Abstract
Background
Dopamine D2 receptors are reported to have high-affinity (D2High) and low-affinity (D2Low) states. Although an increased proportion of D2High has been demonstrated in animal models of schizophrenia, few clinical studies have investigated this alteration of D2High in schizophrenia in vivo.
Methods
Eleven patients with schizophrenia, including 10 antipsychotic-naive and 1 antipsychotic-free individuals, and 17 healthy controls were investigated. Psychopathology was assessed by Positive and Negative Syndrome Scale, and a 5-factor model was used. Two radioligands, [11C]raclopride and [11C]MNPA, were employed to quantify total dopamine D2 receptor and D2High, respectively, in the striatum by measuring their binding potentials. Binding potential values of [11C]raclopride and [11C]MNPA and the binding potential ratio of [11C]MNPA to [11C]raclopride in the striatal subregions were statistically compared between the 2 diagnostic groups using multivariate analysis of covariance controlling for age, gender, and smoking. Correlations between binding potential and Positive and Negative Syndrome Scale scores were also examined.
Results
Multivariate analysis of covariance demonstrated a significant effect of diagnosis (schizophrenia and control) on the binding potential ratio (P=.018), although the effects of diagnosis on binding potential values obtained with either [11C]raclopride or [11C]MNPA were nonsignificant. Posthoc test showed that the binding potential ratio was significantly higher in the putamen of patients (P=.017). The Positive and Negative Syndrome Scale “depressed” factor in patients was positively correlated with binding potential values of both ligands in the caudate.
Conclusions
The present study indicates the possibilities of: (1) a higher proportion of D2High in the putamen despite unaltered amounts of total dopamine D2 receptors; and (2) associations between depressive symptoms and amounts of caudate dopamine D2 receptors in patients with schizophrenia.
Keywords: PET, [11C]raclopride, [11C]MNPA, striatum, high affinity
Significance Statement
It has been documented that dopamine D2 receptors exist in 2 distinct states with high and low affinities for endogenous dopamine. Here, we quantified total and high-affinity-state D2 receptors in the striatal subregions of healthy controls and patients with schizophrenia by PET with a D2-type receptor antagonist, [11C]raclopride, and a D2-type receptor agonist, [11C]MNPA, respectively, and demonstrated a higher proportion of the high-affinity state of D2 receptors in the putamen of the patients. Additionally, the binding of these 2 radioligands in the caudate of the patients was positively correlated with their scores of Positive and Negative Syndrome Scale (PANSS) “depressed” factor. These findings support the utility of PET with a D2-type receptor antagonist and a D2-type receptor agonist in the same individual for investigating a change in the total amount or affinity state of this receptor as a possible molecular basis of symptomatic manifestations in schizophrenia.
Introduction
Dysregulation in dopamine neurotransmission is thought to underlie the pathophysiology of schizophrenia (Meltzer and Stahl, 1976; Davis et al., 1991). This concept has been supported for decades by evidence that the clinical effect of antipsychotics is mediated by dopamine D2 receptor (D2R) blockade (Nord and Farde, 2011).
Previous in vitro studies have suggested that D2R has 2 interconvertible affinity states for endogenous dopamine, referred to as G-protein-coupled high-affinity (D2High) and G-protein-uncoupled low-affinity (D2Low) states (De Lean et al., 1982; Sibley et al., 1982; George et al., 1985; Richfield et al., 1989). D2High is a functionally active state of D2R (George et al., 1985). Elevations of D2High have been reported in several different animal models of schizophrenia, and it has been suggested that an increased proportion of D2High might play an important role in the pathophysiology of schizophrenia (Seeman et al., 2005; Seeman, 2011).
[11C]raclopride is a widely used D2R ligand for visualizing this receptor in the brains of living subjects by positron emission tomography (PET). While [11C]raclopride is a D2-type receptor antagonist with similar affinities for D2High and D2Low (Seneca et al., 2006), the D2-type receptor agonists, exemplified by (-)-N-[11C]propyl-norapomorphine ([11C]NPA) (Hwang et al., 2000), [11]C(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol ([11C]PHNO) (Wilson et al., 2005), and [11C](R)-2-CH3O-N-n-propylnorapomorphine ([11C]MNPA) (Finnema et al., 2005), have been shown to have a higher affinity for D2High than D2Low (Sibley et al., 1982; Seneca et al., 2006; Willeit et al., 2007; Shotbolt et al., 2012; Gallezot et al., 2014).
