ABSTRACT
Radon therapy using radon (222Rn) gas is classified into two types of treatment: inhalation of radon gas and drinking water containing radon. Although short- or long-term intake of spa water is effective in increasing gastric mucosal blood flow, and spa water therapy is useful for treating chronic gastritis and gastric ulcer, the underlying mechanisms for and precise effects of radon protection against mucosal injury are unclear. In the present study, we examined the protective effects of hot spring water drinking and radon inhalation on ethanol-induced gastric mucosal injury in mice. Mice inhaled radon at a concentration of 2000 Bq/m3 for 24 h or were provided with hot spring water for 2 weeks. The activity density of 222Rn ranged from 663 Bq/l (start point of supplying) to 100 Bq/l (end point of supplying). Mice were then orally administered ethanol at three concentrations. The ulcer index (UI), an indicator of mucosal injury, increased in response to the administration of ethanol; however, treatment with either radon inhalation or hot spring water inhibited the elevation in the UI due to ethanol. Although no significant differences in antioxidative enzymes were observed between the radon-treated groups and the non-treated control groups, lipid peroxide levels were significantly lower in the stomachs of mice pre-treated with radon or hot spring water. These results suggest that hot spring water drinking and radon inhalation inhibit ethanol-induced gastric mucosal injury.
Keywords: gastric mucosal injury, radon inhalation, hot spring water drinking, anti-oxidative functions, lipid peroxide level, histological assessment, mouse
INTRODUCTION
Acute gastric mucosal lesion (AGML) is commonly understood as an acute stomach lesion, which is characterized by sudden gastrointestinal bleeding, ischemic injury, acute gastric ulcer (GU), and abdominal pain [1]. The pathogenesis of AGML is multifactorial. Physical stress, psychological stress, tobacco (smoking and chewing), alcohol, drugs and Helicobacter pylori may increase the risk of inflammation and ulcers in the stomach [2–4]. According to Shay and Sun’s Balance Theory, in the healthy stomach, mucosal aggressive factors, such as stomach acid (HCl), pepsin secretion, and mucosal trauma, are balanced by defensive factors, such as mucosal resistance, mucus, local mucosal blood flow, and duodenal brake; however, a disruption in the balance, i.e. aggressive agent enhancement or decline in defense agents via changes in the risk factors, can lead to peptic ulcer [5, 6]. In addition, it has recently been reported that free radicals and reactive oxygen species (ROS), such as superoxide radicals (O2•–), hydrogen peroxide (H2O2) and hydroxyl radicals (•OH), play an important role in the pathogenesis of gastric mucosal injury in the stomach [7–9], and a new balance theory, which considers free radicals and ROS in addition to the aggressive factors, and antioxidants in addition to the defensive factors, has also been proposed [10]. The balance between the generation and loss of radicals maintains homeostasis in the stomach, and gastric mucosal injury occurs when the balance is lost. In other words, drugs and alternative therapies that regulate or eliminate ROS may have defensive effects against gastric mucosa. For example, AGMLs are inhibited by the administration of active oxygen scavengers, such as superoxide dismutase (SOD) and catalase (CAT) [8, 9]. Recent studies have suggested that glutathione (GSH) [11–15] and antioxidants, such as α-tocopherol [16], have roles in gastric protection.
Generally, alcohol (~20% or greater) is a direct cause of gastric mucosa defects, which are more remarkable with high alcohol concentrations. Clinically, alcohol intake induces AGMLs. It has been reported that ethanol has direct effects in terms of destruction of the gastric mucosa, as well as secondary effects such as decreased gastric mucosal blood flow [17]. These effects contribute to the formation of AGMLs. In an ethanol-induced gastric injury model, ulcer formation is not suppressed by gastric acid secretion inhibitors, but it is suppressed by agents that enhance mucosal defensive factors [18]. Therefore, this model is suitable for evaluating gastric mucosa protective effects and anti-ulcer effects.
Therapy using radon hot springs (222Rn) is performed for pain- or respiratory-related diseases such as osteoarthritis [19] and bronchial asthma [20], and gastrointestinal diseases, such as gastroenteritis, at the Misasa Medical Center, Okayama University Hospital, Japan. To clarify the mechanism of the therapeutic effects, we conducted several studies on the effects of radon inhalation in mice. Recently, we reported that radon inhalation induces antioxidant substances in many organs (such as the brain, heart, lung, liver, pancreas, kidney and small intestine) of mice [21], and inhibits carbon tetrachloride (CCl4)-induced hepatopathy [22] and dextran sulfate sodium–induced colitis in mice [23]. These findings suggest that antioxidative functions induced by radon inhalation contribute to the mitigation of ROS- and free radical–related diseases. In addition, we recently reported that radon inhalation and hot spring water drinking inhibit potassium oxonate–induced hyperuricemia in mice [24]. However, significant activation of anti-oxidation mechanisms in response to drinking treatments was not observed, and the mechanisms of action of these treatments, particularly with respect to radon drinking treatments, remain unclear.
If radon treatment activates antioxidative functions in the stomach, it is highly likely to be beneficial against gastric mucosal injury. Tanaka et al. indicated that short- or long-term intake of spa water is effective in increasing gastric mucosal blood flow, and spa water therapy is useful for treating chronic gastritis and gastric ulcer [25, 26]. However, it is not clear whether antioxidative substances are involved in the beneficial effects. The aim of the present study was to investigate the inhibitory effects of radon inhalation and hot spring water treatments in the development of ethanol-induced gastric mucosal injury. We focused on oxidative stress by ethanol-induced gastric mucosal injury because, as described above, ROS and free radicals play important roles in the pathogenesis of mucosal injury in the stomach. We examined the following biochemical parameters and histological changes in the mucosa to assess the effects of radon treatment: SOD activity, CAT activity, lipid peroxide (LPO) levels, and total glutathione content (t-GSH) in the stomach.
MATERIALS AND METHODS
Animals
Male BALB/c mice (8 weeks of age, body weight 25–28 g) were purchased from CLEA Japan Inc. (Tokyo, Japan) for the analyses of radon inhalation and water treatments. They were maintained in plastic cages under controlled temperature (22 ± 2°C), humidity (55 ± 10%) and light conditions (12 h of light, 12 h of dark), and were given free access to food and water. Ethics approval for all protocols and experiments was obtained from the Animal Experimental Committee of Okayama University.
Experimental procedure
Induction of gastric mucosal injury
Gastric mucosal injury was produced by oral administration of ethanol in mice according to the methods of Swarnakar et al., with slight modifications [27]. Briefly, mice were orally administered ethanol after radon inhalation or hot spring water drinking. The mice were fasted overnight (food, but not water) prior to treatment. Ethanol was adjusted with saline solution to obtain concentrations of 30%, 60% or 100% (anhydrous alcohol), and solutions were freshly prepared before administration. Each experimental mouse was given 0.2 ml ethanol by oral gavage. Control mice were manipulated in parallel by oral administration of saline (0.9% NaCl solution).
