Abstract
Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune disease in which the pancreas produces insufficient amounts of insulin. T1DM patients require exogenous sources of insulin to maintain euglycemia. Transplantation of naked or microencapsulated pancreatic islets represents an alternative paradigm to obtain an autonomous regulation of blood glucose levels in a controlled and personalized fashion. However, once transplanted, the fate of these personalized cellular therapeutics is largely unknown, justifying the development of non-invasive tracking techniques.
Areas covered
In vivo imaging of naked pancreatic islet transplantation, monitoring of microencapsulated islet transplantation, visualizing pancreatic inflammation, imaging of molecular-genetic therapeutics, imaging of beta cell function.
Expert commentary
There are still several hurdles to overcome before (microencapsulated) islet cell transplantation will become a mainstay therapy. Non-invasive imaging methods that can track graft volume, graft rejection, graft function (insulin secretion) microcapsule engraftment, microcapsule rupture, and pancreatic inflammation are currently being developed to design the best experimental transplantation paradigms.
Keywords: Diabetes, insulin, inflammation, imaging, islet cells, microencapsulation, personalized medicine, transplantation
1. Introduction
Diabetes mellitus (DM) is a chronic, systemic disease of multiple etiology, characterized by inadequate regulation of blood glucose levels resulting from a reduced or absent release of insulin or the presence of insulin resistence [1]. Among the main risks associated with diabetes are blindness, ulcers, amputation, hypertension, nephropathy, and heart disease [2]. DM can be classified into two major categories, type 1 (T1DM) and type 2 (T2DM). T1DM or juvenile diabetes is characterized by an early onset of autoimmune cell infiltration resulting in chronic pancreatic inflammation and destruction of insulin-producing β-cells [3]. T2DM is characterized by a later onset of the disease, which can result from either inefficient cellular processing of pro-insulin or a premature release of pro-insulin as a result of high insulin demand [4], with a high prevalence in the obese population. For both types, insulin is required to regulate blood glucose levels. While administration of exogenous insulin is now standard practice having been pursued for decades, alternative approaches for treatment of T1DM are now being developed based on personalized medicine principles. These include naked and microencapsulated islet cell transplantation to achieve a continuous on-demand insulin production. Patients in whom pharmaceutical intervention has failed are in need of alternative methods to maintain proper regulation of glucose levels and to prevent complications associated with hyper- and hypoglycemia [5]. Ideally, all patients that are inadequately regulated by insulin injections should be transplanted with a tightly regulating insulin-producing graft. However, in those patients the side effects of immunosuppression do not outweigh the risk of complications of insulin therapy and for that reason it is now only performed in those patients where end-stage renal failure occurred [6]. A combination of a kidney and an islet cell graft is usually given. Islet immunoisolation through encapsulation can avoid the use of immunosuppression and allows cell transplantation long before end-stage renal failure occurs in those diabetic patients where exogenous insulin injections failed. In this expert commentary, we provide an overview of the alternative new strategies that are currently being developed for treatment of T1DM, with special emphasis on the use and tracking of microencapsulated islets as a novel approach towards personalized medicine in islet transplantation.
2. Personalized treatment of T1DM
The primary form of treatment for T1DM is the use of exogenous insulin [7]. However, other options now exist that can provide a finely regulated and on-demand insulin production from within, such as transplantation of the whole pancreas [6, 8], transplantation of isolated, naked pancreatic islets [9, 10], and transplantation of immuno-isolated or microencapsulated pancreatic islets [11, 12].
2.1 Insulin therapy
The use of exogenous insulin to control hyperglycemia requires multiple daily doses, frequent monitoring of glucose levels, and dosage adaptations. A failure to detect fast changes in glucose is associated with episodes of severe hypoglycemia and hyperglycemia which can lead to diabetic ketoacidosis [13]. The use of devices or pumps to monitor continuous changes in glucose controlled insulin release is one solution, but still requires follow-up measurements to assess whether or not insulin has been infused correctly, along with re-calibration of the device [14].
2.2 Whole pancreas transplantation
Transplantation of the whole pancreas avoids the use of exogenous insulin. Over the last five years, pancreatic transplantation has improved in terms of surgical procedures, delay or absence of rejection, and patient outcome [15]. One of the major drawbacks of whole organ transplantation is the life-long use of immunosupressants, which can lead to deleterious side effects [16]. An important early improvement was the introduction of drainage of the exocrine pancreas [17], decreasing the risk of thrombosis and anastomotic insufficiency, but it comes with a higher risk of gastrointestinal bleeding [15]. This therapy is indicated for patients who also receive a kidney transplantation subsequent to transplantation of the pancreas, since the maintenance of euglycemia contributes to a reduction of microvascular complications, increasing the longevity of both grafts [18].
2.3 Unencapsulated islet cell transplantation
A different approach to the treatment of T1DM is the transplantation of isolated, purified β-islet cells of the pancreas [19] to reinstitute the production of insulin in response to increases of glucose in the blood. The first clinical trial on islet transplantation in patients without kidney transplantation was performed in Pittsburgh in 1990 [20]. Ten years later, a successful trial was completed in Edmonton, Canada, in 2000, now seen as a landmark study [21]. The specific criteria for selecting patients for allogeneic islet transplantation are: recipients must be aged between 18 and 65 years, have T1DM for more than 5 years, have a negative fasting C-peptide (<0.3 ng/ml), exhibit hypoglycemia unawareness with glycemic lability, and have elevated HbA1c levels (above 8%) [9, 22]. The Edmonton clinical trial achieved total insulin independence in all transplanted patients for over one year, although this independence remained for 5 years in only 10% of patients [23].
Transplantation of islets is desirable over transplantation of the whole pancreas since the surgery is less invasive and associated with a lower morbidity. However, a pertinent issue to overcome is the significant loss of islets during the immediate post-transplant period. After transplantation, a so-called instant blood-mediated inflammatory reaction (IBMIR) can occur. The IBMIR reaction is characterized by the activation of the complement and coagulation cascades, inducing the formation of clots and an infiltration of leukocytes into the islets, with deleterious effects [24, 25]. Here, successful transplantation depends on the use of external factors to control the IBMIR process using inhibitors of both the complement and coagulation processes [25], with or without immunosuppressants. Although there have been significant improvements in the transplantation of islets since 2000, the use of immunosuppressive therapies are still required. The clinical success after transplantation of islets depends therefore on the capability to sustain immunosuppression levels and the quality of the transplanted islets. A third factor, often overlooked, is the limited availability of histocompatible donors. Furthermore, donor islets must survive the harsh enzymatic and mechanical procedures that are used to isolate them [26]. After transplantation, many islets will die due to ischemia because of hypoxia [27] and the occurrence of inflammation [28]. This will lead to increased antigen presentation, making major histocompatibility complex (MHC) class II matching of donors a crucial factor in graft success or failure.
A personalized approach to overcome the histocompatibility issue is the use of induced pluripotent stem cells (iPSCs) as they are derived from the patients themselves. This approach would allow the use of fully MHC class II-matched donor cells [29]. However, this may not prevent loss of cells due to autoimmunity as the immunogenic epitopes can still be carried by the iPSCs. As the use of iPSCs represents a potentially unlimited source of pancreatic endocrine lineage cells that could be used for the treatment of the diabetic patients [30, 31], immunoisolation by encapsulation still remains a viable option in this scenario.
As stated above, the isolation of islets is a refined process that requires standardization to achieve maximum quality and survival of cells. The National Institutes of Health initiated a clinical islet cell transplantation consortium for a Phase 3 trial of developing a unified process for the manufacturing of highly purified allogeneic human pancreatic islets. The process was performed in eight different manufacturing facilities with a common master production batch record, islet product specifications, certificate of analysis, and test methods, all of them compliant with current good manufacturing practices. The report proved that it is feasible to implement a standardized process at multiple facilities for obtaining a complex cellular product with the desired characteristics and function after transplantation [32].
