Abstract
Background
We conducted a multicenter, 2-stage, open-label, phase II trial to assess the efficacy and safety of dacomitinib in adult patients with recurrent glioblastoma (GB) and epidermal growth factor receptor gene (EGFR) amplification with or without variant III (EGFRvIII) deletion.
Methods
Patients with first recurrence were enrolled in 2 cohorts. Cohort A included patients with EGFR gene amplification without EGFRvIII mutation. Cohort B included patients with EGFR gene amplification and EGFRvIII mutation. Dacomitinib was administered (45 mg/day) until disease progression/unacceptable adverse events (AEs). Primary endpoint was progression-free survival (PFS; RANO criteria) at 6 months (PFS6).
Results
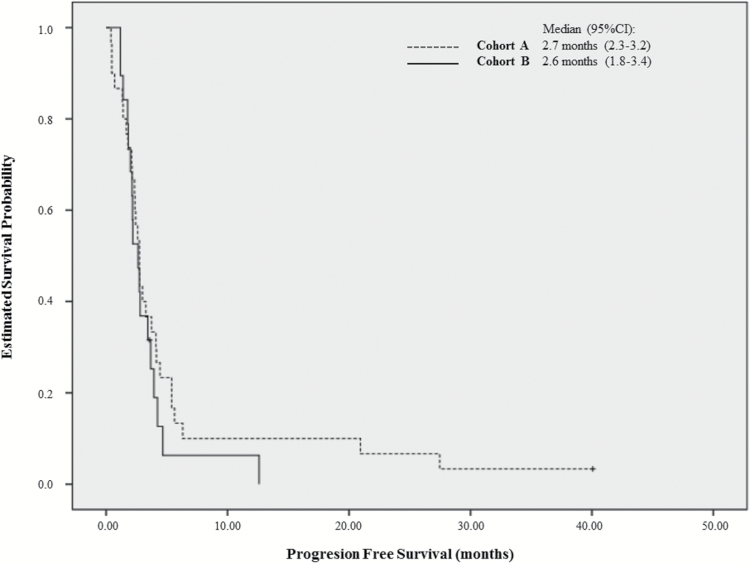
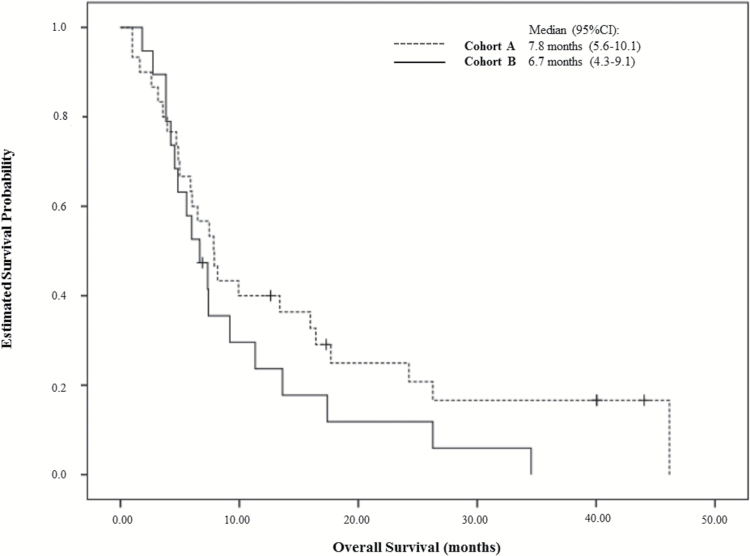
Thirty patients in Cohort A and 19 in Cohort B were enrolled. Median age was 59 years (range 39–81), 65.3% were male, and Eastern Cooperative Oncology Group Performance Status 0/1/2 were 10.2%/65.3%/24.5%, respectively. PFS6 was 10.6% (Cohort A: 13.3%; Cohort B: 5.9%) with a median PFS of 2.7 months (Cohort A: 2.7 mo; Cohort B: 2.6 mo). Four patients were progression free at 6 months and 3 patients were so at 12 months. Median overall survival was 7.4 months (Cohort A: 7.8 mo; Cohort B: 6.7 mo). The best overall response included 1 complete response and 2 partial responses (4.1%). Stable disease was observed in 12 patients (24.5%: eight in Cohort A and four in Cohort B). Diarrhea and rash were the most common AEs; 20 (40.8%) patients experienced grade 3–4 drug-related AEs.
Conclusions
Dacomitinib has a limited single-agent activity in recurrent GB with EGFR amplification. The detailed molecular characterization of the 4 patients with response in this trial can be useful to select patients who could benefit from dacomitinib.
