Abstract
Background
Breast-cancer related lymphedema (BCRL) is a significant complication for women undergoing treatment. We assessed BCRL incidence and risk factors in a large population-based cohort.
Methods
We utilized the Olmsted County Rochester Epidemiology Project Breast Cancer Cohort from 1990–2010 and ascertained BCRL and risk factors. The cumulative incidence estimator was used to estimate the rate of BCRL; competing risks regression was used for multivariable analysis.
Results
1794 patients with stage 0–3 breast cancer with a median of 10 years followup were included. The cumulative incidence of BCRL diagnosis within 5 years was 9.1% (95% CI: 7.8–10.5%). No BCRL events occurred among patients without axillary surgery. In the axillary surgery subset (n=1512), the 5-year incidence of BCRL was 5.3% in sentinel lymph node (SLN) surgery and 15.9% in axillary dissection (ALND) patients (p<0.001). In patients treated with surgery only, BCRL rates were not different between ALND versus SLN (3.5% and 4.1% at 5 years, p=0.36). Addition of breast or chest wall radiation more than doubled the BCRL rate in ALND patients (3.5% versus 9.5% at 5 years, p=0.01). The groups with highest risk (>25% at 5 years) all involved ALND with nodal RT and/or anthracycline/cytoxan+taxane chemotherapy.
In multivariable analysis of patients with any axillary surgery factors significantly associated with BCRL were ALND, chemotherapy, radiation and obesity.
Conclusion
BCRL is a sequelae of multimodal breast cancer treatment and risk is multifactorial. BCRL rates are higher in patients receiving chemotherapy, radiation, ALND, more advanced disease stage, and higher BMI.
Introduction
Breast cancer-related lymphedema (BCRL) is a well-known complication and the most common morbidity resulting from breast cancer treatments. The incidence of lymphedema ranges from 3–65% depending on the treatment, mode of lymphedema diagnosis, and length of follow-up.1–15 Survivors may also experience a substantial degree of functional impairment, psychological morbidity, and diminished quality of life.16–19 Lymphedema occurs when protein-rich fluid accumulates in soft tissues because of interruption of lymphatic flow.20 As a result, factors that influence this disruption have been implicated in the development of lymphedema. Additionally, data indicates that 75% of BCRL cases occur within the first year after surgery and 90% within 3 years, among patients undergoing prospective monitoring.21
Numerous studies have shown that more extensive axillary surgery with axillary lymph node dissection (ALND) significantly increase the risk for development of BCRL compared to sentinel lymph node (SLN) surgery.22 Additionally, regional nodal irradiation (RNI), which involves radiation targeting the axilla, has also been associated with increased rates of BCRL.1 However, other treatment and patient-related factors have not been as strongly linked to BCRL. Knowledge of the incidence of BCRL related to patient and treatment factors is important for patient education. Furthermore, improved recognition of patients at higher risk for BCRL should facilitate early risk-reduction strategies with proactive surveillance and treatment, which has been shown to improve patient outcomes and quality of life.23
In this population-based study, we aimed to determine the incidence of BCRL in a large cohort of patients diagnosed with breast cancer with long-term follow-up and to estimate the association of demographic and clinical characteristics with BCRL.
Methods
We utilized the Olmsted County Rochester Epidemiology Project Breast Cancer Cohort, a population-based sample of all incident breast cancer cases diagnosed in Olmsted County, MN residents in 1990–2010. Trained nurse abstractors performed a comprehensive search of medical records and noted occurrences of the key words “edema”, “heaviness”, “lymphedema”, “puffiness”, and “swelling” affecting the upper extremities. All available clinical notes were examined including those from surgery, oncology, primary care, physical therapy, and lymphedema clinic providers. All cases with definite or probable lymphedema were included as BCRL.
