Abstract
The generation of induced pluripotent stem cells (iPSCs) and differentiation to cells composing major organs has opened up the possibility for a new model system to study adverse toxicities associated with chemotherapy. Therefore, we used human iPSC-derived neurons to study peripheral neuropathy, one of the most common adverse effects of chemotherapy and cause for dose reduction. To determine the utility of these neurons in investigating the effects of neurotoxic chemotherapy, we measured morphological differences in neurite outgrowth, cell viability as determined by ATP levels and apoptosis through measures of caspase 3/7 activation following treatment with clinically relevant concentrations of platinating agents (cisplatin, oxaliplatin and carboplatin), taxanes (paclitaxel, docetaxel and nab-paclitaxel), a targeted proteasome inhibitor (bortezomib), an antiangiogenic compound (thalidomide), and 5-fluorouracil, a chemotherapeutic that does not cause neuropathy. We demonstrate differential sensitivity of neurons to mechanistically distinct classes of chemotherapeutics. We also show a dose-dependent reduction of electrical activity as measured by mean firing rate of the neurons following treatment with paclitaxel. We compared neurite outgrowth and cell viability of iPSC-derived cortical (iCell® Neurons) and peripheral (Peri.4U) neurons to cisplatin, paclitaxel and vincristine. Goshajinkigan, a Japanese herbal neuroprotectant medicine, was protective against paclitaxel-induced neurotoxicity but not oxaliplatin as measured by morphological phenotypes. Thus, we have demonstrated the utility of human iPSC-derived neurons as a useful model to distinguish drug class differences and for studies of a potential neuroprotectant for the prevention of chemotherapy-induced peripheral neuropathy.
Keywords: Neuropathy, Stem cells, IPSC-derived neurons, Taxanes, Platinating agents, Bortezomib, Thalidomide, Goshajinkigan
Graphical abstract
1. Introduction
With an estimate of more than 13.7 million cancer survivors in the United States [Siegel, et al. 2012] there is concern regarding long-term effects of chemotherapy. Chemotherapy-induced peripheral neuropathy (CIPN) is one of the most common and potentially permanent side effects of modern chemotherapy that can result in dose reduction or cessation of therapy [Brewer, et al. 2016]. CIPN may develop in 20–40% of cancer patients as a consequence of treatment with platinum analogues (cisplatin, oxaliplatin, carboplatin), taxanes (paclitaxel, nab-paclitaxel, docetaxel), vinca alkaloids (vincristine), proteasome inhibitors (bortezomib), epothilones or other chemotherapeutics [Chu, et al. 2015, Grisold, et al. 2012]. Differences in structural and mechanistic properties among various chemotherapeutic agents contribute to variations in clinical presentation including numbness, loss of proprioceptive sense, tingling, pins and needles sensations, hyperalgesia or allodynia in the hands or feet in a stocking-glove distribution [Brewer, et al. 2016].
Mechanisms underlying CIPN include direct and indirect effects on sensory nerves such as damage to neuronal cell bodies in the dorsal root ganglion, alteration of the amplitude of the action potential or conduction velocity [Argyriou, et al. 2012, Sisignano, et al. 2014]. Whereas CIPN may be reversible for some cytotoxic drugs (e.g. taxanes), for other agents (e.g. cisplatin), the persistence of CIPN is well documented [Argyriou, et al. 2012, Avan, et al. 2015]. Wide ranges in incidence rates likely reflect not only differences in study populations, drug-related factors (e.g. dose-intensity) and potential confounders, but also genetic susceptibility [Argyriou, et al. 2012, Bhatia 2011]. Patients at high risk could consider alternative chemotherapy regimens with similar efficacy or a treatment strategy that mitigates risk by limiting the cumulative dose of the neurotoxic drug.
For the treatment of painful neuropathies, most drugs fall short of providing adequate relief [Sisignano, et al. 2014]. A systematic evaluation of 48 randomized controlled trials concluded that there are no agents that can be recommended for the prevention of CIPN [Hershman, et al. 2014]. With regard to the treatment of existing CIPN, the best available data support a moderate recommendation for treatment with duloxetine, a selective serotonin and norepinephrine reuptake inhibitor [Smith, et al. 2013]. Goshajinkigan (GJG), a traditional Japanese herbal medicine, has been shown to inhibit the progression of neuropathy or alleviate symptoms of nerve pain resulting from chemotherapy treatment with paclitaxel/carboplatin for ovarian and endometrial cancer patients [Kaku, et al. 2012], docetaxel in breast cancer patients [Abe, et al. 2013], nab-paclitaxel for breast cancer patients [Ohno, et al. 2014] and oxaliplatin in colorectal cancer patients [Nishioka, et al. 2011, Hosokawa, et al. 2012, Yoshida, et al. 2013]. In animal models, GJG has been shown to suppress various transient receptor potential channels that may mitigate the pain responses in the patient [Mizuno, et al. 2014, Kato, et al. 2014, Matsumura, et al. 2014].
Given the paucity of available treatments and increasing number of cancer survivors living with CIPN, there is an urgent need to identify a reasonable model system to identify more effective compounds supporting multiple targets and providing relief to patients undergoing treatment. Previously, we have demonstrated that induced-pluripotent stem cell (iPSC) derived neurons can be used as a preclinical model system to study CIPN [Wheeler, et al. 2015]. In the present manuscript, we extend these studies by: 1) evaluating additional platinating agents (oxaliplatin, carboplatin) and taxane analogs (docetaxel, nab-paclitaxel), a proteasome inhibitor (bortezomib), an antiangiogenic (thalidomide), and a chemotherapeutic that does not cause neuropathy (5-fluorouracil); 2) determining the effect of paclitaxel treatment on electrical activity of neurons; 3) comparing drug sensitivity in iPSC- derived cortical versus peripheral neurons; 4) and testing GJG, as a potential neuroprotectant to counteract the effects of paclitaxel, cisplatin and oxaliplatin by evaluating in neurons and cancer cell lines.
2. Methods
2.1 iCell® Neurons
Commercial human iPSC-derived neurons (iCell® Neurons) were purchased from Cellular Dynamics International (CDI, Madison, WI). The cells have been characterized by CDI to represent a pure neuronal population with >95% pan-neuronal population of GABAergic and to a lesser degree glutamatergic subtype expressing ßIII-Tubulin, MAP-2, peripherin having <1% dopaminergic neurons. iCell® Neurons were determined to express multiple ligand gated and voltage gated ion channels and be characteristically similar to neurons from the neonatal forebrain [Dage, et al. 2014].
2.2 Peri.4U Neurons
Commercial human iPSC-derived neurons (Peri.4U) were purchased from Axiogenesis (Cologne, Germany) with >90% purity and expressing ßIII-Tubulin, MAP-2, peripherin and vGLUT2. These peripheral-like neurons are not DRG nociceptive neurons. All batches of iPSC-derived neurons were tested for sterility, viability, purity, and morphology. Neurons were maintained according to the manufacturers’ protocol.
2.3 Cancer Lines
Ovarian adenocarcinoma, SKOV3 (HTB-77) and non-small cell lung cancer, A549 (CCL-185) was obtained from ATCC (Manassas, VA). Authentication of the cancer cell lines were performed by IDEXX BioResearch (Columbia, MO) for interspecies contamination and misidentification, Case # 10952-2014. This authentication was conducted by measuring short tandem repeat (STR) using the Promega CELL ID System (Madison, WI) (8 STR markers [CSF1PO, D13S317, D16S539, D5S818, D7S820, TH01, TPOX, vWA]) and amelogenin (for gender).
2.4 Compound preparations
Drug stocks were prepared and filtered using a 0.22 µM solvent resistant filter (EMD Millipore, Billerica, MA, USA) for sterility. Paclitaxel (Sigma-Aldrich, St. Louis, MO) and docetaxel (LKT Laboratories Inc., St. Paul, MN) were dissolved in DMSO to obtain a stock solution of 58.4 mM and 60 mM, respectively. Cisplatin and carboplatin (Sigma-Aldrich) were dissolved in DMSO and water, respectively, at a stock solution of 20 mM. Oxaliplatin and 5-fluorouracil (both Sigma-Aldrich), bortezomib and thalidomide (both LKT Laboratories Inc.) were dissolved in DMSO at a stock solution of 100 mM. Abraxane (nab-paclitaxel; 1 part paclitaxel/9 parts human albumin; Celegene, Summit, NJ) was purchased from University of Chicago pharmacy and dissolved in PBS to obtain a stock solution of 1 mM. Nab-paclitaxel could not be tested at 100 µM due to its insolubility at this dose. Vincristine (Sigma-Aldrich) was prepared on ice and in the dark (biological safety and room lights off, samples under cover) with PBS at a stock solution of 100 mM. Hydroxyurea (Sigma-Aldrich) was prepared by dissolving powder in PBS and filtered to obtain a stock solution of 1 M. All stock drugs were serially diluted in media for final concentrations from 1 nM to 100 µM for treatment of iCell® Neurons, 0.01 nM to 100 µM for Peri.4U neurons or 1.56 to 100 nM for treatment of the cancer lines. Vehicle controls for each drug were used at corresponding dilutions of final drug solution (0.1 – 0.2% DMSO).
