Abstract
Background
Malignant glioma (MG) is a devastating neuro-oncologic disease with almost invariably poor prognosis. Prognostic awareness (PA) is the awareness of incurable disease and shortened life expectancy (LE). Accurate PA is associated with favorable psychological outcomes at the end of life (EoL) for patients with cancer; however, little is known about PA or prognostic communication in MG. Moreover, research has yet to evaluate the impact of cognitive impairment on PA and preferred forms of communication.
Methods
Fifty MG patients and 32 paired caregivers were evaluated in this exploratory study with a semi-structured PA assessment aimed to measure their awareness of MG incurability and LE. Full PA was defined as awareness of MG incurability and accurate estimate of LE. The assessment included a survey about preferences for prognostic communication (items from the Prognosis and Treatment Perceptions Questionnaire), neurocognitive assessment (Hopkins Verbal Learning Test–Revised, Trail Making Test Parts A and B, and the Controlled Oral Word Association Test), and measurements of mood (Hospital Anxiety and Depression Scale) and quality of life (Functional Assessment of Cancer Therapy–Brain [FACT-Br]).
Results
Twenty (40%) patients and 22 (69%) caregivers had full PA. Thirty (60%) patients and 23 (72%) caregivers reported that prognostic information was extremely or very important, and 21 (42%) patients and 16 (50%) caregivers desired more prognostic information. Patients with memory impairment more frequently believed that prognostic information was important (P = 0.04, P = 0.03) and desired more information (P = 0.05, P = 0.003) as compared with those without impairment.
Conclusions
Most MG patients were unaware of their LE. Memory impairment may influence preferences for prognostic information.
Keywords: communication, end of life, glioblastoma, malignant brain tumor, quality of life
Importance of the study
This is the first prospective study of PA, including awareness of incurability and of life expectancy in patients with MG, and the first study to examine associations between cognitive function and communication preferences in this patient population. Our data suggest that further research is necessary to understand the underpinnings and implications of inaccurate PA in MG and that such investigation should measure and account for cognitive impairment as it may influence communication outcomes.
For individuals with incurable cancer, optimizing psychosocial well-being, symptom control, quality of life (QoL), and end-of-life (EoL) care is of the utmost importance. Prognostic awareness (PA) is a measurable knowledge state1 reflecting awareness of the incurability of one’s disease and of shortened life expectancy (LE), or in some cases imminent death. Research in recent years has focused on associations between PA and a variety of outcomes related to medical treatments and QoL in cancer. Patients with accurate estimates of LE are more likely to reach acceptance of terminal illness, discuss preferences for treatment at EoL, ultimately receive care enacting those preferences, and have reduced psychological distress and improved QoL at EoL.2–9 Furthermore, it has been demonstrated that disclosure of prognosis by oncologists is associated with accurate understanding of terminal illness and realistic expectations of LE.10,11 There is ample evidence, nonetheless, that oncologists often do not share terminal cancer prognosis with patients5,12 and caregivers,13,14 even though information about EoL is desired by patients10 and felt to embody higher-quality care.15 In several studies,16,17 cancer patients have been found frequently to have inaccurate PA; poor PA was identified in 81% of advanced colorectal cancer patients who believed that their palliative chemotherapy had a curative intent.18
Malignant glioma (MG; World Health Organization [WHO] grade III or IV) is a devastating neurologic illness leading to progressive functional decline, cognitive impairment, and almost invariably death. Despite the universally poor prognosis in MG, referral to hospice within 7 days of death and acute hospitalization within 30 days of death are common, and these outcomes are associated with poor QoL at EoL in other cancers.19,20 There is little systematic research about the landscape of PA and prognostic communication in MG. One systematic review21 identified 6 studies that investigated PA in this population, only 3 of which were prospective.22–24 In these studies, 25%–58% of patients demonstrated “accurate” PA; however, the definition of PA varied widely and none investigated participants’ estimate of LE. Moreover, there is scarce literature about MG patients’ wishes for prognostic information. Some MG patients wish that prognosis was discussed in greater depth and earlier in the disease course, but others do not want to discuss prognosis fully, especially when discussion is experienced as deleterious to maintaining hope.25–27 To date, no study has examined both PA and communication preferences in the same MG population. Furthermore, there has been no investigation of the association between neurocognitive function and PA itself or preferences for communication of prognostic information.
The effect of neurocognitive impairment upon prognostic communication is understudied in cancer generally, although there is modest evidence that even mild impairment can alter EoL decision making.28 Changes in neurocognitive functioning could alter communication dynamics in a variety of ways, including comprehension of prognostic information, wish for information, or physicians’ approach to prognostic disclosure; however, these topics have not been explored in MG, as impaired patients were excluded from prior studies.21 Finally, there has been no examination of MG patients’ prognostic understanding and mood changes such as anxiety and depression.
We present here data on a study of PA and preferences for prognostic communication and neurocognitive function in MG patients and their caregivers. Because systematic research is vitally needed about prognostic communication in MG, and because cognitive impairment is frequent in this disease, we sought to examine PA, including knowledge about LE, in this population. In light of the above-mentioned research demonstrating late hospice referral and hospital admission in MG, this was an exploratory study to examine the frequency of accurate PA in MG. As previous research is scant and conflicting about PA in MG and has not been examined in impaired patients, we did not have an a priori hypothesis about frequency of PA. Also, we sought to explore associations between neurocognitive function and patterns of PA and communication preferences.