Only a limited number of clinical studies have hitherto investigated D2High availability in patients with schizophrenia in vivo, and whether there are alterations in the ligand binding to D2High or in the proportion of D2High in patients with schizophrenia still remains a question. Previous PET studies using [11C]PHNO have shown that there were no significant changes in its binding potential in patients with schizophrenia compared with healthy controls (Graff-Guerrero et al., 2009; Suridjan et al., 2013). However, [11C]PHNO was reported to have a 50-fold higher affinity for dopamine D3 receptor (D3R) than D2R (Freedman et al., 1994; Narendran et al., 2006), potentially hampering sensitive detection of D2High. In contrast to [11C]PHNO, [11C]MNPA has almost identical affinities for D2R and D3R, with dissociation constant (Kd) values of 2.21 nM and 2.02 nM, respectively (Skinbjerg et al., 2009), and thus might be more suitable for assessing D2High (Kodaka et al., 2013). In addition, the binding potential ratio between the 2 ligands, [11C]raclopride and [11C]MNPA, could be a possible index of the proportion of D2High vs total D2Rs, as indicated in a previous study for healthy subjects (Kodaka et al., 2013).
In this study, we aimed to investigate the availability of D2High in antipsychotic-free patients with schizophrenia using PET with both antagonist and agonist ligands.
Methods
Participants
A total of 11 patients with schizophrenia (4 men and 7 women) were recruited from affiliated hospitals or clinics. Seventeen healthy controls (8 men and 9 women) were recruited by the National Institute of Radiological Sciences, Chiba, Japan, for participation in this study.
The patient group was comprised of 10 antipsychotic-naive patients (one had taken benzodiazepines the night before her PET scans) and 1 patient who had been antipsychotic-free for 2 years after 1-year treatment with aripiprazole. All patients fulfilled the diagnostic criteria for schizophrenia according to DSM-IV. None of the patients were comorbid with other neuropsychiatric disorders or had substance abuse. Psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987), and a 5-factor model (Wallwork et al., 2012) was used to calculate PANSS subscale scores (positive, negative, disorganized/concrete, excited, and depressed factors). The healthy control volunteers were recruited by public notices. They had no history of psychiatric disease, neurological injury or disease, severe medical diseases, substance abuse that may affect brain functions, or first-degree relatives suffering from psychotic episodes. All the diagnostic interviews and PANSS ratings were conducted by trained psychiatrists. Table 1 presents the participants’ demographic information.
Table 1.
Demographic and Clinical Characteristics of Subjects
| Patient group (n=11) | Control group (n=17) | Statistics | P | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | ||
| Age (y) | 33.9 | 6.5 | 33.8 | 9.6 | 0.047 | .96 |
| Gender (male/female) | 4/7 | 8/9 | .71 | |||
| Smoker (yes/no) | 4/7 | 4/13 | .67 | |||
| Injected radioactivity of [11C]raclopride (MBq) | 224.8 | 14.4 | 221.5 | 15.3 | -0.57 | .58 |
| Injected radioactivity of [11C]MNPA (MBq) | 220.1 | 17.9 | 221.2 | 16.3 | -0.17 | .87 |
| Specific radioactivity of [11C]raclopride (GBq/mmol) | 178.1 | 87.6 | 206.2 | 140.0 | -0.65 | .52 |
| Specific radioactivity of [11C]MNPA (GBq/mmol) | 173.3 | 99.5 | 182.5 | 166.0 | -0.17 | .87 |
| Age at onset (y) | 31.1 | 5.5 | ||||
| Duration of illness (y) | 3.3 | 4.3 | ||||
| PANSS total score | 77.6 | 22.5 | ||||
| PANSS factor | ||||||
| Positive | 13.5 | 3.0 | ||||
| Negative | 12.4 | 5.5 | ||||
| Disorganized/concrete | 7.3 | 2.6 | ||||
| Excited | 8.5 | 3.5 | ||||
| Depressed | 8.4 | 3.0 | ||||
This study was approved by the Radiation Drug Safety Committee and the Institutional Review Board of the National Institute of Radiological Sciences, Japan, and was carried out in accordance with the Code of Ethics of the World Medical Association. After complete description of the study, written informed consent was obtained from all participants. Data were collected between April 2008 and October 2016.
