Abstract
Background The growing wealth of knowledge on whole-plant genome sequences is highlighting the key role of transposable elements (TEs) in plant evolution, as a driver of drastic changes in genome size and as a source of an important number of new coding and regulatory sequences. Together with polyploidization events, TEs should thus be considered the major players in evolution of plants.
Scope This review outlines the major mechanisms by which TEs impact plant genome evolution and how polyploidy events can affect these impacts, and vice versa. These include direct effects on genes, by providing them with new coding or regulatory sequences, an effect on the epigenetic status of the chromatin close to genes, and more subtle effects by imposing diverse evolutionary constraints to different chromosomal regions. These effects are particularly relevant after polyploidization events. Polyploidization often induces bursts of transposition probably due to a relaxation in their epigenetic control, and, in the short term, this can increase the rate of gene mutations and changes in gene regulation due to the insertion of TEs next to or into genes. Over longer times, TE bursts may induce global changes in genome structure due to inter-element recombination including losses of large genome regions and chromosomal rearrangements that reduce the genome size and the chromosome number as part of a process called diploidization.
Conclusions TEs play an essential role in genome and gene evolution, in particular after polyploidization events. Polyploidization can induce TE activity that may explain part of the new phenotypes observed. TEs may also play a role in the diploidization that follows polyploidization events. However, the extent to which TEs contribute to diploidization and fractionation bias remains unclear. Investigating the multiple factors controlling TE dynamics and the nature of ancient and recent polyploid genomes may shed light on these processes.
Keywords: Transposable element, plant genome, polyploidization, silencing, genome stress, exaptation, genome dominance, diploidization, fractionation bias, neofunctionalization, chromosomal rearrangement
INTRODUCTION
Transposable elements (TEs) are mobile genetic elements present in virtually all genomes. Among all different types of TEs, long terminal repeat (LTR) retrotransposons and miniature inverted transposable elements (MITEs) are in general the most abundant TEs in plant genomes (Casacuberta and Santiago, 2003). The larger size of LTR retrotransposons makes them, by far, the most prevalent in all sequenced plant genomes, comprising between 2·5 % in Utricularia gibba (Ibarra-Laclette et al., 2013) and 90 % of the genome in Fritillaria species (Ambrožová et al., 2011).
Together with polyploidization, TE amplification is considered the main mechanism to increase the plant genome and, more generally, for plant genome evolution (Casacuberta et al., 2016; Wendel et al., 2016). In fact, as discussed below, polyploidization and TE amplification are not two completely independent mechanisms. On the contrary, these two phenomena greatly influence one another, reinforcing their potential to drive plant genome evolution.
The role of TEs in the evolution of plant genes and genomes is not only a key for long-term plant evolution in the wild, but has also been of paramount importance for recent crop domestication and breeding (Olsen and Wendel, 2013). In this article we will review the links between polyploidization and TE dynamics, as well as the role that TEs have played in the evolution of plant genomes both in the wild and during crop domestication and breeding.
LTR RETROTRANSPOSONS AND THE EXPANSION AND CONTRACTION OF PLANT GENOMES
Although all plant genomes contain an important fraction of TEs, with LTR retrotransposons being the most abundant, the prevalence of particular families is highly variable among species and even among varieties of the same species. In many cases, a limited number of TE families have increased their copy number in one lineage (El Baidouri and Panaud, 2013). For example, a single type of LTR retrotransposon explains most of the Capsicum annuum genome expansion (Park et al., 2012), and a single Ty3/gypsy-like retrotransposon, Ogre, makes up approx. 38 % of the genome of Vicia pannonica (Neumann et al., 2006). In some cases, a family’s potential for amplification is shared by several related species (Estep et al., 2013), but it is also usual to observe a TE family with a high copy number in one species that presents a low copy number in a close relative (Hawkins et al., 2009). Moreover, important differences can even be observed among varieties of the same species such as, for example, the Grande LTR retrotransposon (Gómez-Orte et al., 2013) which shows 1450 copies in the maize inbred line B73 whereas 3500 copies are found in ‘Palomero Toluqueño’.
Although the presence of a single or a few highly repetitive TE families in a genome is usual, genomes with several TE families with similar copy numbers have also been observed. For example, although LTR retrotransposons account for almost 50 % of the genome of Pinus taeda (loblolly pine), the three most common repetitive elements represent <5 % the genome (Wegrzyn et al., 2014). All these data suggest that the capacity for TEs to invade genomes may depend on both the element and the genome, with some elements being able to escape the control in a particular genome, and some genomes being more permissive to the TE proliferation. Moreover, the amplification of TEs is not constant during evolution, and periods where TEs are relatively quiescent alternate with periods in which some TEs increase their numbers dramatically, resulting in genome expansions (Qin et al., 2014), suggesting that genome control over TEs is not constant over time. TE activity is tightly controlled by epigenetic mechanisms (Bennetzen and Wang, 2014; Ito and Kakutani, 2014). The permissiveness of some genomes to TEs may be related to a lower silencing efficiency. On the other hand, it is known that silencing can be influenced by the environment, and a transient release of silencing may be one of the reasons behind TE proliferation bursts (Willing et al., 2015).
The differential activity of particular TEs may be due to the capacity of some TEs to counteract genome silencing or to stochastic activation of particular TEs due to general weakening of silencing. Indeed, it has been shown that plant retrotransposons can escape host silencing (Hernández-Pinzón et al., 2012), in some cases by expressing anti-silencing factors (Fu et al., 2013). On the other hand, TE transcription, and in some cases their transposition and amplification, can be reactivated under particular situations such as in particular mutant backgrounds with reduced DNA methylation, some environmental conditions or after genome rearrangements (Vicient, 2010; Ito and Kakutani, 2014). For example, the expression of some TEs is activated in the pollen vegetative nurse cell surrounding the sperm cells, which triggers the production of small interfering RNAs (siRNAs) to ensure the maintenance of the epigenetic silencing of TEs in the following generation (Martínez et al., 2016). In addition, some TEs are activated under different stress conditions. Indeed, biotic and abiotic stresses activated the transcription of the tobacco Tnt1 retrotransposon (Grandbastien et al., 2005), cold and salt stresses activated the amplification of the rice MITE mPing (Naito et al., 2009), heat stress activated the transcription of the Arabidopsis thaliana retrotransposon ONSEN (Cavrak et al., 2014) and its mobilization (Ito et al, 2016), and in vitro culture activated the mobilization of different Oryza sativa (rice) and maize TEs (Hirochika, 1997; Kaeppler et al., 2000). In some of these cases, the presence of stress-associated transcription factor-binding sites (TFBSs) in the TE promoters suggests a transcriptional activation mechanism, but a decrease in silencing associated with stress could also account for the widespread association of stress and TE reactivation (Tittel-Elmer et al., 2010). The stress activation of TEs may produce an increase in TE-related mutations, some of which may result in adaptive mutations to the stress situation, as has been proposed for the arabidopsis ONSEN retrotransposon (Ito et al, 2016). Some changes in the genome, such as interspecific crosses and polyploidization events, have also been shown to lead to global epigenetic changes and activation of TE transcription (Table 1), and have, in some cases, been considered ‘genome stresses’ (Yaakov and Kashkush, 2012). This relationship will be further explored in a dedicated section (see below).
Table 1.