Goshajinkigan (GJG), supplied by Tsumura & Co. (Tokyo, Japan), was stored desiccated at −20°C. Prior to treatment, GJG was dissolved at 10 mg/mL in PBS, sonicated for 10 minutes and diluted to obtain a 50 to 200 µg/mL GJG solution in specific media per cell line.
2.5 Drug Treatment of iCell® Neurons
iCell® Neurons were mixed with 3.3 µg/mL laminin (Sigma-Aldrich) in maintenance media containing 0.025g/L albumin (final concentration) prior to seeding on Poly-D-Lysine coated 96-well Greiner Bio-One plates (Monroe, NC, USA) in 100 µL for a density of 1.33 × 104 cells/well. Four hours following plating, iCell® Neurons were treated with chemotherapeutic drug (1 nM to 100 µM) for 48 and 72 hours and evaluated for morphological changes. For experiments with neuroprotectants, GJG was added at the same time as the chemotherapeutic agent.
2.6 Drug Treatment of Peripheral Neurons
Peri.4U were thawed using Axiogenesis thawing media and suspended in 100 µL complete Peri.4U media containing 0.025g/L albumin (final concentration) prior to seeding (final density 1.0 × 104 cells/well) onto Gel-Trex, reduced-growth factor basement membrane matrix (Life Technologies Inc., Carlsbad, CA) coated Poly-D-Lysine 96-well Greiner Bio-One plates as described above. Four hours following plating, Peri.4U cells were treated with chemotherapeutic drug (0.01 nM to 100 µM) for 48 and 72 hours and evaluated for morphological changes.
2.7 High content imaging of neuronal morphological characteristics
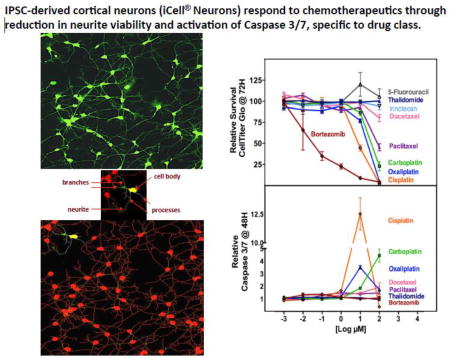
After drug treatments (48 or 72 hours), neurons were stained for 15 minutes at 37°C with 1 µg/mL Hoechst 33342 (Sigma-Aldrich) and 2 µg/mL Calcein AM (Molecular Probes, Life Technologies) then washed twice using dPBS without calcium or magnesium. Imaging was performed at 10× magnification using an ImageXpress Micro imaging device (Molecular Devices, LLC, Sunnyvale, CA) at the University of Chicago Cellular Screening Center. Supplemental Figure 1 illustrates the processing of a representative image used to quantitate individual cell measurements of mean/median/maximum process length, total neurite outgrowth (the sum of the length of all processes), number of processes, number of branches, cell body area, mean outgrowth intensity, straightness and cell numbers using the MetaXpress software Neurite Outgrowth Application Module. At least 1000 cells per dose were imaged in each of three independent experiments.
2.8 Cell viability and apoptosis assays
Cell viability was assessed by ATP measurement 72 hours post drug treatment using the Cell Titer-Glo assay (Promega, Madison, WI) and apoptosis was determined at 48 hours post drug treatment using the Caspase-Glo 3/7 assay (Promega). Three biological replicates of the viability assay and four of the apoptosis assay were performed. At least two wells per drug dose were measured in each experiment.
2.9 Multi-electrode arrays
iCell® Neurons (100,000–125,000 cells) were centered in 10 µL media containing 10 µg/mL laminin onto each well of a PEI (polyethylenimine, Sigma-Aldrich) treated 48-well multi-electrode array plate (MEA, Axion Biosystems, Atlanta, GA). The plates were placed in a humidified 37°C incubator for 30 minutes followed by addition of 300 µL pre-warmed iCell® maintenance media as described in the CDI protocol (#AP-NC120615). Sterile water (2 mL) was added to the area surrounding the wells of the 48-well MEA plate to prevent droplet evaporation and the plate was covered with a sterile, hydrated MicroClime Environmental lid (LabCyte Inc., Sunnyvale, CA), as per manufacture’s instructions. Media was exchanged with pre-warmed Neurobasal A (Gibco) containing 10% FBS (Hyclone) and 1% Penicillin-Streptomycin (Gibco) on day 1 and 50% of media exchanged on days 3 and 5 post-plating. On day 6, paclitaxel was added to obtain a final concentration of 0.01, 0.02 and 1 µM or bicuculline at 10 µM for each of 6 replicates per dose while the control wells received 0.0017% DMSO (paclitaxel) or 0.01% DMSO (bicuculine) in complete Neurobasal A media. Electrical measurements were made with the Axion Maestro multiwell, micro-electrode array (MEA; Axion Biosystems, Atlanta, GA) using the neural datastream settings (200–3000 Hz window with a spike threshold of 5.5 spikes per second and burst detector set to “Poisson surprise”). Four minute MEA recordings were made pre and post drug addition and at 4, 24 and 48 hours thereafter. Electrical measurements at each time point were normalized to the vehicle control and the change of mean firing rate calculated over time then averaged between wells. 2-way ANOVA analysis was performed to compare the drug to vehicle at each dose. At the end of the 48 hours recording, 300 µL CellTiter-Glo was added into the MEA wells, cells lysed for 30 minutes at room temperature with gentle agitation, and 150 µL was transferred to a white assay plate (Costar-Corning, Tewksbury, MA) for viability assay readings performed as described above.
2.10 Effect of Neuroprotectant ± Chemotherapeutic on cancer cell lines
A549 cells were maintained in F-12K media and SKOV3 in McCoy’s 5A. Media were supplemented with 10% FBS (Hyclone, Fisher Scientific) and 1% Penicillin-Streptomycin (Gibco, Life Technologies). Cultures were incubated in a humidified incubator at 37°C with 5% CO2. Effect of treatment on A549 cells was determined following treatment with GJG alone or with paclitaxel. Briefly, 4000 cells per well were plated in 96-well flat bottom plates (Corning) and at 24 hours treated with increasing concentrations of paclitaxel (1.56 to 100 nM) in the presence or absence of GJG at either 50, 100 or 200 µg/mL for 72 hours followed by assay of cell viability with CellTiter-Glo, as described above.
3. Results
3.1 Differential sensitivity of iCell® Neurons to various chemotherapeutic
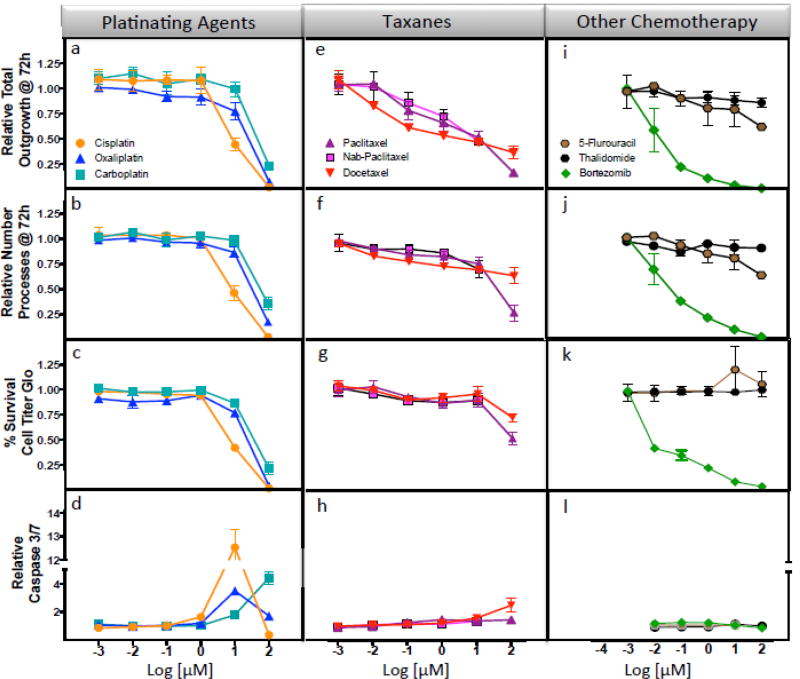
iCell® Neurons (iPSC-derived human cortical neurons) were treated with increasing concentrations of various chemotherapeutics and changes in total neurite outgrowth (sum of the length of all processes), relative number of processes, relative number of branches, relative neurite mean/median/maximum process length, cell body area and straightness of the neurites as quantified using high content image analysis. There were unique patterns of response across drug classes (Figure 1, Supplemental Figure 2). For example, the platinating agents induced changes in neurite outgrowth in a pattern indistinguishable from the effects on cell viability and caspase 3/7 activation, a measure of apoptosis (Figure 1a–d). Neurite outgrowth of cells was inhibited 55%, 23% and 1% for 10 µM cisplatin, oxaliplatin or carboplatin for 72 hours, respectively, compared to control (P<0.05, Figure 1a, Supplemental Table 1). Similarly, at this same dose, neuronal cells were most sensitive to cisplatin compared to carboplatin and oxaliplatin as measured by change in relative number of processes (P<0.005, Figure 1b), cell viability (P<0.05, Figure 1c), apoptosis (p ≤0.05, Figure 1d), as well as relative number of branches, straightness and mean outgrowth intensity (all P<0.05) with no difference in cell body area among platinating agents (Supplemental Figure 2 and Supplemental Table 1). Figure 2 images illustrate changes in the cells are most dramatic at the 10 µM clinically relevant dose for cisplatin and oxaliplatin compared to carboplatin, where some increase in neurite outgrowths can be visualized.
