Abstract
Background
Post-treatment control of viraemia after discontinuation of antiretroviral therapy begun during primary HIV-1 infection is considered a potential path toward a sustained remission of infection.
Methods
Subjects enrolled in an observational primary infection cohort who received at least 11 months of highly active antiretroviral therapy beginning within the first 12 weeks of HIV-1 infection and who subsequently discontinued therapy were evaluated for post-treatment control.
Results
Within a cohort of 389 subjects with primary HIV-1 infection enrolled over 22 years, only 22 met criteria for evaluation of post-treatment control. Among these subjects, 21 (95%) had loss of viral control (HIV-1 RNA>500 copies/ml) within 18 months after treatment discontinuation, and only 1 (4.5%, 95% CI 0.32, 18.9) controlled viral load to levels <500 copies/ml for at least 24 months. The median time to virological failure was 2.17 (IQR 1.18–3.39) months.
Conclusions
Our data suggest a low likelihood of post-treatment control even when highly active antiretroviral therapy is started within 12 weeks of HIV-1 infection.
Introduction
A recent publication [1] described the VISCONTI cohort, a group of individuals treated early during the course of HIV-1 infection whose viraemia remained controlled for several years after discontinuing long-term antiretroviral treatment (ART). Known as post-treatment controllers (PTCs), most of these patients lacked features previously associated with viraemic control such as protective HLA alleles [2,3]. The report estimated the probability of post-treatment control to be as high as 15.3% among patients treated during primary HIV-1 infection (PHI). We undertook a review of our own primary infection cohort to determine the reproducibility of this finding.
Methods
The Seattle Primary Infection Program (SeaPIP) has enrolled individuals with PHI into an observational cohort at the University of Washington Primary Infection Clinic (PIC) since 1992 [4–6]. At the time of cohort entry, all subjects were either HIV-antibody-negative with detectable HIV-1 RNA (acute infection) or HIV-antibody-positive with a negative or indeterminate western blot, negative ‘detuned’ antibody test or negative HIV test within 1 year of screening (early infection). All subjects were enrolled within 240 days after infection, estimated to be the date of onset of seroconversion symptoms or, for asymptomatic subjects, the mid-point between the last negative and first positive HIV tests. From 1992 to May 2014, 389 subjects were enrolled. Over time, some subjects initiated treatment during PHI based on the availability of potent ART, strength of consensus treatment recommendations and personal preferences.
To determine the prevalence of PTCs in the SeaPIP cohort, we identified subjects who satisfied the following conditions: initiation of potent ART within 12 weeks of the estimated date of infection, continuation of potent ART for ≥11 months and subsequent discontinuation of therapy. Drug combinations considered potent were: any three-drug regimen including a boosted or unboosted protease inhibitor, non-nucleoside reverse transcriptase inhibitor, integrase inhibitor or CCR5 inhibitor; the triple nucleoside reverse transcriptase inhibitor combination of zidovudine/lamivudine/abacavir (Trizivir); or any two-drug boosted protease inhibitor regimen. In order to be evaluable for this analysis we required that the subject have HIV-1 RNA less than the lower limit of quantitation prior to their treatment interruption (all but one were <50 copies/ml, one was <90 copies/ml) at least 2 weeks of interruption, and an HIV-1 RNA measurement prior to any treatment re-initiation. Virological control was defined as HIV-1 RNA levels <500 copies/ml for at least 24 months and virological failure as either the first of two consecutive HIV-1 RNA levels >500 copies/ml or one RNA level >500 copies/ml followed by resumption of ART. A Kaplan–Meier time-to-event analysis was used to estimate the probability of remaining off ART without virological failure. A Fisher’s exact test was used to evaluate the association of HLA type and seroconversion symptom severity. Log-rank statistics were used to evaluate association of HLA type and seroconversion symptom severity with virological failure. All tests were two-sided with alpha 0.05. Stata SE version 12.0 was used for all analyses (StataCorp, College Station, TX, USA).
Results
There were 251 treatment-naive subjects whose first antiretroviral regimen was a potent combination (ART) regimen. Of these, 144 initiated treatment within 12 weeks of HIV infection, including 60, 51 and 33 who started treatment within 0–<4 weeks, 4–<8 weeks and 8–<12 weeks, respectively (Figure 1). Nine (15%) of the 60 treated within 4 weeks, 11 (22%) of the 51 treated within 8 weeks, and two (6%) of the 33 treated within 12 weeks met criteria for analysis. Thus, of the 389 subjects enrolled in the SeaPIP cohort over more than 20 years, only 22 (6%) could be evaluated for post-treatment control.