Examples of studies reporting reorganization of, or expression changes related to, the transposable elements after polyploidy in plants
| Species | Auto/Allo | TE | TE type | Effect | Reference |
|---|---|---|---|---|---|
| Synthetic (short-term reorganization) | |||||
| Aegilops charonensis × Triticum monococcum | Allo | Diverse | Diverse | Methylation changes. | Shaked et al. (2001) |
| Aegilops sharonensis × Triticum monococcum | Allo | Wis2-1A | LTR retrotransposon | Transcriptional activation with impact on adjacent genes. | Kashkush et al. (2002, 2003) |
| Arabidopsis thaliana × Arabidopsis arenosa | Allo | Sunfish | En-Spm-like transposon | Transcriptional activation and epigenetic changes. | Madlung et al. (2005) |
| Arabidopsis thaliana × Arabidopsis arenosa | Allo | Diverse | Diverse | Methylation changes and variation in siRNAs in the first generation. | Ha et al. (2009) |
| Arabidopsis thaliana × Arabidopsis arenosa | Allo | Diverse | Diverse | Differential repression of TEs by RNAi in the two sub-genomes. | Chen et al. (2008) |
| Arabidopsis thaliana × Arabidopsis lyrata | Allo | CAC, Ac-III | DNA transposons | No evidence of increased mobility or loss of elements from parental origin and methylation changes. | Beaulieu et al. (2009) |
| Arabidopsis thaliana × Cardaminopsis arenosa | Allo | MITE | MITE | Changes in DNA methylation. | Madlung et al. (2002) |
| Brassica carinata × Brassica rapa | Allo | Diverse | Diverse | Methylation changes. | Xu et al. (2012) |
| Brassica rapa and Brassica oleracea | Allo | Diverse | Diverse | Mobilization in the first generation and reduced in subsequent generations. | An et al. (2014) |
| Brassica rapa × Brassica oleracea | Allo | Diverse | Diverse | Methylation changes. | Xu et al. (2009) |
| Brassica rapa × Brassica oleracea | Allo | Diverse | Diverse | Changes in TE-derived miRNAs. | Fu et al. (2016) |
| Nicotiana sylvestris × Nicotiana tometosiformis | Allo | Tnt1 | LTR retrotransposon | Increase in mobility and loss of elements from parental origin. | Petit et al. (2010) |
| Oryza sativa | Auto | Diverse | Diverse | Hypermethylation that in some cases affects the expression of neighbouring genes. Changes in siRNA abundance. | Zhang et al. (2015) |
| Oryza sativa | Auto | Diverse | Diverse | Changes in miRNAs related to retrotransposons and DNA transposons. | Guo et al. (2017) |
| Spartina alterniflora × Spartina maritima | Allo | Ins2, Cassandra, Wis-like | hAT DNA transposon, TRIM, LTR retrotransposon | Loss of elements especially of maternal origin and epigenetic changes. | Parisod et al. (2009) |
| Triticum turgidum × Aegilops tauschii | Allo | Au | SINE | Mobilization, loss and epigenetic changes (hypermethylation after a few generations). | Ben-David et al. (2013) |
| Triticum turgidum × Aegilops tauschii | Allo | Minos | MITE | Mobilization (but no burst of copy number) and epigenetic changes (hypermethylation after a few generations). | Yaakov and Kashkush (2012) |
| Triticum turgidum × Aegilops tauschii | Allo | Veju | TRIM | Hypomethylated in the first S1 generation and hypermethylated in the S4 generation. | Kraitshtein et al. (2010) |
| Triticum turgidum × Aegilops tauschii | Allo | Diverse | Diverse | No mobilization. | Mestiri et al. (2010) |
| Triticum turgidum × Aegilops tauschii | Allo | Balduin, Apollo,Thalos | DNA transposons | Changes in methylation where hypermethylation was predominant. Lack of massive mobilization. | Yaakov and Kashkush (2011) |
| Triticum turgidum × Aegilops tauschii | Allo | Veju, Wis2-1A | TRIM, LTR-retrotransposon | siRNAs were reduced and CpG methylation decreased. | Kenan-Eichler et al. (2011) |
| Natural (long-term reorganization) | |||||
| Aegilops crassa, Aegilops cylindrical, Aegilops geniculata and Aegilops triuncialis | Allo | Diverse | LTR retrotransposon | Some TE families increase their mobilization and some suffer massive loss, depending on the polyploids. | Senerchia et al. (2014) |
| Arabidopsis suecica and A. arenosa | Auto/Allo | Ac-like | DNA transposon | Differential amplification and fixation of particular elements. | Hazzouri et al. (2008) |
| Arachis spp. | Allo | AhMITE1 | MITE | Recent activation of the element, possibly because of the hybridization followed by allopolyploidization. | Gowda et al. (2011) |
| Biscutella laevigata | Auto | Diverse | LTR retrotransposons | Analyses of the dynamics of LTR retrotransposons following autopolyploidy. | Bardil et al. (2015) |
| Brachiaria decumbens | Auto/Allo | Diverse | LTR retrotransposons | Transcriptional activation. | Santos et al. (2015) |
| Brassica napus | Allo | Diverse | CACTA, LTR retrotransposon | Insertion of a TE in a sub-genome contributed to significant high levels of cytosine methylation and structural divergences between genome orthologues. | Wang et al. (2012) |
| Brassica rapa | Allo | Diverse | Diverse | Biased distribution of TEs among sub-genomes. | Cheng et al. (2016) |
| Brassica rapa × Brassica oleracea | BraSto | MITE | Moderate amplification. | Sarilar et al. (2011) | |
| Brassica rapa × Brassica oleracea | Allo | Athila-like, BraSto, Bot1 | LTR retrotransposonMITECACTA | No massive structural changes. | Sarilar et al. (2013) |
| Brassica spp. | Allo | Diverse | Diverse | Different amplification of TEs depending on the genome. | Liu et al. (2014) |
| Brassica spp. | Allo | Diverse | Diverse | Small RNA-mediated silencing of transposons near genes causes position-effect downregulation. | Woodhouse et al. (2014) |
| Brassica spp. | Allo | Bot1 | CACTA | Differential amplification in the two sub-genomes. | Alix et al. (2008) |
| Capsella bursa-pastoris | Allo | Diverse | Diverse | Increase in copy number but only in the gene-rich regions and not in the centromeres. | Ågren et al. (2016) |
| Coffea arabica | Allo | Diverse | LTR retrotransposon | Differential insertions in the two sub-genomes. | Yu et al. (2011 |
| Coffea canephora × Coffea eugenioides | Allo | Diverse | Diverse | Increase in copy number. | Lopes et al. (2013) |
| Crocus spp. | Allo | Diverse | Diverse | TE markers used to identify allopolyploid parental species. | Alsayied et al. (2015) |
| Glycine max | – | Diverse | Diverse | Differential insertions in the two sub-genomes. | Innes et al. (2008) |
| Glycine max and Phaseolus vulgaris | – | Diverse | Diverse | TE-associated epigenetic gene regulation. | Kim et al. (2015) |
| Gossypium arboretum × Gossypium raimondii | Allo | Diverse | Diverse | Loss of sequences mostly of maternal origin. | Grover et al. (2007) |
| Gossypium hirsutum | Allo | Gorge3, copia, diverse | LTR retrotransposon, LINEs | Deletions in the TE genome fractions and limited transpositions. | Hu et al. (2010) |
| Gossypium hirsutum | Allo | Diverse | Diverse | TE differential activity according to the genome fraction. | Li et al. (2015) |
| Gossypium hirsutum | Allo | CRG | LTR retrotransposon | Differential amplification in the centromere of sub-genomes. | Luo et al. (2012) |
| Gossypium spp. | Allo | Diverse | LTR retrotransposons | Changes in distribution and copy number in centromeres. | Han et al. (2016) |
| Gossypium spp. | Allo | Diverse | Diverse | TE influence on genome fractionation. | Renny-Byfield et al. (2015) |
| Gossypium spp. | Allo | Diverse | Diverse | Spread of TEs in the early stages of polyploidy formation between the genomes from the diploid progenitors of a polyploid. | Zhao et al. (1998) |
| Gossypium spp. | Allo | Diverse | LTR retrotransposon | Differential amplification. | Guo et al. (2014) |
| Helianthus anomalus, Helianthus deserticola and Helianthus paradoxus | Allo | Diverse | LTR retrotransposons | Increase in copy number. | Kawakami et al. (2010) |
| Helianthus anomalus, Helianthus deserticola and Helianthus paradoxus | Allo | Diverse | LTR retrotransposons | Increase in copy number. | Ungerer et al. (2006, 2009); Staton et al. (2009) |
| Nicotiana repanda and Nicotiana nudicaulis | Allo | Diverse | Diverse | Reduction in TE copy numbers depending on species and TE families during diploidization. | Renny-Byfield et al. (2013) |
| Nicotiana spp | Allo | Diverse | SINEs, MITEs and LTR retrotransposons | Increase in copy number and loss of sequences mostly of paternal origin. | Parisod et al. (2012) |
| Nicotiana sylvestris × Nicotiana tomentosiformis | Allo | Tnt1, Tnt2, Tto1 | LTR retrotransposon | Loss of sequences mostly of paternal origin and new insertions. | Petit et al. (2010) |
| Nicotiana tabacum | Allo | Diverse | Diverse | Loss of sequences mostly of paternal origin. | Renny-Byfield et al. (2011) |
| Orobanchaceae gracilis | Auto | Diverse | LTR retrotransposons | Increase in copy number and loss of some TE families. | Piednoël et al. (2013) |
| Orobanche austrohispanica, Orobanche densiflora and Orobanche gracilis | Allo | Diverse | LTR retrotransposons | Increase in copy number. | Piednoël et al. (2015) |
| Oryza minuta | Allo | hAT | DNA transposon | Gene silencing due to DNA methylation differences within promoter regions that were associated with a TE insertion. | Sui et al. (2014) |
| Oryza punctata × Oryza officinalis | Allo | Diverse | Diverse | Loss of sequences mostly of paternal origin and mobility. | Lu et al. (2009) |
| Oryza sativa | Auto | Diverse | Diverse | Changes in siRNAs and methylation associated with TEs. | Li et al. (2014) |
| Spartina angelica | Allo | Skipper | LTR retrotransposons | Transcriptional activation. | Chelaifa et al. (2010) |
| Spartina anglica | Allo | Diverse | Diverse | Few new integration sites were found in the allopolyploid genome compared with the parental genomes. | Baumel et al. (2002) |
| Thinopyrum intermedium | Allo | Diverse | LTR-retrotransposon | Burst of Ty3/gypsy centromeric retrotransposon during allopolyploidization. | Divashuk et al. (2016) |
| Triticum aestivum | Allo | Veju, BARE1 | TRIM, LTR retrotransposons | Methylation changes. | Zhao et al. (2011) |
| Triticum aestivum | Allo | Diverse | Diverse | Increased siRNA density for TEs in one genome. | Li et al. (2014) |
| Triticum aestivum | Allo | Diverse | Diverse | TEs are involved in part of the genomic rearrangements after polyploidization events. | Chantret et al. (2005); Isidore et al. (2005) |
| Triticum aestivum | Allo | CRW, Quinta | LTR retrotransposon | TEs are involved in the centromere rearrangements after polyploidization. | Li et al. (2013) |
| Triticum aestivum | Allo | Sabrina | LTR retrotransposon | Differential amplification in the sub-genomes. | Sehgal et al. (2012) |
| Triticum aestivum | Allo | Fatima | LTR retrotransposon | Differential amplification in the sub-genomes. | Salina et al. (2011) |
| Triticum aestivum | Allo | Diverse | Diverse | TEs are involved in part of the gene specificities among genomes. | Golovnina et al. (2010) |
| Triticum aestivum | Allo | Diverse | Diverse | Differential amplification in the sub-genomes. | Salse et al. (2008) |
| Triticum spp., Aegilops spp. and allopolyploids | Allo | Stowaway-like | MITEs | Genome-specific proliferation and non-additive quantities in the polyploids. | Yaakov et al. (2013a) |
| Triticum spp., Aegilops spp. and allopolyploids | Allo | Diverse | Diverse | Some TE families proliferate in specific genomes reactivated following polyploidization. The changes that occur following polyploidization events are unique to each TE family. | Yaakov et al. (2013b) |
| Triticum turgidum × Aegilops tauschii | Allo | Diverse | Diverse | Predominantly mobility but also loss. | Chantret et al. (2005); Charles et al. (2008) |
| Zea mays | Allo | Ji, Opie | LTR retrotransposons | Increase in copy number. | Estep et al. (2013) |
| Zea mays | Allo | CRM1 | LTR retrotransposon | Expansion associated with polyploidization event. | Sharma et al. (2008) |
| Zea spp and Sorghum spp | Allo | Diverse | Diverse | Spread of TEs in Zea after an ancient genome duplication. | Gaut et al. (2000) |
Although TE amplification leads to larger genomes, their turnover and loss can also occur (Bennetzen and Wang, 2014). Unequal homologous recombination and illegitimate recombination may reduce genome TE content, and differences in their efficiency may contribute to the differences in the TE content between genomes (Bennetzen and Wang, 2014). Homologous recombination between the LTRs of a single retrotransposon results in internal domain removal, leaving behind a single recombinant LTR, or solo-LTR; these are highly abundant in some plant genomes (Vicient et al., 1999). If the recombination occurs between LTRs of two TEs, it may produce not only the loss of TE sequences but also the loss of additional genomic sequences (Vicient et al., 2005) or it may produce chromosomal rearrangements, including duplications, inversions and translocations (Ma et al., 2004).
