Abstract
The aim of this study was to compare the anti-oxidative and anti-inflammatory activities of gamma-irradiated persimmon leaf extract (GPLE) with those of non-irradiated persimmon leaf extract (PLE). Ethanolic extract of persimmon leaf was exposed to gamma irradiation at a dose of 10 kGy. After gamma irradiation, the color of the extract changed from dark brown to light brown. The anti-oxidative and anti-inflammatory activities of GPLE and PLE were assessed from: total polyphenol and total flavonoid contents; 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay; 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assay, and levels of pro-inflammatory mediators such as nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6). The total polyphenol contents of GPLE and PLE were determined to be 224.44 ± 1.54 and 197.33 ± 5.81 mg gallic acid equivalents (GAE)/g, respectively, and the total flavonoid contents of GPLE and PLE were 206.27 ± 1.15 and 167.60 ± 2.00 mg quercetin equivalents (QUE)/g, respectively. The anti-oxidant activities of GPLE and PLE as measured by DPPH assays were 338.33 ± 30.19 μg/ml (IC50) and 388.68 ± 8.45 μg/ml (IC50), respectively, and those measured by ABTS assays were 510.49 ± 15.12 μg/ml (IC50) and 731.30 ± 10.63 μg/ml (IC50), respectively. IC50 is the inhibitor concentration that reduces the response by 50%. GPLE strongly inhibited the production of NO, PGE2 and IL-6 compared with PLE in lipopolysaccharide-stimulated RAW264.7 macrophages. Furthermore, GPLE significantly inhibited the production of TNF-α and IL-6 cytokines compared with PLE in phorbol 12-myristate 13-acetate (PMA) plus A23187-stimulated HMC-1 human mast cells. These results indicate that gamma irradiation of PLE can enhance its anti-oxidative and anti-inflammatory activities through elevation of the phenolic contents. Therefore, gamma-irradiated PLE has potential for use in the food and cosmetic industries.
Keywords: anti-inflammatory, anti-oxidant, gamma irradiation, persimmon leaf
INTRODUCTION
Persimmon (Diospyros kaki) is a widely cultivated plant in East Asia and it is used in herbal teas and traditional medicine in South Korea, Japan and China. It has been extensively studied and is known for its anti-oxidative and anti-inflammatory effects [1]. Yoo et al. [2] studied the in vitro and in vivo activities of persimmon leaf extracts and established that persimmon leaves, due to their phenolic and particularly flavonolic constituents, exhibited anti-oxidative and anti-inflammatory activities. Kim et al. [3] showed that persimmon leaves could inhibit N-methyl-N-nitrosourea (MNU) –induced retinitis in mice. Other studies have shown radical scavenging, neuro-protective, thrombosis inhibitive, and anti-allergic properties of persimmon leaves [4–7]. In China, the well-known NaoXinQing tablet made from persimmon leaf extract has been recommended as a supplement (in the Chinese Pharmacopoeia 2010) for the prevention and cure of coronary heart disease and cerebral arterial sclerosis. Moreover, our laboratory has demonstrated the anti-oxidative and protective effects of persimmon leaves on ultraviolet B (UVB)–induced injury in HacaT keratinocytes that may contribute to recovery from acute and chronic diseases [8, 9].
Gamma irradiation was approved in 1981 by the Food and Drug Administration and the U.S. Department of Agriculture for the preservation of various food products. It was concluded that food irradiated at doses of <50 kGy was safe for human consumption. Since then, gamma irradiation has been widely used for phyto-sanitary food and herbal treatment. This has given rise to research into the effects of irradiation on the bioactive components in plants. For example, various forms of irradiation have been shown to affect the phenolic contents of some plants [10], while the phenolic contents in other plants have remain unaltered [11, 12]. Other studies have shown that gamma irradiation negatively affects the anti-oxidant properties of plant materials [12, 13]. Lee et al. [14] showed that gamma irradiation of centipede grass led to degradation of the plant pigments while the biological activities were maintained or increased. Also, gamma irradiation significantly increased the anti-oxidant activity of Aloe vera, and this was also accompanied by a color change [15]. Even though previous studies have demonstrated the anti-oxidant activities of gamma-irradiated persimmon leaves [16], few or no studies have been done to illustrate their anti-inflammatory potential. The main purpose of this study was to investigate the effect of gamma irradiation on the anti-oxidative and anti-inflammatory activities of persimmon leaf extract (PLE).