PET Procedures
All subjects underwent 2 PET scans on the same day, one with [11C]raclopride and the other with [11C]MNPA, except 1 control subject who took the 2 scans on separate days due to technical trouble. [11C]MNPA-PET preceded [11C]raclopride-PET in 10 patients and 11 controls, and [11C]raclopride-PET was followed by [11C]MNPA-PET in 1 patient and 6 controls. After i.v. rapid bolus injection of [11C]raclopride, a PET scan was performed for 60 minutes. Similarly, after i.v. rapid bolus injection of [11C]MNPA, a PET scan was performed for 90 minutes. The time interval between initiations of the first and second PET scans was 120 minutes or longer. For both PET scans, 3-dimensional dynamic PET data were acquired with a Siemens ECAT Exact HR+ system (CTI/Siemens), which provides 63 sections with an axial field of view of 15.5 cm. PET images were reconstructed with a filtered back-projection method with corrections for attenuation and scatter. The reconstructed in-plane resolution was 7.5 mm full-width at half-maximum. A 10-minute transmission scan using a 68Ge/68Ga line source was performed before each PET scan for attenuation correction. A thermoplastic head fixation device was used to minimize head movement during PET scanning. The dynamic scans consisted of twelve 20-second frames, sixteen 60-second frames, and ten 240-second frames for [11C]raclopride, and nine 20-second frames, five 60-second frames, four 120-second frames, eleven 240-second frames, and six 300-second frames for [11C]MNPA.
MRI Procedures
Because of replacements of MRI machines during this study, 3 types of MRI devices were used depending on the time of participant recruitment. For the initial 4 patients and 7 controls, MR images were acquired with a 1.5-T MR scanner (Intera, Philips Medical Systems). 3D volumetric acquisition of a T1-weighted gradient echo sequence produced a gapless series of thin transverse sections (TE 9.2 ms, TR 21 ms, flip angle 30°, field of view 256 mm, acquisition matrix 256 × 256, slice thickness 1 mm). Then, for the next 6 patients, a 3-T MR scanner (Sigma HDx, General Electric) was used. 3D volumetric acquisition of a T1-weighted 3-dimensional fast spoiled gradient-recalled acquisition in the steady-state sequence produced a gapless series of thin transverse sections (TE 2.8 ms, TR 7.0 ms, flip angle 8°, field of view 260 mm, acquisition matrix 256 × 256, slice thickness 1 mm). Finally, for 1 patient and 10 controls, T1-weighted MR images were obtained with another 3-T MR scanner (MAGNETOM Verio, Siemens). 3D volumetric acquisition of a T1-weighted gradient echo sequence produced a gapless series of thin sagittal sections (TE 1.95 ms, TR 2300 ms, TI 900 ms, flip angle 9°, field of view 250 mm, acquisition matrix 256×256, slice thickness 1 mm).
Region of Interest (ROI) Definition
All ROIs used in our analyses were initially defined on a standard anatomic orientation (MNI standard space; Montreal Neurological Institute). Striatum ROIs (caudate, putamen, and ventral striata) were defined using a striatum anatomical atlas (Tziortzi et al., 2011). A cerebellum ROI was defined with a probabilistic cerebellar atlas (Diedrichsen et al., 2009), excluding the vermis.
These ROIs were transformed into individual MR spaces with transformation matrices calculated with the “normalize” function of statistical parametric mapping software (SPM12; Wellcome Department of Imaging Neuroscience) and were thresholded using FreeSurfer’s automated anatomical segmentation (Fischl et al., 2002) to exclude neighboring white matter, ventricles, and non-brain regions. Time-activity curves for each ROI were extracted by applying these ROIs to individual dynamic PET images based on the transformation parameters for the coregistration of MR images to the PET spaces using PMOD software ver. 3.7 (PMOD Technologies Ltd).
Quantification of Total D2R and D2High Availabilities in the Striatum
Availabilities of striatal total D2R and D2High were quantified as binding potentials relative to the nondisplaceable tissue (BPND) of [11C]raclopride and [11C]MNPA, respectively. [11C]raclopride BPND and [11C]MNPA BPND were calculated for each ROI using a 3-parameter simplified reference tissue model (Lammertsma and Hume, 1996) and cerebellar cortex as reference tissue, which has negligible density of D2R (Suhara et al., 1999). BPND was defined as follows:
| (1) |
where fND is the free fraction of radioligand in the nondisplaceable tissue compartment and Bavail indicates the neuroreceptor density. All kinetic analyses were performed using PMOD software ver. 3.7.
Statistical Analysis
Statistical analyses were conducted with SPSS 23.0 (SPSS Inc). First, MANCOVA was applied to examine group differences in: (1) BPND of [11C]raclopride; (2) BPND of [11C]MNPA; and (3) the BPND ratio (ratio of [11C]MNPA BPND to [11C]raclopride BPND) in 3 striatal ROIs (caudate, putamen, and ventral striata), separately. A fixed factor was diagnosis (patients = 1, controls = 0). Nuisance covariates were defined as age, gender, and smoking state (smoker = 1, nonsmoker = 0) to control for their potential effects on D2Rs (Wong et al., 1997; Okita et al., 2016). Statistical significance threshold was defined as P<.05 (2-tailed).