Materials and Methods
Study Sample
This was an institutional review board–approved, prospective, mixed-methods exploratory study of adult patients with MG admitted to the inpatient neurology service, with a paired caregiver, at Memorial Sloan Kettering Cancer Center. Screening was performed by research staff (J.B.) by review of a list of admitted MG patients generated daily in an automated fashion. Eligibility criteria included age of at least 18 years; diagnosis of MG; intact sensorium defined by full wakefulness (eyes open and following instructions); and orientation to self, place, current month and year, and age. MG diagnosis was based upon radiologic-histopathologic assessment, not molecular characteristics such as status of O6-DNA methylguanine-methyltransferase (MGMT) or isocitrate dehydrogenase. Exclusion criteria included receptive or expressive aphasia interfering with reading the informed consent document and verbalizing cogent understanding of the study’s risks and benefits; hemianopsia; and hemiparesis of the dominant arm that would interfere with completing timed cognitive tests. The study was performed in the inpatient setting because it was unclear how much time the full mixed-methods assessment would require to complete. An eligible informal caregiver was 18 years of age or older and was designated by a patient participant as someone close or important to him/her who provided unpaid help and support. Patients and caregivers completed a separate written informed consent. Caregiver participation was encouraged but not required.
Study Procedures and Assessments
The study assessments were performed in a private, quiet, and uninterrupted setting. Patients and caregivers were assessed separately. Patient assessments were organized as follows: Neurocognitive assessment was performed first to avoid altered performance as a result of discussing prognosis or other sources of emotionality. The selected neurocognitive test battery is frequently implemented in therapeutic trials in patients with primary malignant brain tumors, including MG.30,31 The battery includes tests of verbal memory (Hopkins Verbal Learning Test–Revised [HVLT-R]29) and graphomotor speed and executive functions (Trail Making Test Parts A and B30 and the Controlled Oral Word Association Test [COWA]31), performed in this order. Next, participants completed self-report scales of QoL (Functional Assessment of Cancer Therapy—Brain [FACT-Br]) and mood (Hospital Anxiety and Depression Scale [HADS]).32
The assessment of PA and communication preferences had 2 components. First, the PA assessment tool is a published, semi-structured qualitative interview implemented in the specific context of PA measurement in patients with cancer.2 All assessments were performed by one of 3 trained members of the study team (E.L.D., M.K., or J.B.). Interviews were audio-recorded and transcribed verbatim for analysis by 2 study team members (E.L.D. and A.J.A.). For all interviews, the following questions were asked:
What do you understand about your illness?
How serious do you believe things are?
What have you been told?
Do you have a sense of how much time might be left for you?
For all participants, these prompts were posed with the precise wording above. Unstructured follow-up questions were added to elaborate upon responses and to elicit the participant’s (a) belief whether he/she had a curable or incurable disease and (b) her/his estimation of LE. Furthermore, these follow-up questions encouraged the participant to express both his/her hopes about curability and LE as well as his/her beliefs. The interview emphasized the notion of participants’ hopes for 2 reasons: (i) prior research in MG suggested that prognostic discussions can be most effective and compassionate when not experienced as being at odds with optimism23,33 and (ii) expressions of hope would not be conflated with objective prognostic beliefs in the analysis of interviews. After the PA assessment, the interviewer completed a form indicating whether any PA prompts were not asked verbatim, and if so, the reason for this. All participants were offered psychiatry consultation upon completing the assessment, such that if participation had triggered sadness or worry they could discuss these fully.
Scoring was performed collaboratively by the study leaders (E.L.D. and A.J.A.). Full PA was defined as both (i) awareness of the incurability of MG and (ii) a reasonably accurate estimate of LE. This designation was intrinsically subjective but aimed to favor a score of full PA in a situation of ambiguity by way of the guiding principle that any reference made to standard survival statistics, or a similar estimate, constituted full PA. Specifically, any survival estimate of 3 years (from the time of diagnosis) or less for glioblastoma (GBM) or 7 years or less for grade III tumors was considered full PA, although longer estimates were designated as full PA if prognostic factors (such as MGMT methylation and resection status or oligodendroglioma histology) were cited. Additionally, patients citing longer LE in light of their own personal disease trajectory (ie, a GBM patient alive 5 years from diagnosis) were considered to have full PA. Limited PA was defined as (i) awareness of the incurability of MG but (ii) a fundamentally inaccurate estimate of LE, such as several years beyond standard survival statistics or estimates inconsistent with their disease status (ie, citing 5-year survival in the context of multiply recurrent GBM). No PA was defined as the factual belief that MG is a curable disease. Citing examples of cure or long-term survivorship was viewed as an expression of hope and optimism that was compatible with full PA when expressed with awareness that this is rare. After the PA interview, a short questionnaire was completed selected from the Prognosis and Treatment Perceptions Questionnaire, an assessment used to measure prognostic perceptions in children with cancer and then adapted to the context of adults.5,11,36,37 Here, participants rated (i) how important it was to them to know about their prognosis (phrased as “the likely outcome of your brain tumor over time,” with responses ranging from extremely important, very important, somewhat important, a little bit important, to not at all important); (ii) the amount of information they possessed about their prognosis (wishing they had more information, wishing they had less, or that the information they possessed was as desired); and (iii) the quality of prognostic information they have received thus far (excellent, very good, satisfactory, fair, or poor). Additionally, participants indicated in a binary fashion whether the internet was a source of information and whether a physician or nurse was a source of information.