Figure 1. Effect of chemotherapy agents on iCell® Neurons.
iCell neurons treated with chemotherapy agents were evaluated for cell changes in relative total outgrowth, relative number of processes, cell viability and apoptosis following 48 or 72 hour treatment. With platinating agents, significant decline in (a) neurite outgrowth was most dramatic for cisplatin (orange circle) when compared to oxaliplatin (blue triangle) and carboplatin (teal square) at 10 µM drug in total neurite outgrowth, (b) relative number processes and (c) CellTiter-Glo and (d) caspase 3/7 activation (P ≤ 0.05). For the taxanes, neurons were less sensitive to docetaxel (red inverted triangle) compared to paclitaxel (lilac triangle) at the 100 µM dose for (e) relative total outgrowth, (f) relative number processes and (g) cell viability as measured by CellTiter-Glo (P<0.05); however at the same dose docetaxel resulted in significantly greater (h) caspase 3/7 activation (P<0.05). A t-test was used to compare each dose per phenotype for carboplatin or oxaliplatin against cisplatin (Supplemental Table 1) and also for docetaxel or nab-paclitaxel against paclitaxel. (Supplemental Table 2). The last panel shows the severity of the effects due to bortezomib (green diamond) to (i) total outgrowths, (j) number processes and (k) decreased cell viability without any induction of (l) caspase 3/7 activation. 5-Fluorouracil (brown hexagon) and thalidomide (black hexagon) were not expected to be neurotoxic and did not show significant effects. Each treatment represents three independent experiments and at least 1000 cells for imaged phenotype per drug dose.
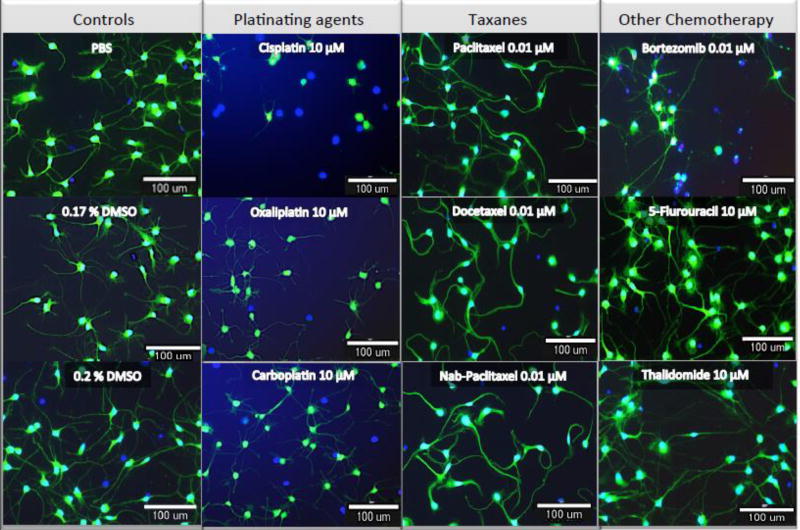
Figure 2. Representative images of iCell® Neurons to drug at clinically relevant dose.
Neurons were stained after 72 hours drug treatment (or vehicle alone) with Calcein AM to highlight the outer membranes and with Hoechst 33342 to detect the nucleus. They were imaged using 10× magnification on the ImageXpress Micro. The panel shows how the effects of the drugs were similar within the drug classes, as expected.
The pattern of neuronal sensitivity for the taxanes (paclitaxel, docetaxel or nab-paclitaxel) was observed to be a gradual reduction of neurite outgrowth parameters at increasing concentrations of drug without an effect on cell viability. Overall, the dose response curves were not significantly different as measured by morphological characteristics of the neurites for the 3 taxanes and determined by 2-way ANOVA (except for straightness) even though at specific concentrations we observed significant differences in some of these phenotypes (Figure 1e–h, Figure 2, Supplemental Figure 2 and Supplemental Table 2). In contrast, there were significant differences in cell viability comparing docetaxel or nab-paclitaxel to paclitaxel (P<0.05, Figure 1g and Supplemental Table 2). Caspase 3/7 activation (P<0.05, Figure 1h and Supplemental Table 2) showed some differential effect only when docetaxel was compared to paclitaxel.
We also evaluated three additional mechanistically distinct drugs: bortezomib, a 26S protease inhibitor, used to treat multiple myeloma and relapsed mantle cell lymphoma; thalidomide, an antiangiogenic compound also used to treat multiple myeloma; and 5-fluorouracil, used to treat colorectal cancer but does not result in neuropathy (negative control). Bortezomib produced a dramatic dose-dependent decline for all the phenotypes measured including relative total outgrowth and number of process per cell (Figure 1i,j and Figure 2) matching its decline in cell viability (Figure 1k) but with no significant effect on apoptosis as determined by lack of caspase 3/7 activation (Figure 1l). In contrast, thalidomide and 5-fluorouracil demonstrated no significant effect on relative total outgrowth, number of processes, cell viability or induction of apoptosis (Figure 1i–l). Additional morphological phenotypes (relative number of branches, max process length, relative mean outgrowth intensity) showed similar patterns of response to those seen for outgrowth measures for bortezomib, thalidomide and 5-fluorouracil (Supplemental Figure 2,i–l). Figure 2 illustrates the dramatic effects with 0.01 µM Bortezomib compared to no distinguishable effects for 1000 times higher concentrations of 5-fluorouracil and thalidomide.
3.2 Effect of paclitaxel on electrical activity of the cells
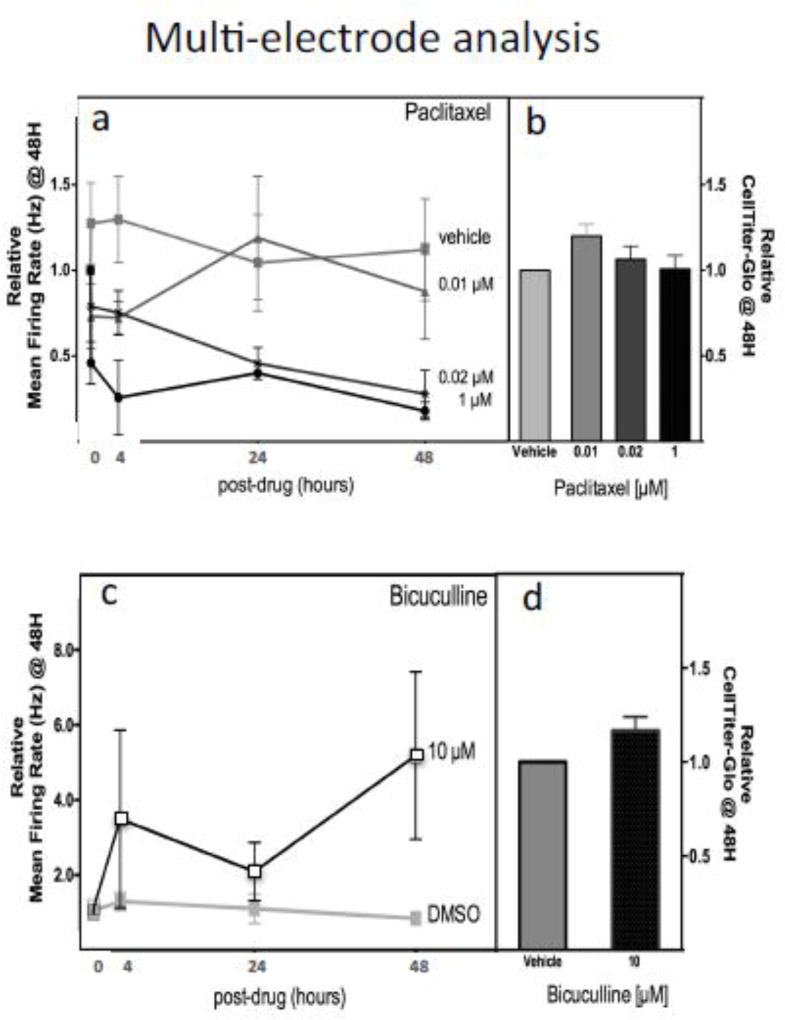
In addition to evaluating morphological changes following chemotherapeutics, neurons forming neuronal networks on multielectrode arrays can be measured using electrophysiological interrogation. We evaluated paclitaxel-induced changes in neuronal network function and observed a significant dose dependent (0.02 µM paclitaxel, P=0.0002; 1 µM paclitaxel, P<0.0001) reduction of mean firing rate (Hz) compared to vehicle control over a 48-hour period (Figure 3a) without loss of cell viability (Figure 3b). Bicuculline, a known GABA-receptor antagonist, was used as a positive control [McConnell, et al. 2012] and was shown to produce the expected increase in mean firing rate (Figure 3c) from 3–7 fold compared to vehicle control over 48 hours without significant reduction in cell viability (Figure 3d).
