Abstract
Pili are filamentous bacterial structures that promote adhesion to host cells. It emerges that a small molecule that inhibits this adhesion can prevent colonization of the mouse gut by a pathogenic bacterium.
The mammalian intestine is a natural habitat for trillions of bacteria, including harmless species called commensals and potentially pathogenic species. However, long-term persistence is a challenge for these resident bacteria, which must resist expulsion by waves of peristalsis — the process that pushes the intestinal contents downstream. The ways in which pathogenic bacteria adhere to host cells at sites of infection have been extensively investigated1, but little is known about how commensal bacteria cling to mucosal surfaces in the gut. Spaulding et al.2 report on page 528 that uropathogenic Escherichia coli (UPEC), a bacterium that is a commensal in the gut but pathogenic in the bladder, persists in the intestine thanks to filamentous protein complexes called pili that project from the bacterium and promote its adhesion to the gut wall. The authors also identify a sugar derivative that can combat pilus-mediated adhesion.
UPEC strains colonize the gut and are shed in faeces. If, from faeces, they gain access to the area around the urethra, the bacteria can work their way up the urethra, causing urinary-tract infections (UTIs). Preventing the colonization of the gut by these bacteria could therefore be a way to stop UTIs (particularly recurrent infections) from arising. Previous studies have demonstrated an essential role for pili in host–UPEC interactions at sites of infection such as the bladder and kidneys3. A type of pilus called the chaperone-usher pathway (CUP) pilus is widespread in bacteria4, including UPEC. Spaulding and colleagues therefore speculated that CUP pili might be involved in the colonization of the gut by UPEC.
To test this hypothesis, the authors generated mutant UPEC strains that lacked each of the nine CUP pili encoded by the bacterium’s genome, and tested the ability of these strains to colonize the mouse gut. Colonization was markedly reduced when either of two types of CUP pilus — type 1 and F17-like — was deleted. The type 1 pilus had previously been shown to be crucial for bladder colonization5, but its role in gut colonization is a new discovery. By contrast, the role of the F17-like pilus is restricted to the gut — mutant UPEC lacking F17-like pili could still colonize the bladder.
Pili promote host–pathogen adhesion through an adhesin protein at their tip, which binds to carbohydrates called glycans that are covalently linked to proteins or lipids on the host-cell surface. Using purified fragments of adhesins from the type 1 and F17-like pili, Spaulding et al. demonstrated that each pilus type binds to distinct host-protein gly-cans — type 1 adhesins bind to ‘N-linked’ glycans that contain the sugar D-mannose (Fig. 1a), and F17-like adhesins bind to ‘O-linked’ glycans. This differential binding diversifies and thereby optimizes UPEC adhesion to the epithelial cells lining the colon, enabling long-term colonization of the gut. The researchers also found that F17-like pili were produced by almost all of the UPEC strains isolated from a cohort of 14 women who had recurrrent UTIs, supporting the link between expression of these pili, UPEC persistence in the gut and the development of recurrent UTIs.
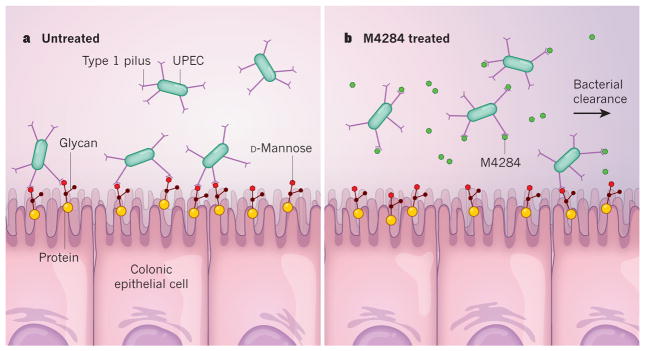
Figure 1. A small molecule clears pathogens from the gut.
a, Spaulding et al.2 report that adhesion of the bacterium uropathogenic Escherichia coli (UPEC) to epithelial cells that line the colon of its host is mediated by type 1 and F17-like pili (pili are filamentous protein complexes; F17-like pili are not shown). Type 1 pili bind carbohydrates called glycans that are linked to proteins on the host-cell surface and contain the sugar D-mannose (the glycan depicted here is a simplified schematic, and does not represent a real glycan structure). b, A small molecule called M4284 has a higher affinity for UPEC type 1 pili than does D-mannose. Spaulding and colleagues show that M4284 inhibits the binding of UPEC to colonic epithelial cells and so facilitates the clearance of these bacteria from the gut.