Hot spring water drinking treatment
A total of 96 male mice were randomly divided into 12 groups of eight each: (1) distilled water (DW) with saline (DW), (2) radon-containing spring water with saline (spring water with Rn), (3) radon-deaerated spring water with saline (spring water without Rn), (4) DW with 30% ethanol (DW + 30% EtOH), (5) radon-containing spring water with 30% ethanol (spring water with Rn + 30% EtOH), (6) radon-deaerated spring water with 30% ethanol (spring water without Rn + 30% EtOH), (7) DW with 60% ethanol (DW + 60% EtOH), (8) radon-containing spring water with 60% ethanol (spring water with Rn + 60% EtOH), (9) radon-deaerated spring water with 60% ethanol (spring water without Rn + 60% EtOH), (10) DW with 100% ethanol (DW + 100% EtOH), (11) radon-containing spring water with 100% ethanol (spring water with Rn + 100% EtOH), and (12) radon-deaerated spring water with 100% ethanol (spring water without Rn + 100% EtOH). Mice had continuous access to distilled water, hot spring water containing radon, or radon-deaeration hot spring water (from which radon was removed) for 2 weeks. Radon-containing hot spring water was obtained from the Misasa Medical Center, Okayama University Hospital, with attention to water foaming and the dissipation of radon. Radon-deaeration hot spring water was obtained by bubbling Rn-containing hot spring water using an air pump for 20 min to dissipate the radon. In order to align the conditions of supply, Rn-containing and Radon-deaeration hot spring water was supplied to mice after 2–3 days storage. The drinking water for all animal groups was supplied at room temperature. Drinking water was replaced three times per week.
The pH of hot spring water was 7.0–7.3, and various chemicals were dissolved in the hot spring water for the drinking treatment [24]. The radon concentration in water was measured using a liquid scintillation counter (LSC-LB5, Aloka, Tokyo, Japan). The radon concentration, water intake volume, and body weight were monitored continuously at 2- or 3-day intervals. The mean radon concentrations in Rn-containing hot spring water were 663 ± 36 Bq/l at the initiation of the treatments (Table 1). One hour after each of the drinking treatments, gastric mucosal injury was induced in mice by oral administration of either 100% ethanol to induce the maximum level of gastric ulcer, 60% ethanol to induce moderate ulcers, or 30% ethanol to induce minimal ulcers. Mice were sacrificed by an overdose of ether anesthesia at 1 h after oral ethanol administration. Blood was drawn from the heart, and the stomach was immediately excised. The stomachs were cut along the greater curvature and rinsed in 10 mM phosphate-buffered saline (PBS; pH 7.4) (Nacalai Tesque, Kyoto, Japan) to examine mucosal lesions and to analyze SOD and CAT activity, t-GSH content, and the levels of LPO and proteins. Samples were preserved at –80°C for subsequent biochemical analyses.
Table 1.
Radon concentrations in hot spring water, body weight, and intake volume
| Parameters | Rn-containing hot spring water | Rn-deaeration hot spring water | Distilled water |
|---|---|---|---|
| Concentrations of water (Bq/l) | |||
| (at the start point of supplying) | 663 ± 36 | Not measured | Not measured |
| (at the end point of supplying) | 100 ± 4.8 | Not measured | Not measured |
| Body weight (g) | |||
| (at the treatment start) | 26.8 ± 1.1 | 26.8 ± 1.2 | 26.6 ± 1.0 |
| (at the time just before fasting) | 28.4 ± 1.0 | 28.7 ± 1.2 | 28.6 ± 1.1 |
| Drinking voume (ml/day/capita) | 3.03 ± 0.13 | 3.10 ± 0.19 | 3.09 ± 0.10 |
Radon inhalation treatment
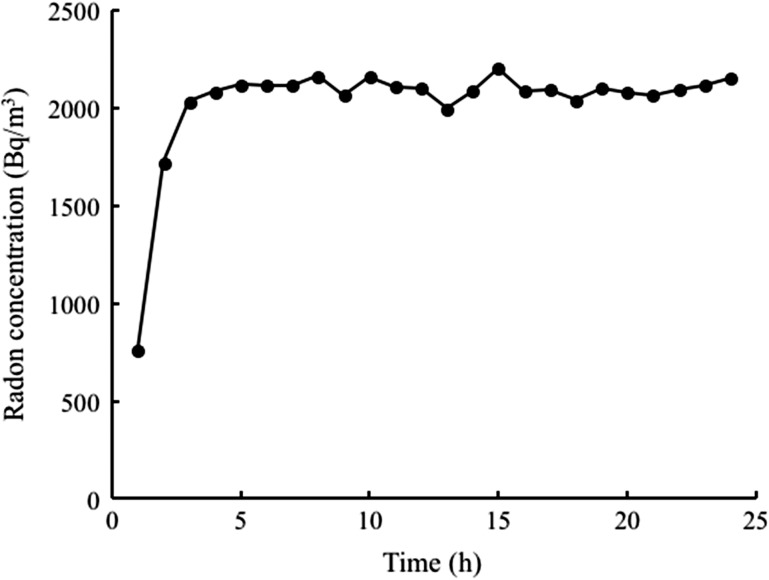
A total of 48 male mice were randomly divided into six groups of eight each: (1) sham inhalation with saline (control), (2) Rn inhalation with saline (Rn), (3) sham inhalation with 30% ethanol (30% EtOH), (4) Rn inhalation with 30% ethanol (Rn + 30% EtOH), (5) sham inhalation with 60% ethanol (60% EtOH) and (6) Rn inhalation with 60% ethanol (Rn + 60% EtOH). Mice were exposed to air only (sham) or radon for 24 h (using the radon exposure system we previously developed) and fed normal drinking water. Briefly, radon at a concentration of 2000 Bq/m3 was blown into a mouse cage [28]. The radon concentration in the cages was determined relative to radon levels used in radon therapy at the Misasa Medical Center, Okayama University Hospital [19, 20]. Radon concentrations were measured using a radon monitor (CMR-510; Femto-Tech Inc., Carlisle, OH, USA). Radon concentrations in the mouse cages are summarized in Fig. 1. The mean radon concentrations for the inhalation treatments were ~2000 Bq/m3 (Fig. 1).
Fig. 1.
Changes in the radon concentration in the mouse cage over the period of radon inhalation using a radon inhalation system.
After sham or radon inhalation, gastric mucosal injury was induced in mice by oral administration of 30% or 60% ethanol (each 0.2 ml/animal). In this experiment, the 100% EtOH group was eliminated to minimize the number of animals because we previously observed that low-dose irradiation does not inhibit severe oxidative stress [29]. Blood was drawn from the heart at 1 h after ethanol administration and the stomach was immediately excised. Specimens were treated using similar procedures to those described for the drinking treatment experiment. Samples were preserved at –80°C for subsequent biochemical analyses.
Assessment of ulcer index scores
To assess the severity of mucosal injury, the ulcer index (UI) was calculated macroscopically. To measure gross gastric mucosal lesions, freshly excised stomachs were laid flat and the mucosal lesions were traced on cork board based on the methods of Birdane et al. [30]. Briefly, gross mucosal lesions were classified as hemorrhages or linear tears (erosions) with damage to the mucosal surface. The area of stomach tissue and gross mucosal lesions were calculated by planimetry using image editing software. The ratio of the total ulcer area to the total gastric area was estimated to determine the UI (%).