2.4 Microencapsulated islet transplantation
An effective method to protect transplanted cells from rejection by the host is the use of a semi-permeable immunoprotective membrane (Figure 1A). Microencapsulation allows free diffusion of certain molecules, including nutrients, glucose, oxygen, and insulin, into and out of the capsule, while preventing the penetration of antibodies or contact with host immune cells [33, 34, 35]. This technology was first used by Lim and Sun in 1980 [36] and has been widely modified and adapted since its first use. Microcapsules must meet several criteria to avoid rapid cell death following encapsulation, with insufficient diffusion of oxygen into the capsule as one of the primary pitfalls. The capsules should have the proper diameter for a sufficient distance of oxygen diffusion, and be close to blood vessels or are able induce new blood supply around the capsule [37, 38].
Figure 1.
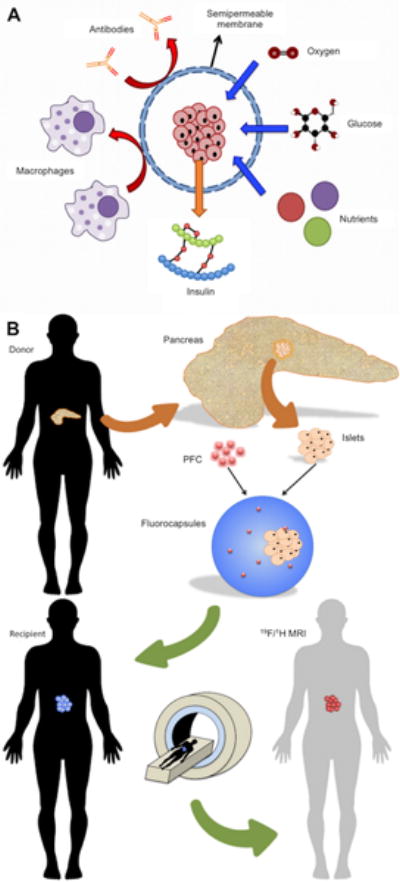
(A) Islet immunoisolation through microencapsulation. A semi-permeable alginate membrane allows diffusion of essential molecules including oxygen, glucose, insulin, and nutrients, while simultaneously protecting the graft from the host immune system. (B) Fluoroencapsulated islet tracking using MRI. A deceased donor pancreas is removed and islets are enzymatically isolated. Islets are then co-encapsulated with perfluorocarbon (PFC), an MRI-detectable tracer, to form fluorocapsules. Fluorocapsules are then transplanted into a T1DM recipient to achieve normoglycemia. The location and persistence of the capsules can be longitudinally monitored using 19F MRI. Reproduced, with permission, from Ref. [35] (A).
In 2006, Calafiore et al. transplanted microencapsulated islets in two TD1M patients, achieving high levels of C-peptide that were still measurable at 1 1/2 year after transplantation without the use of immunosuppression [11]. Another study, performed by Tuch et al. [12], observed a rise in C-peptide levels from one to three months after transplantation. The subsequent reduction of C-peptide levels was attributed to islet cell necrosis and capsule fibrotic overgrowth as demonstrated by biopsy [12]. Microencapsulation offers a possibility to use cells from either an allogeneic or a xenogeneic source, offering a potential solution to a shortage of suitable donor pancreata. However, specific formulation and considerations must be used for both allogeneic and xenogeneic sources. For the latter, the discordance in histocompatibility between the donor and recipient is formidable [39, 40]. Highly immunoreactive epitopes, such as galactosyl residues, can still diffuse out of the microcapsules and activate the complement pathway, leading to neutrophil infiltration and the release of many other cytokines.
There are many polymers that can be used in the preparation of microcapsules, as long as they comply with certain requirements, such as flexibility, softness, mechanical stability, capable diffusion of molecules of interest, and biocompatibility to avoid a native immune response [35, 41]. Among the most studied and characterized polymers are poly(ethylene glycol), polyvinyl alcohol, polyurethane, polyether-sulfone, polypropylene, sodium polystyrene sulfate, polyacrylate, agarose, chitosan, cellulose, collagen, xanthan, and alginate [42, 43]. Alginate is a polymer that has been used in humans in compliance with the regulatory restrictions and ethical aspects of the Italian Ministry of Health [11] and has been studied sufficiently to enable modification of its mechanical characteristics to adapt them to meet the desired purposes. It can be extracted from some species of algae and bacteria and is an unbranched binary copolymer of 1–4 linked β-D-mannuronic acid (M) and α-L-guluronic acid (G) intertwined in different proportions [44]. Alginate consists of G-G blocks, G-M blocks, and M-M blocks, and the repetition and quantities of these blocks give different alginates specific properties that make them more or less suitable for certain applications [35, 42]. The three-dimensional conformational structure of the alginate in the capsule allows incorporation of other polymers, e.g., poly-L-lysine (PLL), to further modify the properties of the microcapsules, including pore size and mechanical stability [45, 46]. Microcapsules are formed when different alginate blocks cross-link with bivalent cations, e.g., Ca2+, Ba2+, and Sr2+, giving specific diffusion, rigidity, and elastic properties to the microcapsules.
Successful use of microcapsules is dependent on the source and kind of alginate, the gelling cation, and the incorporated polyaminoacids and polymers [47]. Many studies have successfully used microencapsulated pancreatic islets to reverse hyperglycemia in small animal models. In large animal models, however, the host immune response against the implanted capsules reportedly remains an issue [40, 48, 49], regardless of implantation site [50].
Proper purification of alginate has been determined as one of the major factors in avoiding a host immune response [51]. Clinical-grade commercialized alginates are tested for the presence of endotoxins, i.e., lipopolysaccharides, in order to render them safe for human use. We have specialized in the development of a platform that enables the production of ultrapure alginate with complete removal of residual endotoxins, such as flagellin, lipoteichoic acid, and peptidoglycans [52]. Those molecules, also known as pathogen-associated molecular patterns (PAMPS) [53, 54, 55, 56], are potent triggers of an immune response upon binding to pattern recognition receptors, particularly Toll-like receptors (TLR) [57, 58].
Another pitfall in microencapsulated islet cell transplantation can be cell death, due to the lack of oxygen or nutrients [37, 38, 59]. Certain molecules, known as danger-associated molecular patterns (DAMPs), e.g., dsDNA, RNA, HMGB1, and uric acid [59], can be present in the intracellular space. After damage occurs, DAMPs are released into the extracellular space, and diffuse out of the capsule where they can be recognized by TLRs of the host cells, inducing an immediate immune reaction [53, 57, 60, 61, 62]. The release and recognition of PAMPs and DAMPs results in the release of cytokines, chemokines, recruitment of immune cells, and localized inflammation that will lead to graft failure. The presence of PAMPs and DAMPs should be avoided to obtain a maximum therapeutic efficacy of islet cell microencapsulation. One solution we have proposed to reduce the amount of DAMPs from necroptotic cells is to co-encapsulate necrostatin-1 as an alginate additive [59], a potent inhibitor of necrosis [63].
2.5. Imaging of transplanted naked islets
Clinically applicable imaging techniques that can be used to track transplanted naked and microencapsulated islet cells include X-ray, computed tomography (CT), single photon emission tomography (SPECT), positron emission tomography (PET) and magnetic resonance imaging (MRI) [64]. The purpose of non-invasive islet cell tracking is, ideally, 4-fold: 1) Monitoring the accuracy of delivery and initial engraftment; 2) Monitoring cell survival and/or cell rejection; 3) Monitoring the occurrence of an innate or adaptive immune response; and 4) Monitoring capsule rupture. All these 4 aspects, of critical importance for a prognosis of treatment outcome, can now be addressed as outlined below.
