Abstract
Glucose is a key metabolite used by cancer cells to generate ATP, maintain redox state and create biomass. Glucose can be catabolized to lactate in the cytoplasm, which is termed glycolysis or alternatively can be catabolized to carbon dioxide and water in the mitochondria via oxidative phosphorylation (OXPHOS). Metabolic heterogeneity exists in a subset of human tumors, with some cells maintaining a glycolytic phenotype while others predominantly utilize OXPHOS. Cells within tumors interact metabolically with transfer of catabolites from supporting stromal cells to adjacent cancer cells. The Reverse Warburg Effect describes when glycolysis in the cancer-associated stroma metabolically supports adjacent cancer cells. This catabolite transfer, which induces stromal-cancer metabolic coupling, allows cancer cells to generate ATP, increase proliferation and reduce cell death. Catabolites implicated in metabolic coupling include the monocarboxylates lactate, pyruvate and ketone bodies. Monocarboxylate transporters (MCT) are critically necessary for release and uptake of these catabolites. MCT4 is involved in the release of monocarboxylates from cells, is regulated by catabolic transcription factors such as hypoxia inducible factor 1 alpha (HIF1A) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and is highly expressed in cancer-associated fibroblasts. Conversely, MCT1 is predominantly involved in the uptake of these catabolites and is highly expressed in a subgroup of cancer cells. MYC and TIGAR, which are genes involved in cellular proliferation and anabolism are inducers of MCT1. Profiling human tumors on the basis of an altered redox balance and intra-tumoral metabolic interactions may have important biomarker and therapeutic implications. Alterations in the redox state and mitochondrial function of cells can induce metabolic coupling. Hence, there is interest in redox and metabolic modulators as anticancer agents. Also, markers of metabolic coupling have been associated with poor outcomes in numerous human malignancies and may be useful prognostic and predictive biomarkers.
Keywords: oxidative phosphorylation, glycolysis, lactate, caveolin 1, TIGAR, hypoxia inducible factor
Introduction
The generation of ATP from glucose is an essential eukaryote cellular process that results in either the production of lactate via glycolysis or carbon dioxide and water via oxidative phosphorylation (OXPHOS) [1]. Also, yeast which are eukaryote facultative anaerobes can perform alcoholic fermentation but this will not be discussed further since the focus of this review is on the metabolism of the different cell types in human tumors. Cells utilize glucose to generate ATP, maintain redox equilibrium or generate biomass [2]. Non-cancer or normal cells and tissues rely primarily on OXPHOS, which takes place in the mitochondria and is the more energetically efficient process; only in the absence of oxygen do non-cancer cells shift to glycolysis [3]. It has long been recognized that cancer cell metabolism differs from that of non-cancer cells [4]. However, the exact nature of the difference continues to be elucidated and debated.
German scientist Otto Warburg proposed his influential theory of tumor cell metabolism in the 1920s. He and his colleagues observed that, even in the presence of adequate oxygen, tumor cells utilized more glucose and produced more lactate than surrounding normal cells, a process that they coined “aerobic glycolysis [5].” As an explanation for why tumor cells would favor this relatively inefficient process, Warburg hypothesized that they must have dysfunctional mitochondria that are irreversibly damaged [6]. A very small group of familial human cancers, which include paragangliomas and renal cell cancers, have irreversible mitochondrial damage due to mutations in the tricarboxylic acid cycle enzymes succinate dehydrogenase and fumarate hydratase [7] [8]. Despite evidence that the majority of cancer cell mitochondria are not dysfunctional and that many different tumor metabolic profiles exist, this theory, known as the “Warburg Effect,” has been a prevailing theory of tumor metabolism [9] [10] [11].
The Warburg Effect only partially explains tumor metabolism. Studies have shown that there is metabolic heterogeneity within tumors, with some cells maintaining a glycolytic phenotype while others predominantly utilize OXPHOS [2] [9] [12]. This is made possible by a complex interplay between different metabolic compartments. Interactions between cancer cells and cells in the tumor microenvironment allows metabolites to be shifted from stromal cells to meet metabolic demands and maintain ATP production in cancer cells [2]. A newer theory, termed the “Reverse Warburg Effect,” describes a two-compartment model in which stromal cells are induced by cancer cells to undergo “aerobic glycolysis” and then transfer the products back to the cancer cells for utilization for mitochondrial OXPHOS [1] [13] [14] [15]. This cellular metabolic coapting of stromal and cancer cells allows tumors to respond to variations in nutrient availability to maximize cellular proliferation and growth [2].
Mitochondrial OXPHOS in the Cancer Cell
Contrary to Warburg’s hypothesis, cancer cells have increased mitochondrial activity in a subgroup of human cancers [4] [11] [16] [17] [18] [19] [20] [21]. TOMM20 (translocase of outer mitochondrial membrane 20) is the receptor subunit of the mitochondrial membrane import pore, which allows the import of nuclear encoded OXPHOS subunits and induces OXPHOS [22]. TOMM20 can be stained by immunohistochemistry, and has been used as a marker of mitochondrial mass and metabolic activity [23]. Studies have confirmed that epithelial cancer cells, including breast, anaplastic thyroid, papillary thyroid, and gastric, express high levels of TOMM20 when compared to adjacent stromal cells [24] [25] [26]. This supports the notion that mitochondrial function in cancer cells is preserved (Figure 1).
