Summary
Background
To combat Neisseria meningitidis serogroup A epidemics in the meningitis belt of sub-Saharan Africa, a meningococcal serogroup A conjugate vaccine (MACV) has been progressively rolled out since 2010. We report the first meningitis epidemic in Niger since the nationwide introduction of MACV.
Methods
We compiled and analysed nationwide case-based meningitis surveillance data in Niger. Cases were confirmed by culture or direct real-time PCR, or both, of cerebrospinal fluid specimens, and whole-genome sequencing was used to characterise isolates. Information on vaccination campaigns was collected by the Niger Ministry of Health and WHO.
Findings
From Jan 1 to June 30, 2015, 9367 suspected meningitis cases and 549 deaths were reported in Niger. Among 4301 cerebrospinal fluid specimens tested, 1603 (37·3%) were positive for a bacterial pathogen, including 1147 (71·5%) that were positive for N meningitidis serogroup C (NmC). Whole-genome sequencing of 77 NmC isolates revealed the strain to be ST-10217. Although vaccination campaigns were limited in scope because of a global vaccine shortage, 1·4 million people were vaccinated from March to June, 2015.
Interpretation
This epidemic represents the largest global NmC outbreak so far and shows the continued threat of N meningitidis in sub-Saharan Africa. The risk of further regional expansion of this novel clone highlights the need for continued strengthening of case-based surveillance. The availability of an affordable, multivalent conjugate vaccine may be important in future epidemic response.
Introduction
In Niger and elsewhere in the meningitis belt of sub- Saharan Africa, endemic meningococcal disease and annual outbreaks have been punctuated every 5–12 years by large-scale epidemics, which were historically caused by Neisseria meningitidis serogroup A (NmA).1 In 2010–11, a novel meningococcal serogroup A conjugate vaccine (MACV), MenAfriVac, was introduced in Niger through preventive mass vaccination campaigns, with the goal of eliminating NmA epidemics. In the 5 years after the introduction of this vaccine, annual cases of suspected meningitis reached the lowest reported levels in Niger, and no NmA cases were confirmed (WHO, unpublished). With the progressive roll-out of MACV in 16 countries in sub-Saharan Africa (as of December, 2015), no NmA epidemics have been reported in vaccinated areas.2
Despite the early success of MACV in the prevention of NmA disease and epidemics, other meningococcal serogroups have caused meningitis epidemics in sub- Saharan Africa. Several epidemics due to N meningitidis serogroup W (NmW) have occurred since the first major epidemic in Africa was reported in 2002 in Burkina Faso, with NmW as the leading meningococcal cause of meningitis in sub-Saharan Africa in the years following MACV introduction.2–7 Additionally, smaller epidemics caused by N meningitidis serogroup X (NmX) have been reported in Burkina Faso, Niger, and Togo.8,9 Although N meningitidis serogroup C (NmC) cases have occasionally been reported in Niger and elsewhere in the meningitis belt, NmC epidemics are rare.1,10
Before 2013, the last known predominantly NmC outbreak in Africa was reported in 1979 in Burkina Faso.11 In 2013–14, outbreaks caused by a novel NmC clone were reported in two neighbouring states of northern Nigeria,12 and were relatively small and focal compared with the large-scale NmA epidemics. In March, 2015, NmC cases were first detected in the Dogon-Doutchi district of Niger along the Nigerian border, with subsequent spread to other regions in Niger. In this report, we describe the emergence of NmC in Niger that resulted in the first large-scale NmC epidemic in sub-Saharan Africa.
Research in context.
Evidence before this study
We searched the PubMed MeSH database for English-language articles published from database inception to April 24, 2016, using the terms “Neisseria meningitidis”, “Neisseria meningitidis, serogroup C”, “meningitis, meningococcal”, “epidemic”, “meningococcal vaccines”, “Africa south of the Sahara”, and “Niger”. Until the introduction of a meningococcal serogroup A conjugate vaccine (MACV), MenAfriVac, in the meningitis belt of sub-Saharan Africa starting in 2010, large meningitis epidemics have primarily been caused by N meningitidis serogroup A (NmA). Sporadic cases and localised epidemics due to N meningitidis serogroup C (NmC) have rarely been reported in the meningitis belt of sub-Saharan Africa, and the sequential NmC outbreaks in Nigeria in 2013–14 were relatively small and focal. No large-scale NmC epidemics have previously been reported.
Added value of this study
9367 suspected meningitis cases, including 1147 confirmed NmC cases, were reported in Niger from Jan 1 to June 30, 2015. We describe the epidemiological and microbiological features of the first large-scale NmC epidemic in sub-Saharan Africa and the largest global NmC outbreak so far. Our report of this NmC epidemic shows the continued threat of meningococcal epidemics despite the successful introduction of MACV in sub-Saharan Africa.