The rate of inter-element recombination is variable among species, LTR retrotransposons and chromosomal regions (Bennetzen and Wang, 2014). For example, heterochromatin has lower recombination rates and, as a consequence, these regions contain lower ratios of solo-LTRs to intact elements (Tian et al., 2009). The processes of LTR retrotransposon removal by recombination seems to be highly efficient because in most plant genomes the majority of intact LTR retrotransposon elements found were recently inserted (Bennetzen and Wang, 2014).
In summary, the TE content of a particular genome is the result of an equilibrium between proliferation and elimination processes, and may result in plant genomes with a very different TE content (from 2·5 to 90 %). Whereas potential advantages and disadvantages of a high TE content have been proposed, the actual phenotypic consequences of this large variability in TE content and genome size are not obvious. It has recently been proposed that the balance between the TE content in different genome regions may be, in fact, more relevant than the total number of TEs in a genome (Freeling et al., 2015).
IMPACT OF TRANSPOSONS IN GENE CODING AND REGULATION IN PLANTS
A significant number of plant genes are derived from TEs in a process known as exaptation, and TEs have also contributed to the evolution of introns, exons and promoters (Zhao et al., 2016). The mechanisms by which TEs can modify genes are diverse (Contreras et al., 2015). The most obvious is the insertional inactivation of the coding or the regulatory regions of the gene. However, the insertion of a TE inside a gene may also generate more subtle mutations such as changes in the protein sequence encoded, changes in the pattern of expression or new splicing variants (Huang et al., 2015). TEs can carry ready-made promoters and/or enhancers, enabling the dissemination of discrete regulatory elements (Rebollo et al., 2012). TEs can amplify and redistribute TFBSs, creating new regulatory networks or rewiring new genes into the existing ones (Hénaff et al., 2014). The mobility of TEs containing transcriptional regulatory elements may endow genomes with a transcriptional plasticity that could be very useful for rapid adaptation to changing conditions.
Transposbale elements may also influence the expression of neighbouring genes by epigenetic effects (Contreras et al., 2015). TEs are the main target of silencing mechanisms which keep their activity under a threshold to avoid compromising genome viability. As a consequence, TEs are usually heavily methylated and are associated with heterochromatic epigenetic marks (Ito and Kakutani, 2014). The insertion of a TE close to a gene can attract silencing epigenetic marks and modify its expression, as, for example, in the case of the repression of the flowering regulator FWA in arabidopsis (Kinoshita et al., 2007) or the regulation of the sex determination gene in Cucumis melo (melon) (Martin et al., 2009). The analysis of maize populations has shown that differences in DNA methylation are associated with changes in the expression of about 300 genes, and that many of the differentially methylated regions are associated with TEs (Eichten et al., 2013). In arabidopsis, a general negative correlation exists between methylation of TEs and expression of the neighbouring genes (Hollister and Gaut, 2009), and it has been proposed that the genome distribution of TEs may contribute to the balanced transcription of gene networks (Freeling et al., 2015). TEs also seem to be at the origin of an important number of microRNA (miRNAs) (Piriyapongsa and Jordan, 2008). For example, many regulatory miRNA genes are derived from TEs in rice (Li et al., 2011) and in the green alga Volvox carteri (Dueck et al., 2016).
The close relationship between stress, TE activation and TE potential to modify gene expression can make these elements important players in plant adaptation to stress conditions. As already explained, TEs usually contain stress-inducible promoters (Cavrak et al., 2014), and their insertion close to genes may confer stress inducibility on them. For example, the rice MITE mPing inserts preferentially upstream of genes, making them stress inducible (Naito et al., 2009), and the stress-induced retrotransposon ONSEN can generate abscisic acid-insensitive mutations in arabidopsis (Ito et al., 2016). A total of 33 % of the genes expressed under stress in maize contain a TE in their promoter region, many of which also respond to stress (Makarevitch et al., 2015). In addition, it has been shown that TEs can regulate stress response genes through TE-derived siRNAs. Indeed, it has been shown that the epigenetic activation of the arabidopsis Athila retrotransposon induces the production of an siRNA that regulates a gene encoding an RNA-binding protein involved in stress granule formation (McCue et al., 2012).
The recent development of bioinformatic tools to detect TE polymorphisms using short reads from re-sequencing data (Ewing, 2015; Hénaff et al., 2015) allows analysis of the prevalence of particular TE insertions in crop varieties or populations. This should help to assess the impact of TEs in crop domestication and breeding. As an example, a recent analysis of melon varieties showed that TEs are responsible for an important part of the variability selected during melon breeding (Sanseverino et al., 2015). The fast growing number of plants and plant varieties for which the genome is available will allow more global evaluation of to what extent TEs are involved in crop domestication and breeding traits.
IMPACT OF TRANSPOSONS ON PLANT GENOME STRUCTURE
In addition to the local impact of transposons on genes, TEs can have a profound impact on genome structure and affect gene expression at a global scale. As already discussed, recombination between two TEs can potentially produce deletions of the interleaving genome sequence, or create chromosomal rearrangements. Examples of such processes have been observed in maize where the Ac element produced deletions, inversions and translocations (Weil and Wessler, 1993), or in arabidopsis where different types of TEs generated segmental duplications that occurred after divergence of the Rosales and Brassicales (Hughes et al., 2003). TE-mediated karyotype differences may be an important mechanism contributing to reproductive isolation, species diversification in plants and crop domestication.
Although there are examples of TEs that insert preferentially in gene-rich chromosomal arms (Du et al., 2010), the regions around the centromeres and telomeres usually contain a higher TE density. This is the result of different combined mechanisms. First, some TEs target heterochromatin for insertion (Contreras et al., 2015). This is frequently the case for Gypsy-like retrotransposons, whereas most Copia-like retrotransposons and most DNA TEs seem to insert preferentially in euchromatin (Contreras et al., 2015). Secondly, selection tends to eliminate deleterious insertions, concentrating TE insertions in gene-poor regions such as the heterochromatic repetitive regions. Thirdly, the rate of elimination of TEs by intra- or inter-element recombination is lower in the heterochromatic repetitive regions because they show a lower recombination rate (Zamudio et al., 2015).
The epigenetic silencing of the TEs accumulating in the heterochromatin reinforces the heterochromatic state of these regions (Bierhoff et al., 2014) which is essential for the normal functioning of these important chromosomal regions (Dernburg et al., 1996). In addition, the concentration of TEs in pericentromeric regions may help centromeres to resist microtubule tension during mitosis and meiosis (Freeling et al., 2015), and retrotransposon insertion into the centromeres contributes to the rapid evolution of the centromere (Han et al., 2016), which is important for the evolution of the species. On the other hand, recent results show that TEs in pericentromeric regions frequently contribute replication origins, somehow compensating for the scarcity of genes which are the preferred source of origins of replication (Vergara et al., unpubl. res.).
The high concentration of TEs near centromeres may also have other important consequences. The size of the heterochromatic pericentromeric regions and the concentration of TEs in them vary among plants. Whereas arabidopsis has relatively small pericentromeric TE-rich regions, the closely related Arabis alpina has a larger genome, with a higher content of retrotransposon elements which seem to have expanded its pericentromeric regions (Willing et al., 2015). Therefore, ancestral genes that have remained in gene-rich regions in arabidopsis may have been incorporated into gene-poor pericentromeric regions in A. alpina, and this may lead to different consequences. The recombination is usually strongly reduced in pericentromeric heterochromatic regions and, in consequence, the evolution of these pairs of orthologous genes may be different in the two species. The larger pericentromeric region of A. alpina correlates with a more important reduction of meiotic recombination in pericentromeric regions as compared with arabidopsis (Willing et al., 2015), which may exacerbate this consequence. Long pericentromeric regions with a high concentration of TEs may therefore constitute particular chromosomal compartments with specific evolutionary constraints which may be well suited for the evolution of particular types of genes. Interestingly, it has been recently shown that the very long heterochromatic pericentromeric regions of Solanum lycopersicum (tomato) are enriched in tomato-specific genes, whereas older genes found in all plants are depleted from these regions (Jouffroy et al., 2016), suggesting that these low-recombining regions may allow the evolution of new gene functions while maintaining the rest of the genome relatively constant. Results from our laboratory suggest that tomato is not an isolated case and other genomes such as melon, which has also expanded its TE-rich pericentromeric regions (Sanseverino et al., 2015), may also concentrate many of its species-specific genes in these regions (C.M.V. and J.M.C., unpubl. res.).
