Abstract
Cancer cells maintain telomere length equilibrium to avoid senescence and apoptosis induced by short telomeres, which trigger the DNA damage response. Limiting the potential for telomere maintenance in cancer cells has been long been proposed as a therapeutic target. Using an unbiased shRNA screen targeting known kinases, we identified bromodomain-containing protein 4 (BRD4) as a telomere length regulator. Four independent BRD4 inhibitors blocked telomere elongation, in a dose-dependent manner, in mouse cells overexpressing telomerase. Long-term treatment with BRD4 inhibitors caused telomere shortening in both mouse and human cells, suggesting BRD4 plays a role in telomere maintenance in vivo. Telomerase enzymatic activity was not directly affected by BRD4 inhibition. BRD4 is in clinical trials for a number of cancers, but its effects on telomere maintenance have not been previously investigated.
INTRODUCTION
Telomere length maintenance is required for long-term division of cells. When telomeres become short, they no longer protect chromosome ends and cells undergo senescence or apoptosis (1–3). This requirement for telomere length maintenance suggested that blocking telomere elongation might block the growth of cancer cells in some settings (4). Telomerase elongates telomeres and maintains a telomere length equilibrium that prevents telomeres from becoming critically short (5). In the setting of insufficient telomerase or other telomere gene mutations in humans, short telomere syndromes manifest as bone marrow failure, immunodeficiency, enteropathy, pulmonary fibrosis and emphysema (6,7). Conversely, most cancer cells upregulate telomerase (8–10). Recent experiments have shown promoter mutations that increase the expression of the telomerase catalytic component TERT are very common in many cancers (11,12). Indeed, germline mutations in the TERT promoter, or POT1, predispose to familial melanoma, glioma or CLL respectively (13–15). This suggests that long telomeres may be a risk factor for cancer (7).
Telomerase inhibitors have been proposed as potential cancer therapeutics for over 25 years (4,10,16,17). BIBR1532 is a potent inhibitor of telomerase in cell extracts and in cell culture (18), but has limited solubility (19,20) and has not progressed to clinical trials. Imetelstat is an anti-sense molecule that inhibits telomerase by binding to the intrinsic RNA template. Imetelstat is an effective in vitro telomerase inhibitor and shortens telomeres in human cultured cells (21,22). However, it has failed phase II clinical trials and the mode of action in some malignancies may be due to off-target effects (21,23,24). With our current understanding of short and long telomere syndromes, and a growing understanding of cancers that rely on telomerase, it is possible to revisit the concept of targeting telomere shortening in cancer with a more nuanced approach.
Several groups identified telomerase regulators through direct screening of compounds or genes that block telomerase enzyme activity (25–27). We took a different approach by identifying pathways that might block telomere elongation without direct inhibition of telomerase activity or telomerase transcription. Telomere elongation is regulated by shelterin proteins (28–30) and by post-translational modification (31–35). To identify kinase pathways that might regulate telomere length, we designed an unbiased shRNA screen against kinases.
In this screen, we identified BRD4 as a novel positive regulator of telomere length. BRD4 is a BET family protein that contains a bromodomain, which binds to acetylated lysines (36). It also has histone acetyl transferase activity (37) and kinase activity (38). BRD4 is a pleiotropic protein with roles in cell cycle regulation, chromatin structure and transcriptional regulation (39). BRD4 has not previously been implicated in telomere length regulation. Because several BRD4 inhibitors are currently in clinical trials for cancer, understanding their potential effects on telomere length will be important to discover the mechanism of action and potential side effects of BRD4 inhibition.
MATERIALS AND METHODS
Cell culture
HeLa cells and mouse fibroblasts were cultured in Dulbecco's modified Eagle's medium (Gibco) with 1% penicillin/streptomycin/glutamine and 10% heat-inactivated fetal bovine serum (Gibco). Drugs were dissolved in dimethyl sulfoxide (DMSO) and added to cell culture media at indicated concentrations.
Lentiviral shRNA kinase library
Decode Pooled Human GIPZ Kinase Library (GE Dharmacon RHS6078) was used to screen for telomere length regulators. This library contained 4675 shRNAs, in pGIPZ lentiviral vectors, directed against 706 kinase and kinase related genes. HeLa cells were transduced with 500-fold representation of the library at an multiplicity of infection (MOI) of 0.1. Experiments were performed in triplicate. After transduction, cells were cultured cells for 7 weeks, at a minimum of 500-fold representation, to allow changes in telomere length over many cell divisions.
Telomere flow-FISH and fluorescence activated cell sorting
We adapted a version of telomere flow-FISH (40) to sort cells with short telomeres. 4 × 107 cells were fixed in 1.5% paraformaldeyhyde for 10 minutes then dehydrated in 100% methanol overnight. Cells were washed with phosphate buffered saline (PBS), hybridized with probe and washed, as described (40). Cells were resuspended at a concentration of 5 × 106/ml in modified propidium iodide staining solution, consisting of PBS with 0.1% Triton X-100, 200 μg/ml RNase A (Sigma), 20 μg/ml propidium iodide (Sigma) and 0.1% sodium dodecyl sulfate (SDS, Bio-Rad). SDS was found to inhibit aggregation of fixed cells during cell sorting. Cells were incubated for 30 min at room temperature, protected from light. Sorting was performed on a MoFlo cell sorter (Becton Dickinson). Cells were first gated on the G1 cell cycle peak to ensure only cells with 2N ploidy were measured. Telomere FITC signal was measured in the linear range, and the cells with the 7% shortest telomeres were collected. A minimum of 2.5 million cells were collected for each fraction. Unsorted cells were collected as a control.
DNA preparation, amplification and sequencing
Genomic DNA was harvested from sorted cells by phenol–chloroform extraction (41). shRNA insert was amplified from common flanking regions by polymerase chain reaction (PCR) as described (42). PCR primers contained indices for multiplexing (42). PCR products were purified with Agencourt AMPure XP Beads (Beckman Coulter), and then sequenced with single end 50 bp reads using Illumina HiSeq 2000.