Keywords: dacomitinib, EGFR, glioblastoma, high-grade glioma
Importance of the study
This study investigates safety and efficacy of dacomitinib, a pan‒human epidermal growth factor receptor (HER) tyrosine kinase inhibitor in patients with relapsed GB. The importance of the study lies in 2 facts—first, the lack of effective therapeutic options in patients with relapsed GB who clearly have unmet needs; second, the antineoplastic agent is a pan-anti-HER related with successful anti-EGFR and anti-HER2 antitumor-targeted agents discovered in the last 15 years. Moreover, about 40% of GB cases carry amplification of the EGFR gene, and half of these patients have EGFRvIII mutation leading to a constitutively activated truncated form of the receptor, and the activation of the EGFR pathway has been related with cell proliferation, migration, and invasiveness of GB cells. This background provided grounds for investigation of this anti-EGFR agent in patients with GB. However, after the negative results of this study, further investigation should take profit from biomarkers based on other EGFR alterations instead of EGFR gene amplification.
Glioblastoma (GB) is the most frequent primary CNS malignancy, with poor survival despite multimodal treatment with surgery, radiotherapy, and chemotherapy with temozolomide.1 Little progress has been made over the past decade and there is an unmet medical need.
Around 40% of GB cases carry amplification of the epidermal growth factor receptor gene (EGFR), and half of these patients have the EGFR variant (v)III mutation, a deletion of exons 2–7 that generates a constitutive activation of the receptor tyrosine kinase domain.2 Since activation of the EGFR pathway increases cell proliferation, migration, and invasiveness of tumor cells,3,4 inhibition of this pathway is an attractive therapeutic target in GB.
Several small molecules and antibodies directed against EGFR have been successfully used in different tumors with EGFR alterations, such as non–small cell lung cancer (NSCLC) and epidermoid head and neck cancers. However, targeted EGFR agents have not shown significant activity in patients with GB.5,6 To date, for patient selection, EGFR inhibitors have been tested in GB without requiring any specific EGFR alteration.
Dacomitinib is a second-generation, oral, irreversible, highly selective pan‒human epidermal growth factor receptor (HER) small-molecule tyrosine kinase inhibitor. Interestingly, dacomitinib is active in erlotinib- and gefitinib-resistant EGFR-mutated NSCLC, suggesting that it could be more active than other EGFR inhibitors in patients with EGFR-amplified GBs.7 In addition, dacomitinib is unlikely to interfere with enzyme-inducing anti-epileptic drug therapies. Dacomitinib, as an inhibitor of other HER family members (dacomitinib inhibits not only EGFR but also HER2 and HER4), could avoid resistance from GB cells to EGFR inhibitor through the activation of HER2 or HER4. In fact, HER2 is commonly overexpressed in GB.8
Preclinical data suggest that dacomitinib has an effect on cell viability, self-renewal, and proliferation in EGFR-amplified GB cells. Moreover, systematic administration of dacomitinib strongly impaired the in vivo tumor growth rates in EGFR-amplified xenograft models.9,10 Unlike previous studies with other molecules,11 the authors’ work showed that dacomitinib was able to dephosphorylate the downstream effectors of EGFR in vivo. The effect is not decreased by the presence of EGFR-mutant isoforms, like EGFRvIII, although it was attenuated in the absence of phosphatase and tensin homolog (PTEN) activity.10 Taking into account these preclinical data and in an attempt to improve the results with first-generation, reversible EGFR inhibitors, the Spanish Group for Research in Neuro-Oncology (GEINO) performed a phase II study of dacomitinib in patients with recurrent EGFR-amplified GB. The drug was tested in 2 independent cohorts with EGFR amplification: patients without EGFRvIII mutation (Cohort A) and patients with EGFRvIII mutation (Cohort B).
The aim of this multicenter, 2-stage, open-label, phase II clinical trial was to assess the efficacy and safety of dacomitinib in adult patients with recurrent GB with EGFR gene amplification with or without EGFRvIII mutation. Patients with or without the EGFRvIII mutation were analyzed independently because a different biological response to dacomitinib could be expected when EGFRvIII mutation was present as previously described in GB preclinical models.12,13
Materials and methods
Study Design and Patient Population
This was a 2-stage, open-label, phase II clinical trial of dacomitinib in patients with GB with EGFR amplification at first recurrence. Patients with EGFR amplification without the EGFRvIII mutation were enrolled in Cohort A and patients with EGFR amplification with EGFRvIII mutation were enrolled in Cohort B. Each cohort was analyzed independently.
Participants from 12 GEINO centers in Spain were enrolled between March 2012 and April 2015. All patients signed an informed consent form before the EGFR test and before enrollment.
The protocol was approved by the institutional review board at each participating site. This study was registered with ClinicalTrials.gov under identifier NCT01520870.
Patients over 18 years of age who had central review histologically confirmed recurrent GB with EGFR amplification (determined by fluorescence in situ hybridization [FISH] assay), also confirmed by central molecular pathology review, were eligible for the study. The assessment of the vIII mutation was made centrally by quantitative PCR.
Molecular analysis of EGFR could be performed before the patient fulfilled the rest of the inclusion criteria.
