Abstract
Background
Hydrogen sulfide (H2S) is a crucial signaling molecule with a wide range of physiological functions. Previously, we confirmed that stress-induced depression is accompanied with disturbance of H2S generation in hippocampus. The present work attempted to investigate the inhibitory effect of H2S on chronic unpredictable mild stress-induced depressive-like behaviors and the underlying mechanism.
Methods
We established the rat model of chronic unpredictable mild stress to simulate depression. Open field test, forced swim test, and tail suspension test were used to assess depressive-like behaviors. The expression of Sirt-1 and three marked proteins related to endoplasmic reticulum stress (GRP-78, CHOP, and cleaved caspase-12) were detected by western blot.
Results
We found that chronic unpredictable mild stress-exposed rats exhibit depression-like behavior responses, including significantly increased immobility time in the forced swim test and tail suspension test, and decreased climbing time and swimming time in the forced swim test. In parallel, chronic unpredictable mild stress-exposed rats showed elevated levels of hippocampal endoplasmic reticulum stress and reduced levels of Sirt-1. However, NaHS (a donor of H2S) not only alleviated chronic unpredictable mild stress-induced depressive-like behaviors and hippocampal endoplasmic reticulum stress, but it also increased the expression of hippocampal Sirt-1 in chronic unpredictable mild stress-exposed rats. Furthermore, Sirtinol, an inhibitor of Sirt-1, reversed the protective effects of H2S against chronic unpredictable mild stress-induced depression-like behaviors and hippocampal endoplasmic reticulum stress.
Conclusion
These results demonstrated that H2S has an antidepressant potential, and the underlying mechanism is involved in the inhibition of hippocampal endoplasmic reticulum stress by upregulation of Sirt-1 in hippocampus. These findings identify H2S as a novel therapeutic target for depression.
Keywords: chronic unpredictable mild stress, depression, endoplasmic reticulum stress, hydrogen sulfide, silent mating type information regulation 2 homolog 1
Significance Statement
Depression is a chronic and recurrent serious mental disorder that affects about 10% of the world population and imposes a substantial societal burden. Therefore, understanding the prevention bases and looking for a future therapeutic target on depression are extremely urgent. The present work is to investigate the inhibitory action of H2S in CUMS-induced depression-like behaviors and the underlying mechanisms. In our study, we found that H2S ameliorated CUMS-induced depression-like behaviors, involving inhibition of hippocampal ER stress, by upregulation of hippocampal Sirt-1. Findings from our present work identify H2S as a potential target for therapeutic intervention in depression.
Introduction
Depression, a chronic and recurrent serious mental disorder characterized by loss of pleasure, mood disturbance, and suicidal tendencies (Tanti and Belzung, 2010), affects over 10% of the world’s population and imposes a substantial societal burden (Ferrari et al., 2014). Moreover, the treatment of depression has attracted great attention around the world (Ferrari et al., 2013). Unfortunately, the existing treatments are too often ineffective and fail to resolve symptoms completely (Liu et al., 2011). Therefore, novel therapeutic targets on depression still need to be addressed.
Hydrogen sulfide (H2S) is recognized as a crucial signaling molecule with a wide range of roles in the development of normal physiological conditions and many kinds of diseases (Kuksis et al., 2014; Sikora et al., 2014; Zhang and Bian, 2014; Wallace and Wang, 2015). Interestingly, H2S has been reported to enhance synaptic transmission and facilitate the induction of long-term potentiation (Abe and Kimura, 1996). It is widely accepted that dysfunction of synaptic plasticity contributes to the pathophysiology of depression (Duman, 2002; Duman and Aghajanian, 2012). Furthermore, our recent results have demonstrated that stress-induced depression is accompanied with disturbance of H2S generation in hippocampus (Tan et al., 2015). Taken together, we hypothesize that H2S might serve as a novel antidepressant gaseous mediator. Thus, in the present work, the antidepressant-like potential of H2S was evaluated by behavioral alterations caused by chronic unpredictable mild stress (CUMS).
Endoplasmic reticulum (ER) stress, a state in which the unfolded and misfolded protein accumulation affects the normal physiological function of cells, refers to the excessive stress caused by dysfunction of the endoplasmic reticulum (Boyce and Yuan, 2006; Kim et al., 2008). Previous studies attested that ER stress is a critical step in the pathology of neurodegeneration disease (Stefani et al., 2012; Roussel et al., 2013) as well as in the pathogenesis of CUMS-elicited depression (Pavlovsky et al., 2013; Zhang et al., 2014). In fact, the potential anti-ER stress effects of H2S has been a focus in the research (Xie et al., 2012; Li et al., 2014; Wei et al., 2014). These results prompted us to investigate whether H2S reserved CUMS-provided ER stress to understand the mechanisms underlying the antidepressant-like action of H2S.
Silent mating type information regulation 2 homolog 1 (Sirt-1), an NAD+-dependent histone deacetylase, is an essential metabolic regulatory transcription factor (Nemoto et al., 2005; Rodgers et al., 2005; Lagouge et al., 2006; Canto et al., 2009) that regulates physiological processes by the acetylation and deacetylation cycle (Yamamoto et al., 2007; Pillarisetti, 2008). In addition to cellular regulation, emerging evidence supported that Sirt-1 has neuroprotective properties (Parker et al., 2005; Kim et al., 2007; Avraham et al., 2010; Ho et al., 2010). Extensive studies have focused on the essential role of Sirt-1 in synaptic plasticity (Gao et al., 2010; Herranz et al., 2010; Michan et al., 2010). Moreover, recent studies demonstrated that chronic stress-induced depression contributes to the reduction of hippocampal Sirt-1 activity (Abe-Higuchi et al., 2016), which indicated that Sirt-1is an anti-depressant-like factor. Additionally, our previous work has proved that H2S inhibits formaldehyde-induced ER stress in PC12 cells via upregulating Sirt-1 expression (Li et al., 2014). Therefore, we assumed that Sirt-1 is indispensable for the protective effect of H2S against CUMS-induced ER stress and depressive-like behaviors.
Therefore, the present study was undertaken to elucidate whether H2S ameliorates depressive-like behaviors in CUMS-exposed rats and whether Sirt-1 mediates the antidepressant-like potential of H2S by suppressing hippocampal ER stress. In this study, we showed that H2S inhibits CUMS-induced depressive-like behaviors and hippocampal ER stress, while it upregulates the expression of hippocampal Sirt-1. We further demonstrated that blocking Sirt-1 reverses the protective effects of H2S in depression-like behaviors and hippocampal ER stress. Our results identified H2S as a potential antidepressant molecule, which is involved in inhibition of hippocampal ER stress via upregulating hippocampal Sirt-1.