Bioinformatic analysis
Illumina reads were aligned to reference sequences using Bowtie2 as described (42,43), and enriched genes were determined by evaluating three biological replicates with MAGeCK analysis (44). MAGeCK first normalizes samples based on median number of reads per sample. Next, it models a relationship for mean number of reads versus variance, based on shRNA read counts across all replicates. Finally, MAGeCK ranks genes by taking into account the enrichment and P-value of each particular shRNA. Since there are multiple shRNAs per gene, genes with multiple highly ranked shRNAs are ranked higher than those with just a few. Statistically significant enriched genes were inspected manually, and prioritized for further characterization based on MAGeCK rank, availability of chemical inhibitors and known role in potential telomere pathways such as DNA damage, cell cycle regulation, checkpoint regulation, DNA replication and chromatin modification.
Small molecular inhibitors
KU-55933 (R&D Systems 3544) was used to inhibit Ataxia Telangiecietasia Mutated (ATM). JQ1 (Selleckchem S7110), OTX015 (Selleckchem S7360), I-BET151 (Selleckchem S2780) and MS436 (Selleckchem S7305) were used to inhibit BRD4. Additional inhibitors tested are listed in Supplementary Table S1.
Secondary screen for inhibitors of telomere elongation
Immortalized CAST/EiJ fibroblasts were treated with drug or vehicle for 24 hours, then transduced with the SVA lentivirus at MOI = 0.5. SVA is a lentiviral vector encoding mTERT and mTR, the catalytic protein and RNA components of telomerase (45). Cells were cultured in the presence of drug or DMSO for 6 days and genomic DNA was isolated at days 2 and 6 post transduction for Southern blot analysis of telomere length as described (46).
Genomic DNA was digested with MseI (NEB), separated on a 0.7% agarose in 1× TAE (40 mM Tris, 20 mM Acetate, 1 mM ethylenediaminetetraacetic acid (EDTA), pH 8.6), denatured in 0.5 M NaOH/1.5 M NaCl and neutralized in 1.5 M NaCl/0.5 M Tris–HCl pH 7.4. DNA was transferred in 20× SSC (Sodium Sodium Citrate: 3M NaCl, 0.34M NaCitrate) to a nylon membrane (Amersham Hybond N+), crosslinked by UV (Stratagene), prehybridized for 2 h in Church buffer (0.5M sodium phosphate, pH7.2, 7% SDS, 1% bovine serum albumin, 1 mM EDTA) and hybridized overnight at 65°C with radiolabeled telomere fragment, generated from JHU821 as described (47), and radiolabeled 2-log DNA ladder (NEB). After washing, the nylon membranes were exposed to Storage Phosphor Screens (GE Healthcare) and scanned on a Storm 825 imager (GE Healthcare). The images were converted using Adobe Photoshop CS6 and adjusted for contrast using the ‘curves’ feature within the software. The 2-log ladder marker shown on the left of the Southern blots indicates the sizes in kb. Image processing software, ImageQuant (GE Healthcare), was used to generate densitometry of Southern blot lanes by graphing pixel counts versus DNA migration distance. The location of the highest pixel counts of each lane is marked on the blots in the Supplementary Figure S1.
Direct telomerase activity assay
Direct telomerase assay was performed on cell lysates as described with modifications (45,48). For stable hTR overexpression, pBluescript II SK(+)U1-hTR (49) was cloned into a FUGW-derived lentiviral backbone where Puro was cloned into the GFP site with BamHI and EcoRI. 293TREx/FRT cells were transduced with the FUPW-hTR lentivirus and selected for clonal hTR overexpressing cells. Polycistronic TERT, POT1 and TPP1 was flipped into a single genomic FRT site using the Flp-in system (Invitrogen). These cells were treated with DMSO, 5 μM IBET151, 0.5 μM JQ1, 25 μM MS436 or 2.5 μM OTX015 for 48 h before lysis in 1× CHAPS buffer. Protein was quantified by Bradford assay (Bio-Rad). Equal amounts of protein were incubated with a5 primer (50) and 0.5 mM dTTP, 0.5 mM dATP, 2.92 μM dGTP and 0.33 μM α32P-dGTP (Perkin Elmer) in telomerase buffer (50 mM Tris-Cl, 30 mM KCl, 1 mM MgCl2, 1 mM spermidine). Reactions were incubated for 15 min at 30°C, terminated with stop buffer (20 mM EDTA, 10 mM Tris) spiked with an end-labeled 18-mer purification control and telomere products were isolated by phenol–chloroform extraction and ethanol precipitation. Reaction products were separated on a 10% polyacrylamide/7M urea sequencing gel, dried and imaged on a Storm 825 imager (GE Healthcare). Telomere repeats were quantitated in ImageQuant TL. Individual telomere repeat bands (+1, +2, etc.) were normalized to the number of radioactive dGTPs incorporated and to the other bands in each lane using the decay method as described in detail in (51). The natural log of the resulting value is plotted against the repeat number and fitted to a linear regression. The slope of this line, m, indicates telomerase processivity. Telomerase catalytic activity was calculated by measuring the intensity of the first telomere repeat relative to the loading control.
Metaphase FISH analysis
Metaphase Fluorescent in situ hybridization (FISH) analysis of signal free ends (SFE) and chromosome fusions was carried out by a method similar to that described previously (52). Cultures were arrested in with Karyomax colcemid (Invitrogen) for 6 h. The cells were washed in PBS, swelled with 0.075M KCl at 37°C for 15 min and fixed in methanol:acetic acid (3:1). Cell suspensions were then dropped onto chilled slides and dried overnight. FISH was performed using a Cy3-labeled (CCCTAA)3 PNA oligonucleotide (PE Biosystems). Metaphase spreads were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Images were acquired using a Nikon Eclipse NI-E microscope and NIS Elements software. Images were examined in Adobe Photoshop. Chromosome ends were scored as signal free when no telomere repeats were detected, even after overexposure.