All patients must have progressive disease on MRI defined by Response Assessment in Neuro-Oncology (RANO) criteria14 after the standard Stupp protocol.1 The time interval for the start of treatment was at least 12 weeks from prior radiotherapy unless there was either histopathologic confirmation of recurrent tumor or new contrast enhancement on MRI outside of the radiotherapy treatment field.
Additional inclusion criteria were KPS ≥70 and adequate hematologic function (hematocrit >28%, leukocytes >3000/μL, absolute neutrophil count >1500 cells/μL, platelets >100000 cells/μL), liver function (bilirubin ≤2X the upper limit of normal [ULN], aspartate aminotransferase [AST] and alanine aminotransferase [ALT] ≤2.5X ULN), and renal function (creatinine clearance >60 mL/min/1.73 m2). Women of childbearing potential and their partners had to use adequate contraception throughout the study period and for 12 weeks after its completion. Patients were not allowed to receive any other investigational agents or have a history of allergic reactions to compounds similar to dacomitinib.
Exclusion criteria were extracranial metastatic disease, Gliadel wafer treatment, severe intercurrent illness presentation, and HIV positivity with a combination antiretroviral therapy.
All patients were required to have pretreatment brain MRI within the 14 days before therapeutic treatment and to have received a stable steroid dose for ≥5 days.
Treatment
Dacomitinib was administered to all patients at an initial oral dose of 45 mg/day. The treatment was the same in both cohorts, Cohort A (EGFR amp/EGFRvIII−) and Cohort B (EGFR amp/EGFRvIII+). Therapy was continued until disease progression, significant clinical decline, unacceptable toxicity, or patient decision. Toxicity was graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
Drug doses were withheld and/or reduced for intolerable grade 2 or grade 3–4 toxicity. A maximum of 2 dose-level reductions was permitted (30 mg, then 15 mg). Dacomitinib administration could be interrupted for a maximum of 14 days.
Study Procedures
Clinical evaluations were scheduled every 2 weeks to screen for toxicity and progression. Surveillance MRI was performed every 12 weeks to assess response to treatment according to RANO criteria.14 The baseline and follow-up scans for each patient were locally and centrally reviewed to determine the overall radiographic response (central review was done at the end of the follow-up). Clinical diagnosis of progressive disease was determined by progressive clinical decline due to the tumor progression.
Molecular Analysis
Fluorescence in situ hybridization
FISH assays were performed on formalin-fixed, paraffin-embedded (FFPE) sections of 4 μm thickness by using the dual color probe LSI EGFR/chromosome 7 enumeration probe (CEP7) from Vysis (Abbott Laboratories). The pretreatment of the FFPE tissue and the hybridization protocol were performed following manufacturer’s instructions.
Fluorescence signals were scored in accordance with the criteria described in previous reports.15,16 For each sample, only well-defined nuclei of at least 50 tumor cells in 4 tumor areas were analyzed, and the numbers of single-copy gene and control probe signals were scored. EGFR gene amplification was considered positive when the EGFR to CEP7 ratio was ≥2, or the presence of EGFR gene clusters of ≥15 gene copies was detected in more than 10% of tumor cells.
Real-time reverse transcription PCR
Total RNA from FFPE tissues of EGFR amplified tumors was extracted using the RNeasy FFPE Kit (Qiagen) according to manufacturer’s protocol. A total of 1 µg of RNA was reverse transcribed for the synthesis of cDNA using the PrimeScript RT kit (Takara Bio) following the manufacturer’s instructions. Real-time PCR to amplify EGFRvIII and hypoxanthine-guanine phosphoribosyltransferase (HPRT1) as the control gene was performed on LightCycler 480 using the SYBR Green method (Roche). EGFRvIII and HPRT1 primer sequences were derived from the Yoshimoto K report.17 Briefly, 2 µL of cDNA product was used as the template in a 10 µL PCR containing 2 µL of LightCycler FastStart DNA MasterPlus SYBR Green I (Roche) and 0.2 µm of each primer. All reactions were performed in duplicate. Amplification protocols were as follows: 95°C for 10 min; 40 cycles of 95°C/10 s, 60°C/10 s, and 72°C/10 s; and 95°C/10 s, 65°C/15 s, and 95°C with 0.06°C/s, 9 acquisitions per degree for melting curve analysis. Threshold cycle number (cross point) was automatically determined by LightCycler software.
High-resolution melting-curve analysis (HRM) and Sanger sequencing
Genomic DNA was extracted from FFPE sections of the GB patients who were enrolled in the clinical trial following manufacturer’s recommendations (Qiagen). The hotspot mutations R132 and R172 of IDH1 and IDH2, respectively, were amplified by PCR by using primers previously described.18,19IDH1 and IDH2 mutations were screened by HRM analysis of the fluorescent melting curves of the amplified fragments on a LightCycler 480 following manufacturer’s instructions. After HRM analysis, mutated samples were purified and Sanger sequenced using the same primers and the Big Dye Terminator v3.1 sequencing kit in an ABI 3130xl genetic analyzer (ThermoFisher).