MATERIALS AND METHODS
Reagents
Gallic acid, chlorogenic acid, rutin, hyperoside, isoquercetin, astragalin, quercetin, lipopolysaccharide (LPS), Greiss reagent, phorbol 12-myristate 13-acetate (PMA) and calcium ionophore A23187 were purchased from Sigma-Aldrich (St Louis, MO, USA). Tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and prostaglandin E2 (PGE2) enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D System (Minneapolis, MN, USA). Dulbecco’s Modified Eagle’s Medium (DMEM) and Iscove’s Modified Dulbecco’s Medium (IMDM) were purchased from Invitrogen (Carlsbad, CA, USA). Other chemicals used in this study were of reagent grade and were purchased from Sigma-Aldrich Chemical Co. Ltd (St Louis, MO, USA), unless otherwise stated.
Plant materials and extract preparation
Persimmon leaves were collected 29 June 2015 from Jeongcheon-myeon, Jinan-gun, Jeollabuk-do, Republic of Korea. The plant was identified and authenticated by Prof. Hong-Jun Kim (College of Oriental Medicine, Woosuk University). A voucher specimen has been deposited in the Department of Health Care & Science, College of Medical Science, Jeonju University. The leaves were washed with distilled water and dried at 50°C. Dried leaves (180 g) were chopped, crushed and extracted in 3.6 l of 70% ethanol at room temperature for 5 days, with constant shaking, and filtered through a membrane filter with a 0.45 μm pore size. The filtrate was freeze-dried to obtain 28.2 g of extract, which was then stored at −20°C until further analysis.
Gamma irradiation
Sample solutions dissolved in ethanol were irradiated at a dose of 10 kGy in a 60cobalt irradiator (point source AECL, IR-79, MDS Nordion International Co., Ltd, Ottawa, ON, Canada) at the Korea Atomic Energy Research Institute (Jeoung-eup, Korea). The irradiated sample was then concentrated under reduced pressure and was freeze-dried to powdered form (26 g). The resulting powder was stored at −20°C until further analysis.
Determination of total polyphenols
The Total Polyphenol (TP) content was determined by the Folin–Ciocalteu method [17]. Briefly, 20 mg/ml of PLE and GPLE were made with DMSO as a solvent, and thereafter serial dilutions (with distilled water) of the extract and standard (gallic acid) were prepared (0 to 1000 μg/ml). Aliquots of 0.1 ml each of the extract and standard were mixed with 0.1 ml Folin–Ciocalteu’s phenol reagent. After 5 min, 1 ml of 4% Na2CO3 was added and the mixture was allowed to stand at room temperature and pressure for 30 min. All determinations were performed in triplicates. The absorbance was read at 600 nm, and the total polyphenol concentration was calculated from a calibration curve (r2 = 0.996) using gallic acid as the standard.
Determination of total flavonoids
The Total Flavonoid (TF) content was determined by the method of Ordoñez et al. [18]. Briefly, 0.5 ml an aliquot of 1 mg/ml PLE or GPLE was added to 0.5 ml of 10% aluminum chloride, thoroughly mixed and allowed to stand at room temperature for 5 min. The absorbance of the supernatant was then taken at 405 nm. All determinations were performed in triplicates. Using quercetin as the standard, the total flavonoid content was calculated from a calibration curve (r2 = 0.996) and expressed as quercetin equivalents in milligram per gram of dry weight (mg·QUE/g).