RESULTS AND DISCUSSION
BRD4 identified in screen for telomere length regulators
To identify new pathways that regulate telomere length, we carried out an unbiased screen of shRNAs that target kinases in HeLa cells. Kinases are attractive therapeutic targets (53), and there is evidence that they affect telomere length. Loss of Tel1 kinase in yeast, or ATM, its homolog in mammals, results in telomere shortening (33,45,54). CDK1 activity is also required for telomere elongation in yeast (31,32). We screened a lentiviral library of shRNAs, targeting 706 kinase and kinase related genes (GE Dharmacon RHS6078) (42). HeLa cells were transduced with the library in triplicate and cultured for 7 weeks to allow for telomere length changes after multiple divisions. We adapted a flow-FISH protocol (40) to hybridize telomere probe to telomeres, in intact cells, and then separate cells based on telomere length, using fluorescence activated cell sorting (FACS). Cells with the shortest telomeres were collected and shRNA inserts were amplified and sequenced. The genes enriched in the short population, compared to the unsorted population, were identified by aligning to reference sequences with Bowtie2 (43) and ranking gene enrichment with MAGeCK (44). The three biological replicates did not show a high degree of concordance in gene enrichment. In retrospect, the inability to do successive enrichment, because flow-FISH requires cell fixation, significantly decreased the power of the selection. However, we chose to follow up on genes that were highly enriched in at least one of the samples and prioritized those that were involved in nuclear localization, cell cycle regulation, checkpoints, DNA replication and chromatin modification. Selected candidates for which small molecule inhibitors were available (Supplementary Table S1) were tested in a secondary screen for inhibition of telomere elongation.
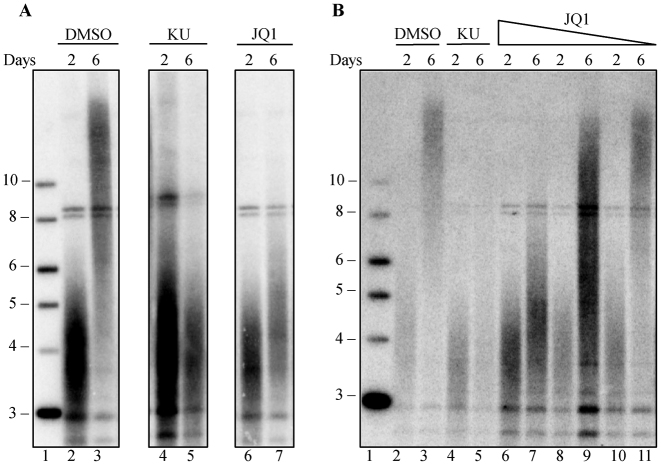
We previously found that inhibition of ATM kinase with the specific inhibitor KU55933 blocked telomere elongation when telomerase was overexpressed (45). Mouse fibroblasts, from a CAST/EiJ strain with short telomeres, transduced with a lentivirus, SVA, encoding both mTR and mTERT, showed robust telomere elongation after 6 days. The ATM inhibitor KU55933 blocked this elongation (45) (Figure 1A). We used this blockage of telomere elongation as a secondary screen to evaluate selected hits from the flow-FISH screen. We tested 31 inhibitors to candidates genes from our screen for their ability to block telomere elongation (Supplementary Table S1). We found that the BRD4 inhibitor, JQ1 (55), effectively blocked telomere elongation (Figure 1A) in a dose-dependent manner from 3.3 to 33 nM (Figure 1B). The degree of inhibition was similar to that seen with the inhibition of ATM by KU55933 (45).
Figure 1.
JQ1 blocks telomere elongation in a dose-dependent manner. Mouse fibroblasts were transduced with SVA lentivirus, encoding for mTERT and mTR and cultured for six days. (A) Southern blot of mouse fibroblast telomeric DNA, at days 2 and 6 post-SVA transduction, grown in the presence of DMSO (lane 2–3), 10 μM KU-55933 (lane 4–5) or 0.1 μM JQ1 (lane 6–7). Lane 1 shows the 2-log ladder marker (NEB) sizes marked in kilobases. (B) Southern blot of mouse fibroblast telomeric DNA, at days 2 and 6 post-SVA transduction, in the presence of DMSO (lane 2–3), 10 μM KU-55933 (lane 4–5) or decreasing concentrations of 33 nM JQ1 (lane 6–7), 11 nM JQ1 (lane 8–9) or 3.3 nM JQ1 (lane 10–11). Lane 1 shows the 2-log ladder marker (NEB) sizes marked are in kilobases.
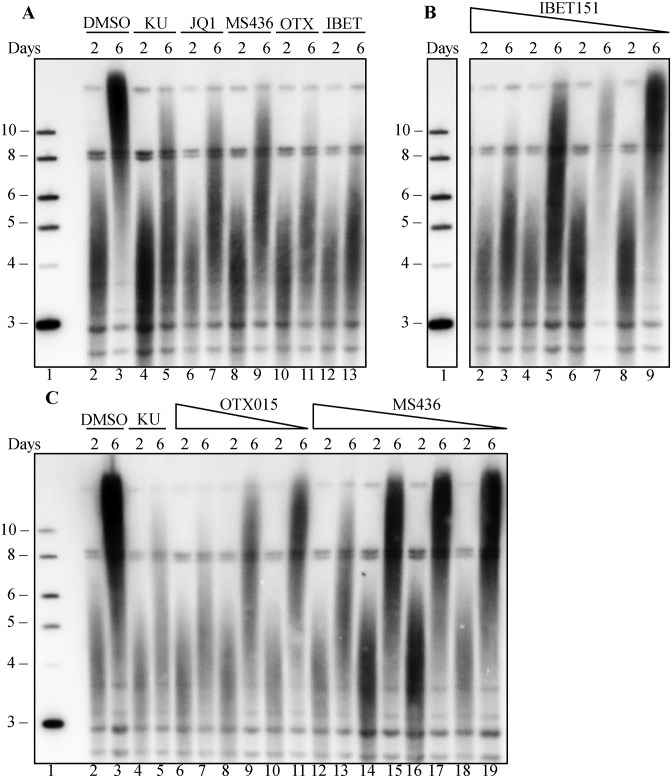
We next tested the effects of three additional BRD4 inhibitors, OTX015, I-BET151 and MS436 on telomere elongation. All of these inhibitors blocked telomere elongation induced by telomerase overexpression in a dose-dependent manner (Figure 2A–C), indicating inhibition is specific to BRD4. All four of these inhibitors target the bromodomain of BRD4 and interfere with its binding to acetyl lysine, thus blocking chromatin binding of BRD4 (56). The inhibitors, however, have distinct mechanisms of inhibition. JQ1, OTX015 and I-BET151 mimic acetyl lysine by directly forming hydrogen bonds with an asparagine in the binding pocket of BRD4 (57). In contrast, MS436 is structurally distinct, and sterically inhibits protein access to the binding site (57). Since multiple BRD4 inhibitors with independent mechanisms all blocked telomere elongation, it is likely that the effect we observed is due to BRD4 inhibition, rather than common off target effects of these inhibitors. Furthermore, the effect on telomere elongation is not likely due to direct transcriptional regulation of telomerase components since, in this screen, mTERT and mTR are highly overexpressed from an exogenous promoter, which is unaffected by BRD4 inhibition.