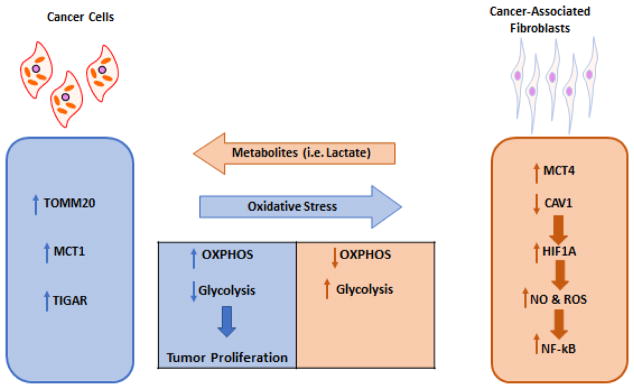
Fig 1. Model of Metabolic Coupling in Cancer.
A model of two-compartment tumor metabolism is shown. Cancer cells have high expression of the translocase of outer mitochondrial membrane 20 (TOMM20), monocarboxylate transporter 1 (MCT1) and TP53 induced glycolysis and apoptosis regulator (TIGAR), which induces high oxidative phosphorylation (OXPHOS) and low glycolysis in these cancer cells with high proliferation. This metabolic reprogramming of cancer cells induces oxidative stress, which in turn upregulates monocarboxylate transporter 4 (MCT4) and reduces caveolin 1 (CAV1) expression driving hypoxia inducible factor 1 alpha (HIF1A), nitric oxide (NO), reactive oxygen species (ROS) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) in cancer-associated fibroblasts.
Further proof of the presence of functional mitochondria in human cancer cells comes from studies of human tumors utilizing functional staining of the mitochondrial OXPHOS complexes [10] [23] [24]. Complex I-NADH dehydrogenase, complex II-succinate dehydrogenase (SDH) and complex IV-cytochrome C oxidase (COX), have all been shown to be hyperactive in human breast cancer cells; compared to tumor stromal cells and normal epithelial ductal cells [24]. In another study in head and neck squamous cell carcinoma, the highest complex IV activity was found in carcinoma cells and this activity was higher than in normal epithelia, normal stroma or tumor associated stroma [23]. These studies demonstrate that there is increased OXPHOS in cancer cells in a subgroup of human malignancies. It has been hypothesized that mitochondrial activity may be required for high biomass production in a subgroup of human cancers.
Monocarboxylate Transporters
The monocarboxylate transporters (MCTs) are a family of proton-linked membrane transporters that are responsible for the movement of single-carboxylate molecules, such as lactate and pyruvate, in and out of cells. 14 MCTs have been identified, however, only MCTs 1–4 are able to transport monocarboxylates bidirectionally [27]. Both MCT1 and MCT4 have been identified as playing an important role in the metabolic relationship between cancer cells and fibroblasts [1] [28] [29] [30] [31]. Specifically, MCT1 has been found to be upregulated in OXPHOS cancer cells that have increased uptake of lactate while MCT4, on the other hand, is upregulated in lactate exporting, glycolytic cells [12] [30] [32]. The upregulation of MCT4, but not MCT1, is driven by hypoxia and mediated by hypoxia inducible factor 1 alpha (HIF1A) [33].
In a study conducted by Whitaker et al., breast cancer cells were co-cultured with fibroblasts to induce the formation of cancer-associated fibroblasts (CAFs) via a mechanism that is mediated by cancer cell driven oxidative stress. The cells were then stained to evaluate for MCT4 expression. They found that neither cancer cells nor fibroblasts expressed MCT4 when stained separately, however, after co-incubation, MCT4 was selectively expressed on CAFs [28]. The same procedure was then performed for MCT1, which was found to be selectively upregulated on MCF7 breast cancer cells and absent on CAFs [28]. High MCT1 expression is found in the majority of carcinoma cells in human breast cancer while as high MCT4 is found in the majority of breast cancer-associated stroma [34] [35]. This supported the authors’ hypothesis that a lactate shuttle may exist in tumors to transfer lactate from the stroma to the cancer cells for utilization [28]. Several clinical studies have evaluated the relationship between MCT1 expression in cancer cells and patient outcomes and most studies have shown that high expression is associated with shorter progression free survival (PFS) or overall survival (OS) (Table 1).
Table 1.
