Abstract
Individuals with schizophrenia are burdened with impairments in functional outcome, despite existing interventions. The lack of understanding of the neurobiological correlates supporting adaptive function in the disorder is a significant barrier to developing more effective treatments. This research conducted a systematic and meta-analytic review of all peer-reviewed studies examining brain-functional outcome relationships in schizophrenia. A total of 53 (37 structural and 16 functional) brain imaging studies examining the neural correlates of functional outcome across 1631 individuals with schizophrenia were identified from literature searches in relevant databases occurring between January, 1968 and December, 2016. Study characteristics and results representing brain-functional outcome relationships were systematically extracted, reviewed, and meta-analyzed. Results indicated that better functional outcome was associated with greater fronto-limbic and whole brain volumes, smaller ventricles, and greater activation, especially during social cognitive processing. Thematic observations revealed that the dorsolateral prefrontal cortex, anterior cingulate, posterior cingulate, parahippocampal gyrus, superior temporal sulcus, and cerebellum may have role in functioning. The neural basis of functional outcome and disability is infrequently studied in schizophrenia. While existing evidence is limited and heterogeneous, these findings suggest that the structural and functional integrity of fronto-limbic brain regions is consistently related to functional outcome in individuals with schizophrenia. Further research is needed to understand the mechanisms and directionality of these relationships, and the potential for identifying neural targets to support functional improvement.
Keywords: meta-analysis, schizophrenia, functional outcome, fronto-limbic, ventricles
Introduction
Schizophrenia is a debilitating psychiatric condition characterized by pronounced disability across many domains of functional outcome,1–3 yet very little is known about the neurobiological correlates that may contribute to poor functional outcomes in the disorder. Presently, a literature search in relevant databases, such as Medline, yields over 30 000 functional magnetic resonance imaging (fMRI) and MRI reports that have provided evidence that schizophrenia has a strong neurobiological etiology.4 Despite this abundance of studies, very few have had a primary goal to understand the association between brain abnormalities and community and daily living in this population. Understanding the association between neurobiology and adaptive function may shed light on the neural targets and associated cognitive and behavioral mechanisms that can support functional improvement. Therefore, a systematic and meta-analytic review of the current literature with the goal of identifying brain correlates of functional outcome in schizophrenia could help identify novel directions for treatment of this domain.
Individuals diagnosed with schizophrenia have poor outcomes in social functioning,5,6 occupational/vocational functioning,7 independent/community living,8 and quality of life.9 Impairments in these domains of functional outcome are observed in the premorbid stage, at first onset, and into the chronic stages of the illness.10,11 Epidemiological studies have shown that compared to other forms of psychosis, functional disability is the most substantial in people diagnosed with schizophrenia.12 In a longitudinal study of long-term outcomes in schizophrenia at the National Institute of Mental Health (NIMH) it was observed at a mean follow-up period of 6 years that only 2 out of 58 patients had good global functioning, 66% were unemployed, and 50% had no social contacts.13 More recently, Harvey and colleagues14 examined the prevalence of impaired functioning by reviewing recipients of disability benefits with schizophrenia-related disorders. They observed that functional disability was stable and common across typical working ages, such that remission from symptomatic outcomes was more frequently observed than remission from functional disability.14
Despite ample evidence that poor functional outcomes are an obvious global burden in schizophrenia, both pharmacological and psychosocial treatments have had only incremental success at rehabilitating community and daily living in this population. The NIMH Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study observed that antipsychotic medications provided only minor improvements in community functioning in people with schizophrenia, and efficacy did not differ between newer and older pharmacological treatments.15 Psychosocial treatments have been moderately successful at improving functional outcome in schizophrenia,16 with notable examples in cognitive remediation,17 cognitive behavior therapy,18 supported employment,19 and other areas, but there remains considerable room for improvement.
Schizophrenia is clearly associated with brain dysfunction,20 which has been linked to impairments in clinical and cognitive outcomes.21–25 Unfortunately, the neural substrates supporting functional outcome in schizophrenia are largely unknown, and such information is essential for developing empirically-driven disorder-relevant treatment targets to improve functioning in this population.
The purpose of this research was to conduct a comprehensive systematic and meta-analytic review of the existing and diverse literature examining brain-functional outcome relationships in schizophrenia. Specifically, we sought to elucidate consistent regional and directional (eg, increased or decreased brain volumes associated with functional outcome) themes of relationships between brain structure, brain function, and functional outcome in schizophrenia, with the goal of identifying neural treatment targets that could support improved adaptive function in this population.
Methods
Literature Search
An extensive literature search was conducted to locate peer-reviewed published studies examining the structural and functional neurobiological correlates of functional outcome in schizophrenia following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.26 This method was implemented by performing keyword database searches in PsycINFO and Medline from January, 1968 until December, 2016. The search strings included (1) “schizophrenia” or “schizoaffective”; (2) “social functioning” or “social dysfunction” or “functional outcome” or “community functioning” or “quality of life” or “life satisfaction” or “well-being” or “social skills” or “social behavior” or “occupational functioning” or “vocational functioning” or “independent living” or “employment”; and (3) “brain” or “MRI” or “imaging” or “activation” or “gray matter”. In addition, references from relevant studies were examined for other neuroimaging reports of functional outcome in schizophrenia.
In total, this review yielded 484 articles. The abstracts from these studies were reviewed and included if they (1) used quantitative structural or functional neuroimaging methodologies that provided specific information about anatomical locations in the brain, (2) reported data on participants diagnosed with schizophrenia, schizoaffective, or schizophreniform disorder only, (3) used a previously validated assessment of functional outcome, and (4) reported quantifiable findings on the relationship between neurobiology and functional outcome. Exclusion criteria were: (1) the sample did not consist only of people diagnosed with schizophrenia, schizoaffective, or schizophreniform; (2) the use of a neuroimaging methodology with low spatial resolution limiting the precise anatomical loci of activation associated with functional outcome (eg, near-infrared spectroscopy [NIRS]); (3) a qualitative approach to examine brain abnormalities related to functional outcome (eg, Galderisi et al27); and (4) not reporting quantitative findings.
The majority of the articles (381) were excluded, as while implications for functional outcome were often suggested, no relationships between measures of neurobiology and functioning were analyzed. After screening out the above 381 articles, 102 full-text studies remained and were reviewed further for eligibility. Of those 102 studies, 22 were excluded because they did not examine functional outcome; 12 were excluded because their sample included other psychotic or mental disorders; 12 used neuroimaging methodologies that could not determine the specific anatomical loci of alteration (eg, NIRS); and 4 were review articles.
Study Coding Procedures
After gathering and organizing the studies included in this review, we systematically extracted relevant study characteristics. The study characteristics gathered included the sample size (N), average age, percent of males, illness duration, clinical status, presence of antipsychotic medication, design of the study (cross-sectional or longitudinal), scan type, measurement of brain structure (eg, gray matter volume, fractional anisotropy) or function (eg, activation, regional cerebral blood flow), the domain of functional outcome assessed, and the functional outcome measure utilized. None of the longitudinal studies were treatment trials, and thus all were naturalistic follow-up examinations. For the structural imaging studies that presented both relative and absolute brain volumes, priority was given to utilizing results from relative volumes due to their adjustment for brain size. The structural study findings were organized by brain lobe as a way to identify regional themes underlying functioning in individuals with schizophrenia. The functional studies were quite varied with regard to the imaging methodology used (10 social-cognitive fMRI; 1 neurocognitive fMRI, 3 resting-state fMRI, and 2 SPECT), cognitive tasks, and findings. Therefore, the functional findings were organized by neuroimaging methodology.
Study Analysis
After coding study characteristics, we extracted the statistics representing the relationships between brain regions and functional outcome (eg, r, pr, β) and/or the differences between patients with good compared to poor functional outcome (eg, t, F). All extracted data were converted to a common r metric using the equations provided in Rosenthal.28 When appropriate, the directional relationship between brain regions and functional outcome was inversed (multiplied by −1), such that higher values represented associations with better functional outcome. Meta-analyses were conducted in R (3.1.2) with the Meta-Analysis Package for R.29 Random-effects meta-analytic models were used to calculate average effect sizes for the various structural lobar locations (whole brain, white matter tracts, ventricles, frontal, limbic, temporal, parietal, occipital, and cerebellum) and functional imaging methodologies (social cognitive fMRI, neurocognitive fMRI, resting-state fMRI, and SPECT). Meta-regression models were also conducted to examine the possible moderating effects of illness duration (first episode vs multiple episode), clinical status (inpatient vs outpatient vs both), and treatment exposure (antipsychotic medication naïve vs presence of antipsychotic medication), wherever such data were available. These characteristics for each study are presented in table 1. Moderator analyses were only conducted on imaging domains with 5 or more studies (accounting for overlapping samples across study reports). Further, sample size was quite diverse (N range: 12–130; table 1) and there were several studies that utilized the same sample or subsample of participants across multiple reports.30–45 Thus, all meta-analytic models accounted for the range of sample sizes by weighting effect sizes by their inverse variances (reflective of sample size)46 and sample dependency by using a sample identifier as the random nesting factor to account for correlated findings across overlapping samples.
Table 1.