Figure 3. Effect of paclitaxel and bicuculline on electrical signaling and cell viability of iCell® Neurons.
Neurons were treated with paclitaxel (0, 0.01, 0.02, 1 µM) and evaluated for (a) mean firing rate of neurons at 0, 4, 24 and 48 hours using measurements from multielectrode array and (b) cell viability at 48 h measured by CellTiter-Glo. Neurons were also treated with a selective GABA antagonist, bicuculline, as a positive control for (c) mean firing rate of neurons at 0, 4, 24 and 48 hours using multi-electrode array measurements and (d) cell viability at 48 hours measured by CellTiter-Glo. Significant decline over time for 0.02 µM paclitaxel (P=0.0002), 1 µM paclitaxel (P<0.0001) and increase firing with 10 µM bicuculline (P=0.0188) was observed. Each drug was tested in 3 independent experiments for multielectrode array and 2 independent experiments for CellTiter-Glo.
3.3 Effect of chemotherapeutics on peripheral neurons
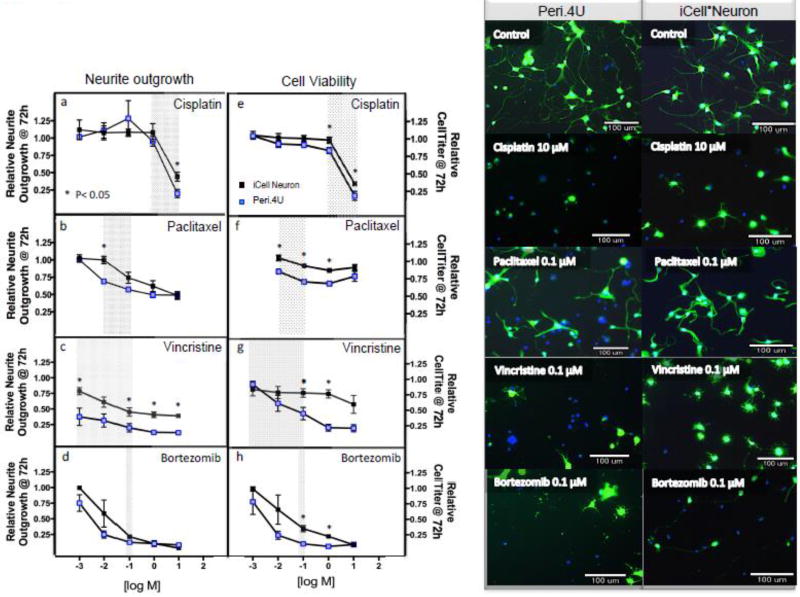
Over the course of this study, peripheral neurons became available through Axiogenesis. We, therefore, chose a subset of chemotherapeutics (cisplatin, paclitaxel, vincristine, and bortezomib, hydroxyurea, 5-fluorouracil) to compare changes in morphological and cell viability in cortical neurons versus peripheral neurons. We chose dose ranges within the clinically relevant plasma concentrations for paclitaxel [Zasadil, et al. 2014], vincristine [Sethi, et al. 1981], cisplatin [Urien and Lokiec 2004] and bortezomib as shown with gray shading on each plot (Figure 4). Within these clinically relevant concentrations, Peri.4U peripheral neurons were found to be more sensitive to cisplatin, paclitaxel and vincristine for at least one concentration as determined by neurite outgrowth and cell viability compared to cortical neurons (Figure 4 a–h). Both types of neurons were equally sensitive to bortezomib-induced relative neurite outgrowth but with increased sensitivity in cell viability for Peri.4U cells (Figure 4 d,h). A representative image of each cell type with a clinically relevant dose for each drug shows similar morphological changes after 72 hours treatment (Figure 4, right panel). Peri.4U effects with cisplatin are visualized in detail with videography in supplemental video 1. In both cortical and peripheral neurons, the effect on cell viability relative to the effect on neurite outgrowth was similar for all four chemotherapeutics (Supplemental Figure 3). Hydroxyurea, a chemotherapeutic not shown to cause CIPN, did not exhibit significant changes for any phenotypes in peripheral neurons (Supplemental Figure 4) or cortical neurons, as expected [Wheeler, et al. 2015]. In contrast 5-fluorouracil, a drug not thought to cause CIPN did produce a slight but significant decline in all phenotypes (P<0.05) except for mean outgrowth intensity for peripheral neurons (Supplemental Figure 4) but not cortical neurons (Figure 1i–l, Supplemental Figure 2).
Figure 4. Comparison of sensitivity of Peri.4U peripheral versus iCell® Cortical Neuron to chemotherapy drugs.
Peri.4U peripheral (blue square) and iCell® cortical (black square) neurons were treated with increasing doses of cisplatin, paclitaxel, vincristine and bortezomib for 72 hours and measured for relative neurite outgrowth (a–d) and CellTiter-Glo (e–h). Included is the clinically relevant plasma range for each drug as shown with gray shading on each plot. All data is representative of three independent experiments per cell line analyzed using multiple t-test. *P<0.05 between the two types of neurons at the dose specified. The right panels illustrate visually between Peri.4U and iCell® Neurons the effects with clinically relevant dose after 72 hours drug shown at 10× magnification and stained with Calcein AM and Hoechsts 33342.
3.4 Effect of potential neuroprotectants on neuronal sensitivity to chemotherapeutics
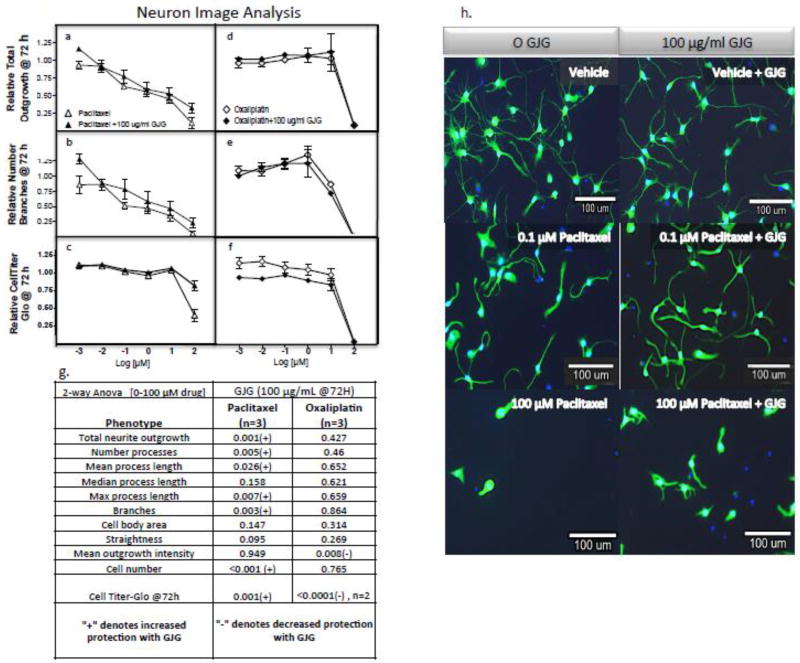
As a result of clinical evidence that GJG has utility as a neuroprotectant when combined with various neurotoxic chemotherapeutics [Kaku, et al. 2012, Abe, et al. 2013, Ohno, et al. 2014, Nishioka, et al. 2011, Hosokawa, et al. 2012, Yoshida, et al. 2013], we treated cortical neurons with paclitaxel or oxaliplatin in the presence or absence of GJG for 72 hours and evaluated neurite changes in total outgrowth and cell viability. When cortical neurons were treated with paclitaxel plus 100 µg/mL GJG for 72 hours, there was a slight but significant decrease in paclitaxel-induced neurotoxicity as measured by relative total outgrowth, mean number of processes, mean/max process length, relative number of branches, cell numbers and cell viability and representative images (Figure 5 a–c). In contrast, GJG did not protect against effects of either oxaliplatin (Figure 5 d–f) or cisplatin treatment (data not shown). Statistical analysis is shown in figure 5g with images of paclitaxel with and without paclitaxel in Figure 5h.
