Abstract
Evidence over the past decades has found that stress, particularly through the corticosterone stress hormones, produces complex changes in glutamatergic signaling in prefrontal cortex, which leads to the alteration of cognitive processes medicated by this brain region. Interestingly, the effects of stress on glutamatergic transmission appear to be “U-shaped,” depending upon the duration and severity of the stressor. These biphasic effects of acute vs chronic stress represent the adaptive vs maladaptive responses to stressful stimuli. Animal studies suggest that the stress-induced modulation of excitatory synaptic transmission involves changes in presynaptic glutamate release, postsynaptic glutamate receptor membrane trafficking and degradation, spine structure and cytoskeleton network, and epigenetic control of gene expression. This review will discuss current findings on the key molecules involved in the stress-induced regulation of prefrontal cortex synaptic physiology and prefrontal cortex-mediated functions. Understanding the molecular and epigenetic mechanisms that underlie the complex effects of stress will help to develop novel strategies to cope with stress-related mental disorders.
Keywords: stress, corticosterone stress hormones, glucocorticoid receptor, prefrontal cortex, glutamatergic transmission, AMPA receptor, NMDA receptor, trafficking, protein ubiquitination, stress-related mental disorders, cognition, emotion, histone deacetylase, E3 ligase
Introduction
Significance Statement
Stress, by activating corticosterone stress hormones, produces profound and diverse effects on cognition and emotion. This review summarizes the key molecules involved in the stress-induced regulation of prefrontal cortex (PFC) synaptic physiology and PFC-mediated functions. Revealing the molecular and epigenetic mechanisms that underlie the complex effects of stress will help to understand the multifaceted role of stress in mental health and disorders.
Trauma or stressful events across the lifespan are associated with many medical comorbidities, such as cardiovascular and immunological illnesses (de Kloet et al., 2005; McEwen, 2008; Iwata et al., 2013). Stress is also a critical predisposing factor for psychiatric disorders, including depression, anxiety, posttraumatic stress disorder, and schizophrenia (Agid et al., 1999; Pittenger and Duman, 2008; McEwen and Morrison, 2013). Corticosteroid, the major stress hormone, plays a key role in emotional and cognitive regulation in the CNS. Interestingly, it induces a “U-shaped” effect to maintain brain homeostasis (Diamond et al., 1992; Joels, 2006). Acutely released or moderate levels of corticosteroid mediate a “flight-or-fight” adaptive response in threatening situations. On the other hand, chronically present or high levels of corticosteroid are considerably harmful to brain functioning and are associated with the maladaptive changes in disease processes (Joels, 2006).
It has been believed that genomic mechanism of corticosteroid is responsible for the delayed and long-lasting effect of stress (de Kloet et al., 2005). Mineralocorticoid and glucocorticoid receptors, the major subtypes of corticosteroid receptor, are also ligand-driven transcription factors. Binding of corticosteroid triggers nuclear translocation of the receptor and subsequently influences the expression of genes involved in stress-mediated pathway (Beato and Sanchez-Pacheco, 1996; Joels et al., 2013). Recent studies suggest that corticosteroid can also rapidly influence synaptic activities via nongenomic mechanisms. It is proposed that release of corticosteroid from the HPA-axis orchestrates an array of crosstalk within the limbic areas, such as amygdala, prefrontal cortex (PFC), and hippocampus, to facilitate behavioral adaptation in response to stress (Groeneweg et al., 2011; Joels et al., 2013). Thus, it is thought that stress hormone has a complex impact on brain functions, largely depending on the exposure duration to stress and the associated levels of corticosterone being released. In agreement with this, experimental data have revealed the biphasic effects of stress on synaptic physiology and cognitive behaviors mediated by PFC, a critical brain region implicated in stress-related diseases (Yuen et al., 2009, 2011, 2012; Popoli et al., 2011; McEwen and Morrison, 2013). This review will discuss current findings on the molecular basis underlying the regulation of synaptic physiology and cognitive functions by corticosteroid stress hormones.
Stress Effects on Cognitive Functions
Human studies support the procognitive effect produced by acute stress. Imaging of functional magnetic resonance imaging (fMRI) shows that acute stress enhances PFC signals during the performance of working memory tasks (Porcelli et al., 2008). Oral administration of hydrocortisone improves working memory performance and elevates dorsolateral PFC activity in humans (Henckens et al., 2011). Moreover, using pharmacological tools to decrease cortisol levels significantly impairs the PFC-dependent cognitive performance, which is restored by hydrocortisone replacement (Lupien et al., 2002a, 2002b). Correlating to human studies, acute stress significantly improves the performance of working memory in young rats (Yuen et al., 2009, 2011). Interestingly, acute stress can also enhance the later ability to acquire new associative memory. Animals exposed to the unescapable tail shock stress have the facilitated classical conditioning of eyeblink responses (Shors et al., 1992; Shors, 2006). Such ability is lost when peripheral adrenal medulla, the glucocorticoid-releasing organ, is removed (Beylin and Shors, 2003).