THE TIGHT LINKS BETWEEN POLYPLOIDY AND TRANSPOSABLE ELEMENT DYNAMICS
Whole-genome duplication (WGD) events, leading to polyploids, are a common theme in plant evolution. With the only exception of Gymnosperms, polyploidy is widespread in plants, either natural or domesticated, and it has been recognized as an important speciation mechanism (Adams and Wendel, 2005; Soltis et al, 2015; Shimizu-Inatsugi et al., 2017). Polyploidyzation has a profound impact on genomes. Reproductive isolation, heterosis, gene redundancy, change in mating systems, changes in cellular architecture, problems in meiosis and mitosis, gene regulatory changes and epistatic instability are some of the possible consequences of polyploidy (Soltis et al., 2015). Duplicated genes can be lost, retained or maintained, often acquiring new functions (Adams and Wendel, 2005). As a result, polyploids often show different phenotypes compared with their diploid progenitors that may contribute to their adaption to the environment or to their utility for agriculture (Gaeta et al., 2007).
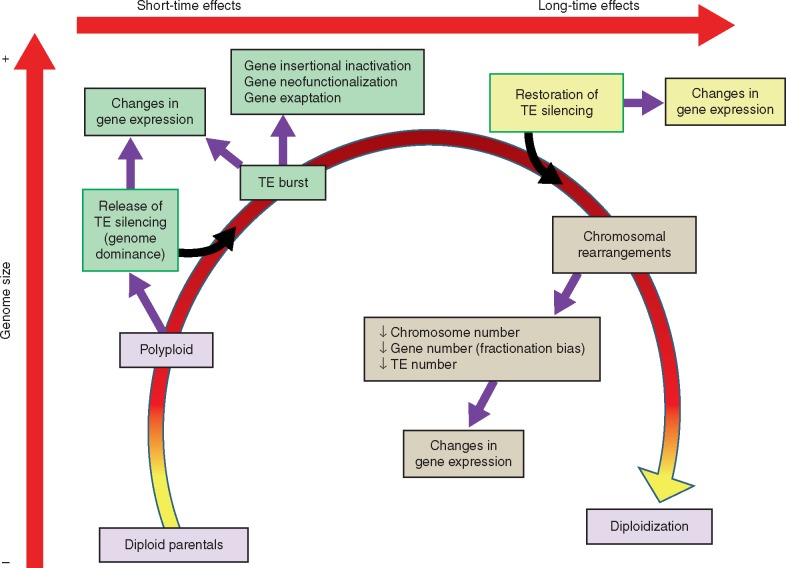
Polyploidization is frequently accompanied by an increase on TE content (Fig. 1) (McClintock, 1984). This can be the result of an induced burst of transposition. However, on the other hand, gene duplication allows genomes to cope with a higher TE activity, as a TE’s mutagenic capacity is buffered by the duplication of essential genes. This increase in TE insertions may lead not only to the inactivation of duplicated genes but also to changes in gene functions. In some cases, as has been described in the allotetraploid Capsella bursa-pastoris, the increase of TE abundance in gene-rich regions seems to be the result of a relaxed selection rather than of an increase in TE activity (Ågren et al, 2016). However, in other cases, an increase of TE activity has also been reported (An et al., 2014).
Fig. 1.
The close connections of polyploidization and TE dynamics. Polyploidization is accompanied by a release of TE silencing, which may be different for parentally or maternally inherited TEs. This release, in addition to activating TE mobilization, may induce changes in the regulation of genes located near TEs. The burst of TEs will produce new TE insertions that can modify the coding capacity of genes or their regulation. The release of TE silencing is reversed after few generations, and TE sequences again become the target of epigenetic silencing mechanisms. The silencing of TEs, including the new insertions resulting from the TE burst, will influence the expression of genes located nearby. This may result in changes of gene expression with respect to the early phases of polyploidy but also with respect to the diploid parents. TEs will also be important for the diploidization of the polyploid genome, as the different TE copies may provide sequence homology for recombination, leading to deletions and chromosome rearrangements.
When two different genomes are combined in an allopolyploid, an induction of TE activity can be the result of the loss of epigenetic silencing associated with this process (Springer et al., 2016). These changes are limited to the first generations after polyploidy which will be followed by the re-establishment of TE silencing. However, the consequences of a TE transposition burst can be extended for many more generations. Even in the absence of new transposition events, recombination between TEs, expected to be more frequent due to their higher abundance, could counteract genome expansion but also induce gene losses, gene mutations and genome restructuring. In summary, under this scenario, TEs play a key role in re-establishing a new equilibrium after genome duplication.
Transcriptional analyses in different allopolyploid plants and their parental diploids suggest that allopolyploidization induces TE transcription (Table 1). For example, an increase in the RNA levels of three En-Spm-like elements and a Ty-1 copia-like retrotransposon was detected in synthetic arabidopsis polyploids compared with the parents Arabidopsis thaliana and Arabidopsis arenosa (Madlung et al., 2005), the Wis2-1a retrotransposon showed high transcriptional activity in newly synthesized wheat amphiploids compared with its diploid parents (Kashkush et al., 2003) and the expression of Tip100 in allopolyploid coffee, Coffea arabica, is higher than in its parents C. eugenioides and C. canephora (Lopes et al., 2013).
Moreover, the copy number of TEs is frequently higher in polyploids than in their related diploid species. This is the case of the Tnt1 retrotransposon in the allotetraploid tobacco (Petit et al., 2010) and the Au SINE in wheat polyploids (Ben-David et al., 2013). Moreover, it has been shown that some TEs proliferate after polyploidization. For example, the Tekay families proliferate after Orobanche gracilis polyploidization (Piednoël et al., 2013) and the Stowaway-like MITEs transpose following allopolyploidization events in wheat and Brassica species (Sarilar et al., 2011; Yaakov and Kashkush, 2012). Moreover, a massive TE derepression was observed after hybridization of three diploid Helianthus species (Kawakami et al., 2010). However, polyploidization is not always be accompanied by an increase of TEs. For example, no significant increase in the copy number of Au SINE was found in newly formed allopolyploid Triticum aestivum (wheat) lines (Ben-David et al., 2013), in the allopolyploid Spartina anglica (Parisod et al., 2009) or in re-synthesized Brassica napus allotetraploids (Sarilar et al., 2013). There may also be differences in activation among different TE families within a single genome, as has been seen after Aegilops allotetraploidy where some gypsy-like retrotransposons proliferate whereas other remained quiescent (Senerchia et al., 2014). However, the effect on a particular TE family may also depend on the parental species, as has been shown for the Sabine retrotransposon that proliferates in particular wheat polyploids and is massively eliminated in others (Senerchia et al., 2014). It seems, therefore, that the response to polyploidization varies among genomes and TE families. Most TEs present in genomes are defective copies no longer able to transpose, and therefore old TE families will probably not respond to an activation stimulus such as the one potentially linked to polyploidization. In addition, different TE families can be regulated differently within a single genome, depending, among others, on the type of TEs, their copy number, chromosome localization and promoter sequences. For example, TEs mainly controlled by promoter methylation may be more prone to reactivation by a polyploidization-related de-methylation than those requiring a more specific transcriptional activation. Also, on the other hand, different genomes differ in their TE control efficiency due, among others, to differences in siRNA populations and methylation status. Finally, a certain degree of stochasticity in TE activation may also contribute to the differences observed on the consequences of polyploidization on TE populations.
An increasing amount of data indeed indicates that polyploidization may induce epigenetic changes, such as modifying DNA methylation at TEs (Parisod and Senerchia, 2012; Zhang et al., 2015). For example, a widespread, DNA methylation variation in TEs was observed in autotetraploid rice accompanied by changes of 24 nucleotide siRNA abundance (Zhang et al., 2015). The demethylation of TEs was observed in newly formed allopolyploids (Parisod et al., 2009; Yaakov and Kashkush, 2011) and, after a few generations, survivors gradually returned to their original TE methylation state (Zhang et al., 2015). This seems to be a general trend. For example, many Veju TRIM sequences were hypomethylated in the first generation of the newly formed wheat allohexaploid, returning to a methylation state similar to the original in the subsequent generations (Kraitshtein et al., 2010). The observed methylation alterations, either hyper- or hypomethylation, depend on the TE family and are reproducible (Yaakov and Kashkush, 2012). For example, in rice and wheat, while retrotransposons showed mainly hypomethylation in the first generation of newly formed allopolyploids, class II DNA elements were hypermethylated (Yaakov and Kashkush, 2011; Zhang et al., 2015).
As a summary, polyploidization may lead to the transient activation of some TEs. The extent of this phenomenon depends on the type of event (auto- or allopoplyploidization) and on the nature of the genome, and will affect particular families of TEs that may be more prone to activation. In addition, the relaxed selection in polyploids, due to the increase of gene copies, may also allow for a higher retention of TE insertions, which will also contribute to an increase in TE copy number.
TRANSPOSABLE ELEMENT-MEDIATED GENE REGULATION IN POLYPLOIDS
As already explained, the epigenetic silencing of TEs can reduce the expression of adjacent genes and therefore changes in TE silencing can generate heritable variations in gene expression. The important changes in TE silencing associated with polyploidization will therefore induce changes in gene expression. Genes located near reactivated TEs after polyploidization could then be under the influence of active TEs instead of silenced ones, which can modify their chromatin status and transcriptional activity. Moreover, the reactivated TEs can generate new copies of themselves (accompanied in some cases by deletions from their original locations). If these altered TE locations are close to genes, this may produce changes in their transcriptional activities. Even if the decreases in TE silencing control are transitory, they may participate in reorganizing the functional genome after polyploidization, as shown in newly synthesized wheat polyploids (Kashkush et al., 2003).