Study Characteristics of the Functional and Structural Neuroimaging Studies Meeting Inclusion Criteria for Systematic Review
| Study | N | Mean Age | % Male | Illness Duration | Clinical Status | Antipsychotic Naïve | Scan Type | Study Design |
|---|---|---|---|---|---|---|---|---|
| Structural studies | ||||||||
| Aoyama et al (2011)47 | 17 | 25.0 (7.0) | 82 | FE: 22.0 (24.0) mo | Outpatient | No | Spectroscopy | Longitudinal |
| Behdinan et al (2015)48 | 30 | 39.3 (12.9) | 60 | ME: 15.4 (13.4) y | Outpatient | No | DTI | Longitudinal |
| Boonstra et al (2011)49 | 57 | 24.0 (5.3) | 89 | FE: 270.6 (238.8) wk | — | No | MRI | Longitudinal |
| Brambilla et al (2013)50 | 67 | 39.4 (12.1) | 66 | ME: 13.3 (10.6) y | Outpatient | No | MRI | Cross-sectional |
| Brickman et al (2004)30 | 52 good outcomea | 40.9 (12.6) | 81 | ME | Inpatient/ outpatient | No | MRI | Cross-sectional |
| 54 poor outcome | 45.1 (11.5) | 80 | ||||||
| Brickman et al (2006)31 | 52 good outcomea | 40.9 (12.6) | 81 | ME | Inpatient/ outpatient | No | MRI | Cross-sectional |
| 54 poor outcome | 45.1 (11.5) | 80 | ||||||
| Buchsbaum et al (2003)32 | 24 good outcomea | 41.1 (12.1) | 75 | ME | Inpatient | No | MRI | Cross-sectional |
| 13 poor outcome | 47.2 (8.9) | 69 | ||||||
| Cahn et al (2002)42 | 34 | 26.2 (5.3) | 85 | FE | — | No | MRI | Longitudinal |
| Cahn et al (2006)43 | 31 | 25.7 (4.9) | 87 | FE | — | No | MRI | Longitudinal |
| Chemerinski et al (2002)51 | 45 | 30.0 (8.9) | 100 | ME: 81.6 (96.9) m | Inpatient | No | MRI | Cross-sectional |
| Davis et al (1998)52 | 31 good outcomea | 38.0 (12.2) | 100 | ME | Inpatient/ outpatient | — | CT | Longitudinal |
| 22 poor outcome | 42.0 (8.6) | |||||||
| Faget-Agius et al (2015)53 | 44 good QoL | 29.7 (6.9) | 100 | ME: 7.7 (4.1) y | Outpatient | No | MRI | Cross-sectional |
| 37 poor QoL | 30.3 (8.2) | ME: 6.6 (5.1) y | ||||||
| Guo et al (2015)54 | 33 | 34.1 (0.6) | 58 | ME | — | No | MRI | Longitudinal |
| Kasparek et al (2009)55 | 21 good functioning (GAF > 60) | 23.8 (4.7) | 100 | FE | Inpatient | No | MRI | Longitudinal |
| 11 poor functioning (GAF ≤ 60) | ||||||||
| Mané et al (2009)56 | 15 | 25.6 (5.8) | 80 | FE | Inpatient | No | MRI | Longitudinal |
| Milev et al (2003)57 | 123 | — | 72 | ME: 1.3 (1.4) y | Inpatient/ outpatient | No | MRI | Longitudinal |
| Mitelman et al (2003)33 | 24 good outcomea | 41.1 (12.1) | 75 | ME | Inpatient/ outpatient | No | MRI | Cross-sectional |
| 13 poor outcome | 47.2 (8.9) | 69 | ||||||
| Mitelman et al (2005)34 | 24 good outcomea | 41.1 (12.1) | 75 | ME | Inpatient/ outpatient | No | MRI | Cross-sectional |
| 13 poor outcome | 47.2 (8.9) | 69 | ||||||
| Mitelman et al (2006)35 | 51 good outcomea | 40.6 (12.6) | 80 | ME | Inpatient/ outpatient | No | DTI | Cross-sectional |
| 53 poor outcome | 44.8 (11.4) | 81 | ||||||
| Mitelman et al (2007a)36 | 51 good outcomea | 40.6 (12.6) | 80 | ME | Inpatient/ outpatient | No | MRI | Cross-sectional |
| 53 poor outcome | 44.8 (11.4) | 81 | ||||||
| Mitelman et al (2007b)37 | 51 good outcomea | 40.6 (12.6) | 80 | ME | Inpatient/ outpatient | No | DTI | Cross-sectional |
| 53 poor outcome | 44.8 (11.4) | 81 | ||||||
| Mitelman et al (2009a)38 | 23 good outcomea | 37.4 (10.7) | 74 | ME: 12.3 (8.8) y | Inpatient/ outpatient | No | MRI | Longitudinal |
| 26 poor outcome | 47.4 (12.0) | 96 | ME: 24.8 (11.7) y | |||||
| Mitelman et al (2009b)39 | 23 good outcomea | 37.4 (10.7) | 74 | ME: 12.3 (8.8) y | Inpatient/ outpatient | No | MRI | Longitudinal |
| 26 poor outcome | 47.4 (12.0) | 96 | ME: 24.8 (11.7) y | |||||
| Mitelman et al (2009c)40 | 23 good outcomea | 37.4 (10.7) | 74 | ME: 12.3 (8.8) y | Inpatient/ outpatient | No | MRI | Longitudinal |
| 26 poor outcome | 47.4 (12.0) | 96 | ME: 24.8 (11.7) y | |||||
| Mitelman et al (2010)41 | 23 good outcomea | 37.4 (10.7) | 74 | ME: 12.3 (8.8) y | Inpatient/ outpatient | No | MRI | Longitudinal |
| 26 poor outcome | 47.4 (12.0) | 96 | ME: 24.8 (11.7) y | |||||
| Molina et al (2010)58 | 18 good outcomea | 36.9 (12.0) | 61 | ME: 10.9 (7.9) y | Inpatient/ outpatient | No | MRI | Cross-sectional |
| 26 poor outcome | 36.3 (11.6) | 65 | ME: 10.4 (8.7) y | |||||
| Prasad et al (2005)59 | 25 | 25.4 (7.8) | 68 | FE | Inpatient/ outpatient | No | MRI | Longitudinal |
| Rossi et al (2000)60 | 38 good functioning: (SCOS > 4) | 32.0 (2.9) | 79 | ME: 7.9 (6.1) y | Inpatient | No | MRI | Cross-sectional |
| 18 poor functioning (SCOS < 4) | ||||||||
| Seidman et al (1987)61 | 15 | 23.5 (5.4) | 53 | ME | Inpatient | — | CT | Cross-sectional |
| Sheng et al (2013)62 | 33 | 22.8 (3.5) | 45 | FE: 8.3 (10.5) mo | Inpatient | No | MRI | Cross-sectional |
| Takayanagi et al (2010)63 | 42 | 28.6 (5.9) | 57 | FE male: 10.5 (12.0) mo | Inpatient | No | MRI | Cross-sectional |
| FE female: 13.7 (13.1) mo | ||||||||
| Tully et al (2014)64 | 26 | 38.7 (10.3) | 62 | ME: 16.4 (12.0) | — | No | MRI | Cross-sectional |
| Uwatoko et al (2015)65 | 33 | 35.7 (9.4) | 58 | ME: 110.5 (15.8) mo | — | No | MRI | Cross-sectional |
| van Haren et al (2008)66 | 47 good outcome [Mean GAF: 66.7 (9.0)] | 32.2 (11.1) | 73 | ME: 11.0 (10.2) y | Inpatient/ outpatient | No | MRI | Longitudinal |
| 47 poor outcome [Mean GAF: 38.2 (10.2)] | ||||||||
| Vita et al (1991)67 | 18 | 30.7 (7.5) | 61 | ME | Inpatient/ outpatient | — | CT | Longitudinal |
| Whitworth et al (2005)68 | 21 | 25.0 (4.8) | 100 | FE: 8.3 (17.7) mo | — | No | MRI | Longitudinal |
| 17 | 28.4 (4.0) | ME: 93.7 (72.6) mo | ||||||
| Wilke et al (2001)69 | 48 | 33.0 (9.1) | 56 | ME: 8.6 (8.5) y | Inpatient | No | MRI | Cross-sectional |
| Functional studies | ||||||||
| Social Cognition | ||||||||
| Das et al (2012)70 | 20 | 34.5 (8.4) | 100 | ME: 9.4 (6.5) y | — | No | fMRI | Cross-sectional |
| Dodell-Feder et al (2013)71 | 20 | 38.8 | 60 | ME: 17.1 (12.2) y | — | No | fMRI | Cross-sectional |
| Lee et al (2006)72 | 14 | 31.7 (7.3) | 93 | ME: 9.8 (5.4) y | Inpatient | No | fMRI | Longitudinal |
| Nelson et al (2015)73 | 14 | 33.4 (9.3) | 71.4 | ME | Outpatient | No | fMRI | Cross-sectional |
| Pinkham et al (2008)74 | 12 NP-SCZ | 28.0 (3.93) | 100 | ME | Outpatient | No | fMRI | Cross-sectional |
| 12 P-SCZ | 26.4 (5.3) | |||||||
| Pinkham et al (2011)75 | 35 | 36.5 (10.7) | 49 | ME: 15.2 (11.1) y | — | No | fMRI | Cross-sectional |
| Shin et al (2015)76 | 17 | 31.0 (6.1) | 65 | ME: 10.9 (6.9) y | Outpatient | No | fMRI | Cross-sectional |
| Smith et al (2015)44 | 30 | 33.6 (7.1) | 60 | ME: 13.6 (7.5) y | Outpatient | No | fMRI | Cross-sectional |
| Taylor et al (2011)77 | 21 | 40.7 (9.3) | 67 | ME: 19.5 (12.3) y | Outpatient | No | fMRI | Cross-sectional |
| Thakkar et al (2014)78 | 16 | 40.2 (9.1) | 56 | ME: 19.4 (9.9) y | Outpatient | No | fMRI | Cross-sectional |
| Neurocognition | ||||||||
| Yoon et al (2008)79 | 25 | 19.6 (3.8) | 68 | FE: < 1 yr. | Outpatient | No | fMRI | Cross-sectional |
| Resting-state connectivity | ||||||||
| Anticevic et al (2015)80 | 25 | 23.6 (9.2) | 20 | ME: 9.10 (13.70) mo | — | No | rsfMRI | Cross-sectional |
| Fox et al (in press)45 | 28 | 33.2 (6.6) | 64 | ME: 14.6 (6.3) y | Outpatient | No | rsfMRI | Cross-sectional |
| Lui et al (2010)81 | 34 | 24.6 (8.5) | 38 | FE: 7.8 (12.4) mo | — | No | rsfMRI | Cross-sectional |
| Single Photon Emission Computed Tomography (SPECT) | ||||||||
| Boyer et al (2012)82 | 19 good QoL | 31.9 (10.0) | 68 | ME: 11.6 (8.5) y | Outpatient | No | SPECT | Cross-sectional |
| 12 poor QoL | 32.8 (11.6) | 75 | ME: 10.8 (8.3) y | |||||
| Faget-Agius et al (2016)83 | 130 | 35.8 (11.1) | 73 | ME: 13.2 (9.2) y | Outpatient | No | SPECT | Cross-sectional |
Note: CT, computerized tomography; DTI, diffusion tensor imaging; FE, first episode; fMRI, functional magnetic resonance imaging; GAF, Global Assessment of Functioning; ME, multiple episode; MRI, magnetic resonance imaging; NP-SCZ, nonparanoid schizophrenia; P-SCZ, paranoid schizophrenia; QoL, quality of life; rsfMRI, resting-state functional magnetic resonance imaging; SCOS, Strauss and Carpenter Outcome Scale.
