Abstract
BACKGROUND
Current US Preventive Services Task Force and other guidelines recommend low-dose aspirin for all pregnant women with pregestational diabetes mellitus to prevent preeclampsia and small-for-gestational-age birth. The Maternal-Fetal Medicine Units High-Risk Aspirin trial did not show a reduction in either preeclampsia or small-for-gestational-age birth in diabetic women.
OBJECTIVE
Our objective was to reassess the impact of aspirin on fetal growth in diabetic pregnancies overall and according to White classification. We hypothesized that aspirin improves fetal growth in pregnancies with vascular complications of diabetes at highest risk for poor fetal growth.
STUDY DESIGN
We conducted secondary analysis of the cohort of diabetic women enrolled in the Maternal-Fetal Medicine Units High-Risk Aspirin trial. The impact of aspirin prophylaxis on birthweight was assessed in the overall cohort and in 2 groups categorized according to White classification as nonvascular (White class B, C, D) or vascular (White class R, F, RF). Birthweight was converted to Z-score normalized for gestational age at delivery and neonatal sex. Difference in birthweight Z-score between aspirin and placebo was tested with a 2-sample t test. The effect of vascular group, aspirin vs placebo randomization, and the interaction of the 2 on normalized birthweight percentile was estimated with linear regression with a multivariable model including covariates body mass index, tobacco use, race, and parity. The percentage of small and large-for-gestational-age newborns born to aspirin- vs placebo-treated women was compared between groups using Pearson exact χ2 analysis, and an adjusted model was estimated by logistic regression.
RESULTS
All 444 women with pregestational diabetes and complete outcome data were included (53 vascular, 391 nonvascular). Aspirin was significantly associated with a higher birthweight Z-score (0.283; 95% confidence interval, 0.023–0.544) in the overall cohort (P = .03). In the adjusted model, the association of aspirin with higher birthweight Z-score was confined to neonates of women with nonvascular diabetes (0.341; 95% confidence interval, 0.677–0.006; P = .044). An opposite but nonsignificant effect was observed among neonates from women with vascular diabetes (−0.416; 95% confidence interval, −1.335 to 0.503; P = .6). This difference in the relationship of aspirin and birthweight Z-score by vascular group was significant at P = .046. Aspirin-randomized women with nonvascular diabetes had more large-for-gestational-age births than those treated with placebo (40.2 vs 26.6%; P = .005). Small-for-gestational-age births occurred at the same frequency with aspirin vs placebo randomization in the overall cohort (8% in each group) and in each vascular group.
CONCLUSION
Inconsistent with our hypothesis, aspirin did not reduce small-for-gestational-age births in the overall cohort or either group. The increased incidence of large-for-gestational-age infants in aspirin-treated diabetic gestations is of potential concern given the known increased maternal and neonatal morbidity associated with macrosomia.
Keywords: aspirin, diabetes, fetal growth, large for gestational age, macrosomia
Introduction
In late 2014, the US Preventive Services Task Force (USPSTF) recommended that low-dose aspirin (60–150 mg/d) be administered to all pregnant women with pregestational type 1 or type 2 diabetes in an effort to reduce the incidence of preeclampsia.1 Additional proposed benefits of aspirin administration are reductions in preterm birth and small-for-gestational-age (SGA) birth. These USPSTF recommendations are in agreement with previously published guidelines from the World Health Organization (WHO)2 and the National Institute for Health and Care Excellence,3 both of which also specifically recommend aspirin for pregnant women with pregestational diabetes (PGDM). The American College of Obstetricians and Gynecologists endorsed these guidelines in 2016.4
PGDM is a known risk factor for preeclampsia.5 The impact of PGDM on fetal growth is more complex and relates to White classification. For example, in a recent report, patients with vascular complications (White classifications R, F, RF, and H) were at increased risk for SGA births (17%), while those with White classification B, C, and D had increased rates of large-for-gestational-age (LGA) newborns (34%, 28%, and 21%, respectively).6
While aspirin may reduce the risk of SGA in pregnancies at risk for preeclampsia in general, the impact of aspirin on fetal growth in diabetic pregnancies is not well understood. Of the 13 studies that formed the basis of the USPSTF conclusion that aspirin reduces SGA, only the Maternal-Fetal Medicine Units (MFMU) High-Risk Aspirin (HRA) trial enrolled women with PGDM.7 In this trial, aspirin was of no benefit in reducing either preeclampsia or SGA. Most other trials explicitly excluded women with PGDM.