In addition to the procognitive effect of acute stress, some human and animal studies also show that acute, uncontrollable stress impairs PFC-mediated cognitive functions (Arnsten, 2009; Hermans et al., 2014; Arnsten et al, 2015). For instance, fMRI studies unveil that healthy participants who are exposed to an experimentally induced acute psychological stress show significantly impaired working memory-related activity in the dorsolateral PFC (Qin et al., 2009). Exposing monkeys to continuous loud noise stress for 30 minutes impairs delayed-response performance in the spatial working memory task, a PFC-mediated cognitive function (Arnsten and Goldman-Rakic, 1998). Rats exposed to acute (15 minute) tail-pinch stress have less accurate recall on a PFC-dependent cognitive task that is sensitive to working memory deficits, and the stress-induced errors are reversed by blockade of glucocorticoid receptors in the medial PFC (Butts et al., 2011). The impairing effect of acute stress often occurs at the very early stage, which is likely due to the fast increase of the concentration of catecholamines, such as norepinephrine and dopamine, in the frontal cortical region (Abercrombie et al., 1989; Finlay et al., 1995; Murphy et al., 1996; Marsteller et al., 2002; Pascucci et al., 2007). High levels of noradrengergic and dopaminergic receptor activation trigger intracellular signaling pathways, which reduces the excitability of PFC neurons, leading to the weakening of PFC function (Birnbaum et al., 2004; Vijayraghavan et al., 2007; Gamo et al., 2015).
To integrate the intricate stress responses, the factor of timing with respect to stressor onset needs to be considered (Hermans et al., 2014). Human studies have found that the PFC activity related to an executive control task decreases shortly following stress induction (Qin et al., 2009) but enhances at a 240-minute delay (Henckens et al., 2011). It has been proposed that the rapid changes in catecholamines / glucocorticoids ratios in response to acute stress may determine the diverse effects on cognitive processes (Hermans et al., 2014). Right after acute stress, the increased catecholamines promote vigilance at the cost of an executive control network. After stress subsides, the increased glucocorticoids enhance higher-order cognitive processes for long-term survival (Hermans et al., 2014).
Importantly, the outcome of stress appears to be determined by the duration and severity of the stressor (de Kloet et al., 2005; Joels M, 2008). Contrary to acute stress, repeated or prolonged unpredictable stress causes the prominent deficit in working memory and recognition memory (Yuen et al., 2012), depression-like phenotypes, including increased immobility in tail suspension test, and increased latency to feed in novelty-suppressed feeding (Seo et al., 2016). In humans, impairment of cognitive function also correlates to the duration of stressor. Imaging studies show that 1-month exposure to psychosocial stressor produces a long-standing but reversible impairment of the PFC-dependent attention shifting task (Liston et al., 2009). However, cumulative life stressful events, such as death or chronic illness of a family member, cause a prominent volume loss in medical PFC and irreversible deficit in spatial working memory (Ansell et al., 2012; Hanson et al., 2012). Taken together, these results suggest that stress exerts complex effects on PFC-mediated functions presumably by linking to divergent molecular pathways.
Stress Effects on Synaptic Physiology
The Impact of Acute Stress on Glutamatergic Transmission
Introducing a short period of stressful stimuli to animals, such as forced swim, foot shock, or restraint, produces significantly enhanced glutamatergic transmission in PFC circuitry. This increased transmission is shown to be related to the enhancement of readily releasable pool of glutamate vesicles via a nongenomic mechanism mediated by membrane receptors (Moghaddam, 1993; Bagley and Moghaddam, 1997; Popoli et al., 2011; Treccani et al., 2014). Such stress-induced increase in glutamate release is caused by the accumulation of presynaptic SNARE complexes in synaptic membranes of PFC neurons (Musazzi et al., 2010; Tardito et al., 2010). Moreover, acute footshock stress increases spine density and excitatory synapses and induces dendritic remodeling in medial PFC, which can be partially blocked by chronic treatment with the antidepressant desipramine (Nava et al., 2014, 2017a; Musazzi et al., 2015). Interestingly, recent study reveals that although both acute stress and in vitro application of corticosterone increase the size of readily releasable pool of synaptic vesicles in PFC, only acute stress enhances depolarization-evoked release of glutamate in PFC, which is positively correlated with phosphorylated synapsin I in PFC synaptic membranes (Musazzi et al., 2010; Treccani et al, 2014).
Besides presynaptic increase of glutamate release, acute stress also produces postsynaptic modification at PFC synapses. Patch-clamp recordings in PFC pyramidal neurons taken from acutely stressed animals shows a delayed and long-lasting potentiation of both NMDAR- and AMPAR-mediated synaptic currents (Yuen et al., 2009, 2011). The delayed time course suggests a genomic mechanism. Serum- and glucocorticoid-inducible kinases (SGKs), an immediate early gene activated by stress hormone, is shown to be involved in the stress-mediated glutamate receptor trafficking in PFC neurons (Yuen et al., 2012). Activation of SGK enhances the activity of Rab4, a small GTPase mediating the trafficking of glutamate receptors from early endosomes to plasma membrane (Liu et al., 2010; Popoli et al., 2011; Yuen et al., 2012). SGK is proposed to be one of the critical regulators of learning and memory. Transfecting SGK facilitates spatial memory performance in rats, and elevated SGK expression levels are found in hippocampus of rats with faster learning (Tsai et al., 2002). On the other hand, reduced SGK expression is found in postmortem brains of PTSD patients, and inhibition of SGK in rat PFC produces helplessness- and anhedonic-like phenotypes (Licznerski et al., 2015).