Outcome characteristics defined by Keefe et al.84
Results
Included Studies
A total of 53 neuroimaging studies (37 structural and 16 functional) met inclusion criteria and were used to examine relationships between brain structure, brain function, and functional outcome in schizophrenia (table 1). There was a total of 1631 individuals with schizophrenia included; 1187 from the structural imaging studies and 444 from the functional imaging studies. A total of 268 findings were extracted across these 53 studies, of which 186 (69%) reported on a significant or trend-level (eg, P ≤ .10) relationship between brain structure or function and functional outcome, and 82 (31%) were nonsignificant (eg, P > .10). Across all samples, the percentage of males ranged from 20%–100%, with the majority (38 [72%]) including more males than females with schizophrenia. The average study age ranged from 19.6 to 43.0, with a minority of the studies (9 [17%]) focused specifically on participants experiencing a first episode of psychosis. The domains of functional outcome examined were classified based on the methodological details contained in each study and included composite functioning (eg, more than one functional outcome domain included in the measure), global functioning (eg, GAF85), social functioning, resource needs, quality of life, socioeconomic status, independent living, employment, and role functioning. Over half (56%) of the studies used a composite measure of functional outcome.
Results of Studies of Brain Structure and Functional Outcome in Schizophrenia
Thirty-seven studies were obtained that reported a total of 199 findings on the relationship between brain structure and functional outcome in schizophrenia (table 2). The findings are summarized according to lobar location. Globally, 21 (21:199) findings indicated that greater whole brain volumes (eg, total brain, gray matter) were associated with better functioning across diverse outcome domains, ranging from global functioning to resource needs to independent living. The average effect size for whole brain findings was significant, but small with significant heterogeneity between the studies (table 3). No moderating relationships were observed for the whole brain findings. A total of 15 findings (15:199) were extracted examining the relationship between white matter tracts and functional outcome. The average effect for this relationship was nonsignificant (table 3). The ventricles (27:199), particularly the lateral ventricles (table 2), showed a consistent relationship with functioning across composite and numerous individual functional outcome domains. The average effect size was medium and significant, indicating that having smaller ventricles was related to better functioning (table 3). Significant heterogeneity was present across the ventricle studies. No moderating effects were observed for the ventricles.
Table 2.
Structural Neuroimaging Studies Examining the Association Between Functional Outcome and the Brain in Schizophrenia
| Study | Brain Region | Type | Effect Size (r) |
|---|---|---|---|
| Whole brain | |||
| Boonstra et al (2011)49 | Total brain | Vol | .34* |
| Total brain | Vol | .29**** | |
| Gray matter | Vol | .31**** | |
| Total brain | Vol | .19 | |
| Total brain | Vol | −.03 | |
| Gray matter | Vol | .10 | |
| Gray matter | Vol | −.08 | |
| Gray matter | Vol | .16 | |
| Cahn et al (2002)42 | Total brain | Vol | .39* |
| Cerebrum | GMV | .50** | |
| van Haren et al (2008)66 | Cerebrum | Vol | .24* |
| Cahn et al (2006)43 | Total brain | Vol | .39* |
| Gray matter | GMV | .46** | |
| Gray matter | GMV | .59*** | |
| Vita et al (1991)67 | Cortex | Vol | .50** |
| Cortex | Vol | .33* | |
| Cortex | Vol | .45* | |
| Mitelman et al (2007a)36 | Total brain | Vol | .17 |
| Whitworth et al (2005)68 | Left hemisphere | Vol | .11 |
| Right hemisphere | Vol | .11 | |
| Faget-Agius et al (2015)53 | White matter | MTR | .07 |
| White matter tracts | |||
| Mitelman et al (2007b)37 | Corpus callosum | FA | .23 |
| Optic radiation | FA | .20 | |
| Internal capsule | FA | .07 | |
| Fronto-occipital fasciculus | FA | .07 | |
| Inferior longitudinal fasciculus | FA | .07 | |
| Arcuate fasciculus | FA | .07 | |
| CiB | FA | .07 | |
| Anterior thalamic radiation | FA | .07 | |
| Mitelman et al (2009b)40 | Corpus callosum | Area | .39** |
| Corpus callosum | Length | −.30* | |
| Behdinan et al (2015)48 | Inferior longitudinal/arcuate fasciculus | FA | .58* |
| Inferior longitudinal/arcuate fasciculus | FA | .78* | |
| UF/IFOF/CiB/gCC/sCC | FA | −.25 | |
| UF/IFOF/CiB/gCC/sCC | FA | −.15 | |
| Brickman et al (2006)31 | Internal capsule | Vol | .07 |
| Ventricles | |||
| Vita et al (1991)67 | Ventricles | VBR | −.43* |
| Ventricles | VBR | −.40**** | |
| Ventricles | VBR | −.25 | |
| Cahn et al (2006)43 | Lateral ventricles | Vol | −.47** |
| Lateral ventricles | Vol | −.39* | |
| Third ventricle | Vol | −.12 | |
| Cahn et al (2002)42 | Lateral ventricles | Vol | −.61** |
| Davis et al (1998)52 | Lateral ventricles | VBR | −.36** |
| Seidman et al (1987)61 | Lateral ventricles | VBR | −.49* |
| Lateral ventricles | VBR | −.48* | |
| Lateral ventricles | VBR | −.58** | |
| Boonstra et al (2011)49 | Lateral ventricles | Vol | −.32* |
| Lateral ventricles | Vol | .13 | |
| Lateral ventricles | Vol | −.23a | |
| Lateral ventricles | Vol | −.03 | |
| Third ventricle | Vol | −.29**** | |
| Third ventricle | Vol | −.09 | |
| Third ventricle | Vol | −.22 | |
| Third ventricle | Vol | .06 | |
| van Haren et al (2008)66 | Lateral ventricles | Vol | −.21* |
| Lateral ventricles | Vol | −.22* | |
| Third ventricle | Vol | −.22* | |
| Rossi et al (2000)60 | Left lateral ventricle | Vol | −.37*** |
| Right lateral ventricle | Vol | −.35*** | |
| Mitelman et al (2010)41 | Lateral ventricles (Posterior horns) | Vol | −.34* |
| Whitworth et al (2005)68 | Left lateral ventricle | Vol | −.11 |
| Right lateral ventricle | Vol | −.11 | |
| Frontal | |||
| Mitelman et al (2003)33 | Frontal lobe | Vol | .35* |
| Mitelman et al (2007b)37 | Frontal lobe white matter | FA | .07 |
| Molina et al (2010)58 | Left frontal lobe | GMV | .50*** |
| Cahn et al (2006)43 | Frontal lobe | Vol | .12 |
| Tully et al (2014)64 | Superior frontal gyrus | Thickness | .34**** |
| Superior frontal gyrus | Thickness | .07 | |
| Kasparek et al (2009)55 | Left prefrontal cortex | GMV | .35* |
| Left prefrontal cortex | GMV | .63*** | |
| Prasad et al (2005)59 | DLPFC | GMV | .37****,b |
| DLPFC | GMV | .38****,b | |
| DLPFC | GMV | .02 | |
| Left DLPFC | GMV | .40** | |
| Left DLPFC | GMV | .42*,b | |
| Left DLPFC | GMV | .44*,b | |
| Right DLPFC | GMV | .23 | |
| Mitelman et al (2009a)49 | Right prefrontal cortex (BA 10) | WMV | −.28* |
| Left DLPFC (BA 46) | WMV | −.28* | |
| Left DLPFC (BA 46) | GMV | .28* | |
| Right DLPFC (BA 46) | WMV | −.28* | |
| Right inferior frontal gyrus (BA 47) | GMV | .28* | |
| Right inferior frontal gyrus (BA 47) | WMV | −.23**** | |
| Right orbital frontal gyrus (BA 12) | WMV | .23**** | |
| Mitelman et al (2006)35 | Left inferior frontal gyrus (BA 44) | FA | .19* |
| Left inferior frontal gyrus (BA 47) | FA | .19* | |
| Left orbital frontal gyrus (BA 12) | FA | .16**** | |
| Right primary motor cortex (BA 4) | FA | .16**** | |
| Wilke et al (2001)69 | Left inferior frontal gyrus | GMV | .59*** |
| Chemerinski et al (2002)51 | Ventral frontal cortex | GMV | .33* |
| Takayanagi et al (2010)63 | Left orbital frontal gyrus | GMV | .36* |
| Left orbital frontal gyrus | GMV | .39**,a | |
| Left orbital frontal gyrus | GMV | .33* | |
| Left orbital frontal gyrus | GMV | .31* | |
| Limbic | |||
| Sheng et al (2013)62 | Insula | Lateralization | .67* |
| Faget-Agius et al (2015)53 | Insula | MTR | .22* |
| Left insula | MTR | .19*** | |
| Right insula | MTR | .20*** | |
| Uwatoko et al (2015)65 | Right insula | GMV | .57*** |
| Right insula | GMV | .48* | |
| Mané et al (2009)56 | Right insula | GMV | .51* |
| Mitelman et al (2005)34 | Anterior cingulate | GMV | −.32* |
| Right anterior cingulate | GMV | −.32* | |
| Posterior cingulate | GMV | .42** | |
| Left posterior cingulate | GMV | .42** | |
| Left posterior cingulate | GMV | .30**** | |
| Right posterior cingulate | GMV | .42** | |
| Mitelman et al (2006)86 | Right anterior cingulate (BA 24) | FA | .16**** |
| Right anterior cingulate (BA 33) | FA | .16**** | |
| Right posterior cingulate (BA 23) | FA | .19* | |
| Right posterior cingulate (BA 29) | FA | .16**** | |
| Mitelman et al (2009a)49 | Left anterior cingulate (BA 25) | GMV | .23**** |
| Right anterior cingulate (BA 25) | GMV | .28* | |
| Right posterior cingulate (BA 26) | WMV | .23**** | |
| Right posterior cingulate (BA 29) | WMV | .23**** | |
| Left parahippocampal gyrus (BA 36) | GMV | .28* | |
| Left parahippocampal gyrus (BA 36) | WMV | .23**** | |
| Right parahippocampal gyrus (BA 36) | WMV | .23**** | |
| Right parahippocampal gyrus (BA 36) | WMV | −.23**** | |
| Right parahippocampal gyrus (BA 34) | WMV | −.23**** | |