Figure 5. Effect of GJG (100 µg/mL) on sensitivity of iCell® Neurons to paclitaxel and oxaliplatin.
Neurons were treated with paclitaxel (open triangle), GJG plus paclitaxel (closed triangle), oxaliplatin (open diamond) and GJG plus oxaliplatin (closed diamond) for 72 hours and measured for (a, d) neurite total outgrowth, relative number of branches (b, e) and cell viability with CellTiter-Glo (c, f). All data is representative of three independent experiments per cell line analyzed using multiple t-test except duplicate experiments to evaluate cell viability for oxaliplatin ± GJG. Using 2-way ANOVA (g), significant differences are observed for GJG plus paclitaxel versus paclitaxel alone for total neurite outgrowth, branches and cell viability (P<0.005), Conversely, neurons are more sensitive to oxaliplatin in the presence of GJG compared to oxaliplatin alone when measured for relative cell viability changes (P<0.0001). Representative images (h) show distinctly the combination effect of GJG and paclitaxel in increasing a number of the outgrowth parameters.
3.5 Effect of potential neuroprotectant on cancer cell sensitivity to paclitaxel
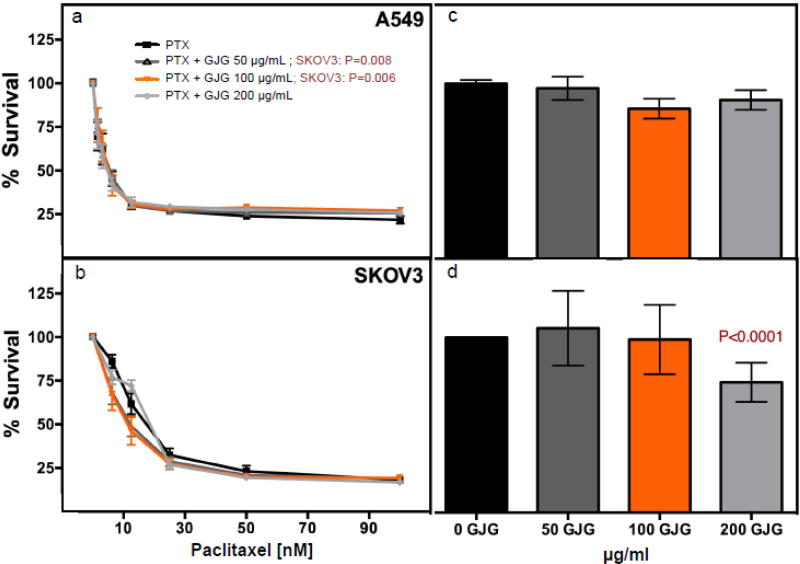
For GJG to be useful in combination with paclitaxel for patients with cancer, the “protectant” effects would need to be specific to neuronal cells and not present in tumor cells. We, therefore, evaluated the effect of GJG (50, 100 or 200 µg/mL) on sensitivity of tumor cell lines representing cancers likely treated with paclitaxel including non-small cell lung cancer (A549) and ovarian cancer (SKOV3) in combination or alone using the CellTiter-Glo assay. GJG did not alter the sensitivity of A549 cells to paclitaxel and slightly increased sensitivity of SKOV3 tumor cells at 50 and 100 µg/ml GJG with paclitaxel (P=0.0081 and 0.006, respectively) (Figure 6 a,b). GJG alone had no effect on A549 cells but a small, albeit significant effect on reducing cell viability for SKOV3 (74% viability with 200 µg/ml GJG, P<0.001) compared to no GJG treatment (Figure 6 c, d).
Figure 6.
Cancer line testing of GJG with or with out paclitaxel at 72 hours post-treatment. NSCLC (non-small cell lung cancer line) A549 and ovarian adenocarcinoma SKOV3 were tested for survival when treated with paclitaxel alone or paclitaxel + 50, 100 or 200 µg/mL GJG as measured by CellTiter-Glo. 2-way Anova analysis showed no significant protection for paclitaxel plus GJG for (a) A549 with any of the GJG doses but (b) SKOV3 was found to have increased sensitivity to paclitaxel with 50 µg/mL GJG (P=0.008) and 100 µg/mL GJG (P=0.006) plus paclitaxel. The sensitivity of the cancer lines to GJG alone revealed no significant reduction in cell viability for (c) A549 with 100 µg/ml GJG but did lower cell viability for (d) SKOV3 with 200 µg/ml (P<0.0001) compared to no GJG treatment as measured in three independent experiments and analyzed by t-test.
4. Discussion
We have demonstrated that induced pluripotent stem cell derived cortical and peripheral neurons provide new opportunities to evaluate neurotoxicity associated with chemotherapeutic agents. Differences in sensitivity to various classes of chemotherapeutics and different drugs within a class are evident. For example, platinating agents (at ≥10 µM) cause a dramatic increase in caspase 3/7 activation in neurons concomitant with a decrease in both cell viability and neurite outgrowth suggesting apoptotic cell death as a mechanism for neurotoxicity. In contrast to platinating agents, other chemotherapeutics evaluated (paclitaxel, nab-paclitaxel, docetaxel, thalidomide and 5-fluorouracil) do not exhibit effects on cell viability through an increase in caspase 3/7 activation. Taxanes had minimal effect on cell viability, yet resulted in a gradual dose dependent inhibition of neurite outgrowth parameters. Bortezomib, a targeted drug, showed the most dramatic effects with increased sensitivity as measured by neurite outgrowths and cellular viability but not in activation of caspase 3/7. Chemotherapeutic drugs not known to cause CIPN, such as 5-fluorouracil and hydroxyurea caused little, to no effect, on neurite formation or cell viability in iPSC-derived cortical neurons. Comparable results as measured by cell viability and neurite outgrowth were observed using either sensory or peripheral neurons for various neurotoxic chemotherapeutics (cisplatin, paclitaxel, vincristine and bortezomib); however peripheral neurons tended to be more sensitive to the effects of chemotherapy. GJG demonstrated some promise as a neuroprotectant for use with paclitaxel, but not with cisplatin or oxaliplatin.
Previously, in vitro studies of CIPN were performed in rat pheochromocytoma or SK-N-SH human neuroblastoma cell lines as model systems to evaluate decreases in neurite outgrowth in response to neurotoxic chemotherapy drugs, such as paclitaxel, vincristine, oxaliplatin and cisplatin [Rovini, et al. 2010, Verstappen, et al. 2004, Wheeler, et al. 2013, Takeshita, et al. 2011, Mendonca, et al. 2013]. Our knowledge of the mechanisms of CIPN has also been enhanced through studies using primary rat and mouse dorsal root ganglion neurons [Xiao, et al. 2012, Xiao, et al. 2011, Cavaletti, et al. 1995, Zheng, et al. 2012, Staff, et al. 2013]. Other models used by researchers include behavioral tests in rodents to assess sensory thresholds to nociceptive stimuli; however, the results, especially regarding cold/heat and mechanical sensitivity, have been, at times, contradictory [Authier, et al. 2009]. There is a lack of consensus regarding which behaviors best represent human manifestations of sensory peripheral neuropathy. Although insights into the mechanism of CIPN have been made through animal models, these studies have not yielded effective drugs to prevent or treat CIPN [Hershman, et al. 2014]. This is likely because rodent models do not reflect the complex genetic interactions that result in CIPN in humans; however they are complementary to neurons because animal studies allow an evaluation of behavior that cannot be studied in vitro.
In efforts to create more relevant models, human neurons have become available through reprogramming skin or blood cells into a state in which the cells have the capability to self-replicate indefinitely and differentiate into many cell types including neurons [Karagiannis and Yamanaka 2014]. Previously, human iPSC-derived neurons have been evaluated to screen for neurotoxic compounds [Ryan, et al. 2016]. Our laboratory has used commercially available iPSC-derived cortical neurons to evaluate their potential as a model of neurotoxicity [Wheeler, et al. 2015] and to functionally validate genes identified in human clinical genome wide association studies of peripheral neuropathy following treatment with paclitaxel [Wheeler, et al. 2015, Komatsu, et al. 2015], vincristine [Diouf, et al. 2015] and docetaxel [Hertz, et al. 2016]. Our work reported here extends previous studies to evaluate mechanistically distinct chemotherapeutics in iPSC-derived cortical and peripheral neurons, for effects on morphological characteristics and electrical activity. Our data suggest that this model has potential for screening neuroprotectants, a much needed area of research. A limitation of our study is that measures of cell viability, neurite outgrowth and apoptosis could be indicators of cellular response to chemotherapeutics, thus other phenotypes such as effects on neuronal hyperexcitability (increased firing in response to a noxious stimulus) may better represent clinical manifestations of peripheral neuropathy. In support of this, studies utilizing rodent sensory neurons suggest that neuronal hyperexcitability is phenotypically linked with CIPN, potentially due to potassium channel dysfunction [Zhang and Dougherty 2014]. Large-scale implementation of these human cells for high throughput characterization will require further optimization experiments. For example, the development of patient derived neurons from individuals who have experienced severe neuropathy after chemotherapeutics to identify in vitro characteristics that recapitulate clinical manifestations of peripheral neuropathy (motor, sensory, pain) would be highly beneficial for the development of appropriate preclinical assays that represent CIPN and to use in drug development. Previous work using patient-specific human iPSC-derived cardiomyocytes in which cellular consequences of drugs were shown to recapitulate the sensitivity and insensitivity to doxorubicin induced cardiotoxicity of individual patients supports this concept [Burridge, et al. 2016].