In 2004, Jirak et al. [65] were the first to label transplanted islets with a superparamagnetic iron oxide (SPIO) contrast agent, that provides hypointensity on MRI [66]. It was shown that, after graft rejection in allogeneic animals, the number of hypointense spots was much reduced over time compare to syngeneic transplants [65, 67, 68]. After the initial MRI cell tracking trials appeared safe [69], MRI islet cell tracking was introduced in the clinic a few years later [70, 71]. However, the initial findings in rodents could not be reproduced, in that the number of MRI-visible spots did not correlate to the initial number of transplanted islets and hence, no good correlation with cell survival could be made. As an alternative to using SPIO as a hypointense MR contrast agent for 1H MRI, perfluorocarbons (PFC) may be used as a “hot spot” tracer for 19F MRI [72], where the quantification of label loss is straightforward and linear [73]. Indeed, PFC-labeled islets have been successfully imaged with sufficient sensitivity using a low-field 3T clinical setup [74]. Now that clinical trials with PFC-labeled cells are being initiated [75], we anticipated fluorinated islet cell tracking to be performed in the near future.
PET has shown that live transplanted cells can take up the tracer [(11)C]5-hydroxytryptophan and that its quantification correlates with islet cell function [76]. Alternative strategies include the use of the PET reporter gene herpes simplex virus thymidine kinase [77], but its use for transplanted islet cells has not yet been used in the clinic. SPECT imaging and quantification of total β islet cell mass in rats following islet transplantation has been demonstrated using the glucagonlike peptide-1 receptor-binding ligand exendin-3, labeled with 111-indium [78], with a good correlation between the radiotracer count and number of islets.
2.6. Imaging of transplanted microencapsulated islets
In comparison to direct labeling of naked islets, it is much easier to label microencapsulated islets: the contrast agent or label is simply added to the alginate, and then automatically embedded within the hydrogel (Figure 1B) [79, 80, 81]. A key difference is that the label is not inside the transplanted islet cell but outside in the gel matrix, ameliorating concerns about potential toxicity. Microencapsulated islets have been labeled with a variety of contrast agents and tracers, including barium and bismuth [82, 83], SPIO nanoparticles [50, 84, 85, 86], Gadolinium-gold nanoparticles [87], and PFCs [88]. The use of PFC is particularly attractive, as these molecules can act as an oxygen sink and bioreactor [89], which may be exploited to reduce the deleterious hypoxic conditions faced after transplantation.
Several of these labeling approaches have enabled multi-modal imaging of the same microcapsules using X-ray/CT, US, and MRI (Figure 2). In one approach, SPIO and gold nanoparticles were first co-encapsulated without cells in a smaller primary capsule, and then co-encapsulated with cells in a secondary larger capsule to create a so-called “capsule-in-capsule” [90]. A demonstration of loss of contrast agents following capsule rupture was shown using SPIO nanoparticles [84]. Recently, micro-CT imaging was employed to image the fine structure of microcapsules using their intrinsic contrast properties without the use of contrast agents [91], using CaCl2 instead of BaCl2 [83]. Barium is particularly attractive, as its high affinity for alginate increases the mechanical strength of the capsules [83, 92], making them suitable for implantation sites that exhibit high mechanical pressure. Moreover, NMR relaxometry can be used to assess the differences in partial oxygen pressure inside the capsules at diverse implantation sites in vivo [93].
Figure 2. Microencapsulated cell tracking using multiple imaging modalities.
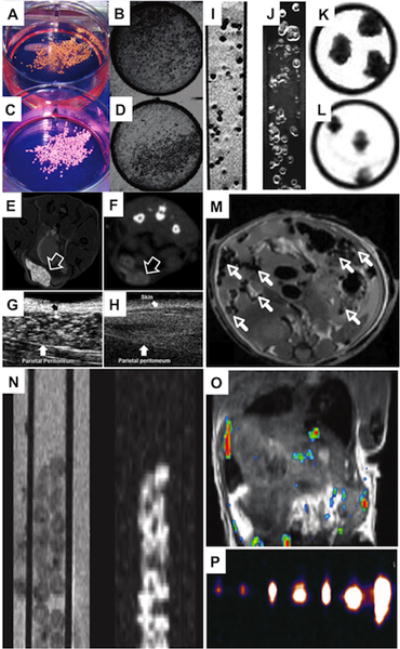
(A–D) Macroscopic (A,C) and radiographic (B,D) images of bismuth (A,B) and barium-loaded (C,D) X-ray-trackable capsules. (E,F) Positive-contrast 1H MR (E) and micro-computed tomography (CT) (F) images of GadoGold microcapsules (arrow) engrafted subcutaneously into a mouse. (G,H) Ultrasound (US) images of the cavity between the abdominal skin and parietal peritoneum (arrow) of a mouse with (G) and without (H) injected GadoGold microcapsules. (I–L) 1H MR images of agarose-embedded magnetocapsules using a (I) T2*-weighted pulse sequence and (J) an inversion recovery on-resonance (IRON) sequence to generate negative and positive contrast, respectively. (K,L) 1H MR images before (K) and after (L) capsule rupture show a decrease of signal due to loss of magnetic label inside capsules. (M) Single magnetocapsules (arrows) can be detected in a mouse following i.p. transplantation. (N) 1H T2-weighted (left) and corresponding 19F (right) MR images of fluorocapsules in a 5 mm glass tube. (M) 1H (grey scale)/19F (hot iron scale) overlaid MR images of a mouse after i.p. transplantation of fluorocapsules. (O) 19F MR images of agarose-embedded fluorocapsules obtained at clinical field strength. Images were obtained for 1, 2, 5, 10, 15, 25, and 50 single capsules, respectively. Reproduced, with permission from [82] (A–D), [87] (E–H), [84] (I–M), and [88] (N–P).
2.7. Imaging of pancreatic inflammation
In T1DM, inflammation may contribute to an early induction and amplification of the auto-immune response against islet cells and, at later stages, to the stabilization and maintenance of insulitis [94]. Hence, imaging of such inflammation may be a valuable adjunct to monitor and evaluate patients at risk for T1DM. Gaglia et al. showed that intravenously injected ultrasmall SPIO nanoparticles localize in the pancreas of T1DM patients and that induced hypointensity may serve as a non-invasive imaging biomarker of islet inflammation (Figure 3) [95, 96]. This increased nanoparticle uptake is believed to result from the phagocytic activity of inflammatory cells, which may be facilitated by the presence of leaky blood vessels known to exist in animal models of T1DM [97].
Figure 3. Imaging of inflammation using iron oxide nanoparticles.