Data Analysis
For patients and caregivers, sex, race, and educational attainment were captured and summarized. Additionally, for patients, WHO tumor grade (III vs IV), location, laterality, recurrence status, and prior trial participation were summarized. Neurocognitive raw test scores were normalized for age and educational attainment and converted into z-scores. For each test, z-scores were dichotomized to impaired versus non-impaired, with impairment defined as a z-score less than or equal to −1.5, which is to say a score ≤1.5 standard deviations below average for individuals of the same age and educational level. The FACT-Br total and subscale scores were captured as a continuous variable. The HADS yielded 2 dichotomous scores for the presence or absence of borderline or greater anxiety and depression, defined as a score on either subscale greater than 8.
For analysis purposes, PA was dichotomized a priori to full PA versus limited or no PA. For the Prognosis and Treatment Perceptions Questionnaire, importance of prognostic information was dichotomized to extremely/very important versus other responses, quantity of information was dichotomized to desiring more information versus other responses, and quality of information was dichotomized to excellent/very good/satisfactory versus other responses. Age was compared between PA groups with the Wilcoxon rank sum test, and race, tumor site, tumor laterality, grade, and recurrence status were compared between PA groups with chi-square tests. Proportions of participants with impaired z-scores for each neurocognitive test, anxiety, and depression were similarly compared among PA groups. Last, the proportion of participants with impaired performance on each cognitive test was compared between dichotomous groups based on importance of prognostic information and wish for more prognostic information.
Results
Patients
Ninety-seven patients were screened; 24 patients were excluded because of aphasia, hemiparesis in the dominant arm, or hemianopsia; 11 were not fully oriented; 2 were not fluent in English. Six were eligible but refused; 54 patients consented to the study, although 2 patients could not complete the cognitive assessment, so the study was aborted. Two patients were discharged before participating; 48 caregivers consented to the study but 16 were unavailable for the assessment. In total, 50 MG patients with 32 matched caregivers participated in the study (Table 1). Thirty-four (68%) patients were men, 36 (72%) were Caucasian; 34 (68%) patients had GBM; 38 (76%) had tumors in the right hemisphere; 26 (58%) patients had multiply recurrent disease. For all patient and caregiver participants, the PA assessment was completed and performed without deviation from the wording of the interview prompts. No participant withdrew consent from the study or aborted the PA assessment, and no participant accepted a psychiatry consultation.
Table 1.
Demographic and clinical characteristics of the study population
| Patients (N = 50) | Caregivers (N = 32) | ||||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age | – | Median: 50 | Range: 18–77 | Median: 51 | Range: 28–81 |
| Sex | Female | 16 | 32 | 19 | 59 |
| Male | 34 | 68 | 13 | 41 | |
| Race | White | 36 | 72 | 23 | 72 |
| Black | 4 | 8 | 2 | 6 | |
| Asian/South Asian | 4 | 8 | 3 | 9 | |
| Unknown | 6 | 12 | 4 | 13 | |
| Education | High school | 5 | 10 | 1 | 3 |
| Vocational training | 6 | 12 | 2 | 6 | |
| Some college | 6 | 12 | 3 | 9 | |
| College graduate | 17 | 34 | 14 | 44 | |
| Postgraduate | 16 | 32 | 12 | 38 | |
| WHO tumor grade | III | 16 | 32 | ||
| IV | 34 | 68 | |||
| Location | Frontal lobe | 26 | 52 | ||
| Occipital | 1 | 2 | |||
| Parietal lobe | 8 | 16 | |||
| Temporal lobe | 10 | 20 | |||
| Nonhemispheric | 5 | 10 | |||
| Side | Left | 11 | 22 | ||
| Right | 38 | 76 | |||
| Bilateral or midline | 1 | 2 | |||
| Disease status | Not recurrent | 17 | 34 | ||
| One recurrence | 9 | 18 | |||
| Multiply (>1) recurrent | 24 | 48 | |||
| Therapeutic trial | Participated | 33 | 66 | ||
| Never participated | 27 | 34 | |||
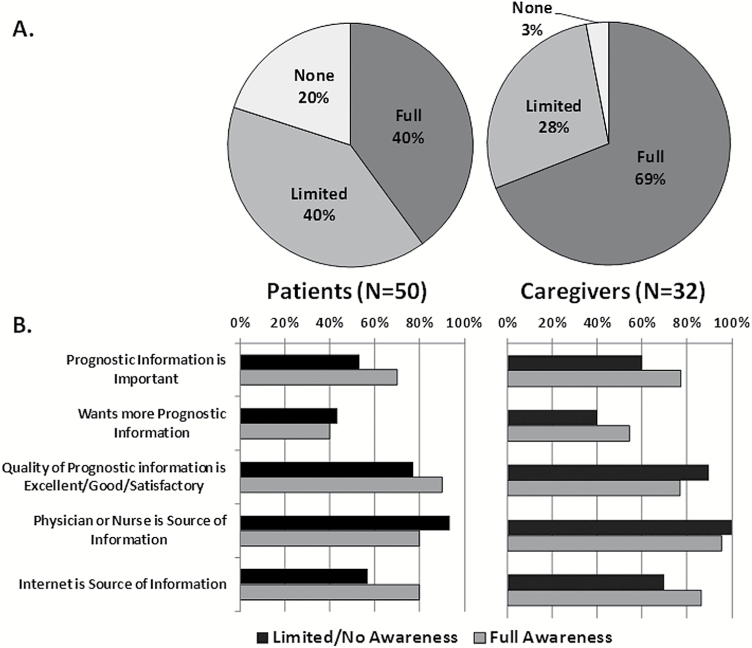
