Abstract
Objective
To determine if endotracheal tube (ETT) insertion depth should be modified in infants with CDH to reduce the risk of main-stem intubation.
Study design
The distance from the thoracic inlet to the carina was measured antenatally by fetal MRI between 20–28 weeks (early) and 30–34 weeks (late) gestation in 30 infants with CDH, and compared with 12 early and 36 late MRIs in control infants without CDH. Postnatal tube position was assessed by chest x-ray in the same 30 CDH infants and compared with 20 control infants with postnatal birth depression.
Results
The carina position was displaced upwards in fetuses and newborns with CDH. Distance from the thoracic inlet to the carina compared with controls was 1.04±0.1cm vs 1.42±0.07cm on early MRI (P < .05), 1.43±0.14cm vs 1.9±0.04cm on late MRI (p<0.01) and 2.36±0.07cm vs 3.28±0.05cm on postnatal x-ray (p<0.01). Adjusting the ETT depth by 1cm resulted in a median distance of 1.27cm from the tip of the endotracheal tube to the carina.
Conclusion
Cephalad displacement of the carina in infants with CDH may predispose to right main-stem intubation and subsequent development of pneumothorax. We speculate that modifying the ETT insertion depth to 5.5cm + weight in term newborns may prevent pneumothoraces and improve outcomes for infants with CDH.
Keywords: Cephalad, pneumothorax, right main-stem intubation, endotracheal tube (ETT)
Congenital diaphragmatic hernia is a severe birth defect, with a prevalence of 1:2000 to 1:3000 live births (1,2). In infants with CDH, a defect in the diaphragm results in herniation of the abdominal contents into the chest and compression of the intrathoracic structures (3). Displacement of the heart and compression of the intrathoracic structures contribute to pulmonary and left ventricular (LV) hypoplasia and abnormal development of the pulmonary vasculature in utero (4–9). These abnormalities cause PPHN and respiratory insufficiency after birth (3,10,11). Initial stabilization of infants with CDH involves endotracheal intubation for management of respiratory failure and prevention of gaseous distention of the intestines and stomach.
Despite advances in neonatal care, mortality in infants with CDH remains high and ranges from 15–80% depending on the severity of the defect (20,21). In addition to the severity of the defect, several postnatal factors contribute to mortality in CDH. Pneumothorax is a complication that occurs frequently in these group of patients, and increases the risk of mortality by 4-fold when it develops pre-repair (12,13). Historically, the development of a pneumothorax pre-repair was thought to be a marker of lung hypoplasia, and representative of overzealous resuscitation and barotrauma (12). However, other factors may contribute to postnatal development of pneumothorax in infants with CDH. With the abdominal organ herniation associated with CDH, it is possible that the carina is displaced cephalad, predisposing the infant to right main-stem intubation during initial endotracheal tube placement. Resuscitation of the newborn infant with CDH with the endotracheal tube in the right main-stem bronchus over-exposes the right lung to the initial inflating pressure used during newborn resuscitation, and impedes emptying of the lung, predisposing the infant to the development of a pneumothorax.
Prenatal MRI at 20–24 weeks and 34 weeks can be used to define the anatomy in infants with CDH, and predict outcome and severity of the defect (17,22). Using these images, the position of the carina can be determined and compared in utero to age matched controls. We hypothesized that the abdominal organ herniation in infants with CDH displaces the carina upward, and this displacement progresses from mid to late gestation and after birth. We examined the position of the carina in fetuses with CDH at 20–28 weeks’ gestation, 32–35 weeks’ gestation, and postnatally, and confirm progressive cephalad displacement of the carina.
Methods
After Institutional Review Board approval, prenatal MRI images at Children’s Hospital Colorado (CHCO) were reviewed on 30 patients with left-sided CDH managed between April 2011 and March 2015. Infants with right-sided defects and Morgagni hernias were excluded from analysis. 30 patients were managed at our institution that met criteria over that time period. At CHCO, fetal MRI is performed on all infants with CDH between 20 and 24 weeks’ gestation (early) and at 34 weeks’ gestation (late) to assess lung volumes (percent predicted lung volume [PPLV] and total lung volume [TLV], degree of liver herniation, the presence of a hernia sac, McGoon index [MGI, diameter of the right pulmonary artery (RPA) + left pulmonary artery (LPA)/diameter of aorta at the level of the diaphragm]) and to determine if other anomalies are present. Early MRI studies were available on 30 infants and late MRI studies available on 24 infants, as 6 infants delivered prior to 34 weeks’ gestation.