HPLC analysis
HPLC (High Performance Liquid Chromatography) was performed using an Agilent 1100 series (Santa Clara, CA, USA), equipped with a binary pump delivery system, a degasser (G1379A), an auto sampler (G1313A) and a diode array detector (G1315B). The separation of compounds was performed on an Agilent Eclipse XDB-C18 column (4.6 × 250 mm, 5 μm particles) through a gradient elution with 0.5% aqueous formic acid (A) and acetonitrile (B): 0 min, 5% B; 10 min, 10% B; 50 min, 40% B; 54 min, 100% B; 64 min, 100% B. The mixture was then held for 10 min before returning to the initial conditions. The mobile phase was retained at a flow rate of 1 ml/min and the column oven was set at a temperature of 30°C. Aliquots of 10 μl of the extracts were injected and UV detection was monitored at 280 nm. All standards (gallic acid, chlorogenic acid, rutin, hyperoside, isoquercetin, astragalin and quercetin) were identified based on retention times, and concentrations were calculated based on comparison with sample peak areas obtained from standards. Stock solutions (1000 μg/ml) were prepared using methanol. Calibration curves were constructed for each standard, using six different concentrations (6.25, 12.5, 25, 50, 100 and 200 μg/ml). A degree of high linearity (r2 > 0.994) was obtained for each standard curve. The integration of each component into the chromatograms was processed using Agilent Chemstation software.
2,2-Diphenyl-1-picrylhydrazyl radical scavenging activity
The radical scavenging activity by 2,2-diphenyl-1-picrylhydrazyl (DPPH) was analyzed using the method of Blois [19]. DPPH free radicals can accept electrons or hydrogen radicals from anti-oxidants to become stable diamagnetic molecules, with discoloration due to the decreasing quantity of the DPPH free radicals in the solution. Therefore, the degree of discoloration can be used to evaluate the scavenging ability of the anti-oxidant [20]. Briefly, 0.1 ml of each extract was mixed with 0.1 ml of 0.3 mM DPPH solution and allowed to stand at room temperature and pressure in the dark for 30 min; the absorbance was measured at 517 nm. The activity was expressed as a percentage, using the following equation:
Radical scavenging activity of 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)
The radical scavenging activity of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) was determined using the method of Re et al. [21], with modifications. Ferryl myoglobin radical will oxidize ABTS, generating a radical cation with a green color. Anti-oxidants will suppress this reaction by donating electrons and inhibiting the formation of the colored ABTS radical [22]. Briefly, ABTS working solution was prepared by diluting 7 mM of ABTS stock solution with distilled water until the absorbance was 0.7 ± 0.02 at a wavelength of 732 nm. From the appropriate ratio of ABTS stock solution and distilled water, the ABTS working solution was prepared. For each extract, an aliquot of 50 µl of the extract was added to 950 µl of ABTS working solution contained in a dark tube, and it was allowed to stand at room temperature and pressure for 30 min. The absorbance was then measured at 732 nm. The activity was expressed as a percentage as follows:
Cell culture
RAW264.7 macrophage cells were purchased from ATCC (Manassas, VA, USA), and human mast cells (HMC-1) were obtained from Jeonju AgroBio-Materials Institute (Jeonju, Korea). The RAW264.7 and HMC-1 cells were grown in DMEM and IMDM culture media, respectively, and supplemented with 10% FBS, 100 units/ml of penicillin and 100 μg/ml of streptomycin (Invitrogen, Carlsbad, CA, USA) in an incubator (37°C, 5% CO2).
Cytokine measurement
RAW 264.7 cells were pre-treated with PLE and GPLE (100 µg/ml) for 1 h and stimulated with 1 µg/ml of LPS for 16 h. HMC-1 cells were pre-treated with PLE and GPLE (50 µg/ml) for 1 h and stimulated with 30 nM of PMA plus 1 µM of calcium ionophore A23187 for 16 h. The quantities of TNF-α and IL-6 in the culture media were measured using an ELISA kit (R&D Systems) according to the manufacturer’s protocol.
Nitric oxide measurement
RAW264.7 cells (2 × 105 cells/ml) were cultured in 96-well plates. They were pre-treated with PLE and GPLE (100 µg/ml) for 1 h and stimulated with 1 µg/ml of LPS (except for the control cells) for 16 h. An aliquote of 100 µl of Greiss reagent was added to 100 µl of culture media supernatant in a 96-well plate and incubated for 15 min at room temperature and pressure. The absorbance was measured spectrophotometrically at 540 nm, and the concentration of nitrite was calculated using a calibration standard curve constructed using sodium nitrite.
Prostaglandin E2 measurement
To measure the PGE2 levels, RAW264.7 cells were pre-treated with PLE and GPLE (100 µg/ml) for 1 h and stimulated with 1 µg/ml LPS for 16 h. Thereafter, the PGE2 levels in the culture supernatant were determined using the PGE2 EIA Kits (R&D System) according to the manufacturer’s protocol.