Second, correlational analyses were performed between the BPND values mentioned above and each of the PANSS factor scores (positive, negative, disorganized/concrete, excited, and depressed factors) in patients. The statistical threshold was set at P<.05 with Bonferroni correction for the 3 striatal subregions (caudate, putamen, and ventral striata), as in previous studies (Talvik et al., 2006; Okita et al., 2016). Because of the small sample size and exploratory nature of this study, corrections were not applied for multiple comparisons of the 5 PANSS factor scores and the 3 binding parameters ([11C]raclopride BPND, [11C]MNPA BPND, and their ratio). In case significant correlations were found, partial correlation analyses were also performed, controlling for age, gender, smoking state, or duration of illness. Statistical significance threshold was defined as P<.05 (2-tailed).
Results
Demographic Data
Demographic data are shown in Table 1. Patients and controls did not significantly differ in terms of age, gender, or proportion of smokers. Injected dose and specific radioactivity of [11C]raclopride and [11C]MNPA were not significantly different between the 2 groups.
Group Comparisons of Radioligand Binding
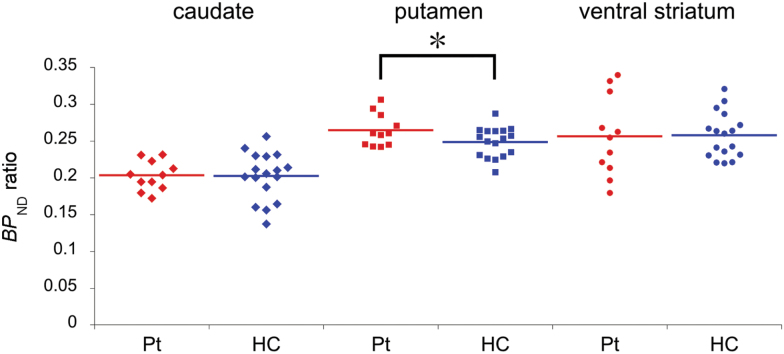
Results of group comparisons of BPND values are shown in Table 2 and Figure 1. MANCOVA demonstrated a significant main effect of diagnosis on the BPND ratio (F = 4.16, P=.018), although the effects of diagnosis on BPND values obtained with either [11C]raclopride or [11C]MNPA were nonsignificant. Posthoc analysis by univariate test revealed a significant effect of diagnosis on the putaminal BPND ratio (F = 6.64, P=.017), indicating that the BPND ratio in the putamen of patients was significantly higher.
Table 2.
Comparisons of BPND of [11C]raclopride, BPND of [11C]MNPA, and BPND Ratio of [11C]MNPA to [11C]raclopride between the Patient and Control Groups
| Patient group (n=11) | Control group (n=17) | Multivariate tests | Between-subject effects (posthoc) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | P | F | P | |
| BP ND of [11C]raclopride | 1.50 | .24 | ||||||
| Caudate | 2.21 | 0.34 | 2.30 | 0.35 | ||||
| Putamen | 2.89 | 0.36 | 3.04 | 0.31 | ||||
| Ventral striatum | 1.98 | 0.36 | 2.12 | 0.22 | ||||
| BP ND of [11C]MNPA | 2.48 | .09 | ||||||
| Caudate | 0.45 | 0.10 | 0.47 | 0.11 | ||||
| Putamen | 0.76 | 0.06 | 0.75 | 0.07 | ||||
| Ventral striatum | 0.49 | 0.09 | 0.54 | 0.07 | ||||
| BP ND ratio | 4.16 | .018a | ||||||
| Caudate | 0.203 | 0.020 | 0.202 | 0.032 | 0.11 | .74 | ||
| Putamen | 0.265 | 0.022 | 0.249 | 0.020 | 6.64 | .017a | ||
| Ventral striatum | 0.256 | 0.054 | 0.258 | 0.031 | 0.00 | .98 | ||
a P < .05.
Figure 1.
A group comparison of binding potential ratio (BPND ratio) ([11C](R)-2-CH3O-N-n-propylnorapomorphine ([11C]MNPA) to [11C]raclopride) in striatal subregions. A significant diagnostic effect was found on the BPND ratio (P=.018; MANCOVA controlled for age, gender, smoking). Univariate posthoc analysis showed that the BPND ratio in the putamen was significantly higher in patients (P=.017). HC, healthy control group; Pt, patient group.
Because age distribution was reasonably matched between the 2 groups both numerically and statistically (Table 1), we repeated the analysis excluding age from nuisance covariates to confirm our results on the BPND ratio. This MANCOVA also showed a significant main effect of diagnosis on the BPND ratio (F = 3.68, P=.028). Univariate posthoc analysis revealed a significant effect of diagnosis on the putaminal BPND ratio (F = 5.83, P=.024), indicating that the BPND ratio, compared with that of controls, was significantly higher in the putamen of patients.