Histological assessment of index of histologic injury scores
Tissue samples were fixed in 10% formalin and embedded in paraffin. Five-micrometer-thick tissue sections were prepared and stained with hematoxylin and eosin (HE) to evaluate mucosal damage. To assess the mucosal injury, histological damage was summarized by a mean damage score, the index of histologic injury (IHI), which represents the average depth of damage in each tissue, based on the methods of Yasue and Whittle et al. [31, 32]. Briefly, photomicrographs of gastric mucosae with a certain width were taken randomly from tissue sections, and cellular damage type and length were determined for each specimen. The relative mucosal length for each grade of damage was then calculated. The IHI was calculated as the sum of the scores multiplied by the fraction of the section for each score, defined as follows: IHI = (% type 1 × 1 + % type 2 × 2 + % type 3 × 3 + % type 4 × 4)/100]. The depth of damage to the mucosa was defined as follows: 0 = all gastric mucosal cells appeared intact and no damage was detected, 1 = luminal surface cells had damage, 2 = luminal surface and gastric pit cells had damage, 3 = damage extended beyond the gastric pits, but involved >50% of the thickness of the gastric mucosa, and 4 = extensive gastric mucosal damage involved <50% of the thickness of the gastric mucosa.
Biochemical assays
The stomachs were homogenized in 10 mM PBS using a vortex-type homogenizer (Shakeman; BioMedical Science Co. Ltd, Tokyo, Japan). The homogenates were centrifuged at 12 000 × g at 4°C for 45 min and the supernatants were used to measure SOD and CAT activities.
SOD activity was measured by the nitroblue tetrazolium (NBT) reduction method [33] using the Wako-SOD test (Wako Pure Chemical Industry Co. Ltd, Osaka, Japan) according to the manufacturer’s recommendations. Briefly, inhibition of the reduction of NBT was measured at 560 nm using a spectrophotometer. One unit of enzyme activity was defined as 50% inhibition of NBT reduction.
CAT activity was measured as the rate of H2O2 reduction at 37°C at 240 nm using a spectrophotometer [34]. The assay mixture consisted of 50 μl of 1 M Tris (tris-hydroxymethyl-aminomethane)-HCl buffer containing 5 mM ethylenediaminetetraacetic acid (pH 7.4), 900 μl of 10 mM H2O2, 30 μl of deionized water, and 20 μl of the supernatant. CAT activity was calculated using a molar extinction coefficient of 7.1 × 10−3 M−1 cm−1.
The t-GSH content was measured using the Bioxytech GSH-420 Assay Kit (Oxis Health Products Inc., Portland, OR, USA) according to the manufacturer’s recommendations. Briefly, stomach samples were suspended in 10 mM PBS, mixed with an ice-cold 7.5% trichloroacetic acid solution, and homogenized. The homogenates were centrifuged at 3000 × g for 10 min. The supernatants were then used in the assay.
These parameters were expressed as units per milligram of protein. Protein concentrations were measured using the Bradford method [35] with the Protein Quantification Kit-Rapid (Dojindo Molecular Technologies Inc., Kumamoto, Japan).
LPO levels were assayed by a colorimetric assay (675 nm) using the K-Assay LPO-CC (lipid peroxides) Kit (K-ASSAY LPO-CC; Kamiya Biomedical Company CC-004, Seattle, WA, USA), following the manufacturer’s instructions [36]. Briefly, the stomach was homogenized and 0.1% Triton X-100 and 0.05% sodium deoxycholate (deoxycholic acid) in saline solution was added to the tissue homogenate with shaking for 15 min on ice in dark conditions. After extraction, the homogenate was centrifuged at 3 000 × g for 10 min at 4°C, and the supernatant was used for the assay. The LPO assay is based on the formation of methylene blue in an equal molar reaction with reduced LPO and chromogenic reagent in the presence of hemoglobin. The optical density of the products was read at 675 nm using a spectrophotometer. The data are expressed as units per milligram of protein. The protein concentrations were estimated by a detergent-compatible assay using the DC Protein Assay Kit (Bio-Rad Laboratories, Inc., Tokyo, Japan).
Statistical analysis
Data are presented as means ± standard error of the mean (SEM). Each experimental group consisted of eight samples. The significance of differences in UI score (total area of gastric mucosal lesions), activities of SOD and CAT, t-GSH contents and LPO levels were determined by Tukey’s test for multiple comparisons. Index of histologic injury score was evaluated by the Steel–Dwass test and the Mann–Whitney U-test.
RESULTS
Intake volume and body weight
No significant differences were observed between treatment groups in fluid intake volumes throughout the experimental period (Table 1).
Body weight increased slightly, reflecting normal mouse growth. On the last day (Day 14), body weight was reduced owing to fasting. However, no significant differences in the increase in body weight were observed between groups (Fig. 2).
Fig. 2.
Changes in mouse body weight. Each value represents a mean ± SEM. Eight mice were included for each experimental point.
Effects of hot spring water on gastric lesions and ulcer index scores
To assess the gross severity of mucosal injury, the UI was determined.
Mucosal injury was induced by ethanol administration. As shown in Fig. 3A, the spring water with/without Rn + EtOH groups showed extensively reduced gastric mucosal injury compared with that of the DW + EtOH group. No macroscopic changes were observed in the DW group or the spring water with/without Rn groups.
Fig. 3.
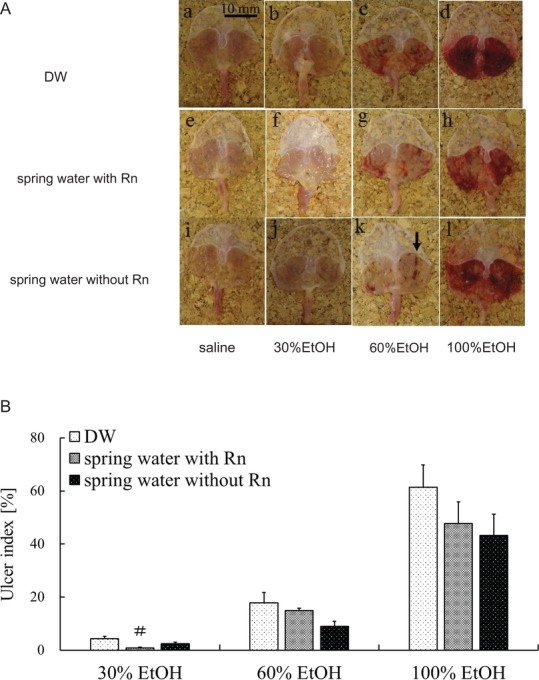
Effects of drinking hot spring water on macroscopic properties and UI in the stomachs of mice with ethanol-induced gastric mucosal injury. (A) Macroscopic appearances of the gastric mucosa in different groups: (a) DW group; (b) DW + 30% EtOH group; (c) DW + 60% EtOH group; (d) DW + 100% EtOH group; (e) spring water with Rn group; (f) spring water with Rn + 30% EtOH group; (g) spring water with Rn + 60% EtOH group; (h) spring water with Rn + 100% EtOH group; (i) spring water without Rn group; (j) spring water without Rn + 30% EtOH group; (k) spring water without Rn + 60% EtOH group; and (l) spring water without Rn + 100% EtOH group. The black arrow in (k) indicates typical ulcer areas. (B) UI values. Each value represents a mean ± SEM. The number of mice per experimental point was eight. #P < 0.05, Rn-containing hot spring water (spring water with Rn) vs DW with ethanol.