High monocarboxylate transporter 1 (MCT1) expression in cancer cells and progression free survival (PFS) or overall survival (OS) as clinical outcomes.
| Cancer type | Number of subjects studied | PFS or OS | Reference: |
|---|---|---|---|
| Renal cell carcinoma | 180 | Shorter PFS | [96] |
| Bladder carcinoma | 360 | Shorter OS | [97] |
| Bladder carcinoma | 111 | Shorter OS | [98] |
| Prostate Cancer | 480 | Shorter PFS | [99] |
| Prostate Cancer | 535 | No association with OS | [44] |
| Breast carcinoma | Not reported | Shorter OS | [100] |
| Breast carcinoma | 257 | Shorter PFS | [34] |
| Non-small Cell Lung Cancer | 335 | Longer OS | [101] |
| Non-small Cell Lung Cancer | 715 | Shorter OS | [100] |
| Small Cell Lung Cancer | 78 | Shorter OS | [102] |
| Oral Cavity Cancer | 135 | Shorter OS | [103] |
| Cutaneous Melanoma | 356 | Shorter OS | [104] |
| Adrenocortical carcinomas | 78 | Shorter OS | [105] |
| Soft tissue sarcomas | 86 | Shorter OS | [106] |
| Gastrointestinal stromal tumors | 64 | Shorter OS | [107] |
Similar findings with metabolic coupling between stromal and cancer cells involving lactate, pyruvate, beta-hydroxybutyrate, acetate, fatty acids, glutamine and alanine have been discovered in many human malignancies including breast, ovarian, prostate, liver, colon, pancreatic and head and neck squamous cell cancer (HNSCC) [23] [29] [36] [37] [38] [39] [40] [41] [42] [43] [44] [45] [46] [47].
Studies have evaluated MCT expression in conjunction with TOMM20 expression in an attempt to correlate cellular transport with metabolic activity. Using biomarker staining, Curry et al. identified three, separate metabolic compartments in HNSCC: (1) proliferating cancer cells expressing high MCT1 and high TOMM20, (2) non-proliferating stromal cells expressing high MCT4, and (3) non-proliferating cancer cells expressing high MCT4, highlighting the metabolic heterogeneity within a tumor [23]. High MCT1 expression in cancer cells and high MCT4 expression in the stroma of multiple human malignancies is associated with poor outcomes [48].
The mechanisms by which cancer cells metabolically reprogram adjacent non-cancer cells are an area of active investigation since it holds promise to determine drivers of cancer aggressiveness, discover prognostic and predictive cancer biomarkers and novel anti-cancer therapies. Caveolin-1 (CAV1), hypoxia inducible factor 1 alpha (HIF1A), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and TP53 induced glycolysis and apoptosis regulator (TIGAR) are all known inducers of cancer-stroma metabolic coupling via modulation of oxidative stress and autophagy (Figure 2).
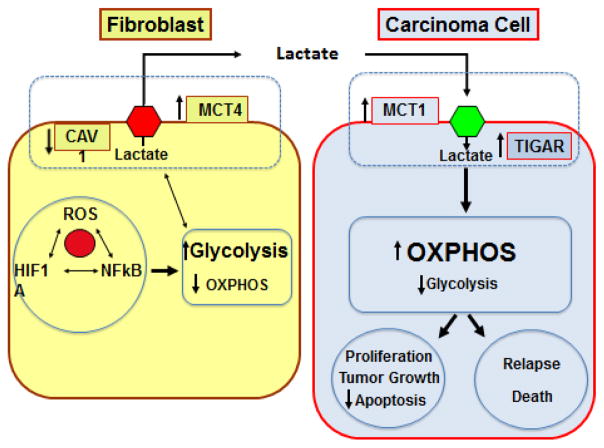
Fig 2. Mechanisms of Metabolic Reprogramming in Cancer.
Reactive oxygen species (ROS), hypoxia inducible factor 1 alpha (HIF1A) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) induce glycolysis with lactate production in cancer-associated fibroblasts which in turn downregulates caveolin 1 (CAV1) and upregulates monocarboxylate transporter 4 (MCT4). Lactate is released from fibroblasts and uptaken by cancer cells via monocarboxylate transporter 1 (MCT1) with upregulation of TP53 induced glycolysis and apoptosis regulator (TIGAR). These cancer cells have high mitochondrial oxidative phosphorylation (OXPHOS) and low glycolysis, which is associated with high proliferation, low apoptosis rates, tumor growth and higher rates of relapse and death.
Caveolin-1, HIF1A, NF-kB
Caveolae are plasma membrane invaginations that are considered a distinct subset of plasma membrane lipid rafts. Coated by unique proteins called caveolins, caveolae are found on multiple different cells types, including endothelial cells, fibroblasts, muscle cells, and adipocytes, and have been shown to be involved in cell signaling, among other functions [49]. The caveolin family of proteins consists of three members, caveolin-1 (CAV1), caveolin-2 (CAV2), and caveolin-3 (CAV3). Here, we focus on CAV1 and its role in cancer-stromal cell metabolism.
CAV1 expression is frequently low in human cancer-associated fibroblasts compared to normal fibroblasts and is mediated by self-digestion or autophagy [14] [50]. Reduced CAV1 expression in fibroblasts reduces mitochondrial function and induces glycolysis [51]. Pavlides et al. performed proteomic analysis of the lysates of Cav1 null fibroblasts and found that loss of Cav1 was associated with the upregulation of eight glycolytic enzymes, including the M-2 isoform of pyruvate kinase and lactate dehydrogenase. They then used immunohistochemistry to stain human breast cancer tissues with CAV1 low fibroblasts for these proteins, confirming the upregulation of these glycolytic enzymes in the tumor stroma [13]. Similarly, Bonuccelli et al. developed a mouse model for evaluating CAV1 null breast cancer associated fibroblasts (CAFS) function and, through proteomic analysis, again demonstrated increases in glycolytic enzymes in CAV1 null CAFs. Furthermore, they showed that treatment of breast cancer xenografts that incorporate these CAV1 null CAFs with glycolytic blocking agents decreased tumor growth [52].