All MRI images were performed in 2 60-inch bore, 1.5 Tesla scanners (Siemens Avanto Scanner, Siemens, Germany) using 2 6-channel surface coils and Philips Ingenia scanner (Philips, Highlands Heights, Ohio) using a 32-channel surface coil. The sequences obtained are similar for both scanners and followed preexisting fetal MRI protocols. All studies were performed in the morning with the pregnant patients under the same nil per os (NPO) status, which consisted of no solid or liquids four hours before the scheduled MRI time.
The best, motion-free coronal plane single shot sequences were used to measure the distance of the carina to the thoracic inlet. Initially, the area of the thoracic inlet (TI) was determined by correlating sagittal, axial, and coronal views of the chest. The line was placed on the coronal plane right above the lung apex which in fetal MRI correlates with the postnatally described thoracic inlet. Because our protocols were the same for early and late MRI images, all the coronal planes were acquired based on a parallel plane to the thoracic spine which corrects for normal fetal thoracic curvature position seen in most fetuses. This provides consistent plane acquisition amongst all the cases although slight variation on the axis on each plane could exist. Once the TI was determined, the distance between the carina to the thoracic inlet was measured in cm only in the coronal plane using the measuring tools built in PACS (Picture archiving computer systems). To prevent bias, the early gestational age group was evaluated first, masking the radiologist to images of the late gestation (and vice versa) as well as to postnatal radiographic measurements. Age matched control fetal MRI studies performed for non-pulmonary related pathology were used as controls. 12 early and 36 late control MRI studies were available for comparison. Indications for control fetal MRIs are presented in Table I (available at www.jpeds.com). The distance (cm) between the thoracic inlet (calculated in the same way as in the CDH cases) and the carina was measured as described above.
Table 1.
(Online Only). Age matched control fetal MRI studies performed for non-pulmonary related pathology.
| Indications for Prenatal MRI | N cases |
|---|---|
| CNS | 13 |
| GI | 6 |
| Renal/GU | 10 |
| Cardiac | 3 |
| TTTS | 2 |
| Skeletal Dysplasia | 2 |
| Genetic Syndrome | 4 |
Postnatally, for infants with CDH, the distance between the thoracic inlet and carina was measured on the first anteroposterior (AP) chest radiograph obtained at our institution. Because infants with CDH are born at CHCO, the first AP chest radiograph was obtained in the delivery room. In our practice, the first chest radiograph is only obtained in AP plane, therefore a lateral view was not available in any of the cases. Evaluating the position of the carina on lateral radiographs (either true lateral or cross table lateral) is challenging in the CDH population due to superimposition of opacities mixed with lucencies (arising from mediastinum, herniated bowel and sometimes liver) and a comparison with normal patient’s chest would have not been consistent. Admission chest radiographs performed in infants admitted for birth depression and selective head cooling were used as a comparison group (normal controls). These initial 30 CDH and 20 control AP chest radiographs were used for comparison. To prevent potential bias and to enhance consistency, we established a consensus on how to identify the thoracic inlet before beginning image analysis. This was defined as a horizontal line placed at the level of the first thoracic vertebral body and the first ribs. A variation in obliquity of the AP plane could not be addressed because these images were already obtained. All postnatal chest x-rays were reviewed by a radiologist and an attending neonatologist. In addition, for each individual chest x-ray, the distance from the thoracic inlet and carina was measured by MLM and JG and the distance compared and correlated to determine if similar measurements were obtained. For all chest x-rays, similar measurements were obtained by.
Demographic information collected for all CDH infants included gestational age at birth, birth weight, sex, presence of anomalies (anatomic/chromosomal), prenatal markers of CDH severity, early percent predicted lung volume (PPLV), late total lung volume (TLV), Mcgoon index (MGI), presence of liver herniation, lung head ratio (LHR) and observed to expected LHR. For controls, gestational age at birth, birth weight, and sex were collected.