Figure 1.
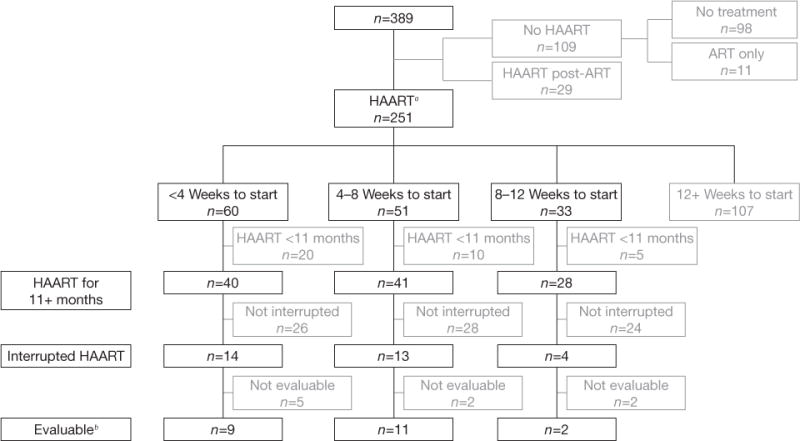
Algorithm showing ART initiation and discontinuation among 389 subjects enrolled with primary HIV-1 infection
aIndicates highly active antiretroviral therapy (HAART) as first treatment with no preceding non-HAART antiretroviral (ART) regimen. bEvaluable subjects had HIV-1 RNA below the limit of assay quantitation prior to interruption, at least 2 weeks of interruption, and an HIV-1 RNA measurement prior to any treatment re-initiation.
Demographic and other baseline characteristics of the 22 evaluable subjects are shown in Table 1. The distribution of baseline characteristics does not mirror the entire cohort as more recent trends have been for earlier identification of infection and for continuation, rather than interruption, of treatment begun during primary infection. Interestingly, HLA B35, associated with rapid HIV-1 progression, is overrepresented among our subjects (7 of 21, 33%) compared with the 8–15% prevalence reported in other HIV-infected populations [7,8], but is similar to the prevalence reported in the VISCONTI cohort. This may be explained by an association of HLA B35 with severity of seroconversion symptoms (42 (62%) of the 68 with HLA B35 had severe symptoms, compared with 95 (39%) of the 247 without HLA B35; P=0.001) making those with HLA B35 more likely to have been identified in primary infection.
Table 1.
Characteristics of subjects evaluated for post-treatment control and overall PIC cohort
| Characteristic | Subjects evaluable for post-treatment control (n=22) | All other PIC subjects (n=367) |
|---|---|---|
| Enrolment year | 2000 (1998–2002) | 2001 (1996–2007) |
| Months from estimated date of infection to ART | 1.1 (0.7–1.6) | Not described for overall cohort |
| Age, years | 35 (30–39) | 33 (27–40) |
| Male | 22 (100) | 357 (97) |
| Race/ethnicity: non-Hispanic White | 20 (91) | 306 (84) |
| Education, years | 16 (14–17) | 15 (13–16) |
| HIV risk group | ||
| MSM | 21 (95) | 305 (83) |
| Heterosexual | 1 (5) | 15 (4) |
| MSM/IDU | 0 | 24 (7) |
| IDU | 0 | 3 (1) |
| Other/unknown | 0 | 20 (5) |
| Seroconversion symptoms | ||
| Asymptomatic | 2 (9) | 56 (15) |
| Mild symptoms, no medical attention | 2 (9) | 62 (17) |
| Mild symptoms, medical attention | 7 (32) | 88 (24) |
| Severe symptoms, medical attention | 10 (45) | 137 (37) |
| Severe symptoms, hospitalized | 1 (5) | 23 (6) |
| Unknown | 0 | 1 (0.3) |
| Fiebig stage at diagnosis of primary infection | ||
| I | 2 (9) | 11 (3) |
| I/IIa | 10 (45) | 88 (24) |
| II | 0 | 13 (4) |
| III | 0 | 8 (2) |
| IV | 3 (14) | 49 (13) |
| V | 4 (18) | 62 (17) |
