Abstract
Due to globalization and sophisticated western and sedentary lifestyle, metabolic syndrome has emerged as a serious public health challenge. Obesity is significantly increasing worldwide because of increased high calorie food intake and decreased physical activity leading to hypertension, dyslipidemia, atherosclerosis, and insulin resistance. Thus, metabolic syndrome constitutes cardiovascular disease, type 2 diabetes, obesity, and nonalcoholic fatty liver disease (NAFLD) and recently some cancers are also considered to be associated with this syndrome. There is increasing evidence of the involvement of natriuretic peptides (NP) in the pathophysiology of metabolic diseases. The natriuretic peptides are cardiac hormones, which are produced in the cardiac atrium, ventricles of the heart and the endothelium. These peptides are involved in the homeostatic control of body water, sodium intake, potassium transport, lipolysis in adipocytes and regulates blood pressure. The three known natriuretic peptide hormones present in the natriuretic system are atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and c-type natriuretic peptide (CNP). These three peptides primarily function as endogenous ligands and mainly act via their membrane receptors such as natriuretic peptide receptor A (NPR-A), natriuretic peptide receptor B (NPR-B) and natriuretic peptide receptor C (NPR-C) and regulate various physiological and metabolic functions. This review will shed light on the structure and function of natriuretic peptides and their receptors and their role in the metabolic syndrome.
Keywords: Natriuretic peptides, Metabolic syndrome, Hypertension, Insulin resistance, Nonalcoholic fatty liver disease
1. Introduction
The term metabolic syndrome is frequently heard in recent years with the rapidly developing changes in global economic scenarios and technologically advanced life styles. Since the past three decades, the population of advanced and developing countries has become more susceptible to metabolic syndrome due to their sedentary life style. The metabolic syndrome is a cluster of disease conditions that increases the risk of type 2 diabetes mellitus and cardiovascular diseases [1]. Previously, Dr. Reaven based on his clinical observations coined the term “Syndrome X” to define and denote these complex clusters of pathological maladies [2]. The main physiological and clinical symptoms and hallmarks of metabolic syndrome are reduced HDL-cholesterol, raised triglycerides, high blood pressure and increased fasting plasma glucose, all of which are directly related to increased adipogenesis and weight gain, specifically intra-abdominal or ectopic fat accumulation and a large waist size and circumference [3]. The main causes for metabolic syndrome are high calorie food intake or over nutrition and lack of exercise or physical inactivity along with some genetic, epigenetic, and environmental factors [4–6]. It has been also shown that metabolic syndrome is associated with less abundance of cardiac hormones such as natriuretic peptides (ANP, BNP and CNP) and altered expression in their receptors [5]. These cardiac natriuretic peptide hormones are a group of structurally and functionally similar but genetically distinct peptides with common cellular membrane receptors [4, 5]. The production and secretion of ANP and BNP takes place in the atrium and the ventricles of the heart and they act as cardiac peptide hormones, whereas CNP is mainly produced and secreted from the endothelium of vasculature and male genital glands and acts as a relaxing peptide [7]. These peptides have three membrane receptors namely NPR-A, NPR-B, and NPR-C via which they regulate various metabolic and physiological functions. The NPR-A and NPR-B receive messages from their ligand hormones and activate downstream signaling pathways, whereas NPR-C is mainly a clearance receptor which is primarily involved in clearance or degradation of these hormones [8].
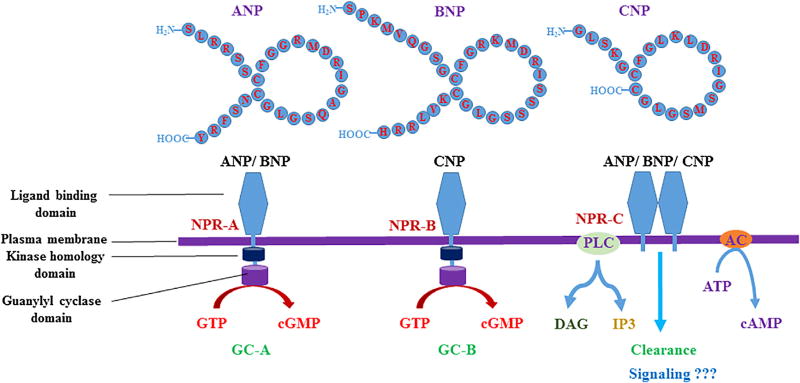
Unlike, NPR-A (Guanylyl cyclase A or GC-A) and NPR-B (Guanylyl cyclase B or GC-B) which are guanylyl cyclase receptors, NPR-C (NPR3) is a non-guanylyl cyclase receptor and is coupled to inhibitory guanine nucleotide regulatory protein (Gi) which leads to inhibition of adenylyl cyclase or activation of PLC and regulates various cellular functions (Fig. 1). This receptor C helps in the maintenance and physiological balance of these natriuretic peptides and any alteration in this balance will lead to pathological conditions in various organs and tissues [5, 8, 9]. There are many epidemiological and clinical studies showing that patients with obesity, insulin resistance and diabetes have reduced levels of plasma or serum ANP and BNP and the deficiency of these peptides may contribute to enhancing their susceptibility to the risk of cardiovascular diseases [10–12, 5]. Thus, studies have clearly shown the importance of natriuretic peptides in homeostatic maintenance of blood pressure, blood glucose, HDL-cholesterol and triglycerides. In this review, we will discuss the major and recent advances that depict the role of natriuretic peptides in metabolic syndrome.
Fig. 1.
The three main types of natriuretic peptides and their receptors.
2. Chemistry, structure and regulation of gene expression and secretion of natriuretic peptides
2.1. Atrial natriuretic peptide (ANP)
ANP is a very well-known and well-studied cardiac natriuretic peptide and has 28 amino acids with a ringed structure formed by intramolecular cysteine disulfide linkages [13]. It was first reported in the early 1980s by De Bold and colleagues from Kingston, Ontario, Canada, wherein they found that rat atrial extracts contained a substance that behaved like a hormone and also increased salt and urine output in the kidney [13, 14]. Later, it was isolated and purified by several other groups and named as atrial natriuretic factor (ANF) or ANP [15–20]. The human ANP gene is located on the short arm of chromosome 1 but its counterpart orthologue mouse ANP gene is located on chromosome 4 [21]. Once ANP gene is expressed, it is known to be produced as 151 amino acid pre-prohormone or pre-pro ANP, which is further processed to a pro hormone of 126 amino acids in the atrial myocytes of the heart [22, 23]. After its release from the cardiac myocytes, ANP circulates in the blood stream and acts on various organs. The secreted pro ANP further undergoes cleavage and suitable post-translational modification by the enzyme corin yielding N-ANP and c-terminal hormone (ANP), the biologically active form in an almost equal ratio [23, 24–28]. The plasma half-life of ANP is very short (2–5 min) and the expression of ANP gene and secretion of mature ANP is regulated by various physiological factors [29–34]. The promoter region of ANP gene contains various transcription factors binding sites such as PPARγ, PPARα, retinoid-X-receptor (RXR), vitamin D receptor (VDR), hypoxia-inducible factor 1-alpha (HIF-1α), activator protein 1 (AP1), serum response factor (SRF), Nkx2–5, GATA binding protein 4 (GATA4), myocyte enhancer factor 2C (Mef2c) and T-Box factors [31–34]. Also, the neuropeptide, calcitonin gene-related peptide (CGRP) increases ANP mRNA in hypertrophying neonatal cardiomyocytes [35]. Glucagon-like peptide-1 (GLP-1), an incretin hormone derived from the transcription product of the proglucagon gene, which is produced from the gut and from pancreatic alpha cells induces ANP expression in cardiac atrium and regulates various physiological functions [36–38]. The stimulus of atrial stretch also induces and increases the ANP release from cardiomyocytes [36]. It is noteworthy that daily exercise and physical activity increases ANP expression and secretion in parallel to the downregulation of NPR-C [5]. Few other known factors modulating ANP gene expression and secretion include α-adrenergic agonists, endothelin, prostaglandin F2α, growth factors, vitamin D, retinoids, glucocorticoids and mechanical strain [39].
2.2. Brain natriuretic peptide (BNP)
BNP is a 32 amino acid peptide having structural similarity with ANP and has 17 common amino acid sequence with that of ANP [23]. It was first isolated and reported from Japan by Sudoh et al., in 1988 from porcine brain extracts [40]. Another group from Japan also isolated mouse BNP gene and generated transgenic mice, which produced high amounts of BNP in cardiac ventricle with chronically elevated plasma BNP concentrations [10, 41, 42]. The human BNP gene is located on chromosome 1 and is a single copy gene consisting of 3 exons and 2 introns whereas mouse BNP gene is located on chromosome 4 [42]. The BNP synthesized by cardiomyocytes (ventricles) as a pre-pro BNP is 134 amino acids in length and undergoes cleavage and modification by enzymes [43]. The enzymes, corin and furin are involved in the conversion of pro-BNP to BNP. Pre-pro BNP modification results in the formation of a signal peptide and a propeptide (pro BNP, 108 amino acids), that is stored as a mature hormone in the human heart [23, 43]. Finally, during secretion from the cardiomyocytes, pro BNP undergoes post-translational modification and is split at a ratio of 1:1 into the physiologically active form of BNP (32 amino acids) which forms C-terminal fragment, and another fragment of biologically inactive N-terminal fragment (NT pro BNP; 76 amino acids) [23, 43]. The plasma half-life of BNP is also very short (12–23 min) and the expression and secretion of BNP are regulated by various physiological factors [44]. The promoter region of BNP gene contains various transcription factors binding sites which include Yin Yang 1 (YY1), GATA-4, GATA-5, GATA-6, MEF-2, dHAND, SRF and Nkx2.5. The bacterial endotoxin lipopolysaccharides (LPS) are also known to induce BNP gene expression in neonatal rat cardiac myocytes via GATA transcription factors [39, 44–53]. Exercise or physical activity has also been shown to have beneficial effects on humans, leading to the up regulation of BNP expression and secretion [30].