Statistical Analyses
The unpaired t-test was used to determine differences in the distance from the thoracic inlet to the carina, antenatally on early and later MRI and postnatally on CXR, with p<0.05, considered significant. To determine if severity of CDH correlated with distance between the thoracic inlet and carina (for each marker of CDH severity, early PPLV, late TLV, McGoon index (MGI), presence of liver herniation, lung head ratio (LHR) and observed to expected LHR) the median was calculated and distance from the thoracic inlet to carina on early and late MRI and postnatal CXR stratified into two groups either greater than or less than the median. Distance from the thoracic inlet to carina was compared using the unpaired t-test with p<0.05 considered significant.
Results
Table 2 shows the median gestational age, 25th percentile, 75th percentile and interquartile ranges for early and late control and CDH MRI and postnatal radiographic studies. For postnatal control and CDH patients, mean birth weight, and sex (male:female ratio) are presented in Table 2.
Table 2.
In control infants, early and late MR’s were performed at a median of 23 4/7 weeks with an interquartile range of 5 and 31 3/7 weeks with an interquartile range of 1. Gestational age at birth in control infants was 37 5/7 weeks with an interquartile range of 2.75, and birth weight of 2885 ± 199g. In CDH infants early and late MRI’s were performed at a median of 24 weeks with an interquartile range of 4 and 34 weeks with an interquartile range of 4.75 respectively. Gestational age at birth in CDH infants was 36 5/7 weeks with an interquartile range of 1.97, and birth weight of 2755 ± 131g. Postnatally, in both control and CDH infants there were equal numbers of male and female infants.
| Early MRI | Late MRI | Postnatal | ||||
|---|---|---|---|---|---|---|
| Control | CDH | Control | CDH | Control | CDH | |
| Gestational Age (Median Weeks) | 23 4/7 | 24 | 31 3/7 | 34*p<0.05 | 37 5/7 | 36 5/7 |
| 25th Percentile | 22 | 23 | 34 | 29 | 36.25 | 36.03 |
| 75th Percentile | 27 | 27 | 35 | 33.75 | 39 | 38 |
| Interquartile Range | 5 | 4 | 1 | 4.75 | 2.75 | 1.97 |
| Birth Weight (Median + SD) | 2885 ± 199g | 2755 ± 131g | ||||
| SEX M:F | 10:10 | 15:15 |
When compared with controls in early and late gestation, the distance from the thoracic inlet to the carina was displaced cephalad in infants with CDH. The distance from the thoracic inlet to the carina was 1.04 ± 0.1 cm vs 1.42 ± 0.07cm in early gestation (p<0.05, 95% confidence interval −0.68 to −0.07), and 1.43 ± 0.14cm vs 1.9 ± 0.04cm in late gestation (p<0.01,95% confidence interval −0.75 to −0.2) in CDH and control fetuses respectively. The carina was displaced cephalad by a 0.37 ± 0.15cm in early gestation, and by 0.47 ± 0.14cm in late gestation (Figures 1 and 3).
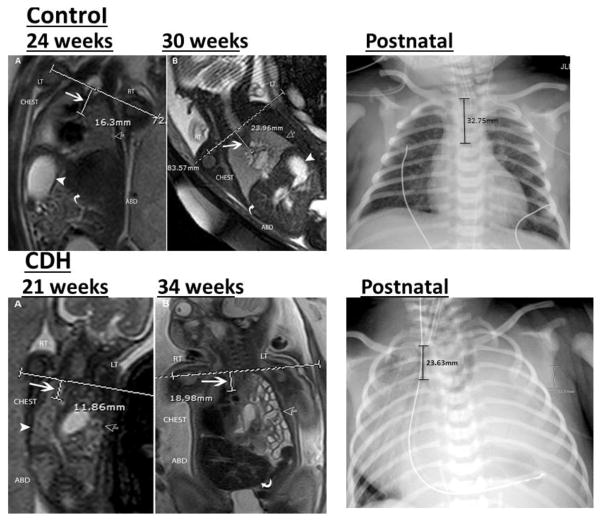
Figure 1.