Time to BCRL was calculated from definitive breast surgery to date of diagnosis of BCRL. Patients in the cohort underwent a range of surgical and adjuvant treatments which allow the relationship of each treatment modality and BCRL to be assessed. Data on other events during follow-up, including dates of recurrence (local and systemic), diagnosis of new contralateral primary breast cancer, last follow-up and death were also collected. Patient, clinicopathologic, and treatment variables collected included age, type of breast and axillary surgery, radiation fields, type(s) of chemotherapy, pathologic stage, and baseline BMI. Breast surgery was classified as breast-conserving surgery (BCS), unilateral mastectomy, or bilateral mastectomy; bilateral mastectomy may have been performed for either bilateral cancer or for unilateral cancer with contralateral prophylactic mastectomy. In patients with synchronous bilateral cancer at baseline, data from the highest stage side with the most extensive treatment was used for analysis. Axillary surgery was classified as the most extensive surgery, so patients who underwent SLN surgery and ALND were classified as ALND.
Statistical Analysis
Time-to-event methods were used to analyze the outcome of definite/probable BCRL with time calculated as the years from the date of definitive cancer surgery to the earliest of the following events: BCRL diagnosis, recurrence, new contralateral primary, last follow-up, or death. The cumulative incidence estimator was used to estimate the proportion with BCRL diagnosis while accounting for the competing risks of death, recurrence, or new contralateral primary. Patients not experiencing any of these events were censored at last follow-up. Fine & Grey competing risks regression was used for multivariable analysis assessing risk factors for BCRL, which are reported with hazard ratios (HRs) and 95% confidence intervals (CIs). Analysis was performed using SAS (Version 9.4, SAS Institute Inc., Cary, NC) and the cmprsk package for R software.24 P-values <0.05 were considered statistically significant.
Results
A total of 1794 patients with stage 0–3 breast cancer with a median of 10 years follow-up were included. Breast cancer was unilateral in 1764 (98%) and bilateral in 30 (2%). Cohort characteristics are summarized in Table 1. Median age at cancer diagnosis was 60 years, and 44% were overweight or obese at baseline. Stage distribution was 17% stage 0, 47% stage I, 28% stage II, and 7% stage III. A majority (58%) underwent BCS, while 28% underwent unilateral mastectomy and 13% bilateral mastectomy. Most patients underwent axillary staging surgery, with 44% undergoing ALND, 40% SLN surgery only, and 16% having no axillary surgery. The median number of lymph nodes examined was 3 for SLN surgery and 16 in ALND. Overall, 57% received radiation and 29% received chemotherapy.
Table 1.
Cohort characteristics
| No Axillary Surgery (N=282) |
Axillary Surgery (N=1512) |
Total (N=1794) |
p value | |
|---|---|---|---|---|
| Age | <0.0011 | |||
| Mean (SD) | 65.7 (14.2) | 59.9 (13.4) | 60.8 (13.7) | |
| Median (Range) | 65.8 (35.6–93.9) | 59.3 (26.0–91.8) | 60.3 (26.0–93.9) | |
| Baseline BMI | 0.781 | |||
| Mean (SD) | 25.1 (5.6) | 25.0 (5.7) | 25.0 (5.7) | |
| Median (Range) | 24.3 (12.2–52.3) | 24.0 (11.0–57.9) | 24.1 (11.0–57.9) | |
| BMI category | 0.362 | |||
| Underweight | 20 (7.1%) | 133 (8.8%) | 153 (8.6%) | |