MATERIALS AND METHODS
Animals
Healthy adult male Wistar rats, weighing 200 to 220 g (n = 180) (SJA Laboratory Animal Co., Ltd) were housed in a temperature-controlled room under a 12-h-light/12-h-dark cycle (lights on at 7:00 am) with free access to food and water. We made efforts to reduce the number and suffering of the animals. Additionally, all experimental operations were carried out in accordance with the National Animal Health and Management Regulations of the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Use and Protection Committee of the University of South China.
Antibodies and Reagents
NaHS (a donor of H2S), Imipramine, dimethyl sulfoxide, specific monoclonal anti-CHOP antibody, and Sirtinol were purchased from Sigma. Imipramine was dissolved in phosphate buffered saline (PBS) as solution and was administered i.p. of 15 mg/kg. Specific monoclonal anti-GRP-78 antibody and anti-Sirt-1 antibody were purchased from Epitomics. Specific monoclonal anti-cleaved caspase-12 antibody was produced by Santa Cruz Biotechnology. Beta-actin antibody, goat anti-mouse antibody, and goat anti-rabbit antibody were purchased from Proteintech.
Drug Treatments and Experimental Design
Rats were randomly divided into 8 treatment groups. In the control group (20 rats), rats were handled without stress for 4 weeks and injected with PBS (i.p.) and artificial CSF (ACSF) (intracerebroventricularly [i.c.v.]) in the last 2 weeks. In the CUMS group (24 rats), CUMS rats were exposed to a stressor daily for 2 weeks and then received 2 weeks of PBS i.p. injections during continuous 2-week CUMS treatment. In the CUMS + NaHS (30 μmol/kg or 100 μmol/kg) group (24 rats/group), CUMS rats were exposed to a stressor for 4 weeks and injected i.p. with 30 μmol/kg/d or 100 μmol/kg/d NaHS for the last 2 weeks. In the CUMS + imipramine (15 mg/kg) group (24 rats), CUMS rats were injected i.p. with 15 mg/kg/d imipramine for the last 2 weeks of the 4-week CUMS exposure. In the CUMS + NaHS (100 μmol/kg) + Sirtinol (10 nmol) group (24 rats), CUMS rats were cotreated with 100 μmol/kg/d NaHS (i.p.) and 10 nmol Sirtinol (i.c.v.) for the last 2 weeks of the 4-week CUMS procedure. In the 100 μmol/kg/d NaHS-alone group and 10 nmol Sirtinol alone-treated group (20 rats/group), rats received conventional treatment for 2 weeks and were injected with NaHS (100 μmol/kg/d, 14 days, i.p.) and Sirtinol (10 nmol, 14 days, i.c.v.), respectively. Sirtinol was diluted in sterilized ACSF/50% dimethyl sulfoxide and i.c.v. injected at a dose of 10 nmol. The depression-like behavioral tests were performed 24 hours after the last injection. Only rats with depression-like behaviors were used for subsequent studies; about 10% of rats were excluded. Then, 1 day after the behavioral tests, the hippocampus region tissues were rapidly collected and stored at −80°C for analysis (Figure 1). Data collection and analysis in the experiments were performed in a blinded fashion.
Figure 1.
Schematic diagram of the experimental schedule. Rats were given the corresponding drug treatment in the last 2 weeks of the 4-week general handling or chronic unpredictable mild stress (CUMS) exposure. ACSF, artificial CSF; OFT, open field test; TST, tail suspension test; FST, forced swimming test; i.c.v., intracerebroventricular injection.
CUMS Procedure
The CUMS procedure was carried out as previously reported (Nollet et al., 2012) with minor modifications. In brief, rats in the CUMS group were exposed to a variety of mild stressors: (1) cold swim at 5°C for 5 minutes, (2) food deprivation for 24 hours, (3) water deprivation for 24 hours, (4) soiled cage (200 mL water in 100 g sawdust bedding) for 12 hours, (5) alteration of light and dark cycles for 24 hours, (6) tail pinching for 1 minute (7) stroboscope (120 flashes/min) for 2 hours; (8) tail suspension for 5 minutes; (9) unpredictable foot shocks (0.4 mA, one shock/0.5 s, 10-second duration) and restricted movement for 10 to 11 seconds. Rats randomly received 3 of these stressors per day in the sequence shown above for 4 weeks. However, the same stressor was not applied continuously to avoid being predicted by rats.
Implantation of the i.c.v. Cannula
Rats were anesthetized with sodium pentobarbital (45 mg/kg, i.p., Sigma) and then mounted on a stereotaxic apparatus for operation. Trimming the incision along the midline of the head and exposing the bregma to determine the lateral ventricle. An aseptically cannula was implanted into the right side of the lateral ventricle for drug administration using the coordinates: AP, -1.0 mm; ML, -1.5 mm; DV, -3.0 mm (Paxinos). To ensure the drug had been completely delivered, the injection needle should be slowly pulled out and kept in position for an additional 2 minutes before being removed. The rats were kept warm under a lamp until they recovered from anesthesia. After the surgery, all rats received antiphlogistic penicillin once per day for 3 days. All the experiments were randomized and observed by an experimenter who was unaware of the treatment.
Behavioral Test
Open Field Test (OFT)
To assess the possible effects of drug treatment on spontaneous locomotor activity, the animals were exposed to the open-field paradigm, as previously described (Cai et al., 2010). The rat was placed in a black box (270 mm × 270 mm × 380 mm) individually into the center of the arena and was permitted free exploration after 12 minutes of adaption. The number of crossings and the total distance were recorded during a 5-minute test. Meanwhile, the box was cleansed with medical alcohol after occupancy by each rat to eliminate the effect of odors. The activities of the rats were analyzed by using spontaneous activity video analysis software.
Forced Swimming Test (FST)
The FST was established according to the protocol described in detail in Porsolt et al. (1977) with some modifications. Rats were individually forced to swim for 15 minutes in a transparent plastic vessel (diameter 18 cm, height 50 cm) filled with 30 cm of water (24 ± 1°C). Twenty-four hours later, an official test was carried out in the same condition to determine the immobility time in 5 minutes. Each rat was forced to swim for 5 minutes, and the duration of immobility, climbing, and swimming was measured during the 5 minutes. The definition of immobility was the absence of all movements except motions required to maintain the rat’s head above the water.