What, then, drives the loss of stromal CAV1? Studies have shown that tumor cells induce oxidative stress in CAFs, which leads to autophagic degradation of CAV1 [53, 54]. The molecular mechanisms underlying this process are linked to two pro-autophagic transcription factors, HIF1A and NF-κB [53, 54].
Autophagy is the process by which cytoplasmic organelles, including mitochondria, are broken down either because they are dysfunctional or because their breakdown products are needed as a source of energy. It has been well described that activation of HIF1A causes autophagy [55] [56]. However, the relationship between HIF1A and the autophagic degradation of CAV1 was not known until a co-culture fibroblast model confirmed that a hypoxia-induced increase in HIF1A directly correlates with a decrease in CAV1 expression [53]. Furthermore, re-oxygenation of these cells restored CAV1 with a concomitant decrease in HIF1A [53].
In addition to promoting autophagy, HIF1A acts as a powerful inducer of glycolysis by increasing the expression and activity of glucose transporters, such as GLUT1 and GLUT3, and glycolytic enzymes, including hexokinase 2 (HK2) and lactate dehydrogenase [57]. When HIF1A is activated in breast cancer associated fibroblasts, it has been shown to cause a measurable decrease in mitochondrial activity and an increase in lactate production, consistent with a glycolytic phenotype, leading to increased tumor growth (via the Reverse Warburg Effect) [58]. Interestingly, HIF1A activation in breast cancer cells has also been shown to cause increased glycolysis, but this acts as a tumor growth suppressor (the Warburg Effect) [58].
CAV1 is a potent inhibitor of nitric oxide synthase (NOS) [59]. When NOS is expressed, nitric oxide (NO) is produced, driving multiple processes including the production of reactive oxygen species (ROS). Both NO and ROS can induce HIF1A, in an oxygen independent and dependent fashion, respectively, leading to enhanced glycolysis [53] [60] [61]. Thus, when CAV1 is lost, production of NO and ROS increases, causing HIF1A to increase, thereby perpetuating the cycle of increased glycolysis and further CAV1 degradation in CAFs [53].
NF-κB activation also plays a role in hypoxia-mediated downregulation of CAV1. Cancer cells can induce NF-κB expression in CAFs [62]. NF-κB activation leads to degradation of CAV1 via autophagy in a similar fashion to HIF1A [53]. This then creates a feed-forward mechanism whereby decreased CAV1 further drives NF-κB expression leading to enhanced degradation of CAV1 [53].
TIGAR
The p53 tumor suppressor gene has long been recognized as a mediator of cell cycle arrest, apoptosis, and the cellular response to hypoxia. More recently, however, p53 has been shown to play a role in cellular metabolism. In 2006, Bensaad et al. described a novel protein called TP53 Induced Glycolysis and Apoptosis Regulator (TIGAR), a glycolytic inhibitor that is regulated by p53 [63]. It functions as a bisphosphatase that decreases the level of the key glycolytic regulators fructose-2,6-bisphosphate and 2,3-bisphosphoglycerate, thereby decreasing glycolysis [63] [64].
Over-expression of TIGAR in breast carcinoma cells in vitro has shown not only to decrease glycolysis, but also to increase OXPHOS in the presence of glutamine and lactate leading to increased ATP generation [65]. Furthermore, when carcinoma cells overexpress TIGAR in co-culture with fibroblasts, a glycolytic phenotype is induced in the fibroblasts and there is increased tumor growth [65]. This supports a two-compartment model of tumor metabolism. In vivo, TIGAR overexpression has been identified in several solid tumor types, including breast, glioblastoma, and non-small cell lung cancer, and in hematologic malignancies such as AML [66] [67] [68] [69]. TP53 mutations induce TIGAR expression more strongly than wild-type TP53 [63]. Consistent with the fact that TIGAR increases OXPHOS [66] [65] and that TIGAR is induced by TP53 mutations [63], subjects with Li-Fraumeni syndrome who have TP53 mutations have increased mitochondrial OXPHOS metabolism [70]. The mechanisms by which TP53 mutations induce organismal OXPHOS are unknown.
Clinical Implications
There are potential clinical implications associated with the expanding knowledge of tumor metabolic heterogeneity. From a prognostic standpoint, markers of metabolism may be useful in predicting disease behavior and outcomes. For example: in triple negative breast cancer, high expression of MCT1 on carcinoma cells has been associated with decreased progression free survival and increased risk of recurrence [34]; in cytogenetically normal acute myeloid leukemia, high TIGAR expression has been correlated with decreased survival [69]; in multiple tumor types (melanoma, colorectal, prostate, breast, gastric) loss of stromal CAV1 has been shown to be an independent predictor of poor prognosis [71] [72] [73] [74] [75]. There is great interest in incorporating metabolic parameters into cancer prognostic models [2]. Future studies should be conducted to determine if markers of metabolic heterogeneity can be used in clinical practice to improve prognostic accuracy.