| V/VIb | 1 (5) | 110 (30) |
| VI | 0 | 10 (3) |
| Missing | 2 (9) | 16 (4) |
| Fiebig stage at treatment initiation | Not described for overall cohort | |
| I/IIa | 2 (9) | |
| II | 2 (9) | |
| III | 0 | |
| IV | 1 (5) | |
| V | 11 (50) | |
| V/VIb | 1 (5) | |
| VI | 4 (18) | |
| Missing | 1 (5) | |
| HLA type (27/35/57)c | ||
| HLA B27 | 0 | 21 (7) |
| HLA B35 | 7 (33) | 61 (21) |
| HLA B57 | 0 | 26 (9) |
| Missing | 1 (5) | 73 (19) |
| First ARV regimen | ||
| None | N/A | 98 (27) |
| NRTI-only | 0 | 40 (11) |
| NNRTI-based | 5 (23) | 80 (22) |
| PI-based | 16 (73) | 111 (30) |
| NNRTI/PI-based | 1 (5) | 16 (4) |
| Integrase-inhibitor-based | 0 | 20 (5) |
| Unknown | 0 | 2 (1) |
| Specific first ARV regimen | Not described for overall cohort | |
| EFV; 3TC; ABC | 2 | |
| EFV; TNV; FTC | 1 | |
| NVP; 3TC; D4T | 2 | |
| NFV; 3TC; D4T | 1 | |
| IDV; 3TC; D4T | 3 | |
| IDV; AZT; 3TC | 4 | |
| IDV; RTV; 3TC; ABC | 3 | |
| LPV; RTV; 3TC; ABC | 1 | |
| LPV; RTV; 3TC; D4T | 1 | |
| LPV; RTV; TNV; FTC | 2 | |
| ATZ; RTV; TNV; FTC | 1 | |
| EFV; IDV; 3TC; ABC | 1 | |
| CD4 lymphocyte count closest to treatment initiation (−15 to +1 days)d | Not described for overall cohort | |
| Absolute CD4+ T-cell count, cells/mm3 | 576 (365–779) | |
| CD4 percentage | 32 (24–42) | |
| HIV-1 RNA, copies/ml | ||
| At earliest time point in primary infection | 505, 412 (26,600–3,717,000) | 86, 807 (17,130–483,690) |
| Closest to treatment initiation (−15 to +1 days) | 119, 807 (12,588–635,000) | Not described for overall cohort |
Data are n (%) or median (IQR).
Fiebig I/II indicates that a distinction could not be made between Fiebig I and Fiebig II as p24 antigen results were not available for the relevant time point.
Fiebig V/VI indicates that a distinction could not be made between Fiebig V and Fiebig VI as western blot bands were not available for the relevant time point.
Data available for 21/22 evaluable and 294/367 other subjects. Among the other subjects, 2 subjects had both HLA B35 and B57, and 1 subject had both HLA B27 and HLA B57 alleles.
Data available for 21/22 evaluable subjects. One subject did not have any CD4 data available until 11 days after treatment initiation. ABC, abacavir; ATZ, atazanavir; ART, antiretroviral therapy; ARV, antiretroviral(s); d4T, stavudine; EFV, efavirenz; FTC, emtricitabine; IDU, injection drug use; IDV, indinavir; LPV, lopinavir; MSM, men who have sex with men; NFV, nelfinavir; NNRTI, non-nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; PIC, Primary Infection Clinic; RTV, ritonavir; TNV, tenofovir disoproxil fumarate; 3TC, lamivudine.