Tail Suspension Test (TST)
The TST was conducted as previously described (Steru et al., 1985). Briefly, each rat was individually suspended 50 cm above the floor by adhesive tape placed approximately 2 cm from the tip of the tail for 5 minutes hanging on a hook. Rats were considered as immobile when they were passively suspended and remained completely motionless. The immobility represents a failure of persistence in escape-directed behavior and was considered to be a struggling helpless state response (Moretti et al., 2014).
Western-Blot Analysis
Rats were sacrificed 1 day after the behavioral test. Hippocampus tissue of the brain was dissected and then homogenized to collect the supernatant. Subsequently, the protein content was assayed by BCA protein assay kit (Beyotime) and then transferred to the polyvinylidene fluoride membrane (Merck Millipore Ltd.) after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein membranes were blocked for 2 hours at room temperature with 5% skim milk in Tris Buffered Saline containing Tween 20. Membranes were incubated with primary antibody (anti-Beta actin, 1:2000; anti-CHOP, 1:500; anti-GRP-78, 1:1000; and anti-cleaved caspase-12, 1:1000) in 5% bovine serum albumin, 1× Tris Buffered Saline, and 0.05% Tween-20 at 4°C with gentle shaking overnight. After that, membranes were washed and then incubated with secondary antibody for 2 hours. Finally, the membranes were visualized by an image analysis system equipped with a software BIO-ID (Vilber Lourmat) and protein band was calculated with the integrated optical density by AlphaImager 2200 software.
Statistical Analysis
Statistical analysis was carried out using SPSS 16.0 software for Windows (SPSS Inc.). All values are expressed as means ± SEM. Data was analyzed by 2-way ANOVA. The level of significance was set at P < .05.
RESULTS
H2S Inhibits CUMS-Induced Depression-Like Behaviors
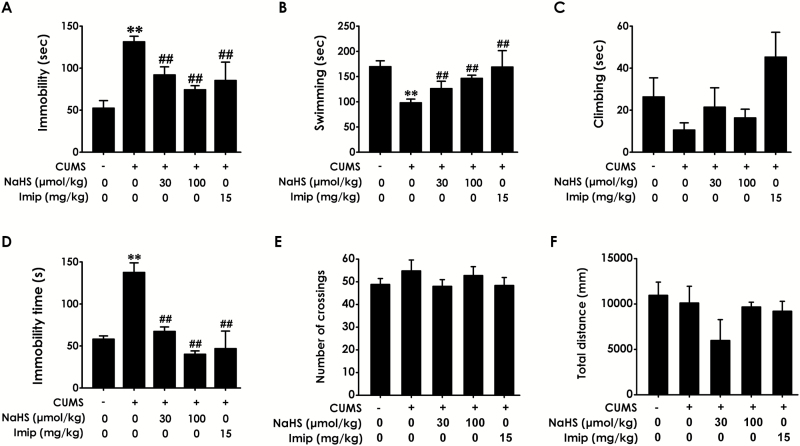
To determine whether H2S inhibits CUMS-induced depression-like behaviors, we evaluated TST and FST. The effects for stress (F1, 60 = 95.81, P<.001; F1, 50 = 24.17, P < .001; F1, 49 = 1.62, P = .209), treatment (F4, 60 = 10.16, P < .001; F4, 50 = 4.57, P = .003; F4, 49 = 1.42, P = .242), and stress × treatment interaction (F4, 60 = 1.87, P = .127; F4, 50 = 2.31, P=.071; F4, 49=2.39, P=.064) (Figure 2A–C) in immobility, swimming, and climbing time of the FST were revealed by 2-way ANOVA, respectively. In the FST, the duration of immobility in CUMS-exposed rats was increased (Figure 2A; P < .01) and the swimming time (Figure 2B; P < .01) was decreased compared with control rats, while the climbing time (Figure 2C, P > .05) was not changed. However, treatment with NaHS (30, 100 μmol/kg/d, 2 weeks) significantly reduced immobility time (P < .01) and increased the swimming time (P < .01) in CUMS-exposed rats as well as treatment with imipramine, indicating that H2S exerts an antidepressant-like effect. In the TST, the 2-way ANOVA revealed significant effects for stress (F1, 64 = 51.58, P < .001) and treatment (F4, 64=11.80, P<.001), but there is no significant stress × treatment interaction (F4, 64=1.16, P=.338) (Figure 2D). NaHS (30, 100 μmol/kg/d, 2 weeks) and imipramine obviously reduced the immobility time in CUMS-exposed rats (Figure 2D; P < .01), which presents evidence to further confirm the antidepressant-like effect of H2S.
Figure 2.
Effects of H2S on the depressive-like behaviors of chronic unpredictable mild stress (CUMS)-exposed rats. After 2-week CUMS exposure, the rats were further treated with CUMS in the absence or presence of NaHS (30, 100 µmol/kg/d, i.p.) or imipramine (15 mg/kg/d, i.p.) for 2 weeks. Rats performed the forced swimming test (FST) (A-C), tail suspension test (TST) (D), and open field test (OFT) (E-F). Values are the mean ± SEM (n = 8-10/group). **P < .01 vs control group; ##P < .01 vs CUMS group.
To exclude the possible effect of H2S on spontaneous locomotor activity, which may contribute to the decreased immobility in the FST and TST, rats were exposed to the open field apparatus for 5 minutes (Zhou et al., 2011). Two-way ANOVA showed no significant effects for stress, treatment, and stress × treatment interaction on either the number of crossing (F1, 64 = 1.18, P = .282; F4, 64 = 0.33, P = .855; F4, 64 = 1.96, P = .111; Figure 2E) or the total distance (F1, 58 = 3.48, P = .067; F4, 58 = 0.87, P = .487; F4, 58 = 1.79, P = 0.144; Figure 2F) in the OFT, which confirmed that antidepressant-like effects of both H2S and imipramine were not a nonspecific consequence of hypolocomotion. Additionally, the original design using H2S alone does not differ from the behavioral test (data not shown).