Implications of all the available evidence
Case-based surveillance and laboratory capacity need to be strengthened to monitor the evolving epidemiology of meningococcal meningitis in the meningitis belt in the post-MACV era and to respond rapidly to disease outbreaks. Additionally, this epidemic highlights the need for an adequate global supply of affordable, multivalent meningococcal vaccines to respond to non-serogroup A meningococcal meningitis epidemics in sub-Saharan Africa.
Methods
Data collection
In this study, we compiled and analysed nationwide case-based surveillance data of all reported meningitis cases in Niger from Jan 1 to June 30, 2015, to describe the epidemiological and microbiological features of the epidemic. We collected epidemiological data of reported meningitis cases through complementary aggregate, case-based, and enhanced meningitis surveillance systems. In the aggregate system, population-based meningitis surveillance is conducted in all 44 functional health districts, with weekly transmission of district-level suspected meningitis cases and death counts. Case-based meningitis surveillance, in which detailed epidemiological and laboratory data are collected for each suspected meningitis case through a standardised case investigation form, is conducted in eight of 44 districts representing 37% of Niger’s population as of December, 2014 (Dogon-Doutchi, Filingué, Madaoua, Madarounfa, Maradi, Niamey I, Say, and Tera). In the nationwide enhanced meningitis surveillance, case-level demographic data are collected and transmitted weekly through a line list, with the same standardised case investigation form used for case-based surveillance, during meningitis epidemics. In each of these surveillance systems, epidemiological data are transmitted from the health facility to the district surveillance office, followed by the regional surveillance office, and finally to the national level.
Procedures and statistical analyses
Laboratory analyses on cerebrospinal fluid specimens from patients with reported meningitis were completed at the Centre de Recherche Médicale et Sanitaire (CERMES), the national meningitis reference laboratory in Niger. We used culture or direct real-time PCR testing, or both, for detection of N meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae.13 Serogroup was deter mined on isolates by slide agglutination and on cerebrospinal fluid specimens that were PCR positive for N meningitidis by serogroup-specific direct real-time PCR. Culture and slide agglutination were also conducted at the Hôpital National de Niamey and a mobile laboratory (LaboMobil, Agence de Médecine Préventive) deployed to a temporary treatment centre in Niamey. External quality assurance of culture and PCR results obtained at CERMES was done collectively by the WHO Collaborating Centres for Meningitis at the US Centers for Disease Control and Prevention, Institut Pasteur, and Norwegian Institute of Public Health, on 111 specimens.
Cases were defined according to WHO guidelines.14 A suspected meningitis case was defined by the presence of sudden onset of fever with headache and one of the following signs: stiff neck, altered consciousness, or other meningeal signs. A probable case is defined as a suspected case with Gram-negative diplococci, Gram-positive diplococci, or Gram-negative bacilli identified by Gram stain. A confirmed case was defined as a suspected or probable case for which N meningitidis, H influenzae, or S pneumoniae was isolated in culture or the DNA of these pathogens was detected by direct real-time PCR. We calculated cumulative incidence as the number of suspected meningitis cases reported per 100 000 people, using age-specific and district-specific population data projected for 2015 from the 2012 national census.15 The WHO alert threshold is defined as a weekly attack rate of three to nine suspected cases per 100 000 people and the epidemic threshold as ten or more suspected cases per 100 000 people.16 We calculated the case fatality rate as the proportion of deaths among reported cases. Details on the reactive vaccination campaigns were obtained from the Niger Ministry of Health and WHO.17
We created a comprehensive, national case-based surveillance database of all suspected, probable, and confirmed meningitis cases reported during the epidemic period from Jan 1 to June 30, 2015, by harmonising case-level data collected from the enhanced and case-based surveillance databases and the databases from the three laboratories. We merged epidemiological and laboratory data using name, age, village, health facility, and onset date, with manual matching, verification, and de-duplication of records. We excluded cases from the database if no epidemiological data were available. Cases were subsequently assigned a unique identifier and the database was anonymised. The number of meningitis cases reported by region was compared with that reported in the aggregate surveillance system to assess sensitivity of the case-based surveillance system.
Isolates were characterised at the three WHO Collaborating Centres using whole-genome sequencing.18 Genome sequencing data were generated with both the PacBio RSII instrument (Pacific Biosciences, Menlo Park, CA, USA) and the Illumina HiSeq 2500 (Illumina, San Diego, CA, USA). Multilocus sequence typing alleles were identified on the basis of a BLAST search of the assembled genomes against the PubMLST allele list.19 Porin A (PorA), porin B (PorB), and ferric enterobactin transport (FetA) were classified according to their respective variable regions.