| Normal | 138 (49.1%) | 712 (47.2%) | 850 (47.5%) | |
| Overweight | 76 (27.0%) | 399 (26.5%) | 475 (26.6%) | |
| Obese | 30 (10.7%) | 187 (12.4%) | 217 (12.1%) | |
| Morbidly Obese | 17 (6.0%) | 76 (5.0%) | 93 (5.2%) | |
| Missing | 1 | 5 | 6 | |
| Pathologic stage | <0.0012 | |||
| 0 | 192 (68.1%) | 121 (8.0%) | 313 (17.4%) | |
| I | 76 (27.0%) | 769 (50.9%) | 845 (47.1%) | |
| II | 12 (4.3%) | 496 (32.8%) | 508 (28.3%) | |
| III | 2 (0.7%) | 126 (8.3%) | 128 (7.1%) | |
| Breast surgery | <0.0013 | |||
| BCS | 242 (85.8%) | 807 (53.4%) | 1049 (58.5%) | |
| Unilateral mastectomy | 27 (9.6%) | 479 (31.7%) | 506 (28.2%) | |
| Bilateral mastectomy | 13 (4.6%) | 226 (14.9%) | 239 (13.3%) | |
| Axillary surgery | <0.0013 | |||
| None | 282 (100.0%) | 0 (0.0%) | 282 (15.7%) | |
| SLN | 0 (0.0%) | 726 (48.0%) | 726 (40.5%) | |
| ALND | 0 (0.0%) | 786 (52.0%) | 786 (43.8%) | |
| Surgery combination | <0.0013 | |||
| BCS | 242 (85.8%) | 0 (0.0%) | 242 (13.5%) | |
| BCS with SLN | 0 (0.0%) | 450 (29.8%) | 450 (25.1%) | |
| BCS with ALND | 0 (0.0%) | 357 (23.6%) | 357 (19.9%) | |
| Mastectomy | 40 (14.2%) | 0 (0.0%) | 40 (2.2%) | |
| Mastectomy with SLN | 0 (0.0%) | 276 (18.3%) | 276 (15.4%) | |
| Mastectomy with ALND | 0 (0.0%) | 429 (28.4%) | 429 (23.9%) | |
| Neoadjuvant chemotherapy | <0.0013 | |||
| No | 282 (100.0%) | 1454 (96.2%) | 1736 (96.8%) | |
| Yes | 0 (0.0%) | 58 (3.8%) | 58 (3.2%) | |
| Chemotherapy type | <0.0013 | |||
| None | 277 (98.2%) | 998 (66.0%) | 1275 (71.1%) | |
| AC+Taxane | 2 (0.7%) | 250 (16.5%) | 252 (14.0%) | |
| AC without Taxane | 2 (0.7%) | 195 (12.9%) | 197 (11.0%) | |
| Taxane without AC | 0 (0.0%) | 24 (1.6%) | 24 (1.3%) | |
| Other agents | 1 (0.4%) | 45 (3.0%) | 46 (2.6%) | |
| Radiation therapy | <0.0013 | |||
| None | 155 (55.0%) | 621 (41.1%) | 776 (43.3%) | |
| Breast or chest wall only | 124 (44.0%) | 652 (43.1%) | 776 (43.3%) | |
| Nodal (± breast or chest wall) | 3 (1.1%) | 239 (15.8%) | 242 (13.5%) |
Wilcoxon rank-sum
Cochran Armitage Trend Test
Chi-Square
Incidence of BCRL
A total of 209 BCRL events were observed during follow-up; the majority (78%, 162/209) occurred within five years of breast cancer surgery. The cumulative incidence of BCRL diagnosis was 6.9% (95% CI: 5.8–8.2%) at 2 years, 9.1% (95% CI: 7.8–10.5%) at 5 years, and 11.4% (95% 10.0–13.0%) at 10 years. No BCRL events were observed among the 282 patients who did not undergo axillary surgery, who had a median of 10.6 years of follow-up. Restricted to the subset with axillary surgery (n=1512), the 2-, 5-, and 10-year rates of BCRL were 8.2% (95% CI: 6.9–9.7%), 10.8% (95% CI: 9.3–12.5%), and 13.5% (95% CI: 11.8–15.3%), respectively. Subsequent analyses of characteristics associated with BCRL were restricted to the axillary surgery subset.
Impact of Patient Factors
Rates of BCRL were higher in patients with BMI ≥25 vs <25 (14.3% vs 8.0% at 5 years, p=0.002). Those overweight (BMI 25–29.99) and class I obesity (BMI 30–34.99) had similar rates (14.4% and 13.0% at 5 years), while those morbidly obese (BMI≥35) had a slightly higher rate at 17.1% (Table 2). Pathologic stage was also significantly associated with BCRL in univariate analysis (p<0.001), with similar 5-year cumulative incidence in stage 0 (4.2%) and stage I (5.4%) but more than double that in stage II (14.1%) and the highest rate in stage III (37.8%).
Table 2.