Posttranslational modification of glutamate receptors may also play a role in the acute footshock stress-induced, time-dependent modification of AMPAR and NMDAR subunits at PFC (Bonini et al., 2016). Phosphorylation of GluR1 at Ser 845, which is linked to the increased AMPAR channel open probability (Wangs et al., 2005), is elevated immediately after stress (Bonini et al., 2016). At 2 hours after start of stress, NR1 and NR2A subunits in postsynaptic spines are markedly upregulated, and phosphorylation of GluR2 at Ser 880, which is involved in promoting AMPAR internalization (Scannevin et al, 2000), is elevated (Bonini et al., 2016). These changes may underlie the early enhancement of AMPAR-mediated currents, followed by the potentiation of NMDAR-mediated currents in animals exposed to footshock stress.
The role of acute stress in glutamate receptor trafficking is supported by additional studies. A single (60 minute) restraint stress enhances the expression of Arc, an activity-dependent cytoskeletal-associated protein involved in AMPAR endocytosis (Fumagalli et al., 2011). Interference of adhesion molecules that anchor glutamate receptors at the synaptic surface abolishes the acute stress-induced enhancement of GluR2 membrane trafficking and memory facilitation (Conboy and Sandi, 2010). In addition, acute footshock stress induces both rapid and sustained alterations of the expression of key genes involved in synaptic plasticity and spine structure, such as Homer, Shank, Spinophilin, Rac1, and downstream target genes Limk1, Cofilin1, and Rock1 (Nava et al., 2017b). Overall, acute stress facilitates postsynaptic signaling molecules or adhesion/cytoskeleton networks that support the synaptic trafficking of glutamate receptors.
The Impact of Chronic Stress on Glutamatergic Transmission
Impairment of cognitive flexibility in chronically stressed individuals has been associated with the suppression of mPFC activity (Liston et al, 2006). A 21-day restraint stress produces impaired dendritic branching, atrophy, and spine loss in PFC pyramidal neurons (Radley et al., 2006; Popoli et al., 2011; Musazzi et al., 2015), and such structural reorganization is found to be reversible after 3-week cessation of stress (Radley et al., 2005). Having a prior chronic exposure to corticosterone causes a reduction of NR2B and GluR2/3 subunit expression in ventromedial PFC (Gourley et al., 2009). Consistently, a prominent loss of GluR1 and NR1 subunit expression has been found in PFC pyramidal neurons from repeatedly stressed animals (Yuen et al., 2012). Such changes lead to a long-lasting depression of both NMDAR- and AMPAR-mediated synaptic currents in PFC.
The loss of glutamate receptor expression in PFC of repeatedly stressed animals is attributable to the increased ubiquitin/proteasome-mediated degradation, which is controlled by E3 ubiquitin ligases Nedd4 and Fbx2. Inhibition of proteasomes or knockdown of Nedd4 and Fbx2 in PFC abolishes the loss of glutamate receptors by repeated stress (Yuen et al., 2012). The transcription of Nedd4 is upregulated by repeated stress via an epigenetic mechanism involving the elevated histone deacetylase 2 (HDAC2). HDAC2 inhibitors prevent the impairment of glutamate receptors and excitatory transmission in PFC of chronically stressed animals (Wei et al., 2016).
Chronic unpredictable stress has also been found to induce extracellular glutamate accumulation and the enhanced NR2B-mediated extrasynaptic response, which is associated with the increased interaction of Death-associated protein kinase 1 (DAPK1) with NMDARs (Li et al., 2017a). Uncoupling of the DAPK-NR2B complex, knockdown of DAPK, and pharmacological blockade of NR2B all produce the rapid antidepressant effects in chronically stressed animals (Li et al., 2017a).
Additional Molecular Players Involved in Stress Effects
A multifunctional protein highly enriched in layer II-III PFC pyramidal neurons, p11, has been found to play an important role in stress-induced depression (Seo et al., 2016). p11 interacts with 5-HT receptors, ion channels, enzymes, and chromatin-remodeling factors and is critically involved in depression-related behaviors and/or antidepressant actions (Svenningsson et al., 2013). Chronic restraint stress induces the selective loss of p11 in PFC. Viral expression of p11 in PFC rescues the stress-induced suppression of glutamatergic transmission and depression-like behaviors (Seo et al., 2016).