2.3. C-type natriuretic peptide (CNP)
CNP was first isolated in 1990 from porcine brain by Sudoh et al., and is known to consist of 22 amino acids [54]. CNP also has ring structure and is highly homologous to both ANP and BNP but lacks the carboxy-terminal extension [55]. The CNP is a highly conserved natriuretic peptide among various species [56]. In humans, CNP gene (Nppc) is located on chromosome 2 and it consists of 2 exons whereas the mouse CNP gene is located on chromosome 1 and it is composed of two exons and one intron [10, 41]. It is also produced as pre-pro CNP from the endothelium, comprising of 126 amino acids, which after cleavage of first 23 amino acids gets converted into pro CNP and is further processed to NCP-53 and CNP by the enzyme furin [57, 58, 23]. In comparison to ANP and BNP, the plasma half-life of CNP is much shorter and is about 2–3 min in humans [59]. The expression and secretion of CNP are also regulated by various cytokines and growth factors such as tumor necrosis factor (TNF), lipopolysaccharide (LPS), basic fibroblast growth factor (bFGF), interleukin-1 (IL-1), transforming growth factor beta (TGF-β) and thrombin which are involved in vascular remodeling and inflammation [60–64]. During endothelial damage, sepsis, hypoxia and chronic renal failure, the levels of CNP are elevated in blood [63]. Shear stress also induces the expression of CNP gene in human endothelial cells [64]. The promoter region of CNP gene has binding sites for the transcription factor TSC-22 [65]. We performed bioinformatics analysis and found that, CNP gene promoter also has binding sites for transcription factors such as NF-κB, STAT1, ATF6 and E2F1.
3. Natriuretic peptide receptors
In the endocrine, paracrine and autocrine system, all the hormones or ligands depend mainly on their receptors for their specific mechanism of action. These receptors may be either membrane bound receptors or nuclear receptors. The peptide hormones ANP, BNP and CNP regulate a variety of physiological and pathological parameters by interacting with their common receptors present on the plasma membrane [39]. The three known receptors for these natriuretic peptides are natriuretic peptide receptor A (NPR-A or NPR1), natriuretic peptide receptor B (NPR-B or NPR2) and natriuretic peptide receptor C (NPR-C or NPR3). The receptors, NPR-A and NPR-B belong to the members of the cellsurface family of guanylyl cyclase receptors, the enzymes that catalyze the synthesis of the intracellular second messenger, cGMP. Hence, NPR-A and NPR-B are sometimes referred by other (previous) names such as guanylyl cyclase-A (GC-A) and guanylyl cyclase-B (GC-B). In contrast, NPR-C is non-guanylyl cyclase receptor, which is mainly involved in clearance of these peptides and it is also coupled to adenylyl cyclase inhibition or phospholipase C activation via inhibitory guanine nucleotide regulatory protein (Gi) [9]. The NPR-A is activated by physiological concentrations of ANP and BNP, but not by CNP. Conversely NPR-B is specifically activated by CNP. Whereas, all the three natriuretic peptides bind to NPR-C (which lacks guanylyl cyclase activity) and undergoes clearance and degradation [66–68].
3.1. NPR-A (NPR1)
Natriuretic peptide receptor A or guanylate cyclase A (atrial natriuretic peptide receptor A or GC-A) is also known as atrial natriuretic peptide receptor 1 (NPR1) [69]. In humans, it is encoded by NPR1 gene located on chromosome 1 and in mouse on chromosome 3. This gene was first cloned by three different groups in three different models such as mouse, rat, and pig [69–71]. The promoter region of NPR-A gene has binding sites for specificity protein 1 (Sp1), Sp3, cGMP response element-binding protein (CREBP), vitamin D receptor (VDR) and these transcription factors regulates NPR-A expression. Angiotensin II, endothelin, osmotic stimuli, endothelial NOS and p38 MAPK also regulate NPR-A expression and secretion [39, 72]. The NPR-A or GC-A is a membrane-bound guanylate cyclase receptor and it functionally serves as the receptor for both the peptides ANP and BNP and further mediates their response by activating downstream signaling pathways [66]. The expression of NPR-A is reported in various organs including heart, kidney, lungs, liver and adipocytes and in some cancers [67]. The expression of NPR-A in various organs shows the possible involvement of this receptor in various physiological and pathological responses.
3.2. NPR-B (NPR2)
This receptor (Natriuretic peptide receptor B) is also known by other names such as guanylate cyclase B (GC-B) or B-type natriuretic peptide receptor 2 (NPR2). In humans, it is encoded by NPR2 gene, which is located on chromosome 9 and in mouse on chromosome 4 [73]. The rat NPR-2 gene was first cloned by Dr. Schulz and colleagues in 1989 [74]. The promoter region of this gene is known to be regulated by various responses including BNP and it has binding sites for Sp1 and Sp3 transcription factors [75]. The expression of NPR-B is reported in various organs such as heart, brain, uterus, ovary, kidney, lungs, liver and adipocytes and in some cancers [74, 76, 77]. The ubiquitous expression of NPR-B signifies its role in many physiological functions.
3.3. NPR-C (NPR3)
Natriuretic peptide receptor C is a clearance receptor encoded by NPR3 gene located on chromosome 5 and chromosome 15 in humans and mouse respectively, and is also known as natriuretic peptide receptor-3 (NPR3) or guanylate cyclase C (atrial natriuretic peptide receptor C) [9, 74]. This gene was first cloned by Yanaka et al., both in human and mouse [78, 79]. The expression of NPR-C is also ubiquitous and is known to be expressed in heart, lung, adrenal gland, heart, cerebral cortex, cerebellum, liver and adipocytes and in some cancers [80, 81]. The expression of NPR-C is influenced by various physiological factors and is reported to be very high in hypertensive, diabetic and obese patients [80]. It is also known that physical inactivity increases the expression of NPR-C, whereas regular physical exercise or activity suppresses its expression. Based on previously reported studies and the bioinformatics analysis performed, the NPR-C gene promoter reveals that it has binding sites for Sp1, AP-2, YY-1, E2F1 and CREB transcription factors [78, 79].
4. Roles of natriuretic peptides in metabolic syndrome
4.1. Natriuretic peptides and cardiovascular disease
The role of Natriuretic peptides, ANP and BNP in cardiovascular diseases are well-studied and well established. These circulating hormones of cardiac origin possess relevant hemodynamic and anti-remodeling actions and play an important role in the regulation of intravascular blood volume and vascular tone [82, 83]. It is reported that there is an elevation in the plasma levels of ANP and BNP during heart failure and these hormones are considered to compensate for heart failure. ANP and BNP have actions of diuresis, natriuresis, vasodilation, inhibition of aldosterone synthesis and renin secretion and hence play an important role as cardio protective hormones under conditions of cardiovascular diseases such as heart failure (HF), coronary artery disease, very high blood pressure (hypertension) and left ventricular hypertrophy and cerebrovascular accidents or stroke [82, 84]. In a recent study, it is shown that high rise in B-type natriuretic peptide acts as a determining factor in cardiovascular diseases [82–86]. Also, there is a report in which disruption of GC-A (NPR-A) gene results in chronic elevations of blood pressure in mice which are fed with normal salt diet [87]. Furthermore, studies have also revealed that acute administration of ANP or BNP can improve left ventricular function in patients with chronic heart failure by its prominent natriuretic, diuretic and vasorelaxant activities, suggesting the potential usefulness of these natriuretic peptides as short-term therapeutic agents [23, 88]. In addition, atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 (MAPK) in human umbilical cord vein vascular endothelial cells (HUVECs) via Rac1 and NAD(P)H oxidase/Nox2-activation [89]. Also, there is a study which shows that ANP inhibits TNF-α-induced endothelial morphology and function [90].
4.2. Natriuretic peptides and type 2 diabetes
The role of these cardiac peptides in diabetes has beneficial effects and any decrease in these peptides has direct link with insulin resistance and decreased glucose tolerance [91, 92]. It is reported that low plasma levels of atrial natriuretic peptide predict the development of diabetes [93]. Also, there is a study which shows that in patients with chronic renal failure and diabetes, there is increased levels of plasma BNP [94]. ANP is known to increase circulating insulin and inhibit glucagon secretion in isolated rat pancreatic islets [91, 95]. It has also been shown that patients with mitral valve disease have increased glucose intolerance and insulin resistance which are associated with higher levels of ANP and free fatty acids, while mitral valve replacement/repair surgery improves these metabolic alterations [94]. The nuclear receptor, LXRα improves myocardial glucose tolerance and reduces cardiac hypertrophy in a mouse model of obesity-induced type 2 diabetes by inducing ANP and BNP [91].