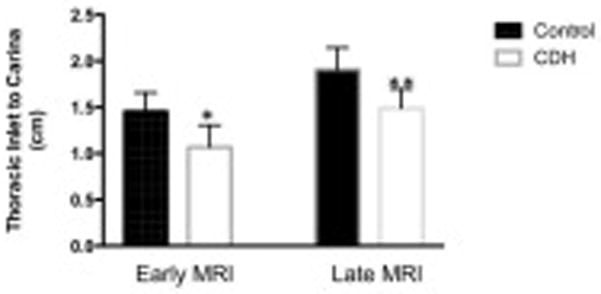
In early and late gestation, the position of the carina was displaced cephalad in infants with CDH. The distance from the thoracic inlet to the carina was 1.04 ± 0.1 cm vs 1.42 ± 0.07cm early (*p<0.05), and 1.43 ± 0.14cm vs 1.9 ± 0.04cm late (**p<0.01) in CDH and control fetuses respectively. In early gestation, the carina was displaced cephalad by a median of 0.37 ± 0.15cm, in late gestation by a median of 0.47 ± 0.14cm
Figure 3.
Coronal T2-weighted MRI of a control fetus and fetus with congenital diaphragmatic hernia.
Top left: A: 24 weeks and B: 30 week images in the same fetus demonstrating the normal appearance of the chest. The horizontal line at the level of the thoracic inlet (TI) and vertical line represents the distance from the TI to the carina (white arrow). Normal lungs are seen (open arrow) as well as normal position of the stomach (white arrowhead) and liver (curved arrow).
Bottom left: A: 21 week and B: 34 week images showing left sided congenital diaphragmatic hernia. hernia (open arrow). Horizontal line shows the level of the thoracic inlet and short vertical line measures the distance from the thoracic inlet to the carina pointed by a large white arrow. The herniated viscera (open arrow) and right lung (arrow head) are seen. B: Same fetus at 34 weeks gestational age shows similar calculation of the distance between the thoracic inlet and the carina (white arrow). Bowel loops are seen herniated into the left chest (open arrow) and the liver is seen in the abdomen (curved arrow).
Top right: Anterior-posterior radiograph of the chest in control infant demonstrating the normal appearance of the chest. The vertical line at the level of clavicles represents the distance from the thoracic inlet to the carina.
Bottom right: Anterior-posterior radiograph of the chest in an infant with left sided congenital diaphragmatic hernia demonstrating herniation of the abdominal viscera into the chest. The vertical line at the level of clavicles represents the distance from the thoracic inlet to the carina.
After birth, the distance from the thoracic inlet to the carina remained decreased in CDH infants, 2.36 ± 0.07cm vs 3.28 ± 0.05 (p<0.01, 95% confidence interval 0.73 to 1.1) in CDH and control infants respectively. Postnatally, the carina was displaced upward by 0.91 ± 0.09cm (Figures 2 and 3).
Figure 2.
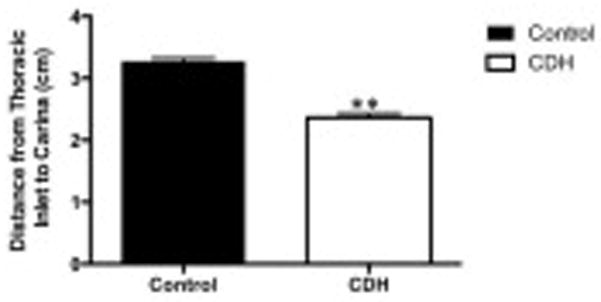
After birth, the distance from the thoracic inlet to the carina remained decreased in CDH infants, 2.36 ± 0.07cm vs 3.28 ± 0.05 (**p<0.01) in CDH and control infants respectively. Postnatally, the carina was displaced upward by a median of 0.91 ± 0.09cm.
For CDH infants, chromosomal anomalies were present in 3 infants and anatomic (cardiac or airway) anomalies present in 5 infants. The median early PPLV was 25%, and late TLV 31ml. The liver was intrathoracic in 17/30 infants, median McGoon index was 1.05, median lung head ratio 1.1 and median O/E LHR 64%. Survival for this subset of infants was 76%.
When the data were stratified based on severity of the CDH, a total lung volume on late MRI less than the median of 31ml predicted a shorter distance from the carina of 1.04 ± 0.03cm vs 1.27 ± 0.06cm (p<0.01) on early MRI and 1.69 ± 0.06 vs. 1.94 ± 0.1cm (p<0.05) on late MRI. In addition, a LHR less than the median of 1.1 predicted a shorter carina distance on late MRI, 1.67 ± 0.07cm vs 1.96 ± 0.11cm (p<0.05). No other marker of CDH severity predicted the distance from the thoracic inlet to the carina. When the distance from the thoracic inlet was compared with early PPLV, MGI, presence of liver herniation, and observed to expected LHR, there was no correlation between CDH severity and distance from the thoracic inlet to the carina.