To better understand the possible impact of aspirin on fetal growth in patients with PGDM, we performed a secondary analysis of the MFMU HRA trial. Our hypothesis was that aspirin would reduce the risk of SGA in women with vascular complications of diabetes.
Materials and Methods
We performed a secondary analysis of the MFMU Network randomized controlled trial of aspirin (60 mg) for the prevention of preeclampsia in high-risk women.7 The original inclusion criteria were pregnancies with at least 1 risk factor for preeclampsia: preexisting insulin-dependent diabetes, chronic hypertension, multiple gestation, or preeclampsia in a previous pregnancy. Women were enrolled into 1 of 4 mutually exclusive high-risk groups defined as: (1) diabetes (with or without prior preeclampsia or chronic hypertension), (2) chronic hypertension (with or without prior preeclampsia), (3) multifetal gestation (with or without prior preeclampsia), and (4) previous preeclampsia (and no other risk factor). The protocol received institutional review board approval at each center, and all participating women provided written informed consent. This secondary analysis was considered exempt by the Colorado Multiple Institutional Review Board.
Full details of the study design are available in the original article. Briefly, enrollment of eligible women occurred in the calendar years 1991 through 1995 during the 13th through 26th week of pregnancy. Women were randomized 1:1 to receive aspirin (60 mg) or placebo in a double-blind, randomized, placebo-controlled trial design to assess the impact of aspirin on the incidence of preeclampsia. Adherence to study drug regimen was measured by questioning of women, counting pills, and measuring thromboxane in serum.
For this analysis, we included all women in the MFMU HRA study with PGDM for whom complete outcome data were available. Women with PGDM and chronic hypertension were class D in accordance with the revised White classification.8
Women were assigned to 1 of 2 mutually exclusive vascular groups: nonvascular (White class B, C, D) or vascular (White class R, F, RF). We used a previously published algorithm for transforming birthweight into a normalized Z-score for gestational age at delivery and neonatal sex.9 From Z-scores, neonates were categorized as SGA, average for gestational age, or LGA.
Body mass index (BMI) calculated from prepregnancy weight was categorized according to WHO classifications with <18.5 kg/m2 underweight, 18.5–24.9 kg/m2 normal, 25.0–29.9 kg/m2 overweight, and ≥30 kg/m2 obese. For some analyses, underweight and normal BMI categories were combined because of the low frequency of women categorized as underweight.
Statistical Methods
The success of randomization within this study population was assessed by comparing demographics between placebo- and aspirin-randomized women, with differences tested using χ2 for categorical and 2-sample t tests for continuous measures.
The effect of vascular group, aspirin vs placebo randomization, and the interaction of the 2 on normalized birthweight percentile was estimated using linear regression with a multivariable model including covariates expected to impact birthweight based on clinical judgement: BMI category, tobacco use, race, and parity. The interaction of vascular group and randomization was parameterized as part of our primary hypothesis.
The percentage of SGA and LGA newborns born to aspirin- vs placebo-treated women was compared between groups using Pearson exact χ2 analysis, and a logistic regression model was estimated to adjust for covariates expected to impact birthweight based on clinical judgement: maternal BMI category, smoking, race, and parity. The adjusted estimates for the overall cohort include vascular group as a covariate but without an interaction with randomization.
In a sensitivity analysis, we assessed the robustness of results on normalized birthweight when gestational age at randomization was week <17 vs week ≥17.