The UI, an indicator of the severity of macroscopic mucosal injury, increased in an ethanol concentration–dependent manner. Regardless of the ethanol concentration, the UI was lower in the spring water with Rn + EtOH and spring water without Rn + EtOH groups than the DW + EtOH group. Specifically, for groups administered a 30% concentration of ethanol, the UI was significantly lower in the spring water with Rn + EtOH group than in the DW + EtOH group (Fig. 3B). The UI values of all mice treated with DW and spring water with/without Rn were 0%, as shown in Fig. 3A (a, e and i).
Effects of hot spring water drinking on histopathological changes and index of histologic injury scores
Histopathological alterations in stomach specimens for each group are shown in Fig. 4A. No severe histological changes were observed in stomach specimens taken from the DW group or the spring water with/without Rn groups, in which the gastric mucosa, luminal surface, and gastric pit cells were intact [Fig. 4A (a, e and i)]. In contrast, ethanol administration to mice caused gastric mucosal injury characterized by luminal surface and gastric pit cell damages [Fig. 4A (b–d)]. Pre-treatment with spring water with/without Rn [Fig. 4A (f–h, j–l)] improved these alterations, and resulted in less mucosal damage compared with that of the DW + EtOH groups. In a histological assessment of gastric ulcers, the IHI, an indicator of ulcer severity (i.e. ulcer depth), increased in an ethanol concentration-dependent manner. The IHI was significantly lower (P < 0.05) in the spring water with Rn + EtOH group than in the EtOH group for 100% ethanol. The IHI was lower in the spring water without Rn + EtOH groups than in the DW + EtOH group, whereas, no significant differences were noted between the DW + EtOH group and the spring water without Rn + EtOH group (Fig. 4B).
Fig. 4.
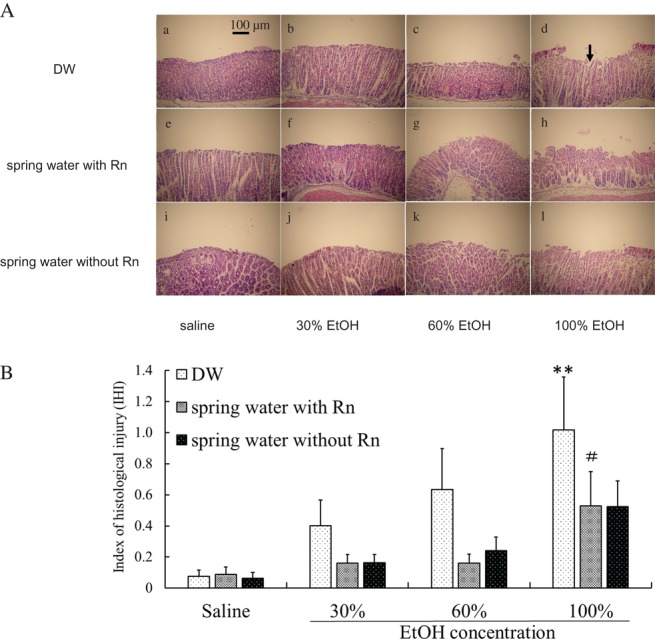
Effects of drinking hot spring water on the microscopic morphology and the IHI in the stomachs of mice with ethanol-induced gastric mucosal injury. (A) Histological appearances of the stomachs of mice in different groups: (a) DW group; (b) DW + 30% EtOH group; (c) DW + 60% EtOH group; (d) DW + 100% EtOH group; (e) spring water with Rn group; (f) spring water with Rn + 30% EtOH group; (g) spring water with Rn + 60% EtOH group; (h) spring water with Rn + 100% EtOH group; (i) spring water without Rn group; (j) spring water without Rn + 30% EtOH group; (k) spring water without Rn + 60% EtOH group; and (l) spring water without Rn + 100% EtOH group. Scale bar = 100 μm. All samples were stained with HE. The black arrow in (d) indicates typical injury areas. (B) IHI scores. Each value represents a mean ± SEM. The number of mice per experimental point was eight. **P < 0.01, DW with ethanol vs DW with saline. #P < 0.05, Rn-containing hot spring water (spring water with Rn) vs DW with ethanol.
Effects of hot spring water drinking on antioxidant-associated substances in the stomachs of mice with ethanol-induced gastric mucosal injury
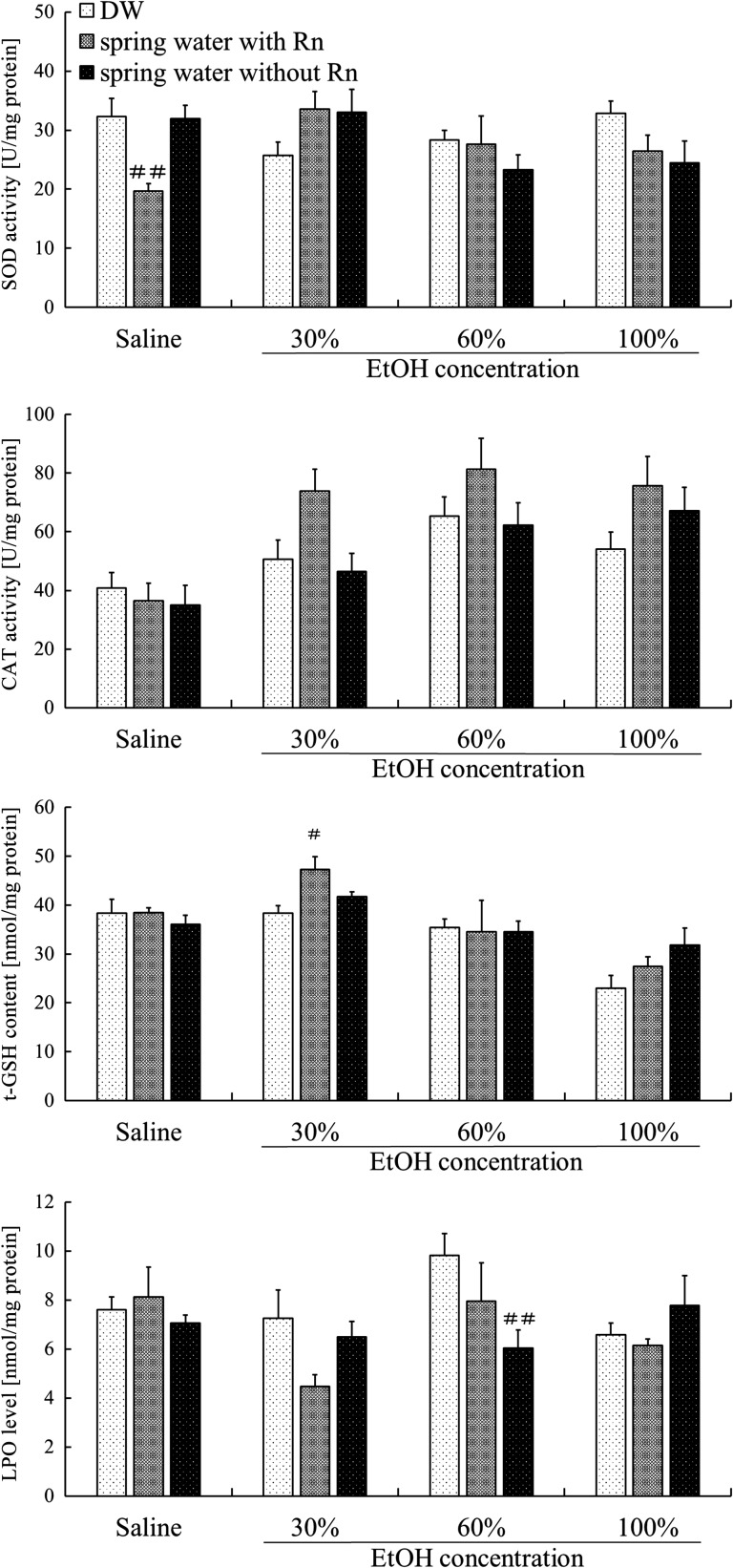
To assess the biological effect of radon inhalation on the gastric mucosa, antioxidant substances in the stomach were assayed. SOD activity was significantly lower (P < 0.01) in the spring water with Rn group than in the DW group. For groups administered 30% ethanol, SOD activity was lower in the DW + EtOH group than in the DW group, but higher in the spring water with/without Rn + EtOH groups than in the DW + EtOH group. However, these differences were not significant (Fig. 5). No statistically significant differences were found between groups with respect to CAT activity. However, regardless of ethanol concentration, CAT activity was higher in all groups pre-treated with Rn-containing hot spring water than in mice pre-treated with distilled water (Fig. 5). The t-GSH content was significantly higher (P < 0.05) in the spring water with Rn + EtOH group than in the DW + EtOH group for a 30% concentration of ethanol. LPO levels were significantly lower (P < 0.01) in the spring water without Rn + EtOH group than in the DW + EtOH group for 60% ethanol (Fig. 5). In addition, among groups administered 30% or 60% of ethanol, LPO levels were lower in the Water with/without Rn + EtOH group than in the DW + EtOH group.