Statistical analysis
Statistical analysis was performed using Student’s t-test; the level of significance was set at P < 0.05. All data shown represent the means of triplicate analyses.
RESULTS AND DISCUSSION
Total polyphenol and total flavonoid contents, and anti-oxidant activities of GPLE and PLE
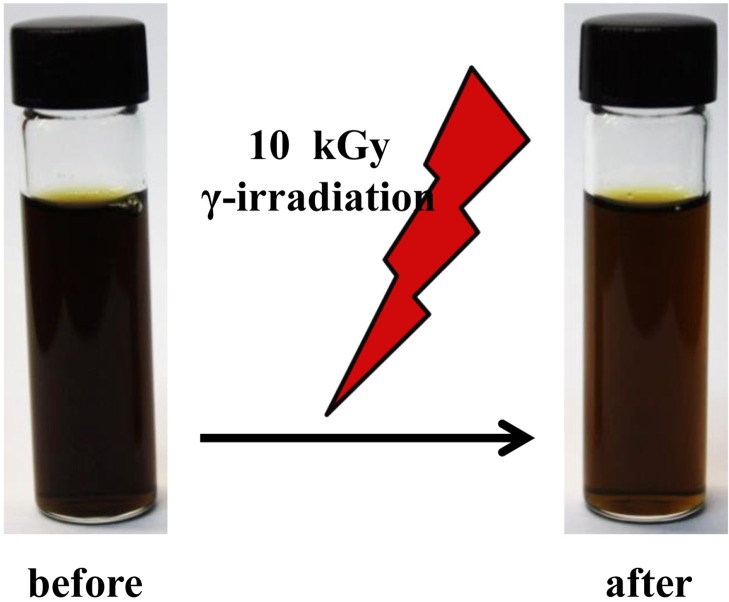
The total polyphenol and flavonoid contents and the ABTS and DPPH radical scavenging activities of 70% ethanol extracts of GPLE and PLE were assessed and the results are shown in Table 1. As seen in Fig. 1, gamma irradiation of PLE led to a marked change in the color of the extract. Though not proven, this could have occurred as a result of the gamma radiolysis of water and ethanol-producing free radicals that are able to destroy pigments in PLE. The phenolic content, measured in milligram·gallic acid equivalents (GAE)/gram, was found to be significantly increased in GPLE (P < 0.05). Also, GPLE had a significantly higher flavonoid content compared with PLE (P < 0.001). Both extracts showed anti-oxidant activities. However, GPLE had significantly improved anti-oxidant activities compared with PLE (P < 0.05). The present study clearly showed that gamma irradiation has significant effects on persimmon leaves in terms of their phenolic and flavonoid contents, thereby increasing the anti-oxidant properties. These results are consistent with those obtained by Jo et al. [16], who recorded development of a bright yellow color after irradiation of PLE at 20 kGy. Different researchers have shown that gamma irradiation increases the phenolic and flavonoid contents and the anti-oxidant activities of plant extracts [14–16]. This was also found to be true in the present study, in which gamma irradiation of PLE increased the biological activities of the extracts. In addition, we can conclude that the color of PLE can be considered by irradiation technologies and maintained by lyophilization after irradiation [18], while increasing its functional properties.
Table 1.
Effect of gamma irradiation on anti-oxidant activity and total polyphenol and flavonoid contents of PLE
| Sample | Total polyphenol (mg·GAE/g) | Total flavonoid (mg·QUE/g) | DPPHa (IC50) | ABTSa (IC50) |
|---|---|---|---|---|
| PLE | 197.33 ± 5.81 | 167.60 ± 2.00 | 388.68 ± 8.45 | 731.30 ± 10.63 |
| GPLE | 224.44 ± 1.54 | 206.27 ± 1.15 | 338.33 ± 30.19 | 510.49 ± 15.12 |
aEach extract was examined in a set of experiments repeated three times. IC50 is the concentration of extract (μg/ml) required to scavenge 50% of DPPH and ABTS radicals. DPPH radical scavenging activity (%) = [1 – (sample absorbance/absorbance of blank)] × 100. ABTS radical scavenging activity (%) = [1 – (sample absorbance/absorbance of blank)] × 100. Sample concentration (μg/ml).