Four (18%) of 22 evaluable subjects began treatment at Fiebig Stage I or II [9]. Following initiation of ART, the 22 subjects were treated for a median of 28.4 (IQR 19.5–37.9) months and then followed off-treatment for a median of 19.0 (IQR 7.2–40.6) months. The reasons for treatment discontinuation included: structured treatment interruption in the setting of a clinical trial (n=4) or as subject decision under clinical care (n=12), lack of access to medical care/insurance coverage (n=3) or antiretroviral side effects (n=3). Twenty-one (95%) of the 22 subjects had loss of viral control (HIV-1 RNA>500 copies/ml) within 18 months after treatment discontinuation (Figure 2). Virological failure occurred in 13 (59.1%) within 3 months, 19 (86.4%) within 6 months, 20 (90.9%) within 9 months, and 21 (95.4%) within 18 months. The median time to virological failure was 2.17 (IQR 1.18–3.39) months. There was a trend toward more rapid loss of virological control among subjects having either HLA B35 (P=0.13) or severe seroconversion symptoms (P=0.076). Within 3 months 5/7 (71%) with HLA B35 and 7/14 (50%) without HLA B35 experienced loss of virological control. Similarly, within 3 months 7/11 (64%) with severe seroconversion symptoms and 6/11 (54%) without severe symptoms experienced loss of virological control. Only 1 subject (4.5%, 95% CI 0.32, 18.9) controlled viral load to levels <500 copies/ml for at least 24 months. This subject is known to be wild-type for CCR5 and does not have HLA type B27 or B57. However, he did have other factors in PHI associated with a favourable prognosis: he was asymptomatic and his pre-therapy HIV-1 RNA was 2, 248 copies/ml within a week after having an indeterminate western blot (Fiebig IV) [9]. This subject is shown with id 63792 in Figure 2: virological control with RNA<500 copies/ml was maintained for 3 years after treatment discontinuation, with subsequent values similar to pre-therapy levels (1,038–3,737 copies/ml) for the ensuing 3 years.
Figure 2.
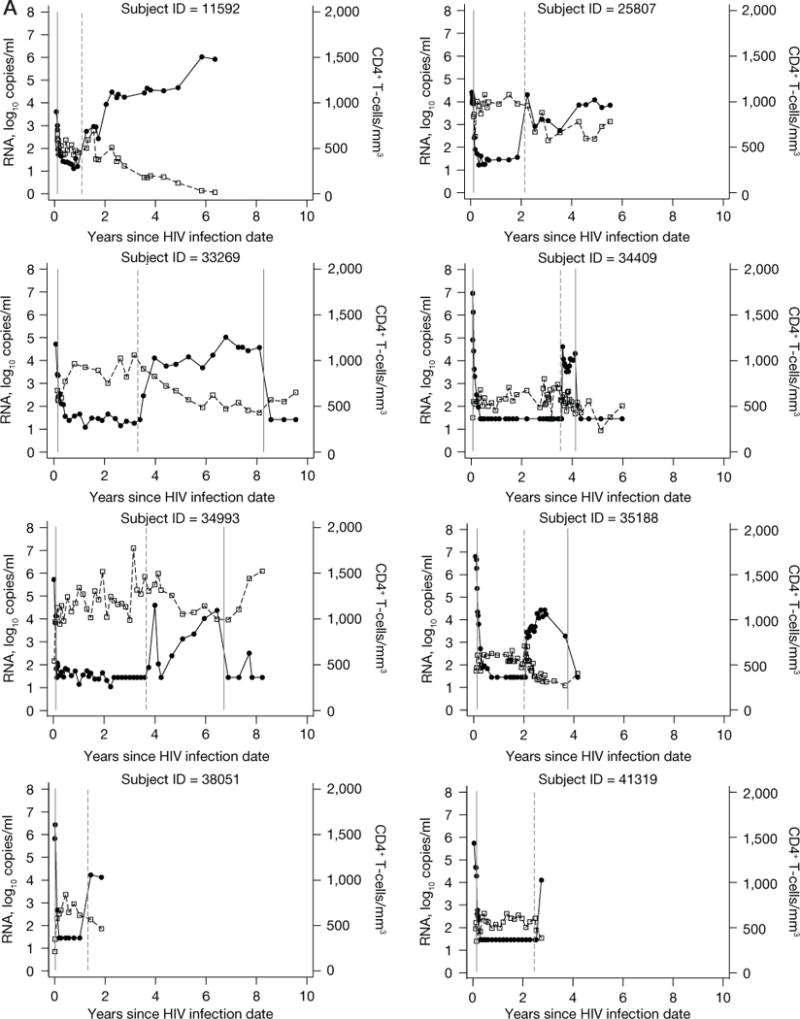