Cumulative incidence of BCRL diagnosis by patient and clinical factors among breast cancer patients undergoing axillary staging surgery (n = 1512)
| N | 2-year Cumulative Incidence | Lower 95% CI |
Upper 95% CI |
5-year Cumulative Incidence | Lower 95% CI |
Upper 95% CI |
|
|---|---|---|---|---|---|---|---|
| BMI category | |||||||
| Underweight or Normal weight (BMI<25) | 845 | 5.7% | 4.3% | 7.4% | 8.0% | 6.3% | 10.0% |
| Overweight (BMI 25–29.99) | 399 | 11.1% | 8.2% | 14.4% | 14.4% | 11.2% | 18.1% |
| Obese (BMI 30–34.99) | 187 | 10.8% | 6.8% | 15.7% | 13.0% | 8.6% | 18.3% |
| Morbidly obese (BMI≥35) | 76 | 15.8% | 8.6% | 24.9% | 17.1% | 9.6% | 26.5% |
| Pathologic stage | |||||||
| 0 | 121 | 3.3% | 1.1% | 7.7% | 4.2% | 1.6% | 8.9% |
| I | 769 | 2.9% | 1.9% | 4.2% | 5.4% | 3.9% | 7.1% |
| II | 496 | 11.8% | 9.1% | 14.8% | 14.1% | 11.1% | 17.3% |
| III | 126 | 32.0% | 24.0% | 40.3% | 37.8% | 29.3% | 46.3% |
| Surgery combination | |||||||
| BCS with SLN | 450 | 5.4% | 3.5% | 7.7% | 6.0% | 4.1% | 8.5% |
| Mastectomy with SLN | 276 | 2.9% | 1.4% | 5.4% | 4.0% | 2.1% | 6.8% |
| BCS with ALND | 357 | 9.6% | 6.8% | 12.9% | 14.7% | 11.2% | 18.6% |
| Mastectomy with ALND | 429 | 13.6% | 10.5% | 17.0% | 17.0% | 13.6% | 20.7% |
| Radiation therapy | |||||||
| None | 621 | 4.2% | 2.8% | 6.0% | 5.9% | 4.2% | 7.9% |
| Breast or chest wall only | 652 | 5.4% | 3.8% | 7.3% | 8.0% | 6.1% | 10.3% |
| Nodal (± breast or chest wall) | 239 | 26.6% | 21.1% | 32.3% | 31.3% | 25.5% | 37.3% |
| Chemotherapy group | |||||||
| AC+Taxane | 250 | 23.4% | 18.3% | 28.8% | 27.2% | 21.8% | 32.9% |
| AC without Taxane | 195 | 9.8% | 6.1% | 14.5% | 13.5% | 9.1% | 18.7% |
| Taxane without AC | 24 | 20.8% | 7.4% | 39.0% | 29.7% | 12.8% | 48.8% |
| Other agents | 45 | 4.6% | 0.8% | 13.8% | 6.9% | 1.7% | 17.0% |
| No chemotherapy | 998 | 4.0% | 2.9% | 5.4% | 6.0% | 4.6% | 7.6% |
| Treatment combination* | |||||||
| SLN only | 220 | 2.7% | 1.1% | 5.6% | 4.1% | 2.0% | 7.4% |
| SLN & breast or chest wall RT only | 312 | 4.5% | 2.6% | 7.2% | 4.8% | 2.8% | 7.6% |
| ALND only | 233 | 1.7% | 0.6% | 4.1% | 3.5% | 1.6% | 6.4% |
| ALND & breast or chest wall RT only | 191 | 4.2% | 2.0% | 7.7% | 9.5% | 5.8% | 14.2% |
| ALND & nodal (± breast or chest wall) RT | 26 | 23.1% | 9.1% | 40.7% | 26.9% | 11.6% | 44.9% |
| ALND & AC+T chemotherapy | 36 | 30.6% | 16.4% | 46.0% | 33.6% | 18.7% | 49.3% |
| ALND & AC w/o Taxane chemotherapy | 49 | 4.2% | 0.7% | 12.7% | 8.4% | 2.6% | 18.5% |
| ALND & breast or chest wall only RT & AC+T chemotherapy | 24 | 25.0% | 9.9% | 43.6% | 33.9% | 15.7% | 53.2% |
| ALND & breast or chest wall only RT & AC w/o Taxane chemotherapy | 32 | 3.1% | 0.2% | 14.0% | 6.4% | 1.1% | 18.7% |
| ALND & nodal (± breast or chest wall) RT & AC+T chemotherapy | 110 | 35.0% | 26.1% | 44.0% | 39.7% | 30.4% | 48.8% |
| ALND & nodal (± breast or chest wall) RT & AC w/o Taxane chemotherapy | 41 | 22.0% | 10.7% | 35.7% | 29.3% | 16.2% | 43.7% |
Only common treatment combinations with sufficient numbers of patients for estimation were included in this table.