Descriptive analyses were done with SAS version 9.3, Microsoft Excel, and ArcGIS.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
From Jan 1 to June 30, 2015 (ie, epidemiological weeks 1–26), 9367 meningitis cases—1604 (17·1%) confirmed cases, 64 (0·7%) probable cases, and 7699 (82·2%) suspected cases—and 549 (5·9%) deaths were reported in the comprehensive case-based surveillance database. Cumulative national attack rate was 50·6 cases per 100 000 people. By comparison, fewer suspected meningitis cases (8661) but more deaths (589 [6·8%]) were reported during the same time period in the aggregate surveillance system. Most cases were from the regions of Niamey (6210 [71·7%], cumulative attack rate 566·5 cases per 100 000 people), Dosso (1228 [14·2%], 41·4 cases per 100 000 people), and Tillaberi (1201 [13·9%], 57·1 cases per 100 000 people).
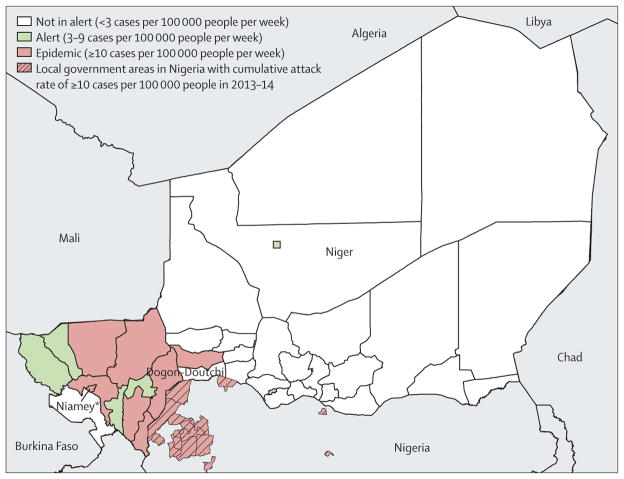
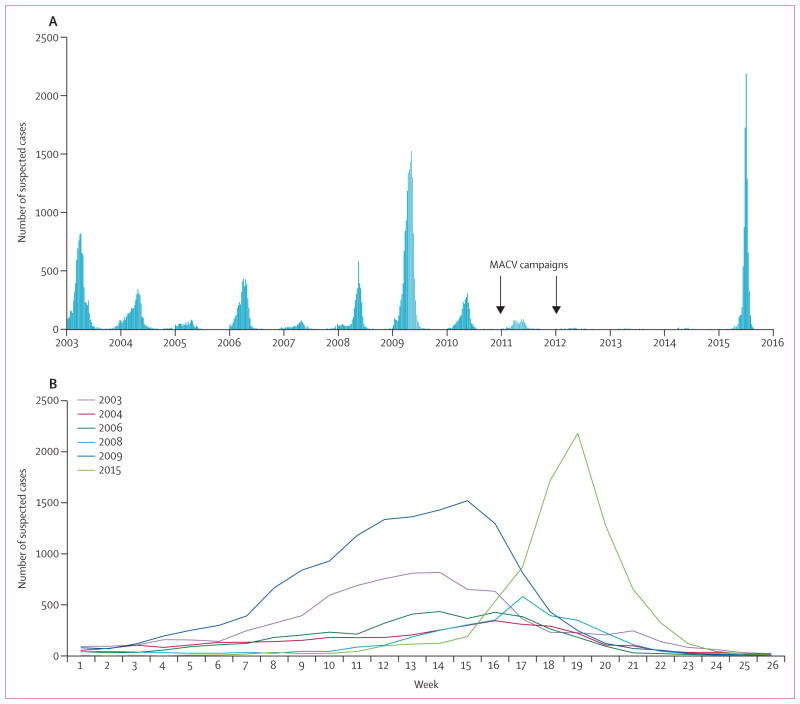
The epidemic started in the Dosso region, when the first sub-district health area in the Dogon-Doutchi district crossed the epidemic threshold in week 7. Sub-district epidemics continued in Dogon-Doutchi through week 12, when the entire district crossed the threshold. The first district in Niamey region, Niamey II, crossed the epidemic threshold in week 13, and by week 17, all five districts in Niamey had crossed the epidemic threshold. The epidemic peaked in week 19; by week 20, 12 of the 44 districts in Niger had crossed the weekly epidemic threshold and six had crossed the weekly alert threshold (figure 1). Among the 9367 total cases, 8453 (90·2%) were reported from epidemic districts, 440 (4·7%) from alert districts, and 474 (5·1%) from districts that remained below the alert threshold. The epidemiological curve was compared with previous epidemics in Niger from 2003 to 2015 (figure 2).
Figure 1. Districts that surpassed epidemic or alert thresholds in Niger, Jan 1 to June 30, 2015.
*Includes five districts: Niamey I, Niamey II, Niamey III, Niamey IV, and Niamey V.
Figure 2. Number of suspected meningitis cases by week in Niger (A) between Jan 1, 2003, and June 30, 2015, and (B) during selected epidemic years.