Correlation Analyses
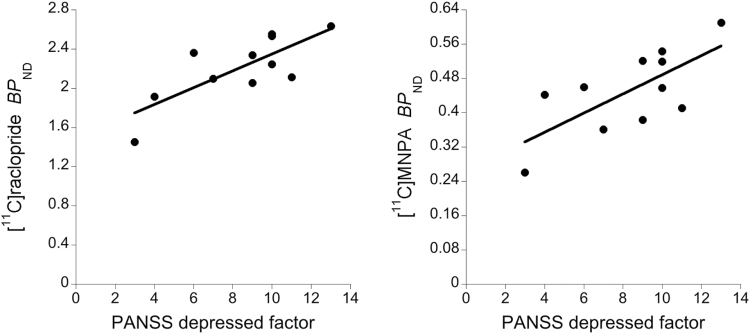
Significant positive correlations were found between PANSS depressed factor and BPND of [11C]raclopride (Pearson’s r = 0.773, P=.005) and of [11C]MNPA (r = 0.701, P=.016) in the caudate (Figure 2). No other significant correlation was found. Partial correlations of PANSS depressed factor with the BPND of both [11C]raclopride and [11C]MNPA in the caudate were significant after controlling for age, gender, smoking state, or duration of illness.
Figure 2.
Scatter plots of binding potential (BPND) values against Positive and Negative Syndrome Scale (PANSS) “depressed” factor and linear regressions in the caudate of patients with schizophrenia. PANSS “depressed” factor was significantly and positively correlated with BPND of [11C]raclopride (left: Pearson’s r = 0.773, P=.005) and of [11C](R)-2-CH3O-N-n-propylnorapomorphine ([11C]MNPA) (right: r = 0.701, P=.016). Statistical significance threshold: P<.05 for the 3 striatal subregions (Bonferroni correction).
Discussion
The binding of [11C]raclopride and [11C]MNPA did not significantly differ between patients and controls. This is consistent with most of the previous D2/3 receptor studies using different radioligands, in which no clear differences were shown between the 2 diagnostic groups (Talvik et al., 2006; Graff-Guerrero et al., 2009; Howes et al., 2012; Brunelin et al., 2013; Suridjan et al., 2013). On the other hand, the current study revealed that the BPND ratio of [11C]MNPA to [11C]raclopride in the putamen was significantly higher in patients with schizophrenia.
In previous studies on animal models of schizophrenia, the proportion of D2High in the striatum was markedly increased (Seeman et al., 2005; Seeman, 2011), while D2R density in this area was not significantly changed. Several different animal models of schizophrenia have been reported, such as amphetamine-sensitized rats, mice deficient in types 2 and 3 metabotropic glutamate receptors, trace amine 1 receptor knockout mice, and rats with a neonatal hippocampus lesion. Interestingly, dopamine super-sensitivity (Lieberman et al., 1987) and marked increase in D2High without significant increase in total D2Rs have been observed in most of these animal models (Bhardwaj et al., 2003; Seeman et al., 2005; Wolinsky et al., 2007; Seeman, 2009; Seeman et al., 2009). These findings imply that there may be a variety of mechanisms that can lead to increases in D2High. As such, it appears that D2High could be one of the common mechanisms of schizophrenia. Although molecular processes triggering this change are not clear, one explanation might be that the constant oscillation between the high- and low-affinity states of D2R is altered in schizophrenia, and the conversion rate of D2High to D2Low may be slower than a normal condition, resulting in an increase in the proportion of D2High (Seeman, 2013). The extent of the group difference in the BPND ratio observed in our study was not as prominent as the increase in the proportion of D2High shown in animal models of psychosis. This might be due to the difference between in vitro tissue assays and in vivo PET imaging (Graff-Guerrero et al., 2009), and it is likely that a low-grade but long-lasting abnormality of D2High over decades may lead to the onset of schizophrenia. There is also a possibility that the contribution of D2High alterations vs other neurochemical factors to the etiology of schizophrenia might be variable among individuals in consideration of the heterogeneity of neuroreceptor statuses in subjects with this disease. The current work indicated a higher proportion of D2High in the putamen of patients with schizophrenia, while it is yet to be elucidated how neurochemical and functional abnormalities in this striatal subregion contribute to the onset of the disease. MRI studies have documented involvements of putaminal volume changes in the evolution of a clinical high risk status for psychosis (Bin Hong et al., 2015) and worsening of clinical outcomes in schizophrenia (Mitelman et al., 2009). Hence, mechanistic links between these morphological alterations and a dysregulated affinity state of D2R in the putamen may need to be investigated in subjects at clinical high risk and with schizophrenia by longitudinal PET and MRI assays.