Fig. 5.
Effects of drinking hot spring water on antioxidant-associated substances in the stomachs of mice with ethanol-induced gastric mucosal injury. Each value represents a mean ± SEM. The number of mice per experimental point is eight. #P < 0.05, ##P < 0.01, Rn-containing hot spring water (spring water with Rn) or Rn-deaeration hot spring water (spring water without Rn) vs DW with saline or DW with ethanol.
Effects of radon inhalation on gastric lesions and ulcer index scores
As shown in Fig. 6A, the Rn + 60% EtOH group showed extensively reduced gastric mucosal injury compared with that of the 60% EtOH group. However, no macroscopic changes were observed in the groups administered 30% ethanol. Similar to the drinking treatment, the severity of mucosal injury was assessed based on the UI. The UIs of all mice in the control group and Rn group were 0, as shown in Fig. 6A (a and d). The UI was significantly lower (P < 0.05) in the Rn + 60% EtOH group than in the 60% EtOH group (Fig. 6B).
Fig. 6.
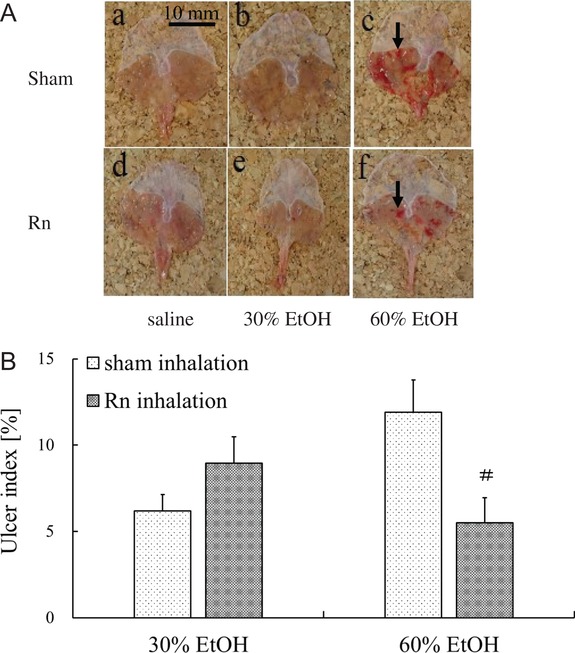
Effects of radon inhalation on the macroscopic properties and UI in the stomachs of mice with ethanol-induced gastric mucosal injury. (A) Macroscopic appearances of the gastric mucosa in different groups: (a) control group; (b) 30% EtOH group; (c) 60% EtOH group; (d) Rn group; (e) Rn + 30% EtOH group; (f) and Rn + 60% EtOH group. The black arrow in (c) indicates typical ulcer areas. (B) UI score. Each value represents a mean ± SEM. The number of mice per experimental point was eight. #P < 0.05, Rn inhalation with ethanol vs sham inhalation with ethanol.
Effects of radon inhalation on histopathological changes and index of histologic injury scores
As shown in Fig. 7A, no severe histological alterations were observed in mouse stomach specimens from the control group and the Rn group [Fig. 7A (a and d)]. In contrast, ethanol administration to mice caused gastric mucosal injury [Fig. 7A (b and c)]; however, Rn + EtOH groups [Fig. 7A (e and f)] exhibited improvements in these alterations, and showed less mucosal damage compared with the EtOH groups.
Fig. 7.
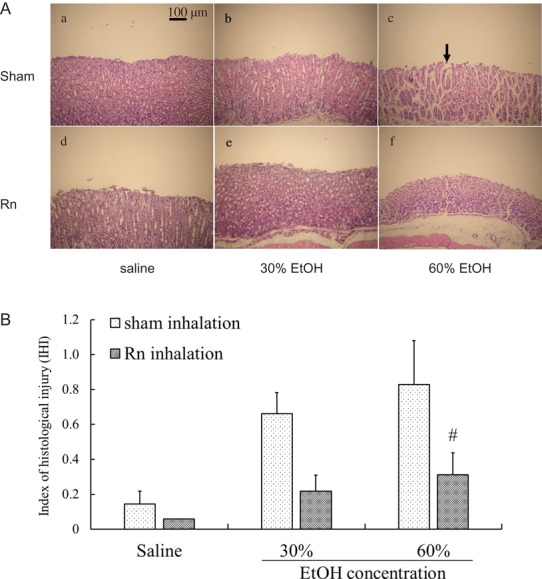
Effects of radon inhalation on the microscopic morphology and IHI in the stomachs of mice with ethanol-induced gastric mucosal injury. (A) Histological appearances of the stomachs of mice in different groups: (a) control group; (b) 30% EtOH group; (c) 60% EtOH group; (d) Rn group; (e) Rn + 30% EtOH group; (f) and Rn + 60% EtOH group. Scale bar = 100 μm. All samples were stained with HE. The black arrow in (c) indicates typical injury area. (B) IHI scores. The number of mice for each experiment and significance are the same as those for Fig. 6.
After ethanol administration, the IHI scores were higher in all groups; however, the IHI score was significantly lower (P < 0.05) in the Rn + 60% EtOH group than in the 60% EtOH group (Fig. 7B).