H2S Prevents the Hippocampal ER Stress in CUMS-Exposed Rats
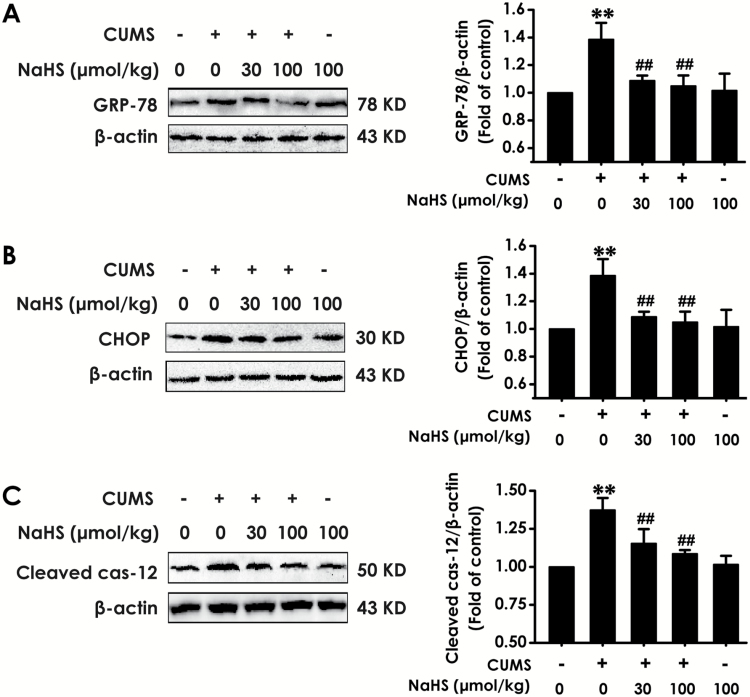
Three marker proteins indicating the ER stress level, GRP-78, CHOP, and cleaved caspase-12, were monitored by western blot to explore the effects of H2S on CUMS-induced hippocampal ER stress. For hippocampal GRP-78, CHOP, and cleaved caspase-12 levels, 2-way ANOVA showed significant effects for stress (F1, 20 = 52.47, P <.001; F1, 20 = 91.16, P<.001; F1, 20 = 30.21, P<.001), treatment (F4, 20 = 11.92, P < .001; F4, 20 = 12.74, P<.001; F4, 20 = 23.82, P<.001), and stress × treatment interaction (F4, 20 = 1.33, P = .294; F4, 20 = 2.28, P = .096; F4, 20 = 0.19, P = .94) (Figure 3), respectively. The GRP-78 (Figure 3A), CHOP (Figure 3B), and cleaved caspase-12 (Figure 3C) expressions in the hippocampus of rats exposed to CUMS for 4 weeks were significantly increased (P < .01), while treatment with NaHS (30, 100 µmol/kg/d, 2 weeks, i.p.) markedly decreased the expressions of GRP-78, CHOP, and cleaved caspase-12 (P < .01); moreover, treatment with NaHS (100 µmol/kg/d, 2 weeks, i.p.) alone did not change the expressions of GRP-78 (P > .05), CHOP (P > .05), and cleaved caspase-12 (P > .05) in control rats, which indicated that H2S could inhibit hippocampal ER stress induced by CUMS.
Figure 3.
Effects of H2S on expressions of CHOP, GRP-78, and cleaved caspase-12 in the hippocampus of chronic unpredictable mild stress (CUMS)-exposed rats. After treatment with CUMS for 2 weeks, the rats were further treated with CUMS in the absence or presence of NaHS (30, 100 µmol/kg/d, i.p.) for 2 weeks. GRP-78 (A), CHOP (B), and cleaved caspase-12 (C) in hippocapus of rats were detected by western blot. Values are the mean ± SEM (n = 3/group). **P < .01 vs control group; ##P < .01 vs CUMS group.
H2S Upregulates Sirt-1 Expression in the Hippocampus of CUMS-Exposed Rats
To explore the role of Sirt-1 in the antidepressant-like actions of H2S, we first investigated the effect of H2S on the expression of Sirt-1 in the hippocampus of rats. As shown in Figure 4, 2-way ANOVA revealed significant effects for stress (F1, 20 = 33.47, P<.001) and treatment (F4, 20=22.33, P<.001), but not for stress × treatment interaction (F4, 20 = 1.19, P = .346) in the hippocampus. After 4-week exposure of CUMS, the expression of Sirt-1 in the hippocampus was significantly decreased (P <.01), while treatment with NaHS (30, 100 µmol/kg/d, 2 wekks, i.p.) reversed the downregulation of Sirt-1 (P < .01) induced by CUMS, indicating that H2S upregulates Sirt-1 expression in the hippocampus of CUMS-exposed rats. In addition, treatment with NaHS (100 µmol/kg/d, 2 weeks, i.p.) did not change the levels of Sirt1 (P > .05) in control rats. These data suggested H2S upregulates the hippocampal Sirt1 in CUMS-exposed rats.
Figure 4.
Effects of H2S on the hippocampal Sirt-1 expression in chronic unpredictable mild stress (CUMS)-exposed rats. After treatment with CUMS for 2 weeks, the rats were further treated with CUMS in the absence or presence of NaHS (30, 100 µmol/kg/d, i.p.) for 2 weeks. Sirt-1 expression in hippocampal was detected by western blot. Values are the mean ± SEM (n = 3/group). **P < .01 vs control group; ##P < .01 vs CUMS group.