| Left entorhinal cortex (BA 28) | WMV | −.28* | |
| Right entorhinal cortex (BA 28) | WMV | −.28* | |
| Mitelman et al (2007a)36 | Left parahippocampal gyrus (BA 35) | GMV | .16**** |
| Left parahippocampal gyrus (BA 27) | GMV | .25** | |
| Right parahippocampal gyrus (BA 35) | GMV | .16**** | |
| Right parahippocampal gyrus (BA 36) | GMV | .16**** | |
| Left entorhinal cortex (BA 28) | GMV | .28** | |
| Right entorhinal cortex (BA 28) | GMV | .25** | |
| Brambilla et al (2013)50 | Left hippocampus | Size | .34** |
| Right hippocampus | Size | .36** | |
| Whitworth et al (2005)68 | Left hippocampus | Vol | .11 |
| Right hippocampus | Vol | .11 | |
| Left amygdala | Vol | .11 | |
| Right amygdala | Vol | .11 | |
| Left hippocampus-amygdala complex | Vol | .11 | |
| Right hippocampus-amygdala complex | Vol | .11 | |
| Aoyama et al (2011)47 | Left thalamus | Metabolites | .69** |
| Left anterior cingulate | Metabolites | .16 | |
| Brickman et al (2004)30 | Right thalamus | Vol | .32*** |
| Molina et al (2010)58 | Left thalamus | GMV | .50*** |
| Right thalamus | GMV | .50*** | |
| Left putamen | GMV | .50*** | |
| Right putamen | GMV | .50*** | |
| Left caudate | GMV | .50*** | |
| Right caudate | GMV | .50*** | |
| Buchsbaum et al (2003)32 | Putamen | Vol | .33* |
| Mitelman et al (2009a)39 | Left putamen | Vol | .37** |
| Temporal | |||
| Mitelman et al (2003)33 | Temporal lobe | GMV | .33* |
| Milev et al (2003)57 | Temporal lobe | Vol | .12 |
| Mitelman et al (2007b)37 | Temporal lobe white matter | FA | .07 |
| Mitelman et al (2007a)36 | Left superior temporal gyrus (BA 42) | GMV | −.19* |
| Left superior temporal gyrus (BA 22) | GMV | .19* | |
| Right superior temporal gyrus (BA 22) | GMV | .16**** | |
| Left middle temporal gyrus (BA 21) | GMV | .25** | |
| Right middle temporal gyrus (BA 21) | GMV | .16**** | |
| Right temporal pole (BA 38) | GMV | .19* | |
| Mitelman et al (2009a)87 | Right superior temporal gyrus (BA 41) | WMV | .28* |
| Right inferior temporal gyrus (BA 20) | WMV | −.28* | |
| Left temporal pole (BA 38) | WMV | −.23**** | |
| Faget-Agius et al (2015)53 | Temporal pole | MTR | .22* |
| Temporal pole | MTR | .21*** | |
| Sheng et al (2013)62 | Middle temporal pole | Lateralization | .65* |
| Mitelman et al (2006)35 | Left inferior temporal | FA | .19* |
| Parietal | |||
| Wilke et al (2001)69 | Left inferior parietal lobule | GMV | .50*** |
| Guo et al (2015) | Right supramarginal | Vol | .37* |
| Mitelman et al (2006)86 | Right supramarginal gyrus | FA | .16**** |
| Left postcentral gyrus | FA | .25** | |
| Mitelman et al (2007a)36 | Left postcentral gyrus (BA 43) | GMV | .25** |
| Mitelman et al (2009a)87 | Left supramarginal gyrus (BA 40) | WMV | −.28* |
| Left supramarginal gyrus (BA 40) | GMV | .23**** | |
| Right supramarginal gyrus (BA 40) | WMV | −.28* | |
| Left angular gyrus (BA 39) | WMV | .23**** | |
| Right angular gyrus (BA 39) | WMV | −.28* | |
| Left postcentral gyrus (BA 43) | GMV | .23**** | |
| Right primary somatosensory (BA 3/1/2) | WMV | .23**** | |
| Right primary somatosensory (BA 3/1/2) | WMV | −.28* | |
| Right somatosensory association (BA 5) | WMV | .23**** | |
| Right somatosensory association (BA 5) | WMV | −.28* | |
| Occipital | |||
| Mitelman et al (2003)33 | Occipital lobe | GMV | .49** |
| Molina et al (2010)58 | Right cuneus | GMV | .50*** |
| Faget-Agius et al (2015)53 | Left secondary visual cortex | MTR | .22* |
| Mitelman et al (2007a)36 | Left primary visual cortex (BA 17) | GMV | .16**** |
| Left secondary visual cortex (BA 18) | GMV | .16**** | |
| Left associative visual cortex (BA 19) | GMV | .19* | |
| Right associative visual cortex (BA 19) | GMV | .16**** | |
| Mitelman et al (2009a)87 | Left secondary visual cortex (BA 18) | WMV | −.28* |
| Left associative visual cortex (BA 19) | WMV | −.28* | |
| Mané et al (2009)56 | Left lingual gyrus | GMV | .51* |
| Cerebellum | |||
| van Haren et al (2008)66 | Cerebellum | Vol | .21* |
| Mané et al (2009)56 | Right cerebellum | GMV | .51* |
| Faget-Agius et al (2015)53 | Right cerebellum | MTR | .22* |
| Right cerebellum | MTR | .14** | |
| Vermis | MTR | .22* | |
| Vermis | MTR | .18*** | |
| Boonstra et al (2011)49 | Cerebellum | Vol | −.11 |
| Cerebellum | Vol | .26 | |
| Cerebellum | Vol | −.03 | |
| Cerebellum | Vol | .20 | |
Note: CiB, cingulum bundle; DLPFC, dorsolateral prefrontal cortex; FA, fractional anisotropy; gCC, corpus callosum genu; GMV, gray matter volume; IFOF, inferior fronto-occipital fasciculus; MTR, magnetization transfer ratio; sCC, corpus callosum splenium; UF, uncinate fasciculus; VBR, ventricle-brain ratio; Vol, volume.
Spearman’s rank correlation (rs).
Partial correlation (pr).
P < .05;
P < .01;
P < .001;
P < .10.
Table 3.
Summary of Brain-Functional Outcome Effect Sizes Across Structural and Functional Neuroimaging Studies in Schizophrenia
| Measure | Number of Studies | Number of Effect Sizes | Effect Size (r) | P | 95% CI | Heterogeneity (QW) |
|---|---|---|---|---|---|---|
| Structural | ||||||
| Whole brain | 8 | 21 | .25 | <.0001 | .13 to .37 | 37.07* |
| White matter tracts | 4 | 15 | .32 | .150 | −.12 to .75 | 112.87*** |
| Ventricles | 10 | 27 | −.31 | <.0001 | −.41 to −.21 | 45.09* |
| Frontal | 12 | 32 | .35 | <.0001 | .22 to .47 | 106.01*** |
| Limbic | 15 | 53 | .38 | <.0001 | .25 to .51 | 166.54*** |
| Temporal | 8 | 16 | .19 | .191 | −.10 to .48 | 54.51*** |
| Parietal | 5 | 15 | .29 | .039 | .01 to .57 | 67.29*** |
| Occipital | 6 | 10 | .30 | .004 | .10 to .50 | 40.80*** |
| Cerebellum | 4 | 10 | .17 | <.0001 | .09 to .26 | 10.96 |
| Functional | ||||||
| Social cognition | 10 | 42 | .25 | .046 | .00 to .49 | 126.46*** |
| Neurocognition | 1 | 1 | .47 | — | — | — |
| Resting-state | 3 | 13 | .38 | <.0001 | .24 to .52 | 8.32 |
| SPECT | 2 | 13 | −.08 | .685 | −.45 to .29 | 170.20*** |
Note: SPECT, single photon emission computed tomography.
P < .05;
P < .01;
P < .001.
Lobar analyses of study findings revealed prominent frontal (32:199) and limbic (53:199) regional themes associated with functional outcome (table 2). A medium average effect size was observed for the association between larger frontal lobe volumes and better functional outcome, with significant heterogeneity between studies (table 3). All moderator analyses were nonsignificant for the frontal lobe. Common frontal regions included the dorsolateral prefrontal cortex (DLPFC; 31%), orbital frontal gyrus (19%), and left inferior frontal gyrus (16%). Greater volume in these frontal regions was associated with better composite functioning, global functioning, and a higher socioeconomic status. With regard to the limbic lobe, having greater volume was significantly related to better functional outcome (composite, global functioning, quality of life, and role functioning). The average effect was medium in size and significant heterogeneity was present among the limbic studies (table 3). Illness duration was observed to significantly moderate the relationship between limbic regions and functional outcome (QB(1) = 4.47, P = .034). A large effect size was demonstrated for the first episode patients (r = .59, P < .0001) and a medium effect size for the multiple episode patients (r = .30, P < .0001). No other significant moderating effects were observed. The limbic brain regions most commonly associated with functional outcome were the parahippocampal gyrus (17%), posterior cingulate (15%), anterior cingulate (13%), and the insula (13%; table 2).
The average effect for the relationship between temporal brain regions (16:199) and functional outcome was not significant. A small, significant average effect was shown between greater parietal regional brain volume (15:199) and better functional outcome, with significant heterogeneity between studies (table 3). Comparatively, fewer findings were observed in the occipital lobe (10:199) and cerebellum (10:199). Greater volume in the occipital lobe (medium average effect size) and cerebellum (small average effect size) was significantly related to better functioning, with significant heterogeneity among studies examining the occipital lobe (table 3). Moderator analyses were not conducted for the white matter tracts, temporal, parietal, and occipital findings due to an insufficient number of studies.
There were several studies (19, 36%) that used a categorical definition of good and poor functional outcome in schizophrenia, with most using outcome criteria from Keefe et al84 (table 1). Using a categorical vs continuous operationalization of functional outcome could have impacted effect size estimates of the association with neurobiological parameters in such studies. However, as can be seen in supplementary table 1, a sensitivity analysis demonstrated that using a continuous vs categorical definition did not bias the association between the major themes of smaller lateral ventricles and greater fronto-limbic gray matter and better functional outcome. The effect sizes remained significant and moderate in size.