Interestingly, the expression of duplicated genes in the progeny of allopolyploids usually shows differences depending on their paternal or maternal origin, a phenomenon called genome dominance. This is reflected, for example, in a differential sub-genome control of morphological traits (Feldman et al., 2012). Genome dominance is a characteristic more usual in ancient polyploids rather than in new synthetic ones, indicating that it takes some generations to be established (Woodhouse et al., 2014). In addition, although most ancient polyploids, which probably are allopolyploids, show genome dominance, some, which probably are autopolyploids, do not (Woodhouse et al., 2014). Different mechanisms have been proposed for such inter-genomic suppression of gene activity, including chromatin modifications and the differential suppression of genes near TEs (Feldman et al., 2012).
The process of suppression of the genes near TEs by induced methylation in a polyploid genome is generally higher in one of the two parental genomes. This may be due to the fact that only the female parent contributes to cytoplasmic TE-repressing factors (e.g. siRNAs) and, as a consequence, TEs in the maternal genome are expected to have a higher repression, at least in the very early phases of polyploidy (Zhang et al., 2015). Another possibility is that the two parental genomes have different TE repression efficiencies; for example, if one of the parental genomes has a greater TE content and/or if the TEs are closer to the genes, it will become the recessive sub-genome in the stabilized allotetraploid (Garsmeur et al., 2014). In B.rapa, transposon-derived 24 nucleotide RNAs target the upstream region of genes preferentially located in the recessive sub-genome (Woodhouse et al., 2014). This has led to the hypothesis that the parental genome with the lowest TE content may become the dominant genome in the polyploid (Woodhouse et al., 2014). Whatever the initial reason is, this difference initiates a cascade of processes based on the fact that a gene that is less transcribed is a gene that can be mutated or altered more easily without phenotypic consequences. These effects will be more important the more divergent the parental species are. Thus, whereas in an autopolyploid no differences are expected, in an allopolyploid from species of different genera this difference will be very important (Cheng et al., 2016).
ROLE OF TRANSPOSABLE ELEMENTS IN DIPLOIDIZATION
Although all plant genomes present signatures of one or more polyploidy events during their evolution, they do not exhibit chromosome numbers or genome sizes proportional to such duplication processes, indicating that polyploidy is, at least in part, reversible by a process called diploidization (Soltis et al., 2015). The mechanisms governing diploidization are largely unknown, although TEs are likely to be pivotal players through transposition but also by inducing recombination and various types of chromosomal rearrangements involving reductions in chromosome number and large-scale loss of repetitive sequences and duplicated genes. It is known that TEs may have played a major role during diploidization in Nicotiana (Lim et al., 2007) and maize (Bruggmann et al., 2006). Although intra-element recombination only produces relatively small deletions, a high number of these events may represent a major process in genome restructuring during diploidization (Vicient et al., 1999).
During diploidization, usually one of the parental genomes experiences greater sequence loss than the other, as was found in Nicotiana (Renny-Byfield et al., 2011), arabidopsis (Freeling and Thomas, 2006) and maize (Woodhouse et al., 2010). This phenomenon is called fractionation bias and can be explained, at least in part, by the bias in TE insertions when comparing sub-genomes. As already explained, it has been proposed that a different TE content between the two parental genomes may lead to the dominance, and the preferential gene retention, of the genome with the lowest TE load (Woodhouse et al., 2014).
The TE-associated epigenetic changes and DNA recombination events during diploidization may produce a high number of new alleles that could allow for adaptive evolution and, following a chaotic tetraploid period, some of the duplicated genes may suffer sub-functionalization or neofunctionalization. For example, the insertion of a non-autonomous Helitron element into the promoter of the self-incompatibility male-determining gene BnSP11-1 had led to its loss of function in B. napus (B. rapa × B. oleracea) and an alteration in its mating system from self-incompatible to self-compatible, which had a great impact on the reproduction of the species (Gao et al., 2016). Moreover, different recombination events involving TEs have driven the deletion of the hardness locus, which controls grain hardness, in different sub-genomes of various polyploid wheat species (Chantret et al., 2005).
CONCLUDING REMARKS
The growing wealth of knowledge on whole-genome sequences for plant species and varieties is highlighting the major role played by TEs in the evolution of wild and domesticated plants. The impact of TEs in plant genomes includes direct effects on genes, by providing them with new coding or regulatory sequences, a more indirect effect on the epigenetic status of the chromatin close to genes, but also more subtle effects by imposing different evolutionary constraints on different chromosomal regions. Because of this, TEs are considered together with polyploidy as the major drivers of plant gene evolution. However, these are not two independent sources of variability, as polyploidy can induce TE activity and TEs explain some of the new variability associated with polyploidy. In addition, genomes tend to diploidize after polyploidization. The extent to which TEs contribute to diploidization and fractionation bias remains an open question, but it is clear that polyploid speciation is a promising model to investigate the multiple factors controlling TE dynamics, and that understanding TE activity will shed light on the dynamics of polyploid genomes.
ACKNOWLEDGEMENTS
We apologize to those authors whose primary works could not be cited owing to space limitations. Work done at the laboratoy of J.M.C. and C.M.V. was supported by by the Spanish Ministerio de Economía y Competitividad [grants AGL2013-43244-R to J.M.C., AGL2016-78992-R to J.M.C. and C.M.V., and SEV-2015-0533 to J.M.C. and C.M.V.].
LITERATURE CITED
- Adams KL, Wendel JF.. 2005. Polyploidy and genome evolution in plants. Current Opinion in Plant Biology 8: 135–141. [DOI] [PubMed] [Google Scholar]
- Ågren JA, Huang HR, Wright SI.. 2016. Transposable element evolution in the allotetraploid Capsella bursa-pastoris. American Journal of Botany 103: 1197–1202.3 [DOI] [PubMed] [Google Scholar]
- Alix K, Joets J, Ryder CD, et al. 2008. The CACTA transposon Bot1 played a major role in Brassica genome divergence and gene proliferation. The Plant Journal 56: 1030–1044. [DOI] [PubMed] [Google Scholar]
- Alsayied NF, Fernández JA, Schwarzacher T, Heslop-Harrison JS.. 2015. Diversity and relationships of Crocus sativus and its relatives analysed by inter-retroelement amplified polymorphism (IRAP). Annals of Botany 116: 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrožová K, Mandáková T, Bureš P, et al. 2011. Diverse retrotransposon families and an AT-rich satellite DNA revealed in giant genomes of Fritillaria lilies. Annals of Botany 107: 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Z, Tang Z, Ma B, et al. 2014. Transposon variation by order during allopolyploidisation between Brassica oleracea and Brassica rapa. Plant Biology (Stuttgart) 16: 825–835. [DOI] [PubMed] [Google Scholar]
- Bardil A, Tayalé A, Parisod C.. 2015. Evolutionary dynamics of retrotransposons following autopolyploidy in the Buckler Mustard species complex. The Plant Journal 82: 621–631. [DOI] [PubMed] [Google Scholar]
- Baumel A, Ainouche M, Kalendar R, Schulman AH.. 2002. Retrotransposons and genomic stability in populations of the young allopolyploid species Spartina anglica C.E. Hubbard (Poaceae). Molecular Biology and Evolution 19: 1218–1227. [DOI] [PubMed] [Google Scholar]
- Beaulieu J, Jean M, Belzile F.. 2009. The allotetraploid Arabidopsis thaliana–Arabidopsis lyrata subsp. petraea as an alternative model system for the study of polyploidy in plants. Molecular Genetics and Genomics 281: 421–435. [DOI] [PubMed] [Google Scholar]
- Ben-David S, Yaakov B, Kashkush K.. 2013. Genome-wide analysis of short interspersed nuclear elements SINES revealed high sequence conservation, gene association and retrotranspositional activity in wheat. The Plant Journal 76: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen JL, Wang H.. 2014. The contributions of transposable elements to the structure, function, and evolution of plant genomes. Annual Review of Plant Biology 65: 505–530. [DOI] [PubMed] [Google Scholar]
- Bierhoff H, Postepska-Igielska A, Grummt I.. 2014. Noisy silence: non-coding RNA and heterochromatin formation at repetitive elements. Epigenetics 9: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggmann R, Bharti AK, Gundlach H, et al. 2006. Uneven chromosome contraction and expansion in the maize genome. Genome Research 16: 1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casacuberta JM, Santiago N.. 2003. Plant LTR-retrotransposons and MITEs: control of transposition and impact on the evolution of plant genes and genomes. Gene 311: 1–11. [DOI] [PubMed] [Google Scholar]