For the D2R/D3R selectivity of radioligands, unlike [11C]PHNO, both [11C]raclopride and [11C]MNPA have similar affinities to D2R and D3R according to an in vitro study (Skinbjerg et al., 2009). Because D2Rs are predominant and only low levels of D3R have been detected in the caudate and putamen by previous binding assays or postmortem studies (Murray et al., 1994; Seeman et al., 2006), and [11C]MNPA has a higher affinity for D2High than D2Low (Seneca et al., 2006), the BPND ratio of [11C]MNPA to [11C]raclopride in these regions could reflect the proportion of D2High relative to total D2Rs with minimal influence of the D3R status.
PANSS depressed factor was positively correlated with both BPND of [11C]raclopride and [11C]MNPA in the caudate. A previous PET study with [11C]raclopride has reported its elevated binding in the caudate and putamen in medication-free patients with depression compared with healthy subjects (Meyer et al., 2006), suggesting the possible involvement of dopaminergic neurotransmission in depression and depressed subjects. Our results might indicate specific associations between depressive symptoms in schizophrenia and D2R availability in the caudate, in a manner independent of its affinity states. Since BPND values of both antagonistic [11C]raclopride and agonistic [11C]MNPA were correlated with the depression score, densities of D2Rs rather than occupancy of D2R by endogenous dopamine may increase in association with a depressive state. This dopaminergic modulation might not efficiently compensate for the mood change, in light of somewhat limited therapeutic application of dopaminergic stimulants to depressive conditions (Hardy, 2009). Accordingly, further studies with a larger sample size will be needed to better understand the current observations and to confirm them on the basis of more rigorous statistical criteria.
Several technical limitations of the current study should be taken into account. First, the sample size is relatively small, and the demographic compositions of gender and smokers vs nonsmokers are not equal between the 2 groups numerically, if not statistically. In addition, a previous PET study in a healthy population reported that central striatal D2R/D3R availability was negatively correlated with recent and lifetime smoking, and also with nicotine dependence (Okita et al., 2016). Another PET study reported a significant gender-by-smoking interaction on D2R/D3R availability in the caudate and putamen (Brown et al., 2012). These findings provide a rationale for the inclusion of gender and smoking as covariates of no interest, but an expanded analysis of patients and controls with a larger sample size and minimal inter-group differences in these factors will be required for more robust proof of the current results. Second, the temporal sequence of PET scans with [11C]raclopride and [11C]MNPA differed among the subjects. However, the occupancy of D2R by these ligands was <1% based on their injected mass doses, and the interval between the initiations of the first and second PET scans was longer than 120 minutes in all subjects. Hence, effects of the sequence of PET scans with the 2 ligands on quantitative data are considered negligible. Third, 3 different MRI devices were used to acquire anatomical information on the brains of the subjects. To minimize a possible confounding effect of MRI qualities on kinetic analyses, we used the standard MNI space to define ROIs, which were eventually transformed into individual PET spaces. Finally, we used only PANSS to evaluate symptoms in patients with schizophrenia. Using multiple scales to assess delusion, hallucination, depression, and other symptoms of schizophrenia would be more desirable.
In conclusion, the present study has provided in vivo clinical evidence that a modulated proportion of D2High is implicated in the molecular etiology of schizophrenia. Relationships between depressive symptoms and D2R levels in schizophrenia patients have also been demonstrated by the consistent observations with 2 radioligands. The combined use of [11C]raclopride and [11C]MNPA validated here could be applied to a PET study on a larger scale to further clarify involvement of the dopaminergic statuses in schizophrenia as a molecular basis of symptomatic manifestations.
Funding
This work was supported in part by a Grant-in-Aid for Research Activity Start-up (15H06873 to M.K.) from the Japan Society for the Promotion of Science; and the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) and the Strategic Research Program for Brain Sciences to T.S. from the Japan Agency for Medical Research and Development, AMED. These agencies had no further role in the study design, collection, analysis or interpretation of the data, writing of the manuscript, or in the decision to submit the manuscript for publication.
Statement of Interest
None.
Acknowledgments
We thank Kazuko Suzuki and Shizuko Kawakami for their assistance as clinical coordinators, Hiromi Sano and Naoto Sato for their support with MRI scans, and the staff of the Department of Radiopharmaceutics Development for the radioligand synthesis.