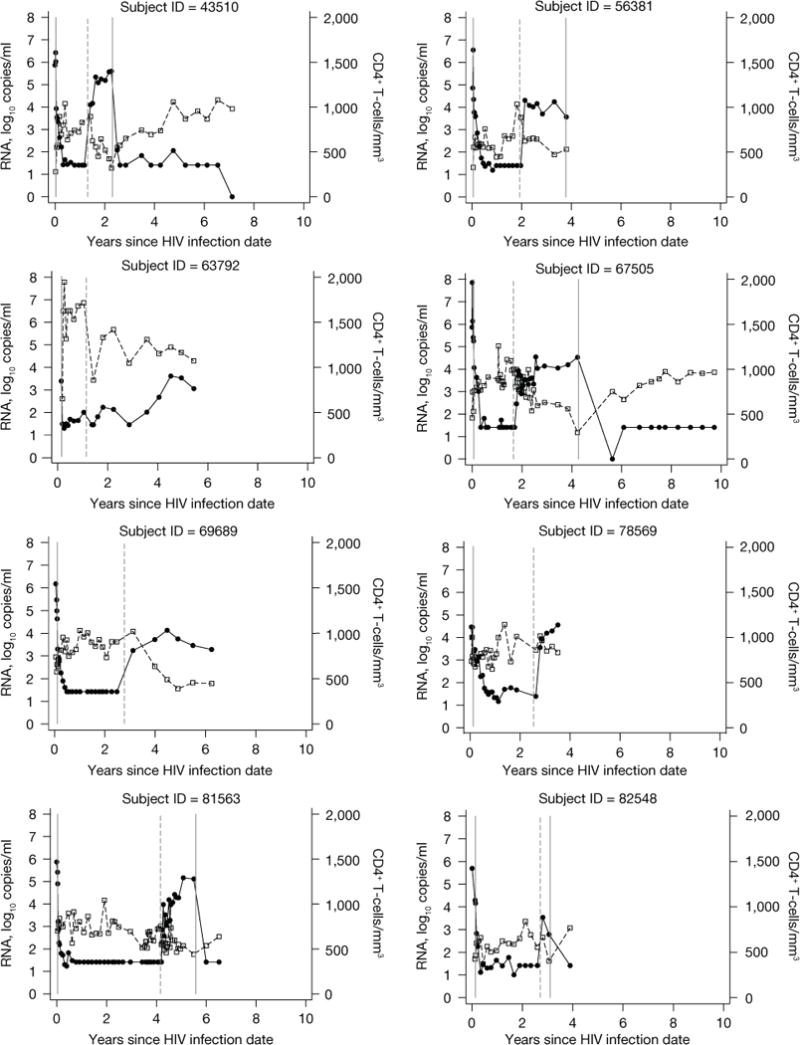
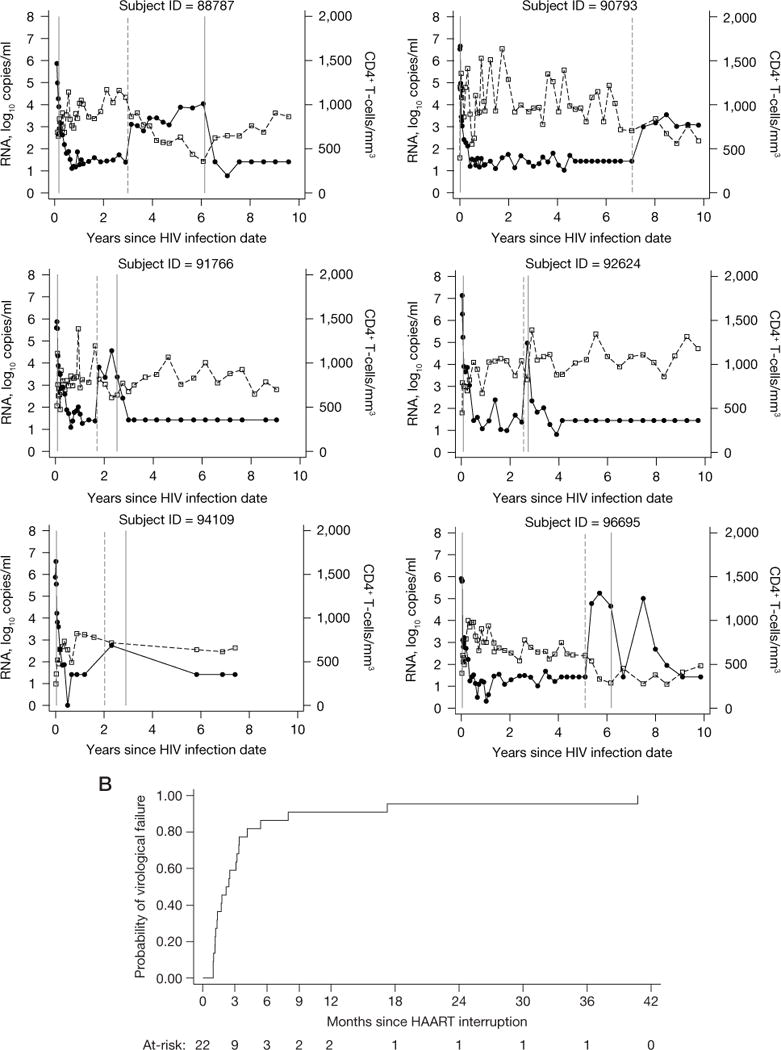
Data from 22 subjects evaluated for post-treatment control and Kaplan–Meier curve showing the cumulative probability of virological failure
(A) Data from 22 subjects evaluated for post-treatment control are shown. HIV-1 RNA data shown as closed circles. CD4 lymphocyte data shown as open squares. The first solid vertical line indicates highly active antiretroviral therapy (HAART) initiation in all subjects. The dashed vertical line indicates HAART interruption. The last solid vertical line indicates treatment re-initiation (for those subjects who re-started antiretroviral therapy while still in follow-up). (B) A Kaplan–Meier curve showing the cumulative probability of virological failure. The number of subjects at-risk at each time point is shown below the figure.
Several of the evaluable subjects had some degree of virological control not meeting the stringent criterion of <500 copies/ml, including one subject with HIV-1 RNA<1,000 copies/ml for 7 months before losing control, another who had a pretreatment HIV-1 RNA value of 4.4 million copies/ml and showed post-treatment levels of <4,000 copies/ml for the nearly 4 years he was followed, and a third who had a pretreatment HIV-1 RNA of 750,000 copies/ml and post-treatment levels <3,000 copies/ml for nearly 2 years until RNA increased and he restarted potent ART. Of interest as well is a subject with an initial post-treatment RNA increase to 37,345 copies/ml, followed by evidence of substantial post-treatment control (<300 copies/ml) at three time points over 7 months before RNA increased and potent ART was subsequently restarted.
Our last observation relates to four subjects who had frequent HIV-1 RNA sampling done after treatment discontinuation in the context of a therapeutic vaccination protocol [10]. Each of these subjects (two vaccine and two placebo recipients) showed HIV-1 RNA levels of under 50 copies/ml for 2–4 weeks off treatment, and then a rebound to detectable levels. These data are consistent with that from studies of patients with established HIV infection that typically demonstrate trajectories of viral rebound within 2–4 weeks of treatment interruption [11,12].