Figure 2.
Three additional BRD4 inhibitors block telomere inhibition in a dose-dependent manner. Mouse fibroblasts transduced with SVA lentivirus were treated with four different BRD4 inhibitors: JQ1, IBET151, MS436 OTX015. (A) Southern blot of mouse fibroblast telomeric DNA, at days 2 and 6 post SVA transduction, grown in the presence of DMSO (lane 2–3), 10 μM KU-55933 (lane 4–5), 0.1 μM JQ1 (lane 6–7), 5 μM MS436 (lane 8–9), 0.5 μM OTX015 (lane 10–11) or 1 μM IBET151 (lane 12–13). (B) Dose dependence of IBET151. Southern blot of mouse fibroblast telomeric DNA, at days 2 and 6 post SVA transduction, in the presence of 1 μM (lane 2–3), 0.5 μM (lane 4–5), 0.25 (lane 6–7) or 0.125 μM (lane 8–9) IBET151. (C) Dose dependence of OTX015 and MS436. Southern blot of mouse fibroblast telomeric DNA, at days 2 and 6 post SVA transduction, in the presence of DMSO (lane 2–3), 10 μM KU-55933 (lane 4–5), 250 nM (lane 6–7), 125 nM (lane 8–9) or 62.5 nM OTX015 (lane 10–11), 5 μM (lane 12–13), 2.5 μM (lane 14–15), 1.25 μM (lane 16–17) or 0.625 μM (lane 18–19) MS436. Lane 1 in all panels shows the 2-log ladder marker (NEB), sizes marked are in kilobases. In Panel B, other lanes between the marker and IBET treated lanes were removed.
BRD4 does not affect telomerase enzymatic activity
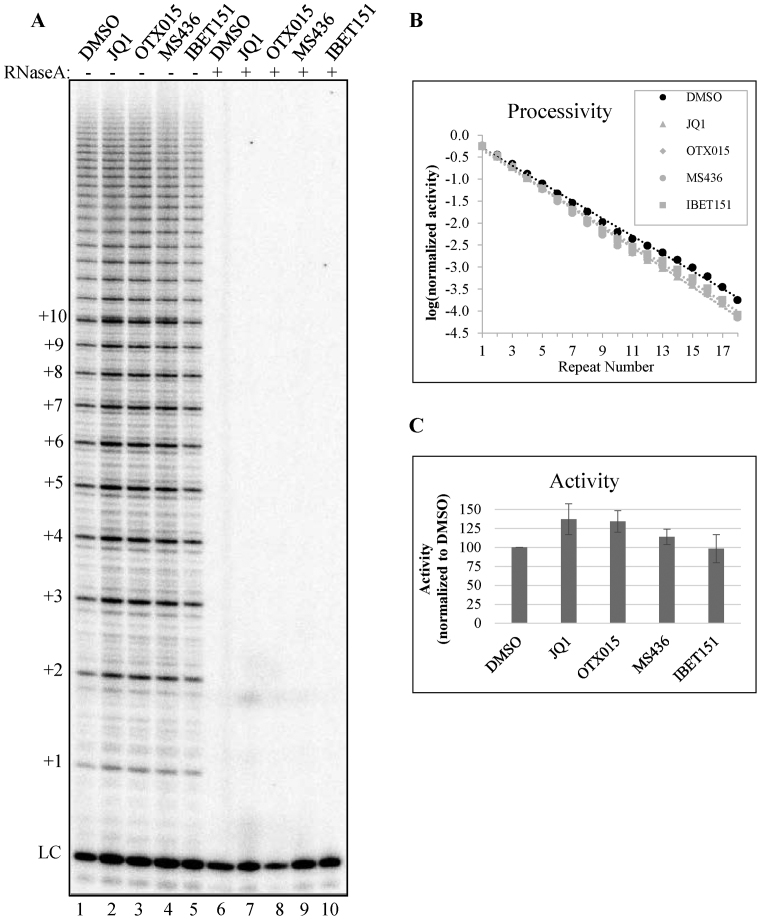
To examine whether the BRD4 inhibitors had a direct effect on telomerase enzyme activity, we used a quantitative direct activity assay (45). 293T cells overexpressing telomerase components were treated with the highest tolerated dose of each of the four BRD4 inhibitors JQ1, OTX015, I-BET151 and MS436. There was no effect of any of these compounds on telomerase activity or processivity in vitro (Figure 3A and B), suggesting that BRD4 inhibitors do not block telomere elongation through direct inhibition of telomerase enzymatic activity.
Figure 3.
BRD4 inhibition does not affect telomerase enzyme activity. (A) Direct telomerase assay using whole cell lysates of 293TRex cells overexpressing hTR, TERT, POT1 and TPP1, which were treated with DMSO (lane 1), 0.5 μM JQ1 (lane 2), 2.5 μM OTX015 (lane 3), 25 μM MS436 (lane 4) or 5 μM IBET151 (lane 5). Lanes 6–10 show extracts pretreated with RNase A to show activity due to RNase sensitive telomerase enzyme. (B) Quantification of telomerase processivity and (C) Quantification of telomerase catalytic activity as described in ‘Materials and Methods’ section. Values in (B) and (C) are averages of two technical replicates. Error bars in (C) represent the standard deviation.