Methylation-specific (MS) PCR
Extracted DNA (1 μg) from the FFPE tissue sections was subjected to bisulfite modification using the Epitect Bisulfite Kit (Qiagen) following manufacturer’s recommendations. The methylation status of the promoter region of the O6-DNA methylguanine-methyltransferase gene (MGMT) was determined by MS-PCR by using a nested, 2-stage PCR approach and the primers previously described by Palmisano and coworkers.20 Positive and negative methylated DNA were obtained from normal lymphocytes of peripheral blood of healthy individuals. Methylated and unmethylated products were separated on a 3% agarose gel and examined under ultraviolet illumination.
Statistical Analysis
The primary endpoint was the rate of progression-free survival (PFS) at 6 months (PFS6) according to RANO criteria.14 Response was radiographically assessed by investigators and by an independent blinded radiologist.
Secondary endpoints were safety and tolerability of dacomitinib, overall survival (OS), and antitumor response assessed by RANO criteria.14
The sample size of each cohort was estimated according to the Simon 2-stage phase II design: P0 = 15% PFS6; P1 = 35% PFS6. To achieve an (ɑ,β) error of (0.1, 0.1), a total of 32 patients for each cohort was required.
According to the Simon design, the primary endpoint would be considered not reached in each cohort if ≤2 patients were progression free at 6 months among the first evaluable 17 patients of each cohort, or if ≤7 patients were progression free at 6 months among 32 patients of each cohort in the second stage.
PFS6 and response rate were based on the proportion of patients known to have achieved that endpoint using the intention to treat concept. Median PFS and OS were calculated from the Kaplan–Meier curves. The time was measured from registration date. All patients receiving treatment per protocol were included in the safety assessment. The analysis of toxicity was reported using CTCAE v4.0.
Results
Patient Disposition and Characteristics
Molecular screening was performed in 178 patients (121 had no EGFR amplification and 57 had EGFR amplification). Eight of these patients with EGFR amplification did not have disease progression when the study was closed and were not finally enrolled in the study. All the tissues from patients finally enrolled in the trial were obtained from the surgery at diagnosis.
Forty-nine patients were enrolled in the study: 30 in Cohort A (EGFR amp/EGFRvIII−) and 19 in Cohort B (EGFR amp/EGFRvIII+). Two patients in Cohort B were not evaluable for the primary endpoint (PFS6): one because the patient, with stable disease in the first MRI, decided not to continue treatment after 3 months. The other patient decided not to continue in the study after 1 month of treatment because he changed his place of residence. None of these patients had any grade 2 toxicities.
The demographic, molecular, and clinical baseline characteristics of the patients by each cohort are described in Table 1. Detailed characteristics of each individual patient are displayed in Supplementary Table S1.
Table 1.
Demographic, clinical, and molecular characteristics at baseline
| Cohort A (EGFR amp/EGFRvIII−) n = 30 | Cohort B (EGFR amp/EGFRvIII+) n = 19 | Total (n = 49) | |
|---|---|---|---|
| Age, y, median (range) | 62.5 (41–81) | 52.0 (39–72) | 59 (39–81) |
| Gender, n (%) | |||
| Male | 20 (66.7) | 12 (63.2) | 32 (65.3) |
| Female | 10 (33.3) | 7 (36.8) | 17 (34.7) |
| ECOG-PS, n (%) | |||
| 0 | 3 (10.0) | 2 (10.5) | 5 (10.2) |
| 1 | 19 (63.3) | 13 (68.4) | 32 (65.3) |
| 2 | 8 (26.7) | 4 (21.1) | 12 (24.5) |
| MGMT methylation status, n (%) | |||
| Methylated | 9 (30) | 1 (5.3) | 10 (20) |
| Not methylated | 7 (23.3) | 10 (52.6) | 17 (34.7) |
| Not determined | 14 (46.6) | 8 (42.1) | 22 (44.9) |
| IDH1/2 mutations, n (%) | |||
| IDH1 mut | 2 (6.6) | 0 | 2 (4.1) |
| IDH2 mut | 0 | 0 | 0 |
| IDH1/2 not mutated | 19 (63.3) | 15 (79) | 34 (69.4) |
| Not determined | 9 (30) | 4 (21) | 13 (26.5) |
ECOG-PS: Eastern Cooperative Oncology Group Performance Status.
Progression-free Survival at 6 Months
Progression-free survival rate at 6 months defined using RANO criteria was the primary endpoint. A Simon 2-stage design (PFS6 P0 = 15%; P1 = 35%; α = 0.1; β = 0.1) required that at least 3 patients be progression free at 6 months in the first 17 evaluable patients in order to expand to a second step. This goal was achieved by Cohort A (EGFR amp/EGFRvIII−) but not by Cohort B (EGFR amp/EGFRvIII+), where only 1 patient was progression free at 6 months among the first 17 evaluable patients, and this cohort was closed after this first stage.