Fig. 1.
Color change effect of gamma irradiation on persimmon leaf extracts.
HPLC analysis of GPLE and PLE
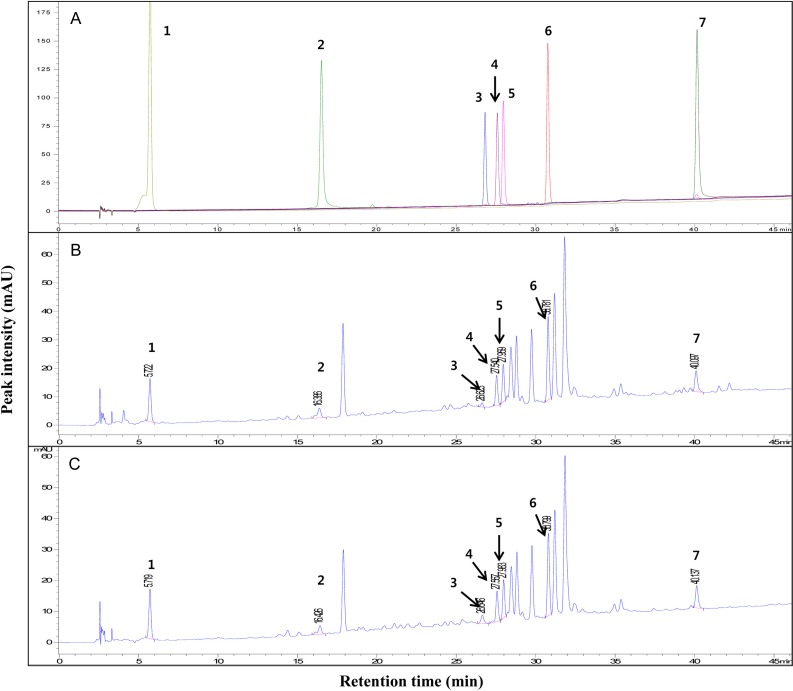
The HPLC chromatograms of GPLE and PLE at 280 nm are shown in Fig. 2. The chromatogram showed more than seven major peaks, seven of which were identified using standard compounds as follows: 1-gallic acid (RT 5.728 min), 2-chlorogenic acid (RT 16.517 min), 3-rutin (RT 26.814 min), 4-hyperoside (RT 27.586 min), 5-isoquercetin (RT 27.967 min), 6-astragalin (RT 30.764 min) and 7-quercetin (RT 40.161 min). The chromatogram of PLE was consistent with the results of previous studies [23, 24]. In order to compare the contents of GPLE and PLE, the concentrations of gallic acid, isoquercetin and astragalin in PLE were measured (in milligrams/gram) and compared with the concentrations in the gamma-irradiated counterpart (GPLE). Even though gamma irradiation increased the concentration of gallic acid and decreased the concentrations of isoquercetin and astragalin (Table 2), the differences were not statistically significant. We therefore speculated that the higher levels of phenolic and flavonoid contents (and hence of anti-oxidant activities) in GPLE could be due to the generation of free radicals by gamma irradiation [25], which could break the chemical bonds of higher molecular weight compounds, thus yielding other phenolic compounds. Other analytical systems such as NMR or LC-MS will be required for further identification of the specific compounds formed as a result of gamma irradiation of persimmon leaves
Fig. 2.
HPLC chromatogram of standard compounds (A), PLE (B) and GPLE (C) at 280 nm. Peaks: 1, gallic acid; 2, chlorogenic acid; 3, rutin; 4, hyperoside; 5, isoquercetin; 6, astragalin; 7, quercetin.
Table 2.
Effect of gamma irradiation on content (mg/g) of phenolic components of PLE
| Sample | Gallic acid | Isoquercetin | Astragalin |
|---|---|---|---|
| PLE | 1.55 ± 0.27a | 3.59 ± 0.31 | 4.80 ± 0.33 |
| GPLE | 1.64 ± 0.12 | 3.43 ± 0.18 | 4.38 ± 0.36 |
aAll extracts were examined in a set of experiments repeated three times.