Neurotrophic factors, such as brain derived trophic factor (BDNF), vascular endothelial growth factor, fibroblast growth factor 2, and insulin-like growth factor 1 (IGF1) are suggested as one of the important players in synaptic plasticity induced by long-term stress (Hill et al., 2011; Musazzi et al., 2011; Duman et al., 2016). Individuals carrying the Val66met allele of the BDNF gene have increased vulnerability to stress and antidepressant responses (Yu et al., 2012; Nava et al., 2014, 2015). Such a polymorphism shows the decreased activity-dependent BDNF secretion (Egan et al., 2003). BDNF expression is suppressed in animals exposed to various stress paradigms (Vaidya et al., 1997; Treccani et al., 2014; Musazzi et al., 2016). Application of corticosterone decreases BDNF expression (Schaaf et al., 1998) but increases BDNF in animals undergoing adrenalectomy (Chao et al., 1998). BDNF overexpression increases dendritic arborization in hippocampal neurons (Tolwani et al., 2002), blocks chronic stress-induced hippocampal atrophy, and improves depression-like behaviors (Govindarajan et al., 2006). Chronic stress is also known for suppressing neurogenesis, a process promoting proliferation and survival of newborn neurons in adult brain (Duman, 2004). Antidepressant treatment reverses the stress-induced downregulation of neurogenesis (Duman, 2004), which is likely through BDNF-mediated tyrosine kinase-regulated signal transduction (Duman and Monteggia, 2006).
Many other molecular targets of stress are also involved in synaptic alteration. Animals exposed to chronic unpredictable stress have the decreased expression of Neuritin, a synaptic activity-dependent gene, which is reversed by antidepressant treatment. Viral knockdown of Neurtitin prevents the stress-induced atrophy of dendrites and spines and the depression-like behaviors (Son et al., 2012).
Another stress-activated molecule, mTORC (also known as mammalian target of rapamycin complex), also receives much attention in the field. The mTORC signaling is found to be suppressed by cellular stress (Corradetti et al., 2005). Decreased levels of mTORC are reported in postmortem brains of individuals with stress-related mood disorders (Jernigan et al., 2011), whereas the rapid-acting antidepressant ketamine increases mTORC signaling in rat PFC (Li et al., 2010). REDD1 (regulated in development and DNA damage responses-1) is an endogenous inhibitor of mTOR. Enhanced expression of REDD1, together with inhibition of downstream cascades of mTOR, are concomitantly found in the PFC of animals exposed to 21-day unpredictable stress (Ota et al., 2014). Animals with REDD1 knockdown have greater resilience to the chronic stress-induced spine shrinkage and AMPAR current reduction (Ota et al., 2014). Moreover, REDD1 level is found to be significantly elevated in postpartum depressed human brains and is thought to play a key role in the stress-induced depressive phenotypes (Ota et al., 2014).
Recent studies propose a new concept that inflammatory cytokine can be a central mediator linking stress to psychiatric disorders and other systemic diseases (Musazzi et al., 2011; Iwata et al., 2013; Duman et al., 2016). Supporting this theory, depressed patients show elevated proinflammatory cytokines, such as tumor necrosis factor and interleukin 1β, which are reversed by antidepressant treatment (Pascucci et al., 2007; Arnsten, 2009; Dowlati et al., 2010). Pharmacological blockade or genetic knockout of caspase-1, an interleukin 1β-converting enzyme, prevents the chronic restraint stress-induced, depressive-like phenotypes in mice by stabilizing surface AMPARs (Li et al., 2017b). Interestingly, levels of cytokine also demonstrate a biphasic relationship with synaptic transmission. It is suggested that intact glutamatergic transmission requires a moderate level of inflammatory molecules. A low level of cytokine promotes new AMPAR insertion and glutamate release from astrocyte in an activity-dependent synaptic modification (Santello and Volterra, 2012). However, “too much” inflammatory cytokine leads to impairment of long-term potentiation and synaptic loss (Finlay et al., 1995; Boulanger, 2009; Arnsten et al., 2015). Blocking the activation of cytokine reverses anhedonic phenotypes induced by chronic unpredictable stress (Iwata et al., 2016).
Epigenetic Factors in Stress Effects
It has been a fascinating question whether stressful experience or its phenotype can be transmitted across generations. If so, what are the molecular substrates to determine vulnerability or resilience to stress? It is shown that chronic maternal separation alters the profile of DNA methylation at particular genes in the sperm of stressed animals (Franklin et al., 2010) and alters the HPA stress responsivity of offspring (Rodgers et al., 2013). Interestingly, injecting sperm RNAs from stressed males into wild-type oocytes creates offspring with behavioral and metabolic phenotypes similar to the stressed father (Gapp et al., 2014). Recent studies have identified genes that contribute to stress susceptibility, including the ones within the HPA axis (Polanczyk et al., 2009), serotonin receptors (Yu et al., 2012; Nava et al., 2014, 2015), and neuropeptide Y (Finlay et al., 1995; Marsteller et al., 2002; Domschke et al., 2010; Liu et al., 2010).