In Cohort A, only 4 of 30 patients were progression free at 6 months, and the study was closed at this point when the goal of 7 patients without progression at 6 months could not be reached in the second stage. The PFS6 was 13.3% (95% CI: 0–26.5%) and the median PFS was 2.7 months (95% CI: 2.3–3.2). The Kaplan–Meier curve of PFS in Cohort A is displayed in Fig. 1. Interestingly, the 3 patients without progression at 6 months were also progression free at 1 year and one of them was also progression free at 40 months of treatment, at the time of study closure.
Fig. 1.
Progression-free survival rate at 6 months for Cohort A (EGFR amp/EGFRvIII−) and Cohort B (EGFR amp/EGFRvIII+).
As stated above, Cohort B was closed to enrollment in the first stage (19 patients were included with EGFR amp/EGFR vIII+ and 17 of them were evaluable). The PFS6 was 5.9% (95% CI: 0–18%) and the median PFS was 2.6 months (95% CI: 1.8–3.4). The Kaplan–Meier curve for Cohort B is shown in Fig. 1.
Globally, including both cohorts, the PFS6 was 10.6% (95% CI: 2%–19.6%), with a median PFS of 2.7 months (95% CI: 2.3–3.1).
Results obtained from the central radiological evaluation were very similar to those obtained from the local evaluation. In Cohort A, the PFS6 was 13.6% (95% CI: 0–31.4%) and the median PFS was 2.9 months (95% CI: 1.03–4.07), while in Cohort B the results were 5.9% (95% CI: 0–18%) and 2.4 months (95% CI: 1.9–2.8), respectively. Median PFS for both cohorts was 2.8 months (95% CI: 2.6–3.0).
Cases that Met the Primary Endpoint
Three patients in Cohort A were progression free at 6 months and at 1 year of treatment. In Cohort B only one patient had clear medical benefit, with a PFS of 12.6 months. Tissue from the surgery at diagnosis was available in 3 of these 5 cases with longer PFS and OS. IDH1, IDH2, and MGMT methylation status are shown in Table 2 together with the main demographic and clinical data of these long-term survivors. Neither MGMT methylation status nor the presence of isocitrate dehydrogenase (IDH) 1 and 2 mutations seems to preclude a clinical benefit with dacomitinib.
Table 2.
Patients achieving progression-free status at 6 months
| Patient No 1 | Patient No 16 | Patient No 20 | Patient No 23 | Patient No 39 | |
|---|---|---|---|---|---|
| Age, y | 59 | 61 | 68 | 65 | 59 |
| Sex | Male | Female | Female | Male | Male |
| EGFR Status (Cohort) | EGFRamp/EGFRvIII−(Cohort A) | EGFRamp/EGFRvIII−(Cohort A) | EGFRamp/EGFRvIII−(Cohort A) | EGFRamp/EGFRvIII+(Cohort B) | EGFRamp/EGFRvIII−(Cohort A) |
| IDH1/IDH2 status | Wild type | Wild type | IDH1 mutation (R132H) | Tissue not available | Tissue not available |
| MGMT methylation status | Not methylated | Methylated | Methylated | Tissue not available | Tissue not available |
| Time from diagnosis to dacomitinib initiation (mo) | 17 | 18 | 11 | 7 | 3 |
| Time from the end of radiotherapy to dacomitinib initiation (mo) | 14 | 14 | 9 | 3.5 | 1 |
| Best response | SD | PR | CR | SD | SD |
| Time to disease progression (mo) | 27.5 | 21 | Progression-free after 40 mo | 12.6 | 6 |
| Follow-up (mo) | 46.2 | 44 | 40 | 34.5 | 13 |
| Outcome at the end of follow-up | Dead | Alive | Alive, on treatment | Dead | Dead |
| Surgery after progression to dacomitinib? | Yes. Loss of EGFR amplification. | No | No | No | No |
| Comments | — | Radiological evolution in Supplementary Figure S1 | Radiological evolution in Supplementary Figure S1 | — | — |
EGFRamp/EGFRvIII−: EGFR amplification without EGFRvIII mutation.
EGFRamp/EGFRvIII+: EGFR amplification with EGFRvIII mutation.
SD: stable disease; PR: partial response. CR: complete response.
One patient on Cohort A had tumor progression after 27 months of treatment and a second resection was performed. Tissue from this second surgery was available and, interestingly, EGFR amplification was not found at this time.
Overall Survival
For Cohort A, the median OS was 7.8 months (95% CI: 5.6–10.1) and for Cohort B, 6.7 months (95% CI: 4.3–9.1). The OS curve for both cohorts is shown in Fig. 2. For the total population, median OS was 7.4 months (95% CI: 5.6–9.2).
Fig. 2.
Overall survival (mo) for Cohort A (EGFR amp/EGFRvIII−) and Cohort B (EGFR amp/EGFRvIII+).