Overall, the structural results suggest a potentially central relationship between better functional outcome and greater whole brain and fronto-limbic gray matter and smaller ventricles in individuals with schizophrenia.
Results of Studies of Brain Function and Functional Outcome in Schizophrenia
Sixteen studies reported a total of 69 findings on the relationship between brain function and functional outcome in schizophrenia. These findings were organized by neuroimaging methodology and presented in table 4. There were a total of 42 (42:69) findings extracted from the social cognitive fMRI studies that had a significant, but small relationship with functional outcome (table 3). The social cognitive fMRI findings were significantly heterogeneous, but no significant moderators were observed. The most frequent observations were that greater activation in the medial prefrontal cortex (MPFC; 12%), anterior cingulate (12%), and amygdala (12%) during social cognitive processing was associated with better composite, social, and role functioning. Only 1 (1:69) study examined the association between neurocognitive-related brain functioning and functional outcome. Greater resting-state connectivity (13:69), mostly in frontal areas, was significantly related to better global and social functioning with a medium effect size and homogeneity among the findings (table 3). Regional cerebral blood flow, mostly in the superior temporal sulcus, measured by SPECT was not significantly related to functional outcome in schizophrenia. There were an insufficient number of SPECT studies to examine moderator effects.
Table 4.
Funcitonal Neuroimanging Studies Displaying Relationships Between Functional Outcome and Regional Brain Activation/Connecitivty in Schizophrenia
| Study | Brain Region | Type | Effect Size (r) |
|---|---|---|---|
| Social cognition | |||
| Dodell-Feder et al (2013)71 | MPFC | Activation | .44* |
| MPFC | Activation | .11 | |
| Ventral medial prefrontal cortex | Activation | −.05 | |
| Ventral medial prefrontal cortex | Activation | −.19 | |
| Left temporoparietal junction | Activation | .21 | |
| Left temporoparietal junction | Activation | .04 | |
| Right temporoparietal junction | Activation | .23 | |
| Right temporoparietal junction | Activation | .09 | |
| Lee et al (2006)72 | Left MPFC | Activation | .51**** |
| Shin et al (2015)76 | Left DLPFC | Activation | .69* |
| Das et al (2012)70 | Right inferior frontal gyrus | Activation | .53* |
| Right superior temporal gyrus | Activation | .39 | |
| Nelson et al (2015)73 | Anterior cingulate | Activation | .75** |
| Anterior cingulate | Activation | .01 | |
| Anterior cingulate | Activation | .28 | |
| Anterior cingulate | Activation | −.21 | |
| Smith et al (2015)44 | Right suppl motor/middle cingulate | Activation | .46* |
| Right cuneus | Activation | −.40* | |
| Right suppl motor/middle cingulate | Activation | .46* | |
| Right precuneal sulcus | Activation | .35**** | |
| Pinkham et al (2011)75 | Amygdala | Activation | .59* |
| Pinkham et al (2008)74 | Left amygdala | Activation | .50* (P-SCZ)a |
| Left fusiform | Activation | .45**** (P-SCZ)a | |
| Right fusiform | Activation | .44**** (P-SCZ)a | |
| MPFC | Activation | .26 (P-SCZ)a | |
| Left ventrolateral prefrontal cortex | Activation | .37 (P-SCZ)a | |
| Right ventrolateral prefrontal cortex | Activation | .12 (P-SCZ)a | |
| Right amygdala | Activation | .36 (P-SCZ)a | |
| Left superior temporal sulcus | Activation | .40 (P-SCZ)a | |
| Right superior temporal sulcus | Activation | .39 (P-SCZ)a | |
| MPFC | Activation | .26 (NP-SCZ)a | |
| Left ventrolateral prefrontal cortex | Activation | .34 (NP-SCZ)a | |
| Right ventrolateral prefrontal cortex | Activation | .22 (NP-SCZ)a | |
| Left amygdala | Activation | −.10 (NP-SCZ)a | |
| Right amygdala | Activation | −.03 (NP-SCZ)a | |
| Left superior temporal sulcus | Activation | −.05 (NP-SCZ)a | |
| Right superior temporal sulcus | Activation | .07 (NP-SCZ)a | |
| Left fusiform gyrus | Activation | .21 (NP-SCZ)a | |
| Right fusiform gyrus | Activation | .35 (NP-SCZ)a | |
| Thakkar et al (2014)78 | Right inferior parietal lobule | Activation | −.57*,a |
| Taylor et al (2011)77 | Calcarine/precuneus/lingual | Activation | −.43* |
| Anterior cingulate | Activation | −.28 | |
| Neurocognition | |||
| Yoon et al (2008)79 | DLPFC | Connectivity | .47* |
| Resting-state | |||
| Anticevic et al (2015)80 | Prefrontal cortex | Hyper-connectivity | .48* |
| Prefrontal cortex | Hyper-connectivity normalization | .51** | |
| Fox et al (in press)45 | MPFC to Posterior Cingulate | Connectivity | .44* |
| MPFC to Posterior Cingulate | Connectivity | .45* | |
| Lui et al (2010)81 | R inferior frontal gyrus | Connectivity | .34* |
| Left prefrontal cortex | Connectivity | .34* | |
| Right MPFC | Connectivity | .34* | |
| Left superior temporal gyrus | Connectivity | .34* | |
| Left superior parietal lobule | Connectivity | .44** | |
| Right inferior parietal lobule | Connectivity | .34* | |
| Left superior frontal gyrus | Connectivity | .12 | |
| Right middle frontal gyrus | Connectivity | .12 | |
| Right caudate | Connectivity | .12 | |
| SPECT | |||
| Faget-Agius et al (2016)83 | Right superior frontal gyrus | rCBF | −.38* |
| Right superior temporal gyrus | rCBF | .43* | |
| Left supramarginal gyrus | rCBF | .32* | |
| Right precentral gyrus | rCBF | .33* | |
| Left precuneus | rCBF | −.39* | |
| Right cuneus | rCBF | .36* | |
| Boyer et al (2012)82 | Left superior temporal sulcus | rCBF | −.42* |
| Right superior temporal sulcus | rCBF | −.39* | |
| Left superior temporal sulcus | rCBF | −.40*,a | |
| Left superior temporal sulcus | rCBF | .15a | |
| Left superior temporal sulcus | rCBF | −.23a | |
| Left superior temporal sulcus | rCBF | −.21a | |
| Left superior temporal sulcus | rCBF | −.24a | |
Note: DLPFC, dorsolateral prefrontal cortex; MPFC, medial prefrontal cortex; NP-SCZ, nonparanoid schizophrenia; P-SCZ, paranoid schizophrenia; rCBF, regional cerebral blood flow; SPECT, single photon emission computed tomography.
Spearman’s rank correlation (rs).
P < .05;
P < .01;
P < .001;
P < .10.
Overall, the studies of brain function suggest that individuals with schizophrenia who elicit greater brain activation while performing social cognitive tasks or brain connectivity at rest have better functional outcome (r = .25, P = .004; QW(66) = 359.62, P < .0001). No significant moderators were observed for the overall effect of the functional imaging studies.
Neurobiological Correlates of Functional Outcomes in Schizophrenia
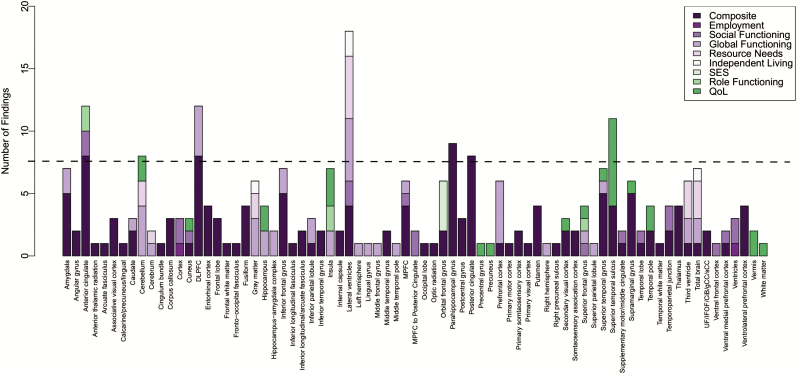
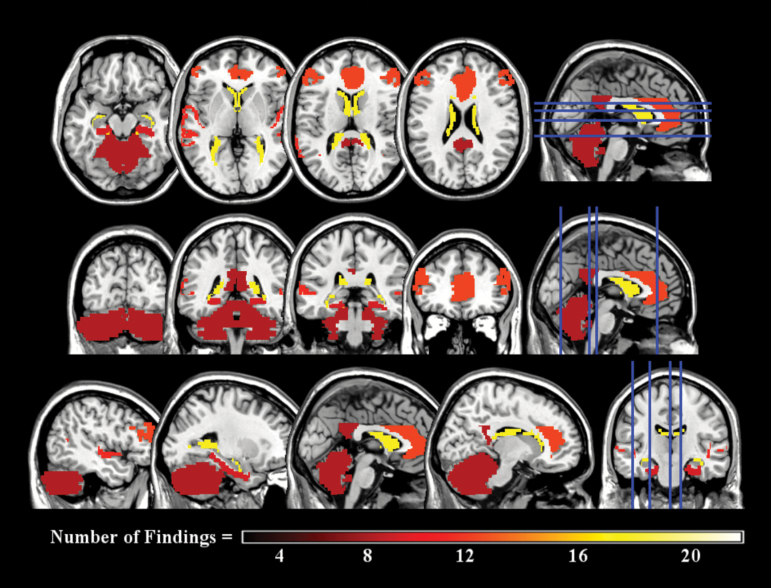
In figure 1, we combined the structural and functional imaging findings and their domains of functional outcome in order to visually inspect brain-functional outcome themes in schizophrenia across all the 268 findings. Brain regions and the number of findings contributing to that brain region were subjected to a scree plot, which indicated that brain-functional outcome relationships with 8 or more findings were the most frequent. An overlay of these regions across a T1 template brain is presented in figure 2. The brain regions most commonly associated with functional outcome across all studies and modalities were the lateral ventricles (18:268), anterior cingulate (12:268), DLPFC (12:268), superior temporal sulcus (11:268), parahippocampal gyrus (9:268), posterior cingulate (8:268), and cerebellum (8:268; figure 2). The domains of functional outcome related to these regions included composite, global functioning, social functioning, resource needs, independent living, role functioning, and quality of life (figure 1). The measures of composite functioning dominated these relationships making associations between brain regions and specific domains of functional outcome inconclusive. Prominent directional themes suggested that having (1) smaller lateral ventricles; (2) greater parahippocampal gyrus, posterior cingulate, and cerebellum gray matter volume; and (3) greater activation and/or gray matter volume in the anterior cingulate, DLPFC and superior temporal sulcus were related to better functional outcome in the illness.