Of the patients, 20 (40%) had full PA, 20 (40%) had limited PA, and 10 (20%) had no PA (Table 2). Of note, 17 (57%) of the patients with multiply recurrent MG had limited or no PA. Representative verbatim passages from PA interviews, demonstrating participants’ beliefs about MG curability and LE, are presented in Table 3. Thirty (60%) patients reported that information about prognosis was extremely or very important to have, 21 (42%) indicated that they wanted more prognostic information, and 42 (84%) indicated that the prognostic information they had received was excellent, very good, or satisfactory. Furthermore, 33 (66%) listed the internet as a source of prognostic information and 44 (88%) indicated that a physician or nurse was a source of information. Borderline or greater anxiety and depression was present in 19 (38%) and 19 (38%) patients, respectively. The median FACT-Br score was 112 (SD 24) and median Emotional subscale score was 15 (SD 5).
Table 2.
Prognostic awareness and information preferences for the entire study population
| Patients (N = 50) | Caregivers (N = 32) | ||||
|---|---|---|---|---|---|
| N | % | N | % | ||
| PA | Full PA | 20 | 40 | 22 | 69 |
| Limited PA | 20 | 40 | 9 | 28 | |
| No PA | 10 | 20 | 1 | 3 | |
| Communication preferences | Prognostic information is extremely or very important to know | 30 | 60 | 23 | 72 |
| Wants more prognostic information | 21 | 42 | 16 | 50 | |
| Quality of information is fair or poor | 8 | 16 | 6 | 19 | |
| Information sources | Internet is source of information | 33 | 66 | 26 | 81 |
| Physician or nurse is source of information | 44 | 88 | 31 | 97 | |
| Cognitive impairment | TMT Part A: z ≤ −1.5 | 33 | 66 | ||
| TMT Part B: z ≤ −1.5 | 35 | 70 | |||
| COWA Total: z ≤ −1.5 | 28 | 56 | |||
| HVLT-R Total: z ≤ −1.5 | 32 | 64 | |||
| HVLT-R Delay: z ≤ −1.5 | 38 | 74 | |||
| HVLT-R Discrimination Index: z ≤ −1.5 | 33 | 66 | |||
| Mood | Anxiety (HADS ≥8) | 19 | 38 | ||
| Depression (HADS ≥8) | 19 | 38 | |||
| Quality of life | FACT-Br Total Score | Median: 112 | SD 24 | ||
| FACT-Br Emotional Subscale | Median: 15 | SD: 5 | |||
Abbreviations: TMT, Trail-Making Test; COWA, Controlled Oral Word Association; HVLT-R, Hopkins Verbal Learning Test–Revised; HADS, Hospital Anxiety and Depression Scale; FACT-Br, Functional Assessment of Cancer Therapy–Brain.
Table 3.
Representative verbatim quotations from PA interviews
| No Awareness | Limited Awareness | Full Awareness | |
|---|---|---|---|
| Curability | “If I follow the protocols and keep a good state of mind, I think I have a good chance of beating this.” | “I don’t believe this is curable.” “95% lethal, 5% survive. I need to have good luck.” |
“It’s an aggressive tumor. They never said it’s not going to come back.” “It’s awful. For cancer, it’s the worst of the worst.” |
| Life expectancy | “Lifespan? Do I have a sense? About as much as a normal person.” | “An estimated time, I’m looking at maybe 10 years.” | “Um, probably the end of October. Today is May, June . . . I would start worrying in about 6 months.” “Maybe 2–3 years, 2 years probably.” |
Comparing patients with full PA to those with limited or no PA, there were no differences with respect to age, sex, race, or any tumor characteristic (Table 4; Fig. 1). Thirty-seven percent of patients with full PA had participated in a therapeutic clinical trial, compared with 60% of those with limited or no PA (P = 0.10). There were no significant differences between patients with full PA versus limited/no PA with respect to information preferences or sources of information. The proportion of patients with full PA and anxiety, depression, or impaired performance on any of the neurocognitive tests was not significantly different compared with those with limited or no PA. Statistically significant differences were observed with respect to neurocognitive test performance and preferences for prognostic information (Table 5). Patients who reported that prognostic information was important to know were significantly more impaired on tests of memory (77% vs 47% on the HVLT-Total, P = 0.04; 60% vs 28% on the HVLT-Discrimination Index, P = 0.03); the results approached significance for delayed recall (87% vs 63% on the HVLT-Delay, P = 0.06). Furthermore, patients who indicated a wish for more prognostic information were more frequently impaired on tests of memory (81% vs 54% on the HVLT-Total, P = 0.05; 71% vs 28% on the HVLT-Discrimination Index, P = 0.003).