Response Rate
Four patients (3 in Cohort A and 1 in Cohort B) were not evaluable for this endpoint because of clinical decline before the radiologic assessment.
One patient in Cohort A achieved a complete response after 6 months of treatment. One patient in each cohort had a partial response as the best overall response (see Supplementary Figure S1). Eight patients from Cohort A and 4 patients from Cohort B had stable disease as the best overall response. However, most patients had progressive disease in the first MRI performed. Central radiological evaluation was performed, subsequently validating and verifying all patient response.
The response rates are shown in Table 3.
Table 3.
Best overall response
| Cohort A (EGFR amp/EGFRvIII− n = 30, n (%) | Cohort B (EGFR amp/EGFRvIII+) n = 19, n (%) | Total (n = 49) n (%) | |
|---|---|---|---|
| Complete response | 1 (3.3) | 0 (0.0) | 1 (2.0) |
| Partial response | 1 (3.3) | 1 (5.3) | 2 (4.1) |
| Stable disease | 8 (26.7) | 4 (21.1) | 12 (24.5) |
| Progressive disease | 17 (56.7) | 13 (68.4) | 30 (61.2) |
| Not evaluable | 3 (10.0) | 1 (5.3) | 4 (8.2) |
EGFRamp/EGFRvIII−: EGFR amplification without EGFRvIII mutation.
EGFRamp/EGFRvIII+: EGFR amplification with EGFRvIII mutation.
Safety and Tolerability
During the study, 47 patients (96%) reported at least one adverse event (AE) potentially related to dacomitinib. Twenty (41%) patients experienced CTCAE grade 3 or 4. The most common AEs related to the study drug were: rash (82%), diarrhea (67%), fatigue (22.4%), and nausea (8.2%) (Table 4). One patient had ALT/AST grade 3 elevation.
Table 4.
Summary of adverse events
| Adverse Event by CTCAE 4.0 | All Grades n (%) | Grade ≥ 3 n (%) |
|---|---|---|
| Patients with adverse events | 47 (95.9) | 20 (40.8) |
| Adverse events | ||
| Rash | 40 (81.6) | 11 (22.4) |
| Diarrhea | 33 (67.3) | 3 (6.1) |
| Asthenia | 11 (22.4) | 2 (4.1) |
| Nausea/vomiting | 4 (8.2) | 0 (0.0) |
A total of 16 dose reductions in 14 patients and 6 treatment interruptions in 5 patients were reported.
Discussion
Despite the rationale to target EGFR in GB, dacomitinib, a pan-HER tyrosine kinase inhibitor, has a limited single-agent activity in recurrent GB patients with EGFR amplification. The response to EGFR inhibition in relapsed GB had also been disappointing previously with either erlotinib or gefitinib as first-generation oral EGFR inhibitors.5,6,21 The results of the European Organisation for Research and Treatment of Cancer randomized phase II trial, which included 110 patients (54 treated with erlotinib and 56 with chemotherapy), showed PFS6 of 12% for erlotinib and 24% for the control arm, with similar OS in both arms.22 Results with afatinib, a newer EGFR tyrosine kinase inhibitor that is able to irreversibly block EGFR and to inhibit HER2 and HER4, were also negative in recurrent GB.23
One of the major issues of the clinical trials with EGFR inhibitors is the fact that these drugs have been tested without any patient selection according to EGFR status. In fact, to date only one clinical trial developed by GEINO has evaluated an EGFR inhibitor after selecting patients by their EGFR status. In this study, erlotinib was tested in recurrent GB with expression of EGFRvIII and PTEN by immunohistochemistry. The study showed no significant activity, with a PFS6 of 20% and only one partial response.5
The results of our clinical trial show that dacomitinib has limited activity in recurrent GB, even though the drug was administered to a selected population with EGFR amplification. Indeed, the administration of dacomitinib did not meet the prespecified threshold for PFS6, neither in cases with EGFRvIII mutation nor in cases without this mutation. The observed antitumor activity with dacomitinib was comparable to that observed previously in trials with reversible EGFR tyrosine kinase inhibitors in nonmolecularly selected recurrent GB. Erlotinib alone or in combination with chemotherapy achieved partial response rates of 6%–25% with modest impact on PFS or OS.24–26 Gefitinib in recurrent GB showed response rates of 0–13%, median PFS of 2 months, and PFS6 of 9%–13%.6,11,27,28
Despite these disappointing global results, 4 patients had significant benefit from this treatment: 4 patients were progression free at 6 months, 3 were progression free after 12 months, and 1 patient was still progression free after 40 months of treatment.
In relation to toxicity, dacomitinib exhibited a profile consistent with previous reports,29–31 but this drug is clearly more toxic than erlotinib or gefitinib. The most frequently reported AEs with dacomitinib included rash and diarrhea.
In order to interpret our results, it is important to notice that intratumoral pharmacokinetic assessment of dacomitinib was not incorporated and it is not possible to determine whether insufficient intratumoral delivery was the cause of the poor therapeutic benefit.