Inhibition of Sirt-1 by Sirtinol Reverses the Antidepressant-Like Effects of H2S
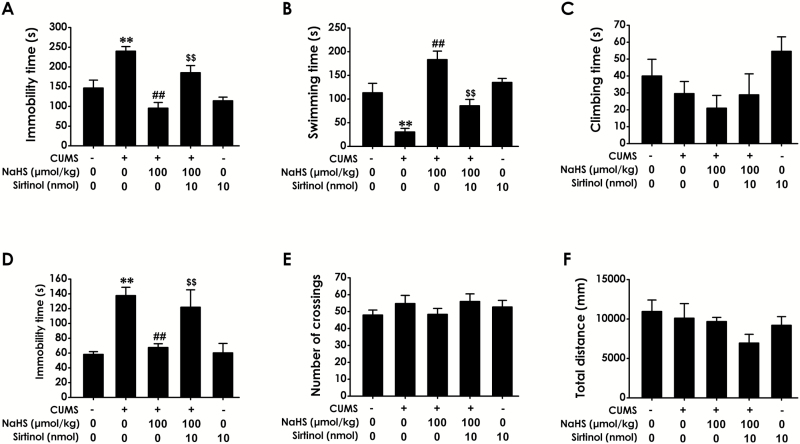
To further confirm the mediatory role of Sirt-1 in the antidepressant-like effects of H2S, we investigated whether Sirtinol, an inhibitor of Sirt-1, reverses the antidepressant-like effects of H2S. Rats were exposed to CUMS for 4 weeks and treated with NaHS (100 µmol/kg/d, i.p.) in the absence or presence of Sirtinol (10 nmol/d, i.c.v.) for the last 2 weeks of 4-week CUMS. For immobility, swimming, and climbing time in the FST, 2-way ANOVA showed significant effects for Sirtinol (F1, 28 =11.45, P =.026; F1, 33 = 6.05, P =.019; F1, 32 = 0.70, P = .409), H2S (F1, 28 = 7.12, P = .013; F1, 33 = 11.01, P = .002; F1, 32 = 2.74, P = .108), and Sirtinol × H2S interaction (F1, 28 =11.45, P =.002; F1, 33 = 15.58, P < .001; F1, 32 = 0.001, P = .975) (Figure 3), respectively (Figure 5A–C). Sirtinol (10 nmol/d, i.c.v.) dramatically prevented H2S from shortening immobility (Figure 5A; P < .01) and increasing the duration of swimming (Figure 5B; P < .01) in the FST as well as shortening immobility in the TST (Fig. 5D; P<.01), whereas it did not change the climbing time (Figure 5C; P>.05) in the FST. For the TST, 2-way ANOVA revealed significant main effects for Sirtinol (F1, 31 = 7.98, P = .008) and H2S (F1, 31 = 2.91, P = .098) with a significant Sirtinol × H2S interaction (F1, 31 = 29.27, P < .001) (Figure 5D). Additionally, Sirtinol (10 nmol/d, 2 weeks, i.c.v.) treatment alone did not affect both the immobility, swimming, and climbing time in FST and the immobility time in the TST, indicating that the inhibitory role of Sirtinol on the antidepressive action of NaHS was not due to the possibility that Sirtinol affected the normal ability of swimming and climbing. These data indicated that inhibition of Sirt-1 by Sirtinol reverses the antidepressant-like effects of H2S. Moreover, treatment with Sirtinol did not affect the total distance of spontaneous activity and the number of crossing in the OFT (Figure 5E,F; P>.05), which further rule out nonspecific motoric effects of Sirtinol that influence activity in the FST and TST.
Figure 5.
Effects of Sirtinol on the antidepressant-like actions of H2S in chronic unpredictable mild stress (CUMS)-exposed rats. Rats were exposed to CUMS for 4 weeks and treated with NaHS (100 µmol/kg/d, i.p.) in the absence or presence of Sirtinol (10 nmol/d, i.c.v.) for the last 2 weeks of 4-week CUMS. The forced swimming test (FST) (A-C), tail suspension test (TST) (D), and open field test (OFT) (E-F) were used to assess the depressive-like behaviors of rats. Values are the mean ± SEM (n = 8–10/group). **P < .01 vs control group; ##P < .01 vs CUMS group; $$P < .01 vs cotreatment with CUMS and NaHS group.
Inhibition of Sirt-1 by Sirtinol Attenuates the Protective Effect of H2S against CUMS-Induced ER Stress
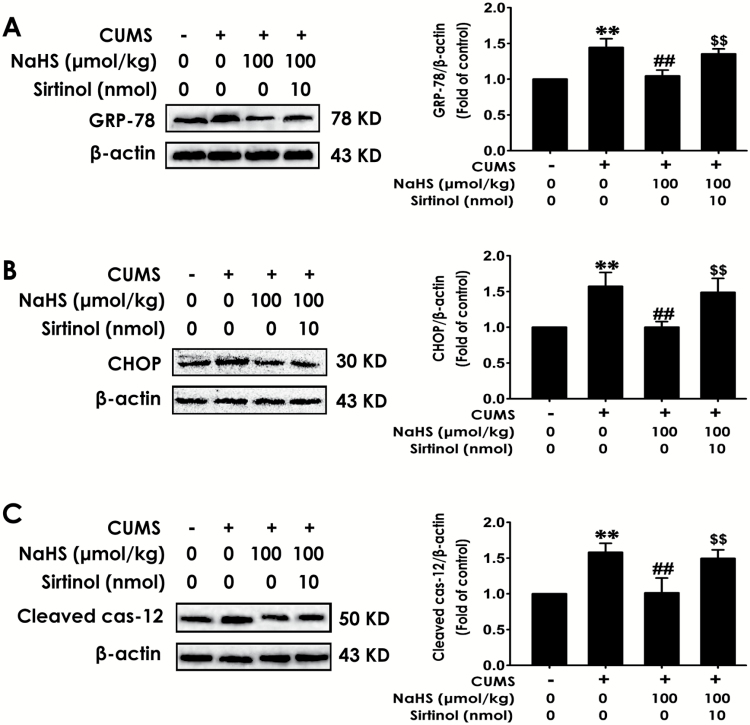
To elaborate the mediatory role of Sirt-1 in the protection of H2S against CUMS-induced hippocampal ER stress, we detected whether Sirtinol, an inhibitor of Sirt-1, abolishes the protective role of H2S against hippocampal ER stress in CUMS-exposed rats. After exposing to CUMS for 2 weeks, rats were cotreated with CUMS and NaHS (100 µmol/kg/d, i.p.) in the absence or presence of Sirtinol (10 nmol/d, i.c.v.) for 2 weeks. For ER stress-related proteins, GRP78, CHOP, and cleaved caspase-12, 2-way ANOVA showed significant effects for Sirtinol (F1, 11 = 13.63, P = .004; F1, 11 = 10.78, P = .007; F1, 11 = 20.74, P = .001), H2S (F1, 11 = 5.94, P = .033; F1, 11 = 20.33, P = .001; F1, 11 = 13.12, P = .004), and Sirtinol × H2S interaction (F1, 11 = 8.75, P = .013; F1, 11 = 44.37, P < .001; F1, 11 = 50.27, P < .001) (Figure 6), respectively. Sirtinol (10 nmol/d, 2 weeks, i.c.v.) significantly eliminated NaHS (100 µmol/kg/d, 2 weeks, i.p.), reduced the expressions of GRP-78 (Figure 6A, P<.01) and CHOP (Figure 6B, P<.01), and cleaved caspase-12 (Figure 6C, P < .01) in hippocampus of CUMS-exposed rats, while treatment with Sirtinol (10 nmol/d, 2 weeks, i.c.v.) alone did not affect the GRP-78 (Figure 6A, P>.05), CHOP (Figure 6B, P>.05), and cleaved caspase-12 (Figure 6C, P >.05) levels in control rats, which documented that inhibition of Sirt-1 reverses the antidepressive effect of H2S on CUMS-induced hippocampal ER stress.
Figure 6.