Anti-inflammatory properties of GPLE and PLE
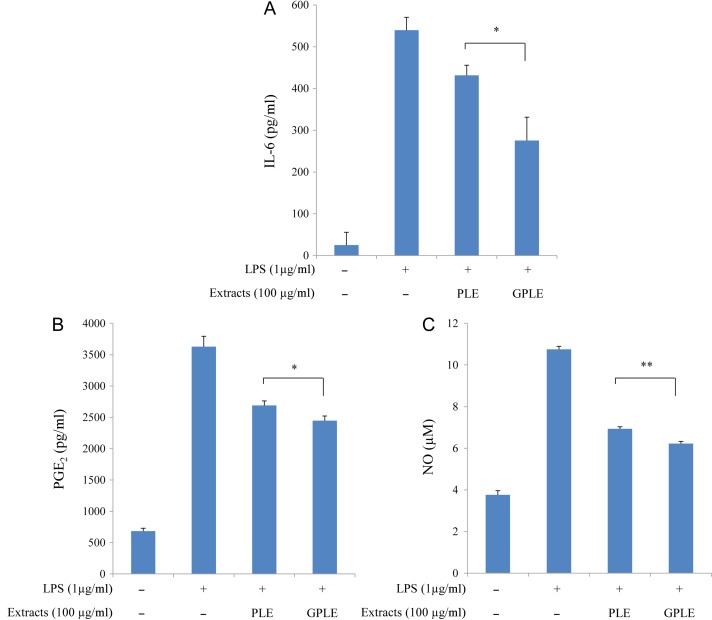
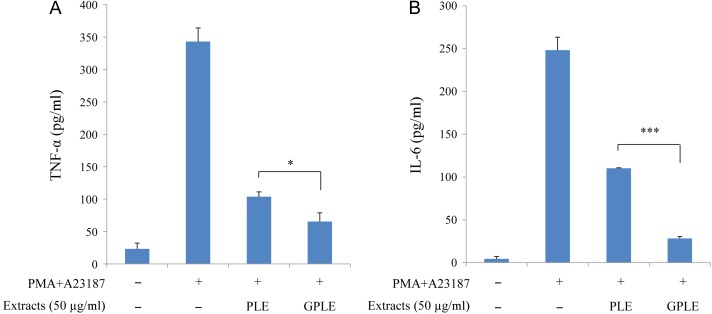
We investigated and compared the anti-inflammatory effects of GPLE and PLE on the inhibition of IL-6, TNF-α, PGE2 and NO production by LPS-stimulated RAW264.7 cells and/or PMA plus A23187-stimulated HMC-1 cells (Figs 3 and 4). First of all, to rule out possible cytotoxicity of GPLE and PLE on these cells, WST cell viability was performed, which showed that GPLE and PLE (in a solvent of <0.1% DMSO) had no cytotoxicity on RAW264.7 cells at up to 150 µg/ml and on HMC-1 cells at up to 50 µg/ml (data not shown). GPLE significantly reduced the production of IL-6, PGE2 and NO compared with PLE in LPS-stimulated RAW264.7 cells. The production of TNF-α by PMA plus A23187-stimulated HMC-1 cells was significantly reduced by GPLE compared with PLE. IL-6 production was greatly reduced by GPLE compared with PLE in PMA plus A23187-stimulated HMC-1 cells. Correlations have been reported between several inflammatory cytokines (especially TNF-α and IL-6) and inflammatory diseases (such as asthma, rheumatoid arthritis, inflammatory bowel diseases and allergic diseases) [26, 27]. NO can also influence the outcome of the immune response during infection [28]. Thus, inhibiting production of these cytokines and of NO and PGE2 could be effective therapeutically in preventing inflammatory responses and diseases [29]. Our results have shown that GPLE can be much more effective than PLE in inhibiting PGE2, NO and inflammatory cytokines production and can therefore be targeted as an important method for improving the potencies of PLE that could be used in treating inflammatory diseases. Several studies have investigated the inhibitory effects of PLE with respect to cytokine, NO and PGE2 production, and Koichi et al. [30] reported a significant decrease in TNFα production when they pretreated human serum albumin and Nε-carboxymethyl lysine-stimulated RAW264.7 cells with PLE. Astragalin from persimmon inhibits the expression of pro-inflammatory mediators through the inhibition of NF-κB in macrophages [31]. Little or no work has been done on inhibitory effects of gamma irradiation of PLE in inflammatory disease response. Our study is the first to show these effects of gamma irradiation on PLE.