To investigate how binding of type 1 pili to the gut might be disrupted, Spaulding and colleagues looked at the compound M4284. This is a mannoside — a mannose-linked small molecule — and has 100,000 times greater affinity than D-mannose for the adhesin on type 1 pili. Spaulding and colleagues found that oral treatment of mice with M4284 significantly reduced intestinal colonization by UPEC. Thus, M4284 effectively competes with D-mannose on the surface of colonic epithelial cells for binding to type 1 pili (Fig. 1b). Bacteria that are unable to bind to host epithelial cells are cleared from the gut.
Could this molecule be used to combat UTIs? The authors reported that the M4284 administered to their mice facilitated clearance of UPEC bladder infection by directly interfering with the interactions between type 1 pili and urothelial cells in the bladder. Presumably, M4284 was absorbed into the bloodstream from the gut, and transported to the bladder. However, it is likely that a reduction in the density of UPEC in the intestine would also help to reduce UTI rates, by reducing the levels of UPEC available to colonize the urinary tract. Indeed, using a mouse model in which UPEC was introduced directly into the urinary tract, the researchers demonstrated that lower densities of UPEC in the gut resulted in lower rates of UTIs.
We are currently facing an antibiotic-resistance crisis, as acquisition of resistance by many pathogens outpaces the development of new antibiotics6. Spaulding and colleagues’ findings, which point to a potential antibiotic-independent approach to reduce infections, are therefore welcome. Reducing adhesion of bacteria to host cells may provide new approaches to prevent, and even treat, UPEC infections7.
Long experience and extensive study tell us that antibiotics inhibit not only pathogens but also the population of commensal bacteria in the gut. This microbiota provides a natural barrier to intestinal colonization by pathogenic bacteria, but the barrier can be compromised by antibiotic-mediated destruction of commensal microbes8. By contrast, anti-adhesion therapies that use small molecules such as M4284 might preserve the commensals. Encouragingly, Spaulding et al. did not observe any major changes in the gut microbiota following M4284 treatment. Nevertheless, as researchers study the mechanisms by which pathogens cling to our surfaces, they should also investigate how the harmless and health-promoting members of our microbiota remain in the gut. Only through this understanding will we be able to displace the troublemakers and keep the good guys around.
Contributor Information
HEA-JIN JUNG, Immunology Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, New York 10065, USA.
ERIC G. PAMER, Immunology Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, New York 10065, USA. Infectious Diseases Service in the Department of Medicine and the Center for Microbes, Inflammation and Cancer, Memorial Sloan Kettering Cancer Center
References
- 1.Ringot-Destrez B, et al. Biochem Soc Trans. 2017;45:389–399. doi: 10.1042/BST20160167. [DOI] [PubMed] [Google Scholar]
- 2.Spaulding CN, et al. Nature. 2017;546:528–532. doi: 10.1038/nature22972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribet D, Cossart P. Microbes Infect. 2015;17:173–183. doi: 10.1016/j.micinf.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Busch A, Waksman G. Phil Trans R Soc B. 2012;367:1112–1122. doi: 10.1098/rstb.2011.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Nature Rev Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ventola CL. Pharm Therapeut. 2015;40:277–283. [Google Scholar]
- 7.Krachler AM, Orth K. Virulence. 2013;4:284–294. doi: 10.4161/viru.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Littman DR, Pamer EG. Cell Host Microbe. 2011;10:311–323. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]