Although the use of iPSC-derived neurons offer a number of advantages because they are human derived and more closely resemble neurons than tumor cell lines, there are some limitations which should be considered [Gurwitz 2016]. They are expensive, do not grow indefinitely and require some level of expertise to use. Currently, large cohorts of genetically diverse iPSC-derived neurons for genotype-phenotype studies are not available. A limitation related to their use in studies of CIPN is that CIPN may not be entirely due to a direct effect of chemotherapy on neuronal tissue. Other cell types/tissues (e.g. vascular endothelium, cellular immunity) or serum factors (proinflammatory cytokines) may play an intermediary role in the pathophysiology of CIPN [Brewer, et al. 2016, Grisold, et al. 2012, Sisignano, et al. 2014]. These factors are missing from pure neuronal cultures in vitro. To overcome this limitation, there have been efforts to develop culture systems that integrate multiple cell types into a complex organoid structure that allow for a microenvironment that supports the formation of cell-cell interactions and cell-extracellular matrix interactions [Hunsberger, et al. 2015]. These 3D cell culture models have demonstrated closer physiological similarity over 2D cultures to in vivo conditions for voltage-gated ion channel functionality, resting membrane potentials, intracellular Ca+ dynamics, compound action potential and anatomically relevant neural growth [Huval, et al. 2015]. Although organoid cultures have great potential for high throughput screenings [Fatehullah, et al. 2016], limitations that complicate the analysis of drug toxicity and efficacy include: 1) the limited presence of stromal components, including immune cells; 2) variable drug penetration and; 3) intrinsic heterogeneity in terms of viability, size and shape [Fatehullah, et al. 2016]. The 3D organoid system is a step towards testing multiple variables in play in human disease complementing both 2D cell culture models that have utility for mechanistic studies and animal models that provide interacting organ systems.
Clinical manifestations of neuropathy differ with different classes of chemotherapeutics. For example, platinum-induced peripheral neurotoxicity can present as two clinically distinct syndromes [Brewer, et al. 2016, Argyriou, et al. 2012, Cavaletti and Marmiroli 2010]. The acute transient paresthesia in the distal extremities, which is commonly seen with oxaliplatin, usually occurs within the early phase of drug administration. In contrast, cisplatin is associated with worsening CIPN that occurs after the discontinuation of the platinum agent, a phenomenon called “coasting” [Avan, et al. 2015]. Our data with platinating agents is consistent with previous data showing that cisplatin and carboplatin harm mainly peripheral nerves and dorsal root ganglia neurons, through progressive DNA-adduct accumulation and/or oxidative stress, both resulting in apoptosis [Avan, et al. 2015].
Thalidomide and bortezomib, mechanistically distinct agents are both used to treat multiple myeloma, with about half of newly diagnosed patients experiencing neuropathy [Morawska, et al. 2015]. Thalidomide affects sensory and sensorimotor and bortezomib affects sensory neurons [Morawska, et al. 2015]. The mechanism of thalidomide is thought to be through its antiangiogenic properties explaining why in our neuronal system we did not observe a significant effect on cell viability or neurite outgrowth. Neuronal cell models that can recapitulate the multi-tissue environment such as the 3D organoid model would have utility for evaluating drugs with this mechanism. In contrast, bortezomib interferes with cellular process such as transcription, nuclear processing and transport, and cytoplasmic translation of messenger RNA in dorsal root ganglion neurons [Casafont, et al. 2010]. In our system, bortezomib exhibited the most dramatic effect on neurites outgrowth concomitant with effects cell viability, but not through caspase 3/7 apoptosis.
Multi-electrode array approaches have been proposed as a tool for detecting functional changes in electrically excitable cells, including neurons, exposed to drugs or toxins and allow use in high throughput studies [McConnell, et al. 2012]. Although there are a number of electrophysiological measures to evaluate, mean firing rate has been shown to be sensitive, robust and accurate for the identification of the effect of compounds on neural network function [McConnell, et al. 2012, Novellino, et al. 2011, Vassallo, et al. 2016, Defranchi, et al. 2011]. Recent investigations with human iPSC-derived neuronal cultures appear to be useful for high throughput screening studies [Rosenkopf 1989]. In our studies, we were able to measure significant decreases in mean firing rate in iCell® Neurons indicative of neurotoxicity for paclitaxel and, as expected, increases following treatment with bicuculline, a GABA antagonist used as a positive control [McConnell, et al. 2012]. Mean firing rate could be another phenotype to evaluate potential neuroprotectants.
There is a great need for discovery of agents to prevent peripheral neuropathy in patients at risk. One such pharmacological herbal mixture, GJG, has been shown in animal studies and small clinical studies to prevent CIPN [Schroder, et al. 2013, Tawata, et al. 1994]. GJG alleviates paclitaxel induced hyperalgesia by preventing degeneration of the ganglion cells and suppressing TRPV4 expression [Matsumura, et al. 2014], bortezomib-induced mechanical allodynia through the kappa opioid receptor [Higuchi, et al. 2015] and oxaliplatin through attenuation of the generation of oxaliplatin-induced reactive oxygen species [Kono, et al. 2015]. Our research has demonstrated the potentiality of GJG to protect human iPSC-derived cortical neurons against paclitaxel-induced neuropathy without causing decreased sensitivity of particular cancer cells (i.e. A549, SKOV3) to paclitaxel. However, we did not observe neuroprotection of oxaliplatin with GJG consistent with lack of clinical evidence from a randomized phase III study of GJG combined with oxaliplatin [Oki, et al. 2015].
In summary, human iPSC- derived neurons offer a new model for studies related to CIPN. Evaluation of morphological characteristics and/or electrical activity following chemotherapy provides potential phenotypes for high throughput screening of compounds that may prevent or treat existing peripheral neuropathy.
Supplementary Material
Highlights.
Nine drugs in 5 mechanistically distinct classes of chemotherapeutics including platinating agents (cisplatin, oxaliplatin and carboplatin), taxanes (paclitaxel, docetaxel and nab-paclitaxel), a 26s proteasome targeting drug (bortezomib), an anti-angiogenic (thalidomide) and 5-fluorouracil, demonstrated different degrees of inhibition of neurite outgrowth following exposure of two independent commercial stem cell derived neurons (iCell® Neurons vs. Peri.4U) to these agents.
Platinating agents inhibited neurite outgrowth and cell viability through caspase 3/7 activation; in contrast to taxanes, vincristine and bortezomib that inhibited neurite outgrowth with no significant changes in caspase 3/7 activation.
Agents not known to cause peripheral neuropathy in patients (5-fluorouracil) or whose mechanism of action involved anti-angiogenesis (thalidomide) did not result in inhibition of neurite outgrowth, affect cell viability or increase caspase 3/7 activation.
Commercial stem cell- derived peripheral neurons (Peri.4U) were generally more sensitive to cisplatin, paclitaxel, vincristine and bortezomib than the commercial stem cell- derived cortical neurons (iCell® Neurons) as measured by inhibition of neurite outgrowth as well as reduction in cell viability.
Goshajinkigan (GJG) protected neurons against paclitaxel-induced effects but did not alter neuronal sensitivity to oxaliplatin or cisplatin.
Paclitaxel, at nontoxic dosages, resulted in a dose dependent decrease in mean firing rate as measured using multi electrode array.
Acknowledgments
Funding Source: This work is supported, in part, by the NIH/NIGMS Pharmacogenomics of Anticancer Agents Research Grant U01 GM61393 (M.E.D.), the University of Chicago Comprehensive Cancer Center (CA14599) and Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust.