- Casacuberta JM, Jackson S, Panaud O, Purugganan M, Wendel J.. 2016. Evolution of plant phenotypes, from genomes to traits. G3 (Bethesda) 6: 775–778.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrak VV, Lettner N, Jamge S, Kosarewicz A, Bayer LM, Mittelsten Scheid O.. 2014. How a retrotransposon exploits the plant’s heat stress response for its activation. PLoS Genetics 10: e1004115. doi:10.1371/journal.pgen.1004115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantret N, Salse J, Sabot F, et al. 2005. Molecular basis of evolutionary events that shaped the hardness locus in diploid and polyploid wheat species (Triticum and Aegilops). The Plant Cell 17: 1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles M, Belcram H, Just J, et al. 2008. Dynamics and differential proliferation of transposable elements during the evolution of the B and A genomes of wheat. Genetics 180: 1071–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelaifa H, Monnier A, Ainouche M.. 2010. Transcriptomic changes following recent natural hybridization and allopolyploidy in the salt marsh species Spartina×townsendii and Spartina anglica (Poaceae). New Phytologist 186: 161–174. [DOI] [PubMed] [Google Scholar]
- Chen M, Ha M, Lackey E, Wang J, Chen ZJ.. 2008. RNAi of met1 reduces DNA methylation and induces genome-specific changes in gene expression and centromeric small RNA accumulation in Arabidopsis allopolyploids. Genetics 178: 1845–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Sun C, Wu J, et al. , 2016. Epigenetic regulation of subgenome dominance following whole genome triplication in Brassica rapa. New Phytologist 211: 288–299. [DOI] [PubMed] [Google Scholar]
- Contreras B, Vives C, Castells R, Casacuberta JM.. 2015. The impact of transposable elements in the evolution of plant genomes: from selfish elements to key players In: Pontarotti P, ed. Evolutionary biology: biodiversification from genotype to phenotype. Cham: Springer International Publishing, 93–105. [Google Scholar]
- Dernburg AF, Sedat JW, Hawley RS.. 1996. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86: 135–146. [DOI] [PubMed] [Google Scholar]
- Divashuk MG, Khuat TM, Kroupin PY, et al. 2016. Variation in copy number of Ty3/Gypsy centromeric retrotransposons in the genomes of Thinopyrum intermedium and its diploid progenitors. PLoS One 11: e0154241. doi: 10.1371/journal.pone.0154241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Tian Z, Hans CS, et al. 2010. Evolutionary conservation, diversity and specificity of LTR-retrotransposons in flowering plants: insights from genome-wide analysis and multi-specific comparison. The Plant Journal 63: 584–598. [DOI] [PubMed] [Google Scholar]
- Dueck A, Evers M, Henz SR, et al. 2016. Gene silencing pathways found in the green alga Volvox carteri reveal insights into evolution and origins of small RNA systems in plants. BMC Genomics 17: 853. doi: 10.1186/s12864-016-3202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichten SR, Briskine R, Song J, et al. 2013. Epigenetic and genetic influences on DNA methylation variation in maize populations. The Plant Cell 25: 2783–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Baidouri M, Panaud O.. 2013. Comparative genomic paleontology across plant kingdom reveals the dynamics of TE-driven genome evolution. Genome Biology and Evolution 5: 954–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estep MC, DeBarry JD, Bennetzen JL.. 2013. The dynamics of LTR retrotransposon accumulation across 25 million years of panicoid grass evolution. Heredity (Edinburgh) 110: 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing AD. 2015. Transposable element detection from whole genome sequence data. Mobile DNA 29: 24. doi:10.1186/s13100-015-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M, Levy AA, Fahima T, Korol A.. 2012. Genomic asymmetry in allopolyploid plants: wheat as a model. Journal of Experimental Botany 63: 5045–5059. [DOI] [PubMed] [Google Scholar]
- Freeling M, Thomas BC.. 2006. Gene-balanced duplications, like tetraploidy, provide predictable drive to increase morphological complexity. Genome Research 16: 805–814. [DOI] [PubMed] [Google Scholar]
- Freeling M, Xu J, Woodhouse M, Lisch D.. 2015. A solution to the c-value paradox and the function of junk DNA: the genome balance hypothesis. Molecular Plant 8: 899–910. [DOI] [PubMed] [Google Scholar]
- Fu Y, Kawabe A, Etcheverry M, et al. 2013. Mobilization of a plant transposon by expression of the transposon-encoded anti-silencing factor. EMBO Journal 32: 2407–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Xiao M, Yu H, et al. 2016. Small RNA changes in synthetic Brassica napus. Planta 244: 607–622. [DOI] [PubMed] [Google Scholar]
- Gaeta RT, Pires JC, Iniguez-Luy F, Leon E, Osborn TC.. 2007. Genomic changes in resynthesized Brassica napus and their effect on gene expression and phenotype. The Plant Cell 19: 3403–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Zhou G, Ma C, et al. 2016. Helitron-like transposons contributed to the mating system transition from out-crossing to self-fertilizing in polyploid Brassica napus L. Science Reports 6: 33785. doi:10.1038/srep33785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsmeur O, Schnable JC, Almeida A, Jourda C, D’Hont A, Freeling M.. 2014. Two evolutionarily distinct classes of paleopolyploidy. Molecular Biology and Evolution 31: 448–454. [DOI] [PubMed] [Google Scholar]
- Gaut BS, Le Thierry d’Ennequin M, Peek AS, Sawkins MC.. 2000. Maize as a model for the evolution of plant nuclear genomes. Proceedings of the National Academy of Sciences, USA 97: 7008–7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovnina KA, Kondratenko EY, Blinov AG, Goncharov NP.. 2010. Molecular characterization of vernalization loci VRN1 in wild and cultivated wheats. BMC Plant Biology 10: 168. doi:10.1186/1471-2229-10-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Orte E, Vicient CM, Martínez-Izquierdo JA.. 2013. Grande retrotransposons contain an accessory gene in the unusually long 3′-internal region that encodes a nuclear protein transcribed from its own promoter. Plant Molecular Biology 81: 541–551. [DOI] [PubMed] [Google Scholar]
- Gowda MVC, Bhat RS, Sujay V, et al. 2011. Characterization of AhMITE1 transposition and its association with the mutational and evolutionary origin of botanical types in peanut (Arachis spp.). Plant Systematics and Evolution 291: 153–158. [Google Scholar]
- Grandbastien MA, Audeon C, Bonnivard E, et al. 2005. Stress activation and genomic impact of Tnt1 retrotransposons in Solanaceae. Cytogenetic and Genome Research 110: 229–241. [DOI] [PubMed] [Google Scholar]
- Grover CE, Kim H, Wing RA, Paterson AH, Wendel JF.. 2007. Microcolinearity and genome evolution in the AdhA region of diploid and polyploid cotton (Gossypium). The Plant Journal 50: 995–1006. [DOI] [PubMed] [Google Scholar]
- Guo H, Wang X, Gundlach H, et al. 2014. Extensive and biased intergenomic nonreciprocal DNA exchanges shaped a nascent polyploid genome, Gossypium (cotton). Genetics 197: 1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Mendrikahy JN, Xie L, et al. 2017. Transcriptome analysis of neo-tetraploid rice reveals specific differential gene expressions associated with fertility and heterosis. Scientific Reports 7: 40139. doi: 10.1038/srep40139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M, Lu J, Tian L, Ramachandran V, Kasschau K.. 2009. Small RNAs serve as a genetic buffer against genomic shock in Arabidopsis interspecific hybrids and allopolyploids. Proceedings of the National Academy of Sciences, USA 106: 17835–17840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Masonbrink RE, Shan W, et al. 2016. Rapid proliferation and nucleolar organizer targeting centromeric retrotransposons in cotton. The Plant Journal 88: 992–1005, [DOI] [PubMed] [Google Scholar]
- Hawkins JS, Proulx SR, Rapp RA, Wendel JF.. 2009. Rapid DNA loss as a counterbalance to genome expansion through retrotransposon proliferation in plants. Proceedings of the National Academy of Sciences, USA 106: 17811–17816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazzouri KM, Mohajer A, Dejak SI, Otto SP, Wright SI.. 2008. Contrasting patterns of transposable-element insertion polymorphism and nucleotide diversity in autotetraploid and allotetraploid Arabidopsis species. Genetics 179: 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hénaff E, Vives C, Desvoyes B, et al. 2014. Extensive amplification of the E2F transcription factor binding sites by transposons during evolution of Brassica species. The Plant Journal 77: 852–862. [DOI] [PubMed] [Google Scholar]
- Hénaff E, Zapata L, Casacuberta JM, Ossowski S.. 2015. Jitterbug: somatic and germline transposon insertion detection at single-nucleotide resolution. BMC Genomics 16: 768. doi: 10.1186/s12864-015-1975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Pinzón I, Cifuentes M, Hénaff E, Santiago N, Espinás ML, Casacuberta JM.. 2012. The Tnt1 retrotransposon escapes silencing in tobacco, its natural host. PLoS One 7: e33816. doi:10.1371/journal.pone.0033816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirochika H. 1997. Retrotransposons of rice: their regulation and use for genome analysis. Plant Molecular Biology 35: 231–240. [PubMed] [Google Scholar]
- Hollister JD, Gaut BS.. 2009. Epigenetic silencing of transposable elements: a trade-off between reduced transposition and deleterious effects on neighboring gene expression. Genome Research 19: 1419–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Hawkins JS, Grover CE, Wendel JF.. 2010. The history and disposition of transposable elements in polyploid Gossypium. Genome 53: 599–607. [DOI] [PubMed] [Google Scholar]
- Huang J, Gao Y, Jia H, Liu L, Zhang D, Zhang Z.. 2015. Comparative transcriptomics uncovers alternative splicing changes and signatures of selection from maize improvement. BMC Genomics 16: 363. doi:10.1186/s12864-015-1582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AL, Friedman R, Ekollu V, Rose JR.. 2003. Non-random association of transposable elements with duplicated genomic blocks in Arabidopsis thaliana. Molecular Phylogenetics and Evolution 29: 410–416. [DOI] [PubMed] [Google Scholar]