4.3. Natriuretic peptides and obesity
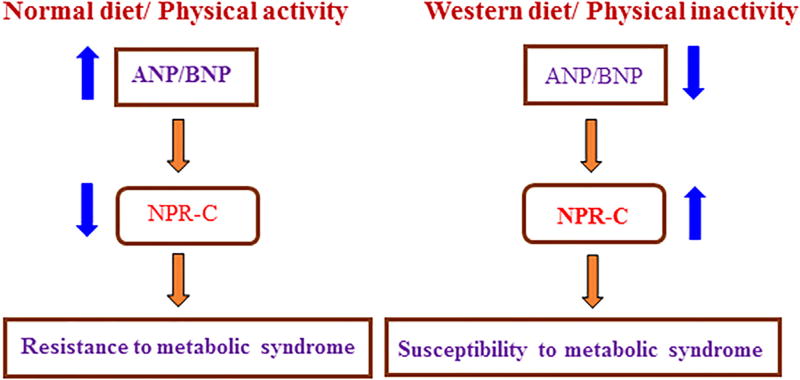
There are many reports which show low circulating natriuretic peptide levels in obese individuals, which may indirectly contribute to their susceptibility to hypertension and hypertension-related cardiovascular disorders [96]. A Recent study shows that C-type natriuretic peptide is closely associated with obesity in Caucasian adolescents [97]. It has been shown that physical exercise has huge impact and acutely increases ANP release in obese patients. Normal diet (low calorie food) and regular physical activity results in increased ANP and BNP levels due to reduced NPR-C expression. Whereas, increased NPR-C expression in conditions of obesity (western diet with reduced physical activity) results in reduced levels of ANP and BNP (Fig. 2). In adipocytes of obese subjects, there is increased expression of NPR-C (clearance of all three natriuretic peptides) resulting in obesity, diabetes, hypertension, and nonalcoholic fatty liver disease [98, 99, 5]. Increased NPR-C expression is also known to result in the suppression of ANP mediated lipolysis [5, 100].
Fig. 2.
The role of natriuretic peptides in association with the diet and physical activity.
4.4. Natriuretic peptides and renal disease
There is a balance between vasoconstrictor/sodium-retaining and vasodilator/natriuretic systems and this balance is very essential for maintaining proper nephro-cardiac functions, body fluid and electrolyte homeostasis [101]. Natriuretic peptides belong to the vasodilator family and are involved in natriuresis (excretion of sodium in the urine) [8]. It is also known that the action of ANP and its role is to contribute in the increased renal excretion of sodium rich urine, i.e. natriuresis [102]. Another study shows that BNP as an appropriate biomarker in screening for cardiac dysfunctions in chronic kidney disease [103]. It is also shown that in patients with type 2 diabetes, there is reduced basal plasma ANP concentration which are inversely correlated to kidney function. In contrast to normal (non-diabetic) controls, ANP in type 2 diabetes does not rise in response to feeding, clearly showing the significance of natriuretic peptide signaling cascades in renal pathophysiology [104]. BNP and pro-BNP play an important role in the renal function, which affects the plasmatic levels of these natriuretic peptides and this may limit their utility as hemodynamic biomarkers in renal failure [105]. Hence, all these studies prove the significance of natriuretic peptides and shows that there exists a direct connection in the function between cardiac and renal system.
4.5. Natriuretic peptides and liver disease
There are studies which show the possible involvement of natriuretic peptides in liver diseases such as nonalcoholic fatty liver disease and cirrhosis [106]. There was a study published in 2013 showing that higher natriuretic peptide levels were independently associated with a favorable adiposity profile (that is, more lipolysis in adipocytes), which is characterized by decreased visceral and liver fat and increased lower body fat, suggesting a link between the cardio-hepatic regulation with adipose tissue as a connecting link for natriuretic peptides function [107]. There are many indirect studies which predict the involvement of natriuretic peptides in liver disease [108, 109]. A study using BNP as a biomarker shows that the plasma BNP level is correlated significantly with the severity of liver disease in cirrhotic patients and thus high plasma BNP seems to be a good prognostic marker of liver cirrhosis related deaths [109, 110]. There exists a link between cardiac dysfunction and nonalcoholic liver disease wherein advanced cardiac dysfunctions lead to increased BNP levels and also liver cirrhosis [111]. In addition, it is also seen that plasma BNP level increases in post hepatitis C cirrhotic patients but tends to decrease in fatty liver disease patients [112]. This may be due to the direct link with obesity and fatty liver disease where adipocytes in obese patients have high NPR-C expression that results in the clearance of ANP and BNP (low ANP and BNP levels). This is supported by the expression of all the three natriuretic peptide receptors in the normal liver [113]. Suitable and elegant studies are in need to better understand the complete role of natriuretic peptides and their receptors, which may provide new avenues for the treatment of various types of liver diseases.
4.6. Natriuretic peptides and cancer
There are many reports that have been published regarding anti-inflammatory and anticancer activity of natriuretic peptides [114–116]. The NPR-A has been reported to be expressed in various types of cancer, such as lung cancer, ovarian cancer, gastric cancer and prostate cancer [117–119]. Another study shows the involvement of NPR-A in lung cancer-induced vascular endothelial growth factor (VEGF) expression and angiogenesis [118]. In addition, NPR-A is also known to be involved in stem cell recruitment, angiogenesis, and inflammation along with the regulation of self-renewal and pluripotency of embryonic stem cells [120]. Studies have shown that appropriate BNP expression and its abundance are essential for the maintenance of embryonic stem cells propagation establishing the role of BNP as a novel endogenous regulator of embryonic stem cell proliferation and it may also activate NPR-A which helps in self renewal and pluripotency of embryonic stem cells [121, 122]. Also, ANP is known for its antitumor activity by inhibiting DNA synthesis [123]. Recently, its antitumor activity was tested on cells derived from patients with various types of cancers such as pancreas, prostate, lung and kidney cancer [124–130]. There is a strong potential interaction between ANP and its specific receptors such as NPR-A, NPR-B and NPR-C, which are tissue and organ specific (Fig. 3) and are expressed at different levels in various tumor cells [4, 8, 9, 117–120]. Also, in recent years there is a strong association between metabolic syndrome and various types of cancers [131–133]. Therefore, proper scientific knowledge and better understanding of the molecular mechanisms underlying cardiac hormone function and their interaction with specific receptors in various organs and tissue will aid in targeting cancers which are mediated by metabolic syndrome (Table. 1).
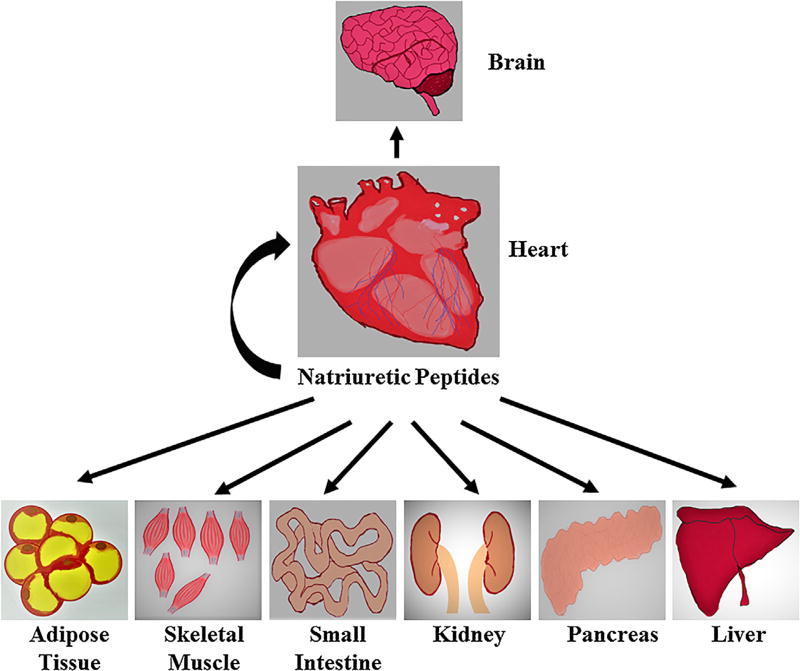
Fig. 3.
Action of natriuretic peptides on different target organs in the human body.
Table 1.