This study has a few limitations. First, EGFR amplification, which has been used as a primary laboratory assessment, was tested in the primary tumor, and we have no evidence of the stability of this alteration in the recurrent GBs. However, the analysis of a cohort of 55 pairs of primary and recurrent GBs showed more than 80% of cases in which EGFR amplification remained stable.32 In any case, the presence of the amplification alone has not been found helpful in the use of other anti-EGFR agents in lung cancer or colorectal cancer. Instead, recent successes in this field have been based on the presence of point mutations associated with a constitutive activation of the pathway. However, these point mutations are not commonly found in GBs.
Other important limitations of our study are the small sample size and the nonrandomized design, which preclude drawing firm conclusions. Related to this, the 2-stage design has resulted in a very small sample size in the cohort of patients who could have provided the more interesting results: those with an EGFRvIII mutation.
Recent technological innovations have allowed the analysis of cancer genetics to be conducted on the single-cell level in every case. Parker et al,33 for example, profiled 430 cells from 5 GBs and found that individual cells could be classified as different types of GB according to The Cancer Genome Atlas classification scheme. Other studies confirmed the observation of heterogeneous amplification of EGFR and other receptor tyrosine kinases in GB.34 GB tumor heterogeneity may be behind the lack of efficacy of targeted therapies in GB because the treatment would be only active in a group of tumor cells. Another feasible explanation could be the presence of other alterations that could activate the EGFR pathway downstream of the receptor. In fact, preclinical data suggest that the lack of PTEN renders the tumors less sensitive to dacomitinib10 and that phosphatidylinositol-3 kinase (PI3K) inhibitors synergize with EGFR inhibition. One of our patients with significant clinical benefit suffered a tumor regrowth that was removed. We did not find either EGFR amplification or other EGFR alterations in the relapse. This case suggests that a loss of the EGFR alterations, or a clonal selection of cells without EGFR modifications, may be a mechanism of secondary resistance to EGFR inhibitors in GB. However, the lack of tissue sample at the start of dacomitinib administration does not allow us to conclude that the loss of EGFR amplification was induced by the experimental treatment.
Long-term survival with dacomitinib was not related to MGMT promoter methylation status or the presence of IDH1/2 mutations, showing that the benefit with the EGFR inhibitor was independent of known good prognosis factors.
Blood–brain barrier (BBB) penetrance remains a challenge in small-molecule–based antiglioma therapy. Available information about the ability of dacomitinib to achieve adequate concentrations in brain parenchyma is scarce, since patients with brain metastases have been excluded from clinical trials in NSCLC and other tumors. However, preclinical studies conducted in mice suggest that dacomitinib can cross the BBB, since oral administration of dacomitinib 25 mg/kg is followed by an overall brain-to-plasma ratio (based on AUC72 values) of 1.21, and rats receiving a single 4.98 mg/kg p.o. dose of [14C]dacomitinib achieved CNS and CSF radioequivalents over the time course of 2 to 48 hours (data not published/investigator brochure provided by Pfizer). Additionally, in vivo models of GB showed that dacomitinib inhibits tumor growth not only in patient-derived xenografts implanted into the flanks of immunodeficient mice but also in intracranial patient-derived xenografts.10 The results obtained in the flanks with strong downregulation of the phosphorylation of EGFR and its downstream target were confirmed in the intracranial models in the same study.
In conclusion, the administration of dacomitinib in recurrent GB and EGFR amplification showed minimal activity in both cases, with or without vIII mutation. The detailed molecular characterization of the 4 patients with response in this trial can help to narrow the possible predictive markers of dacomitinib effectiveness. Further studies should benefit from a more profound molecular profiling of EGFR/PI3K/PTEN status.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This study was supported by an unrestricted educational grant from Pfizer Oncology.
Conflict of interest statement. J.M.S-S. has received a grant from Pfizer for this study. The remaining authors declare no conflict of interest.