Table 4.
Comparison of demographic characteristics, clinical characteristics, and cognitive function between patients with full prognostic awareness versus limited or no prognostic awareness
| Characteristic | Full Awareness (n = 20) | Limited/No Awareness (n = 30) | P-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Sex | |||||
| Female | 5 | 25 | 11 | 37 | 0.39 |
| Male | 15 | 75 | 19 | 63 | |
| Age (continuous) | Median: 51.5 | Range: 28–70 | Median: 50 | Range: 18–77 | 0.24 |
| Race | |||||
| White | 14 | 70 | 22 | 73 | 0.80 |
| Nonwhite | 6 | 30 | 8 | 27 | |
| Subsite | |||||
| Frontal lobe | 10 | 50 | 13 | 43 | 0.64 |
| Other | 10 | 50 | 17 | 57 | |
| Side | |||||
| Left or bilateral | 5 | 25 | 7 | 23 | 1.0 |
| Right | 15 | 75 | 23 | 77 | |
| WHO Tumor Grade | |||||
| III (non-GBM) | 5 | 25 | 11 | 37 | 0.54 |
| IV | 15 | 75 | 19 | 63 | |
| Tumor Status | |||||
| >1 recurrence | 11 | 55 | 13 | 43 | 0.42 |
| ≤1 recurrence | 9 | 45 | 17 | 57 | |
| Clinical trial participation | 12 | 60 | 11 | 37 | 0.10 |
| TMT Part A: z ≤ −1.5 | 11 | 55 | 22 | 76 | 0.13 |
| TMT Part B: z ≤ −1.5 | 12 | 60 | 23 | 79 | 0.14 |
| COWA Total: z ≤ −1.5 | 9 | 45 | 19 | 66 | 0.15 |
| HVLT-R Total: z ≤ −1.5 | 12 | 60 | 20 | 69 | 0.52 |
| HVLT-R Delay: z ≤ −1.5 | 14 | 70 | 24 | 83 | 0.32 |
| HVLT-R Discrimination: z ≤ −1.5 | 11 | 55 | 12 | 43 | 0.41 |
| Anxiety (HADS ≥8) | 7 | 37 | 12 | 40 | 0.96 |
| Depression (HADS ≥8) | 8 | 42 | 11 | 37 | 0.70 |
| FACT-Br Total Score | Median: 111 | SD: 25 | Median: 114 | SD 23 | 0.98 |
| FACT-Br Emotional Subscale | Median: 16 | SD: 5 | Median: 15 | SD: 5 | 0.72 |
Abbreviations: TMT, Trail-Making Test; COWA, Controlled Oral Word Association; HVLT-R, Hopkins Verbal Learning Test–Revised; HADS, Hospital Anxiety and Depression Scale; FACT-Br, Functional Assessment of Cancer Therapy–Brain.
Fig. 1.
(A) Prognostic awareness (full, limited, and none) is demonstrated for patients and caregivers. (B) Communication preferences and sources of information by PA group.
Table 5.
Cognitive impairment and information preferences in patients with malignant glioma
| Prognosis Is Important to Know (N = 30) | Prognosis Not Important to Know (N = 20) | P-value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| TMT Part A: z ≤ −1.5 | 20 | 67 | 13 | 68 | 0.90 |
| TMT Part B: z ≤ −1.5 | 22 | 73 | 13 | 68 | 0.71 |
| COWA Total | 19 | 63 | 9 | 47 | 0.27 |
| HVLT-R Total: z ≤ −1.5 | 23 | 77 | 9 | 47 | 0.04 |
| HVLT-R Delay: z ≤ −1.5 | 26 | 87 | 12 | 63 | 0.06 |
| HVLT-R Discrimination Index: z < −1.5 | 18 | 60 | 5 | 28 | 0.03 |
| Wants More Prognostic Information (N = 21) | Has Enough Prognostic Information (N = 29) | P-value | |||
| N | % | N | % | ||
| TMT Part A: z ≤ −1.5 | 15 | 71 | 17 | 65 | 0.66 |
| TMT Part B: z ≤ −1.5 | 16 | 76 | 18 | 60 | 0.60 |
| COWA Total | 13 | 62 | 14 | 54 | 0.58 |
| HVLT-R Total: z ≤ −1.5 | 17 | 81 | 14 | 54 | 0.05 |
| HVLT-R Delay: z ≤ −1.5 | 18 | 86 | 20 | 76 | 0.71 |
| HVLT-R Discrimination Index: z ≤ −1.5 | 15 | 71 | 7 | 28 | 0.003 |
Abbreviations: TMT, Trail-Making Test; COWA, Controlled Oral Word Association; HVLT-R, Hopkins Verbal Learning Test–Revised.
Caregivers
Thirteen (41%) caregivers were men, and 23 (72%) were Caucasian; 22 (69%) had full PA, 9 (28%) had limited PA, and 1 (3%) had no PA; 23 (72%) caregivers felt that information about prognosis was extremely or very important to have, 16 (50%) indicated that they wanted more prognostic information, and 26 (81%) rated the information they had received as excellent, very good, or satisfactory; 26 (81%) listed the internet as a source of prognostic information and 31 (97%) indicated that a physician or nurse was a source of information. There was no significant difference between full PA and limited or no PA with respect to sources of prognostic information or information preferences.