Impact of Type of Surgery
Overall, mastectomy versus lumpectomy was not associated with higher rates of BCRL (p=0.42). The 5-year incidence of BCRL was 5.3% (95% CI: 3.8–7.1%) in SLN surgery and 15.9% (95% CI: 13.5–18.6%) in ALND patients (p<0.001). Figure 1 shows cumulative incidence curves for combinations of breast and axillary surgery. In patients treated with surgery only (no radiation and no chemotherapy), BCRL rates were not different between ALND versus SLN surgery (3.5% and 4.1% at 5 years, p=0.36).
Figure 1.
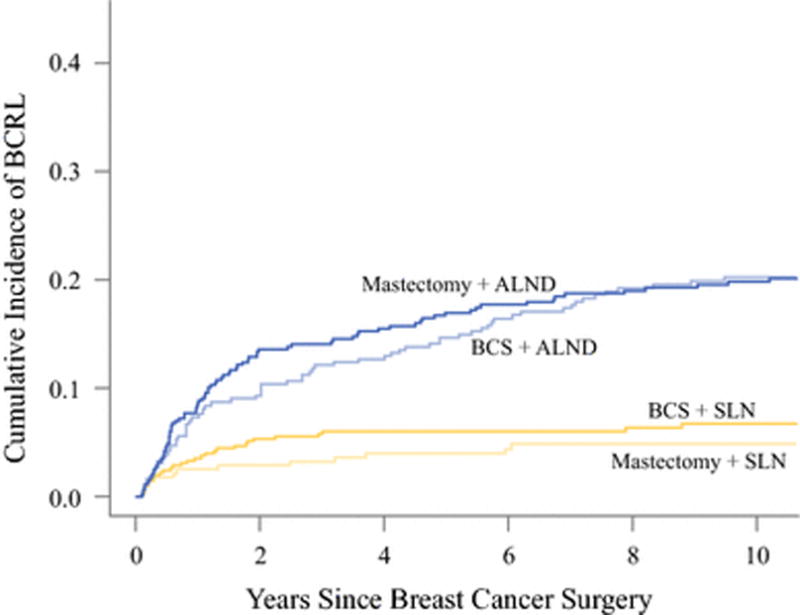
Cumulative incidence curves by surgery type combinations.
Impact of Radiation Therapy and Chemotherapy
In patients without chemotherapy, addition of breast or chest wall radiation to SLN surgery did not increase BCRL substantially (4.8% vs 4.1% at 5 years, p=0.79) but did more than double the rate in ALND patients (9.5% vs 3.5% at 5 years, p=0.01). Nodal radiation following ALND resulted in a marked increase in BCRL incidence to 26.9% at 5 years (Table 2, Figure 2). As only a small number of SLN surgery patients received nodal radiation, we were unable to estimate the effect of nodal radiation in this subset. Chemotherapy including taxane and to a lesser degree anthracycline also increased the incidence of BCRL in ALND patients. Addition of anthracycline chemotherapy (without RT) increased the 5-year incidence of BCRL from 3.5% (ALND surgery only) to 8.4% (ALND & AC without taxane chemotherapy), p=0.10, while ALND & AC+T chemotherapy had a much higher 5-year incidence of 33.6% (p<0.001). The highest 5-year rates of BCRL were seen in patients treated with ALND plus nodal RT (26.9%), ALND plus nodal RT and AC chemotherapy (29.3%), ALND and AC+T chemotherapy (33.6%), ALND plus breast or chest wall only RT & AC+T chemotherapy (33.9%), and ALND with nodal RT and AC+T chemotherapy (39.7%). (Table 2, Figure 2).
Figure 2.