Fig. 1.
Findings of neurobiological correlates associated with functional outcome domains across structural and functional imaging reports. Note: DLPFC, dorsolateral prefrontal cortex; MPFC, medial prefrontal cortex; CiB, cingulum bundle; gCC, corpus callosum genu; IFOF, inferior fronto-occipital fasciculus; sCC, corpus callosum splenium; UF, uncinate fasciculus.
Fig. 2.
Common frontal-limbic regions associated with functional outcome in schizophrenia.
Discussion
Individuals diagnosed with schizophrenia have pronounced impairments across many domains of functional disability1,2,13,14 that have only been modestly responsive to pharmacological15 and psychosocial interventions.16,88 A critical barrier to providing a more complete improvement in functional outcome is the limited understanding of the link between neurobiology and functional outcome in schizophrenia. Therefore, this research sought to systematically examine previous neuroimaging studies in order to elucidate common themes of brain-functional outcome relationships in the illness. Our goal was to establish an initial neurobiological basis of functional outcome that could function as a treatment biomarker and serve to direct future investigations.
A total of 53 neuroimaging studies were identified that examined functional outcome in schizophrenia: 37 used structural and 16 used functional neuroimaging. The studies yielded a total of 1631 individuals with schizophrenia and 268 findings of brain-functional outcome relationships, of which 199 were structural and 69 were functional. The studies of brain structure suggested that individuals with schizophrenia who displayed better functional outcome had smaller ventricles and greater gray matter volumes across the cortex and in fronto-limbic regions. The relationship between functional outcome and limbic volume was significantly moderated by illness duration, suggesting that interventions, such as cognitive remediation, applied early in the illness may be important for restoring or enhancing functional abilities.89 Results from the functional studies were quite heterogeneous, but did indicate that individuals with schizophrenia who had better functional outcome had greater activation during social cognitive processing and/or greater connectivity while at rest. No significant moderators emerged in the functional imaging findings. Thematic observations across all the findings revealed that smaller lateral ventricles, greater parahippocampal gyrus, posterior cingulate, and cerebellum gray matter volume; and greater activation and/or gray matter volume in the anterior cingulate, DLPFC and superior temporal sulcus were most consistently associated with better functioning (figure 2). The commonality of using a composite measure of functional outcome among the studies made specific brain-functional outcome domain (eg, employment or social functioning) relationships inconclusive.
The consistency of ventricular, fronto-limbic, and temporal results identified in this review preliminarily suggests neurocircuitry is associated with functional outcome in schizophrenia. Larger ventricles have generally been a key feature of poor functional outcome in the illness41,52,61 and are reflective of progressive gray matter atrophy.90 For example, Gaser and colleagues91 showed that reductions in gray matter in the insula, thalamus, and putamen were related to larger ventricular volumes in patients with schizophrenia. Gray matter loss has been consistently reported in the disorder for many of the regions identified in this review.92 Furthermore, impairments in social and nonsocial cognition are associated with reduced gray matter across fronto-limbic and temporal regions, including the medial/dorsolateral prefrontal, cingulate, superior/middle temporal, and hippocampal cortices.93–96 There is substantial overlap among these brain regions associated with cognitive impairments and the regions observed to be associated with functional outcome in this review. This is likely reflective of an important neurobiological link that could be targeted through intervention, specifically cognitive remediation, to promote functional recovery from schizophrenia. Indeed, a well-known and robust relationship exists between cognition and functional outcome in the illness.97–99 Eack and colleagues have demonstrated improved cognition mediated the beneficial effects of cognitive remediation on functional outcome,100 in addition to protecting against gray matter loss101 and increasing resting-state network connectivity102 that was associated with improved cognition. Examining treatment-related brain alterations and their mediating/moderating role in functional treatment goals (ie, independent living, maintaining employment) is an important direction for future research and for discerning the specificity of these findings.
The findings from this review suggest that the ability to maintain interpersonal relationships, live independently in the community, and work, eg, all may be partially related to having sufficient fronto-limbic brain resources. The onset of schizophrenia-spectrum disorders typically occurs during late adolescence or early adulthood and triggers substantial alterations to neuromaturation (eg, reduced gray matter103), which could lead to major disruptions in meeting early adult developmental milestones. The insult to functional outcome made by reduced gray matter in fronto-limbic regions may be reflective of excessive synaptic pruning104–106 that results in significant tissue loss107 and broad cognitive dysfunction that extends to cognitive abilities that directly support adaptive function (eg, shifting from one task to the next, maintaining focus to understand information). Without treatment,101,108 impairments in fronto-limbic brain integrity may persist and have a distributed impact on many domains of community and daily living. Specific conclusions regarding the role of the fronto-limbic regions associated with functional outcome are limited with this being the first comprehensive review of a diverse and relatively small literature. These findings do imply, however, that fronto-limbic, cortical, and ventricle parameters are potentially consistent correlates of changes to functional outcome. Preservation and restoration of fronto-limbic structure and function may be profitable for rehabilitating functional disability in schizophrenia. Greater baseline limbic and temporal gray matter volumes have been shown to be predictive of better outcomes following treatment in patients schizophrenia109,110 (“brain reserve” model111).
Despite the evidence for a neurobiological basis of functional outcome in schizophrenia, several limitations of this research should be considered. First, there was a large discrepancy between the number of findings from the structural (k = 199) and functional (k = 69) imaging studies. This gave more weight to fronto-limbic brain-structural outcome relationships and allowed for a more stable structural pattern to emerge. The functional imaging studies also varied in scanner tasks, imaging modalities (eg, activation, connectivity), and measured a different neural process (blood flow) than structural imaging studies (primarily volume). These differences likely accounted for variations in brain-functional outcome findings between the structural and functional imaging methodologies. Although there were a limited number of functional neuroimaging findings, file drawer analyses indicated that a publication bias was not likely to be present for either the structural or functional imaging findings. A total of 42 135 unpublished null structural imaging findings and 1989 unpublished null functional imaging findings would be required to reduce meta-analytic results to non-significant levels.
Second, very few studies in this review had a primary aim to examine brain-functional outcome relationships. Most studies examined this relationship post-hoc after restricting brain regions to differences between groups (eg, controls or comparison groups) or hypothesized brain regions of interest. Such restrictions may be overlooking other central brain regions outside the frontal-limbic theme observed. Third, the studies revealed that a large variety of measures of functional outcome were used, with most utilizing a composite assessment that limited conclusions that could be made about specific neurobiological associations with different domains of functioning (eg, social functioning, employment, independent living). Finally, given that few studies reviewed were longitudinal (18, 34%), the time-ordered direction of brain-functional outcome associations remains unknown. Based on the pathophysiology of schizophrenia,105 it is perhaps predicted that neurobiology impacts community functioning. However, such an assumption would ignore existing evidence indicating that functional disability can contribute to neural dysfunction, especially in cases where functional impairment leads to deprivation.112 In the context of schizophrenia, it is possible that early sensitivity to stress can lead to social isolation, which then interrupts healthy neurobiological development.86 An alternative approach to examining brain-functional outcome relationships in schizophrenia would be to examine premorbid functional changes, such as social isolation, that can negatively impact neurobiology.
Overall, the findings of this research indicate that there is a consistent relationship between brain structure and function, and functional outcome in people living with schizophrenia. The aforementioned limitations largely preclude further specification of these relationships beyond regional themes, but give clear direction for future research. First, a priori studies are greatly needed that are not limited to regions of interest and analyses based on post hoc decisions. Second, the collection of longitudinal data is essential, given the likelihood of bidirectional relationships between the brain and functional outcome. Longitudinal studies of first episode patients will be especially vital, given the need to provide prognostic information to patients and families, as well as allocating needed resources to those most likely to experience adverse outcomes. Third, the standardization of functional outcome measures would greatly improve the interpretability of this literature, and enable clearer conclusions regarding specific outcome domains. We are excited by work progressing in this area and encourage its use.87,113 Fourth, greater focus on functional neuroimaging modalities are needed, given the small number of functional imaging studies and heterogeneous results observed in this review. It will be important to consider the possibility of performance-based measures of functional capacity that could be adapted for use in the scanner, as well as neurocognitive tasks that may mediate the relationship between brain function and outcome. Finally, given the likelihood of several downstream mechanisms in the relationship between brain and functional outcome (eg, “brain reserve,” neurocognitive function, social cognition), it will be important for future studies to conduct mediator analyses and begin to construct models that describe the direct and indirect effects of neural variables on functioning.