This work is supported by NIH/NIGMS Pharmacogenomics of Anticancer Agents Research Grant U01GM61393 (M.E.D.), NIH/NCI R01 CA136765 (M.E.D.) and NIH/NCI R01 CA157823 (M.E.D.). The authors would like to thank Won Huh, Megan Opferman, Haley Budigan, and Nicole Favre for their technical assistance. In addition, the authors are grateful to Carter Cliff, Rachel Llanas, Susan DeLaura, Dr. Genie Jones, and Dr. Kyle Mangan from Cellular Dynamics International and Brian Murphy and Dr. Gregory Luerman from Axiogenesis for intellectual advice in the neuron experimental protocols. The authors are also grateful to Dr. Stacie Chavel and Anthony Nicolini from Axion Biosystems for their technical assistance on the MEA maestro system.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- Brewer JR, Morrison G, Dolan ME, Fleming GF. Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol Oncol. 2016;140:176–183. doi: 10.1016/j.ygyno.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SH, Lee YJ, Lee ES, Geng Y, Wang XS, Cleeland CS. Current use of drugs affecting the central nervous system for chemotherapy-induced peripheral neuropathy in cancer patients: a systematic review. Support Care Cancer. 2015;23:513–524. doi: 10.1007/s00520-014-2408-8. [DOI] [PubMed] [Google Scholar]
- Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 2012;14(Suppl 4):iv45–54. doi: 10.1093/neuonc/nos203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82:51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Sisignano M, Baron R, Scholich K, Geisslinger G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol. 2014;10:694–707. doi: 10.1038/nrneurol.2014.211. [DOI] [PubMed] [Google Scholar]
- Avan A, Postma TJ, Ceresa C, Avan A, Cavaletti G, Giovannetti E, Peters GJ. Platinum-induced neurotoxicity and preventive strategies: past, present, and future. Oncologist. 2015;20:411–432. doi: 10.1634/theoncologist.2014-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia S. Role of genetic susceptibility in development of treatment-related adverse outcomes in cancer survivors. Cancer Epidemiol Biomarkers Prev. 2011;20:2048–2067. doi: 10.1158/1055-9965.EPI-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL. O. American Society of Clinical, Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL. O. Alliance for Clinical Trials in, Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309:1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku H, Kumagai S, Onoue H, Takada A, Shoji T, Miura F, Yoshizaki A, Sato S, Kigawa J, Arai T, Tsunoda S, Tominaga E, Aoki D, Sugiyama T. Objective evaluation of the alleviating effects of Goshajinkigan on peripheral neuropathy induced by paclitaxel/carboplatin therapy: A multicenter collaborative study. Exp Ther Med. 2012;3:60–65. doi: 10.3892/etm.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Kawai Y, Mori T, Tomida K, Kubota Y, Umeda T, Tani T. The Kampo medicine Goshajinkigan prevents neuropathy in breast cancer patients treated with docetaxel. Asian Pac J Cancer Prev. 2013;14:6351–6356. doi: 10.7314/apjcp.2013.14.11.6351. [DOI] [PubMed] [Google Scholar]
- Ohno T, Mine T, Yoshioka H, Kosaka M, Matsuda S, De Kerckhove M, De Kerckhove C, Irie J, Inoue K, Haraguchi M, Kitajima M, Shinichiro I, Tokai H, Tanaka T, Izumida R. Management of peripheral neuropathy induced by nab-paclitaxel treatment for breast cancer. Anticancer Res. 2014;34:4213–4216. [PubMed] [Google Scholar]
- Nishioka M, Shimada M, Kurita N, Iwata T, Morimoto S, Yoshikawa K, Higashijima J, Miyatani T, Kono T. The Kampo medicine, Goshajinkigan, prevents neuropathy in patients treated by FOLFOX regimen. Int J Clin Oncol. 2011;16:322–327. doi: 10.1007/s10147-010-0183-1. [DOI] [PubMed] [Google Scholar]
- Hosokawa A, Ogawa K, Ando T, Suzuki N, Ueda A, Kajiura S, Kobayashi Y, Tsukioka Y, Horikawa N, Yabushita K, Fukuoka J, Sugiyama T. Preventive effect of traditional Japanese medicine on neurotoxicity of FOLFOX for metastatic colorectal cancer: a multicenter retrospective study. Anticancer Res. 2012;32:2545–2550. [PubMed] [Google Scholar]
- Yoshida N, Hosokawa T, Ishikawa T, Yagi N, Kokura S, Naito Y, Nakanishi M, Kokuba Y, Otsuji E, Kuroboshi H, Taniwaki M, Taguchi T, Hosoi H, Nakamura T, Miki T. Efficacy of goshajinkigan for oxaliplatin-induced peripheral neuropathy in colorectal cancer patients. J Oncol. 2013;2013:139740. doi: 10.1155/2013/139740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Kono T, Suzuki Y, Miyagi C, Omiya Y, Miyano K, Kase Y, Uezono Y. Goshajinkigan, a traditional Japanese medicine, prevents oxaliplatin-induced acute peripheral neuropathy by suppressing functional alteration of TRP channels in rat. J Pharmacol Sci. 2014;125:91–98. doi: 10.1254/jphs.13244fp. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tateai Y, Ohkubo M, Saito Y, Amagai SY, Kimura YS, Iimura N, Okada M, Matsumoto A, Mano Y, Hirosawa I, Ohuchi K, Tajima M, Asahi M, Kotaki H, Yamada H. Gosha-jinki-gan reduced oxaliplatin-induced hypersensitivity to cold sensation and its effect would be related to suppression of the expression of TRPM8 and TRPA1 in rats. Anticancer Drugs. 2014;25:39–43. doi: 10.1097/CAD.0000000000000022. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Yokoyama Y, Hirakawa H, Shigeto T, Futagami M, Mizunuma H. The prophylactic effects of a traditional Japanese medicine, goshajinkigan, on paclitaxel-induced peripheral neuropathy and its mechanism of action. Mol Pain. 2014;10:61. doi: 10.1186/1744-8069-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler HE, Wing C, Delaney SM, Komatsu M, Dolan ME. Modeling chemotherapeutic neurotoxicity with human induced pluripotent stem cell-derived neuronal cells. PLoS One. 2015;10:e0118020. doi: 10.1371/journal.pone.0118020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dage JL, Colvin EM, Fouillet A, Langron E, Roell WC, Li J, Mathur SX, Mogg AJ, Schmitt MG, Felder CC, Merchant KM, Isaac J, Broad LM, Sher E, Ursu D. Pharmacological characterisation of ligand- and voltage-gated ion channels expressed in human iPSC-derived forebrain neurons. Psychopharmacology (Berl. 2014;231:1105–1124. doi: 10.1007/s00213-013-3384-2. [DOI] [PubMed] [Google Scholar]
- McConnell ER, McClain MA, Ross J, Lefew WR, Shafer TJ. Evaluation of multi-well microelectrode arrays for neurotoxicity screening using a chemical training set. Neurotoxicology. 2012;33:1048–1057. doi: 10.1016/j.neuro.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasadil LM, Andersen KA, Yeum D, Rocque GB, Wilke LG, Tevaarwerk AJ, Raines RT, Burkard ME, Weaver BA. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra243. doi: 10.1126/scitranslmed.3007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi VS, Jackson DV, Jr, White DR, Richards F, 2nd, Stuart JJ, Muss HB, Cooper MR, Spurr CL. Pharmacokinetics of vincristine sulfate in adult cancer patients. Cancer Res. 1981;41:3551–3555. [PubMed] [Google Scholar]
- Urien S, Lokiec F. Population pharmacokinetics of total and unbound plasma cisplatin in adult patients. Br J Clin Pharmacol. 2004;57:756–763. doi: 10.1111/j.1365-2125.2004.02082.x. Dailymed, Journal, pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovini A, Carre M, Bordet T, Pruss RM, Braguer D. Olesoxime prevents microtubule-targeting drug neurotoxicity: selective preservation of EB comets in differentiated neuronal cells. Biochem Pharmacol. 2010;80:884–894. doi: 10.1016/j.bcp.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Verstappen CC, Postma TJ, Geldof AA, Heimans JJ. Amifostine protects against chemotherapy-induced neurotoxicity: an in vitro investigation. Anticancer Res. 2004;24:2337–2341. [PubMed] [Google Scholar]
- Wheeler HE, Gamazon ER, Wing C, Njiaju UO, Njoku C, Baldwin RM, Owzar K, Jiang C, Watson D, Shterev I, Kubo M, Zembutsu H, Winer EP, Hudis CA, Shulman LN, Nakamura Y, Ratain MJ, Kroetz DL, Cancer, B. Leukemia Group. Cox NJ, Dolan ME. Integration of cell line and clinical trial genome-wide analyses supports a polygenic architecture of Paclitaxel-induced sensory peripheral neuropathy. Clin Cancer Res. 2013;19:491–499. doi: 10.1158/1078-0432.CCR-12-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita M, Banno Y, Nakamura M, Otsuka M, Teramachi H, Tsuchiya T, Itoh Y. The pivotal role of intracellular calcium in oxaliplatin-induced inhibition of neurite outgrowth but not cell death in differentiated PC12 cells. Chem Res Toxicol. 2011;24:1845–1852. doi: 10.1021/tx200160g. [DOI] [PubMed] [Google Scholar]