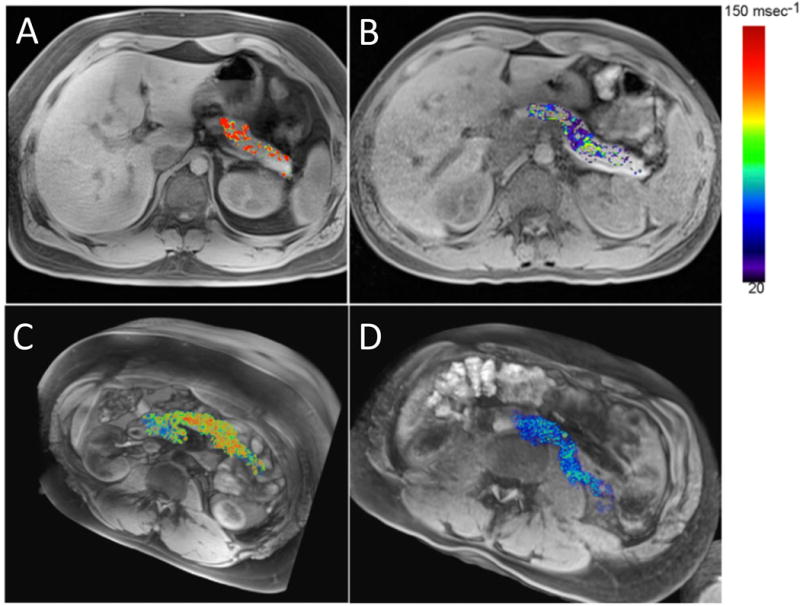
Single-slice (A,B) and 3D volume sets (C,D) of a representative patient with recently diagnosed T1D (A,C) and a normal control subject (B,D). An increased pancreatic nanoparticle accumulation in patients with T1D can be observed. Hot iron color map represents changes in R2* (transversion relaxation rate) values after injection of iron oxide nanoparticles. Reproduced, with permission, from Ref. [96].
2.8. Combining siRNA with SPIO nanoparticles: Towards developing theranostics
As a theranostic approach, magnetic nanoparticles can be linked to small interference (si) RNA and incorporated into islets before transplantation. In this way, the engraftment (and survival) of naked islets can be tracked while biological processes that drive apoptosis or cell rejection can be modulated through molecular-genetic engineering (Figure 4A) [98]. By labeling islets with SPIO linked to Caspase-3 siRNA, a protective effect with increased cell survival could be achieved as a result of inhibition of apoptosis [99]. Similarly, when human islets were labeled pre-transplantation with a siRNA SPIO probe that targets beta (2) microglobulin, a key component of major histocompatibility complex class I, a significantly improved preservation of graft volume could be observed, along with a delayed onset of hyperglycemia following an adoptive transfer of autoimmune T cells (Figure 4B) [100].
Figure 4.
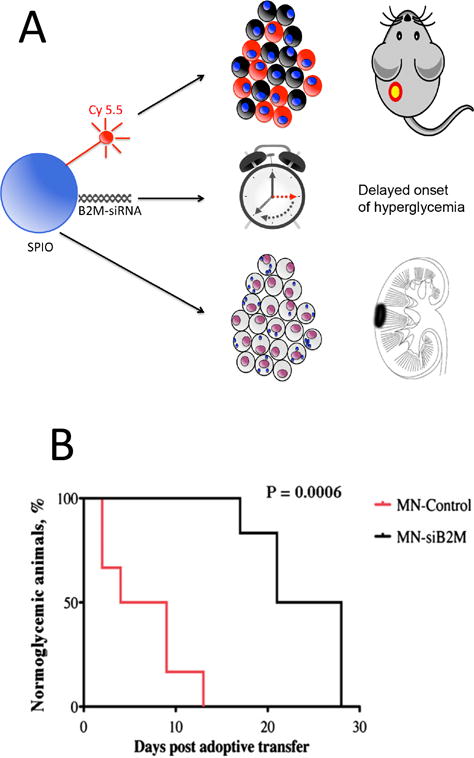
(A) Theranostic nanoparticle platform. The main core of the particle contains SPIO as MR contrast agent. The second component is Cy 5.5 for optical detection. B2M-siRNA is the therapeutic arm of the formulation, inhibiting the expression of B2M and the immunogenicity of transplanted islets. The theranostic particles are introduced into islet cells by simply adding them to the culture medium before transplantation. Cellular uptake is verified by Cy 5.5 fluorescence or SPIO-positive Prussian Blue staining. In vivo, labeled islets can be visualized by optical imaging and MRI. (B) Delayed onset of hyperglycemia for SPIO-siB2M-labeled islets. Kaplan-Meier curve shows animals transplanted with control (red) and MN-siB2M-labeled (black) islets. Development of diabetes was defined as the occurrence of hyperglycemia (blood glucose level >250 mg/dL on two consecutive measurements, n=6; P=0.0006). Reproduced, with permission, from Ref. [98] (A) and [100] (B,C).
2.9. Imaging of insulin production using a zinc-responsive contrast agent
Upon binding of certain metal ions, paramagnetic contrast agents may undergo structural changes that affect their access to water protons and hence 1H MR contrast. The Zn2+ -responsive T 1 agent, (GdDOTA-diBPEN) was found to be able to enhance the pancreas following glucose stimulation in normal mice, but not diabetic mice, as a result of concomitant insulin release (Figure 5) [101]. Insulin is pre-formed in vesicles containing zinc; upon the secretion of these exosomes Zn2+ is instantly released in the surrounding extracellular space. This approach may be further used for non-invasive monitoring of β -cell function in vivo during development of T2DM or after (microencapsulated) islet cell transplantation. Alternative approaches for imaging Zn2+ using a non-paramagnetic zinc-responsive MR contrast agent have also been recently developed. One is a hyperpolarized 129Xe gas-based sensor, where the resonance frequency of xenon encapsulated in a cryptophane that bears a nitrilotriacetic ligand moiety varies when Zn2+ ions are present. A second one is to perform 19F MRI with fluorinated (1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid) (BAPTA) agents, which are derivatives of common fluorescent metal dyes, and which change their chemical exchange saturation transfer properties upon Zn2+ binding [102]. For the latter two approaches, no exogenously introduced paramagnetic metal is needed, which may facilitate clinical translation.
Figure 5. Functional imaging of insulin release using a zinc-responsive agent.
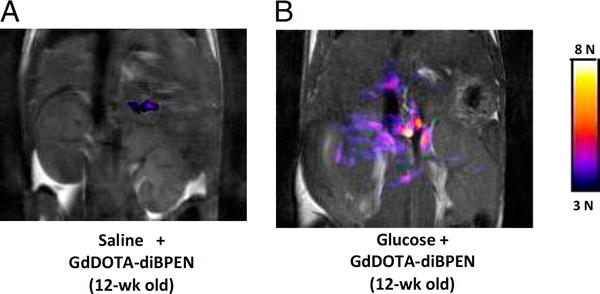
Grayscale T1-weighted MR images of the pancreas of 12-wk-old non-diabetic mice after intravenous injection of either saline (A) or glucose (B) followed by the zinc-responsive MR contrast agent GdDOTA-diBPEN. Colored overlays represent pixels where the MR signal intensity increased by threefold or more after injection of saline plus agent or glucose plus agent. Reproduced, with permission, from Ref. [101].
3. Expert Commentary
Transplantation of naked and microencapsulated islets represents a form of personalized medicine that has considerable potential to supplement and perhaps even replace conventional exogenous insulin therapy. Since their introduction in 1980, microencapsulation of islets has seen many improvements. Among those are a better control of the physicochemical properties of the capsule, with specific molecular weight porosity cutoff values to prevent the diffusion of non-desired molecules, and the formulation of capsules with smooth surfaces that decrease the attachment of immune cells.
Despite these advances, there are still several key issues that need to be addressed before a widespread use of microcapsules in clinical practice can be considered. Many studies cannot not be reproduced by others, and many large animal studies failed to produce robust results, which is believed to be largely due to differences in the use of specific biomaterials and proper purification of alginate. Alginate is a well-studied polymer that offers great flexibility when combined with other molecules and cross-linking cations; however, the relative amount of guluronic and mannuronic blocks and their viscosity, molecular weight, cross-linking cations, use of perm-selective polycations, and source and purity of alginate are major determinants of the specific mechanical and chemical capsule that do not need to be necessarily the same for different implantation sites.