In the epidemics of 2003, 2004, 2008, and 2009, Neisseria meningitidis serogroup A was the predominant serogroup. In the 2006 epidemic, N meningitidis serogroup X was the predominant serogroup. In the epidemic of 2015, N meningitidis serogroup C was the predominant serogroup. MACV=serogroup A conjugate meningococcal vaccine.
Of 5059 (54·0%) individuals with suspected meningitis who were reported to have had lumbar puncture for cerebrospinal fluid collection, 4301 (85·0%) had their cerebrospinal fluid specimens received by a laboratory performing confirmatory testing and had a case report form available. 1603 (37·3%) specimens tested positive: 1147 (71·5%) were positive for NmC, 236 (14·7%) NmW, 90 (5·6%) undetermined N meningitidis, one (0·06%) NmX, 121 (7·5%) S pneumoniae, and eight (0·5%) H influenzae serotype b. Of the 4136 (96·2%) specimens analysed at CERMES, 4132 (99·9%) were analysed by PCR and 353 (8·5%) by culture. External quality assurance testing on 111 specimens showed 100% concordance with results obtained at CERMES.
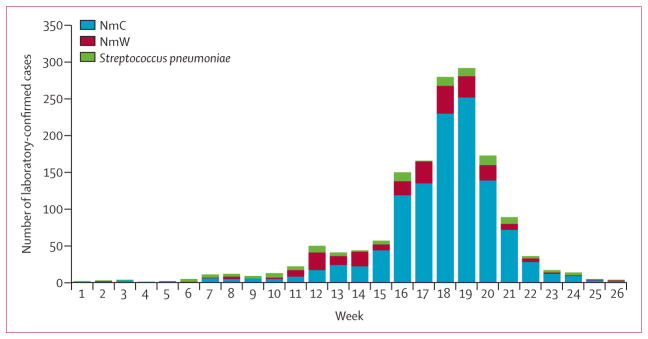
At the beginning of the epidemic (weeks 11–14), the proportion of NmC and NmW cases among N meningitidis cases with known serogroup was roughly equal. From week 15 onwards, the proportion of weekly NmC cases predominated (82–90%; figure 3). This predominance of NmC cases was only observed in the 18 districts that exceeded the alert or epidemic threshold, whereas in districts that remained below the thresholds, the distribution of NmC and NmW cases remained roughly equal (53·6% vs 46·4%). The age distribution differed in individuals with confirmed NmC cases and in those with confirmed NmW and S pneumoniae cases (table).
Figure 3. Laboratory-confirmed cases by epidemiological week and pathogen, Jan 1 to June 30, 2015.
NmC=Neisseria meningitidis serogroup C. NmW=N meningitidis serogroup W.
Table.
Characteristics and outcome of all cases and confirmed cases between Jan 1 and June 30, 2015, by serogroup
| All cases (n=9367) | Confirmed cases | ||||
|---|---|---|---|---|---|
|
|
|||||
| All (n=1603)* | NmC (n=1147) | NmW (n=236) | Streptococcus pneumoniae (n=121) | ||
| Age group, years† | |||||
| <1 | 376 (4·2%) | 61 (4·0%) | 22 (2·0%) | 14 (6·1%) | 18 (15·4%) |
| 1–4 | 1666 (18·5%) | 294 (19·3%) | 172 (15·9%) | 76 (33·0%) | 19 (16·2%) |
| 5–9 | 1694 (18·8%) | 396 (26·0%) | 304 (28·1%) | 60 (26·1%) | 16 (13·7%) |
| 10–14 | 1875 (20·8%) | 393 (25·8%) | 309 (28·6%) | 46 (20·0%) | 21 (17·9%) |
| 15–29 | 2562 (28·4%) | 305 (20·0%) | 236 (21·8%) | 27 (11·7%) | 21 (17·9%) |
| ≥30 | 855 (9·5%) | 76 (5·0%) | 39 (3·6%) | 7 (3·0%) | 22 (18·8%) |
|
| |||||
| Male sex | 4917 (52·5%)‡ | 994 (62·0%) | 715 (62·3%) | 138 (58·5%) | 81 (66·9%) |
|
| |||||
| Known outcome | 2224 (23·7%) | 603 (37·6%) | 428 (37·3%) | 106 (44·9%) | 50 (41·3%) |
| Deaths | 549 (24·7%) | 137 (22·7%) | 92 (21·5%) | 19 (17·9%) | 18 (36·0%) |
|
| |||||
| Case fatality rate among all reported cases | 5·9% | 8·5% | 8·0% | 8·1% | 14·9% |
NmC=Neisseria meningitidis serogroup C. NmW=N meningitidis serogroup W. NmX=N meningitidis serogroup X.
Includes all cases confirmed as NmC, NmW, NmX, indeterminant N meningitidis, S pneumoniae, and Haemophilus influenzae serotype b.