BRD4 inhibition has pleiotropic phenotypes on the cell cycle by affecting its role as a mitotic bookmark (58), a transcriptional scaffold (58,59) or altering its HAT activity (37). The mechanism by which BRD4 inhibition blocks telomere elongation is not yet clear. However, it is unlikely that telomerase transcription is a major factor as BRD4 inhibitors block elongation when telomerase is overexpressed from an exogenous promoter. In addition, we have shown that telomerase activity is not affected by the inhibitors. Inhibition of BRD4 may either block telomerase access to the telomere, modify a telomere binding protein or regulate levels of telomere binding proteins.
BRD4 inhibition shortens telomeres in long-term growth assays
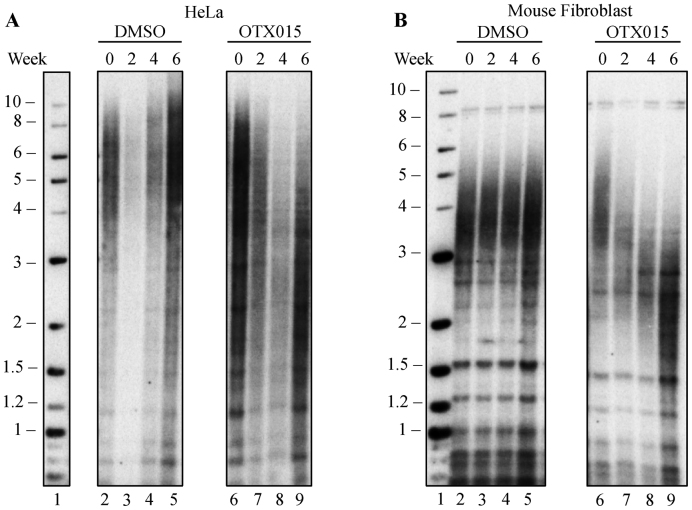
The short-term lentiviral screen is a powerful, rapid method to identify inhibitors that block telomere elongation. To examine whether inhibition of BRD4 causes telomere shortening in a more physiological setting, we cultured cells for 6 weeks and examined telomere length by Southern blot. We chose OTX015 to inhibit BRD4 because it showed the greatest inhibition in blocking telomere elongation. OTX015 is also of particular interest because it is currently being tested in clinical trials to treat acute leukemia (60), lymphoma and multiple myeloma (61). Treatment with OTX015 caused significant telomere shortening in both human HeLa cells (Figure 4A), and mouse CAST/EiJ fibroblasts (Figure 4B). Densitometry scanning of the lanes was used to identify the midpoint of the length distribution (Supplementary Figure S1). DMSO-treated cells showed no change in telomere length over the same period.
Figure 4.
BRD4 inhibition causes telomere shortening in human and mouse cells in culture. (A) Southern blot of telomeric DNA from HeLa cells treated with DMSO (lane 2–5), or 2.5 μM OTX015 (lane 6–9) for 6 weeks, with samples taken at 2, 4 and 6 weeks of treatment. (B) Southern blot of telomeric DNA from mouse fibroblast cells, which were treated with DMSO (lane 2–5), or 0.5 μM OTX015 (lane 6–9) for 6 weeks, with samples taken at 2, 4 and 6 weeks of treatment.
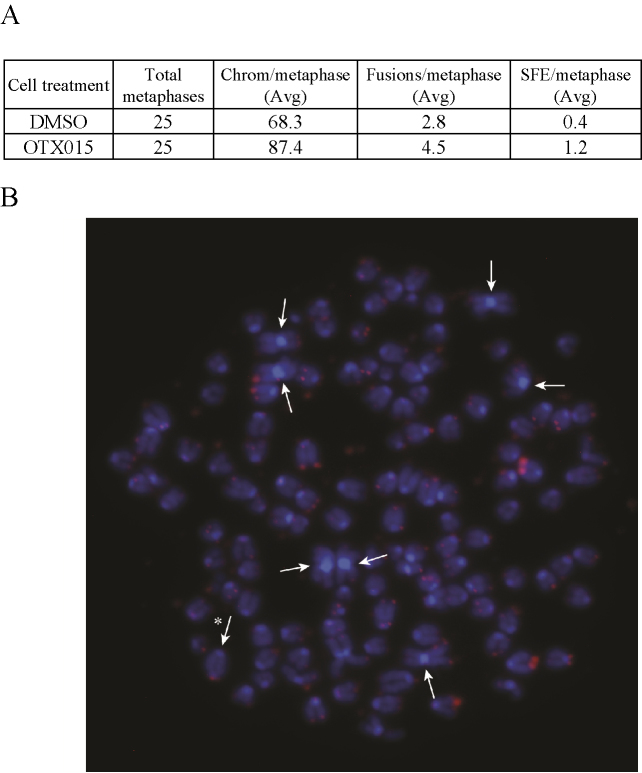
To determine whether the telomere shortening seen with OTX015 was significant enough to cause telomere dysfunction, we cultured the mouse fibroblasts from the 6 week time point shown in Figure 4B and examined metaphases from cultures treated with OTX015 or DMSO. As we have documented previously, the mouse fibroblasts become aneuploid and show some chromosome fusions upon culturing even with no treatment (62). Comparison between the OTX015 and DMSO treatment showed an increase in fusions from 2.8 to 4.5 per metaphase and an increase in SFE from 0.4 to 1.2 (Figure 5). This suggests that the degree of telomere shortening caused by BRD4 inhibition is sufficient to cause telomere dysfunction.
Figure 5.
BRD4 inhibition increases chromosome fusions. Mouse fibroblast cells shown in Figure 4B from 6 weeks of growth were further grown in OTX015 or DMSO and metaphases were processed for telomere FISH. (A) There was a significant increase in fusions per metaphase from 2.8 to 4.5 (P = 0.003 t-test) and a significant increase in signal free ends from 0.4 to 1.2 (P = 0.016 t-test). (B) Representative Metaphase showing chromosome fusions. Arrows indicate chromosome p-arm fusions and asterisk indicates a q-arm fusion.