All analyses were intent to treat, using software (SAS 9.4; SAS Institute Inc, Cary, NC). Graphic results were created using software (GraphPad Prism 6.07; GraphPad, La Jolla, CA).
Results
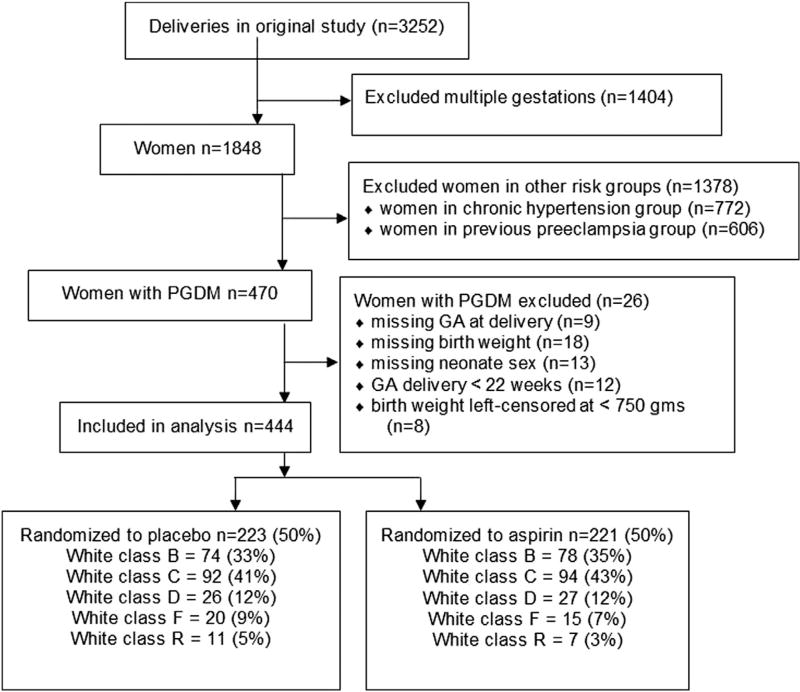
Of the 444 women with PGDM enrolled in the HRA trial, 391 had nonvascular diabetes (White class B, C, or D) and 53 had vascular diabetes (White class R, F, or RF) as shown in Figure 1. Demographics for randomization groups and vascular and nonvascular groups are tabulated in the Table. Women with vascular diabetes were less likely to be parous (P <.001). Black race was more common among women with nonvascular PGDM. There were no significant differences in other demographic characteristics including age, BMI, and tobacco use between vascular and nonvascular groups (Table).
FIGURE 1.
Patient selection from Maternal-Fetal Medicine Units High-Risk Aspirin trial
GA, gestational age; PGDM, pregestational diabetes.
Adkins et al. Aspirin and fetal growth in diabetic pregnancies. Am J Obstet Gynecol 2017.
TABLE.
Demographic characteristics by randomization and vascular diabetes categorization
| Characteristic | Aspirin n = 221 |
Placebo n = 223 |
P | Vascular n = 53 |
Nonvascular n = 391 |
P |
|---|---|---|---|---|---|---|
| Parous | 107 (48.4) | 114 (51.1) | .569 | 15 (28.3) | 206 (52.7) | <.001 |
| Age, y | ||||||
| <35 | 204 (92.7) | 197 (88.3) | .115 | 50 (94.3) | 351 (90.0) | .312 |
| ≥35 | 16 (7.3) | 26 (11.7) | 3 (5.7) | 39 (10.0) | ||
| Body mass index | ||||||
| Underweight | 3 (1.4) | 3 (1.4) | .712 | 1 (1.9) | 5 (1.3) | .672 |
| Normal | 103 (46.8) | 95 (43.2) | 25 (47.2) | 173 (44.7) | ||
| Overweight | 44 (20.0) | 54 (24.6) | 14 (26.4) | 84 (21.7) | ||
| Obese | 70 (31.8) | 68 (30.9) | 13 (24.5) | 125 (32.3) | ||
| Black race | 89 (40.2) | 86 (38.6) | .713 | 13 (24.5) | 162 (41.4) | .018 |
| Education ≤12 y | 130 (58.8) | 144 (64.6) | .213 | 29 (54.7) | 245 (62.7) | .264 |
| Gestational age, wk, at randomization, mean (SD) | 18.5 (0.3) | 18.1 (0.3) | .329 | 18.4 (0.6) | 18.3 (0.2) | .878 |