Discussion
This was a study investigating PA and preferences for prognostic information and neurocognitive function in 50 MG patients and 32 matched caregivers; 40% of patient participants demonstrated full PA, which is to say that 60% did not have an accurate estimate of LE; 20% of patients believed that MG is a curable disease. Full PA was no more common in patients with multiply recurrent MG, that is, more advanced disease with shorter LE at the time of the study assessment. We would have hypothesized that patients with multiply recurrent disease would more frequently possess PA by virtue of having more opportunities to discuss what the future holds for MG, but this finding may suggest that prognostic discussions do not necessarily take place as a function of recurrence status. The opinion that prognostic information is important and the wish for more information were expressed by 60% and 42% of the patients, respectively. Overall QoL and emotional well-being scores were essentially identical between those with full versus limited/no PA. Patients with impairment on tests of memory more frequently, to a statistically significant degree, believed that prognostic information was important and desired more of it, suggesting that acquisition and recall of new information may influence some aspects of PA. Caregivers were more prognostically aware than patients, with 69% possessing full PA and only one participant believing that MG is a curable disease; 88% of patients and 97% of caregivers indicated that the health care team was one of their sources of prognostic information.
This study raises many considerations about PA and prognostic communication in MG. On the one hand, 60% of patients, including many with advanced disease, were unaware of their LE, and this could represent an absence of prognostic communication; this is especially important in a disease where many patients experience late hospital admission and late referral to hospice.20,21 On the other hand, 80% of our participants knew that MG is incurable, and 40% were aware of LE, suggesting that accurate PA may be more frequent in MG than in other cancers. Findings in other cancers vary widely, compounded by variability in how PA is measured and defined1; in one prospective multicenter study of patients with advanced cancer measuring both PA and assessment of LE, only 5% demonstrated accurate illness understanding, referring to awareness of incurability and accurate estimate of LE.14 In another prospective study, 54% of advanced cancer patients believed their illness was curable, but LE was not estimated.34 In another multicenter study, 51% of patients were willing to estimate their LE; however, only 15% were accurate.13 In a meta-analysis of 34 articles about PA in cancer, approximately 50% of aggregated patients had accurate prognostic understanding, defined predominantly as awareness of incurable disease.35 It is not clear why MG patients would be more aware of disease incurability than other cancer patients. Underlying reasons could include different (ie, more candid) communication within neuro-oncologic practice compared with other cancers, greater public awareness that brain cancer is lethal, or others. It is also not clear from our study why caregivers possess more PA than patients; this could reflect discrepant understanding of shared communication with the health care team about prognosis, caregivers’ seeking prognostic information from the health care team independently of the patient who they may think is too cognitively impaired to process the information, or obtaining information from other sources (such as the internet). Further dedicated attention to preferences and dynamics of communication for MG caregivers is merited.
Our findings highlight that there are distinct components of prognostic understanding. In MG, awareness that the disease is incurable doesn’t necessarily imply awareness of LE. Furthermore, our data suggest that clinicians cannot assume that patients with more advanced disease have more accurate PA than those earlier in the MG trajectory. Rather, in the process of communicating with all patients and caregivers about MG prognosis, clinicians must elicit and address curability and LE distinctly, along with their implications for treatment preferences. Our findings point to deeper and unanswered questions about the relationship between awareness of incurability, knowledge of LE, EoL outcomes such as acute hospitalization and hospice referral in MG, and QoL at EoL. Addressing these associations requires a larger prospective study.
Our study also suggests that prognostic communication may represent an unmet need for some MG patients and caregivers, particularly those with impaired performance on memory tests. The finding that MG patients with memory impairment more frequently believe that prognostic information is important and desire more information suggests that memory abilities may influence some aspects of PA. This study suggests, on the contrary, that patients with memory problems may require more dedicated efforts at prognostic communication and discussion of treatment preferences and EoL care, reiteration of the information on subsequent encounters, and the provision of written information. There is scarce research about how cognitive impairment alters dynamics of prognostic or EoL communication in cancer, although one study suggests that in the setting of patients’ cognitive impairment, treatment preferences of caregivers disproportionately influence EoL outcomes in comparison to patients’ preferences.28 Further study is necessary to investigate whether the pattern and extent of cognitive dysfunction in MG are associated with particular patterns of EoL outcomes and whether this is mediated by magnification of caregiver preferences or other mechanisms. Such research is especially important in light of the relevance of both cognitive function and PA for informed decision making, as it has been previously found that cognitive impairment can diminish decision-making capacity.36 Furthermore, advanced care planning before later stages of disease would best take place at a junction when cognitive function and a patient’s disease understanding are optimally aligned for such discussion.
While these data suggest that there are MG patients and caregivers who desire more prognostic information, they also suggest that there are those who do not wish to have more information, and this is a psychological reality that merits reflection and investigation. Our study does not suggest that possessing accurate PA is associated with anxiety, depression, or worsened QoL. Therefore, the key question that must be addressed by further investigation is whether accurate PA and inaccurate PA are associated with different preferences for MG treatment and EoL outcomes, such as late acute medical care and late hospice referral, which have been shown to be deleterious to QoL at EoL in other cancers. Communication strategies and interventions to augment PA in MG would be desirable and beneficial with respect to EoL outcomes, even for individuals who do not actively desire such information. These domains have not been studied in MG, and moreover our study suggests that this kind of future research must measure and account for cognitive impairment, particularly memory problems, which may alter information preferences and information-seeking behavior.