Effects of Sirtinol on the inhibitory role of H2S on chronic unpredictable mild stress (CUMS)-induced hippocampal endoplasmic reticulum (ER) stress. After treatment with CUMS for 2 weeks, rats were cotreated with CUMS and NaHS (100 µmol/kg/d, i.p.) in the absence or presence of Sirtinol (10 µmol/d, i.c.v.) for 2 weeks. The expressions of GRP-78 (A), CHOP (B), and cleaved caspase-12 (C) in hippocampus were detected by western blot. Values are the mean ± SEM (n = 3/group). **P < .01 vs control group; ##P < .01 vs CUMS group; $$P < .01 vs cotreatment with CUMS and NaHS group.
Discussion
The limited efficacy and serious side effects of current antidepressant drugs emphasize the demand for better approaches to the treatment of depression (Tanti and Belzung, 2010; Ferrari et al., 2014). The present study establishes the antidepressant potential action of H2S in CUMS-induced depressive-like behaviors. Our findings are as follows: (1) H2S attenuates CUMS-induced depressive-like behaviors; (2) H2S prevents the level of ER stress in the hippocampus of CUMS-exposed rats; (3) H2S upregulates the expression of Sirt-1 in the hippocampus of CUMS-exposed rats; and (4) inhibited Sirt-1 interrupts H2S-offered antidepressant and anti-ER stress effects in CUMS-exposed rats. These results identify H2S is a potential inhibitor of CUMS-induced depressive-like behaviors as a result of attenuating hippocampal ER stress via upregulation of hippocampal Sirt-1.
H2S involves a range of biological and physiological functions, especially in the neurodegenerative diseases (Jia et al., 2013; Liu et al., 2013; Kuksis et al., 2014; Sikora et al., 2014; Wei et al., 2014; Zhang and Bian, 2014). In the present work, we performed a rat model of CUMS to confirm the antidepressant-like properties of H2S and the underling mechanism. We found that exposure of rats to CUMS significantly increased the immobility time in FST and TST, and decreased the climbing time and swimming time in FST, which indicated that CUMS causes depressive-like behaviors. Novelty, NaHS, a donor of H2S, suppressed these depressive-like behaviors in CUMS-exposed rats, which verified the antidepressant-like activities of H2S. Our results strengthen and expand the previously established links between H2S and depression (Chen et al., 2013; Tang et al., 2015).
It has been confirmed that there are significant relationships between ER stress response and neuropsychiatric disorders such as major depressive disorder (Bown et al., 2000) and bipolar disorder (So et al., 2007; Hayashi et al., 2009). Recently, evidence demonstrated that ER stress-related proteins are also increased in the hippocampus of chronic social defeat stress- (Huang et al., 2013) and CUMS- (Tan et al., 2015) treated animal depression models. These previous findings suggest the hippocampal ER stress is involved in the pathological process of exogenous stress-induced depression. Furthermore, Ishisaka et al., 2011) reported that the antidepressant-like role of luteolin is via suppressing ER stress in hippocampus (Ishisaka et al., 2011). Interestingly, increasing evidence suggested that H2S is a protective gaseous signaling molecule by preventing ER stress (Wei et al., 2010; Chen et al., 2011; Wang et al., 2012). Therefore, regulation of ER stress is critical for understanding the contribution of H2S to the inhibition in CUMS-induced depressive-like behaviors. We detected the influence of H2S on the expression of ER stress-marked proteins, such as GRP-78, CHOP, and cleaved caspase-12, in CUMS rat model. We found that H2S alleviates the upregulation of CHOP, GRP-78, and cleaved caspase-12 in the hippocampus of rats exposed to CUMS. Our outcomes indicated that the antidepressant potential action of H2S is endowed with its significant neuroprotective properties antagonistic CUMS-offered hippocampal ER stress.
Recently, Sirt-1 has been identified to act as a defender of ER stress (Li et al., 2011; Jung et al., 2012) and function as a neuroprotectant (Han, 2009; Zhang et al., 2011; Donmez, 2012). Additionally, overexpression of Sirt-1 prevents axonal degeneration (Araki et al., 2004), but the suppression of Sirt-1 significantly exacerbates neuronal damage (Chen et al., 2005) and disturbs the information of synaptic plasticity (Michan et al., 2010). Moreover, it has been suggested that dysfunction of Sirt-1 may be implicated in the pathophysiology of psychiatric disorders (Herskovits and Guarente, 2014). Interestingly, the recent findings from Abe-Higuchi et al. (2016) indicated that inhibition of hippocampal Sirt-1 function leads to an increase in depression-like behaviors. Thus, we speculate that upregulation of Sirt-1 might be a potential target for an antidepressant drug. In our study, we found that H2S markedly upregulates the expression of Sirt-1 in the hippocampus of CUMS-exposed rats. Moreover, blocking Sirt-1 pathway with Sirtinol, a specific inhibitor of Sirt-1, reverses H2S-elicited protective effect against CUMS-induced hippocampal ER stress and inhibitory role in depression-like behavior. These results indicated that the antidepressant-like properties of H2S is via upregulation of hippocampal Sirt-1. It has been demonstrated that resveratrol, a Sirt-1 activator, has an antidepressant-like effect (Ge et al., 2013; Hurley et al., 2014; Liu et al., 2014). These previous findings provide a reasonable interpretation for our present results. Therefore, our present work not only suggests the essential mediatory role of Sirt-1 in the antidepressant-like properties of H2S but also extends the previous observations on the importance of the Sirt-1 pathway as a key target for antidepressant (Abe-Higuchi et al., 2016).
To summarize, our present study demonstrated that H2S attenuates CUMS-induced depression-like behaviors and ER stress. H2S upregulates Sirt1 expression in CUMS-exposed rats. Moreover, inhibition of Sirt1 reverses H2S-elicited protection against the ER stress and depression-like behaviors induced by CUMS. These findings suggest that H2S produces an antidepressive effect by inhibition of hippocampal ER stress as a result of upregulation of Sirt1 in hippocampus. Our findings provide a comprehensive view on the antidepressive effect of H2S that partially identify a new approach for the treatment of depression. Additionally, to enhance and expand our findings, S-sulfhydration would no doubt be a promising strategy for the important and significant role of H2S with regard to the regulation of Sirt1 in our further study, given the evidence that H2S elicits its biological functions by S-sulfhydration (Mustafa et al., 2009, 2011; Krishnan et al., 2011; Paul and Snyder, 2012).
Statement of Interest
None.