Effects of radon inhalation on antioxidant-associated substances in the stomach of mice with ethanol-induced gastric mucosal injury
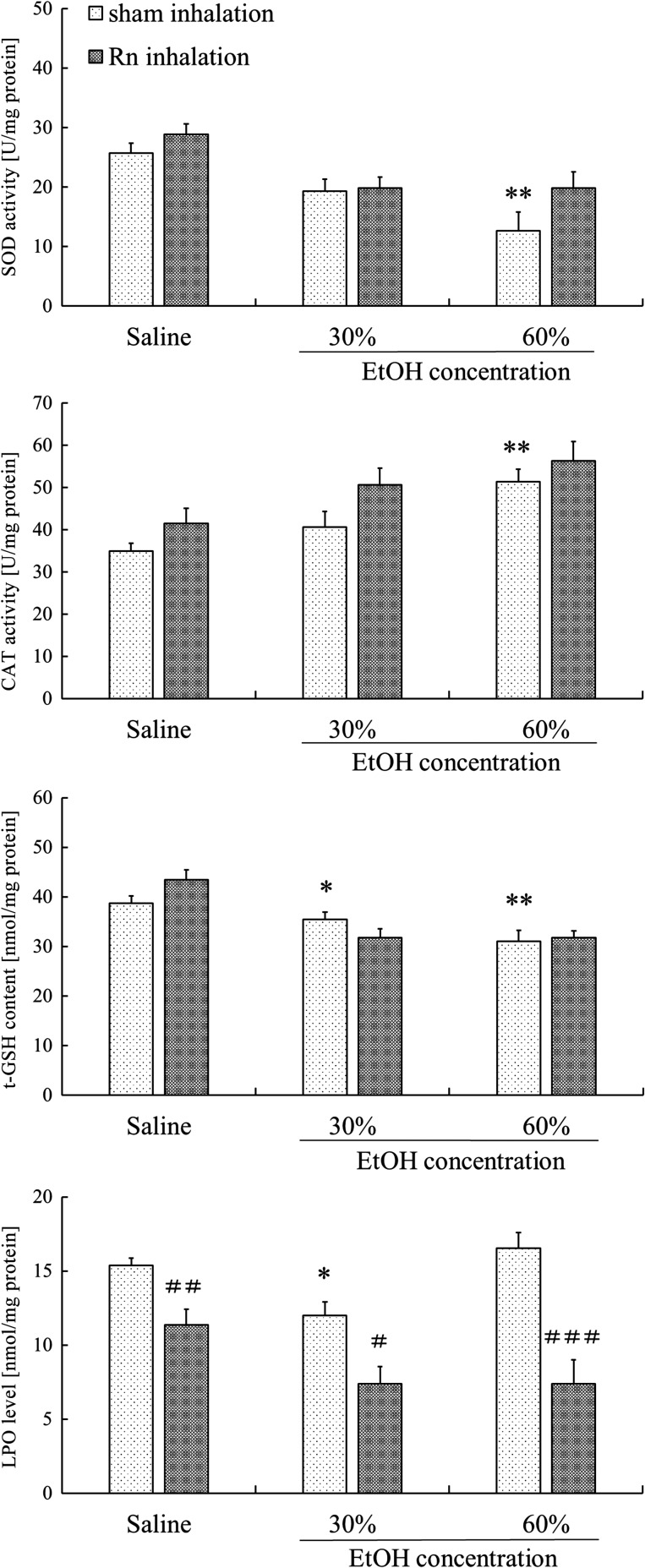
Although SOD activity in the stomach was significantly lower (P < 0.01) in the 60% EtOH group than in the control group, SOD activity was higher in the Rn + EtOH group than in the EtOH group; however, this difference was not significant (Fig. 8). CAT activity in the stomach was significantly higher (P < 0.01) in the EtOH group after the administration of 60% ethanol than in the control group (Fig. 8). However, regardless of whether ethanol was administered, CAT activity showed a tendency to increase in all groups pre-treated with Rn inhalation than in groups pre-treated with sham inhalation; no significant difference was indicated. The t-GSH content was significantly lower in the EtOH group (30%, P < 0.05; 60%, P < 0.01) than in the control group (Fig. 8). Regardless of ethanol administration, LPO was significantly lower (Saline P < 0.01, 30% ethanol P < 0.05, 60% ethanol P < 0.001) in all groups pre-treated with Rn inhalation compared with in sham inhalation groups (Fig. 8).
Fig. 8.
Effects of radon inhalation on antioxidant-associated substances in the stomachs of mice with ethanol-induced gastric mucosal injury. Each value represents a mean ± SEM. The number of mice per experimental point was eight. *P < 0.05, **P < 0.01, sham inhalation with ethanol vs sham inhalation with saline. #P < 0.05, ##P < 0.01, ###P < 0.001, radon inhalation (with EtOH) vs sham inhalation (with ethanol).
DISCUSSION
The mechanism of action of radon therapy is thought to involve the entrance of radon dissolved in the blood into the gas exchange compartment and its subsequent transport to many tissues through the systemic circulation, resulting in stimulatory effects in these tissues [37]. In fact, we previously demonstrated that radon inhalation protects a number of tissue types from chemically induced oxidative damage [21, 38]. Although several studies have reported effects of hot spring water intake on the stomach, it is not clear whether antioxidative substances are involved in the beneficial effects.
In this study, mice were orally administered ethanol at three concentrations (30%, 60% and 100%) to examine the mechanisms of pathogenesis. Ischemia following the administration of a low concentration of ethanol does not play an important role in ethanol-induced gastric mucosal injury. ROS are involved in the pathogenesis following the administration of a medium concentration of ethanol, but lipid peroxidation is not affected. ROS and lipid peroxidation both play important roles in the pathogenesis following the administration of a high concentration of ethanol [15, 39–42]. Our results showed that all concentrations of ethanol elevated the UI, indicating that the gastric mucosa was damaged. For the drinking treatment, a lower UI was observed in the spring water with/without Rn + EtOH groups than in the DW + EtOH group, and a significantly lower UI was observed in the spring water with Rn + EtOH group than in the DW + EtOH group for the 30% ethanol groups. Furthermore, IHI values obtained by pathological observation of the gastric mucosa suggest protective effects of hot spring water drinking on the gastric mucosa. The IHI was lower in the spring water with/without Rn + EtOH groups than in the DW + EtOH group. These findings suggested that hot spring water drinking inhibits ethanol-induced gastric ulcers in mice. In addition, no significant differences were observed between the spring water with Rn + EtOH group and the spring water without Rn + EtOH group. There are several reports on the in vivo effects of radon ingestion via drinking water [43, 44]. The radioactivity ingested by drinking radon-treated spring water per mouse was estimated to be a maximum of ~2 Bq in one day, excluding the half life of radioactivity from consideration. This calculation gives a whole-body dose of 5.5 nGy, assuming that the absorbed dose in the mouse is same as the 2.70 μGy/Bq of ingested radon in humans [45]. It is believed that the stomach takes up most of the effective dose when ingesting radon with drinking water [46]. However, there is no data available on the biokinetics of radon ingested via drinking water in small animals such as mice, and it is difficult to estimate the actual stomach-absorbed dose. In this study, the total intake of radon by drinking is estimated to be extremely small because it seems difficult to induce radiobiological reactions. These findings indicate that radon in water does not influence the inhibitory effects, but probably reflects that the pharmacological effects are due to other chemicals dissolved in the hot spring water, rather than radon.