The number of patients with known age was 9028 (all cases), 1525 (all confirmed cases), 1082 (NmC), 230 (NmW), and 117 (S pneumoniae).
9366 patients had available data.
549 deaths were reported, although 7143 (76·3%) of 9367 patients had unknown outcome. In all reported cases, the case fatality rate was 5·9%. However, when the analysis was restricted to the 2224 cases with known outcome, the case fatality rate was much higher (24·7%). The case fatality rates were 8·5% in the 1604 laboratory-confirmed cases and 22·7% in the 603 laboratory-confirmed cases with known outcomes (table).
Reactive vaccination campaigns were introduced at the sub-district level of 13 districts from March to June, 2015 (weeks 13–26). 1·4 million doses of meningococcal vaccines were made available from the national stockpile, from a neighbouring country’s stockpile, and through five separate requests to the global emergency stockpile managed by the International Coordinating Group on Vaccine Provision for Epidemic Meningitis Control. 12 districts rolled out trivalent (serogroups A, C, and W) or tetravalent (serogroups A, C, W, and Y) polysaccharide meningococcal vaccines in children aged 2–15 years, achieving an overall administrative coverage in targeted subgroups of 62·4% (619 435 of 992 085). The 13th district, Ouallam, was the first to use the quadrivalent conjugate meningococcal vaccine MenACWY-D (serogroups A, C, Y, and W) for outbreak response in Africa, and the campaign achieved a 96·1% (188 129 of 195 772) coverage in children aged 2–19 years. However, a global shortage in polysaccharide meningococcal vaccines limited the scope and, in some districts, the timeliness of the reactive campaigns. Vaccination campaigns were restricted to children aged 2–15 years or 2–19 years instead of the entire at-risk population (ie, those aged 2–29 years), and sub-district health areas were targeted instead of the entire district. Children younger than 2 years were not targeted for vaccination because polysaccharide vaccines are not immunogenic in this age group and the need for a two-dose conjugate MenACWY-D series in children aged 9–23 months was not feasible. Roughly one-third of the total vaccine doses became available in June, 2015, after the epidemic had largely subsided.
We used whole-genome sequencing to characterise 98 isolates. All 77 NmC isolates were PorA P1·21-15,16, PorB 3-463, FetA 1-7, and ST-10127, belonging to an unassigned clonal complex.18 ST-10127 is distinct and has only been previously identified from outbreaks in Nigeria in 2013–14.12 All 21 NmW isolates were ST-11 that belonged to clonal complex 11, with identical PorA (P1·5,2) but different PorB and FetA sequences. 14 NmW isolates had PorB 2-2, six had PorB 2-277, and one had PorB 2-60. 15 NmW isolates had FetA 1-1, and six had FetA type 1-84. All NmW isolates were closely related to other recent isolates from sub-Saharan Africa.18 Detailed description of NadA, NhbA, and FHbp sequences, as well as phylogenetic analysis of strains, is presented elsewhere.18
Discussion
The occurrence of a large-scale NmC epidemic in sub- Saharan Africa in the years following successful mass vaccination with MACV shows the continued risk of meningitis epidemics in the region. As the largest known NmC epidemic so far on a global scale and the first major NmC epidemic in Africa, the 2015 epidemic in Niger is further evidence of the evolving epidemiology of bacterial meningitis in the meningitis belt. Although it is too early to fully describe the epidemic potential of NmC in sub- Saharan Africa, evidence shows regional spread, with NmC cases reported in 2016 in Burkina Faso, Mali, Côte d’Ivoire, and Ghana, in addition to further localised outbreaks in Niger and Nigeria.20
Compared with epidemics caused by serogroups A, W, and X in the past 15 years in Niger, this NmC epidemic is most notable for the rapid acceleration to its peak, which occurred late in the epidemic season, following several years of historically low case counts. Although the NmC epidemic did not match the magnitude of the largest NmA epidemics in Niger (eg, the 2009 epidemic with nearly 14 000 cases reported),21 greater numbers of cases were reported during the NmC epidemic (9367 total cases) than in the 2010 mixed NmA–NmW epidemic (nearly 3000 total cases4) and the 2006 mixed NmA–NmX epidemic (more than 4000 total cases).8 The age distribution of NmC cases, with the highest proportions observed in adolescents and young adults, is similar to that reported in previous NmA epidemics,22 whereas the predominance of NmW cases in children younger than 5 years has been reported in other NmW epidemics.4,6 The overall case fatality rate of 5·9% in this NmC epidemic is consistent with that reported in several NmA, NmW, and NmX epidemics in Niger (roughly 4–8%),4,8,21–23 although the incompleteness of case outcome in the surveillance data limits the interpretation of these results.