Emerging evidence indicates that aberrant gene transcription via chromatin remodeling or histone modifications contributes to the stress-induced maladaptive changes, including neuronal plasticity, synaptic neurotransmission, as well as cognitive processes. In response to chronic stress, histone acetylation level is robustly changed in different brain regions. In nucleus accumbens, decreased HDAC2 and HDAC5 expression is observed in depressed animals or humans (Renthal et al., 2007; Covington et al., 2009). In the hippocampus of stressed animals, global acety-H3K14 shows a transient increase, followed by a persistent decrease, which is associated with changes in BDNF gene expression (Tsankova et al., 2006; Covington et al., 2011). In amygdala, H3K14 acetylation is found to be transiently increased after social defeat stress, while HDAC5 is significantly decreased after unpredictable stress (Covington et al., 2011; Sterrenburg et al., 2011). Repeated stress increases HDAC2 in rat PFC, which causes the epigenetic alteration of Nedd4, an E3 ubiquitin ligase for AMPAR degradation, leading to the impairment of AMPAR expression and cognitive function (Wei et al., 2016). In addition, various HDAC inhibitors are implicated in antidepressant responses in stressed animals (Covington et al., 2009; Sun et al., 2013; Bagot et al., 2014; Wei et al., 2016).
In addition to histone acetylation, DNA methylation is found to be altered in stressed animals. Animals with better maternal care are found to have decreased DNA methylation of the glucocorticoid receptor gene, leading to the increased expression of the receptors and more resilient stress response in adult (Weaver et al., 2005). Such resilience can be reversed by introducing hypermethylation of glucocorticoid receptors in adult rats (Marsteller et al., 2002; Tsai et al., 2002; Weaver et al., 2005; Liu et al., 2010). Genes controlling HPA axis adaptation have also been found to contribute to stress resilience. The expression of Corticotropin-Releasing Hormone is elevated in the hypothalamus of animals that develop social avoidance after exposure to chronic social defect stress (Elliott et al., 2010). However, in the subset of animals that do not show stress-induced social avoidance, their Corticotropin-Releasing Hormone gene is hypermethylated. Environmental enrichment reduces basal ACTH and stress responses (Moncek et al., 2004), suggesting a possible link among epigenetic, genetic, and environmental factors contributing to the HPA stress response.
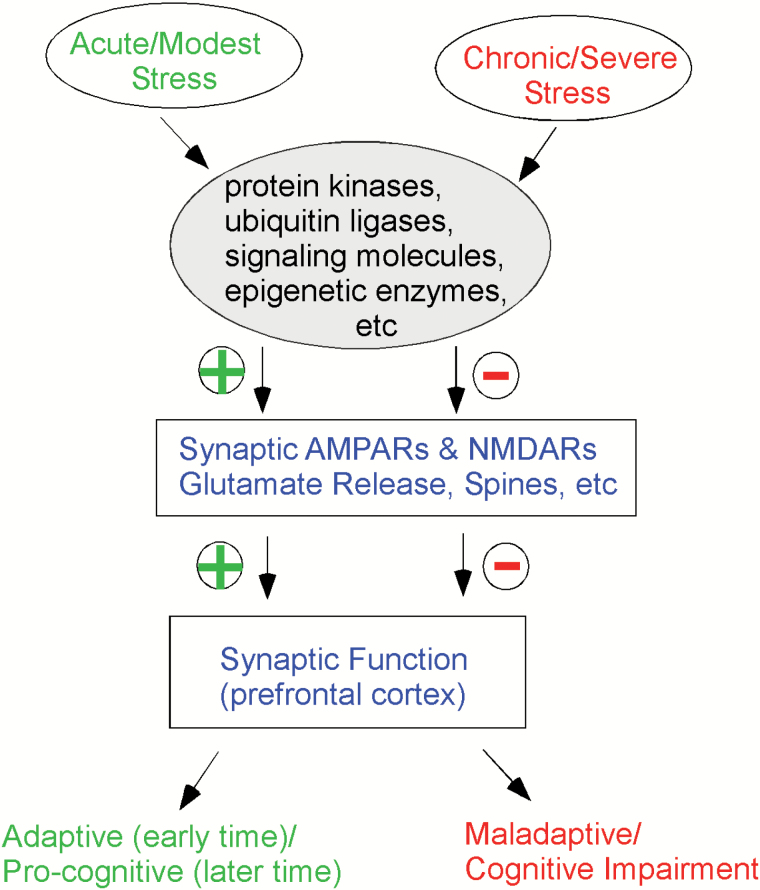
In conclusion, recent studies introduce the concept of biphasic stress responses in cognitive processes mediated by prefrontal cortex, through modulating molecular substrates at glutamatergic synapses (Figure 1). Changes in presynaptic glutamate release, postsynaptic glutamate receptor trafficking and expression, spine structure and cytoskeleton network, and epigenetic control of plasticity genes all contribute to the complex effects of stress. Despite the richness of information in this field, there are still key questions waiting to be answered. For example, how is the adaptive response to short-term modest stress switched to the maladaptive response to long-term severe stress? Why does the vulnerability to stress differ a lot among individuals? How much translational value do the results from animal studies have? Understanding the mechanisms that regulate glutamatergic synaptic function in PFC may shed light on identifying pathophysiology and novel pharmacological intervention for stress-related psychiatric disorders.