On the other hand, for the inhalation treatments, a significantly lower UI was observed in the Rn + 60% EtOH group than in the 60% EtOH group. Moreover, the IHI was significantly lower in the Rn + EtOH group than in the EtOH group at 60% concentrations of ethanol. These findings substantiated the protective effects of radon inhalation on the gastric mucosa, as for the hot spring water drinking treatment. The absorbed dose level of the mouse stomach during the inhalation treatment was estimated as 0.1 μGy by using the absorbed dose rate of radon inhalation reported by Sakoda et al. [37]. They showed that the absorbed dose of the stomach in radon inhalation is almost the same level as that of other major organs. Our previous report also showed that SOD activity was increased in major organs other than the stomach by the same inhalation conditions [21]. Also in this study, the dose level was enough to induce radiobiological reactions in the stomach, because the absorbed dose was similar to that of other organs. These findings suggest that the mechanisms responsible for the inhibitory effects of drinking water and radon inhalation on ethanol-induced gastric ulcer differ.
ROS are involved in ethanol-induced gastric mucosal injury [47]. In addition, ethanol-induced gastric mucosal injury is suppressed by the prior administration of SOD, O2•– removal enzymes, and scavengers of •OH [39]. Thus, O2•– and •OH are involved in gastric mucosal injury induced by ethanol. Additionally, lipid peroxidation following the generation of ROS plays an important role in ROS-induced cellular damage. Although the degree of injury differs, gastric mucosal injury is induced by alcohol administration. Regardless of the concentration of ethanol, ROS are involved in the induction of gastric mucosal injury. On the other hand, SOD plays an important role in protecting cells from oxidative damage by converting O2•– into H2O2. CAT transforms H2O2 into H2O as well as GSH. Many studies have demonstrated that antioxidant substances are beneficial against gastric mucosal injury [15, 16, 46]. Therefore, to clarify the roles of antioxidative substances that suppress mucosal injury, SOD and CAT activity, t-GSH content, and LPO levels in the stomach were investigated.
In the case of drinking treatments, our results showed that antioxidative functions, as indicated by SOD (30% EtOH), CAT (100% EtOH) and t-GSH (30% EtOH) levels, in the stomach were higher, but not significantly different, in the water with/without Rn + EtOH group than in the DW + EtOH group. In addition, for groups administered a 30% or 60% EtOH, CAT activity was higher only in the water with Rn + EtOH group compared with the DW + EtOH group. LPO levels (30%, 60% EtOH) were lower in the water with/without Rn + EtOH group than in the DW + EtOH group.
In the case of inhalation treatments, antioxidative functions, as evidenced by CAT (30%, 60% EtOH) and SOD activity (60% EtOH), in the stomach were higher in the Rn + EtOH group than in the EtOH group, but these differences were not significant. LPO levels were lower in the Rn + EtOH group than in the EtOH group. Higher CAT activity in the drinking treatment groups was similar to that observed in the inhalation groups. These findings suggest that CAT plays an important role in the inhibition of ethanol-induced gastric ulcer. Since many of these differences were not significant, the activation of antioxidative functions in the stomach is less effective than in other tissues, as reported in our previous study [21]. However, LPO levels in the stomach were clearly suppressed. Other antioxidants such as glutathione peroxidase that were not measured in this study may be involved in inhibiting gastric mucosal injury.
We focused on changes in antioxidative substances to clarify the inhibitory effects of ethanol-induced gastric mucosal injury. In conclusion, there is a possibility that hot spring water drinking and radon inhalation suppress ethanol-induced gastric mucosal injury, via the activation of antioxidative enzymes, specifically CAT. Although the activation of antioxidative mechanisms after hot spring water intake and radon inhalation was less effective in the stomach than in other tissues (based on our previous study), oxidative stress induced by ethanol was clearly suppressed. In this study, the effects of radon inhalation and drinking on the blood flow in the stomach were not examined. It is highly possible that activation of antioxidant function is one of the mechanisms of radon therapy; however, more detailed studies are necessary to further clarify the mechanism by which radon affects the stomach.
Generally, the gastrointestinal epithelium is sensitive to radiation; however, in the present study, morphological changes in the gastric mucosa were not observed in the Water with/without Rn group and the Rn group. Radon therapy is performed for various diseases at the Misasa Medical Center, Okayama University Hospital, Japan. However, the mechanisms underlying the health effects have not been investigated. Our results suggest the potential of radon therapy for the prevention of gastric mucosal injury.
ACKNOWLEDGEMENTS
The authors thank the staffs of the Departments of Animal Resources and Radiation Research, Shikata Laboratory Advanced Science Research Center (Okayama University) for technical support in this work. The results of this study were presented at the Atomic Energy Society of Japan 2016 Annual Meeting.
CONFLICT OF INTEREST
The authors state that there are no conflicts of interest.
FUNDING
This work was supported by Okayama University and Japan Atomic Energy Agency.
REFERENCES
- 1. Katz D. Erosive Gastritis and Acute Gastric Mucosal Lesion. New York: Grune & Stratton, 1968. [Google Scholar]
- 2. Levenstein S. Peptic ulcer at the end of the 20th century: biological and psychological risk factors. Can J Gastroenterol 1999;13:753–9. [DOI] [PubMed] [Google Scholar]
- 3. Malfertheiner P, Chasn FK, McColl KE. Peptic ulcer disease. Lancet 2009;374:1449–61. [DOI] [PubMed] [Google Scholar]
- 4. Friedman GD, Siegelaub AB, Seltzer CC. Cigarettes, alcohol, coffee and peptic ulcer. N Engl J Med 1974;290:469–73. [DOI] [PubMed] [Google Scholar]
- 5. Shay H. Etiology of peptic ulcer. Am J Dig Dis 1961;6:29–49. [DOI] [PubMed] [Google Scholar]
- 6. Sun DCH. Etiology and pathology of peptic ulcer In: Bockus HL. (ed). Gastroenterology, 3rd edn Philadelphia: Saunders, 1974, 579–610. [Google Scholar]
- 7. Itoh M, Guth PH. Role of oxygen-derived free radicals in hemorrhagic shock–induced gastric lesions in the rat. Gastroenterology 1985;88:1162–7. [DOI] [PubMed] [Google Scholar]
- 8. Yoshikawa T, Ueda S, Naito Y et al. Role of oxygen-derived free radicals in gastric mucosal injury induced by ischemia or ischemia-reperfusion in rats. Free Radic Res Commun 1989;7:285–91. [DOI] [PubMed] [Google Scholar]
- 9. Yoshikawa T, Naito Y, Kishi A et al. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut 1993;34:732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshikawa T, Takahashi S, Naito Y et al. Antioxidants in gastric mucosal injury. J Nutr Sci Vitaminol (Tokyo) 1992;38:556–9. [DOI] [PubMed] [Google Scholar]