Analyses of strains from countries in the meningitis belt from the past 50 years show that most epidemics have been caused by the introduction of successive clonal waves from hyper-invasive lineages in a naive population.24 Similar to previous reports,25 the novel NmC clone causing this epidemic emerged following clonal waves of serogroup A. Thus, immunity against NmC in this population is expected to be low or nonexistent because of the infrequency of sporadic cases or epidemics, low NmC carriage,26 and the lack of recent vaccinations in the region with serogroup C-containing vaccines. Since the ST-10217 strain causing this epidemic is highly distinct from encapsulated meningococcal strains of any serogroup in global strain collections, there is no evidence for vaccine-induced capsular switching as a factor in this epidemic.18 Although it is too early to determine whether serogroup replacement, in which non-vaccine serogroups expand in the ecological niche left after MACV-induced reductions in serogroup A meningococcal carriage, is a factor in the emergence of NmC epidemics in Niger and Nigeria, the overall incidence of suspected meningitis in the meningitis belt, excluding the NmC epidemic in Niger, has decreased since the introduction of MACV.2,27,28 Thus, the rapid spread of this epidemic is likely to result from the introduction of this novel hypervirulent clone in an immunologically naive population. However, molecular surveillance and additional carriage studies are needed for continued monitoring of the changing epidemiology of meningococcal disease and epidemics and of the long-term effects of MACV in the meningitis belt.
The prevention of NmC and other non-serogroup A meningitis epidemics in Africa remains an elusive goal because affordable multivalent conjugate vaccines are not available. Compared with conjugate vaccines, polysaccharide vaccines have a shorter estimated duration of protection and do not reduce acquisition of naso-pharyngeal carriage of N meningitidis, making them ineffective for the prevention of meningococcal outbreaks. 29 The current quadrivalent meningococcal conjugate (MenACWY) vaccines, marketed at more than US$100 per dose in the USA,30 are unaffordable for widespread use in Africa, even with a substantially reduced price such as that negotiated for the campaign in Ouallam of $25 per dose. Preventive vaccination is therefore prohibitively expensive. An affordable pentavalent conjugate meningococcal vaccine (MenACWXY), which is being developed for use in Africa, will not be licensed until 2019 at the earliest.31
The NmC epidemic in Niger shows the importance of case-based surveillance as a complement to enhanced and aggregate meningitis surveillance during epidemics. The massive scale-up effort of epidemic response activities in a very short time period, along with the ongoing shortage of polysaccharide meningococcal vaccines, highlights the need for rapid detection, confirmation, and characterisation of the epidemic at the sub-district level to optimise public health interventions, including reactive vaccination campaigns, that target the population at highest risk. Additionally, as the epidemic evolved from an equally mixed NmC–NmW epidemic to a predominantly NmC epidemic, strong capacity for culture and PCR testing at referral laboratories with linkage to epidemiological data is crucial for real-time decision making of vaccination campaigns and appropriate stewardship of limited vaccine stocks.
Although the continued absence of NmA in the 5 years since Niger successfully introduced MACV highlights the remarkable success of this vaccine, the NmC epidemic in Niger shows the continued threat of meningococcal epidemics in the meningitis belt. The risk of further regional expansion of NmC underscores the need for continued strengthening of case-based surveillance with rapid laboratory confirmation and molecular characterisation of strains. Additionally, the availability of an affordable multivalent conjugate vaccine in the global emergency stockpile to respond to non-serogroup A meningococcal epidemics may be important in future epidemic response.
Acknowledgments
Funding: MenAfriNet consortium, a partnership between the US Centers for Disease Control and Prevention, WHO, and Agence de Médecine Preventive, through a grant from the Bill & Melinda Gates Foundation.
Meningitis case-based surveillance in Niger is supported in part by the MenAfriNet consortium, a partnership between the US Centers for Disease Control and Prevention, WHO, and Agence de Médecine Preventive, through a grant from the Bill & Melinda Gates Foundation. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Footnotes
See Comment page 1212
Contributors
All authors contributed to the conception of this analysis. FS, MZ, IA, SSc, BI, RO, CL, AT, FA, SSa, SO, JZ, IS, DM, and OA were directly responsible for the collection of epidemiological data or analyses of laboratory results. FS, MZ, CL, RO, and SM were responsible for the analysis of results. All authors interpreted the data. FS, MZ, OOMO-B, and SM were responsible for the preparation of the manuscript. All authors critically revised the manuscript, gave approval for publication, and agreed to be held accountable for the integrity of the work.
Declaration of interests
We declare no competing interests.
Contributor Information
Fati Sidikou, Centre de Recherche Médicale et Sanitaire (CERMES), Ministry of Public Health, Institut Pasteur International Network, Niamey, Niger.
Maman Zaneidou, Direction de la Surveillance et Riposte aux Epidémies, Ministry of Health, Niamey, Niger.
Ibrahim Alkassoum, Direction de la Surveillance et Riposte aux Epidémies, Ministry of Health, Niamey, Niger.