- Mendonca LM, da Silva Machado C, Teixeira CC, de Freitas LA, Bianchi Mde L, Antunes LM. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology. 2013;34:205–211. doi: 10.1016/j.neuro.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Xiao WH, Zheng H, Bennett GJ. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience. 2012;203:194–206. doi: 10.1016/j.neuroscience.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WH, Zheng H, Zheng FY, Nuydens R, Meert TF, Bennett GJ. Mitochondrial abnormality in sensory, but not motor, axons in paclitaxel-evoked painful peripheral neuropathy in the rat. Neuroscience. 2011;199:461–469. doi: 10.1016/j.neuroscience.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaletti G, Tredici G, Braga M, Tazzari S. Experimental peripheral neuropathy induced in adult rats by repeated intraperitoneal administration of taxol. Exp Neurol. 1995;133:64–72. doi: 10.1006/exnr.1995.1008. [DOI] [PubMed] [Google Scholar]
- Zheng H, Xiao WH, Bennett GJ. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp Neurol. 2012;238:225–234. doi: 10.1016/j.expneurol.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Staff NP, Podratz JL, Grassner L, Bader M, Paz J, Knight AM, Loprinzi CL, Trushina E, Windebank AJ. Bortezomib alters microtubule polymerization and axonal transport in rat dorsal root ganglion neurons. Neurotoxicology. 2013;39:124–131. doi: 10.1016/j.neuro.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authier N, Balayssac D, Marchand F, Ling B, Zangarelli A, Descoeur J, Coudore F, Bourinet E, Eschalier A. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics. 2009;6:620–629. doi: 10.1016/j.nurt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis P, Yamanaka S. The fate of cell reprogramming. Nat Methods. 2014;11:1006–1008. doi: 10.1038/nmeth.3109. [DOI] [PubMed] [Google Scholar]
- Ryan KR, Sirenko O, Parham F, Hsieh JH, Cromwell EF, Tice RR, Behl M. Neurite outgrowth in human induced pluripotent stem cell-derived neurons as a high-throughput screen for developmental neurotoxicity or neurotoxicity. Neurotoxicology. 2016;53:271–281. doi: 10.1016/j.neuro.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Wheeler HE, Chung S, Low SK, Wing C, Delaney SM, Gorsic LK, Takahashi A, Kubo M, Kroetz DL, Zhang W, Nakamura Y, Dolan ME. Pharmacoethnicity in Paclitaxel-Induced Sensory Peripheral Neuropathy. Clin Cancer Res. 2015;21:4337–4346. doi: 10.1158/1078-0432.CCR-15-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diouf B, Crews KR, Lew G, Pei D, Cheng C, Bao J, Zheng JJ, Yang W, Fan Y, Wheeler HE, Wing C, Delaney SM, Komatsu M, Paugh SW, McCorkle JR, Lu X, Winick NJ, Carroll WL, Loh ML, Hunger SP, Devidas M, Pui CH, Dolan ME, Relling MV, Evans WE. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA. 2015;313:815–823. doi: 10.1001/jama.2015.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz DL, Owzar K, Lessans S, Wing C, Jiang C, Kelly WK, Patel JN, Halabi S, Furukawa Y, Wheeler HE, Sibley A, Lassiter C, Weisman LS, Watson D, Krens SD, Mulkey F, Renn CL, Small EJ, Febbo PG, Shterev I, Kroetz D, Friedman PN, Mahoney JF, Carducci MA, Kelley MJ, Nakamura Y, Kubo M, Dorsey SG, Dolan ME, Morris MJ, Ratain MJ, McLeod HL. Pharmacogenetic Discovery in CALGB (Alliance) 90401 and Mechanistic Validation of a VAC14 Polymorphism That Increases Risk of Docetaxel-Induced Neuropathy. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Dougherty PM. Enhanced excitability of primary sensory neurons and altered gene expression of neuronal ion channels in dorsal root ganglion in paclitaxel-induced peripheral neuropathy. Anesthesiology. 2014;120:1463–1475. doi: 10.1097/ALN.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge PW, Li YF, Matsa E, Wu H, Ong SG, Sharma A, Holmstrom A, Chang AC, Coronado MJ, Ebert AD, Knowles JW, Telli ML, Witteles RM, Blau HM, Bernstein D, Altman RB, Wu JC. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med. 2016;22:547–556. doi: 10.1038/nm.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D. Human iPSC-derived neurons and lymphoblastoid cells for personalized medicine research in neuropsychiatric disorders. Dialogues Clin Neurosci. 2016;18:267–276. doi: 10.31887/DCNS.2016.18.3/dgurwitz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsberger JG, Efthymiou AG, Malik N, Behl M, Mead IL, Zeng X, Simeonov A, Rao M. Induced Pluripotent Stem Cell Models to Enable In Vitro Models for Screening in the Central Nervous System. Stem Cells Dev. 2015;24:1852–1864. doi: 10.1089/scd.2014.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huval RM, Miller OH, Curley JL, Fan Y, Hall BJ, Moore MJ. Microengineered peripheral nerve-on-a-chip for preclinical physiological testing. Lab Chip. 2015;15:2221–2232. doi: 10.1039/c4lc01513d. [DOI] [PubMed] [Google Scholar]
- Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6:657–666. doi: 10.1038/nrneurol.2010.160. [DOI] [PubMed] [Google Scholar]
- Morawska M, Grzasko N, Kostyra M, Wojciechowicz J, Hus M. Therapy-related peripheral neuropathy in multiple myeloma patients. Hematol Oncol. 2015;33:113–119. doi: 10.1002/hon.2149. [DOI] [PubMed] [Google Scholar]
- Casafont I, Berciano MT, Lafarga M. Bortezomib induces the formation of nuclear poly(A) RNA granules enriched in Sam68 and PABPN1 in sensory ganglia neurons. Neurotox Res. 2010;17:167–178. doi: 10.1007/s12640-009-9086-1. [DOI] [PubMed] [Google Scholar]
- Novellino A, Scelfo B, Palosaari T, Price A, Sobanski T, Shafer TJ, Johnstone AF, Gross GW, Gramowski A, Schroeder O, Jugelt K, Chiappalone M, Benfenati F, Martinoia S, Tedesco MT, Defranchi E, D'Angelo P, Whelan M. Development of microelectrode array based tests for neurotoxicity: assessment of interlaboratory reproducibility with neuroactive chemicals. Front Neuroeng. 2011;4:4. doi: 10.3389/fneng.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo A, Chiappalone M, De Camargos Lopes R, Scelfo B, Novellino A, Defranchi E, Palosaari T, Weisschu T, Ramirez T, Martinoia S, Johnstone AF, Mack CM, Landsiedel R, Whelan M, Bal-Price A, Shafer TJ. A multi-laboratory evaluation of microelectrode array-based measurements of neural network activity for acute neurotoxicity testing. Neurotoxicology. 2016 doi: 10.1016/j.neuro.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Defranchi E, Novellino A, Whelan M, Vogel S, Ramirez T, van Ravenzwaay B, Landsiedel R. Feasibility Assessment of Micro-Electrode Chip Assay as a Method of Detecting Neurotoxicity in vitro. Front Neuroeng. 2011;4:6. doi: 10.3389/fneng.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkopf KL. Current concepts concerning the etiology and treatment of trigeminal neuralgia. Cranio. 1989;7:312–318. doi: 10.1080/08869634.1989.11746272. [DOI] [PubMed] [Google Scholar]
- Schroder S, Beckmann K, Franconi G, Meyer-Hamme G, Friedemann T, Greten HJ, Rostock M, Efferth T. Can medical herbs stimulate regeneration or neuroprotection and treat neuropathic pain in chemotherapy-induced peripheral neuropathy? Evid Based Complement Alternat Med. 2013;2013:423713. doi: 10.1155/2013/423713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawata M, Kurihara A, Nitta K, Iwase E, Gan N, Onaya T. The effects of goshajinkigan, a herbal medicine, on subjective symptoms and vibratory threshold in patients with diabetic neuropathy. Diabetes Res Clin Pract. 1994;26:121–128. doi: 10.1016/0168-8227(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Yamamoto S, Ushio S, Kawashiri T, Egashira N. Goshajinkigan reduces bortezomib-induced mechanical allodynia in rats: Possible involvement of kappa opioid receptor. J Pharmacol Sci. 2015;129:196–199. doi: 10.1016/j.jphs.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Kono T, Suzuki Y, Mizuno K, Miyagi C, Omiya Y, Sekine H, Mizuhara Y, Miyano K, Kase Y, Uezono Y. Preventive effect of oral goshajinkigan on chronic oxaliplatin-induced hypoesthesia in rats. Sci Rep. 2015;5:16078. doi: 10.1038/srep16078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki E, Emi Y, Kojima H, Higashijima J, Kato T, Miyake Y, Kon M, Ogata Y, Takahashi K, Ishida H, Saeki H, Sakaguchi Y, Yamanaka T, Kono T, Tomita N, Baba H, Shirabe K, Kakeji Y, Maehara Y. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo-controlled, double-blind, randomized phase III study. Int J Clin Oncol. 2015;20:767–775. doi: 10.1007/s10147-015-0784-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.