):169–173. [DOI] [PubMed] [Google Scholar]
- Deb R, Nagotu S.. 2017. Versatility of peroxisomes: an evolving concept. Tissue Cell. 49(2 Pt B):209–226. [DOI] [PubMed] [Google Scholar]
- Ding M, Clayton C, Soldati D.. 2000. Toxoplasma gondii catalase: are there peroxisomes in toxoplasma? J Cell Sci. 113(Pt 13):2409–2419. [DOI] [PubMed] [Google Scholar]
- Ding M, Kwok LY, Schlüter D, Clayton C, Soldati D.. 2004. The antioxidant systems in Toxoplasma gondii and the role of cytosolic catalase in defence against oxidative injury. Mol Microbiol. 51(1):47–61. [DOI] [PubMed] [Google Scholar]
- Dodt G, Warren D, Becker E, Rehling P, Gould SJ.. 2001. Domain mapping of human PEX5 reveals functional and structural similarities to Saccharomyces cerevisiae Pex18p and Pex21p. J Biol Chem. 276(45):41769–41781. [DOI] [PubMed] [Google Scholar]
- Doerig C, Rayner JC, Scherf A, Tobin AB.. 2015. Post-translational protein modifications in malaria parasites. Nat Rev Microbiol. 13(3):160–172.http://dx.doi.org/10.1038/nrmicro3402 [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Speer CA.. 1998. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 11(2):267–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt NA. 2005. Peroxisomal citrate synthase provides exit route from fatty acid metabolism in oilseeds. Plant Cell. 17(7):1863–1865.http://dx.doi.org/10.1105/tpc.105.034843 [Google Scholar]
- Effelsberg D, Cruz-Zaragoza LD, Schliebs W, Erdmann R.. 2016. Pex9p is a new yeast peroxisomal import receptor for PTS1-containing proteins. J Cell Sci. 129(21):4057–4066. [DOI] [PubMed] [Google Scholar]
- El Magraoui F, et al. 2014. The cytosolic domain of Pex22p stimulates the Pex4p-dependent ubiquitination of the PTS1-receptor. PLoS ONE. 9(8):e105894.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G.. 2000. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 300(4):1005–1016. [DOI] [PubMed] [Google Scholar]
- Freitag J, Ast J, Bölker M.. 2012. Cryptic peroxisomal targeting via alternative splicing and stop codon read-through in fungi. Nature 485(7399):522–525. [DOI] [PubMed] [Google Scholar]
- Fritz HM, Bowyer PW, Bogyo M, Conrad PA, Boothroyd JC.. 2012. Proteomic analysis of fractionated Toxoplasma oocysts reveals clues to their environmental resistance. PLoS ONE. 7(1):e29955.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaldon T. 2010. Peroxisome diversity and evolution. Philos Trans R Soc Lond B Biol Sci. 365(1541):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaldon T, Ginger ML, Michels PA.. 2016. Peroxisomes in parasitic protists. Mol Biochem Parasitol. 209(1–2):35–45. [DOI] [PubMed] [Google Scholar]
- Gabaldon T, et al. 2006. Origin and evolution of the peroxisomal proteome. Biol Direct. 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland N, Michels PA.. 2010. Comparison of the peroxisomal matrix protein import system of different organisms. Exploration of possibilities for developing inhibitors of the import system of trypanosomatids for anti-parasite chemotherapy. Eur J Cell Biol. 89(9):621–637.http://dx.doi.org/10.1016/j.ejcb.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Ginger ML. 2006. Niche metabolism in parasitic protozoa. Philos Trans R Soc Lond B Biol Sci. 361(1465):101–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez NH, et al. 2011. A single peroxisomal targeting signal mediates matrix protein import in diatoms. PLoS ONE. 6(9):e25316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ, Valle D.. 2000. Peroxisome biogenesis disorders: genetics and cell biology. Trends Genet. 16(8):340–345.http://dx.doi.org/10.1016/S0168-9525(00)02056-4 [DOI] [PubMed] [Google Scholar]
- Haanstra JR, Gonzalez-Marcano EB, Gualdron-Lopez M, Michels PA.. 2016. Biogenesis, maintenance and dynamics of glycosomes in trypanosomatid parasites. Biochim Biophys Acta. 1863(5):1038–1048. [DOI] [PubMed] [Google Scholar]
- Kaasch AJ, Joiner KA.. 2000. Targeting and subcellular localization of Toxoplasma gondii catalase. Identification of peroxisomes in an apicomplexan parasite. J Biol Chem. 275(2):1112–1118. [DOI] [PubMed] [Google Scholar]
- Kienle N, Kloepper TH, Fasshauer D.. 2016. Shedding light on the expansion and diversification of the Cdc48 protein family during the rise of the eukaryotic cell. BMC Evol Biol. 16(1):215..http://dx.doi.org/10.1186/s12862-016-0790-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PK, Hettema EH.. 2015. Multiple pathways for protein transport to peroxisomes. J Mol Biol. 427(6 Pt A):1176–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL.. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 305(3):567–580. [DOI] [PubMed] [Google Scholar]