Overall, amongst the 21 subjects who did not maintain control, 7 did not re-start treatment over a median duration of additional follow-up of 40.6 (6.4–44.0) months prior to censoring. The remaining 14 re-initiated treatment at a median time of 15.1 (7.2–31.4) months from the time of treatment discontinuation.
Discussion
The VISCONTI cohort has generated renewed excitement about the possibility of virological control after ART started during primary infection [1]. Review of our primary infection cohort identified a smaller proportion of individuals who met the definition of a PTC; however, the 95% CI for our data contains the percentage (15%) of treated patients observed by the ANRS VISCONTI report to be able to control viraemia for at least 24 months. Given our single controller’s relatively low HIV-1 RNA levels during primary infection, however, we question whether he might have become a natural controller, regardless of initiation of early therapy. Nonetheless, under the scenario where this subject is considered non-evaluable and therefore excluded, then all subjects lose virological control by 24 months and the upper bound of the exact binomial 95% CI is 16.1%, remaining consistent with the ANRS VISCONTI data [1]. We also conducted an analysis to determine whether our conclusions would be affected by including subjects who started therapy slightly later, up to 20 weeks, into primary infection. This subsequent analysis identified 7 additional subjects, such that among all 29 subjects, the percentage of those who controlled HIV-1 RNA to levels <500 copies/ml for at least 24 months was 6.9% (95% CI 1.2, 19.8). The only additional subject who had features of post-treatment control in this expanded analysis similarly showed features that might be associated with natural control, including low HIV-1 RNA (3,428 copies/ml) during primary infection and HLA B57.