The proteins involved in tumor-stroma metabolic coupling also represent novel targets for anti-cancer therapies. The antidiabetic drug metformin is known to alter cellular metabolism through mitochondrial OXPHOS complex I inhibition [76] [77] [78] [79] [80]. Researchers have attempted to harness this anti-metabolic effect to treat a variety of cancers [81]. In a pilot study by Curry et al., patients with squamous cell carcinoma of the head and neck were treated with metformin during the time between their initial diagnostic biopsy and surgical resection. Biopsies pre- and post-treatment with metformin were stained for CAV1 and MCT4, along with other metabolic markers. Patients who received metformin were found to have increased stromal CAV1 staining intensity, from 25.7% pre-treatment to 62.8% post-treatment; control patients showed no difference in staining. No difference was seen in MCT4 expression [82]. Metformin induced carcinoma cell apoptosis 2.8 fold by TUNEL staining in this trial, which was not seen in control patients and increased lactate levels 2.4 fold in carcinoma cells [82]. This can be viewed as proof of principle that metformin can be used to modify the metabolic milieu of tumor stroma. Metformin has shown promising activity in multiple human malignancies including breast, non-small cell lung, prostate, endometrial, pancreatic, colorectal, and renal cell cancers and clinical trials of metformin alone and in combination with standard therapies are underway in multiple tumor types [83] [84] [85].
N-acetylcysteine (NAC) is an antioxidant that is commonly used to treat acetaminophen overdoses [86]. It has also been studied in a variety of other conditions such as psychiatric illnesses, cardiac diseases, and chronic lung disorders [87] [88] [89]. NAC’s antioxidant properties are related to its role as a precursor for glutathione, the main intracellular antioxidant responsible for free radical and ROS scavenging, buffering, and many other cellular reactions [90]. When given therapeutically, NAC increases glutathione production thereby decreasing oxidative stress. Since NAC preferentially targets cells with abnormal glycolysis, it has been postulated that it would be an effective anti-cancer therapy, specifically targeting the tumor stroma. A clinical trial evaluating the effect of NAC treatment on early stage breast cancer metabolic markers, proliferation and apoptosis has been conducted. Proliferation of carcinoma cells was reduced after treatment with NAC as measured by Ki-67 staining. MCT4 staining of cancer associated stromal cells was also reduced after NAC.
Additional methods of targeting tumor-stroma metabolic coupling are in varying stages of pre-clinical and clinical development. In an effort to alter lactate transport, small molecule inhibitors have been designed against both MCT1 and MCT4 [91] [92]. Drugs that inhibit mitochondrial OXPHOS are in early phase clinical trials in diseases such as AML, prostate cancer, and colorectal cancer [93] [94] [95]. TIGAR and CAV1 have also been proposed as possible targets, however, directed therapies have yet to be designed and tested in clinical trials.
Conclusion
The metabolic heterogeneity that exists within a tumor allows cancer and stromal cells to couple and transfer metabolites between them to support maximal cellular growth. Recognition of this complex dynamic has led to the development of a model of tumor metabolism, referred to as the “Reverse Warburg Effect.” Along with it, new prognostic markers and therapeutic targets have been identified. Exploiting the metabolic differences between cancer and stromal cells may have a therapeutic effect that needs to be tested in clinical trials.