| Chronic hypertension | 21 (9.5) | 30 (13.5) | .192 | 16 (30.2) | 35 (9.0) | <.001 |
| Cigarette smoking | ||||||
| Never | 155 (70.1) | 150 (67.6) | .626 | 39 (73.6) | 266 (68.2) | .564 |
| Quit during pregnancy | 18 (8.1) | 24 (10.8) | 3 (5.7) | 39 (10.0) | ||
| Yes, current | 48 (21.7) | 48 (21.6) | 11 (20.8) | 85 (21.8) | ||
| White classification | ||||||
| B | 78 (35.3) | 74 (33.2) | .783 | 0 (0.0) | 152 (38.9) | |
| C | 94 (42.5) | 92 (41.3) | 0 (0.0) | 186 (47.6) | ||
| D | 27 (12.2) | 26 (11.7) | 0 (0.0) | 53 (13.6) | ||
| F | 15 (6.8) | 20 (9.0) | 35 (66.0) | 0 (0.0) | ||
| R | 7 (3.2) | 11 (4.9) | 18 (34.0) | 0 (0.0) |
Values are n (%) unless otherwise noted.
Adkins et al. Aspirin and fetal growth in diabetic pregnancies. Am J Obstet Gynecol 2017.
Aspirin was significantly associated with a higher birthweight Z-score (0.283; 95% confidence interval [CI], 0.023–0.544) in the overall cohort (P = .03). In the adjusted model, the association of aspirin with higher birthweight Z-score was confined to neonates of women with nonvascular diabetes (0.341; 95% CI, 0.677–0.006; P =.044). An opposite but nonsignificant effect was observed among neonates from women with vascular diabetes (−0.416; 95% CI, −1.335 to 0.503; P = .6). This difference in the relationship of aspirin and birthweight Z-score by vascular group was significant at P = .046 (Figure 2). As 198 participants (45%) were enrolled week <17, and 246 (55%) were enrolled week ≥17, sensitivity analysis was conducted to explore the existence of an impact of gestational age at randomization on these findings. There was a negligible difference in the impact of aspirin on birthweight Z-score for vascular vs nonvascular diabetics who enrolled <17 vs ≥17 weeks; the 3-way interaction term was not significant (P = .4, data not shown).
FIGURE 2.
Effect of aspirin on adjusted birthweight Z-scores for pregestational diabetic gestations overall and by vascular group
Adkins et al. Aspirin and fetal growth in diabetic pregnancies. Am J Obstet Gynecol 2017.