Stephanie Schwartz, Meningitis and Vaccine Preventable Diseases Branch, National Center for Immunization and Respiratory Diseases, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Bassira Issaka, Centre de Recherche Médicale et Sanitaire (CERMES), Ministry of Public Health, Institut Pasteur International Network, Niamey, Niger.
Ricardo Obama, World Health Organization—Niger, Niamey, Niger.
Clement Lingani, World Health Organization Intercountry Support Team for West Africa, Ouagadougou, Burkina Faso.
Ashley Tate, Meningitis and Vaccine Preventable Diseases Branch, National Center for Immunization and Respiratory Diseases, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Flavien Ake, Davycas Consulting, Gounghin Petit-Paris, Ouagadougou, Burkina Faso.
Souleymane Sakande, Agence de Médecine Préventive—Ouagadougou, Ouagadougou, Burkina Faso.
Sani Ousmane, Centre de Recherche Médicale et Sanitaire (CERMES), Ministry of Public Health, Institut Pasteur International Network, Niamey, Niger.
Jibir Zanguina, Centre de Recherche Médicale et Sanitaire (CERMES), Ministry of Public Health, Institut Pasteur International Network, Niamey, Niger.
Issaka Seidou, Centre de Recherche Médicale et Sanitaire (CERMES), Ministry of Public Health, Institut Pasteur International Network, Niamey, Niger.
Innocent Nzeyimana, World Health Organization—Niger, Niamey, Niger.
Didier Mounkoro, Agence de Médecine Préventive—Togo, Dapaong, Togo.
Oubote Abodji, Agence de Médecine Préventive—Togo, Dapaong, Togo.
Xin Wang, Meningitis and Vaccine Preventable Diseases Branch, National Center for Immunization and Respiratory Diseases, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Muhamed-Kheir Taha, Institut Pasteur, Paris, France.
Jean Paul Moulia-Pelat, Centre de Recherche Médicale et Sanitaire (CERMES), Ministry of Public Health, Institut Pasteur International Network, Niamey, Niger.
Assimawe Pana, World Health Organization—Niger, Niamey, Niger.
Goumbi Kadade, Direction de la Surveillance et Riposte aux Epidémies, Ministry of Health, Niamey, Niger.
Olivier Ronveaux, World Health Organization, Geneva, Switzerland.
Ryan Novak, Meningitis and Vaccine Preventable Diseases Branch, National Center for Immunization and Respiratory Diseases, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Odile Ouwe Missi Oukem-Boyer, Centre de Recherche Médicale et Sanitaire (CERMES), Ministry of Public Health, Institut Pasteur International Network, Niamey, Niger.
Sarah Meyer, Meningitis and Vaccine Preventable Diseases Branch, National Center for Immunization and Respiratory Diseases, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
References
- 1.Greenwood B. Manson lecture. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg. 1999;93:341–53. doi: 10.1016/s0035-9203(99)90106-2. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Meningococcal disease control in countries of the African meningitis belt, 2014. Wkly Epidemiol Rec. 2015;90:123–31. [PubMed] [Google Scholar]
- 3.WHO. Meningococcal meningitis. Wkly Epidemiol Rec. 2003;78:294–96. [PubMed] [Google Scholar]
- 4.Collard JM, Maman Z, Yacouba H, et al. Increase in Neisseria meningitidis serogroup W135, Niger, 2010. Emerg Infect Dis. 2010;16:1496–98. doi: 10.3201/eid1609.100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forgor AA, Leimkugel J, Hodgson A, et al. Emergence of W135 meningococcal meningitis in Ghana. Trop Med Int Health. 2005;10:1229–34. doi: 10.1111/j.1365-3156.2005.01520.x. [DOI] [PubMed] [Google Scholar]
- 6.MacNeil JR, Medah I, Koussoube D, et al. Neisseria meningitidis serogroup W, Burkina Faso, 2012. Emerg Infect Dis. 2014;20:394–99. doi: 10.3201/eid2003.131407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan N, Rose AM, Legros D, et al. Meningitis serogroup W135 outbreak, Burkina Faso, 2002. Emerg Infect Dis. 2007;13:920–23. doi: 10.3201/eid1306.060940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boisier P, Nicolas P, Djibo S, et al. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis. 2007;44:657–63. doi: 10.1086/511646. [DOI] [PubMed] [Google Scholar]
- 9.Delrieu I, Yaro S, Tamekloe TA, et al. Emergence of epidemic Neisseria meningitidis serogroup X meningitis in Togo and Burkina Faso. PLoS One. 2011;6:e19513. doi: 10.1371/journal.pone.0019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campagne G, Schuchat A, Djibo S, Ousseini A, Cisse L, Chippaux JP. Epidemiology of bacterial meningitis in Niamey, Niger, 1981–96. Bull World Health Organ. 1999;77:499–508. [PMC free article] [PubMed] [Google Scholar]