Organ and tissue specific function of natriuretic peptides in human body
| Brain | Sympathetic nervous system regulation of food intake? |
| Heart | Water and sodium balance, cardiovascular homeostasis and prevention of cardiac fibrosis |
| Skeletal muscle | Increase in mitochondrial respiration and fatty acid oxidation |
| Adipose tissue | Increase in HSL-mediated lipolysis, mitochondrial biogenesis, fatty acid oxidation WAT “browning”, adiponectin release and decrease in leptin release |
| Pancreas | Increase in glucose stimulated insulin secretion |
| Kidney | Increase in natriuresis and diuresis and decrease in RAAS activation |
| Small Intestine | Relaxation of smooth muscle cells and GLP-1 release? |
| Liver | Unknown function |
5. Therapeutic and clinical applications of natriuretic peptides
Based on their major role in obesity, diabetes, cardiovascular diseases and cancer, natriuretic peptides have several therapeutic and clinical applications. As we know that natriuretic peptides are hormones acting via autocrine, paracrine or endocrine mechanism, they regulate various physiological functions and play an important role in glucose homeostasis, energy expenditure, fat metabolism, intravascular volume, blood pressure, natriuresis, diuresis, inflammation and cancer [114–123]. There are many elegant studies signifying the important clinical and therapeutic uses of natriuretic peptides [134–136]. Recent studies show that supra physiological infusion of ANP raises plasma insulin levels in human volunteers and has a very beneficial effect on diabetic patients [137]. Due to their cardiac origin, studies have examined the useful effects of these peptides as potential therapeutic agents for the treatment of congestive heart failure, hypertension and later in kidney diseases [134–136]. Many clinical studies have also revealed that the infusion of synthetic ANP (also known as anaritide or ANF 1 V or carperitide, a peptide of 25 amino acids in length, lacking the first three amino terminal residues of the 28 amino acids of mature or active form of ANP) into patients with cardiac diseases such as hypertension or chronic heart failure has lots of beneficial effects and have resulted in elevated sodium and water excretion and decreased blood pressure [23, 80, 138–140]. ANP infusion is known to improve cardiac function by altering loading conditions for the left ventricle and significantly decreasing pulmonary capillary wedge pressure and systemic vascular resistance [141]. The long term (48 h) infusions of anaritide to patients with acute heart failure have shown strong beneficial hemodynamic responses without tolerance, suggesting that ANP injections may be clinically useful for the treatment of heart failure [142]. The government of Japan has approved anaritide or carperitide for the commercial and clinical use for the treatment of acute decompensated heart failure in 1995 [142, 143]. Furthermore, ANP treatment has also been shown to act as an effective adjunctive therapy in patients with acute myocardial infarction and continuous infusion of low-dose ANP from the start of cardiopulmonary bypass effectively maintained post-operative kidney function and prevents early post-operative acute kidney failure and helps to achieve safer cardiac surgery [143, 144]. Also, the ANP infusion has been shown to increase lipolysis in obese women with a low calorie diet [144]. The natriuretic peptides also have beneficial effects on kidney function. It has been shown that anaritide may improve dialysis-free survival in patients with oliguria (the production of abnormally small amounts of urine) and may worsen it in patients without oliguria who have acute tubular necrosis [145, 146]. Studies have also been performed to investigate the ability of anaritide to prevent radio-contrast-induced nephropathy [147].
The BNP hormone is also known to be clinically useful as of ANP and the human recombinant BNP, clinically also known as nesiritide (trade name Natrecor) mimics and has same actions as that of endogenous BNP [148–150]. It has been shown to cause a potential decrease in vascular pressure and a reduction in tension in the walls of blood vessels, which are the main hallmarks of vasorelaxation [149]. Furthermore, natrecor treatment is also accompanied with an increase in natriuresis and diuresis, as well as decrease in plasma aldosterone and endothelin levels in patients with acute heart failure [150]. The intravenous BNP infusion is shown to reduce total and plasma acylated ghrelin concentrations in healthy volunteers [151]. Another interesting study shows that low dose continuous ANP treatment maintains and prevents renal deterioration in patients with renal dysfunctions before cardiac surgery [152]. Thus, based on the beneficial effects of BNP, it has emerged as an important diagnostic, prognostic, and therapeutic agents in patients with heart failure [153–155]. Also, in 2001 the U.S. Food and Drug Administration (FDA) approved the use of BNP (nesiritide) for the treatment of acutely decompensated heart failure [156]. Due to its significantly longer half-life in comparison to anaritide (ANP), it was found to be more clinically useful [138]. The N-terminal region of BNP has proline, lysine, glycine, methionine, valine and glutamine and these amino acids add more stability to BNP and is less susceptible to NPR-C mediated degradation. Thus, the stable nature of BNP makes it to be more beneficial and can be used as a suitable therapeutic drug in the treatment of metabolic syndrome. But unfortunately, due to the increased risk of renal dysfunction and other side effects and high mortality rates in patients, BNP treatment has dampened the interest of widespread use of this drug and recently it has been under scrutiny for this deleterious side effect [157, 80]. More and stronger additional studies and clinical trials are necessary to evaluate the efficacy of this drug during this type of situation and more importantly to define the beneficial effects, major risks, and other parameters required for optimal usage of this drug [158].
The endothelium derived peptide CNP is also a useful biomarker for the cardiovascular diseases [159]. It is used as a urine biomarker for acute decompensated heart failure and elevated levels predict adverse outcomes [159, 160]. The beneficial action of this peptide is also used in coronary artery bypass surgery patients requiring auto-transplantation [161]. New strategies exploiting desirable and beneficial metabolic effects of these natriuretic peptides for the prevention and treatment of metabolic syndrome are the primary concerns in ongoing basic, preclinical, and clinical research. Thus, in the future, researchers should focus on the ideal way to clinically manipulate the natriuretic peptide system and exploit it for clinical and pharmacological applications [4, 162].
6. Conclusion
The altered balance of natriuretic peptides expression and secretion have been associated with various maladies [80, 138]. These peptides have both direct and indirect effects on metabolic syndrome. Also, there is a direct link that exists between the gut and heart in cardio metabolic regulation through which GLP-1 induces ANP secretion and regulates blood pressure [37]. This secreted ANP also acts on various other organs and cells including endothelial cells and vascular smooth muscle cells of blood vessels, inner medullary collecting duct cells in the kidney and adipocytes in fat pads and cardiomyocytes in heart and beta cells of islets of Langerhans of pancreas [4]. All these complex interconnecting molecular networks reveal the importance of natriuretic peptides in health and diseases. Therefore, better understanding of these natriuretic peptides and their receptors have potential clinical applications in the treatment of metabolic syndrome.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK081450 and T32 07150-40 to Arun J. Sanyal.
Footnotes
Conflict of interest
No conflicts to disclose: Prasanna K. Santhekadur, Divya P. Kumar, Mulugeta Seneshaw.
Faridoddin Mirshahi: No conflicts to disclose. He has ownership interest in Sanyal Biotechnology.
Dr. Arun Sanyal: None for this project. Dr. Sanyal is President, Chairman and Chief medical officer of Sanyal Biotechnology and has stock options in Genfit. He has served as a consultant to AbVie, Astra Zeneca, Nitto Denko, Nimbus, Salix, Tobira, Takeda, Fibrogen, Lilly, Zafgen, Novartis, Pfizer, Immuron, Exhalenz and Genfit. He has been an unpaid consultant to Intercept, Echosens, Immuron, Amarin, Ardelyx, Fractyl, Syntlogic, Nordic Bioscience and Bristol Myers Squibb. His institution has received grant support from Gilead, Salix, Tobira, Intercept, Merck, Astra Zeneca and Novartis.