In summary, this comprehensive systematic and meta-analytic review was conducted to investigate neurobiological themes of functional outcome in schizophrenia. Overall, the meta-analytic results demonstrated that individuals with schizophrenia who had greater whole brain and fronto-limbic volumes, smaller ventricles, and greater activation during social cognitive processing had better functional outcome. Thematic observations revealed that better functional outcome was associated with smaller lateral ventricles and larger gray matter volumes in the parahippocampal gyrus, posterior cingulate, and cerebellum. Additionally, greater volume and/or activation in the DLPFC, anterior cingulate, and superior temporal sulcus were associated with better functional outcome. The results of this review provide preliminary evidence that fronto-limbic neurocircuitry is involved in functional outcome in schizophrenia, despite this literature base being limited and heterogeneous. Such findings support continuing to research and establish the neural correlates of functional outcome in schizophrenia, which is advantageous for intervention development and optimizing the capacity for full functional recovery from this illness.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
National Institutes of Health (MH 95783 to S.M.E.).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Hegarty JD, Baldessarini RJ, Tohen M, Waternaux C, Oepen G. One hundred years of schizophrenia: a meta-analysis of the outcome literature. Am J Psychiatry. 1994;151:1409–1416. [DOI] [PubMed] [Google Scholar]
- 2. Bromley E, Brekke JS. Assessing function and functional outcome in schizophrenia. Curr Top Behav Neurosci. 2010;4:3–21. [DOI] [PubMed] [Google Scholar]
- 3. Strauss JS, Carpenter WT., Jr The prediction of outcome in schizophrenia. I. Characteristics of outcome. Arch Gen Psychiatry. 1972;27:739–746. [DOI] [PubMed] [Google Scholar]
- 4. Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. [DOI] [PubMed] [Google Scholar]
- 5. Burns T, Patrick D. Social functioning as an outcome measure in schizophrenia studies. Acta Psychiatr Scand. 2007;116:403–418. [DOI] [PubMed] [Google Scholar]
- 6. Morrison RL, Bellack AS. Social functioning of schizophrenic patients: clinical and research issues. Schizophr Bull. 1987;13:715–725. [DOI] [PubMed] [Google Scholar]
- 7. Tsang HW, Leung AY, Chung RC, Bell M, Cheung WM. Review on vocational predictors: a systematic review of predictors of vocational outcomes among individuals with schizophrenia: an update since 1998. Aust N Z J Psychiatry. 2010;44:495–504. [DOI] [PubMed] [Google Scholar]
- 8. Hansson L, Middelboe T, Sørgaard KW, et al. Living situation, subjective quality of life and social network among individuals with schizophrenia living in community settings. Acta Psychiatr Scand. 2002;106:343–350. [DOI] [PubMed] [Google Scholar]
- 9. Eack SM, Newhill CE. Psychiatric symptoms and quality of life in schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:1225–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bromet EJ, Naz B, Fochtmann LJ, Carlson GA, Tanenberg-Karant M. Long-term diagnostic stability and outcome in recent first-episode cohort studies of schizophrenia. Schizophr Bull. 2005;31:639–649. [DOI] [PubMed] [Google Scholar]
- 11. McClure MM, Bowie CR, Patterson TL, et al. Correlations of functional capacity and neuropsychological performance in older patients with schizophrenia: evidence for specificity of relationships? Schizophr Res. 2007;89:330–338. [DOI] [PubMed] [Google Scholar]
- 12. Morgan VA, McGrath JJ, Jablensky A, et al. Psychosis prevalence and physical, metabolic and cognitive co-morbidity: data from the second Australian national survey of psychosis. Psychol Med. 2014;44:2163–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Breier A, Schreiber JL, Dyer J, Pickar D. National Institute of Mental Health longitudinal study of chronic schizophrenia. Prognosis and predictors of outcome. Arch Gen Psychiatry. 1991;48:239–246. [DOI] [PubMed] [Google Scholar]
- 14. Harvey PD, Heaton RK, Carpenter WT, Jr, Green MF, Gold JM, Schoenbaum M. Functional impairment in people with schizophrenia: focus on employability and eligibility for disability compensation. Schizophr Res. 2012;140:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Swartz MS, Perkins DO, Stroup TS, et al. ; CATIE Investigators. Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. Am J Psychiatry. 2007;164:428–436. [DOI] [PubMed] [Google Scholar]
- 16. Pfammatter M, Junghan UM, Brenner HD. Efficacy of psychological therapy in schizophrenia: conclusions from meta-analyses. Schizophr Bull. 2006;32(suppl 1):S64–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. [DOI] [PubMed] [Google Scholar]
- 18. Grant PM, Huh GA, Perivoliotis D, Stolar NM, Beck AT. Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Arch Gen Psychiatry. 2012;69:121–127. [DOI] [PubMed] [Google Scholar]
- 19. Bond GR, Drake RE, Becker DR. An update on randomized controlled trials of evidence-based supported employment. Psychiatr Rehabil J. 2008;31:280–290. [DOI] [PubMed] [Google Scholar]
- 20. Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bellani M, Dusi N, Brambilla P. Longitudinal imaging studies in schizophrenia: the relationship between brain morphology and outcome measures. Epidemiol Psichiatr Soc. 2010;19:207–210. [PubMed] [Google Scholar]
- 23. Gur RE, Cowell P, Turetsky BI, et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55:145–152. [DOI] [PubMed] [Google Scholar]
- 24. Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. [DOI] [PubMed] [Google Scholar]
- 25. Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galderisi S, Vita A, Rossi A, et al. Qualitative MRI findings in patients with schizophrenia: a controlled study. Psychiatry Res. 2000;98:117–126. [DOI] [PubMed] [Google Scholar]
- 28. Rosenthal R. Meta-analytic Procedures for Social Research Vol 6. Beverly Hill, CA: Sage; 1984. [Google Scholar]
- 29. Viechtbauer W. Meta-Analysis Package for R (“metafor”) [computer program], Version 1.9–9. 2016. http://www.metafor-project.org. Accessed October 5, 2016.
- 30. Brickman AM, Buchsbaum MS, Shihabuddin L, et al. Thalamus size and outcome in schizophrenia. Schizophr Res. 2004;71:473–484. [DOI] [PubMed] [Google Scholar]
- 31. Brickman AM, Buchsbaum MS, Ivanov Z, et al. Internal capsule size in good-outcome and poor-outcome schizophrenia. J Neuropsychiatry Clin Neurosci. 2006;18:364–376. [DOI] [PubMed] [Google Scholar]
- 32. Buchsbaum MS, Shihabuddin L, Brickman AM, et al. Caudate and putamen volumes in good and poor outcome patients with schizophrenia. Schizophr Res. 2003;64:53–62. [DOI] [PubMed] [Google Scholar]
- 33. Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. MRI assessment of gray and white matter distribution in Brodmann’s areas of the cortex in patients with schizophrenia with good and poor outcomes. Am J Psychiatry. 2003;160:2154–2168. [DOI] [PubMed] [Google Scholar]
- 34. Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophr Res. 2005;72:91–108. [DOI] [PubMed] [Google Scholar]
- 35. Mitelman SA, Newmark RE, Torosjan Y, et al. White matter fractional anisotropy and outcome in schizophrenia. Schizophr Res. 2006;87:138–159. [DOI] [PubMed] [Google Scholar]
- 36. Mitelman SA, Brickman AM, Shihabuddin L, et al. A comprehensive assessment of gray and white matter volumes and their relationship to outcome and severity in schizophrenia. Neuroimage. 2007a;37:449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mitelman SA, Torosjan Y, Newmark RE, et al. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophr Res. 2007b;92:211–224. [DOI] [PubMed] [Google Scholar]
- 38. Mitelman SA, Canfield EL, Newmark RE, et al. Longitudinal assessment of gray and white matter in chronic schizophrenia: A combined diffusion-tensor and structural Mmagnetic resonance imaging study. Open Neuroimag J. 2009a;3:31–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mitelman SA, Canfield EL, Chu KW, et al. Poor outcome in chronic schizophrenia is associated with progressive loss of volume of the putamen. Schizophr Res. 2009b;113:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mitelman SA, Nikiforova YK, Canfield EL, et al. A longitudinal study of the corpus callosum in chronic schizophrenia. Schizophr Res. 2009c;114:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mitelman SA, Canfield EL, Brickman AM, Shihabuddin L, Hazlett EA, Buchsbaum MS. Progressive ventricular expansion in chronic poor-outcome schizophrenia. Cogn Behav Neurol. 2010;23:85–88. [DOI] [PubMed] [Google Scholar]
- 42. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59:1002–1010. [DOI] [PubMed] [Google Scholar]
- 43. Cahn W, van Haren NE, Hulshoff Pol HE, et al. Brain volume changes in the first year of illness and 5-year outcome of schizophrenia. Br J Psychiatry. 2006;189:381–382. [DOI] [PubMed] [Google Scholar]
- 44. Smith MJ, Schroeder MP, Abram SV, et al. Alterations in brain activation during cognitive empathy are related to social functioning in schizophrenia. Schizophr Bull. 2015;41:211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fox JM, Abram SV, Reilly JL, et al. Default mode functional connectivity is associated with social functioning in schizophrenia. J Abnorm Psychol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Borenstein M, Hedges LV, Higgins J, Rothstein HR. Introduction to Meta-Analysis. West Sussex, UK: John Wiley & Sons Ltd; 2009. [Google Scholar]
- 47. Aoyama N, Théberge J, Drost DJ, et al. Grey matter and social functioning correlates of glutamatergic metabolite loss in schizophrenia. Br J Psychiatry. 2011;198:448–456. [DOI] [PubMed] [Google Scholar]
- 48. Behdinan T, Foussias G, Wheeler AL, et al. Neuroimaging predictors of functional outcomes in schizophrenia at baseline and 6-month follow-up. Schizophr Res. 2015;169:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boonstra G, Cahn W, Schnack HG, et al. Duration of untreated illness in schizophrenia is not associated with 5-year brain volume change. Schizophr Res. 2011;132:84–90. [DOI] [PubMed] [Google Scholar]
- 50. Brambilla P, Perlini C, Rajagopalan P, et al. Schizophrenia severity, social functioning and hippocampal neuroanatomy: three-dimensional mapping study. Br J Psychiatry. 2013;202:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chemerinski E, Nopoulos PC, Crespo-Facorro B, Andreasen NC, Magnotta V. Morphology of the ventral frontal cortex in schizophrenia: relationship with social dysfunction. Biol Psychiatry. 2002;52:1–8. [DOI] [PubMed] [Google Scholar]
- 52. Davis KL, Buchsbaum MS, Shihabuddin L, et al. Ventricular enlargement in poor-outcome schizophrenia. Biol Psychiatry. 1998;43:783–793. [DOI] [PubMed] [Google Scholar]
- 53. Faget-Agius C, Catherine FA, Boyer L, et al. Neural substrate of quality of life in patients with schizophrenia: a magnetisation transfer imaging study. Sci Rep. 2015;5:17650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guo JY, Huhtaniska S, Miettunen J, et al. Longitudinal regional brain volume loss in schizophrenia: relationship to antipsychotic medication and change in social function. Schizophr Res. 2015;168:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kasparek T, Prikryl R, Schwarz D, et al. Gray matter morphology and the level of functioning in one-year follow-up of first-episode schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1438–1446. [DOI] [PubMed] [Google Scholar]