There are several limitations to this study. First, the relatively small number of patients may have limited the power to detect statistically significant differences between compared groups, and this limits the interpretation of negative comparative findings. For example, 60% of those with limited/no PA had participated in a clinical trial compared with 37% of those with full PA (P = 0.10). Clinical trial participation predicts late hospitalization in GBM, so these data support this association despite the lack of statistical validation.19 Second, this study was done at a single institution in a population that was of limited racial diversity; this limits the generalizability of our findings. Additionally, we studied admitted patients who are more acutely ill and perhaps more likely to be impaired, although delirium was excluded, as all participants were fully oriented and participated cogently in the consenting process. There were relatively few patients with newly diagnosed disease, likely a function of this being an inpatient study; this may influence the study results, although we did not see more frequent PA in those with multiply recurrent disease. The exclusion of aphasic patients and those with hemiparesis of the dominant arm did select for patients with right-sided tumors. We also did not measure patients’ coping strategies such as acceptance and denial, which are relevant to communication dynamics and psychological states.37 Additionally, a significant proportion of caregivers who enrolled in the study did not ultimately participate, and this should be considered a possible source of bias. It is possible that caregivers who were not able to be present for the study assessment were less engaged in the patient’s care or prognostic communication and may have possessed a different perspective on the study’s concerns than those who participated. Finally, there is the matter of PA assessment itself, which is intrinsically subjective and challenging to reproduce. We believe that combining a structured instrument (asking all participants the same unambiguous prompts) with open-ended questions to probe awareness of curability and LE, while conscientiously allowing for expressions of optimism, was a rich and meaningful method in this patient population. Moreover, verbatim transcripts were analyzed by both a neuro-oncologist and a psychologist with particular expertise in the measurement of PA. Altogether, we believe that we obtained detailed and believable PA assessments and that future studies may consider a similar approach.
This pilot study suggests that PA is measurable in MG patients, even those with cognitive impairment, and that while the majority of participants are aware that MG is incurable, a minority are aware of their LE. Many, but not all, MG patients believe that prognostic information is important to possess and desire more information; patients with memory impairment are more likely to possess these beliefs and clinicians should be mindful of this in their communication approach. Further research is needed to examine associations between PA states and attitudes such as treatment preferences as well as EoL outcomes.
Funding
This work was supported by a Translation and Integrative Medicine Award and the Brain Tumor Center at Memorial Sloan Kettering Cancer Center (to E.L.D. and A.J.A.); the National Institutes of Health/National Cancer Institute Core Grant (P30 CA008748) awarded to Memorial Sloan Kettering Cancer Center; Conquer Cancer Foundation of the American Society of Clinical Oncology (to E.L.D.).
Acknowledgments
Drs Diamond, Prigerson, Correa, Panageas, and Applebaum and Ms Reiner had full access to all of the data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest statement. No author reports a conflict of interest.
References
- 1. Applebaum AJ, Kolva EA, Kulikowski JR et al. . Conceptualizing prognostic awareness in advanced cancer: a systematic review. J Health Psychol. 2014;19(9):1103–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chochinov HM, Tataryn DJ, Wilson KG, Ennis M, Lander S. Prognostic awareness and the terminally ill. Psychosomatics. 2000;41(6): 500–504. [DOI] [PubMed] [Google Scholar]
- 3. Dunn SM, Patterson PU, Butow PN, Smartt HH, McCarthy WH, Tattersall MH. Cancer by another name: a randomized trial of the effects of euphemism and uncertainty in communicating with cancer patients. J Clin Oncol. 1993;11(5):989–996. [DOI] [PubMed] [Google Scholar]
- 4. Lichtenthal WG, Nilsson M, Zhang B et al. . Do rates of mental disorders and existential distress among advanced stage cancer patients increase as death approaches? Psychooncology. 2009;18(1):50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wright AA, Zhang B, Ray A et al. . Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mack JW, Wolfe J, Grier HE, Cleary PD, Weeks JC. Communication about prognosis between parents and physicians of children with cancer: parent preferences and the impact of prognostic information. J Clin Oncol. 2006;24(33):5265–5270. [DOI] [PubMed] [Google Scholar]
- 7. Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hebert RS, Schulz R, Copeland VC, Arnold RM. Preparing family caregivers for death and bereavement. Insights from caregivers of terminally ill patients. J Pain Symptom Manage. 2009;37(1):3–12. [DOI] [PubMed] [Google Scholar]
- 9. Ray A, Block SD, Friedlander RJ, Zhang B, Maciejewski PK, Prigerson HG. Peaceful awareness in patients with advanced cancer. J Palliat Med. 2006;9(6):1359–1368. [DOI] [PubMed] [Google Scholar]