Fig. 3.
Effect of PLE and GPLE on IL-6 (A), PGE2 (B) and NO (C) production in LPS-stimulated RAW264.7 cells. The results are expressed as mean ± SD. *P < 0.05, **P < 0.01.
Fig. 4.
Effect of PLE and GPLE on TNF-α (A) and IL-6 (B) production in PMA plus A23187-stimulated HMC-1 cells. The results are expressed as mean ± SD. *P < 0.05, ***P < 0.001.
CONCLUSION
The present study provided evidence that gamma-irradiated PLE possesses anti-oxidant and anti-inflammatory properties through the structural changes it induces in PLE. It is thought that these structural changes increase the phenolic content of PLE, thereby making gamma irradiation a potentially useful tool in the food and cosmetic industry. Further research should be carried out to determine the actual compounds in GPLE that lead to its increased biological activities. It would be desirable to investigate the effects of higher irradiation doses, and mass spectrometry could be used to detect new compounds formed after the gamma irradiation.
ACKNOWLEDGEMENTS
We would like to thank Dr Chang Hyun Jin (Advanced Radiation Technology Institute, Korea Atomic Energy Research Institute, Republic of Korea) and Mr Hyo-Young Kim (Advanced Radiation Technology Institute, Korea Atomic Energy Research Institute, Republic of Korea) for HPLC analysis and gamma irradiation.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest regarding this article.
REFERENCES
- 1. Kim AH, Kim HJ, Ryu R et al. . A mixture of ethanol extracts of persimmon leaf and Citrus junos Sieb improves blood coagulation parameters and ameliorates lipid metabolism disturbances caused by diet-induced obesity in C57BL/6J mice. J Microbiol Biotechnol 2016;26:295–308. [DOI] [PubMed] [Google Scholar]
- 2. Yoo KH, Jeong JM. Antioxidative and antiallergic effect of persimmon leaf extracts. J Korean Soc Food Sci Nutr 2009;38:1691–8. [Google Scholar]
- 3. Kim KA, Kang SW, Ahn HR et al. . Leaves of persimmon (Diospyros kaki Thunb.) ameliorate N-methyl-N-nitrosourea (MNU)-induced retinal degeneration in mice. J Agric Food Chem 2015;63:7750–9. [DOI] [PubMed] [Google Scholar]
- 4. Matsumoto M, Kotani M, Fujita A et al. . Oral administration of persimmon leaf extract ameliorates skin symptoms and transepidermal water loss in atopic dermatitis model mice, NC/Nga. Br J Dermatol 2002;146:221–7. [DOI] [PubMed] [Google Scholar]
- 5. Kotani M, Matsumoto M, Fujita A et al. . Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. J Allergy Clin Immunol 2000;106:159–66. [DOI] [PubMed] [Google Scholar]
- 6. Tanaka T, Higa S, Hirano T et al. . Flavonoids as potential anti-allergic substances. Curr Med Chem Antiinflamm Antiallergy Agents 2003;2:57–65. [Google Scholar]
- 7. Sakanaka S, Tachibana Y, Okada Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem 2005;89:569–75. [Google Scholar]
- 8. Jeong SI, Cho JK, Mok JY et al. . Antioxidant activity of persimmon leaves during growth. Kor J Pharmacogn 2010;41:255–63. [Google Scholar]
- 9. Cho JK, Park JM, Jeon IH et al. . Effect of persimmon leaf extract on utraviolet B–induced inflammation in HaCaT keratinocytes and mice. J Korean Soc Appl Biol Chem 2011;54:583–90. [Google Scholar]
- 10. Variyar PS, Bandyopadhyay C, Thomas P. Effect of gamma-irradiation on the phenolic acids of some Indian spices. Int J Food Sci Technol 1998;33:533–7. [Google Scholar]
- 11. Cantos E, García-Viguera C, de Pascual-Teresa S et al. . Effect of postharvest ultraviolet irradiation on resveratrol and other phenolics of cv. Napoleon table grapes. J Agric Food Chem 2000;48:4606–12. [DOI] [PubMed] [Google Scholar]