Acknowledgments
Xiao-Qing Tang and Wei Zou designed the study. Hai-Ying Zeng and Li-Yuan Kan, Shu-Yun Liu, and Dan Li collected samples. Ping Zhang and Hong-Feng Gu conducted the analysis. Shu-Yun Liu and Dan Li wrote the first version of the manuscript. Xiao-Qing Tang has made necessary modifications to the manuscript. All authors have made a substantial intellectual contribution to the work and have approved the final manuscript.
This study was supported by the project of Economic Services Bureau of Shenzhen Longhua New District (20140917A1030046), Natural Science Foundation of Guangdong Province of China (2014A030313738), Natural Science Foundation of China (81371485), Zhengxiang Scholar Program of University of South China (2014-004), and the Construct Program of the Key Discipline in Hunan Province.
References
- Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe-Higuchi N, Uchida S, Yamagata H, Higuchi F, Hobara T, Hara K, Kobayashi A, Watanabe Y (2016) Hippocampal Sirtuin 1 signaling mediates depression-like behavior. Biol Psychiatry 80:815–826. [DOI] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J (2004) Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305:1010–1013. [DOI] [PubMed] [Google Scholar]
- Avraham Y, Davidi N, Porat M, Chernoguz D, Magen I, Vorobeiv L, Berry EM, Leker RR (2010) Leptin reduces infarct size in association with enhanced expression of CB2, TRPV1, SIRT-1 and leptin receptor. Curr Neurovasc Res 7:136–143. [DOI] [PubMed] [Google Scholar]
- Bown C, Wang JF, MacQueen G, Young LT (2000) Increased temporal cortex ER stress proteins in depressed subjects who died by suicide. Neuropsychopharmacology 22:327–332. [DOI] [PubMed] [Google Scholar]
- Boyce M, Yuan J (2006) Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ 13:363–373. [DOI] [PubMed] [Google Scholar]
- Cai L, Yan XB, Chen XN, Meng QY, Zhou JN (2010) Chronic all-trans retinoic acid administration induced hyperactivity of HPA axis and behavioral changes in young rats. Eur Neuropsychopharmacol 20:839–847. [DOI] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458:1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L (2005) SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Bio Chem 280:40364–40374. [DOI] [PubMed] [Google Scholar]
- Chen WL, Xie B, Zhang C, Xu KL, Niu YY, Tang XQ, Zhang P, Zou W, Hu B, Tian Y (2013) Antidepressant-like and anxiolytic-like effects of hydrogen sulfide in behavioral models of depression and anxiety. Behav Pharmacol 24:590–597. [DOI] [PubMed] [Google Scholar]
- Chen ZF, Zhao B, Tang XY, Li W, Zhu LL, Tang CS, Du JB, Jin HF (2011) Hydrogen sulfide regulates vascular endoplasmic reticulum stress in apolipoprotein E knockout mice. Chin Med J 124:3460–3467. [PubMed] [Google Scholar]
- Donmez G. (2012) The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol Sci 33:494–501. [DOI] [PubMed] [Google Scholar]
- Duman RS. (2002) Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry 17:306–310. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA (2013) Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med 10:e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari AJ, Norman RE, Freedman G, Baxter AJ, Pirkis JE, Harris MG, Page A, Carnahan E, Degenhardt L, Vos T, Whiteford HA (2014) The burden attributable to mental and substance use disorders as risk factors for suicide: findings from the Global Burden of Disease Study 2010. PloS One 9:e91936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH (2010) A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466:1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge JF, Peng L, Cheng JQ, Pan CX, Tang J, Chen FH, Li J (2013) Antidepressant-like effect of resveratrol: involvement of antioxidant effect and peripheral regulation on HPA axis. Pharmacol Biochem Behav 114–115:64–69. [DOI] [PubMed] [Google Scholar]
- Han SH. (2009) Potential role of sirtuin as a therapeutic target for neurodegenerative diseases. J Clin Neurol 5:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Kasahara T, Kametani M, Toyota T, Yoshikawa T, Kato T (2009) Aberrant endoplasmic reticulum stress response in lymphoblastoid cells from patients with bipolar disorder. Int J Neuropsychopharmacol 12:33–43. [DOI] [PubMed] [Google Scholar]
- Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M (2010) Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits AZ, Guarente L (2014) SIRT1 in neurodevelopment and brain senescence. Neuron 81:471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF (2010) Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Exp Neurol 225:74–84. [DOI] [PubMed] [Google Scholar]
- Huang GB, Zhao T, Muna SS, Bagalkot TR, Jin HM, Chae HJ, Chung YC (2013) Effects of chronic social defeat stress on behaviour, endoplasmic reticulum proteins and choline acetyltransferase in adolescent mice. Int J Neuropsychopharmacol 16:1635–1647. [DOI] [PubMed] [Google Scholar]
- Hurley LL, Akinfiresoye L, Kalejaiye O, Tizabi Y (2014) Antidepressant effects of resveratrol in an animal model of depression. Behav Brain Res 268:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishisaka M, Kakefuda K, Yamauchi M, Tsuruma K, Shimazawa M, Tsuruta A, Hara H (2011) Luteolin shows an antidepressant-like effect via suppressing endoplasmic reticulum stress. Biol Pharm Bull 34:1481–1486. [DOI] [PubMed] [Google Scholar]
- Jia J, Xiao Y, Wang W, Qing L, Xu Y, Song H, Zhen X, Ao G, Alkayed NJ, Cheng J (2013) Differential mechanisms underlying neuroprotection of hydrogen sulfide donors against oxidative stress. Neurochem Int 62:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TW, Lee KT, Lee MW, Ka KH (2012) SIRT1 attenuates palmitate-induced endoplasmic reticulum stress and insulin resistance in HepG2 cells via induction of oxygen-regulated protein 150. Biochem Biophys Res Commun 422:229–232. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J 26:3169–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7:1013–1030. [DOI] [PubMed] [Google Scholar]