- 10. Enzinger AC, Zhang B, Schrag D, Prigerson HG. Outcomes of prognostic disclosure: associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol. 2015;33(32):3809–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epstein AS, Prigerson HG, O’Reilly EM, Maciejewski PK. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol. 2016;34(20):2398–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daugherty CK, Hlubocky FJ. What are terminally ill cancer patients told about their expected deaths? A study of cancer physicians’ self-reports of prognosis disclosure. J Clin Oncol. 2008;26(36): 5988–5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fumis RR, De Camargo B, Del Giglio A. Physician, patient and family attitudes regarding information on prognosis: a Brazilian survey. Ann Oncol. 2012;23(1):205–211. [DOI] [PubMed] [Google Scholar]
- 14. Oh DY, Kim JE, Lee CH et al. . Discrepancies among patients, family members, and physicians in Korea in terms of values regarding the withholding of treatment from patients with terminal malignancies. Cancer. 2004;100(9):1961–1966. [DOI] [PubMed] [Google Scholar]
- 15. Mack JW, Cronin A, Keating NL et al. . Associations between end-of-life discussion characteristics and care received near death: a prospective cohort study. J Clin Oncol. 2012;30(35):4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morasso G, Alberisio A, Capelli M, Rossi C, Baracco G, Costantini M. Illness awareness in cancer patients: a conceptual framework and a preliminary classification hypothesis. Psychooncology. 1997;6(3):212–217. [DOI] [PubMed] [Google Scholar]
- 17. Burns CM, Broom DH, Smith WT, Dear K, Craft PS. Fluctuating awareness of treatment goals among patients and their caregivers: a longitudinal study of a dynamic process. Support Care Cancer. 2007;15(2):187–196. [DOI] [PubMed] [Google Scholar]
- 18. Weeks JC, Catalano PJ, Cronin A et al. . Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diamond EL, Panageas KS, Dallara A et al. . Frequency and predictors of acute hospitalization before death in patients with glioblastoma. J Pain Symptom Manage. 2017;53(2):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diamond EL, Russell D, Kryza-Lacombe M et al. . Rates and risks for late referral to hospice in patients with primary malignant brain tumors. Neuro Oncol. 2016;18(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diamond EL, Corner GW, De Rosa A, Breitbart W, Applebaum AJ. Prognostic awareness and communication of prognostic information in malignant glioma: a systematic review. J Neurooncol. 2014;119(2):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davies E, Clarke C, Hopkins A. Malignant cerebral glioma—II: perspectives of patients and relatives on the value of radiotherapy. BMJ. 1996;313(7071):1512–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salander P, Bergenheim T, Henriksson R. The creation of protection and hope in patients with malignant brain tumours. Soc Sci Med. 1996;42(7):985–996. [DOI] [PubMed] [Google Scholar]
- 24. Anderson SI, Taylor R, Whittle IR. Mood disorders in patients after treatment for primary intracranial tumours. Br J Neurosurg. 1999;13(5):480–485. [PubMed] [Google Scholar]
- 25. Janda M, Eakin EG, Bailey L, Walker D, Troy K. Supportive care needs of people with brain tumours and their carers. Support Care Cancer. 2006;14(11):1094–1103. [DOI] [PubMed] [Google Scholar]
- 26. Lobb EA, Halkett GK, Nowak AK. Patient and caregiver perceptions of communication of prognosis in high grade glioma. J Neurooncol. 2011;104(1):315–322. [DOI] [PubMed] [Google Scholar]
- 27. Díaz JL, Barreto P, Gallego JM, Barbero J, Bayés R, Barcia JA. Proper information during the surgical decision-making process lowers the anxiety of patients with high-grade gliomas. Acta Neurochir (Wien). 2009;151(4):357–362. [DOI] [PubMed] [Google Scholar]
- 28. Gao X, Prigerson HG, Diamond EL et al. . Minor cognitive impairments in cancer patients magnify the effect of caregiver preferences on end-of-life care. J Pain Symptom Manage. 2013;45(4):650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13(3):348–358. [DOI] [PubMed] [Google Scholar]
- 30. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8(3):271–276. [Google Scholar]
- 31. Ross TP, Calhoun E, Cox T, Wenner C, Kono W, Pleasant M. The reliability and validity of qualitative scores for the Controlled Oral Word Association Test. Arch Clin Neuropsychol. 2007;22(4):475–488. [DOI] [PubMed] [Google Scholar]
- 32. Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363–370. [DOI] [PubMed] [Google Scholar]
- 33. Rosenblum ML, Kalkanis S, Goldberg W et al. . Odyssey of hope: a physician’s guide to communicating with brain tumor patients across the continuum of care. J Neurooncol. 2009;92(3):241–251. [DOI] [PubMed] [Google Scholar]
- 34. El-Jawahri A, Traeger L, Park ER et al. . Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer. 2014;120(2):278–285. [DOI] [PubMed] [Google Scholar]
- 35. Chen CH, Kuo SC, Tang ST. Current status of accurate prognostic awareness in advanced/terminally ill cancer patients: systematic review and meta-regression analysis. Palliat Med. 2017;31(5):406–418. [DOI] [PubMed] [Google Scholar]
- 36. Triebel KL, Martin RC, Nabors LB, Marson DC. Medical decision-making capacity in patients with malignant glioma. Neurology. 2009;73(24):2086–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nipp RD, El-Jawahri A, Fishbein JN et al. . The relationship between coping strategies, quality of life, and mood in patients with incurable cancer. Cancer. 2016;122(13):2110–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]