- 56. Mané A, Falcon C, Mateos JJ, et al. Progressive gray matter changes in first episode schizophrenia: a 4-year longitudinal magnetic resonance study using VBM. Schizophr Res. 2009;114:136–143. [DOI] [PubMed] [Google Scholar]
- 57. Milev P, Ho BC, Arndt S, Nopoulos P, Andreasen NC. Initial magnetic resonance imaging volumetric brain measurements and outcome in schizophrenia: a prospective longitudinal study with 5-year follow-up. Biol Psychiatry. 2003;54:608–615. [DOI] [PubMed] [Google Scholar]
- 58. Molina V, Hernández JA, Sanz J, et al. Subcortical and cortical gray matter differences between Kraepelinian and non-Kraepelinian schizophrenia patients identified using voxel-based morphometry. Psychiatry Res. 2010;184:16–22. [DOI] [PubMed] [Google Scholar]
- 59. Prasad KM, Sahni SD, Rohm BR, Keshavan MS. Dorsolateral prefrontal cortex morphology and short-term outcome in first-episode schizophrenia. Psychiatry Res: Neuroimaging. 2005;140:147–155. [DOI] [PubMed] [Google Scholar]
- 60. Rossi A, Bustini M, Prosperini P, et al. Neuromorphological abnormalities in schizophrenic patients with good and poor outcome. Acta Psychiatr Scand. 2000;101:161–166. [DOI] [PubMed] [Google Scholar]
- 61. Seidman LJ, Sokolove RL, McElroy C, Knapp PH, Sabin T. Lateral ventricular size and social network differentiation in young, nonchronic schizophrenic patients. Am J Psychiatry. 1987;144:512–514. [DOI] [PubMed] [Google Scholar]
- 62. Sheng J, Zhu Y, Lu Z, et al. Altered volume and lateralization of language-related regions in first-episode schizophrenia. Schizophr Res. 2013;148:168–174. [DOI] [PubMed] [Google Scholar]
- 63. Takayanagi Y, Takahashi T, Orikabe L, et al. Volume reduction and altered sulco-gyral pattern of the orbitofrontal cortex in first-episode schizophrenia. Schizophr Res. 2010;121:55–65. [DOI] [PubMed] [Google Scholar]
- 64. Tully LM, Lincoln SH, Liyanage-Don N, Hooker CI. Impaired cognitive control mediates the relationship between cortical thickness of the superior frontal gyrus and role functioning in schizophrenia. Schizophr Res. 2014;152:358–364. [DOI] [PubMed] [Google Scholar]
- 65. Uwatoko T, Yoshizumi M, Miyata J, et al. Insular gray matter volume and objective quality of life in schizophrenia. PLoS One. 2015;10:e0142018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. van Haren NE, Hulshoff Pol HE, Schnack HG, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63:106–113. [DOI] [PubMed] [Google Scholar]
- 67. Vita A, Dieci M, Giobbio GM, et al. CT scan abnormalities and outcome of chronic schizophrenia. Am J Psychiatry. 1991;148:1577–1579. [DOI] [PubMed] [Google Scholar]
- 68. Whitworth AB, Kemmler G, Honeder M, et al. Longitudinal volumetric MRI study in first- and multiple-episode male schizophrenia patients. Psychiatry Res: Neuroimaging. 2005;140:225–237. [DOI] [PubMed] [Google Scholar]
- 69. Wilke M, Kaufmann C, Grabner A, Pütz B, Wetter TC, Auer DP. Gray matter-changes and correlates of disease severity in schizophrenia: a statistical parametric mapping study. Neuroimage. 2001;13:814–824. [DOI] [PubMed] [Google Scholar]
- 70. Das P, Lagopoulos J, Coulston CM, Henderson AF, Malhi GS. Mentalizing impairment in schizophrenia: a functional MRI study. Schizophr Res. 2012;134:158–164. [DOI] [PubMed] [Google Scholar]
- 71. Dodell-Feder D, Tully LM, Lincoln SH, Hooker CI. The neural basis of theory of mind and its relationship to social functioning and social anhedonia in individuals with schizophrenia. Neuroimage Clin. 2014;4:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee KH, Brown WH, Egleston PN, et al. A functional magnetic resonance imaging study of social cognition in schizophrenia during an acute episode and after recovery. Am J Psychiatry. 2006;163:1926–1933. [DOI] [PubMed] [Google Scholar]
- 73. Nelson BD, Bjorkquist OA, Olsen EK, Herbener ES. Schizophrenia symptom and functional correlates of anterior cingulate cortex activation to emotion stimuli: an fMRI investigation. Psychiatry Res: Neuroimaging. 2015;234:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pinkham AE, Hopfinger JB, Ruparel K, Penn DL. An investigation of the relationship between activation of a social cognitive neural network and social functioning. Schizophr Bull. 2008;34:688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pinkham AE, Loughead J, Ruparel K, Overton E, Gur RE, Gur RC. Abnormal modulation of amygdala activity in schizophrenia in response to direct- and averted-gaze threat-related facial expressions. Am J Psychiatry. 2011;168:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shin JE, Choi SH, Lee H, Shin YS, Jang DP, Kim JJ. Involvement of the dorsolateral prefrontal cortex and superior temporal sulcus in impaired social perception in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:81–88. [DOI] [PubMed] [Google Scholar]
- 77. Taylor SF, Chen AC, Tso IF, Liberzon I, Welsh RC. Social appraisal in chronic psychosis: role of medial frontal and occipital networks. J Psychiatr Res. 2011;45:526–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Thakkar KN, Peterman JS, Park S. Altered brain activation during action imitation and observation in schizophrenia: a translational approach to investigating social dysfunction in schizophrenia. Am J Psychiatry. 2014;171:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yoon JH, Minzenberg MJ, Ursu S, et al. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry. 2008;165:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Anticevic A, Hu X, Xiao Y, et al. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci. 2015;35:267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. [DOI] [PubMed] [Google Scholar]
- 82. Boyer L, Richieri R, Faget C, et al. Functional involvement of superior temporal sulcus in quality of life of patients with schizophrenia. Psychiatry Res: Neuroimaging 2012;202:155–160. [DOI] [PubMed] [Google Scholar]
- 83. Faget-Agius C, Boyer L, Richieri R, Auquier P, Lançon C, Guedj E. Functional brain substrate of quality of life in patients with schizophrenia: a brain SPECT multidimensional analysis. Psychiatry Res: Neuroimaging. 2016;249:67–75. [DOI] [PubMed] [Google Scholar]
- 84. Keefe RS, Mohs RC, Losonczy MF, et al. Characteristics of very poor outcome schizophrenia. Am J Psychiatry. 1987;144:889–895. [DOI] [PubMed] [Google Scholar]
- 85. Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF). Br J Psychiatry. 1995;166:654–659. [DOI] [PubMed] [Google Scholar]
- 86. Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Harvey PD, Raykov T, Twamley EW, Vella L, Heaton RK, Patterson TL. Validating the measurement of real-world functional outcomes: phase I results of the VALERO study. Am J Psychiatry. 2011;168:1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hogarty GE, Greenwald D, Ulrich RF, et al. Three-year trials of personal therapy among schizophrenic patients living with or independent of family, II: effects on adjustment of patients. Am J Psychiatry. 1997;154:1514–1524. [DOI] [PubMed] [Google Scholar]
- 89. Bowie CR, Grossman M, Gupta M, Oyewumi LK, Harvey PD. Cognitive remediation in schizophrenia: efficacy and effectiveness in patients with early versus long-term course of illness. Early Interv Psychiatry. 2014;8:32–38. [DOI] [PubMed] [Google Scholar]
- 90. Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70:88–96. [DOI] [PubMed] [Google Scholar]
- 91. Gaser C, Nenadic I, Buchsbaum BR, Hazlett EA, Buchsbaum MS. Ventricular enlargement in schizophrenia related to volume reduction of the thalamus, striatum, and superior temporal cortex. Am J Psychiatry. 2004;161:154–156. [DOI] [PubMed] [Google Scholar]
- 92. Bora E, Fornito A, Radua J, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127:46–57. [DOI] [PubMed] [Google Scholar]
- 93. Wolf RC, Höse A, Frasch K, Walter H, Vasic N. Volumetric abnormalities associated with cognitive deficits in patients with schizophrenia. Eur Psychiatry. 2008;23:541–548. [DOI] [PubMed] [Google Scholar]
- 94. Yamada M, Hirao K, Namiki C, et al. Social cognition and frontal lobe pathology in schizophrenia: a voxel-based morphometric study. Neuroimage. 2007;35:292–298. [DOI] [PubMed] [Google Scholar]
- 95. Antonova E, Kumari V, Morris R, et al. The relationship of structural alterations to cognitive deficits in schizophrenia: a voxel-based morphometry study. Biol Psychiatry. 2005;58:457–467. [DOI] [PubMed] [Google Scholar]
- 96. Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. [DOI] [PubMed] [Google Scholar]
- 97. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. [DOI] [PubMed] [Google Scholar]
- 98. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia. Schizophr Bull. 2000;26:119–136. [DOI] [PubMed] [Google Scholar]
- 99. Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. [DOI] [PubMed] [Google Scholar]
- 100. Eack SM, Pogue-Geile MF, Greenwald DP, Hogarty SS, Keshavan MS. Mechanisms of functional improvement in a 2-year trial of cognitive enhancement therapy for early schizophrenia. Psychol Med. 2011;41:1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Eack SM, Hogarty GE, Cho RY, et al. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia. Arch Gen Psychiatry. 2010;67:674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Eack SM, Newhill CE, Keshavan MS. Cognitive enhancement therapy improves resting-state functional connectivity in early course schizophrenia. J Soc Social Work Res. 2016;7:211–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38:1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Faludi G, Mirnics K. Synaptic changes in the brain of subjects with schizophrenia. Int J Dev Neurosci. 2011;29:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Keshavan MS, Anderson S, Pettergrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–265. [DOI] [PubMed] [Google Scholar]
- 106. Sekar A, Bialas AR, de Rivera H, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cannon TD, Chung Y, He G, et al. ; North American Prodrome Longitudinal Study Consortium. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ramsay IS, MacDonald AW. Brain correlates of cognitive remediation in schizophrenia: activation likelihood analysis shows preliminary evidence of neural target engagement. Schizophr Bull. 2015;41:1276–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Keshavan MS, Eack SM, Wojtalik JA, et al. A broad cortical reserve accelerates response to cognitive enhancement therapy in early course schizophrenia. Schizophr Res. 2011;130:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kurtz MM. Cognitive remediation for schizophrenia: current status, biological correlates and predictors of response. Expert Rev Neurother. 2012;12:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Satz P. Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology. 1993;7:273–295. [Google Scholar]
- 112. Nelson CA, III, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318:1937–1940. [DOI] [PubMed] [Google Scholar]
- 113. Leifker FR, Patterson TL, Heaton RK, Harvey PD. Validating measures of real-world outcome: the results of the VALERO expert survey and RAND panel. Schizophr Bull. 2011;37:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.