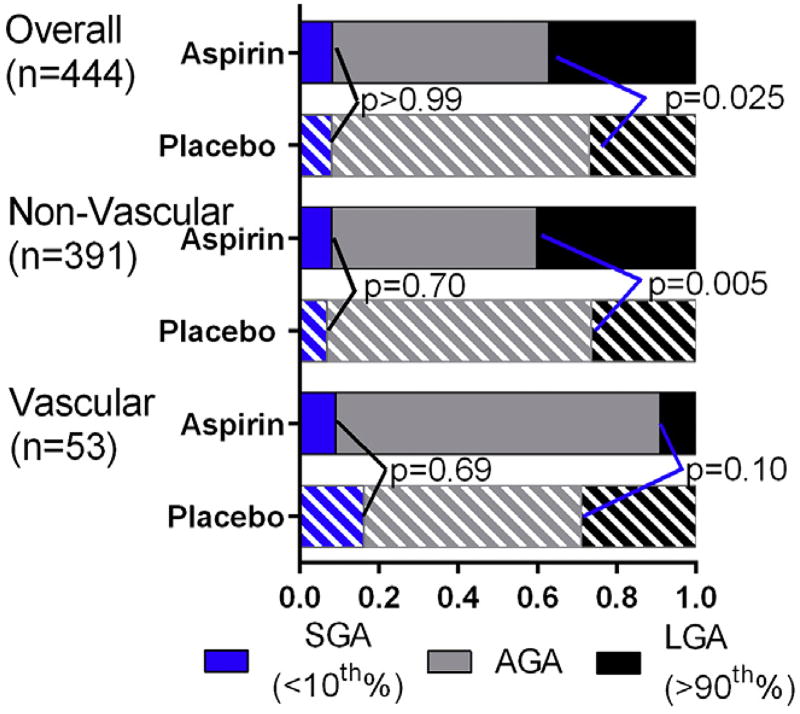
Among all gestations included in the analysis, 32% (n = 142) were LGA and 8% (n = 36) were SGA. There were significantly more LGA births among aspirin-treated pregnancies as compared to those randomized to placebo (37% vs 27%, P = .025). For the nonvascular diabetics, those treated with aspirin had significantly more LGA births (40.2%) compared to those in the placebo group (26.6%) (P = .005). Among women with vascular diabetes, the difference in rates of LGA birth between the aspirin (9%) and placebo (29%) groups was not significant (P = .10). Aspirin was not significantly associated with differences in SGA in the nonvascular group (8% with aspirin, 7% with placebo, P =.70), the vascular group (9% with aspirin, 16% with placebo, P = .69), or in the overall cohort (8% with both aspirin and placebo) (Figure 3).
FIGURE 3.
Effect of aspirin on proportions of small-for-gestational-age (SGA), appropriate-for-gestational-age (AGA), and large-for-gestational-age (LGA) infants in unadjusted model
Adkins et al. Aspirin and fetal growth in diabetic pregnancies. Am J Obstet Gynecol 2017.
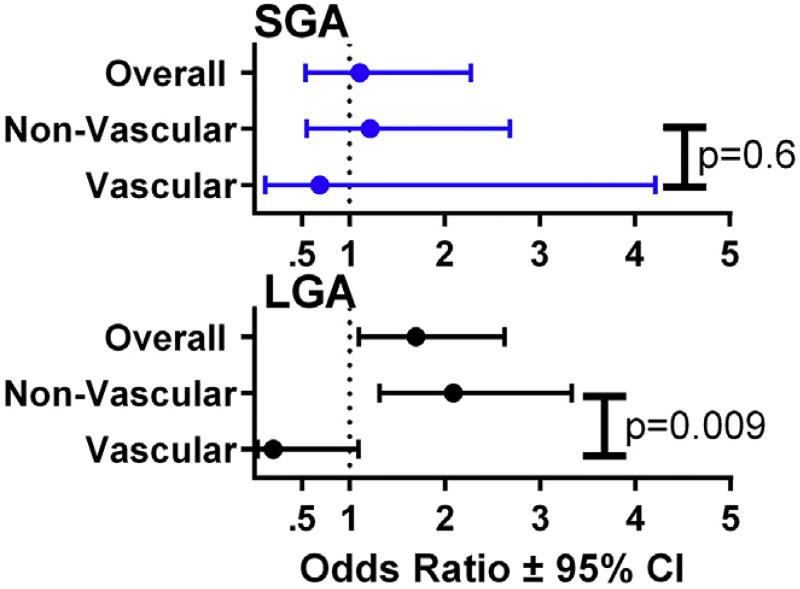
In a multivariate analysis adjusted for BMI, tobacco use, race, and parity, the association of aspirin with more LGA births persisted among women in the overall cohort (odds ratio [OR], 1.696; 95% CI, 1.096–2.625) and in the nonvascular group (OR, 2.09; 95% CI, 1.31–3.33). Aspirin was not associated with a significant difference in SGA in the overall cohort or within either vascular group in the adjusted analysis (overall cohort OR, 1.11; 95% CI, 0.54–2.28, nonvascular OR, 1.22; 95% CI, 0.55–2.69, vascular OR, 0.69; 95% CI, 0.113–4.22) (Figure 4).