BRD4 translocations are linked to cancer, and BRD4 inhibition reduces cell proliferation in acute myeloid leukemia (63,64), potentially by blocking BRD4 mediated transcription of c-MYC. Our finding, that BRD4 inhibition shortens telomeres in human and mouse cells in culture, suggests that part of the mechanism by which BRD4 inhibitors block cancer cell growth (64–66) may be through telomere shortening.
BRD4 inhibitors are currently in early phase clinical trials for treatment of hematopoietic cancers, including acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), multiple myeloma and lymphoma (67). If telomere shortening also occurs in vivo in a clinical trial setting, telomere shortening might accentuate the anti-cancer effects of BRD4. BRD4 also affects immune cell function through its interaction with NF-κB (68), and BRD4 inhibitors have been proposed as anti-inflammatory agents, including in liver fibrosis and idiopathic pulmonary fibrosis (69,70). However, these approaches should be carefully considered, because short telomeres in humans lead to telomere syndromes that can manifest as bone marrow failure, pulmonary fibrosis, liver fibrosis and other diseases (71–73). Telomere shortening in either cancer clinical trials or fibrosis may exacerbate underlying short telomere syndromes. Stratifying patients by telomere length before treatment may help identify individuals who might be at risk from side effects caused by further telomere shortening.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Hao Zhang at the Johns Hopkins School of Public Health Cell Sorting Facility, and John Weger at the UC Riverside High-Throughput Sequencing Center for many helpful discussions on the design and application of FACS and Illumina sequencing.
Footnotes
Present address: Stella S. Lee, Office of the Commissioner, Food and Drug Administration, Silver Spring, MD 20993, USA.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Institute of Health (NIH) [R37AG009383, 1R35CA209974 to C.W.G.]; Johns Hopkins Telomere Center. Funding for open access charge: NIH [R37AG009383, 1R35CA209974].
Conflict of interest statement. None declared.
REFERENCES
- 1. d’Adda di Fagagna F., Reaper P.M., Clay-Farrace L., Fiegler H., Carr P., Von Zglinicki T., Saretzki G., Carter N.P., Jackson S.P.. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003; 426:194–198. [DOI] [PubMed] [Google Scholar]
- 2. IJpma A., Greider C.W.. Short telomeres induce a DNA damage response in Saccharomyces cerevisiae. Mol. Biol. Cell. 2003; 14:987–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Enomoto S., Glowczewski L., Berman J.. MEC3, MEC1, and DDC2 are essential components of a telomere checkpoint pathway required for cell cycle arrest during senescence in Saccharomyces cerevisiae. Mol. Biol. Cell. 2002; 13:2626–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greider C.W. Telomeres, telomerase and senescence. Bioessays. 1990; 12:363–369. [DOI] [PubMed] [Google Scholar]
- 5. Greider C.W., Blackburn E.H.. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985; 43:405–413. [DOI] [PubMed] [Google Scholar]
- 6. Armanios M., Chen J.L., Chang Y.P., Brodsky R.A., Hawkins A., Griffin C.A., Eshleman J.R., Cohen A.R., Chakravarti A., Hamosh A. et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc. Natl. Acad. Sci. U.S.A. 2005; 102:15960–15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stanley S.E., Armanios M.. The short and long telomere syndromes: paired paradigms for molecular medicine. Curr. Opin. Genet. Dev. 2015; 33:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Counter C.M., Gupta J., Harley C.B., Leber B., Bacchetti S.. Telomerase activity in normal leukocytes and in hematologic malignancies. Blood. 1995; 85:2315–2320. [PubMed] [Google Scholar]
- 9. Hiyama E., Gollahon L., Kataoka T., Kuroi K., Yokoyama T., Gazdar A.F., Hiyama, Piatyszek M.A., Shay J.W.. Telomerase activity in human breast tumors. J. Natl. Cancer Inst. 1996; 88:116–122. [DOI] [PubMed] [Google Scholar]
- 10. Greider C.W. Telomerase activation: one step on the road to cancer?. Trends Genet. 1999; 15:109–112. [DOI] [PubMed] [Google Scholar]
- 11. Killela P.J., Reitman Z.J., Jiao Y., Bettegowda C., Agrawal N., Diaz L.A. Jr, Friedman A.H., Friedman H., Gallia G.L., Giovanella B.C. et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bell R.J., Rube H.T., Xavier-Magalhaes A., Costa B.M., Mancini A., Song J.S., Costello J.F.. Understanding TERT promoter mutations: a common path to immortality. Mol. Cancer Res. 2016; 14:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horn S., Figl A., Rachakonda P.S., Fischer C., Sucker A., Gast A., Kadel S., Moll I., Nagore E., Hemminki K. et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013; 339:959–961. [DOI] [PubMed] [Google Scholar]
- 14. Huang F.W., Hodis E., Xu M.J., Kryukov G.V., Chin L., Garraway L.A.. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013; 339:957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramsay A.J., Quesada V., Foronda M., Conde L., Martinez-Trillos A., Villamor N., Rodriguez D., Kwarciak A., Garabaya C., Gallardo M. et al. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat. Genet. 2013; 45:526–530. [DOI] [PubMed] [Google Scholar]
- 16. Harley C.B., Kim N.W., Prowse K.R., Weinrich S.L., Hirsch K.S., West M.D., Bacchetti S., Hirte H.W., Counter C.M., Greider C.W. et al. Telomerase, cell immortality, and cancer. Cold Spring Harb. Symp. Quant. Biol. 1994; 59:307–315. [DOI] [PubMed] [Google Scholar]
- 17. Sharma H.W., Maltese J.Y., Zhu X., Kaiser H.E., Narayanan R.. Telomeres, telomerase and cancer: is the magic bullet real?. Anticancer Res. 1996; 16:511–515. [PubMed] [Google Scholar]