References
- Bhardwaj SK, Beaudry G, Quirion R, Levesque D, Srivastava LK (2003) Neonatal ventral hippocampus lesion leads to reductions in nerve growth factor inducible-B mRNA in the prefrontal cortex and increased amphetamine response in the nucleus accumbens and dorsal striatum. Neuroscience 122:669–676. [DOI] [PubMed] [Google Scholar]
- Bin Hong S, Lee TY, Bin Kwak Y, Kim SN, Kwon JS (2015) Baseline putamen volume as a predictor of positive symptom reduction in patients at clinical high risk for psychosis: a preliminary study. Schizophr Res 169:178–185. [DOI] [PubMed] [Google Scholar]
- Brown AK, Mandelkern MA, Farahi J, Robertson C, Ghahremani DG, Sumerel B, Moallem N, London ED (2012) Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. Int J Neuropsychopharm 15:989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelin J, Fecteau S, Suaud-Chagny MF (2013) Abnormal striatal dopamine transmission in schizophrenia. Curr Med Chem 20:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M (1991) Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 148:1474–1486. [DOI] [PubMed] [Google Scholar]
- De Lean A, Kilpatrick BF, Caron MG (1982) Dopamine receptor of the porcine anterior pituitary gland. Evidence for two affinity states discriminated by both agonists and antagonists. Mol Pharmacol 22:290–297. [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N (2009) A probabilistic MR atlas of the human cerebellum. NeuroImage 46:39–46. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Seneca N, Farde L, Shchukin E, Sovago J, Gulyás B, Wikström HV, Innis RB, Neumeyer JL, Halldin C (2005) A preliminary PET evaluation of the new dopamine D2 receptor agonist [11C]MNPA in cynomolgus monkey. Nucl Med Biol 32:353–360. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Patel S, Marwood R, Emms F, Seabrook GR, Knowles MR, McAllister G (1994) Expression and pharmacological characterization of the human D3 dopamine receptor. J Pharmacol Exp Ther 268:417–426. [PubMed] [Google Scholar]
- Gallezot J- D, Kloczynski T, Weinzimmer D, Labaree D, Zheng M- Q, Lim K, Rabiner EA, Ridler K, Pittman B, Huang Y, Carson RE, Morris ED, Cosgrove KP (2014) Imaging nicotine- and amphetamine-induced dopamine release in rhesus monkeys with [11C]PHNO vs [11C]raclopride PET. Neuropsychopharmacology 39:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Watanabe M, Di Paolo T, Falardeau P, Labrie F, Seeman P (1985) The functional state of the dopamine receptor in the anterior pituitary is in the high affinity form. Endocrinology 117:690–697. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Mizrahi R, Agid O, Marcon H, Barsoum P, Rusjan P, Wilson AA, Zipursky R, Kapur S (2009) The dopamine D2 receptors in high-affinity state and D3 receptors in schizophrenia: a clinical [11C]-(+)-PHNO PET study. Neuropsychopharmacology 34:1078–1086. [DOI] [PubMed] [Google Scholar]
- Hardy SE. (2009) Methylphenidate for the treatment of depressive symptoms, including fatigue and apathy, in medically ill older adults and terminally ill adults. Am J Geriatr Pharmacother 7:34–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S (2012) The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry 69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DR, Kegeles LS, Laruelle M (2000) (-)-N-[11C]propyl-norapomorphine: a positron-labeled dopamine agonist for PET imaging of D2 receptors. Nucl Med Biol 27:533–539. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Lewis OA (1987) The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Kodaka F, Ito H, Kimura Y, Fujie S, Takano H, Fujiwara H, Sasaki T, Nakayama K, Halldin C, Farde L, Suhara T (2013) Test-retest reproducibility of dopamine D2/3 receptor binding in human brain measured by PET with [11C]MNPA and [11C]raclopride. Eur J Nucl Med Mol Imaging 40:574–579. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP (1996) Simplified reference tissue model for PET receptor studies. NeuroImage 4:153–158. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Kane JM, Alvir J (1987) Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology 91:415–433. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Stahl SM (1976) the dopamine hypothesis of schizophrenia. Schizophr Bull 2:19–76. [DOI] [PubMed] [Google Scholar]
- Meyer JH, McNeely HE, Sagrati S, Boovariwala A, Martin K, Verhoeff NP, Wilson AA, Houle S (2006) Elevated putamen D2 receptor binding potential in major depression with motor retardation: an [11C]raclopride positron emission tomography study. Am J Psychiatry 163:1594–1602. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Canfield EL, Chu K- W, Brickman AM, Shihabuddin L, Hazlett EA, Buchsbaum MS (2009) Poor outcome in chronic schizophrenia is associated with progressive loss of volume of the putamen. Schizophr Res 113:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AM, Ryoo HL, Gurevich E, Joyce JN (1994) Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci 91:11271–11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, Reeder S, Rabiner E, Laruelle M (2006) Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse 60:485–495. [DOI] [PubMed] [Google Scholar]