It is, at present, not known how long transplanted islet survive. A halt of insulin production may be a good indirect indicator of islet cell death, but even if there is sufficient production of insulin, it is possible that it is derived from only a fraction of islets that survive. A possible route for increasing cell survival could be pre-conditioning of islets with hypoxia, and/or label them with siRNA to inhibit apoptosis or to reduce immunogenicity.
A major weakness for clinical management and further improvement of (microencapsulated) islet transplantation is the current lack of suitable non-invasive methods to monitor the fate of these cellular therapeutics following engraftment. However, over the last decade or so, clinically applicable cellular imaging techniques have been developed that can be used to track graft volume, graft rejection, graft function, microcapsule engraftment, microcapsule rupture, and pancreatic inflammation.
4. Five-year view
Additional actions are needed to implement (microencapsulated) islets successfully on a larger scale. (Stem) cell engineering approaches are currently being pursued that may yield sufficient amounts of beta cell mimicking designer cells in a controlled and potentially unlimited fashion [103], avoiding the current problem of the limited availability of donor/graft -matched islet cells. We need to call for a standardization of alginates that are used for microencapsulation including source, purification, endotoxin testing, relative amount of M and G alginate building blocks, size, and porosity. We can also expect more efforts towards engineering implantable encapsulation devices that have the advantage of being able to be retrieved.
Cell and capsule tracking techniques will be implemented by the (interventional) radiologist working side-by-side with the endocrinologist and surgeon. Immediate verification of accurate delivery and engraftment is of paramount importance. It is also desirable to identify specific biomarkers that can report on the active release of DAMPs as a consequence of cell death. A comprehensive screening of patients for cytokines, chemokines, and adipokines should be performed both before and after transplantation in order to determine if their presence correlates to the clinical outcome of transplantation [104]. A non-invasive follow-up of the fate of the implants, for which imaging techniques are now being developed, is highly desirable as well. Probe development for imaging of pancreatic inflammation may tell us when and why some people develop T1DM and some not, given an equal genetic predisposition. Targeted imaging probes will be more and more combined with therapeutics that create a less hostile environment for transplanted islets, leading to a new era of personalized medicine. Novel imaging agents that are either targeted to pro-insulin or change their properties upon insulin (zinc) release may tell us what fraction of transplanted islet cells are functional. To make this happen, biomaterial scientists, cell engineers, imaging scientists, and endocrinologists/islet cell biologists will need to work together as one interdiscplinary team.
Key issues.
Islet cell transplantation has shown great potential for treatment of T1DM, but the shortage of donors and stringent host immunosuppression calls for alternative ways of immunoprotection. Alginate encapsulation is one such approach, but major improvements are needed to ensure long-term biocompatibility of microencapsulated islets.
We need imaging probes that can report on pancreatic inflammation, beta cell mass, islet cell survival, and capsule biocompatibility.
The use of personalized medicine for imaging and treatment of T1DM is still a largely unexplored area.
Acknowledgments
Funding
The authors are supported, in part, by NIH R01 DK106972.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Contributor Information
Genaro A. Paredes-Juarez, Russell H. Morgan Department of Radiology, Division of Magnetic Resonance Research, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA Cellular Imaging Section and Vascular Biology Program, Institute for Cell Engineering, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA. 733 N. Broadway, 21205-1832, Baltimore, Maryland, USA.
Paul de Vos, University Medical Center Groningen (UMCG), Department of Pathology and Medical Biology, Section Immunoendocrinology. Hanzeplein 1, 9713 GZ, Groningen, The Netherlands.
Jeff W.M. Bulte, Russell H. Morgan Department of Radiology, Division of Magnetic Resonance Research, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA Department of Biomedical Engineering, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Department of Chemical & Biomolecular Engineering, The Johns Hopkins Whiting School of Engineering, Baltimore, Maryland, USA; Department of Oncology, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Cellular Imaging Section and Vascular Biology Program, Institute for Cell Engineering, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA. 733 N. Broadway, Baltimore, Maryland 21205, USA.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Muoio DM, Newgard CB. Mechanisms of disease:Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 2.Shamoon H, Duffy H, Fleischer N, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes-mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Notkins AL, Lernmark A. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest. 2001;108:1247–52. doi: 10.1172/JCI14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, Hu Y, Zhou Z, Yan X, Tian H, Ran X, Luo Z, Xian J, Yan L, Li F, Zeng L, Chen Y, Yang L, Yan S, Liu J, Li M, Fu Z, Cheng H. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–60. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 5.de Vos P, Spasojevic M, Faas MM. In: Treatment of diabetes with encapsulated islets. Pedraz JL, Orive G, editors. Vol. 670. Austin, Texas: Springer New York; 2010. pp. 38–53. [DOI] [PubMed] [Google Scholar]
- 6.Mittal S, Johnson P, Friend P. Pancreas transplantation: solid organ and islet. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahn A, Miccoli R, Dardano A, Del Prato S. New forms of insulin and insulin therapies for the treatment of type 2 diabetes. The Lancet Diabetes & Endocrinology. 2015;8587:1–15. doi: 10.1016/S2213-8587(15)00097-2. [DOI] [PubMed] [Google Scholar]
- 8.Frank A, Deng S, Huang X, Velidedeoglu E, Bae YS, Liu C, Abt P, Stephenson R, Mohiuddin M, Thambipillai T, Markmann E, Palanjian M, Sellers M, Naji A, Barker CF, Markmann JF. Transplantation for type I diabetes: comparison of vascularized whole-organ pancreas with isolated pancreatic islets. Ann Surg. 2004;240:631–40. doi: 10.1097/01.sla.0000140754.26575.2a. discussion 40-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, Pattou F, Berney T, Secchi A, Messinger S, Senior PA, Maffi P, Posselt A, Stock PG, Kaufman DB, Luo X, Kandeel F, Cagliero E, Turgeon NA, Witkowski P, Naji A, O’Connell PJ, Greenbaum C, Kudva YC, Brayman KL, Aull MJ, Larsen C, Kay TWH, Fernandez LA, Vantyghem M-C, Bellin M, Shapiro AMJ. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care. 2012;35:1436–45. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rickels MR, Pelekis AJ, Markmann E, Dalton-Bakes C, Kong SM, Teff KL, Naji A. Long-term improvement in glucose control and counterregulation by islet transplantation for type 1 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2016:jc.2016–1649. doi: 10.1210/jc.2016-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, Racanicchi L, Mancuso F, Brunetti P. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care. 2006;29:137–8. doi: 10.2337/diacare.29.1.137. [DOI] [PubMed] [Google Scholar]