Discussion
We describe cephalad displacement of the carina in mid and late gestation in fetuses with CDH, and postnatally in newborn infants with CDH. Throughout gestation, herniation of the abdominal contents into the chest displaces the carina upwards, and this distance increases progressively from mid to late gestation and after birth. These findings support adjusting the depth of ETT placement after birth in infants with CDH, and suggests that right main-stem intubation during resuscitation may contribute to the development of pneumothorax in CDH infants after birth.
The rate of pneumothorax has been decreasing in infants with CDH, with studies reporting an incidence of 46% prior to 1997 and 26% after 1997 (12), 13.5% in the mid-2000s (19) and more recent reports describing an incidence of 7.3% in infants less than 34 weeks and 4.5% in infants greater than 34 weeks (13). It is likely that the decrease in the rate of pneumothorax is due to the widespread adoption of gentle ventilation strategies in the management of CDH (20,21), however even with this practice, infants with CDH continue to develop pneumothorax that is associated with increased mortality. Despite the decreasing incidence of pneumothorax in infants with CDH, the need for thoracostomy tube placement prior to CDH repair remains a significant risk factor for mortality (12,13,19). Early reports describe a 4-fold mortality with pneumothorax pre-repair (12) and more recently, a mortality rate of 55% has been described with the development of pneumothorax in infants with CDH (19).
Historically, the development of a pneumothorax pre-repair has been thought of as a marker of disease severity and degree of lung hypoplasia (12,19) and represents overzealous resuscitation and injury to the lung with barotrauma (12). Although the presence of lung hypoplasia likely contributes to the development of pneumothorax, other factors may be contributory.
Our findings of an abnormal carina position in infants with CDH could significantly impact the approach to the initial resuscitation and ETT placement. We describe progressive cephalad displacement of the carina from mid to late gestation and after birth, with almost 1cm upward displacement of the carina postnatally. The 7th edition of NRP recommends three potential methods for estimating proper ETT placement. The first is a simple recommendation to use printed depth markers to place the ETT 1 to 2 cm below the vocal cords. The second is an update to the 6th edition gestational age/weight-based chart in which Tochen’s formula (6cm+weight in kg rounded to the closest integer) is now rounded to the nearest 0.5 cm mark. The third method estimates proper ETT depth based on the nasal tragus length (NTL)+1 cm formula, where the NTL is measured as the distance between the tragus of the ear and the base of the nasal septum (14). Published reports have validated the use of the NTL + 1cm formula demonstrating appropriate placement in 98% of patients over a broad range of gestational ages and weights. A more recent report found that the NTL+1 cm estimate performed well for infants weighing < 2.5 kg, but for infants weighing > 2.5 kg, the NTL+0.5 cm was needed for appropriate ETT placement (16). Despite this, the 7th edition of NRP now endorses NTL-based estimates of neonatal ETT depth specifically using the NTL+1 cm formula (14). Whether this recommendation is appropriate for infants with CDH is unknown.
In an effort to predict which infants might be at greater risk for cephalad displacement of the carina, we used antenatal predictors of CDH severity, and found that total lung volume on late MRI <31ml and LHR < 1.1 predicted a shorter distance to the carina. This finding fits with the hypothesis that more extensive organ herniation results in greater upward displacement of the carina, putting infants with more severe CDH at greater risk for this abnormality. At our institution, it is standard to adjust for the upward displacement of the carina, and with the initial resuscitation in term newborns, the ETT is inserted to a depth of 5cm + baby’s weight. To validate this approach, we measured the distance from the tip of the endotracheal tube to the carina. In this subset of infants, the median distance from the tip of the endotracheal tube to the carina was 1.27cm with an interquartile range of 0.6cm. Based on these findings, it is our recommendation, especially in the most severe infants with CDH to adjust the ETT depth by 0.5cm, inserting the ETT to a depth of 5.5cm + the weight to prevent right main-stem intubation and minimize the risk for pneumothorax.