- 11. Body SC, Sasame HA, Body MR. High concentrations of glutathione in glandular stomach: possible implications for carcinogenesis. Science 1979;205:1010–12. [DOI] [PubMed] [Google Scholar]
- 12. Boyd SC, Sasame HA, Boyd MR. Gastric glutathione depletion and acute ulcerogenesis by diethylmaleate given subcutaneously to rats. Life Sci 1981;28:2987–92. [DOI] [PubMed] [Google Scholar]
- 13. Szabo S, Trier JS, Frankel PW. Sulfhydryl compounds may mediate gastric cytoprotection. Science 1981;214:200–202. [DOI] [PubMed] [Google Scholar]
- 14. Hirota M, Inoue M, Ando Y et al. Inhibition of stress-induced gastric injury in the rat by glutathione. Gastroenterology 1989;97:853–9. [DOI] [PubMed] [Google Scholar]
- 15. Mutoh H, Hiraishi H, Ota S et al. Protective role of intracellular glutathione against ethanol-induced damage in cultured rat gastric mucosal cells. Gastroenterology 1990;98:1452–9. [DOI] [PubMed] [Google Scholar]
- 16. Yoshikawa T, Yasuda M, Ueda S et al. Vitamin E in gastric mucosal injury induced by ischemia-reperfusion. Am J Clin Nutr 1991;53:210–4. [DOI] [PubMed] [Google Scholar]
- 17. Masuda E, Kawano S, Nagano K et al. Role of endogenous endothelin in pathogenesis of ethanol-induced gastric mucosal injury in rats. Am J Physiol 1993;265:474–81. [DOI] [PubMed] [Google Scholar]
- 18. Watanabe Y, Okumura T, Onodera S et al. Intracisternal injection of basic fibroblast growth factor reduces the severity of gastric mucosal lesions evoked by ethanol in rats. Jpn J Physiol 1997;47:231–3. [DOI] [PubMed] [Google Scholar]
- 19. Yamaoka K, Mitsunobu F, Hanamoto K et al. Study on biologic effects of radon and thermal therapy on osteoarthritis. J Pain 2004;5:20–5. [DOI] [PubMed] [Google Scholar]
- 20. Mitsunobu F, Yamaoka K, Hanamoto K et al. Elevation of antioxidant enzymes in the clinical effects of radon and thermal therapy for bronchial asthma. J Radiat Res 2003;44:95–9. [DOI] [PubMed] [Google Scholar]
- 21. Kataoka T, Sakoda A, Ishimori Y et al. Study of the response of superoxide dismutase in mouse organs to radon using a new large-scale facility for exposing small animals to radon. J Radiat Res 2011;52:775–81. [DOI] [PubMed] [Google Scholar]
- 22. Kataoka T, Nishiyama Y, Toyota T et al. Radon inhalation protects mice from carbon-tetrachloride–induced hepatic and renal damage. Inflammation 2011;34:559–67. [DOI] [PubMed] [Google Scholar]
- 23. Nishiyama Y, Kataoka T, Yamato K et al. Suppression of dextran sulfate sodium–induced colitis in mice by radon inhalation. Mediators Inflamm 2012;2012:239617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Etani R, Kataoka T, Kanzaki N et al. Difference in the action mechanism of radon inhalation and radon hot spring water drinking in suppression of hyperuricemia in mice. J Radiat Res 2016;57:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanaka J, Matsumoto S, Senou T et al. Short-term effect of thermal water on gastric mucosal blood flow. J Soc Balneol Climatol Phys Med Jpn 1988;51:153–6 (in Japanese). [Google Scholar]
- 26. Tanaka J, Matsumoto S, Senou T et al. Short-term effect of thermal water on gastric mucosal blood flow. J Soc Balneol Climatol Phys Med Jpn 1988;52:127–30 (in Japanese) . [Google Scholar]
- 27. Swarnakar S, Mishra A, Ganguly K et al. Matrix metalloproteinase-9 activity and expression is reduced by melatonin during prevention of ethanol-induced gastric ulcer in mice. J Pineal Res 2007;43:56–64. [DOI] [PubMed] [Google Scholar]
- 28. Kataoka T, Teraoka J, Sakoda A et al. Protective effects of radon inhalation on carrageenan-induced inflammatory paw edema in mice. Inflammation 2012;35:713–22. [DOI] [PubMed] [Google Scholar]
- 29. Kataoka T, Mizuguchi Y, Yoshimoto M et al. Inhibitory effects of prior low-dose X-irradiation on ischemia-reperfusion injury in mouse paw. J Radiat Res 2007;48:505–13. [DOI] [PubMed] [Google Scholar]
- 30. Birdane FM, Cemek M, Birdane YO et al. Beneficial effects of Foeniculum vulgare on ethanol-induced acute gastric mucosal injury in rats. World J Gastroenterol 2007;13:607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whittle BJ, Lopez-Belmonte J, Moncada S. Regulation of gastric mucosal integrity by endogenous nitric oxide: interactions with prostanoids and sensory neuropeptides in the rat. Br J Pharmacol 1990;99:607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yasue N, Guth PH. Role of exogenous acid and retransfusion in hemorrhagic shock–induced gastric lesions in the rat. Gastroenterology 1988;94:1135–43. [DOI] [PubMed] [Google Scholar]
- 33. Baehner RL, Murrmann SK, Davis J et al. The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J Clin Invest 1975;56:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aebi H, Wyss SR, Scherz B et al. Properties of erythrocyte catalase from homozygotes and heterozygotes for Swiss-type acatalasemia. Biochem Genet 1976;14:791–807. [DOI] [PubMed] [Google Scholar]
- 35. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 36. Cervantes-Munguía R, Valle-Razo FP, Gómez-Contreras PC et al. Serum lipoperoxide in a pregnant woman and in term and premature neonates. Presse Med 2000;29:788. [PubMed] [Google Scholar]
- 37. Sakoda A, Ishimori Y, Kawabe A et al. Physiologically based pharmacokinetic modeling of inhaled radon to calculate absorbed doses in mice, rats, and humans. J Nucl Sci Technol 2010;47:731–8. [Google Scholar]
- 38. Nishiyama Y, Kataoka T, Teraoka J et al. Inhibitory effects of pre and post radon inhalation on carbon tetrachloride–induced oxidative damage in mouse organs. Radioisotopes 2012;61:231–41. [Google Scholar]
- 39. Terano A, Hiraishi H, Ota S et al. Role of superoxide and hydroxyl radicals in rat gastric mucosal injury induced by ethanol. Gastroenterol Jpn 1989;24:488–93. [DOI] [PubMed] [Google Scholar]
- 40. Ueda S. Effects of Ebselen on Acute Gastric Mucosal Injury in Rats. Amsterdam: Elsevier, 1989. [Google Scholar]
- 41. Mizui T, Doteuchi M. Lipid peroxidation: a possible role in gastric damage induced by ethanol in rats. Life Sci 1986;38:2163–7. [DOI] [PubMed] [Google Scholar]
- 42. Mizui T, Sato H, Hirose F et al. Effect of antiperoxidative drugs on gastric damage induced by ethanol in rats. Life Sci 1987;41:755–63. [DOI] [PubMed] [Google Scholar]
- 43. Hursh JB, Morken DA, Davis TP et al. The fate of radon ingested by man. Health Phys 1965;11:465–76. [DOI] [PubMed] [Google Scholar]
- 44. Gosink TA, Baskaran M, Holleman DF et al. Radon in the human body from drinking water. Health Phys 1990;59:919–24. [DOI] [PubMed] [Google Scholar]
- 45. Brown WL, Hess CT. Measurement of the biotransfer and time constant of radon from ingested water by human breath analysis. Health Phys 1992;62:162–70. [DOI] [PubMed] [Google Scholar]
- 46. Sharma N, Hess CT, Thrall KD. A compartmental model of water radon contamination in the human body. Health Phys 1997;72:261–8. [DOI] [PubMed] [Google Scholar]
- 47. Szelenyi I, Brune K. Possible role of oxygen free radicals in ethanol-induced gastric mucosal damage in rats. Dig Dis Sci 1988;33:865–71. [DOI] [PubMed] [Google Scholar]