- Kunze M, et al. 2015. Mechanistic insights into PTS2-mediated peroxisomal protein import: the co-receptor PEX5L drastically increases the interaction strength between the cargo protein and the receptor PEX7. J Biol Chem. 290(8):4928–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lametschwandtner G, et al. 1998. The difference in recognition of terminal tripeptides as peroxisomal targeting signal 1 between yeast and human is due to different affinities of their receptor Pex5p to the cognate signal and to residues adjacent to it. J Biol Chem. 273(50):33635–33643.http://dx.doi.org/10.1074/jbc.273.50.33635 [DOI] [PubMed] [Google Scholar]
- Li X, Gould SJ.. 2002. PEX11 promotes peroxisome division independently of peroxisome metabolism. J Cell Biol. 156(4):643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lige B, Jayabalasingham B, Zhang H, Pypaert M, Coppens I.. 2009. Role of an ancestral d-bifunctional protein containing two sterol-carrier protein-2 domains in lipid uptake and trafficking in Toxoplasma. Mol Biol Cell. 20(2):658–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay DS, Dubey JP, Blagburn BL.. 1997. Biology of Isospora spp. from humans, nonhuman primates, and domestic animals. Clin Microbiol Rev. 10(1):19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, et al. 2016. Comparative genomics reveals Cyclospora cayetanensis possesses coccidia-like metabolism and invasion components but unique surface antigens. BMC Genomics. 17:316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi IJ, Semenkovich CF.. 2014. Peroxisomes: a nexus for lipid metabolism and cellular signaling. Cell Metab. 19(3):380–392.http://dx.doi.org/10.1016/j.cmet.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons RE, McLeod R, Roberts CW.. 2002. Toxoplasma gondii tachyzoite–bradyzoite interconversion. Trends Parasitol. 18(5):198–201.http://dx.doi.org/10.1016/S1471-4922(02)02248-1 [DOI] [PubMed] [Google Scholar]
- Madsen L, Seeger M, Semple CA, Hartmann-Petersen R.. 2009. New ATPase regulators – p97 goes to the PUB. Int J Biochem Cell Biol. 41(12):2380–2388. [DOI] [PubMed] [Google Scholar]
- McFadden GI. 2014. Apicoplast. Curr Biol. 24(7):R262–R263. [DOI] [PubMed] [Google Scholar]
- Meinecke M, Bartsch P, Wagner R.. 2016. Peroxisomal protein import pores. Biochim Biophys Acta. 1863(5):821–827. [DOI] [PubMed] [Google Scholar]
- Menard R, et al. 2013. Looking under the skin: the first steps in malarial infection and immunity. Nat Rev Microbiol. 11:701–712.http://dx.doi.org/10.1038/nrmicro3111 [DOI] [PubMed] [Google Scholar]
- Moog D, Rensing SA, Archibald JM, Maier UG, Ullrich KK.. 2015. Localization and evolution of putative triose phosphate translocators in the diatom Phaeodactylum tricornutum. Genome Biol Evol. 7(11):2955–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger G, Maurer-Stroh S, Eisenhaber B, Hartig A, Eisenhaber F.. 2003. Prediction of peroxisomal targeting signal 1 containing proteins from amino acid sequence. J Mol Biol. 328(3):581–592. [DOI] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, von Heijne G.. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10(1):1–6.http://dx.doi.org/10.1093/protein/10.1.1 [DOI] [PubMed] [Google Scholar]
- Olszewski KL, Llinas M.. 2011. Central carbon metabolism of Plasmodium parasites. Mol Biochem Parasitol. 175(2):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RD, et al. 2014. BCKDH: the missing link in apicomplexan mitochondrial metabolism is required for full virulence of Toxoplasma gondii and Plasmodium berghei. PLoS Pathog. 10(7):e1004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri N, et al. 2017. The genome of the protozoan parasite Cystoisospora suis and a reverse vaccinology approach to identify vaccine candidates. Int J Parasitol. 47(4):189–202.http://dx.doi.org/10.1016/j.ijpara.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H.. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 8(10):785–786.http://dx.doi.org/10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- Petsalaki EI, Bagos PG, Litou ZI, Hamodrakas SJ.. 2006. PredSL: a tool for the N-terminal sequence-based prediction of protein subcellular localization. Genomics Proteomics Bioinformatics. 4(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platta HW, Hagen S, Reidick C, Erdmann R.. 2014. The peroxisomal receptor dislocation pathway: to the exportomer and beyond. Biochimie 98:16–28.http://dx.doi.org/10.1016/j.biochi.2013.12.009 [DOI] [PubMed] [Google Scholar]