- Krishnan N, Fu C, Pappin DJ, Tonks NK (2011) H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal 4:ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuksis M, Smith PM, Ferguson AV (2014) Hydrogen sulfide regulates cardiovascular function by influencing the excitability of subfornical organ neurons. PloS One 9:e105772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127:1109–1122. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang KY, Zhang P, Chen LX, Wang L, Xie M, Wang CY, Tang XQ (2014) Hydrogen sulfide inhibits formaldehyde-induced endoplasmic reticulum stress in PC12 cells by upregulation of SIRT-1. PloS One 9:e89856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X, Donmez G, Li J, Luo Z, Walsh K, Guarente L, Zang M (2011) Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J 25:1664–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Xie K, Yang X, Gu J, Ge L, Wang X, Wang Z (2014) Resveratrol reverses the effects of chronic unpredictable mild stress on behavior, serum corticosterone levels and BDNF expression in rats. Behav Brain Res 264:9–16. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang N, Hao W, Zhao Q, Ying T, Liu S, Li Q, Liang Y, Wang T, Dong Y, Ji C, Zuo P (2011) Dynamic proteomic analysis of protein expression profiles in whole brain of Balb/C mice subjected to unpredictable chronic mild stress: implications for depressive disorders and future therapies. Neurochem Int 58:904–913. [DOI] [PubMed] [Google Scholar]
- Liu YY, Nagpure BV, Wong PT, Bian JS (2013) Hydrogen sulfide protects SH-SY5Y neuronal cells against d-galactose induced cell injury by suppression of advanced glycation end products formation and oxidative stress. Neurochem Int 62:603–609. [DOI] [PubMed] [Google Scholar]
- Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD (2010) SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci 30:9695–9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti M, Budni J, Freitas AE, Rosa PB, Rodrigues AL (2014) Antidepressant-like effect of ascorbic acid is associated with the modulation of mammalian target of rapamycin pathway. J Psychiatr Res 48:16–24. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH (2009) H2S signals through protein S-sulfhydration. Sci Signal 2:ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH (2011) Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109:1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T (2005) SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J Biol Chem 280:16456–16460. [DOI] [PubMed] [Google Scholar]
- Nollet M, Gaillard P, Tanti A, Girault V, Belzung C, Leman S (2012) Neurogenesis-independent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology 37:2210–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Neri C (2005) Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet 37:349–350. [DOI] [PubMed] [Google Scholar]
- Paul BD, Snyder SH (2012) H(2)S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13:499–507. [DOI] [PubMed] [Google Scholar]
- Pavlovsky AA, Boehning D, Li D, Zhang Y, Fan X, Green TA (2013) Psychological stress, cocaine and natural reward each induce endoplasmic reticulum stress genes in rat brain. Neuroscience 246:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GW C. (1998) The Rat Brain in Stereotaxic Coordinates. San Diego; (CA): Academic Press. [Google Scholar]
- Pillarisetti S. (2008) A review of Sirt1 and Sirt1 modulators in cardiovascular and metabolic diseases. Recent Pat Cardiovasc Drug Discov 3:156–164. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434:113–118. [DOI] [PubMed] [Google Scholar]
- Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ (2013) Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol 12:105–118. [DOI] [PubMed] [Google Scholar]
- Sikora M, Drapala A, Ufnal M (2014) Exogenous hydrogen sulfide causes different hemodynamic effects in normotensive and hypertensive rats via neurogenic mechanisms. Pharmacol Rep 66:751–758. [DOI] [PubMed] [Google Scholar]
- So J, Warsh JJ, Li PP (2007) Impaired endoplasmic reticulum stress response in B-lymphoblasts from patients with bipolar-I disorder. Biol Psychiatry 62:141–147. [DOI] [PubMed] [Google Scholar]
- Stefani IC, Wright D, Polizzi KM, Kontoravdi C (2012) The role of ER stress-induced apoptosis in neurodegeneration. Curr Alzheimer Res 9:373–387. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367–370. [DOI] [PubMed] [Google Scholar]
- Tan H, Zou W, Jiang J, Tian Y, Xiao Z, Bi L, Zeng H, Tang X (2015) Disturbance of hippocampal H2S generation contributes to CUMS-induced depression-like behavior: involvement in endoplasmic reticulum stress of hippocampus. Acta Biochim Biophy Sin 47:285–291. [DOI] [PubMed] [Google Scholar]
- Tang ZJ, Zou W, Yuan J, Zhang P, Tian Y, Xiao ZF, Li MH, Wei HJ, Tang XQ (2015) Antidepressant-like and anxiolytic-like effects of hydrogen sulfide in streptozotocin-induced diabetic rats through inhibition of hippocampal oxidative stress. Behav Pharmacol 26:427–435. [DOI] [PubMed] [Google Scholar]
- Tanti A, Belzung C (2010) Open questions in current models of antidepressant action. Br J Pharmacol 159:1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL, Wang R (2015) Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov 14:329–345. [DOI] [PubMed] [Google Scholar]
- Wang XY, Yang CT, Zheng DD, Mo LQ, Lan AP, Yang ZL, Hu F, Chen PX, Liao XX, Feng JQ (2012) Hydrogen sulfide protects H9c2 cells against doxorubicin-induced cardiotoxicity through inhibition of endoplasmic reticulum stress. Mol Cell Biochem 363:419–426. [DOI] [PubMed] [Google Scholar]
- Wei H, Zhang R, Jin H, Liu D, Tang X, Tang C, Du J (2010) Hydrogen sulfide attenuates hyperhomocysteinemia-induced cardiomyocytic endoplasmic reticulum stress in rats. Antioxid Redox Signal 12:1079–1091. [DOI] [PubMed] [Google Scholar]
- Wei HJ, Xu JH, Li MH, Tang JP, Zou W, Zhang P, Wang L, Wang CY, Tang XQ (2014) Hydrogen sulfide inhibits homocysteine-induced endoplasmic reticulum stress and neuronal apoptosis in rat hippocampus via upregulation of the BDNF-TrkB pathway. Acta pharmacol Sin 35:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Tiong CX, Bian JS (2012) Hydrogen sulfide protects SH-SY5Y cells against 6-hydroxydopamine-induced endoplasmic reticulum stress. Am J Physiol Cell Physiol 303:C81–91. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Schoonjans K, Auwerx J (2007) Sirtuin functions in health and disease. Mol Endocrinol 21:1745–1755. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Gan L, Vosler PS, Gao Y, Zigmond MJ, Chen J (2011) Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol 95:373–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bian JS (2014) Hydrogen sulfide: a neuromodulator and neuroprotectant in the central nervous system. ACS Chem Neurosci 5:876–883. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu W, Zhou Y, Ma C, Li S, Cong B (2014) Endoplasmic reticulum stress is involved in restraint stress-induced hippocampal apoptosis and cognitive impairments in rats. Physiol Behav 131:41–48. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lu L, Li Z, Gao X, Tian J, Zhang L, Wu B, Qin X (2011) Antidepressant-like effects of the fractions of Xiaoyaosan on rat model of chronic unpredictable mild stress. J Ethnopharmacol 137:236–244. [DOI] [PubMed] [Google Scholar]