- 12.Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V, Philips R. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32:1887–9. doi: 10.2337/dc09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfsdorf J, Glaser N, Sperling MA, American Diabetes A. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1150–9. doi: 10.2337/diacare.2951150. [DOI] [PubMed] [Google Scholar]
- 14.Cescon M, DeSalvo DJ, Ly TT, Maahs DM, Messer LH, Buckingham BA, Doyle FJ, Dassau E. Early Detection of Infusion Set Failure During Insulin Pump Therapy in Type 1 Diabetes. J Diabetes Sci Technol. 2016 doi: 10.1177/1932296816663962. 1932296816663962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stites E, Kennealey P, Wiseman AC. Current status of pancreas transplantation. Curr Opin Nephrol Hypertens. 2016;150:1. doi: 10.1097/MNH.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 16.Denton MD, Magee CC, Sayegh MH. Immunosuppressive strategies in transplantation. Lancet. 1999;353:1083–91. doi: 10.1016/S0140-6736(98)07493-5. [DOI] [PubMed] [Google Scholar]
- 17.Sollinger HW, Cook K, Kamps D, Glass NR, Belzer FO. Clinical and experimental experience with pancreaticocystostomy for exocrine pancreatic drainage in pancreas transplantation. Transplant Proc. 1984;16:749–51. [PubMed] [Google Scholar]
- 18.Stites E, Wiseman AC. Multiorgan transplantation. Transplant Rev. 2016;30:253–60. doi: 10.1016/j.trre.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Vantyghem MC, Defrance F, Quintin D, Leroy C, Raverdi V, Prévost G, Caiazzo R, Kerr-Conte J, Glowacki F, Hazzan M, Noel C, Pattou F. Treating diabetes with islet transplantation: Lessons from the past decade in Lille. Diabetes & metabolism. 2014;40:108–19. doi: 10.1016/j.diabet.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Tzakis AG, Ricordi C, Alejandro R, Zeng Y, Fung JJ, Todo S, Demetris AJ, Mintz DH, Starzl TE. Pancreatic islet transplantation after upper abdominal exenteration and liver replacement. Lancet. 1990;336:402–5. doi: 10.1016/0140-6736(90)91946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. The New England journal of medicine. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 22.Vantyghem MC, Defrance F, Quintin D, Leroy C, Raverdi V, Prevost G, Caiazzo R, Kerr-Conte J, Glowacki F, Hazzan M, Noel C, Pattou F, Diamenord AS, Bresson R, Bourdelle-Hego MF, Cazaubiel M, Cordonnier M, Delefosse D, Dorey F, Fayard A, Fermon C, Fontaine P, Gillot C, Haye S, Le Guillou AC, Karrouz W, Lemaire C, Lepeut M, Leroy R, Mycinski B, Parent E, Siame C, Sterkers A, Torres F, Verier-Mine O, Verlet E, groups Gw. Desailloud R, Durrbach A, Godin M, Lalau JD, Lukas-Croisier C, Thervet E, Toupance O, Reznik Y, Westeel PF. Treating diabetes with islet transplantation: lessons from the past decade in Lille. Diabetes Metab. 2014;40:108–19. doi: 10.1016/j.diabet.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JRT, Shapiro AMJ. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–9. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 24.Goto M, Tjernberg J, Dufrane D, Elgue G, Brandhorst D, Ekdahl KN, Brandhorst H, Wennberg L, Kurokawa Y, Satomi S, Lambris JD, Gianello P, Korsgren O, Nilsson B. Dissecting the instant blood-mediated inflammatory reaction in islet xenotransplantation. Xenotransplantation. 2008;15:225–34. doi: 10.1111/j.1399-3089.2008.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson B, Ekdahl KN, Korsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant. 2011;16:620–6. doi: 10.1097/MOT.0b013e32834c2393. [DOI] [PubMed] [Google Scholar]
- 26.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–20. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 27.Giuliani M, Moritz W, Bodmer E, Dindo D, Kugelmeier P, Lehmann R, Gassmann M, Groscurth P, Weber M. Central necrosis in isolated hypoxic human pancreatic islets: evidence for postisolation ischemia. Cell Transplant. 2005;14:67–76. doi: 10.3727/000000005783983287. [DOI] [PubMed] [Google Scholar]
- 28.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587–97. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 29.Makhlouf L, Kishimoto K, Smith RN, Abdi R, Koulmanda M, Winn HJ, Auchincloss H, Sayegh MH. The Role of Autoimmunity in Islet Allograft Destruction: Major Histocompatibility Complex Class II Matching Is Necessary for Autoimmune Destruction of Allogeneic Islet Transplants After T-Cell Costimulatory Blockade. Diabetes. 2002;51:3202–10. doi: 10.2337/diabetes.51.11.3202. [DOI] [PubMed] [Google Scholar]
- 30.Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A. 2009;106:15768–73. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricordi C, Goldstein JS, Balamurugan AN, Szot GL, Kin T, Liu C, Czarniecki CW, Barbaro B, Bridges ND, Cano J, Clarke WR, Eggerman TL, Hunsicker LG, Kaufman DB, Khan A, Lafontant DE, Linetsky E, Luo X, Markmann JF, Naji A, Korsgren O, Oberholzer J, Turgeon NA, Brandhorst D, Friberg AS, Lei J, Wang LJ, Wilhelm JJ, Willits J, Zhang X, Hering BJ, Posselt AM, Stock PG, Shapiro AM. National Institutes of Health-Sponsored Clinical Islet Transplantation Consortium Phase 3 Trial: Manufacture of a Complex Cellular Product at Eight Processing Facilities. Diabetes. 2016;65:3418–28. doi: 10.2337/db16-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vos P, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27:5603–17. doi: 10.1016/j.biomaterials.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Steele JAM, Hallé JP, Poncelet D, Neufeld RJ. Therapeutic cell encapsulation techniques and applications in diabetes. Advanced drug delivery reviews. 2014;67–68:74–83. doi: 10.1016/j.addr.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Paredes Juarez GA, Spasojevic M, Faas MM, de Vos P. Immunological and Technical Considerations in Application of Alginate-Based Microencapsulation Systems. Frontiers in Bioengineering and Biotechnology. 2014;2:1–15. doi: 10.3389/fbioe.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim F, Sun A. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–10. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 37.Colton CK. Oxygen supply to encapsulated therapeutic cells. Advanced drug delivery reviews. 2014;67–68C:93–110. doi: 10.1016/j.addr.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 38.de Groot M, Schuurs Ta, Keizer PPM, Fekken S, Leuvenink HGD, van Schilfgaarde R. Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant. 2003;12:867–75. doi: 10.3727/000000003771000219. [DOI] [PubMed] [Google Scholar]
- 39.Anderson DJ, Kirk AD. Primate models in organ transplantation. Cold Spring Harb Perspect Med. 2013;3:a015503–a. doi: 10.1101/cshperspect.a015503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dufrane D, Gianello P. Macro- or microencapsulation of pig islets to cure type 1 diabetes. World journal of gastroenterology: WJG. 2012;18:6885–93. doi: 10.3748/wjg.v18.i47.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bidarra SJ, Barrias CC, Granja PL. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomaterialia. 2014;10:1646–62. doi: 10.1016/j.actbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 42.de Vos P, Lazarjani HA, Poncelet D, Faas MM. Polymers in cell encapsulation from an enveloped cell perspective. Advanced drug delivery reviews. 2014;67–68:15–34. doi: 10.1016/j.addr.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Orive G, Santos E, Poncelet D, Hernández RM, Pedraz JL, Wahlberg LU, De Vos P, Emerich D. Cell encapsulation: technical and clinical advances. Trends Pharmacol Sci. 2015;36:537–46. doi: 10.1016/j.tips.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Draget KI. In: Alginates. Phillips GO, A WP, editors. Cambridge: Woodhead Publishing Limited; 2000. pp. 379–95. [Google Scholar]