- Ibarra-Laclette E, Lyons E, Hernández-Guzmán G, et al. 2013. Architecture and evolution of a minute plant genome. Nature 498: 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes RW, Ameline-Torregrosa C, Ashfield T, et al. 2008. Differential accumulation of retroelements and diversification of NB-LRR disease resistance genes in duplicated regions following polyploidy in the ancestor of soybean. Plant Physiology 148: 1740–1759, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidore E, Scherrer B, Chalhoub B, Feuillet C, Keller B.. 2005. Ancient haplotypes resulting from extensive molecular rearrangements in the wheat A genome have been maintained in species of three different ploidy levels. Genome Research 15: 526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Kakutani T.. 2014. Control of transposable elements in Arabidopsis thaliana. Chromosome Research 22: 217–223. [DOI] [PubMed] [Google Scholar]
- Ito H, Kim J-M, Matsunaga W, et al. 2016. A stress-activated transposon in Arabidopsis induces transgenerational abscisic acid insensitivity. Scientific Reports 6: 23181. doi: 10.1038/srep23181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouffroy O, Saha S, Mueller L, et al. 2016. Comprehensive repeatome annotation reveals strong potential impact of repetitive elements on tomato ripening. BMC Genomics 17: 624. doi: 10.1186/s12864-016-2980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeppler SM, Kaeppler HF, Rhee Y.. 2000. Epigenetic aspects of somaclonal variation in plants. Plant Molecular Biology 43: 179–188. [DOI] [PubMed] [Google Scholar]
- Kashkush K, Feldman M, Levy AA.. 2002. Gene loss, silencing and activation in a newly synthesized wheat allotetraploid. Genetics 160: 1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashkush K, Feldman M, Levy AA.. 2003. Transcriptional activation of retrotransposons alters the expression of adjacent genes in wheat. Nature Genetics 33: 102–106. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Strakosh SC, Zhen Y, Ungerer MC.. 2010. Different scales of Ty1/copia-like retrotransposon proliferation in the genomes of three diploid hybrid sunflower species. Heredity 104: 341–350. [DOI] [PubMed] [Google Scholar]
- Kenan-Eichler M, Leshkowitz D, Tal L, et al. 2011. Wheat hybridization and polyploidization results in deregulation of small RNAs. Genetics 188: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KD, El Baidouri M, Abernathy B, et al. 2015. A comparative epigenomic analysis of polyploidy-derived genes in soybean and common bean. Plant Physiology 168: 1433–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita Y, Saze H, Kinoshita T, et al. 2007. Control of FWA gene silencing in Arabidopsis thaliana by SINE-related direct repeats. The Plant Journal 49: 38–45. [DOI] [PubMed] [Google Scholar]
- Kraitshtein Z, Yaakov B, Khasdan V, Kashkush K.. 2010. Genetic and epigenetic dynamics of a retrotransposon after allopolyploidization of wheat. Genetics 186: 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Liu D, Wu J, et al. 2014. mRNA and small RNA transcriptomes reveal insights into dynamic homoeolog regulation of allopolyploid heterosis in nascent hexaploid wheat. The Plant Cell 26: 1878–1900, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Choulet F, Heng Y, et al. 2013. Wheat centromeric retrotransposons: the new ones take a major role in centromeric structure. The Plant Journal 73: 952–965. [DOI] [PubMed] [Google Scholar]
- Li F, Fan G, Lu C, et al. 2015. Genome sequence of cultivated Upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nature Biotechnology 33: 524–530. [DOI] [PubMed] [Google Scholar]
- Li Y, Li C, Xia J, Jin Y.. 2011. Domestication of transposable elements into microRNA genes in plants. PLoS One 6: e19212. doi:10.1371/journal.pone.0019212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KY, Kovarik A, Matyasek R, et al. 2007. Sequence of events leading to near-complete genome turnover in allopolyploid Nicotiana within five million years. New Phytologist 175: 756–763. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu Y, Yang X, et al.2014. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nature Communications 5: 3930. doi: 10.1038/ncomms4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes FR, Jjingo D, da Silva CR, et al. 2013. Transcriptional activity, chromosomal distribution and expression effects of transposable elements in Coffea genomes. PLoS One 8: e78931. doi:10.1371/journal.pone.0078931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Ammiraju JS, Sanyal A, et al. 2009. Comparative sequence analysis of MONOCULM1-orthologous regions in 14 Oryza genomes. Proceedings of the National Academy of Sciences, USA 106: 2071–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Mach J, Abramson B, et al. 2012. The cotton centromere contains a Ty3-gypsy-like LTR retroelement. PLoS One 7: e35261. doi:10.1371/journal.pone.0035261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Devos KM, Bennetzen JL.. 2004. Analyses of LTR-retrotransposon structures reveal recent and rapid genomic DNA loss in rice. Genome Research 14: 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlung A, Masuelli RW, Watson B, Reynolds SH, Davison J, Comai L.. 2002. Remodeling of DNA methylation and phenotypic and transcriptional changes in synthetic Arabidopsis allotetraploids. Plant Physiology 129: 733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlung A, Tyagi AP, Watson B, Jiang H, Kagochi T, Doerge RW, Martienssen R, Comai L. 2005. Genomic changes in synthetic Arabidopsis polyploids. The Plant Journal 41: 221–230. [DOI] [PubMed] [Google Scholar]
- Makarevitch I, Waters AJ, West PT, et al. 2015. Transposable elements contribute to activation of maize genes in response to abiotic stress. PLoS Genetics 11: e1004915. doi: 10.1371/journal.pgen.1004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Troadec C, Boualem A, et al. 2009. A transposon-induced epigenetic change leads to sex determination in melon. Nature 461: 1135–1138. [DOI] [PubMed] [Google Scholar]
- Martínez G, Panda K, Köhler C, Slotkin RK.. 2016. Silencing in sperm cells is directed by RNA movement from the surrounding nurse cell. Nature Plants 2: 16030. doi: 10.1038/nplants.2016.30. [DOI] [PubMed] [Google Scholar]
- McClintock B. 1984. The significance of responses of the genome to challenge. Science 226: 792–801. [DOI] [PubMed] [Google Scholar]
- McCue AD, Nuthikattu S, Reeder SH, Slotkin RK.. 2012. Gene expression and stress response mediated by the epigenetic regulation of a transposable element small RNA. PLoS Genetics 8: e1002474. doi:10.1371/journal.pgen.1002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestiri I, Chague V, Tanguy A-M, Huneau C, Huteau V.. 2010. Newly synthesized wheat allohexaploids display progenitor-dependent meiotic stability and aneuploidy but structural genomic additivity. New Phytologist 186: 86–101. [DOI] [PubMed] [Google Scholar]
- Naito K, Zhang F, Tsukiyama T, et al. 2009. Unexpected consequences of a sudden and massive transposon amplification on rice gene expression. Nature 461: 1130–1134. [DOI] [PubMed] [Google Scholar]
- Neumann P, Koblížková A, Navrátilová A, Macas J.. 2006. Significant expansion of Vicia pannonica genome size mediated by amplification of a single type of giant retroelement. Genetics 173: 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen KM, Wendel JF.. 2013. A bountiful harvest: genomic insights into crop domestication phenotypes. Annual Review of Plant Biology 64: 47–70. [DOI] [PubMed] [Google Scholar]
- Parisod C, Senerchia N.. 2012. Responses of transposable elements to polyploidy In: Grandbastien MA, Casacuberta JM eds. Plant transposable elements. Berlin: Springer, 147–168. [Google Scholar]
- Parisod C, Salmon A, Zerjal T, Tenaillon M, Grandbastien MA, Ainouche M.. 2009. Rapid structural and epigenetic reorganization near transposable elements in hybrid and allopolyploid genomes in Spartina. New Phytologist 184: 1003–1015. [DOI] [PubMed] [Google Scholar]
- Parisod C, Mhiri C, Lim KY, et al. 2012. Differential dynamics of transposable elements during long-term diploidization of Nicotiana section Repandae (Solanaceae) allopolyploid genomes. PLoS One 7: e50352. doi: 10.1371/journal.pone.0050352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Park J, Kim S, et al. 2012. Evolution of the large genome in Capsicum annuum occurred through accumulation of single-type long terminal repeat retrotransposons and their derivatives. The Plant Journal 69: 1018–1029. [DOI] [PubMed] [Google Scholar]
- Petit M, Guidat C, Daniel J, et al. 2010. Mobilization of retrotransposons in synthetic allotetraploid tobacco. New Phytologist 186: 135–147. [DOI] [PubMed] [Google Scholar]
- Piednoël M, Carrete-Vega G, Renner SS.. 2013. Characterization of the LTR retrotransposon repertoire of a plant clade of six diploid and one tetraploid species. The Plant Journal 75: 699–709. [DOI] [PubMed] [Google Scholar]
- Piednoël M, Sousa A, Renner SS.. 2015. Transposable elements in a clade of three tetraploids and a diploid relative, focusing on Gypsy amplification. Mobile DNA 25: 6: 5. doi: 10.1186/s13100-015-0034.8/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piriyapongsa J, Jordan IK.. 2008. Dual coding of siRNAs and miRNAs by plant transposable elements. RNA 14: 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Yu C, Shen Y, et al. 2014. Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proceedings of the National Academy of Sciences, USA 111: 5135–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo R, Romanish MT, Mager DL.. 2012. Transposable elements: an abundant and natural source of regulatory sequences for host genes. Annual Review of Genetics 46: 21–42, [DOI] [PubMed] [Google Scholar]
- Renny-Byfield S, Chester M, Kovařík A, et al. 2011. Next generation sequencing reveals genome downsizing in allotetraploid Nicotiana tabacum, predominantly through the elimination of paternally derived repetitive DNAs. Molecular Biology and Evolution 28: 2843–2854. [DOI] [PubMed] [Google Scholar]