- 11.Broome CV, Rugh MA, Yada AA, et al. Epidemic group C meningococcal meningitis in Upper Volta, 1979. Bull World Health Organ. 1983;61:325–30. [PMC free article] [PubMed] [Google Scholar]
- 12.Funk A, Uadiale K, Kamau C, Caugant DA, Ango U, Greig J. Sequential outbreaks due to a new strain of Neisseria meningitidis serogroup C in northern Nigeria, 2013–14. PLoS Curr. 2014 doi: 10.1371/currents.outbreaks. published online Dec 29. b50c2aaf1032b3ccade0fca0b63ee518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vuong J, Collard JM, Whaley MJ, et al. Development of real-time PCR methods for the detection of bacterial meningitis pathogens without DNA extraction. PLoS One. 2016;11:e0147765. doi: 10.1371/journal.pone.0147765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. Standard operating procedures for enhanced meningitis surveillance in Africa. Geneva: World Health Organization; 2009. [Google Scholar]
- 15.Institut National de la Statistique. Présentation des résultats globaux définitifs du Quatrième Recensement Général de la Population et de l’Habitat de 2012. Niamey: Institut National de la Statistique; 2013. [accessed Aug 12, 2016]. http://www.stat-niger.org/statistique/file/rgph2012.pdf. French. [Google Scholar]
- 16.WHO. Revised guidance on meningitis outbreak response in sub-Saharan Africa. Wkly Epidemiol Rec. 2014;89:580–86. [PubMed] [Google Scholar]
- 17.Niger Ministry of Health. Rapport de la gestion de l’épidémie de 2015 au Niger. Niamey: Niger Ministry of Health; 2015. (in French) [Google Scholar]
- 18.Kretz CB, Retchless A, Sidikou F, et al. Whole genome characterization of the emerging epidemic meningococcal serogroup C and resurgence of serogroup W in Niger, 2015. Emerg Infect Diseases. doi: 10.3201/eid2210.160468. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolley KA, Maiden MC. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meningitis weekly bulletin. Ouagadougou: World Health Organization Intercountry Support Team for West Africa; 2016. [Google Scholar]
- 21.Collard JM, Maman Z, Abani A, et al. Microbiological and epidemiological investigation of the Neisseria meningitidis serogroup A epidemic in Niger in 2009: last wave before the introduction of the serogroup A meningococcal conjugate vaccine? Epidemiol Infect. 2011;139:1656–60. doi: 10.1017/S0950268810003092. [DOI] [PubMed] [Google Scholar]
- 22.Collard JM, Issaka B, Zaneidou M, et al. Epidemiological changes in meningococcal meningitis in Niger from 2008 to 2011 and the impact of vaccination. BMC Infect Dis. 2013;13:576. doi: 10.1186/1471-2334-13-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boisier P, Djibo S, Sidikou F, et al. Epidemiological patterns of meningococcal meningitis in Niger in 2003 and 2004: under the threat of N. meningitidis serogroup W135. Trop Med Int Health. 2005;10:435–43. doi: 10.1111/j.1365-3156.2005.01394.x. [DOI] [PubMed] [Google Scholar]
- 24.Caugant DA, Nicolas P. Molecular surveillance of meningococcal meningitis in Africa. Vaccine. 2007;25(suppl 1):A8–11. doi: 10.1016/j.vaccine.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Shao Z, Li W, Ren J, et al. Identification of a new Neisseria meningitidis serogroup C clone from Anhui province, China. Lancet. 2006;367:419–23. doi: 10.1016/S0140-6736(06)68141-5. [DOI] [PubMed] [Google Scholar]
- 26.MenAfriCar consortium. The diversity of meningococcal carriage across the African meningitis belt and the impact of vaccination with a group A meningococcal conjugate vaccine. J Infect Dis. 2015;212:1298–307. doi: 10.1093/infdis/jiv211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lingani C, Bergeron-Caron C, Stuart JM, et al. Meningococcal meningitis surveillance in the African meningitis belt, 2004–2013. Clin Infect Dis. 2015;61(suppl 5):S410–15. doi: 10.1093/cid/civ597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Meningitis control in countries of the African meningitis belt, 2015. Wkly Epidemiol Rec. 2016;91:209–16. [PubMed] [Google Scholar]
- 29.Granoff DM, Pelton S, Harrison LH. Meningococcal vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6. London: Elsevier; 2013. pp. 338–418. [Google Scholar]
- 30.CDC vaccine price list. Atlanta: US Centers for Disease Control and Prevention; 2015. [accessed June 2, 2015]. http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list. [Google Scholar]
- 31.LaForce M, Kulkarni P. Development update on a new African pentavalent ACWYX conjugate vaccine. Meningitis Vaccine Project Closure Conference; Addis Ababa, Ethiopia. Feb 22–25, 2016. [Google Scholar]