- 45.de Vos P, de Haan BJ, Kamps JAAM, Faas MM, Kitano T. Zeta-potentials of alginate-PLL capsules: a predictive measure for biocompatibility? Journal of biomedical materials research Part A. 2007;80:813–9. doi: 10.1002/jbm.a.30979. [DOI] [PubMed] [Google Scholar]
- 46.Bünger CM, Gerlach C, Freier T, Schmitz KP, Pilz M, Werner C, Jonas L, Schareck W, Hopt UT, de Vos P. Biocompatibility and surface structure of chemically modified immunoisolating alginate-PLL capsules. Journal of biomedical materials research Part A. 2003;67:1219–27. doi: 10.1002/jbm.a.10094. [DOI] [PubMed] [Google Scholar]
- 47.Mallett AG, Korbutt GS. Alginate modification improves long-term survival and function of transplanted encapsulated islets. Tissue engineering Part A. 2009;15:1301–9. doi: 10.1089/ten.tea.2008.0118. [DOI] [PubMed] [Google Scholar]
- 48.Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, Fenton P, Kang JW, Hollister-Locke J, Bochenek MA, Chiu A, Siebert S, Tang K, Jhunjhunwala S, Aresta-Dasilva S, Dholakia N, Thakrar R, Vietti T, Chen M, Cohen J, Siniakowicz K, Qi M, McGarrigle J, Lyle S, Harlan DM, Greiner DL, Oberholzer J, Weir GC, Langer R, Anderson DG. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol. 2016 doi: 10.1038/nbt.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Vos P, Marchetti P. Encapsulation of pancreatic islets for transplantation in diabetes: the untouchable islets. Trends Mol Med. 2002;8:363–6. doi: 10.1016/s1471-4914(02)02381-x. [DOI] [PubMed] [Google Scholar]
- 50.Arifin DR, Valdeig S, Anders RA, Bulte JW, Weiss CR. Magnetoencapsulated human islets xenotransplanted into swine: a comparison of different transplantation sites. Xenotransplantation. 2016;23:211–21. doi: 10.1111/xen.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paredes-Juarez GA, de Haan BJ, Faas MM, de Vos P. The role of pathogen-associated molecular patterns in inflammatory responses against alginate based microcapsules. Journal of controlled release: official journal of the Controlled Release Society. 2013;172:983–92. doi: 10.1016/j.jconrel.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Paredes-Juarez G, de Haan B, Faas M, de Vos P. A Technology Platform to Test the Efficacy of Purification of Alginate. Materials. 2014;7:2087–103. doi: 10.3390/ma7032087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 54.Greenfield EM, Beidelschies Ma, Tatro JM, Goldberg VM, Hise AG. Bacterial pathogen-associated molecular patterns stimulate biological activity of orthopaedic wear particles by activating cognate Toll-like receptors. The Journal of biological chemistry. 2010;285:32378–84. doi: 10.1074/jbc.M110.136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar S, Ingle H, Prasad DVR, Kumar H. Recognition of bacterial infection by innate immune sensors. Crit Rev Microbiol. 2012:1–18. doi: 10.3109/1040841X.2012.706249. [DOI] [PubMed] [Google Scholar]
- 56.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 57.Pearl JI, Ma T, Irani AR, Huang Z, Robinson WH, Smith RL, Goodman SB. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials. 2011;32:5535–42. doi: 10.1016/j.biomaterials.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oberbarnscheidt MH, Zecher D, Lakkis FG. The innate immune system in transplantation. Semin Immunol. 2011;23:264–72. doi: 10.1016/j.smim.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paredes-Juarez GA, Sahasrabudhe NM, Tjoelker RS, de Haan BJ, Engelse MA, de Koning EJP, Faas MM, de Vos P. DAMP production by human islets under low oxygen and nutrients in the presence or absence of an immunoisolating-capsule and necrostatin-1. Sci Rep. 2015;5:14623. doi: 10.1038/srep14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piccinini AM, Midwood KS. DAMPening Inflammation by Modulating TLR Signalling. Mediators Inflamm. 2010;2010:1–21. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. Journal of the American Society of Nephrology: JASN. 2011;22:416–25. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nature reviews Nephrology. 2015;12:13–26. doi: 10.1038/nrneph.2015.175. [DOI] [PubMed] [Google Scholar]
- 63.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–23. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Arifin DR, Bulte JW. Imaging of pancreatic islet cells. Diabetes Metab Res Rev. 2011;27:761–6. doi: 10.1002/dmrr.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jirak D, Kriz J, Herynek V, Andersson B, Girman P, Burian M, Saudek F, Hajek M. MRI of transplanted pancreatic islets. Magn Reson Med. 2004;52:1228–33. doi: 10.1002/mrm.20282. [DOI] [PubMed] [Google Scholar]
- 66.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–99. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 67.Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A. In vivo imaging of islet transplantation. Nat Med. 2006;12:144–8. doi: 10.1038/nm1316. [DOI] [PubMed] [Google Scholar]
- 68.Jirak D, Kriz J, Strzelecki M, Yang J, Hasilo C, White DJ, Foster PJ. Monitoring the survival of islet transplants by MRI using a novel technique for their automated detection and quantification. MAGMA. 2009;22:257–65. doi: 10.1007/s10334-009-0172-4. [DOI] [PubMed] [Google Scholar]
- 69.Bulte JW. In vivo MRI cell tracking: clinical studies. AJR Am J Roentgenol. 2009;193:314–25. doi: 10.2214/AJR.09.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saudek F, Jirak D, Girman P, Herynek V, Dezortova M, Kriz J, Peregrin J, Berkova Z, Zacharovova K, Hajek M. Magnetic resonance imaging of pancreatic islets transplanted into the liver in humans. Transplantation. 2010;90:1602–6. doi: 10.1097/tp.0b013e3181ffba5e. [DOI] [PubMed] [Google Scholar]
- 71.Toso C, Vallee JP, Morel P, Ris F, Demuylder-Mischler S, Lepetit-Coiffe M, Marangon N, Saudek F, James Shapiro AM, Bosco D, Berney T. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant. 2008;8:701–6. doi: 10.1111/j.1600-6143.2007.02120.x. [DOI] [PubMed] [Google Scholar]
- 72.Bulte JW. Hot spot MRI emerges from the background. Nat Biotechnol. 2005;23:945–6. doi: 10.1038/nbt0805-945. [DOI] [PubMed] [Google Scholar]
- 73.Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JW. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011;24:114–29. doi: 10.1002/nbm.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barnett BP, Ruiz-Cabello J, Hota P, Ouwerkerk R, Shamblott MJ, Lauzon C, Walczak P, Gilson WD, Chacko VP, Kraitchman DL, Arepally A, Bulte JW. Use of perfluorocarbon nanoparticles for non-invasive multimodal cell tracking of human pancreatic islets. Contrast Media Mol Imaging. 2011;6:251–9. doi: 10.1002/cmmi.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rose LC, Kadayakkara DK, Wang G, Bar-Shir A, Helfer BM, O’Hanlon CF, Kraitchman DL, Rodriguez RL, Bulte JW. Fluorine-19 Labeling of Stromal Vascular Fraction Cells for Clinical Imaging Applications. Stem Cells Transl Med. 2015;4:1472–81. doi: 10.5966/sctm.2015-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eriksson O, Selvaraju R, Eich T, Willny M, Brismar TB, Carlbom L, Ahlstrom H, Tufvesson G, Lundgren T, Korsgren O. Positron Emission Tomography to Assess the Outcome of Intraportal Islet Transplantation. Diabetes. 2016;65:2482–9. doi: 10.2337/db16-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu Y, Dang H, Middleton B, Campbell-Thompson M, Atkinson MA, Gambhir SS, Tian J, Kaufman DL. Long-term monitoring of transplanted islets using positron emission tomography. Mol Ther. 2006;14:851–6. doi: 10.1016/j.ymthe.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 78.van der Kroon I, Andralojc K, Willekens SM, Bos D, Joosten L, Boerman OC, Brom M, Gotthardt M. Noninvasive Imaging of Islet Transplants with 111In-Exendin-3 SPECT/CT. J Nucl Med. 2016;57:799–804. doi: 10.2967/jnumed.115.166330. [DOI] [PubMed] [Google Scholar]