Limitations to this study include differences in gestational age between the control and CDH group with respect to the late MRI studies (median of 31.5 weeks in controls versus 34 weeks in CDH). These differences do not explain the difference in the distance between the thoracic inlet and carina, as MRI studies were performed earlier in the controls, likely decreasing the distance between the thoracic inlet and carina, and minimizing the difference between control and CDH studies.
CDH displaces the carina cephalad, predisposing the newborn to right main-stem intubation, which may contribute to abnormalities in gas exchange and increased need for ECMO, as well as the development of pneumothorax. We recommend adjusting the depth of ETT insertion by 0.5cm, inserting the ETT to a depth of 5.5cm + the weight to ensure optimal ETT placement in infants with CDH.
Acknowledgments
Supported by the National Institutes of Health (NIH) (5K08HL102261 to J.G.) and the NIH/ National Center for Advancing Translational Sciences Colorado Clinical & Translational Sciences Institute (UL1 TR000154).
We thank the CDH team at CHCO (Timothy Crombleholme, MD, Kenneth Liechty, MD, and Ahmed Marwan, MD,) for their contribution to the comprehensive care of the patients described in this report.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torfs CP, Curry CJ, Bateson TF, Honore LH. A population-based study of congenital diaphragmatic hernia. Teratology. 1992 Dec;46(6):555–565. doi: 10.1002/tera.1420460605. [DOI] [PubMed] [Google Scholar]
- 2.Skari H, Bjornland K, Haugen G, Egeland T, Emblem R. Congenital diaphragmatic hernia: A meta-analysis of mortality factors. J Pediatr Surg. 2000 Aug;35(8):1187–97. doi: 10.1053/jpsu.2000.8725. [DOI] [PubMed] [Google Scholar]
- 3.Harrison MR, Adzick NS, Nakayama DK, deLorimier AA. Fetal diaphragmatic hernia: pathophysiology, natural history, and outcome. Clin Obstet Gynecol. 1986 Sep;29(3):490–501. [PubMed] [Google Scholar]
- 4.Downard CD, Jaksic T, Garza JJ, Dzakovic A, Nemes L, Jennings RW, et al. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg. 2003 May;38(5):729–32. doi: 10.1016/jpsu.2003.50194. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz SM, Vermillion RP, Hirschl RB. Evaluation of left ventricular mass in children with left-sided congenital diaphragmatic hernia. J Pediatr. 1994 Sep;125(3):447–51. doi: 10.1016/s0022-3476(05)83293-7. [DOI] [PubMed] [Google Scholar]
- 6.Breaux CW, Jr, Rouse TM, Cain WS, Georgeson KE. Congenital diaphragmatic hernia in an era of delayed repair after medical and/or extracorporeal membrane oxygenation stabilization: A prognostic and management classification. J Pediatr Surg. 1992 Sep;27(9):1192–6. doi: 10.1016/0022-3468(92)90785-6. [DOI] [PubMed] [Google Scholar]
- 7.Vogel M, McElhinney DB, Marcus E, Morash D, Jennings RW, Tworetzky W. Significance and outcome of left heart hypoplasia in fetal congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2010 Mar;35(3):310–7. doi: 10.1002/uog.7497. [DOI] [PubMed] [Google Scholar]
- 8.Taira Y, Yamataka T, Miyazaki E, Puri P. Comparison of the pulmonary vasculature in newborns and stillborns with congenital diaphragmatic hernia. Pediatr Surg Int. 1998 Nov;14(1–2):30–5. doi: 10.1007/s003830050429. [DOI] [PubMed] [Google Scholar]
- 9.Van Loenhout RB, De Krijger RR, Van de Ven CP, Van der Horst IW, Beurskens LW, Tibboel D, et al. Postmortem biopsy to obtain lung tissue in congenital diaphragmatic hernia. Neonatology. 2013 Mar;103(3):213–7. doi: 10.1159/000345921. [DOI] [PubMed] [Google Scholar]
- 10.Reiss I, Schaible T, van den Hout L, Capolupo I, Allegaert K, van Heijst A, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: The CDH Euro Consortium consensus. Neonatology. 2010 Nov;98(4):354–364. doi: 10.1159/000320622. [DOI] [PubMed] [Google Scholar]