Supplementary Material
Acknowledgments
Manuscript writing was provided by Neus Valveny, PhD, from TFS Develop with financial support provided by Pfizer. The authors would like to thank María Victoria Bolós (Pfizer Oncology) for her collaboration in the study development. This paper in part was presented at the European Cancer Conference 2015: Abstract No. 2902. The authors are fully responsible for all content and editorial decisions.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–2024. [DOI] [PubMed] [Google Scholar]
- 3. Talasila KM, Soentgerath A, Euskirchen P, et al. EGFR wild-type amplification and activation promote invasion and development of glioblastoma independent of angiogenesis. Acta Neuropathol. 2013;125(5):683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lal A, Glazer CA, Martinson HM, et al. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62(12):3335–3339. [PubMed] [Google Scholar]
- 5. Gallego O, Cuatrecasas M, Benavides M, et al. Efficacy of erlotinib in patients with relapsed gliobastoma multiforme who expressed EGFRVIII and PTEN determined by immunohistochemistry. J Neurooncol. 2014;116(2):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rich JN, Reardon DA, Peery T, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22(1):133–142. [DOI] [PubMed] [Google Scholar]
- 7. Engelman JA, Zejnullahu K, Gale CM, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67(24):11924–11932. [DOI] [PubMed] [Google Scholar]
- 8. Mineo JF, Bordron A, Baroncini M, et al. Low HER2-expressing glioblastomas are more often secondary to anaplastic transformation of low-grade glioma. J Neurooncol. 2007;85(3):281–287. [DOI] [PubMed] [Google Scholar]
- 9. Zhu Y, Shah K. Multiple lesions in receptor tyrosine kinase pathway determine glioblastoma response to pan-ERBB inhibitor PF-00299804 and PI3K/mTOR dual inhibitor PF-05212384. Cancer Biol Ther. 2014;15(6):815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zahonero C, Aguilera P, Ramírez-Castillejo C, et al. Preclinical test of dacomitinib, an irreversible EGFR inhibitor, confirms its effectiveness for glioblastoma. Mol Cancer Ther. 2015;14(7):1548–1558. [DOI] [PubMed] [Google Scholar]
- 11. Hegi ME, Diserens AC, Bady P, et al. Pathway analysis of glioblastoma tissue after preoperative treatment with the EGFR tyrosine kinase inhibitor gefitinib—a phase II trial. Mol Cancer Ther. 2011;10(6):1102–1112. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka K, Babic I, Nathanson D, et al. Oncogenic EGFR signaling activates an mTORC2-NF-κB pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1(6):524–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramnarain DB, Park S, Lee DY, et al. Differential gene expression analysis reveals generation of an autocrine loop by a mutant epidermal growth factor receptor in glioma cells. Cancer Res. 2006;66(2):867–874. [DOI] [PubMed] [Google Scholar]
- 14. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment In Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 15. Hirsch FR, Herbst RS, Olsen C, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapy. J Clin Oncol. 2008;26(20):3351–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Varella-Garcia M. Stratification of non-small cell lung cancer patients for therapy with epidermal growth factor receptor inhibitors: the EGFR fluorescence in situ hybridization assay. Diagn Pathol. 2006;1:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshimoto K, Dang J, Zhu S, et al. Development of a real-time RT-PCR assay for detecting EGFRvIII in glioblastoma samples. Clin Cancer Res. 2008;14(2):488–493. [DOI] [PubMed] [Google Scholar]
- 18. Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116(6):597–602. [DOI] [PubMed] [Google Scholar]
- 19. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319–1325. [DOI] [PubMed] [Google Scholar]
- 20. Palmisano WA, Divine KK, Saccomanno G, et al. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 2000;60(21):5954–5958. [PubMed] [Google Scholar]
- 21. Yung WK, Vredenburgh JJ, Cloughesy TF, et al. Safety and efficacy of erlotinib in first-relapse glioblastoma: a phase II open-label study. Neuro Oncol. 2010;12(10):1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27(8):1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reardon DA, Nabors LB, Mason WP, et al. ; BI 1200 36 Trial Group and the Canadian Brain Tumour Consortium. Phase I/randomized phase II study of afatinib, an irreversible ErbB family blocker, with or without protracted temozolomide in adults with recurrent glioblastoma. Neuro Oncol. 2015;17(3):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reardon DA, Desjardins A, Vredenburgh JJ, et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J Neurooncol. 2010;96(2):219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown PD, Krishnan S, Sarkaria JN, et al. ; North Central Cancer Treatment Group Study N0177. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26(34):5603–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Groot JF, Gilbert MR, Aldape K, et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J Neurooncol. 2008;90(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kreisl TN, Lassman AB, Mischel PS, et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J Neurooncol. 2009;92(1):99–105. [DOI] [PubMed] [Google Scholar]
- 28. Franceschi E, Cavallo G, Lonardi S, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br J Cancer. 2007;96(7):1047–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jänne PA, Ou SH, Kim DW, et al. Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: a multicentre, open-label, phase 2 trial. Lancet Oncol. 2014;15(13):1433–1441. [DOI] [PubMed] [Google Scholar]
- 30. Ellis PM, Shepherd FA, Millward M, et al. ; NCIC CTG; Australasian Lung Cancer Trials Group; NCI Naples Clinical Trials Unit. Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR.26): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2014;15(12):1379–1388. [DOI] [PubMed] [Google Scholar]
- 31. Ramalingam SS, Blackhall F, Krzakowski M, et al. Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2012;30(27):3337–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van den Bent MJ, Gao Y, Kerkhof M, et al. Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro Oncol. 2015;17(7):935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parker NR, Khong P, Parkinson JF, Howell VM, Wheeler HR. Molecular heterogeneity in glioblastoma: potential clinical implications. Front Oncol. 2015;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Szerlip NJ, Pedraza A, Chakravarty D, et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci U S A. 2012;109(8):3041–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.