- 79.Barnett BP, Arepally A, Stuber M, Arifin DR, Kraitchman DL, Bulte JW. Synthesis of magnetic resonance-, X-ray- and ultrasound-visible alginate microcapsules for immunoisolation and noninvasive imaging of cellular therapeutics. Nat Protoc. 2011;6:1142–51. doi: 10.1038/nprot.2011.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arifin DR, Kedziorek DA, Fu Y, Chan KW, McMahon MT, Weiss CR, Kraitchman DL, Bulte JW. Microencapsulated cell tracking. NMR Biomed. 2013;26:850–9. doi: 10.1002/nbm.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paredes-Juarez GA, Barnett BP, Bulte JW. Noninvasive Tracking of Alginate-Microencapsulated Cells. Methods Mol Biol. 2017;1479:143–55. doi: 10.1007/978-1-4939-6364-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barnett BP, Kraitchman DL, Lauzon C, Magee CA, Walczak P, Gilson WD, Arepally A, Bulte JW. Radiopaque alginate microcapsules for X-ray visualization and immunoprotection of cellular therapeutics. Mol Pharm. 2006;3:531–8. doi: 10.1021/mp060056l. [DOI] [PubMed] [Google Scholar]
- 83.Arifin DR, Manek S, Call E, Arepally A, Bulte JWM. Microcapsules with intrinsic barium radiopacity for immunoprotection and X-ray/CT imaging of pancreatic islet cells. Biomaterials. 2012;33:4681–9. doi: 10.1016/j.biomaterials.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barnett BP, Arepally A, Karmarkar PV, Qian D, Gilson WD, Walczak P, Howland V, Lawler L, Lauzon C, Stuber M, Kraitchman DL, Bulte JWM. Magnetic resonance–guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat Med. 2007;13:986–91. doi: 10.1038/nm1581. [DOI] [PubMed] [Google Scholar]
- 85.Link TW, Woodrum D, Gilson WD, Pan L, Qian D, Kraitchman DL, Bulte JW, Arepally A, Weiss CR. MR-guided portal vein delivery and monitoring of magnetocapsules: assessment of physiologic effects on the liver. J Vasc Interv Radiol. 2011;22:1335–40. doi: 10.1016/j.jvir.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mills PH, Hitchens TK, Foley LM, Link T, Ye Q, Weiss CR, Thompson JD, Gilson WD, Arepally A, Melick JA, Kochanek PM, Ho C, Bulte JW, Ahrens ET. Automated detection and characterization of SPIO-labeled cells and capsules using magnetic field perturbations. Magn Reson Med. 2012;67:278–89. doi: 10.1002/mrm.22998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arifin DR, Long CM, Gilad AA, Alric C, Roux S, Tillement O, Link TW, Arepally A, Bulte JW. Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked by using US, CT, and positive-contrast MR imaging. Radiology. 2011;260:790–8. doi: 10.1148/radiol.11101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barnett BP, Ruiz-Cabello J, Hota P, Liddell R, Walczak P, Howland V, Chacko VP, Kraitchman DL, Arepally A, Bulte JW. Fluorocapsules for improved function, immunoprotection, and visualization of cellular therapeutics with MR, US, and CT imaging. Radiology. 2011;258:182–91. doi: 10.1148/radiol.10092339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.White JC, Stoppel WL, Roberts SC, Bhatia SR. Addition of perfluorocarbons to alginate hydrogels significantly impacts molecular transport and fracture stress. Journal of Biomedical Materials Research - Part A. 2013;101 A:438–46. doi: 10.1002/jbm.a.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim J, Arifin DR, Muja N, Kim T, Gilad AA, Kim H, Arepally A, Hyeon T, Bulte JW. Multifunctional capsule-in-capsules for immunoprotection and trimodal imaging. Angew Chem Int Ed Engl. 2011;50:2317–21. doi: 10.1002/anie.201007494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Appel AA, Ibarra V, Somo SI, Larson JC, Garson AB, 3rd, Guan H, McQuilling JP, Zhong Z, Anastasio MA, Opara EC, Brey EM. Imaging of Hydrogel Microsphere Structure and Foreign Body Response Based on Endogenous X-Ray Phase Contrast. Tissue Eng Part C Methods. 2016;22:1038–48. doi: 10.1089/ten.tec.2016.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vaithilingam V, Kollarikova G, Qi M, Lacik I, Oberholzer J, Guillemin GJ, Tuch BE. Effect of prolonged gelling time on the intrinsic properties of barium alginate microcapsules and its biocompatibility. J Microencapsul. 2011;28:499–507. doi: 10.3109/02652048.2011.586067. [DOI] [PubMed] [Google Scholar]
- 93.Nöth U, Gröhn P, Jork A, Zimmermann U, Haase A, Lutz J. 19F-MRI in vivo determination of the partial oxygen pressure in perfluorocarbon-loaded alginate capsules implanted into the peritoneal cavity and different tissues. Magn Reson Med. 1999;42:1039–47. doi: 10.1002/(sici)1522-2594(199912)42:6<1039::aid-mrm8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 94.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–26. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 95.Gaglia JL, Guimaraes AR, Harisinghani M, Turvey SE, Jackson R, Benoist C, Mathis D, Weissleder R. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest. 2011;121:442–5. doi: 10.1172/JCI44339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gaglia JL, Harisinghani M, Aganj I, Wojtkiewicz GR, Hedgire S, Benoist C, Mathis D, Weissleder R. Noninvasive mapping of pancreatic inflammation in recent-onset type-1 diabetes patients. Proc Natl Acad Sci U S A. 2015;112:2139–44. doi: 10.1073/pnas.1424993112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang P, Moore A. Theranostic magnetic resonance imaging of type 1 diabetes and pancreatic islet transplantation. Quantitative imaging in medicine and surgery. 2012;2:151–62. doi: 10.3978/j.issn.2223-4292.2012.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bulte JW. The magnetic appeal of silencing theranostics. Diabetes. 2012;61:3068–9. doi: 10.2337/db12-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang P, Yigit MV, Medarova Z, Wei L, Dai G, Schuetz C, Moore A. Combined small interfering RNA therapy and in vivo magnetic resonance imaging in islet transplantation. Diabetes. 2011;60:565–71. doi: 10.2337/db10-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang P, Yigit MV, Ran C, Ross A, Wei L, Dai G, Medarova Z, Moore A. A theranostic small interfering RNA nanoprobe protects pancreatic islet grafts from adoptively transferred immune rejection. Diabetes. 2012;61:3247–54. doi: 10.2337/db12-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lubag AJ, De Leon-Rodriguez LM, Burgess SC, Sherry AD. Noninvasive MRI of beta-cell function using a Zn2+-responsive contrast agent. Proc Natl Acad Sci U S A. 2011;108:18400–5. doi: 10.1073/pnas.1109649108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bar-Shir A, Yadav NN, Gilad AA, van Zijl PC, McMahon MT, Bulte JW. Single (19)F probe for simultaneous detection of multiple metal ions using miCEST MRI. J Am Chem Soc. 2015;137:78–81. doi: 10.1021/ja511313k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xie M, Ye H, Wang H, Charpin-El Hamri G, Lormeau C, Saxena P, Stelling J, Fussenegger M. beta-cell-mimetic designer cells provide closed-loop glycemic control. Science. 2016;354:1296–301. doi: 10.1126/science.aaf4006. [DOI] [PubMed] [Google Scholar]
- 104.van der Torren CR, Verrijn Stuart AA, Lee D, Meerding J, van de Velde U, Pipeleers D, Gillard P, Keymeulen B, de Jager W, Roep BO. Serum Cytokines as Biomarkers in Islet Cell Transplantation for Type 1 Diabetes. PLoS One. 2016;11:e0146649. doi: 10.1371/journal.pone.0146649. [DOI] [PMC free article] [PubMed] [Google Scholar]