FIGURE 4.
Effect of aspirin on adjusted odds ratios for small-for-gestational-age (SGA) vs large-for-gestational-age (LGA) infants overall and by vascular group
CI, confidence interval.
Adkins et al. Aspirin and fetal growth in diabetic pregnancies. Am J Obstet Gynecol 2017.
Comment
While aspirin was associated with an increase in fetal growth in this cohort of women with PGDM, this effect was due to an increase in LGA birth, and this effect was confined to women with nonvascular PGDM already at risk for fetal overgrowth. Contrary to our hypothesis, aspirin did not improve fetal growth in vascular PGDM pregnancies at highest risk for SGA, nor did it reduce SGA in the nonvascular group or the overall cohort. These observations have potential importance in further refining what populations might benefit from aspirin prophylaxis. Macrosomia is one of the central complications of diabetes in pregnancy. Given the increased maternal and neonatal morbidity associated with LGA birth these observations are of concern.10–13
The strengths of this study are the prospective data collection that occurred at the time of the original trial by trained research nurses. Our primary outcome of gestational age-specific birthweight percentiles that relies on the objective measures of gestational age at delivery and birthweight allows rigorous comparison. Our results were robust to gestational age at enrollment. We are limited in assessing aspirin vs placebo within the vascular diabetic group because of the low frequency of vascular diabetes, thus seemingly large trends towards lower risk of both SGA and LGA with aspirin occur in the absence of statistical significance.
An additional limitation of our study is our inability to report results for women according to type 1 vs type 2 diabetes or according to medications used to treat diabetes in pregnancy, as these variables were not captured in the original data set. Women with type 2 diabetes as a group may be more likely to be obese, an additional risk factor for LGA birth. However, we controlled for maternal BMI in our adjusted analysis and our findings persisted. In addition, as a secondary analysis of a randomized controlled trial, we would anticipate that type 1 and type 2 diabetics would be evenly allocated between treatment groups. The recent study by Bennett et al6 confirms the strong association between birthweight and White classification. Although not a primary outcome of this study, we also found, among placebo-randomized women, a higher proportion of LGA births in the nonvascular group (26%) and a higher proportion of SGA births in the vascular group (16%). Together, these observations lend support to our vascular vs nonvascular grouping as biologically valid. We are unaware of similar data on abnormal growth as stratified by type 1 vs type 2 PGDM.
The observation that aspirin increased the chance of LGA in the overall cohort and nonvascular PGDM gestations must be interpreted with caution. As an unplanned secondary analysis, such an observation should be viewed as hypothesis-generating. Is this a biologically plausible hypothesis? Fetal growth is a complex interaction of genetics, growth factors, substrate availability, and other factors. Given that PGDM pregnancies are at risk for preeclampsia,5 a disease characterized by altered placental perfusion,14,15 and given that aspirin acts by improving placental perfusion (among other mechanisms),16,17 we submit that aspirin could further augment the already increased substrate availability in PGDM pregnancies and further augment fetal growth.
As aspirin has gained increased support as prophylaxis for preeclampsia, SGA and preterm birth, the inclusion criteria for prophylaxis have also broadened. However, the inclusion of women with PGDM in these recommendations may not be evidence-based, beyond the mere fact that PGDM patients are at increased risk for preeclampsia. The MFMU HRA trial is the only aspirin intervention study to specifically include women with PGDM, and most other trials specifically excluded these women. The MFMU HRA trial did not show a reduction in preeclampsia or SGA overall and there was no reduction in preeclampsia in the PGDM subgroup in particular. Our failure to detect a benefit (no reduction in SGA) and finding of potential harm (increased risk of LGA) thus provide incremental information regarding the appropriate use of aspirin in pregnancy. These findings may warrant further investigation as well as consideration in future recommendations regarding the use of aspirin in pregnancy.