Our findings highlight the difficulty of assessing outcomes of very early therapy even within a relatively large primary infection cohort. Specifically, even within a cohort of this size, there were only a small number of individuals treated within the earliest stages of primary infection and only a small proportion of those subsequently discontinued antiretrovirals. Our data are then limited by the fact that the cohort is followed through an observational study and has variable censoring.
Nonetheless, important implications can be derived from the data collected across primary infection studies [1,13–18] suggesting that treatment of HIV-1 infection during the very earliest stages of infection may impact immunological responses and limit the development of viral reservoirs. Given the relevance of reservoir development to possible functional cure, additional data from our cohort and others should help clarify why a limited subset of those treated during primary infection show evidence of post-treatment control. Understanding the defining characteristics of this subset could prove key in strategies to control viraemia and provide a path toward functional cure of HIV infection.
Acknowledgments
The authors would like to thank the Primary Infection Clinic subjects who participated in this project, Terri Smith for data management, and our many past and current collaborators in the Seattle Primary Infection Program. Grant support for this project was provided by NIH P01 AI057005 and the University of Washington Center for AIDS Research (P30 AI027757).
Footnotes
Disclosure statement
All authors declare no competing interests.
References
- 1.Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI study. PLoS Pathog. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaslow RA, Carrington M, Apple R, et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 3.Migueles SA, Sabbaghian MS, Shupert WL, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A. 2000;97:2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Schacker TW, Hughes JP, Shea T, Coombs RW, Corey L. Biological and virologic characteristics of primary HIV infection. Ann Intern Med. 1998;128:613–620. doi: 10.7326/0003-4819-128-8-199804150-00001. [DOI] [PubMed] [Google Scholar]
- 6.Stekler JD, Wellman R, Holte S, et al. Are there benefits to starting antiretroviral therapy during primary HIV infection? Conclusions from the Seattle Primary Infection Cohort vary by control group. Int J STD AIDS. 2012;23:201–206. doi: 10.1258/ijsa.2011.011178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrington M, O’Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–551. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- 8.Itescu S, Mathur-Wagh U, Skovron ML, et al. HLA-B35 is associated with accelerated progression to AIDS. J Acquir Immune Defic Syndr. 1992;5:37–45. [PubMed] [Google Scholar]
- 9.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg ES, Graham BS, Chan ES, et al. Safety and immunogenicity of therapeutic DNA vaccination in individuals treated with antiretroviral therapy during acute/early HIV-1 infection. PLoS ONE. 2010;5:e10555. doi: 10.1371/journal.pone.0010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davey RT, Jr, Bhat N, Yoder C, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J. Analysis of analytic treatment interruption in past ACTG studies. Symposium II, Intensely monitored antiretroviral pause. Annual ACTG Network Meeting; 23–24 June 2014; Washington, DC, USA. [Google Scholar]
- 13.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 14.Ngo-Giang-Huong N, Deveau C, Da Silva I, et al. Proviral HIV-1 DNA in subjects followed since primary HIV-1 infection who suppress plasma viral load after one year of highly active antiretroviral therapy. AIDS. 2001;15:665–673. doi: 10.1097/00002030-200104130-00001. [DOI] [PubMed] [Google Scholar]
- 15.Lodi S, Meyer L, Kelleher AD, et al. Immunovirologic control 24 months after interruption of antiretroviral therapy initiated close to HIV seroconversion. Arch Intern Med. 2012;172:1252–1255. doi: 10.1001/archinternmed.2012.2719. [DOI] [PubMed] [Google Scholar]
- 16.Williams JP, Hurst J, Stohr W, et al. HIV-1 DNA predicts disease progression and post-treatment virological control. eLIFE. 2014;3:e03821. doi: 10.7554/eLife.03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ananworanich J, Schuetz A, Vandergeeten C, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS ONE. 2012;7:e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott-Algara D, Didier C, Arnold V, et al. Post-treatment controllers have particular NK cells with high anti-HIV capacity: VISCONTI study. Conference on Retroviruses and Opportunistic Infections; 23–26 February 2015; Seattle, WA, USA. Abstract 52. [Google Scholar]