- 18. Damm K., Hemmann U., Garin-Chesa P., Hauel N., Kauffmann I., Priepke H., Niestroj C., Daiber C., Enenkel B., Guilliard B. et al. A highly selective telomerase inhibitor limiting human cancer cell proliferation. EMBO J. 2001; 20:6958–6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El-Daly H., Kull M., Zimmermann S., Pantic M., Waller C.F., Martens U.M.. Selective cytotoxicity and telomere damage in leukemia cells using the telomerase inhibitor BIBR1532. Blood. 2005; 105:1742–1749. [DOI] [PubMed] [Google Scholar]
- 20. Pascolo E., Wenz C., Lingner J., Hauel N., Priepke H., Kauffmann I., Garin-Chesa P., Rettig W.J., Damm K., Schnapp A.. Mechanism of human telomerase inhibition by BIBR1532, a synthetic, non-nucleosidic drug candidate. J. Biol. Chem. 2002; 277:15566–15572. [DOI] [PubMed] [Google Scholar]
- 21. Roth A., Harley C.B., Baerlocher G.M.. Imetelstat (GRN163L)–telomerase-based cancer therapy. Recent Results Cancer Res. 2010; 184:221–234. [DOI] [PubMed] [Google Scholar]
- 22. Harley C.B. Telomerase and cancer therapeutics. Nat. Rev. Cancer. 2008; 8:167–179. [DOI] [PubMed] [Google Scholar]
- 23. Chiappori A.A., Kolevska T., Spigel D.R., Hager S., Rarick M., Gadgeel S., Blais N., Von Pawel J., Hart L., Reck M. et al. A randomized phase II study of the telomerase inhibitor imetelstat as maintenance therapy for advanced non-small-cell lung cancer. Ann. Oncol. 2015; 26:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Armanios M., Greider C.W.. Treating myeloproliferation–on target or off. N. Engl. J. Med. 2015; 373:965–966. [DOI] [PubMed] [Google Scholar]
- 25. Cerone M.A., Burgess D.J., Naceur-Lombardelli C., Lord C.J., Ashworth A.. High-throughput RNAi screening reveals novel regulators of telomerase. Cancer Res. 2011; 71:3328–3340. [DOI] [PubMed] [Google Scholar]
- 26. Coussens M., Davy P., Brown L., Foster C., Andrews W.H., Nagata M., Allsopp R.. RNAi screen for telomerase reverse transcriptase transcriptional regulators identifies HIF1alpha as critical for telomerase function in murine embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 2010; 107:13842–13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong L.H., Unciti-Broceta A., Spitzer M., White R., Tyers M., Harrington L.. A yeast chemical genetic screen identifies inhibitors of human telomerase. Chem. Biol. 2013; 20:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smogorzewska A., van Steensel B., Bianchi A., Oelmann S., Schaefer M.R., Schnapp G., de Lange T.. Control of human telomere length by TRF1 and TRF2. Mol. Cell. Biol. 2000; 20:1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smogorzewska A., de Lange T.. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 2004; 73:177–208. [DOI] [PubMed] [Google Scholar]
- 30. Palm W., de Lange T.. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008; 42:301–334. [DOI] [PubMed] [Google Scholar]
- 31. Vodenicharov M.D., Wellinger R.J.. DNA degradation at unprotected telomeres in yeast is regulated by the CDK1 (Cdc28/Clb) cell-cycle kinase. Mol. Cell. 2006; 24:127–137. [DOI] [PubMed] [Google Scholar]
- 32. Frank C.J., Hyde M., Greider C.W.. Regulation of telomere elongation by the cyclin-dependent kinase CDK1. Mol. Cell. 2006; 24:423–432. [DOI] [PubMed] [Google Scholar]
- 33. Greenwell P.W., Kronmal S.L., Porter S.E., Gassenhuber J., Obermaier B., Petes T.D.. TEL1, a gene involved in controlling telomere length in S. cerevisiae, is homologous to the human ataxia telangiectasia gene. Cell. 1995; 82:823–829. [DOI] [PubMed] [Google Scholar]
- 34. Smith S., Giriat I., Schmitt A., de Lange T.. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science. 1998; 282:1484–1487. [DOI] [PubMed] [Google Scholar]
- 35. Peuscher M.H., Jacobs J.J.. DNA-damage response and repair activities at uncapped telomeres depend on RNF8. Nat. Cell Biol. 2011; 13:1139–1145. [DOI] [PubMed] [Google Scholar]
- 36. Wu S.Y., Chiang C.M.. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J. Biol. Chem. 2007; 282:13141–13145. [DOI] [PubMed] [Google Scholar]
- 37. Devaiah B.N., Case-Borden C., Gegonne A., Hsu C.H., Chen Q., Meerzaman D., Dey A., Ozato K., Singer D.S.. BRD4 is a histone acetyltransferase that evicts nucleosomes from chromatin. Nat. Struct. Mol. Biol. 2016; 23:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Devaiah B.N., Lewis B.A., Cherman N., Hewitt M.C., Albrecht B.K., Robey P.G., Ozato K., Sims R.J. 3rd, Singer D.S.. BRD4 is an atypical kinase that phosphorylates serine2 of the RNA polymerase II carboxy-terminal domain. Proc. Natl. Acad. Sci. U.S.A. 2012; 109:6927–6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Devaiah B.N., Gegonne A., Singer D.S.. Bromodomain 4: a cellular Swiss army knife. J. Leukoc. Biol. 2016; 100:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baerlocher G.M., Vulto I., de Jong G., Lansdorp P.M.. Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nat. Protoc. 2006; 1:2365–2376. [DOI] [PubMed] [Google Scholar]
- 41. Green M., Sambrook J.. Molecular Cloning: A Laboratory Manual. 2012; 4th edn, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 42. Strezoska Z., Licon A., Haimes J., Spayd K.J., Patel K.M., Sullivan K., Jastrzebski K., Simpson K.J., Leake D., van Brabant Smith A. et al. Optimized PCR conditions and increased shRNA fold representation improve reproducibility of pooled shRNA screens. PLoS One. 2012; 7:e42341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Langmead B., Salzberg S.L.. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012; 9:357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li W., Xu H., Xiao T., Cong L., Love M.I., Zhang F., Irizarry R.A., Liu J.S., Brown M., Liu X.S.. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 2014; 15:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee S.S., Bohrson C., Pike A.M., Wheelan S.J., Greider C.W.. ATM Kinase is required for telomere elongation in mouse and human cells. Cell Rep. 2015; 13:1623–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harley C.B., Futcher A.B., Greider C.W.. Telomeres shorten during ageing of human fibroblasts. Nature. 1990; 345:458–460. [DOI] [PubMed] [Google Scholar]