References
- 1.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu. Rev. Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Banting lecture, Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Han TS, Lean ME. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis. 2016;25:1–13. doi: 10.1177/2048004016633371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlueter N, de Sterke A, Willmes DM, Spranger J, Jordan J, Birkenfeld AL. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol. Ther. 2014;144:12–27. doi: 10.1016/j.pharmthera.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Moro C, Smith SR. Natriuretic peptides: new players in energy homeostasis. Diabetes. 2009;58:2726–2728. doi: 10.2337/db09-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Wu Z, Li D, Li N, Dindot SV, Satterfield MC, Bazer FW, Wu G. Nutrition, epigenetics, and metabolic syndrome. Antioxid. Redox Signal. 2012;17:282–301. doi: 10.1089/ars.2011.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suga SI, Itoh H, Komatsu Y, Ishida H, Igaki T, Yamashita J, Doi K, et al. Regulation of endothelial production of C-type natriuretic peptide by interaction between endothelial cells and macrophages. Endocrinology. 1998;139:1920–1926. doi: 10.1210/endo.139.4.5918. [DOI] [PubMed] [Google Scholar]
- 8.Itoh H, Nakao K. Natriuretic peptide system. Nihon Rinsho. 1997;55:1923–1936. [PubMed] [Google Scholar]
- 9.Anand-Srivastava MB. Natriuretic peptide Receptor-C signaling and regulation. Peptides. 2005;26:1044–1059. doi: 10.1016/j.peptides.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa Y, Itoh H, Tamura N, et al. Molecular cloning of the cDNA and gene that encode mouse brain natriuretic peptide gene and generation of transgenic mice that overexpress the brain natriuretic peptide gene. The Journal of Clinical Investigation. 1994;93:1911–1921. doi: 10.1172/JCI117182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medina-Gomez G, Vidal-Puig A. Gateway to the metabolic syndrome. Nat. Med. 2005;11:602–603. doi: 10.1038/nm0605-602. [DOI] [PubMed] [Google Scholar]
- 12.Ruskoaho H. Cardiac hormones as diagnostic tools in heart failure. Endocr. Rev. 2003;24:341–356. doi: 10.1210/er.2003-0006. [DOI] [PubMed] [Google Scholar]
- 13.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 14.de Bold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- 15.Kangawa K, Matsuo H. Purification and complete amino acid sequence of α-human atrial natriuretic polypeptide (α-hANP) Biochem. Biophys. Res. Commun. 1984;118:131–139. doi: 10.1016/0006-291x(84)91077-5. [DOI] [PubMed] [Google Scholar]
- 16.Kangawa K, Fukuda A, Minamino N, Matsuo H. Purification and complete amino acid sequence of β-rat atrial natriuretic polypeptide (β-rANP) of 5, 000 daltons. Biochem. Biophys. Res. Commun. 1984;119:933–940. doi: 10.1016/0006-291x(84)90863-5. [DOI] [PubMed] [Google Scholar]
- 17.Kangawa K, Fukuda A, Matsuo H. Structural identification of β-and γ-human atrial natriuretic polypeptides. Nature. 1985;313:397–400. doi: 10.1038/313397a0. [DOI] [PubMed] [Google Scholar]
- 18.Currie MG, Geller DM, Cole BR, Boylan JG, YuSheng W, Holmberg SW, Needleman P. Bioactive cardiac substances: potent vasorelaxant activity in mammalian atria. Science. 1983;221:71–73. doi: 10.1126/science.6857267. [DOI] [PubMed] [Google Scholar]
- 19.Geller DM, Currie MG, Wakitani K, Cole BR, Adams SP, Fok KF, Siegel NR, Eubanks SR, Galluppi GR, Needleman P. Atriopeptins: a family of potent biologically active peptides derived from mammalian atria. Biochem. Biophys. Res. Commun. 1984;120:333–338. doi: 10.1016/0006-291x(84)91258-0. [DOI] [PubMed] [Google Scholar]
- 20.Atlas SA, Kleinert HD, Camargo MJ, Januszewicz A, Sealey JE, Laragh JH, et al. Purification. sequencing and synthesis of natriuretic and vasoactive rat atrial peptide. Nature. 1984;309:717–719. doi: 10.1038/309717a0. [DOI] [PubMed] [Google Scholar]
- 21.Tamura N, Ogawa Y, Yasoda A, Itoh H, Saito Y, Nakao K. Two cardiac natriuretic peptide genes (atrial natriuretic peptide and brain natriuretic peptide) are organized in tandem in the mouse and human genomes. J. Mol. Cell. Cardiol. 1996;28:1811–1815. doi: 10.1006/jmcc.1996.0170. [DOI] [PubMed] [Google Scholar]
- 22.Mathisen P, Hall C, Simonsen S. Comparative study of atrial peptides ANF (1–98) and ANF (99–126) as diagnostic markers of atrial distention in patients with cardiac disease. Scand. J. Clin. Lab. Invest. 1993;53:41–49. doi: 10.1080/00365519309092530. [DOI] [PubMed] [Google Scholar]
- 23.Chopra S, Cherian D, Verghese PP, Jacob JJ. Physiology and clinical significance of natriuretic hormones. Indian J. Endocrinol. Metab. 2013;17:83–90. doi: 10.4103/2230-8210.107869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song W, Wang H, Wu Q. Atrial natriuretic peptide in cardiovascular biology and disease (NPPA) Gene. 2015;10:1–6. doi: 10.1016/j.gene.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pemberton CJ, Siriwardena M, Kleffmann T, Ruygrok P, Palmer SC, Yandle TG, Richards AM. First identification of circulating prepro-A-type natriuretic peptide (preproANP) signal peptide fragments in humans: initial assessment as cardiovascular biomarkers. Clin. Chem. 2012;58:757–767. doi: 10.1373/clinchem.2011.176990. [DOI] [PubMed] [Google Scholar]
- 26.Wu F, Yan W, Pan J, Morser J, Wu Q. Processing of pro-atrial natriuretic peptide by corin in cardiac myocytes. J. Biol. Chem. 2002;277:16900–16905. doi: 10.1074/jbc.M201503200. [DOI] [PubMed] [Google Scholar]
- 27.Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75:142–146. doi: 10.1038/ki.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J. Biol. Chem. 1999;274:14926–14935. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- 29.Numata Y, Dohi K, Furukawa A, Kikuoka S, et al. Immunoradiometric assay for the N-terminal fragment of proatrial natriuretic peptide in human plasma. Clin. Chem. 1998;44:1008–1013. [PubMed] [Google Scholar]
- 30.Tanaka M, Ishizaka Y, et al. Exercise-induced secretion of brain natriuretic peptide in essential hypertension and normal subjects. Hypertens. Res. 1995;18:159–166. doi: 10.1291/hypres.18.159. [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Nakamura K, Gardner DG. 1,25-dihydroxyvitamin D inhibits human ANP gene promoter activity. Regul. Pept. 2005;128:197–202. doi: 10.1016/j.regpep.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 32.Chun YS, Hyun JY, Kwak YG, Kim IS, Kim CH, Choi E, Kim MS, Park JW. Hypoxic activation of the atrial natriuretic peptide gene promoter through direct and indirect actions of hypoxia-inducible factor-1. Biochem. J. 2003;370:149–157. doi: 10.1042/BJ20021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornelius T, Holmer SR, Müller FU, Riegger GA, Schunkert H. Regulation of the rat atrial natriuretic peptide gene after acute imposition of left ventricular pressure overload. Hypertension. 1997;30:1348–1355. doi: 10.1161/01.hyp.30.6.1348. [DOI] [PubMed] [Google Scholar]
- 34.Houweling AC, van Borren MM, Moorman AF, Christoffels VM. Expression and regulation of the atrial natriuretic factor encoding gene Nppa during development and disease. Cardiovasc. Res. 2005;67:583–593. doi: 10.1016/j.cardiores.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Bell D, Tamamori M, Marumo F, Hiroe M, McDermott BJ, Ito H. Calcitonin gene-related peptide (CGRP) increases cell surface area and induces expression of skeletal α-actin ANP mRNA in hypertrophying neonatal cardiomyocytes. Regul. Pept. 1997;71:1–7. doi: 10.1016/s0167-0115(97)01015-x. [DOI] [PubMed] [Google Scholar]
- 36.Kim M, Platt MJ, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat. Med. 2013;19:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 37.Buglioni A, Burnett JC., Jr A gut-heart connection in cardiometabolic regulation. Nat. Med. 2013;19:534–536. doi: 10.1038/nm.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whalley NM, Pritchard LE, Smith DM, White A. Processing of proglucagon to GLP-1 in pancreatic (-cells: is this a paracrine mechanism enabling GLP-1 to act on β-cells? J. Endocrinol. 2011;211:99–106. doi: 10.1530/JOE-11-0094. [DOI] [PubMed] [Google Scholar]
- 39.Gardner DG, Chen S, Glenn DJ, Grigsby CL. Molecular biology of the natriuretic peptide system: implications for physiology and hypertension. Hypertension. 2007;49:419–426. doi: 10.1161/01.HYP.0000258532.07418.fa. [DOI] [PubMed] [Google Scholar]
- 40.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa Y, Itoh H, Yoshitake Y, Inoue M, Yoshimasa T, Serikawa T, Nakao K. Molecular cloning and chromosomal assignment of the mouse C-type natriuretic peptide (CNP) gene (Nppc): comparison with the human CNP gene (NPPC), Genomics. 1994;15(24):383–387. doi: 10.1006/geno.1994.1633. [DOI] [PubMed] [Google Scholar]
- 42.Semenov AG, Tamm NN, Seferian KR, Postnikov AB, Karpova NS, Serebryanaya DV, Koshkina EV, Krasnoselsky MI, Katrukha AG. Processing of pro-B-type natriuretic peptide: furin and corin as candidate convertases. Clin. Chem. 2010;56:1166–1176. doi: 10.1373/clinchem.2010.143883. [DOI] [PubMed] [Google Scholar]