- 12. Pinela J, Antonio AL, Barros L et al. . Combined effects of gamma-irradiation and preparation method on antioxidant activity and phenolic composition of Tuberaria lignosa. RSC Adv 2015;5:14756–67. [Google Scholar]
- 13. Ahn HJ, Kim JH, Jo C et al. . Comparison of irradiated phytic acid and other antioxidants for antioxidant activity. Food Chem 2004;88:173–8. [Google Scholar]
- 14. Lee EM, Lee SS, Bai HW et al. . Effect of gamma irradiation on the pigments and the biological activities of methanolic extracts from leaves of centipedegrass (Eremochloa ophiuroides Munro). Radiat Phys Chem 2013;91:108–13. [Google Scholar]
- 15. Lee EM, Bai HW, Lee SS et al. . Gamma irradiation improves the antioxidant activity of Aloe vera (Aloe barbadensis Miller) extracts. Radiat Phys Chem 2012;81:1029–32. [Google Scholar]
- 16. Jo C, Son JH, Shin MG et al. . Irradiation effects on color and functional properties of persimmon (Diospyros kaki L. folium) leaf extract and licorice (Glycyrrhiza uralensis Fischer) root extract during storage Radiat Phys Chem 2003;67:143–8. [Google Scholar]
- 17. Peterson DM, Emmons CL, Hibbs AH. Phenolic antioxidants and antioxidant activity in pearling fractions of oat groats. J Cereal Sci 2001;33:97–103. [Google Scholar]
- 18. Ordoñez AAL, Gomez JD, Vattuone MA et al. . Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem 2006;97:452–8. [Google Scholar]
- 19. Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;181:1199–200. [Google Scholar]
- 20. Miller NJ, Rice-Evans C, Davies MJ et al. . A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci 1993;84:407–12. [DOI] [PubMed] [Google Scholar]
- 21. Re R, Pellegrini N, Proteggente A et al. . Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26:1231–7. [DOI] [PubMed] [Google Scholar]
- 22. Soares JR, Dins TCP, Cunha AP et al. . Antioxidant activity of some extracts of Thymus zygis. J Free Radic Res 1997;26:469–78. [DOI] [PubMed] [Google Scholar]
- 23. Kim KA, Kang SW, Ahn HR et al. . Leaves of persimmon (Diospyros kaki Thunb.) ameliorate N‑Methyl‑N‑nitrosourea (MNU)-induced retinal degeneration in mice. J Agric Food Chem 2015;63:7750–9. [DOI] [PubMed] [Google Scholar]
- 24. Kawakami K, Shibukura Y, Kanno T et al. . Identification of 2″-galloylated flavonol 3-O-glycosides accumulating in developing leaves of persimmon. Phytochem Anal 2011;22:403–10. [DOI] [PubMed] [Google Scholar]
- 25. Getoff N, Ritter A, Schworer F et al. . Primary yields of CH3•O and •CH2OH radicals resulting in the radiolysis of high purity methanol. Radiat Phys Chem 1993;41:797–801. [Google Scholar]
- 26. Ohkubo K, Ikeda M, Pawankar R et al. . Mechanisms of IL-6, IL-8, and GM-CSF release in nasal secretions of allergic patients after nasal challenge. Rhinology 1998;36:156–61. [PubMed] [Google Scholar]
- 27. Eigler A, Sinha B, Hartmann G et al. . Taming TNF: strategies to restrain this proinflammatory cytokine. Immunol Today 1997;18:487–92. [DOI] [PubMed] [Google Scholar]
- 28. Hibbs JB Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science 1987;235:473–6. [DOI] [PubMed] [Google Scholar]
- 29. MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol 1997;15:323–50. [DOI] [PubMed] [Google Scholar]
- 30. Koichi S, Masayuki Y, Wakako T et al. . Inhibitory effect of plant extract on tumor necrosis factor-α formation from carboxymethyllysine stimulated macrophages. Glycative Stress Res 2015;2:191–6. [Google Scholar]
- 31. Kim MS, Kim SH. Inhibitory effect of astragalin on expression of lipopolysaccharide-induced inflammatory mediators through NF-κB in macrophages. Arch Pharm Res 2011;34:2101–7. [DOI] [PubMed] [Google Scholar]