Footnotes
The authors disclose no potential conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Martinez-Outschoorn UE, et al. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14(1):11–31. doi: 10.1038/nrclinonc.2016.60. [DOI] [PubMed] [Google Scholar]
- 2.Danhier P, et al. Cancer metabolism in space and time: Beyond the Warburg effect. Biochim Biophys Acta. 2017;1858(8):556–572. doi: 10.1016/j.bbabio.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Ahn CS, Metallo CM. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 2015;3(1):1. doi: 10.1186/s40170-015-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeNicola GM, Cantley LC. Cancer’s Fuel Choice: New Flavors for a Picky Eater. Mol Cell. 2015;60(4):514–23. doi: 10.1016/j.molcel.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 6.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–70. [PubMed] [Google Scholar]
- 7.DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell. 2012;148(6):1132–44. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sciacovelli M, et al. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature. 2016;537(7621):544–547. doi: 10.1038/nature19353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12(10):685–98. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2(5):e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinberg F, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107(19):8788–93. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonveaux P, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118(12):3930–42. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavlides S, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8(23):3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Caveolae and signalling in cancer. Nat Rev Cancer. 2015;15(4):225–37. doi: 10.1038/nrc3915. [DOI] [PubMed] [Google Scholar]
- 15.Wu D, Zhuo L, Wang X. Metabolic reprogramming of carcinoma-associated fibroblasts and its impact on metabolic heterogeneity of tumors. Semin Cell Dev Biol. 2017;64:125–131. doi: 10.1016/j.semcdb.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17(4):351–9. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marin-Valencia I, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15(6):827–37. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caro P, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. 2012;22(4):547–60. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viale A, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514(7524):628–32. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birsoy K, et al. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell. 2015;162(3):540–51. doi: 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hensley CT, et al. Metabolic Heterogeneity in Human Lung Tumors. Cell. 2016;164(4):681–94. doi: 10.1016/j.cell.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wurm CA, et al. Nanoscale distribution of mitochondrial import receptor Tom20 is adjusted to cellular conditions and exhibits an inner-cellular gradient. Proc Natl Acad Sci U S A. 2011;108(33):13546–51. doi: 10.1073/pnas.1107553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curry JM, et al. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle. 2013;12(9):1371–84. doi: 10.4161/cc.24092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitaker-Menezes D, et al. Hyperactivation of oxidative mitochondrial metabolism in epithelial cancer cells in situ: visualizing the therapeutic effects of metformin in tumor tissue. Cell Cycle. 2011;10(23):4047–64. doi: 10.4161/cc.10.23.18151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Z, et al. Stromal-epithelial metabolic coupling in gastric cancer: stromal MCT4 and mitochondrial TOMM20 as poor prognostic factors. Eur J Surg Oncol. 2014;40(10):1361–8. doi: 10.1016/j.ejso.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JM, et al. Mitochondrial Metabolism as a Treatment Target in Anaplastic Thyroid Cancer. Semin Oncol. 2015;42(6):915–22. doi: 10.1053/j.seminoncol.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halestrap AP. Monocarboxylic acid transport. Compr Physiol. 2013;3(4):1611–43. doi: 10.1002/cphy.c130008. [DOI] [PubMed] [Google Scholar]
- 28.Whitaker-Menezes D, et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10(11):1772–83. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiaschi T, et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72(19):5130–40. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- 30.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123(9):3685–92. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodwin ML, et al. Modeling alveolar soft part sarcomagenesis in the mouse: a role for lactate in the tumor microenvironment. Cancer Cell. 2014;26(6):851–62. doi: 10.1016/j.ccell.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doherty JR, et al. Blocking lactate export by inhibiting the Myc target MCT1 Disables glycolysis and glutathione synthesis. Cancer Res. 2014;74(3):908–20. doi: 10.1158/0008-5472.CAN-13-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem. 2006;281(14):9030–7. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 34.Johnson JM, et al. MCT1 in Invasive Ductal Carcinoma: Monocarboxylate Metabolism and Aggressive Breast Cancer. Front Cell Dev Biol. 2017;5:27. doi: 10.3389/fcell.2017.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witkiewicz AK, et al. Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle. 2012;11(6):1108–17. doi: 10.4161/cc.11.6.19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang CK, et al. Adipocytes promote malignant growth of breast tumours with monocarboxylate transporter 2 expression via beta-hydroxybutyrate. Nat Commun. 2017;8:14706. doi: 10.1038/ncomms14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang, et al. Hepatocellular carcinoma redirects to ketolysis for progression under nutrition deprivation stress. Cell Res. 2016;26(10):1112–1130. doi: 10.1038/cr.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diers AR, et al. Pyruvate fuels mitochondrial respiration and proliferation of breast cancer cells: effect of monocarboxylate transporter inhibition. Biochem J. 2012;444(3):561–71. doi: 10.1042/BJ20120294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko YH, et al. Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells: implications for preventing chemotherapy resistance. Cancer Biol Ther. 2011;12(12):1085–97. doi: 10.4161/cbt.12.12.18671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L, et al. Targeting Stromal Glutamine Synthetase in Tumors Disrupts Tumor Microenvironment-Regulated Cancer Cell Growth. Cell Metab. 2016;24(5):685–700. doi: 10.1016/j.cmet.