Figure 1.
A diagram illustrating the complex effects of stress on prefrontal cortex (PFC) synaptic physiology and PFC-mediated functions. Acute/modest stress or chronic/severe stress induces divergent changes on protein kinases, ubiquitin ligases, signaling molecules, and epigenetic enzymes, which leads to the convergent and opposite alterations of postsynaptic glutamate receptors, presynaptic glutamate release, and dendritic spine structure. Consequently, glutamatergic synaptic function in prefrontal cortex is bi-directionally changed, resulting in adaptive or maladaptive effects on cognitive processes. In response to acute stress, executive control can be compromised at the early time point as a result of the promoted emotional reactivity for short-term adaptation, while higher-order cognitive processes are enhanced at later time points for long-term survival.
Statement of Interest
None.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants (MH108842 and DA037618) and a generous gift from E. F. Trachtman to Z.Y.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ (1989) Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem 52:1655–1658. [DOI] [PubMed] [Google Scholar]
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B (1999) Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry 4:163–172. [DOI] [PubMed] [Google Scholar]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R (2012) Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry 72:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. (2009) Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS (1998) Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry 55:362–368. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Raskind MA, Taylor FB, Connor DF (2015) The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress 1:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley J, Moghaddam B (1997) Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience 77:65–73. [DOI] [PubMed] [Google Scholar]
- Bagot RC, Labonté B, Peña CJ, Nestler EJ (2014) Epigenetic signaling in psychiatric disorders: stress and depression. Dialogues Clin Neurosci 16:281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M, Sanchez-Pacheco A (1996) Interaction of steroid hormone receptors with the transcription initiation complex. Endocr Rev 17:587–609. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Shors TJ (2003) Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Horm Behav 43:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, Gobeske KT, Sweatt JD, Manji HK, Arnsten AF (2004) Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science 306:882–884. [DOI] [PubMed] [Google Scholar]
- Bonini D, Mora C, Tornese P, Sala N, Filippini A, La Via L, Milanese M, Calza S, Bonanno G, Racagni G, Gennarelli M, Popoli M, Musazzi L, Barbon A (2016) Acute footshock stress induces time-dependent modifications of AMPA/NMDA protein expression and AMPA phosphorylation. Neural Plast 2016:7267865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger LM. (2009) Immune proteins in brain development and synaptic plasticity. Neuron 64:93–109. [DOI] [PubMed] [Google Scholar]
- Butts KA, Weinberg J, Young AH, Phillips AG (2011) Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc Natl Acad Sci USA 108:18459–18464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HM, Sakai RR, Ma LY, McEwen BS (1998) Adrenal steroid regulation of neurotrophic factor expression in the rat hippocampus. Endocrinology 139:3112–3118. [DOI] [PubMed] [Google Scholar]
- Conboy L, Sandi C (2010) Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology 35:674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradetti MN, Inoki K, Guan KL (2005) The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem 280:9769–9772. [DOI] [PubMed] [Google Scholar]
- Covington HE 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ (2009) Antidepressant actions of histone deacetylase inhibitors. J Neurosci 29:11451–11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE 3rd, Maze I, Sun H, Bomze HM, DeMaio KD, Wu EY, Dietz DM, Lobo MK, Ghose S, Mouzon E, Neve RL, Tamminga CA, Nestler EJ (2011) A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron 71:656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, Vialou VF, LaPlant Q, Ohnishi YN, Nestler EJ (2011) Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neurosci Lett 493:122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E R, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM (1992) Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus 2:421–430. [DOI] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U, Hohoff C, Ohrmann P, Bauer J, Kugel H, Zwanzger P, Heindel W, Deckert J, Arolt V, Suslow T, Baune BT (2010) Neuropeptide Y (NPY) gene: Impact on emotional processing and treatment response in anxious depression. Eur Neuropsychopharmacol 20:301–309. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457. [DOI] [PubMed] [Google Scholar]
- Duman RS. (2004) Depression: a case of neuronal life and death? Biol Psychiatry 56:140–145. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257–269. [DOI] [PubMed] [Google Scholar]
- Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A (2010) Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci 13:1351–1353. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED (1995) Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience 64:619–628. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, Vizi S, Mansuy IM (2010) Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 68:408–415. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Caffino L, Vogt MA, Frasca A, Racagni G, Sprengel R, Gass P, Riva MA (2011) AMPA GluR-A receptor subunit mediates hippocampal responsiveness in mice exposed to stress. Hippocampus 21:1028–1035. [DOI] [PubMed] [Google Scholar]