- 47. Morrish T.A., Greider C.W.. Short telomeres initiate telomere recombination in primary and tumor cells. PLoS Genet. 2009; 5:e1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nandakumar J., Bell C.F., Weidenfeld I., Zaug A.J., Leinwand L.A., Cech T.R.. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature. 2012; 492:285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cristofari G., Lingner J.. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 2006; 25:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang F., Podell E.R., Zaug A.J., Yang Y., Baciu P., Cech T.R., Lei M.. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature. 2007; 445:506–510. [DOI] [PubMed] [Google Scholar]
- 51. Latrick C.M., Cech T.R.. POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation. EMBO J. 2010; 29:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hemann M.T., Strong M., Hao L.-Y., Greider C.W.. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001; 107:67–77. [DOI] [PubMed] [Google Scholar]
- 53. Zhang J., Yang P.L., Gray N.S.. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer. 2009; 9:28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tong A.S., Stern J.L., Sfeir A., Kartawinata M., de Lange T., Zhu X.D., Bryan T.M.. ATM and ATR signaling regulate the recruitment of human telomerase to telomeres. Cell Rep. 2015; 13:1633–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W.B., Fedorov O., Morse E.M., Keates T., Hickman T.T., Felletar I. et al. Selective inhibition of BET bromodomains. Nature. 2010; 468:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang W., Zheng X., Yang Y., Wang X., Shen Z.. An overview on small molecule inhibitors of BRD4. Mini Rev. Med. Chem. 2016; 16:1403–1414. [DOI] [PubMed] [Google Scholar]
- 57. Filippakopoulos P., Knapp S.. Targeting bromodomains: epigenetic readers of lysine acetylation. Nat. Rev. Drug Discov. 2014; 13:337–356. [DOI] [PubMed] [Google Scholar]
- 58. Dey A., Nishiyama A., Karpova T., McNally J., Ozato K.. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol. Biol. Cell. 2009; 20:4899–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhao R., Nakamura T., Fu Y., Lazar Z., Spector D.L.. Gene bookmarking accelerates the kinetics of post-mitotic transcriptional re-activation. Nat. Cell Biol. 2011; 13:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Berthon C., Raffoux E., Thomas X., Vey N., Gomez-Roca C., Yee K., Taussig D.C., Rezai K., Roumier C., Herait P. et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Haematol. 2016; 3:e186–195. [DOI] [PubMed] [Google Scholar]
- 61. Amorim S., Stathis A., Gleeson M., Iyengar S., Magarotto V., Leleu X., Morschhauser F., Karlin L., Broussais F., Rezai K. et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 2016; 3:e196–204. [DOI] [PubMed] [Google Scholar]
- 62. Hao L.Y., Greider C.W.. Genomic instability in both wild-type and telomerase null MEFs. Chromosoma. 2004; 113:62–68. [DOI] [PubMed] [Google Scholar]
- 63. French C.A., Miyoshi I., Aster J.C., Kubonishi I., Kroll T.G., Dal Cin P., Vargas S.O., Perez-Atayde A.R., Fletcher J.A.. BRD4 bromodomain gene rearrangement in aggressive carcinoma with translocation t(15;19). Am. J. Pathol. 2001; 159:1987–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zuber J., Shi J., Wang E., Rappaport A.R., Herrmann H., Sison E.A., Magoon D., Qi J., Blatt K., Wunderlich M. et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011; 478:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mertz J.A., Conery A.R., Bryant B.M., Sandy P., Balasubramanian S., Mele D.A., Bergeron L., Sims R.J. 3rd. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc. Natl. Acad. Sci. U.S.A. 2011; 108:16669–16674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Delmore J.E., Issa G.C., Lemieux M.E., Rahl P.B., Shi J., Jacobs H.M., Kastritis E., Gilpatrick T., Paranal R.M., Qi J. et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011; 146:904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chaidos A., Caputo V., Karadimitris A.. Inhibition of bromodomain and extra-terminal proteins (BET) as a potential therapeutic approach in haematological malignancies: emerging preclinical and clinical evidence. Ther. Adv. Hematol. 2015; 6:128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nicodeme E., Jeffrey K.L., Schaefer U., Beinke S., Dewell S., Chung C.W., Chandwani R., Marazzi I., Wilson P., Coste H. et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010; 468:1119–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tang X., Peng R., Phillips J.E., Deguzman J., Ren Y., Apparsundaram S., Luo Q., Bauer C.M., Fuentes M.E., DeMartino J.A. et al. Assessment of Brd4 inhibition in idiopathic pulmonary fibrosis lung fibroblasts and in vivo models of lung fibrosis. Am. J. Pathol. 2013; 183:470–479. [DOI] [PubMed] [Google Scholar]
- 70. Ding N., Hah N., Yu R.T., Sherman M.H., Benner C., Leblanc M., He M., Liddle C., Downes M., Evans R.M.. BRD4 is a novel therapeutic target for liver fibrosis. Proc. Natl. Acad. Sci. U.S.A. 2015; 112:15713–15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Armanios M.Y., Chen J.J., Cogan J.D., Alder J.K., Ingersoll R.G., Markin C., Lawson W.E., Xie M., Vulto I., Phillips J.A. 3rd et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2007; 356:1317–1326. [DOI] [PubMed] [Google Scholar]
- 72. Armanios M., Blackburn E.H.. The telomere syndromes. Nat. Rev. Genet. 2012; 13:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Armanios M. Telomeres and age-related disease: how telomere biology informs clinical paradigms. J. Clin. Invest. 2013; 123:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.