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colegio OR, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–63. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schug ZT, et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell. 2015;27(1):57–71. doi: 10.1016/j.ccell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen S, et al. Organized metabolic crime in prostate cancer: The coexpression of MCT1 in tumor and MCT4 in stroma is an independent prognosticator for biochemical failure. Urol Oncol. 2015;33(8):338e9–17. doi: 10.1016/j.urolonc.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Valencia T, et al. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell. 2014;26(1):121–135. doi: 10.1016/j.ccr.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koukourakis MI, et al. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66(2):632–7. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 47.Sousa CM, et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536(7617):479–83. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bovenzi CD, et al. Prognostic Indications of Elevated MCT4 and CD147 across Cancer Types: A Meta-Analysis. Biomed Res Int. 2015;2015:242437. doi: 10.1155/2015/242437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sotgia F, et al. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol. 2012;7:423–67. doi: 10.1146/annurev-pathol-011811-120856. [DOI] [PubMed] [Google Scholar]
- 50.Martinez-Outschoorn UE, et al. Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation: implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle. 2010;9(12):2423–33. doi: 10.4161/cc.9.12.12048. [DOI] [PubMed] [Google Scholar]
- 51.Asterholm IW, et al. Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metab. 2012;15(2):171–85. doi: 10.1016/j.cmet.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonuccelli G, et al. The reverse Warburg effect: glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle. 2010;9(10):1960–71. doi: 10.4161/cc.9.10.11601. [DOI] [PubMed] [Google Scholar]
- 53.Martinez-Outschoorn UE, et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFkappaB activation in the tumor stromal microenvironment. Cell Cycle. 2010;9(17):3515–33. doi: 10.4161/cc.9.17.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez-Outschoorn UE, et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9(16):3256–76. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283(16):10892–903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Bellot G, et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29(10):2570–81. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeung SJ, Pan J, Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer. Cell Mol Life Sci. 2008;65(24):3981–99. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiavarina B, et al. Metabolic reprogramming and two-compartment tumor metabolism: opposing role(s) of HIF1alpha and HIF2alpha in tumor-associated fibroblasts and human breast cancer cells. Cell Cycle. 2012;11(17):3280–9. doi: 10.4161/cc.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Cardena G, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272(41):25437–40. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 60.Chandel NS, et al. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95(20):11715–20. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmer LA, Gaston B, Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox-dependent effect of nitrogen oxides. Mol Pharmacol. 2000;58(6):1197–203. doi: 10.1124/mol.58.6.1197. [DOI] [PubMed] [Google Scholar]
- 62.Erez N, et al. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17(2):135–47. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 63.Bensaad K, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–20. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 64.Gerin I, et al. Identification of TP53-induced glycolysis and apoptosis regulator (TIGAR) as the phosphoglycolate-independent 2,3-bisphosphoglycerate phosphatase. Biochem J. 2014;458(3):439–48. doi: 10.1042/BJ20130841. [DOI] [PubMed] [Google Scholar]
- 65.Ko YH, et al. TIGAR Metabolically Reprograms Carcinoma and Stromal Cells in Breast Cancer. J Biol Chem. 2016 doi: 10.1074/jbc.M116.740209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wanka C, Steinbach JP, Rieger J. Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by up-regulating respiration and improving cellular redox homeostasis. J Biol Chem. 2012;287(40):33436–46. doi: 10.1074/jbc.M112.384578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Won KY, et al. Regulatory role of p53 in cancer metabolism via SCO2 and TIGAR in human breast cancer. Hum Pathol. 2012;43(2):221–8. doi: 10.1016/j.humpath.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 68.Zhou X, et al. TIGAR is correlated with maximal standardized uptake value on FDG-PET and survival in non-small cell lung cancer. PLoS One. 2013;8(12):e80576. doi: 10.1371/journal.pone.0080576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian S, et al. TIGAR cooperated with glycolysis to inhibit the apoptosis of leukemia cells and associated with poor prognosis in patients with cytogenetically normal acute myeloid leukemia. J Hematol Oncol. 2016;9(1):128. doi: 10.1186/s13045-016-0360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang PY, et al. Increased oxidative metabolism in the Li-Fraumeni syndrome. N Engl J Med. 2013;368(11):1027–32. doi: 10.1056/NEJMoa1214091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu KN, et al. Loss of stromal caveolin-1 expression in malignant melanoma metastases predicts poor survival. Cell Cycle. 2011;10(24):4250–5. doi: 10.4161/cc.10.24.18551. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Z, et al. Loss of stromal caveolin-1 expression in colorectal cancer predicts poor survival. World J Gastroenterol. 2015;21(4):1140–7. doi: 10.3748/wjg.v21.i4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ayala G, et al. Loss of caveolin-1 in prostate cancer stroma correlates with reduced relapse-free survival and is functionally relevant to tumour progression. J Pathol. 2013;231(1):77–87. doi: 10.1002/path.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Witkiewicz AK, et al. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009;174(6):2023–34. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao X, et al. Caveolin-1 expression level in cancer associated fibroblasts predicts outcome in gastric cancer. PLoS One. 2013;8(3):e59102. doi: 10.1371/journal.pone.0059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–14. [PMC free article] [PubMed] [Google Scholar]