Acknowledgments
The authors appreciate the assistance of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and Maternal-Fetal Medicine Units (MFMU) Network in making the database from the MFMU High-Risk Aspirin trial available for secondary analysis.
Dr Metz is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) under award no. 2K12HD001271-16. The contents of this report represent the views of the authors and do not necessarily represent the views of the NICHD Maternal-Fetal Medicine Units Network or the National Institutes of Health.
Footnotes
The authors report no conflict of interest.
Presented at poster session III of the 37th Annual Pregnancy Meeting of the Society for Maternal-Fetal Medicine, Las Vegas, NV, Jan. 27, 2017.
References
- 1.LeFevre ML, US Preventive Services Task Force Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:819–26. doi: 10.7326/M14-1884. [DOI] [PubMed] [Google Scholar]
- 2.Organization WH. WHO recommendations for prevention and treatment of preeclampsia and eclampsia. Geneva: World Health Organization; 2014. [Google Scholar]
- 3.Redman CW. Hypertension in pregnancy: the NICE guidelines. Heart. 2011;97:1967–9. doi: 10.1136/heartjnl-2011-300949. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists. Practice advisory on low-dose aspirin and prevention of preeclampsia: updated recommendations. [Accessed July 12, 2017];2016 Available at: https://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-Low-Dose-Aspirinand-Prevention-of-Preeclampsia-Updated-Recommendations.
- 5.Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160:695–703. doi: 10.7326/M13-2844. [DOI] [PubMed] [Google Scholar]
- 6.Bennett SN, Tita A, Owen J, Biggio JR, Harper LM. Assessing White’s classification of pregestational diabetes in a contemporary diabetic population. Obstet Gynecol. 2015;125:1217–23. doi: 10.1097/AOG.0000000000000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338:701–5. doi: 10.1056/NEJM199803123381101. [DOI] [PubMed] [Google Scholar]
- 8.Hare JW, White P. Gestational diabetes and the White classification. Diabetes Care. 1980;3:394. [Google Scholar]
- 9.Talge NM, Mudd LM, Sikorskii A, Basso O. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics. 2014;133:844–53. doi: 10.1542/peds.2013-3285. [DOI] [PubMed] [Google Scholar]
- 10.Nesbitt TS, Gilbert WM, Herrchen B. Shoulder dystocia and associated risk factors with macrosomic infants born in California. Am J Obstet Gynecol. 1998;179:476–80. doi: 10.1016/s0002-9378(98)70382-5. [DOI] [PubMed] [Google Scholar]
- 11.Oral E, Cagdas A, Gezer A, Kaleli S, Aydinli K, Ocer F. Perinatal and maternal outcomes of fetal macrosomia. Eur J Obstet Gynecol Reprod Biol. 2001;99:167–71. doi: 10.1016/s0301-2115(01)00416-x. [DOI] [PubMed] [Google Scholar]
- 12.Stotland NE, Caughey AB, Breed EM, Escobar GJ. Risk factors and obstetric complications associated with macrosomia. Int J Gynaecol Obstet. 2004;87:220–6. doi: 10.1016/j.ijgo.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Weissmann-Brenner A, Simchen MJ, Zilberberg E, et al. Maternal and neonatal outcomes of large for gestational age pregnancies. Acta Obstet Gynecol Scand. 2012;91:844–9. doi: 10.1111/j.1600-0412.2012.01412.x. [DOI] [PubMed] [Google Scholar]
- 14.Campbell S. Placental vasculature as visualized by 3D power Doppler angiography and 3D color Doppler imaging. Ultrasound Obstet Gynecol. 2007;30:917–20. doi: 10.1002/uog.5195. [DOI] [PubMed] [Google Scholar]
- 15.Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol. 2015;213:S115–22. doi: 10.1016/j.ajog.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179:1359–75. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 17.Walsh SW. Physiology of low-dose aspirin therapy for the prevention of preeclampsia. Semin Perinatol. 1990;14:152–70. [PubMed] [Google Scholar]