- 11.Dillon PW, Cilley RE, Mauger D, Zachary C, Meier A. The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J Pediatr Surg. 2004 Mar;39(3):307–12. doi: 10.1016/j.jpedsurg.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Casaccia G, Ravà L, Bagolan P, di Ciommo VM. Predictors and statistical models in congenital diaphragmatic hernia. Pediatr Surg Int. 2008 Apr;24(4):411–4. doi: 10.1007/s00383-008-2108-x. [DOI] [PubMed] [Google Scholar]
- 13.Grover TR, Murthy K, Brozanski B, Gien J, Rintoul N, Keene S, et al. Children’s Hospitals Neonatal Consortium. Short-term outcomes and medical and surgical interventions in infants with congenital diaphragmatic hernia. Am J Perinatol. 2015 Sep;32(11):1038–44. doi: 10.1055/s-0035-1548729. [DOI] [PubMed] [Google Scholar]
- 14.Kattwinkel J, Niermeyer S, Nadkarni V, Tibballs J, Phillips B, Zideman D, et al. Textbook of Neonatal Resuscitation. 7. American Academy of Pediatrics and American Heart Association; 2016. [Google Scholar]
- 15.Shukla HK, Hendricks-Munoz KD, Atakent Y, Rapaport S. Rapid estimation of insertional length of endotracheal intubation in newborn infants. J Pediatr. 1997 Oct;131(4):561–4. doi: 10.1016/s0022-3476(97)70062-3. [DOI] [PubMed] [Google Scholar]
- 16.Wang T-C, Kuo L-L, Lee C-Y. Utilizing nasal-tragus length to estimate optimal endotracheal tube depth for neonates in Taiwan. Indian J Pediatr. 2011 Mar;78(3):296–300. doi: 10.1007/s12098-010-0278-8. [DOI] [PubMed] [Google Scholar]
- 17.Zamora IJ, Olutoye OO, Cass DL, Fallon SC, Lazar DA, Cassady CI, et al. Prenatal MRI fetal lung volumes and percent liver herniation predict pulmonary morbidity in congenital diaphragmatic hernia (CDH) J Pediatr Surg. 2014 May;49(5):688–93. doi: 10.1016/j.jpedsurg.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 18.Lee TC, Lim FY, Keswani SG, Frischer JS, Haberman B, Kingma PS, et al. Late gestation fetal magnetic resonance imaging-derived total lung volume predicts postnatal survival and need for extracorporeal membrane oxygenation support in isolated congenital diaphragmatic hernia. J Pediatr Surg. 2011 Jun;46(6):1165–71. doi: 10.1016/j.jpedsurg.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usui N, Nagata K, Hayakawa M, Okuyama H, Kanamori Y, Takahashi S, et al. Pneumothoraces as a fatal complication of congenital diaphragmatic hernia in the era of gentle ventilation. Eur J Pediatr Surg. 2014 Feb;24(1):31–8. doi: 10.1055/s-0033-1357753. [DOI] [PubMed] [Google Scholar]
- 20.Frenckner B, Ehrén H, Granholm T, Lindén V, Palmér K. Improved results in patients who have congenital diaphragmatic hernia using preoperative stabilization, extracorporeal membrane oxygenation, and delayed surgery. J Pediatr Surg. 1997 Aug;32(8):1185–9. doi: 10.1016/s0022-3468(97)90679-5. [DOI] [PubMed] [Google Scholar]
- 21.Wung J, James LS, Kilchevsky E, James E. Management of infants with severe respiratory failure and persistence of the fetal circulation, without hyperventilation. Pediatrics. 1985 Oct;76(4):488–94. [PubMed] [Google Scholar]
- 22.Le LD, Keswani SG, Biesiada J, Lim FY, Kingma PS, Haberman BE, et al. The congenital diaphragmatic hernia composite prognostic index correlates with survival in left- sided congenital diaphragmatic hernia. J Pediatr Surg. 2012 Jan;47(1):57–62. doi: 10.1016/j.jpedsurg.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deprest J, Brady P, Nicolaides K, Benachi A, Berg C, Vermeesch J, et al. Prenatal management of the fetus with isolated congenital diaphragmatic hernia in the era of the TOTAL trial. Semin Fetal Neonatal Med. 2014 Dec;19(6):338–48. doi: 10.1016/j.siny.2014.09.006. [DOI] [PubMed] [Google Scholar]