- 43.Spevack DM, Schwartzbard A. B-type natriuretic peptide measurement in heart failure. Clin. Cardiol. 2004;27:489–494. doi: 10.1002/clc.4960270903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomaru KK, Arai M, Yokoyama T, Aihara Y, Sekiguchi KK, et al. Transcriptional activation of the BNP gene by lipopolysaccharide is mediated through GATA elements in neonatal rat cardiac myocytes. J. Mol. Cell. Cardiol. 2002;34:649–659. doi: 10.1006/jmcc.2002.2005. [DOI] [PubMed] [Google Scholar]
- 45.Glenn DJ, Wang F, Chen S, Nishimoto M, Gardner DG. Endothelin-stimulated human B-type natriuretic peptide gene expression is mediated by Yin Yang 1 in association with histone deacetylase 2. Hypertension. 2009;53:549–555. doi: 10.1161/HYPERTENSIONAHA.108.125088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charron F, Paradis P, Bronchain O, Nemer G, Nemer M. Cooperative interaction between GATA-4 and GATA-6 regulates myocardial gene expression. Mol. Cell. Biol. 1999;19:4355–4365. doi: 10.1128/mcb.19.6.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morin S, Charron F, Robitaille L, Nemer M. GATA-dependent recruitment of MEF2 proteins to target promoters. EMBO J. 2000;19:2046–2055. doi: 10.1093/emboj/19.9.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cserjesi P, Markham BE, Molkentin JD. The transcription factors GATA4 and dHAND physically interact to synergistically activate cardiac gene expression through a p300-dependent mechanism. J. Biol. Chem. 2002;277:24390–24398. doi: 10.1074/jbc.M202490200. [DOI] [PubMed] [Google Scholar]
- 49.Morin S, Paradis P, Aries A, Nemer M. Serum response factor-GATA ternary complex required for nuclear signaling bya G-protein-coupled receptor. Mol. Cell. Biol. 2001;21:1036–1044. doi: 10.1128/MCB.21.4.1036-1044.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiojima I, Komuro I, Oka T, Hiroi Y, Mizuno T, et al. Context dependent transcriptional cooperation mediated by cardiac transcription factors Csx/ Nkx-2.5 and GATA-4. J. Biol. Chem. 1999;274:8231–8239. doi: 10.1074/jbc.274.12.8231. [DOI] [PubMed] [Google Scholar]
- 51.Durocher D, Charron F, Warren R, Schwartz RJ, Nemer M. The cardiac transcription factors Nkx2–5 and GATA-4 are mutual cofactors. EMBO J. 1997;16:5687–5696. doi: 10.1093/emboj/16.18.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhalla SS, Robitaille L, Nemer M. Cooperative activation by GATA-4 and YY1 of the cardiac B-type natriuretic peptide promoter. J. Biol. Chem. 2001;276:11439–11445. doi: 10.1074/jbc.M100208200. [DOI] [PubMed] [Google Scholar]
- 53.Kakita T, Hasegawa K, Morimoto T, Kaburagi S, Wada H, Sasayama S. p300 protein as a coactivator of GATA-5 in the transcription of cardiac restricted atrial natriuretic factor gene. J. Biol. Chem. 1999;274:34096–34102. doi: 10.1074/jbc.274.48.34096. [DOI] [PubMed] [Google Scholar]
- 54.Sudoh T, Maekawa K, Kojima M, Minamino N, Kangawa K, Matsuo H. Cloning and sequence analysis of cDNA encoding a precursor for human brain natriuretic peptide. Biochem. Biophys. Res. Commun. 1989;159:1427–1434. doi: 10.1016/0006-291x(89)92269-9. [DOI] [PubMed] [Google Scholar]
- 55.Hunt PJ, Richards AM, Espiner EA, Nicholls MG, Yandle TG. Bioactivity and metabolism of C-type natriuretic peptide in normal man. J. Clin. Endocrinol. Metab. 1994;78:1428–1435. doi: 10.1210/jcem.78.6.8200946. [DOI] [PubMed] [Google Scholar]
- 56.Imura H, Nakao K, Itoh H. The natriuretic peptide system in the brain: implications in the central control of cardiovascular and neuroendocrine functions. Front. Neuroendocrinol. 1992;13:217–249. [PubMed] [Google Scholar]
- 57.Lumsden NG, Khambata RS, Hobbs AJ. C-type natriuretic peptide (CNP): cardiovascular roles and potential as a therapeutic target. Curr. Pharm. Des. 2010;16:4080–4088. doi: 10.2174/138161210794519237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu C, Wu F, Pan J, Morser J, Wu Q. Furin-mediated processing of Pro-C-type natriuretic peptide. J. Biol. Chem. 2003;278:25847–25852. doi: 10.1074/jbc.M301223200. [DOI] [PubMed] [Google Scholar]
- 59.Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011;278:1808–1817. doi: 10.1111/j.1742-4658.2011.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suga S, Itoh H, Komatsu Y, Ogawa Y, Hama N, Yoshimasa T, et al. Cytokine-induced C-type natriuretic peptide (CNP) secretion from vascular endothelial cells - evidence for CNP as a novel autocrine/paracrine regulator from endothelial cells. Endocrinology. 1993;133:3038–3041. doi: 10.1210/endo.133.6.8243333. [DOI] [PubMed] [Google Scholar]
- 61.Suga S, Nakao K, Itoh H, Komatsu Y, Hosoda K, Suga S, et al. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-β – possible existence of vascular natriuretic system. J. Clin. Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodard GE, Rosado JA, Brown J. Expression and control of C-type natriuretic peptide in rat vascular smooth muscle cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R156–R165. doi: 10.1152/ajpregu.2002.282.1.R156. [DOI] [PubMed] [Google Scholar]
- 63.Hama N, Itoh H, Shirakami G, Suga S, Komatsu Y, Yoshimasa T, et al. Detection of C-type natriuretic peptide in human circulation and marked increase of plasma CNP level in septic shock patients. Biochem. Biophys. Res. Commun. 1994;198:1177–1182. doi: 10.1006/bbrc.1994.1166. [DOI] [PubMed] [Google Scholar]
- 64.Okahara K, Kambayashi J, Ohnishi T, Fujiwara Y, Kawasaki T, Monden M. Shear stress also induces expression of CNP gene in human endothelial cells. Shear stress induces expression of CNP genein human endothelial cells. FEBS Lett. 1995;373:108–110. doi: 10.1016/0014-5793(95)01027-c. [DOI] [PubMed] [Google Scholar]
- 65.Sellitti DF, Koles N, Mendonça MC. Regulation of C-type natriuretic peptide expression. Peptides. 2011;32:1964–1971. doi: 10.1016/j.peptides.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 66.Koller KJ, Lowe DG, Bennett GL, Minamino N, et al. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP) Science. 1991;252:120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- 67.Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, et al. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology. 1992;130:229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- 68.Potter LR, Hunter T. Guanylyl cyclase-linked natriuretic peptide receptors: structure and regulation. J. Biol. Chem. 2001;276:6057–6060. doi: 10.1074/jbc.R000033200. [DOI] [PubMed] [Google Scholar]
- 69.Chinkers M, Garbers DL. The protein kinase domain of the ANP receptor is required for signaling. Science. 1989;245:1392–1394. doi: 10.1126/science.2571188. [DOI] [PubMed] [Google Scholar]
- 70.Chang MS, Lowe DG, Lewis M, Hellmiss R, Chen E, Goeddel DV. Differential activation by atrial and brain natriuretic peptides of two different receptor guanylate cyclases. Nature. 1989;341:68–72. doi: 10.1038/341068a0. [DOI] [PubMed] [Google Scholar]
- 71.Pandey KN, Singh S. Molecular cloning and expression of murine guanylate cyclase/atrial natriuretic factor receptor cDNA. J. Biol. Chem. 1990;265:12342–12348. [PubMed] [Google Scholar]
- 72.Theilig F, Wu Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am. J. Physiol. Renal Physiol. 2015;308:F1047–F1055. doi: 10.1152/ajprenal.00164.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nuglozeh E, Kozak LP. Genetic mapping of the C-type natriuretic peptide receptor (Npr2) gene to mouse chromosome 4. Mamm. Genome. 1997;8:624–625. doi: 10.1007/s003359900523. [DOI] [PubMed] [Google Scholar]
- 74.Schulz S, Singh S, Bellet RA, Singh G, et al. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989;58:1155–1162. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- 75.Rahmutula D, Cui J, Chen S, Gardner DG. Transcriptional regulation of type B human natriuretic Peptide receptor gene promoter: dependence on Sp1. Hypertension. 2004;44:283–288. doi: 10.1161/01.HYP.0000136908.60317.92. [DOI] [PubMed] [Google Scholar]
- 76.Nagase M, Katafuchi T, Hirose S, Fujita T. Tissue distribution and localization of natriuretic peptide receptor subtypes in stroke-prone spontaneously hypertensive rats. J. Hypertens. 1997;15:1235–1243. doi: 10.1097/00004872-199715110-00007. [DOI] [PubMed] [Google Scholar]
- 77.Chrisman TD, Schulz S, Potter LR, Garbers DL. Seminal plasma factors that cause large elevations in cellular cyclic GMP are C-type natriuretic peptides. J. Biol. Chem. 1993;268:3698–3703. [PubMed] [Google Scholar]
- 78.Yanaka N, Kotera J, Omori K. Isolation and characterization of the 5’-flanking regulatory region of the human natriuretic peptide receptor C gene. Endocrinology. 1998;139:1389–1400. doi: 10.1210/endo.139.3.5781. [DOI] [PubMed] [Google Scholar]
- 79.Yanaka N, Kotera J, Taguchi I, et al. Structure of the 5’-flanking regulatory region of the mouse gene encoding the clearance receptor for atrial natriuretic peptide. Eur. J. Biochem. 1996;237:25–34. doi: 10.1111/j.1432-1033.1996.0025n.x. [DOI] [PubMed] [Google Scholar]