- Gamo NJ, Lur G, Higley MJ, Wang M, Paspalas CD, Vijayraghavan S, Yang Y, Ramos BP, Peng K, Kata A, Boven L, Lin F, Roman L, Lee D, Arnsten AF (2015) Stress Impairs Prefrontal Cortical Function via D1 Dopamine Receptor Interactions With Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels. Biol Psychiatry 78:860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM (2014) Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 17:667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR (2009) A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology 34:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S (2006) Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci U S A 103:13208–13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joels M (2011) Rapid non-genomic effects of corticosteroids and their role in the central stress response. J Endocrinol 209:153–167. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Rudolph KD, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD (2012) Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. J Neurosci 32:7917–7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, van Wingen GA, Joëls M, Fernández G (2011) Time-dependent corticosteroid modulation of prefrontal working memory processing. Proc Natl Acad Sci U S A 108:580–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, Henckens MJ, Joëls M, Fernández G (2014) Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci 37:304–314. [DOI] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Duman RS (2013) The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun 31:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, Banasr M, Duric V, Yamanashi T, Kaneko K, Rasmussen K, Glasebrook A, Koester A, Song D, Jones KA, Zorn S, Smagin G, Duman RS (2016) Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol Psychiatry 80:12–22. [DOI] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B (2011) The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 35:1774–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M. (2006) Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci 27:244–250. [DOI] [PubMed] [Google Scholar]
- Joels M. (2008) Functional actions of corticosteroids in the hippocampus. Eur J Pharmacol 583:312–321. [DOI] [PubMed] [Google Scholar]
- Joels M, Pasricha N, Karst H (2013) The interplay between rapid and slow corticosteroid actions in brain. Eur J Pharmacol 719:44–52. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Han Y, Xu LZ, Yuan K, Zhang RX, Sun CY, Xu DF, Yuan M, Deng JH, Meng SQ, Gao XJ, Wen Q, Liu LJ, Zhu WL, Xue YX, Zhao M, Shi J, Lu L (2017a) Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects. Molecular Psychiatry (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MX, Zheng HL, Luo Y, He JG, Wang W, Han J, Zhang L, Wang X, Ni L, Zhou HY, Hu ZL, Wu PF, Jin Y, Long LH, Zhang H, Hu G, Chen JG, Wang F (2017b) Gene deficiency and pharmacological inhibition of caspase-1 confers resilience to chronic social defeat stress via regulating the stability of surface AMPARs. Molecular Psychiatry (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licznerski P, Duric V, Banasr M, Alavian KN, Ota KT, Kang HJ, Jonas EA, Ursano R, Krystal JH, Duman RS; Traumatic Stress Brain Study Group (2015) Decreased SGK1 expression and function contributes to behavioral deficits induced by traumatic stress. PLoS Biol 13:e1002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ (2009) Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A 106:912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS (2006) Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 26:7870–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Yuen EY, Yan Z (2010) The stress hormone corticosterone increases synaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors via serum- and glucocorticoid-inducible kinase (SGK) regulation of the GDI-Rab4 complex. J Biol Chem 285:6101–6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Brière S, Ménard C, Ng Ying Kin NM, Nair NP (2002a) The modulatory effects of corticosteroids on cognition: studies in young human populations. Psychoneuroendocrinology 27:401–416. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Brière S, Ng Ying Kin NM, Meaney MJ, Nair NP (2002b) Acute modulation of aged human memory by pharmacological manipulation of glucocorticoids. J Clin Endocrinol Metab 87:3798–3807. [DOI] [PubMed] [Google Scholar]
- Marsteller DA, Gerasimov MR, Schiffer WK, Geiger JM, Barnett CR, Schaich Borg J, Scott S, Ceccarelli J, Volkow ND, Molina PE, Alexoff DL, Dewey SL (2002) Acute handling stress modulates methylphenidate-induced catecholamine overflow in the medial prefrontal cortex. Neuropsychopharmacology 27:163–170. [DOI] [PubMed] [Google Scholar]
- McEwen BS. (2008) Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583:174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH (2013) The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B. (1993) Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem 60:1650–1657. [DOI] [PubMed] [Google Scholar]
- Moncek F, Duncko R, Johansson BB, Jezova D (2004) Effect of environmental enrichment on stress related systems in rats. J Neuroendocrinol 16:423–431. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH (1996) Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci U S A 93:1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, Bonifacino T, Mallei A, Baldelli P, Racagni G, Raiteri M, Benfenati F, Bonanno G, Popoli M (2010) Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One 5:e8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L, Treccani G, Popoli M (2015) Functional and structural remodeling of glutamate synapses in prefrontal and frontal cortex induced by behavioral stress. Front Psychiatry 6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava N, Treccani G, Liebenberg N, Chen F, Popoli M, Wegener G, Nyengaard JR (2014) Chronic desipramine prevents acute stress-induced reorganization of medial prefrontal cortex architecture by blocking glutamate vesicle accumulation and excitatory synapse increase. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava N, Treccani G, Alabsi A, Kaastrup Mueller H, Elfving B, Popoli M, Wegener G, Nyengaard JR (2017a) Temporal dynamics of acute stress-induced dendritic remodeling in medial prefrontal cortex and the protective effect of desipramine. Cereb Cortex 27:694–705. [DOI] [PubMed] [Google Scholar]
- Nava N, Treccani G, Müller HK, Popoli M, Wegener G, Elfving B (2017b) The expression of plasticity-related genes in an acute model of stress is modulated by chronic desipramine in a time-dependent manner within medial prefrontal cortex. Eur Neuropsychopharmacol 27:19–28. [DOI] [PubMed] [Google Scholar]