- 77.El-Mir MY, et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275(1):223–8. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 78.Wheaton WW, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gui DY, et al. Environment Dictates Dependence on Mitochondrial Complex I for NAD+ and Aspartate Production and Determines Cancer Cell Sensitivity to Metformin. Cell Metab. 2016;24(5):716–727. doi: 10.1016/j.cmet.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu X, et al. Metformin Targets Central Carbon Metabolism and Reveals Mitochondrial Requirements in Human Cancers. Cell Metab. 2016;24(5):728–739. doi: 10.1016/j.cmet.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heckman-Stoddard BM, et al. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia. 2017 doi: 10.1007/s00125-017-4372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Curry J, et al. Metformin effects on head and neck squamous carcinoma microenvironment: Window of opportunity trial. Laryngoscope. 2017 doi: 10.1002/lary.26489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Incio J, et al. Metformin Reduces Desmoplasia in Pancreatic Cancer by Reprogramming Stellate Cells and Tumor-Associated Macrophages. PLoS One. 2015;10(12):e0141392. doi: 10.1371/journal.pone.0141392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sonnenblick A, et al. Impact of Diabetes, Insulin, and Metformin Use on the Outcome of Patients With Human Epidermal Growth Factor Receptor 2-Positive Primary Breast Cancer: Analysis From the ALTTO Phase III Randomized Trial. J Clin Oncol. 2017;35(13):1421–1429. doi: 10.1200/JCO.2016.69.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bradley CA. Diabetes: Metformin in breast cancer. Nat Rev Endocrinol. 2017;13(5):251. doi: 10.1038/nrendo.2017.37. [DOI] [PubMed] [Google Scholar]
- 86.Marks DJB, et al. Outcomes from massive paracetamol overdose: a retrospective observational study. Br J Clin Pharmacol. 2017;83(6):1263–1272. doi: 10.1111/bcp.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Minarini A, et al. N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin Drug Metab Toxicol. 2017;13(3):279–292. doi: 10.1080/17425255.2017.1251580. [DOI] [PubMed] [Google Scholar]
- 88.Pasupathy S, et al. Early use of N-Acetylcysteine (NAC) with Nitrate Therapy in Patients Undergoing Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction Reduces Myocardial Infarct Size (The NACIAM Trial) Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.117.027575. [DOI] [PubMed] [Google Scholar]
- 89.Fowdar K, et al. The effect of N-acetylcysteine on exacerbations of chronic obstructive pulmonary disease: A meta-analysis and systematic review. Heart Lung. 2017;46(2):120–128. doi: 10.1016/j.hrtlng.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 90.Wu G, et al. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 91.Bola BM, et al. Inhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transport. Mol Cancer Ther. 2014;13(12):2805–16. doi: 10.1158/1535-7163.MCT-13-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee JY, et al. MCT4 as a potential therapeutic target for metastatic gastric cancer with peritoneal carcinomatosis. Oncotarget. 2016;7(28):43492–43503. doi: 10.18632/oncotarget.9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Skrtic M, et al. Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell. 2011;20(5):674–88. doi: 10.1016/j.ccr.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rico-Bautista E, et al. Small molecule-induced mitochondrial disruption directs prostate cancer inhibition via UPR signaling. Oncotarget. 2013;4(8):1212–29. doi: 10.18632/oncotarget.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X, et al. Induction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised microenvironments. Nat Commun. 2014;5:3295. doi: 10.1038/ncomms4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim Y, et al. Expression of lactate/H(+) symporters MCT1 and MCT4 and their chaperone CD147 predicts tumor progression in clear cell renal cell carcinoma: immunohistochemical and The Cancer Genome Atlas data analyses. Hum Pathol. 2015;46(1):104–12. doi: 10.1016/j.humpath.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 97.Choi JW, et al. Prognostic significance of lactate/proton symporters MCT1, MCT4, and their chaperone CD147 expressions in urothelial carcinoma of the bladder. Urology. 2014;84(1):245e9–15. doi: 10.1016/j.urology.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 98.Afonso J, et al. Metabolic coupling in urothelial bladder cancer compartments and its correlation to tumor aggressiveness. Cell Cycle. 2016;15(3):368–80. doi: 10.1080/15384101.2015.1121329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pertega-Gomes N, et al. A lactate shuttle system between tumour and stromal cells is associated with poor prognosis in prostate cancer. BMC Cancer. 2014;14:352. doi: 10.1186/1471-2407-14-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hong CS, et al. MCT1 Modulates Cancer Cell Pyruvate Export and Growth of Tumors that Co-express MCT1 and MCT4. Cell Rep. 2016;14(7):1590–601. doi: 10.1016/j.celrep.2016.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eilertsen M, et al. Monocarboxylate transporters 1–4 in NSCLC: MCT1 is an independent prognostic marker for survival. PLoS One. 2014;9(9):e105038. doi: 10.1371/journal.pone.0105038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Polanski R, et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin Cancer Res. 2014;20(4):926–937. doi: 10.1158/1078-0432.CCR-13-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simoes-Sousa S, et al. Prognostic significance of monocarboxylate transporter expression in oral cavity tumors. Cell Cycle. 2016;15(14):1865–73. doi: 10.1080/15384101.2016.1188239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pinheiro C, et al. The metabolic microenvironment of melanomas: Prognostic value of MCT1 and MCT4. Cell Cycle. 2016;15(11):1462–70. doi: 10.1080/15384101.2016.1175258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pinheiro C, et al. Metabolic reprogramming: a new relevant pathway in adult adrenocortical tumors. Oncotarget. 2015;6(42):44403–21. doi: 10.18632/oncotarget.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pinheiro C, et al. Characterization of monocarboxylate transporters (MCTs) expression in soft tissue sarcomas: distinct prognostic impact of MCT1 sub-cellular localization. J Transl Med. 2014;12:118. doi: 10.1186/1479-5876-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Oliveira AT, et al. Co-expression of monocarboxylate transporter 1 (MCT1) and its chaperone (CD147) is associated with low survival in patients with gastrointestinal stromal tumors (GISTs) J Bioenerg Biomembr. 2012;44(1):171–8. doi: 10.1007/s10863-012-9408-5. [DOI] [PubMed] [Google Scholar]