- Renny-Byfield S, Kovarik A, Kelly LJ, et al. 2013. Diploidization and genome size change in allopolyploids is associated with differential dynamics of low- and high-copy sequences. The Plant Journal 74: 829–839. [DOI] [PubMed] [Google Scholar]
- Renny-Byfield S, Gong L, Gallagher JP, Wendel JF.. 2015. Persistence of subgenomes in paleopolyploid cotton after 60 my of evolution. Molecular Biology and Evolution 32: 1063–1071. [DOI] [PubMed] [Google Scholar]
- Salina EA, Sergeeva EM, Adonina IG, et al. 2011. The impact of Ty3-gypsy group LTR retrotransposons Fatima on B-genome specificity of polyploid wheats. BMC Plant Biology 11: 99. doi: 10.1186/1471-2229-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salse J, Chagué V, Bolot S, et al. 2008. New insights into the origin of the B genome of hexaploid wheat: evolutionary relationships at the SPA genomic region with the S genome of the diploid relative Aegilops speltoides. BMC Genomics 9: 555. doi: 10.1186/1471-2164-9-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanseverino W, Hénaff E, Vives C, et al. 2015. Transposon insertion, structural variations and SNPs contribute to the evolution of the melon genome. Molecular Biology and Evolution 32: 2760–2774. [DOI] [PubMed] [Google Scholar]
- Santos FC, Guyot R, do Valle CB, et al. 2015. Chromosomal distribution and evolution of abundant retrotransposons in plants: gypsy elements in diploid and polyploid Brachiaria forage grasses. Chromosome Research 23: 571–582. [DOI] [PubMed] [Google Scholar]
- Sarilar V, Marmagne A, Brabant P, Joets J, Alix K.. 2011. BraSto, a Stowaway MITE from Brassica: recently active copies preferentially accumulate in the gene space. Plant Molecular Biology 77: 59–75. [DOI] [PubMed] [Google Scholar]
- Sarilar V, Palacios PM, Rousselet A, et al. 2013. Allopolyploidy has a moderate impact on restructuring at three contrasting transposable element insertion sites in resynthesized Brassica napus allotetraploids. New Phytologist 198: 593–604. [DOI] [PubMed] [Google Scholar]
- Sehgal SK, Li W, Rabinowicz PD, et al. 2012. Chromosome arm-specific BAC end sequences permit comparative analysis of homoeologous chromosomes and genomes of polyploid wheat. BMC Plant Biology 12: 64. doi: 10.1186/1471-2229-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senerchia N, Felber F, Parisod C.. 2014. Contrasting evolutionary trajectories of multiple retrotransposons following independent allopolyploidy in wild wheats. New Phytologist 202: 975–985.. [DOI] [PubMed] [Google Scholar]
- Shaked H, Kashkush K, Ozkan H, Feldman M, Levy AA.. 2001. Sequence elimination and cytosine methylation are rapid and reproducible responses of the genome to wide hybridization and allopolyploidy in wheat. The Plant Cell 13: 1749–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Schneider KL, Presting GG.. 2008. Sustained retrotransposition is mediated by nucleotide deletions and interelement recombinations. Proceedings of the National Academy of Sciences, USA 105: 15470–15474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Inatsugi R, Terada A, Hirose K, Kudoh H, Sese J, Shimizu KK.. 2017. Plant adaptive radiation mediated by polyploid plasticity in transcriptomes. Molecular Ecololgy 26: 193–207. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Marchant DB, Van de Peer Y, Soltis DE.. 2015. Polyploidy and genome evolution in plants. Current Opinion in Genetics and Development 35: 119–125. [DOI] [PubMed] [Google Scholar]
- Springer NM, Li Q, Lisch D.. 2016. Creating order from chaos: epigenome dynamics in plants with complex genomes. The Plant Cell 28: 314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton SE, Ungerer MC, Moore RC.. 2009. The genomic organization of Ty3/gypsy-like retrotransposons in Helianthus (Asteraceae) homoploid hybrid species. American Journal of Botany 96: 1646–1655. [DOI] [PubMed] [Google Scholar]
- Sui Y, Li B, Shi J, Chen M.. 2014. Genomic, regulatory and epigenetic mechanisms underlying duplicated gene evolution in the natural allotetraploid Oryza minuta. BMC Genomics 15: 11. doi: 10.1186/1471-2164-15-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z, Rizzon C, Du J, et al. 2009. Do genetic recombination and gene density shape the pattern of DNA elimination in rice long terminal repeat retrotransposons? Genome Research 19: 2221–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittel-Elmer M, Bucher E, Broger L, Mathieu O, Paszkowski J, Vaillant I.. 2010. Stress-induced activation of heterochromatic transcription. PLoS Genetics 6: e1001175. doi: 10.1371/journal.pgen.1001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerer MC, Strakosh SC, Zhen Y.. 2006. Genome expansion in three hybrid sunflower species is associated with retrotransposon proliferation. Current Biology 16: R872–R873. [DOI] [PubMed] [Google Scholar]
- Ungerer MC, Strakosh SC, Stimpson KM.. 2009. Proliferation of Ty3/gypsy-like retrotransposons in hybrid sunflower taxa inferred from phylogenetic data. BMC Biology 7: 40. doi: 10.1186/1741-7007-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicient CM. 2010. Transcriptional activity of transposable elements in maize. BMC Genomics 11: 601. doi: 10.1186/1471-2164-11-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicient C, Suoniemi A, Anamthawat-Jónsson K, et al. 1999. Retrotransposon BARE-1 and its role in genome evolution in the genus Hordeum. The Plant Cell 11: 1769–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicient CM, Kalendar R, Schulman AH.. 2005. Variability, recombination, and mosaic evolution of the barley BARE-1 retrotransposon. Journal of Molecular Evolution 61: 275–291. [DOI] [PubMed] [Google Scholar]
- Wang J, Hopkins CJ, Hou J, et al. 2012. Promoter variation and transcript divergence in Brassicaceae lineages of FLOWERING LOCUS T. PLoS One 7: e47127. doi: 10.1371/journal.pone.0047127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegrzyn JL, Liechty JD, Stevens KA, et al. 2014. Unique features of the loblolly pine (Pinus taeda L.) megagenome revealed through sequence annotation. Genetics 196: 891–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil CF, Wessler SR.. 1993. Molecular evidence that chromosome breakage by Ds elements is caused by aberrant transposition. The Plant Cell 5: 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel JF, Jackson SA, Meyers BC, Wing RA.. 2016. Evolution of plant genome architecture. Genome Biology 17: 37. doi: 10.1186/s13059-016-0908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing EM, Rawat V, Mandáková T, et al. 2015. Genome expansion of Arabis alpina linked with retrotransposition and reduced symmetric DNA methylation. Nature Plants 1: 14023. doi: 10.1038/nplants.2014.23. [DOI] [PubMed] [Google Scholar]
- Woodhouse MR, Schnable JC, Pedersen BS, et al. 2010. Following tetraploidy in maize, a short deletion mechanism removed genes preferentially from one of the two homologs. PLoS Biology 8: e1000409. doi: 10.1371/journal.pbio.1000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse MR, Cheng F, Pires JC, Lisch D, Freeling M, Wang X.. 2014. Origin, inheritance, and gene regulatory consequences of genome dominance in polyploids. Proceedings of the National Academy of Sciences, USA 111: 5283–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhong L, Wu X, Fang X, Wang J.. 2009. Rapid alterations of gene expression and cytosine methylation in newly synthesized Brassica napus allopolyploids. Planta 229: 471–483. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhao Q, Mei S, Wang J.. 2012. Genomic and transcriptomic alterations following hybridisation and genome doubling in trigenomic allohexaploid Brassica carinata × Brassica rapa. Plant Biology (Stuttgart) 14: 734–744. [DOI] [PubMed] [Google Scholar]
- Yaakov B, Kashkush K.. 2011. Massive alterations of the methylation patterns around DNA transposons in the first four generations of a newly formed wheat allohexaploid. Genome 54: 42–49. [DOI] [PubMed] [Google Scholar]
- Yaakov B, Kashkush K.. 2012. Mobilization of Stowaway-like MITEs in newly formed allohexaploid wheat species. Plant Molecular Biology 80: 419–427. [DOI] [PubMed] [Google Scholar]
- Yaakov B, Ben-David S, Kashkush K.. 2013a. Genome-wide analysis of Stowaway-like MITEs in wheat reveals high sequence conservation, gene association, and genomic diversification. Plant Physiology 161: 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaakov B, Meyer K, Ben-David S, Kashkush K.. 2013b. Copy number variation of transposable elements in Triticum–Aegilops genus suggests evolutionary and revolutionary dynamics following allopolyploidization. Plant Cell Reports 32: 1615–1624. [DOI] [PubMed] [Google Scholar]
- Yu Q, Guyot R, de Kochko A, et al. 2011. Micro-collinearity and genome evolution in the vicinity of an ethylene receptor gene of cultivated diploid and allotetraploid coffee species (Coffea). The Plant Journal 67: 305–317. [DOI] [PubMed] [Google Scholar]
- Zamudio N, Barau J, Teissandier A, et al. 2015. DNA methylation restrains transposons from adopting a chromatin signature permissive for meiotic recombination. Genes and Development 29: 1256–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu Y, Xia EH, Yao QY, Liu XD, Gao LZ.. 2015. Autotetraploid rice methylome analysis reveals methylation variation of transposable elements and their effects on gene expression. Proceedings of the National Academy of Sciences, USA 112: E7022–E7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Ferguson AA, Jiang N.. 2016. What makes up plant genomes: the vanishing line between transposable elements and genes. Biochimica et Biophysica Acta 1859: 366–380. [DOI] [PubMed] [Google Scholar]
- Zhao N, Zhu B, Li M, et al. 2011. Extensive and heritable epigenetic remodeling and genetic stability accompany allohexaploidization of wheat. Genetics 188: 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XP, Si Y, Hanson RE, et al. 1998. Dispersed repetitive DNA has spread to new genomes since polyploid formation in cotton. Genome Research 8: 479–492. [DOI] [PubMed] [Google Scholar]