- Ota KT, Liu RJ, Voleti B, Maldonado-Aviles JG, Duric V, Iwata M, Dutheil S, Duman C, Boikess S, Lewis DA, Stockmeier CA, DiLeone RJ, Rex C, Aghajanian GK, Duman RS (2014) REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med 20:531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascucci T, Ventura R, Latagliata EC, Cabib S, Puglisi-Allegra S (2007) The medial prefrontal cortex determines the accumbens dopamine response to stress through the opposing influences of norepinephrine and dopamine. Cereb Cortex 17:2796–2804. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33:88–109. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE (2009) Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry 66:978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G (2011) The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli AJ, Cruz D, Wenberg K, Patterson MD, Biswal BB, Rypma B (2008) The effects of acute stress on human prefrontal working memory systems. Physiol Behav 95:282–289. [DOI] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJ, Luo J, Fernández G (2009) Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry 66:25–32. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH (2005) Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol 196:199–203. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH (2006) Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 16:313–320. [DOI] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE 3rd, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, Kerstetter KA, Neve RL, Haggarty SJ, McKinsey TA, Bassel-Duby R, Olson EN, Nestler EJ (2007) Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron 56:517–529. [DOI] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL (2013) Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 33:9003–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Volterra A (2012) TNFalpha in synaptic function: switching gears. Trends Neurosci 35:638–647. [DOI] [PubMed] [Google Scholar]
- Scannevin RH, Huganir RL (2000) Postsynaptic organization and regulation of excitatory synapses. Nat Rev Neurosci 1:133–141. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E (1998) Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res 813:112–120. [DOI] [PubMed] [Google Scholar]
- Seo JS, Wei J, Qin L, Kim Y, Yan Z, Greengard P (2016) Cellular and molecular basis for stress-induced depression. Mol Psychiatry. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. (2006) Stressful experience and learning across the lifespan. Annu Rev Psychol 57:55–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Weiss C, Thompson RF (1992) Stress-induced facilitation of classical conditioning. Science 257:537–539. [DOI] [PubMed] [Google Scholar]
- Son H, Banasr M, Choi M, Chae SY, Licznerski P, Lee B, Voleti B, Li N, Lepack A, Fournier NM, Lee KR, Lee IY, Kim J, Kim JH, Kim YH, Jung SJ, Duman RS (2012) Neuritin produces antidepressant actions and blocks the neuronal and behavioral deficits caused by chronic stress. Proc Natl Acad Sci U S A 109:11378–11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, Chen A, Peeters BW, Roubos EW, Kozicz T (2011) Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS One 6:e28128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Kennedy PJ, Nestler EJ (2013) Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology 38:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Kim Y, Warner-Schmidt J, Oh YS, Greengard P (2013) p11 and its role in depression and therapeutic responses to antidepressants. Nat Rev Neurosci 14:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardito D, Milanese M, Bonifacino T, Musazzi L, Grilli M, Mallei A, Mocaer E, Gabriel-Gracia C, Racagni G, Popoli M, Bonanno G (2010) Blockade of stress-induced increase of glutamate release in the rat prefrontal/frontal cortex by agomelatine involves synergy between melatonergic and 5-HT2C receptor-dependent pathways. BMC Neurosci 11:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwani RJ, Buckmaster PS, Varma S, Cosgaya JM, Wu Y, Suri C, Shooter EM (2002) BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience 114:795–805. [DOI] [PubMed] [Google Scholar]
- Treccani G, Musazzi L, Perego C, Milanese M, Nava N, Bonifacino T, Lamanna J, Malgaroli A, Drago F, Racagni G, Nyengaard JR, Wegener G, Bonanno G, Popoli M (2014) Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol Psychiatry 19:433–443. [DOI] [PubMed] [Google Scholar]
- Tsai KJ, Chen SK, Ma YL, Hsu WL, Lee EH (2002) sgk, a primary glucocorticoid-induced gene, facilitates memory consolidation of spatial learning in rats. Proc Natl Acad Sci U S A 99:3990–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ (2006) Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9:519–525. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, Duman RS (1997) 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci 17:2785–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF (2007) Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 10:376–384. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Arora A, Yang L, Parelkar NK, Zhang G, Liu X, Choe ES, Mao L (2005) Phosphorylation of AMPA receptors: mechanisms and synaptic plasticity. Mol Neurobiol 32:237–249. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M (2005) Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci 25:11045–11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Xiong Z, Lee JB, Cheng J, Duffney LJ, Matas E, Yan Z (2016) Histone Modification of Nedd4 Ubiquitin Ligase Controls the Loss of AMPA Receptors and Cognitive Impairment Induced by Repeated Stress. J Neurosci 36:2119–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY (2012) Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci 32:4092–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z (2009) Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A 106:14075–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z (2011) Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry 16:156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z (2012) Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73:962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]