- 80.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr. Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 81.Santhekadur PK, Akiel M, Emdad L, Gredler R, et al. Staphylococcal nuclease domain containing-1 (SND1) promotes migration and invasion via angiotensin II type 1 receptor (AT1R) and TGFβ signaling. FEBS Open Bio. 2014;4:353–361. doi: 10.1016/j.fob.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardio protection. Cardiovasc. Res. 2006;69:318–328. doi: 10.1016/j.cardiores.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 83.Volpe M. Natriuretic peptides and cardio-renal disease. Int. J. Cardiol. 2014;176:630–639. doi: 10.1016/j.ijcard.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 84.Yoshimura M, Yasue H, Ogawa H. Pathophysiological significance and clinical application of ANP and BNP in patients with heart failure. Can. J. Physiol. Pharmacol. 2001;79:730–735. [PubMed] [Google Scholar]
- 85.Yoshimura M, Yasue H, Morita E, Sakaino N, Jougasaki M, et al. Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure. Circulation. 1991;84:1581–1588. doi: 10.1161/01.cir.84.4.1581. [DOI] [PubMed] [Google Scholar]
- 86.Saito Y, Nakao K, Nishimura K, et al. Clinical application of atrial natriuretic polypeptide in patients with congestive heart failure: beneficial effects on left ventricular function. Circulation. 1987;76:115–124. doi: 10.1161/01.cir.76.1.115. [DOI] [PubMed] [Google Scholar]
- 87.Lopez MJ, Wong SK, Kishimoto I, et al. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 88.Pandit K, Mukhopadhyay P, Ghosh S, Chowdhury S. Natriuretic peptides: diagnostic and therapeutic use. Indian J Endocrinol Metab. 2011;15:S345–S353. doi: 10.4103/2230-8210.86978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Furst R, Brueckl CWM, et al. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase 1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ. Res. 2005;96:43–53. doi: 10.1161/01.RES.0000151983.01148.06. [DOI] [PubMed] [Google Scholar]
- 90.Kiemer AK, Weber NC, Furst R, Bildner N, et al. Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF - induced actin polymerization and endothelial permeability. Circ. Res. 2002;90:874–881. doi: 10.1161/01.res.0000017068.58856.f3. [DOI] [PubMed] [Google Scholar]
- 91.Cannon MV, Sillje HH, Sijbesma JW, Khan MA, et al. LXRα improves myocardial glucose tolerance and reduces cardiac hypertrophy in a mouse model of obesity-induced type 2 diabetes. Diabetologia. 2016;59:634–643. doi: 10.1007/s00125-015-3827-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jujic APM, Engström G, Hedblad B, Melander O, Magnusson M. Atrial natriuretic peptide and type 2 diabetes development biomarker and genotype association study. PLoS One. 2014;9:e89201. doi: 10.1371/journal.pone.0089201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Magnusson M, Jujic A, Hedblad B, Engström G, Persson M, et al. Low plasma level of atrial natriuretic peptide predicts development of diabetes: the prospective Malmo diet and cancer study. J. Clin. Endocrinol. Metab. 2012;97:638–645. doi: 10.1210/jc.2011-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Monti LD, Lucotti PC, Setola E, Rossodivita A, Pala MG, Galluccio E, et al. Effects of chronic elevation of atrial natriuretic peptide and free fatty acid levels in the induction of type 2 diabetes mellitus and insulin resistance in patients with mitral valve disease. Nutr. Metab. Cardiovasc. Dis. 2012;22:58–65. doi: 10.1016/j.numecd.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 95.Verspohl EJ, Bernemann IK. Atrial natriuretic peptide (ANP)-induced inhibition of glucagon secretion: mechanism of action in isolated rat pancreatic islets. Peptides. 1996;17:1023–1029. doi: 10.1016/0196-9781(96)00152-0. [DOI] [PubMed] [Google Scholar]
- 96.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 97.Del RS, Cabiati M, Bianchi V, Caponi L, et al. C-type natriuretic peptide is closely associated to obesity in Caucasian adolescents. Clin. Chim. Acta. 2016;460:172–177. doi: 10.1016/j.cca.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 98.Haufe S, Kaminski J, Utz W, Haas V, et al. Differential response of the natriuretic peptide system to weight loss and exercise in overweight or obese patients. J. Hypertens. 2015;33:1458–1464. doi: 10.1097/HJH.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 99.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Fulgheri PD, Zhang C, Takahashi N, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Theilig F, Wu Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am. J. Physiol. Renal Physiol. 2015;308:F1047–F1055. doi: 10.1152/ajprenal.00164.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ballermann BJ, Brenner BM. Atrial natriuretic peptide and the kidney. Am. J. Kidney Dis. 1987;10:7–12. [PubMed] [Google Scholar]
- 102.Tagore R, Lieng H, Yang LH, Daw HY, et al. Natriuretic peptides in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2008;6:1644–1651. doi: 10.2215/CJN.00850208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chattington PD, Anderson JV, Rees LH, et al. Atrial natriuretic peptide in type 2 diabetes mellitus: response to a physiological mixed meal and relationship to renal function. Diabet. Med. 1998;15:375–379. doi: 10.1002/(SICI)1096-9136(199805)15:5<375::AID-DIA585>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 104.Theilig F, Wu Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am. J. Physiol. Renal Physiol. 2015;308:F1047–F1055. doi: 10.1152/ajprenal.00164.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Araujo SC, Moreira AL, Pestana M. Clinical value of natriuretic peptides in chronic kidney disease. Nefrologia. 2015;35:227–233. doi: 10.1016/j.nefro.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 106.Metwaly A, Khalik AA, Nasr FM, et al. Brain natriuretic peptide in liver cirrhosis and fatty liver: correlation with cardiac performance. Electron Physician. 2016;8:1984–1993. doi: 10.19082/1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Neeland IJ, Winders BR, Ayers CR, et al. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. J. Am. Coll. Cardiol. 2013;62:752–760. doi: 10.1016/j.jacc.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yildiz R, Yildirim B, Karincaoglu M, Harputluoglu M, Hilmioglu F. Brain natriuretic peptide and severity of disease in non-alcoholic cirrhotic patients. J. Gastroenterol. Hepatol. 2005;20:1115–1120. doi: 10.1111/j.1440-1746.2005.03906.x. [DOI] [PubMed] [Google Scholar]
- 109.Gerbes AL. The role of atrial natriuretic peptide (ANP) in chronic liver disease. Pharmacol. Ther. 1993;58:381–390. doi: 10.1016/0163-7258(93)90028-c. [DOI] [PubMed] [Google Scholar]
- 110.Woo JJ, Koh YY, Kim HJ, et al. N-terminal pro B-type natriuretic peptide and the evaluation of cardiac dysfunction and severity of disease in cirrhotic patients. Yonsei Med. J. 2008;49:625–631. doi: 10.3349/ymj.2008.49.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Metwaly A, Khalik AA, Nasr FM, Sabry AI, et al. Brain natriuretic peptide in liver cirrhosis and fatty liver: correlation with cardiac performance. Electron Physician. 2016;8:1984–1993. doi: 10.19082/1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramos HR, Birkenfeld AL, de Bold AJ. Interacting disciplines: cardiac natriuretic peptides and obesity: perspectives from an endocrinologist and a cardiologist. Endocr Connect. 2015;4:R25–R36. doi: 10.1530/EC-15-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nojiri T, Hosoda H, Tokudome T, et al. Atrial natriuretic peptide prevents cancer metastasis through vascular endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 2015;112:4086–4091. doi: 10.1073/pnas.1417273112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Hu G, uang X, Zhang K, Jiang H, Hu X. Anti-inflammatory effect of B-type natriuretic peptide postconditioning during myocardial ischemia-reperfusion: involvement of PI3 K/Akt signaling pathway. Inflammation. 2014;37:1669–1674. doi: 10.1007/s10753-014-9895-0. [DOI] [PubMed] [Google Scholar]
- 115.Parissis JT, Farmakis D, Nikolaou M, Birmpa D, et al. Plasma B-type natriuretic peptide and anti-inflammatory cytokine interleukin-10 levels predict adverse clinical outcome in chronic heart failure patients with depressive symptoms: a 1-year follow-up study. Eur. J. Heart Fail. 2009;11:967–972. doi: 10.1093/eurjhf/hfp125. [DOI] [PubMed] [Google Scholar]
- 116.Zhang J, Li M, Yang Y, Yan Y, et al. NPR-A: a therapeutic target in inflammation and cancer. Crit. Rev. Eukaryot. Gene Expr. 2015;25:41–46. doi: 10.1615/critreveukaryotgeneexpr.2015012447. [DOI] [PubMed] [Google Scholar]
- 117.Kong X, Wang X, Xu W, et al. Natriuretic peptide receptor a as a novel anticancer target. Cancer Res. 2008;68:249–256. doi: 10.1158/0008-5472.CAN-07-3086. [DOI] [PubMed] [Google Scholar]
- 118.Zhao Z, Zhang J, Li M, Yang Y, et al. ANP-NPRA signaling pathway?a potential therapeutic target for the treatment of malignancy. Crit. Rev. Eukaryot. Gene Expr. 2013;23:93–101. doi: 10.1615/critreveukargeneexpr.2013006641. [DOI] [PubMed] [Google Scholar]