- Nord M, Farde L (2011) Antipsychotic occupancy of dopamine receptors in schizophrenia. CNS Neurosci Ther 17:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Mandelkern MA, London ED (2016) Cigarette use and striatal dopamine D2/3 receptors: possible role in the link between smoking and nicotine dependence. Int J Neuropsychopharm 19:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB (1989) Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience 30:767–777. [DOI] [PubMed] [Google Scholar]
- Seeman P. (2009) Dopamine D2High receptors measured ex vivo are elevated in amphetamine-sensitized animals. Synapse 63:186–192. [DOI] [PubMed] [Google Scholar]
- Seeman P. (2011) All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2High receptors. CNS Neurosci Ther 17:118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. (2013) Are dopamine D2 receptors out of control in psychosis? Prog Neuropsychopharmacol Biol Psychiatry 46:146–152. [DOI] [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, O’Dowd BF, George SR, Perreault ML, Männistö PT, Robinson S, Palmiter RD, Tallerico T (2005) Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci 102:3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Wilson A, Gmeiner P, Kapur S (2006) Dopamine D2 and D3 receptors in human putamen, caudate nucleus, and globus pallidus. Synapse 60:205–211. [DOI] [PubMed] [Google Scholar]
- Seeman P, Battaglia G, Corti C, Corsi M, Bruno V (2009) Glutamate receptor mGlu2 and mGlu3 knockout striata are dopamine supersensitive, with elevated D2High receptors and marked supersensitivity to the dopamine agonist (+)PHNO. Synapse 63:247–251. [DOI] [PubMed] [Google Scholar]
- Seneca N, Finnema SJ, Farde L, Gulyás B, Wikström HV, Halldin C, Innis RB (2006) Effect of amphetamine on dopamine D2 receptor binding in nonhuman primate brain: a comparison of the agonist radioligand [11C]MNPA and antagonist [11C]raclopride. Synapse 59:260–269. [DOI] [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, Plisson C, Miller SR, Huiban M, Beaver JD, Gunn RN, Laruelle M, Rabiner EA (2012) Within-subject comparison of [11C]-(+)-PHNO and [11C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab 32:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR, De Lean A, Creese I (1982) Anterior pituitary dopamine receptors. J Biol Chem 257:6351–6361. [PubMed] [Google Scholar]
- Skinbjerg M, Namkung Y, Halldin C, Innis RB, Sibley DR (2009) Pharmacological characterization of 2-methoxy-N-propylnorapomorphine’s interactions with D2 and D3 dopamine receptors. Synapse 63:462–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhara T, Sudo Y, Okauchi T, Maeda J, Kawabe K, Suzuki K, Okubo Y, Nakashima Y, Ito H, Tanada S, Halldin C, Farde L (1999) Extrastriatal dopamine D2 receptor density and affinity in the human brain measured by 3D PET. Int J Neuropsychopharm 2:73–82. [DOI] [PubMed] [Google Scholar]
- Suridjan I, Rusjan P, Addington J, Wilson A, Houle S, Mizrahi R (2013) Dopamine D2 and D3 binding in people at clinical high risk for schizophrenia, antipsychotic-naive patients and healthy controls while performing a cognitive task. J Psychiatry Neurosci 38:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talvik M, Nordström A- L, Okubo Y, Olsson H, Borg J, Halldin C, Farde L (2006) Dopamine D2 receptor binding in drug-naïve patients with schizophrenia examined with raclopride-C11 and positron emission tomography. Psychiatry Res 148:165–173. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN (2011) Imaging dopamine receptors in humans with [11C]-(+)-PHNO: Dissection of D3 signal and anatomy. NeuroImage 54:264–277. [DOI] [PubMed] [Google Scholar]
- Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D (2012) Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res 137:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S, Seeman P, Wilson AA, Kapur S (2007) First human evidence of d-Amphetamine induced displacement of a D2/3 Agonist radioligand: a [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology 33:279–289. [DOI] [PubMed] [Google Scholar]
- Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, Houle S, Seeman P, Ginovart N (2005) Radiosynthesis and evaluation of [11C]-(+)-4-Propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem 48:4153–4160. [DOI] [PubMed] [Google Scholar]
- Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P, Branchek T, Gerald CP (2007) The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes Brain Behav 6:628–639. [DOI] [PubMed] [Google Scholar]
- Wong DF, Young D, Wilson PD, Meltzer CC, Gjedde A (1997) Quantification of neuroreceptors in the living human brain: III. D2-like dopamine receptors: theory, validation, and changes during normal aging. J Cereb Blood Flow Metab 17:316–330. [DOI] [PubMed] [Google Scholar]