- Poirier Y, Antonenkov VD, Glumoff T, Hiltunen JK.. 2006. Peroxisomal beta-oxidation – a metabolic pathway with multiple functions. Biochim Biophys Acta. 1763(12):1413–1426. [DOI] [PubMed] [Google Scholar]
- Possenti A, et al. 2013. Global proteomic analysis of the oocyst/sporozoite of Toxoplasma gondii reveals commitment to a host-independent lifestyle. BMC Genomics. 14:183.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan S, et al. 2012. Apicoplast and endoplasmic reticulum cooperate in fatty acid biosynthesis in apicomplexan parasite Toxoplasma gondii. J Biol Chem. 287(7):4957–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucktäschel R, Girzalsky W, Erdmann R.. 2011. Protein import machineries of peroxisomes. Biochim Biophys Acta. 1808(3):892–900. [DOI] [PubMed] [Google Scholar]
- Saryi NA, et al. 2017. Pnc1 piggy-back import into peroxisomes relies on Gpd1 homodimerisation. Sci Rep. 7:42579.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliebs W, Kunau WH.. 2006. PTS2 co-receptors: diverse proteins with common features. Biochim Biophys Acta. 1763(12):1605–1612. [DOI] [PubMed] [Google Scholar]
- Schliebs W, et al. 1999. Recombinant human peroxisomal targeting signal receptor PEX5. Structural basis for interaction of PEX5 with PEX14. J Biol Chem. 274(9):5666–5673. [DOI] [PubMed] [Google Scholar]
- Schlüter A, et al. 2006. The evolutionary origin of peroxisomes: an ER-peroxisome connection. Mol Biol Evol. 23(4):838–845. [DOI] [PubMed] [Google Scholar]
- Schlüter A, et al. 2007. PeroxisomeDB: a database for the peroxisomal proteome, functional genomics and disease. Nucleic Acids Res. 35(Database issue):D815–D822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M, Costello J, Godinho LF, Islinger M.. 2015. Peroxisome-mitochondria interplay and disease. J Inherit Metab Dis. 38(4):681–702. [DOI] [PubMed] [Google Scholar]
- Schrader M, Fahimi HD.. 2006. Peroxisomes and oxidative stress. Biochim Biophys Acta. 1763(12):1755–1766. [DOI] [PubMed] [Google Scholar]
- Simdyanov TG, et al. 2017. A new view on the morphology and phylogeny of eugregarines suggested by the evidence from the gregarine Ancora sagittata (Leuckart, 1860) Labbe, 1899 (Apicomplexa: Eugregarinida). PeerJ 5:e3354.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I, Peeters N, Legeai F, Lurin C.. 2004. Predotar: a tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 4(6):1581–1590.http://dx.doi.org/10.1002/pmic.200300776 [DOI] [PubMed] [Google Scholar]
- Smith JJ, Aitchison JD.. 2013. Peroxisomes take shape. Nat Rev Mol Cell Biol. 14(12):803–817.http://dx.doi.org/10.1038/nrm3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A, Mattie S, Prudent J, McBride HM.. 2017. Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature 542(7640):251–254.http://dx.doi.org/10.1038/nature21375 [DOI] [PubMed] [Google Scholar]
- Szöör B, Haanstra JR, Gualdron-Lopez M, Michels PA.. 2014. Evolution, dynamics and specialized functions of glycosomes in metabolism and development of trypanosomatids. Curr Opin Microbiol. 22:79–87. [DOI] [PubMed] [Google Scholar]
- Tenter AM, Heckeroth AR, Weiss LM.. 2000. Toxoplasma gondii: from animals to humans. Int J Parasitol. 30(12–13):1217–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toso MA, Omoto CK.. 2007. Ultrastructure of the Gregarina niphandrodes nucleus through stages from unassociated trophozoites to gamonts in syzygy and the syzygy junction. J Parasitol. 93(3):479–484.http://dx.doi.org/10.1645/GE-1028R1.1 [DOI] [PubMed] [Google Scholar]
- Williams C, et al. 2012. Insights into ubiquitin-conjugating enzyme/co-activator interactions from the structure of the Pex4p: Pex22p complex. Embo J. 31(2):391–402.http://dx.doi.org/10.1038/emboj.2011.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YH, et al. 2015. Chromerid genomes reveal the evolutionary path from photosynthetic algae to obligate intracellular parasites. Elife 4:e06974.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarsky V, Tachezy J.. 2015. Evolutionary loss of peroxisomes – not limited to parasites. Biol Direct. 10:74.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Monroe-Augustus M, Silva ID, Bartel B.. 2005. Identification and functional characterization of Arabidopsis PEROXIN4 and the interacting protein PEROXIN22. Plant Cell. 17(12):3422–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.