- 119.Mallela J, Ravi S, Louis FJ, et al. Natriuretic peptide receptor A signaling regulates stem cell recruitment and angiogenesis: a model to study linkage between inflammation and tumorigenesis. Stem Cells. 2013;31:1321–1329. doi: 10.1002/stem.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abdelalim EM, Tooyama I. BNP signaling is crucial for embryonic stem cell proliferation. PLoS One. 2009;4:e534. doi: 10.1371/journal.pone.0005341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pandey KN. Guanylyl cyclase/atrial natriuretic peptide receptor-A: role in the pathophysiology of cardiovascular regulation. Can. J. Physiol. Pharmacol. 2011;89:557–573. doi: 10.1139/y11-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Serafino A, Pierimarchi P. Atrial natriuretic peptide: a magic bullet for cancer therapy targeting Wnt signaling and cellular pH regulators. Curr. Med. Chem. 2014;21:2401–2409. doi: 10.2174/0929867321666140205140152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vesely BA, McAfee Q, Gower WR, Jr, Vesely DL. Four peptides decrease the number of human pancreatic adenocarcinoma cells. Eur. J. Clin. Invest. 2003;33:998–1005. doi: 10.1046/j.1365-2362.2003.01262.x. [DOI] [PubMed] [Google Scholar]
- 124.Vesely BA, Song S, Ramos JS, Fitz SR, et al. Four peptide hormones decrease the number of human breast adenocarcinoma cells. Eur. J. Clin. Invest. 2005;35:60–69. doi: 10.1111/j.1365-2362.2005.01444.x. [DOI] [PubMed] [Google Scholar]
- 125.Vesely BA, Fitz SR, Gower WR, Jr, Vesely DL., Jr Vessel dilator Most potent of the atrial natriuretic peptides in decreasing the number and DNA synthesis of human squamous lung cancer cells. Cancer Lett. 2006;233:226–231. doi: 10.1016/j.canlet.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 126.Vesely BA, Song S, Ramos JS, et al. Five cardiac hormones decrease the number of human small-cell lung cancer cells. Eur. J Clin Invest. 2005;35:388–398. doi: 10.1111/j.1365-2362.2005.01501.x. [DOI] [PubMed] [Google Scholar]
- 127.Gower WR, Vesely BA, Alli AA, Vesely DL. Four peptides decrease human colon adenocarcinoma cell number and DNA synthesis via cyclic GMP. Int. J. Gastrointest. Cancer. 2005;36:77–87. doi: 10.1385/IJGC:36:2:77. [DOI] [PubMed] [Google Scholar]
- 128.Vesely BA, Eichelbaum EJ, Alli AA, et al. Urodilatin and four cardiac hormones decrease human renal carcinoma cell numbers. Eur. J. Clin Invest. 2006;36:810–819. doi: 10.1111/j.1365-2362.2006.01721.x. [DOI] [PubMed] [Google Scholar]
- 129.Vesely DL. Cardiac and renal hormones anticancer effects in vitro and in vivo. J. Investig Med. 2009;57:22–28. doi: 10.2310/JIM.0b013e3181948b25. [DOI] [PubMed] [Google Scholar]
- 130.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 131.van Kruijsdijk RC, Wall EVD, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol. Biomarkers Prev. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 132.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moro C. Targeting cardiac natriuretic peptides in the therapy of diabetes and obesity. Expert Opin. Ther. Targets. 2016;20:1445–1452. doi: 10.1080/14728222.2016.1254198. [DOI] [PubMed] [Google Scholar]
- 134.Feng Y, Wang D, Bi H, Zhang H. The role of natriuretic peptides in diabetes and its complications. Biomed. Pharmacother. 2016;16:30987–30988. doi: 10.1016/j.biopha.2016.10.089. [DOI] [PubMed] [Google Scholar]
- 135.Namdari M, Eatemadi A, Negahdari B. Natriuretic peptides and their therapeutic potential in heart failure treatment: an updated review. Cell. Mol. Biol. 2016;62:1–7. [PubMed] [Google Scholar]
- 136.Uehlinger DE, Weidmann P, Gnädinger MP, Hasler L, Bachmann C, et al. Increase in circulating insulin induced by atrial natriuretic peptide in normal humans. J. Cardiovasc. Pharmacol. 1986;8:1122–1129. doi: 10.1097/00005344-198611000-00005. [DOI] [PubMed] [Google Scholar]
- 137.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb. Exp. Pharmacol. 2009;191:341–366. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weder AB, Sekkarie MA, Takiyyuddin M, Schork NJ, Julius S. Antihypertensive and hypotensive effects of atrial natriuretic factor in men. Hypertension. 1987;10:582–589. doi: 10.1161/01.hyp.10.6.582. [DOI] [PubMed] [Google Scholar]
- 139.Fifer MA, Molina CR, Quiroz AC, Giles TD, et al. Hemodynamic and renal effects of atrial natriuretic peptide in congestive heart failure. Am. J. Cardiol. 1990;65:211–216. doi: 10.1016/0002-9149(90)90087-h. [DOI] [PubMed] [Google Scholar]
- 140.Saito Y, Nakao K, Nishimura K, et al. Clinical applications of atrial natriuretic polypeptide in patients with congestive heart failure: beneficial effects on left ventricular function. Circulation. 1987;76:115–124. doi: 10.1161/01.cir.76.1.115. [DOI] [PubMed] [Google Scholar]
- 141.Kitashiro S, Sugiura T, Takayama Y, et al. Long-term administration of atrial natriuretic peptide in patients with acute heart failure. J. Cardiovasc. Pharmacol. 1999;33:948–952. doi: 10.1097/00005344-199906000-00016. [DOI] [PubMed] [Google Scholar]
- 142.Konishi M, Ishida J, Springer J, et al. Heart failure epidemiology and novel treatments in Japan: facts and numbers. ESC Heart Fail. 2016;3:145–151. doi: 10.1002/ehf2.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sezai A, Hata M, Niino T, Yoshitake I, Unosawa S, et al. Influence of continuous infusion of low-dose human atrial natriuretic peptide on renal function during cardiac surgery: a randomized controlled study. J. Am. Coll. Cardiol. 2009;54:1058–1064. doi: 10.1016/j.jacc.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 144.Sengenes C, Stich V, Berlan M, Hejnova J, Lafontan M, Pariskova Z, Galitzky J. Increased lipolysis in adipose tissue and lipid mobilization to natriuretic peptides during low-calorie diet in obese women. Int. J. Obes. Relat. Metab. Disord. 2002;26:24–32. doi: 10.1038/sj.ijo.0801845. [DOI] [PubMed] [Google Scholar]
- 145.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N. Engl. J. Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 146.Krämer BK, Kammerl M, Schweda F, Schreiber M. A primer in radiocontrast-induced nephropathy. Nephrol. Dial. Transplant. 1999;14:2830–2834. doi: 10.1093/ndt/14.12.2830. [DOI] [PubMed] [Google Scholar]
- 147.Potter LR. Encycl. Biol. Chem. Vol. 3. Elsevier Inc; 2004. Natriuretic peptides and their receptors. [Google Scholar]
- 148.Corti R, Burnett JC, Jr, Rouleau JL, et al. Vasopeptidase inhibitors: a new therapeutic concept in cardiovascular disease? Circulation. 2001;104:1856–1862. doi: 10.1161/hc4001.097191. [DOI] [PubMed] [Google Scholar]
- 149.Keating GM, Goa KL. Nesiritide:a review of its use in acute decompensated heart failure. Drugs. 2003;63:47–70. doi: 10.2165/00003495-200363010-00004. [DOI] [PubMed] [Google Scholar]
- 150.Vila G, Grimm G, Resl M, Heinisch B, Einwallner E, et al. B-type natriuretic peptide modulates ghrelin, hunger, and satiety in healthy men. Diabetes. 2012;61:2592–2596. doi: 10.2337/db11-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Izumi K, Eishi K, Yamachika S, Hashizume K, et al. The efficacy of human atrial natriuretic peptide in patients with renal dysfunction undergoing cardiac surgery. Ann. Thorac. Cardiovasc. Surg. 2008;14:294–302. [PubMed] [Google Scholar]
- 152.Yap LB, Mukerjee D, Timms PM, et al. Natriuretic peptides, respiratory disease, and the right heart. Chest. 2004;126:1330–1336. doi: 10.1378/chest.126.4.1330. [DOI] [PubMed] [Google Scholar]
- 153.Denus SD, Pharand C, Williamson DR. Brain natriuretic peptide in the management of heart failure: the versatile neuro hormone. Chest. 2004;125:652–668. doi: 10.1378/chest.125.2.652. [DOI] [PubMed] [Google Scholar]
- 154.Boerrigter G, Burnett JC., Jr Recent advances in natriuretic peptides in congestive heart failure. Expert Opin. Investig. Drugs. 2004;13:643–652. doi: 10.1517/13543784.13.6.643. [DOI] [PubMed] [Google Scholar]
- 155.Kesselheim AS, Fischer MA, Avorn J. The rise and fall of Natrecor for congestive heart failure: implications for drug policy. Health Aff. (Millwood) 2006;25:1095–1102. doi: 10.1377/hlthaff.25.4.1095. [DOI] [PubMed] [Google Scholar]
- 156.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 157.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 158.Lin J, Han Z, Li H, Chen SY, et al. Plasma C-type natriuretic peptide as a predictor for therapeutic response to metoprolol in children with postural tachycardia syndrome. PLoS One. 2015;10:e0121913. doi: 10.1371/journal.pone.0121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zakeri R, Sangaralingham SJ, Sandberg SM, Heublein DM, et al. Urinary C-type natriuretic peptide: a new heart failure biomarker. JACC Heart Fail. 2013;1:170–177. [Google Scholar]
- 160.Lumsden NG, Khambata RS, Hobbs AJ. C-type natriuretic peptide (CNP): cardiovascular roles and potential as a therapeutic target. Curr. Pharm. Des. 2010;16:4080–4088. doi: 10.2174/138161210794519237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dickey DM, Potter LR. Dendroaspis natriuretic peptide and the designer natriuretic peptide CD-NP, are resistant to proteolytic inactivation. J. Mol. Cell. Cardiol. 2011;51:67–71. doi: 10.1016/j.yjmcc.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ogawa T, de Bold AJ. The heart as an endocrine organ. Endocr Connect. 2014;15:R31–44. doi: 10.1530/EC-14-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]