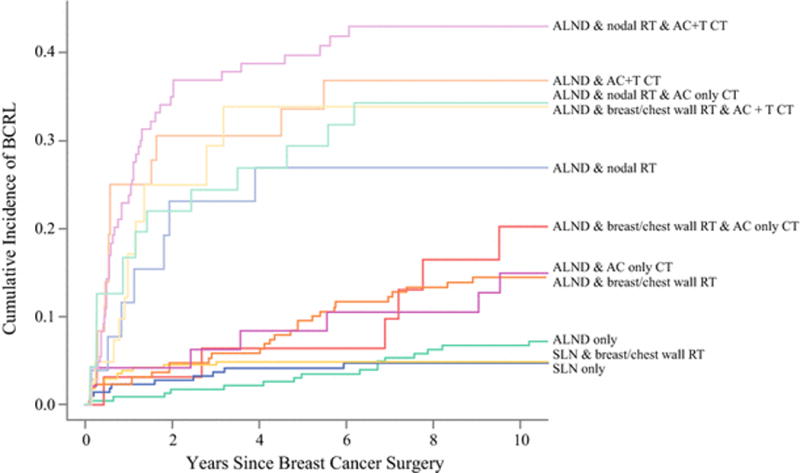
Cumulative incidence curves by major multimodality treatment combinations.
Multivariable Analysis
In multivariable analysis among patients with axillary surgery (Table 3), adjuvant nodal radiation and ALND remained independently associated with BCRL with adjusted HRs of 1.91 (p=0.008) for adjuvant radiation and 2.69 (p<0.001) for ALND. Chemotherapy was also associated with BCRL in multivariate analyses. Evaluating the different chemotherapy agents showed that taxane chemotherapy conferred the highest increase in BCRL risk: anthracycline + taxane chemotherapy (HR 2.25, p=0.001) and taxane without anthracycline (HR 2.65, p=0.02), while anthracycline without taxane showed a smaller but still significant increase in risk (HR 1.68, p=0.04) compared to no chemotherapy. Pathologic stage was not significant in multivariable analysis after adjusting for treatment variables. Patients with BMI≥35 (HR 1.9, p=0.03) or BMI 25–34.99 (HR 1.5, p=0.006) had higher rates of BCRL than those with BMI<25.
Table 3.
Multivariable analysis of factors associated with BCRL among patients undergoing any axillary surgery (n = 1512).
| Hazard Ratio | Lower 95% CI |
Upper 95% CI |
p-value | |
|---|---|---|---|---|
| Age, per 1 year | 1.00 | 0.992 | 1.017 | 0.51 |
| BMI | ||||
| < 25 | 1.0 (reference) | |||
| 25–34.99 | 1.49 | 1.12 | 1.98 | 0.006 |
| ≥35 | 1.92 | 1.03 | 3.31 | 0.03 |
| Bilateral breast cancer, yes vs no | 1.34 | 0.50 | 2.88 | 0.51 |
| Pathologic stage | ||||
| 0 | 1.0 (reference) | |||
| I | 1.18 | 0.53 | 3.24 | 0.72 |
| II | 1.37 | 0.58 | 3.89 | 0.52 |
| III | 2.08 | 0.79 | 6.37 | 0.17 |
| Breast surgery, mastectomy vs BCS | 1.04 | 0.69 | 1.58 | 0.86 |
| Axillary surgery, ALND vs SLN surgery | 2.69 | 1.88 | 3.92 | <0.001 |
| Radiation therapy | ||||
| None | 1.0 (reference) | |||
| Breast or chest wall only | 1.55 | 0.94 | 2.59 | 0.09 |
| Nodal (± breast or chest wall) | 1.91 | 1.19 | 3.08 | 0.008 |
| Chemotherapy | ||||
| None | 1.0 (reference) | |||
| AC+Taxane | 2.25 | 1.38 | 3.68 | 0.001 |
| AC without Taxane | 1.68 | 1.03 | 2.74 | 0.04 |
| Taxane without AC | 2.65 | 1.07 | 5.76 | 0.02 |
| Other agents | 0.70 | 0.19 | 1.83 | 0.52 |
Discussion
Our study showed that the risk for developing BCRL is multifactorial and not solely related to the extent of axillary surgery. Factors influencing BCRL rates include the delivery of chemotherapy, use of radiation, ALND, more advanced stage of disease, and higher BMI.
More extensive axillary surgery was associated with higher rates of BCRL, as patients who had ALND had a 15.9% rate of incidence of BCRL over 5 years compared to 5.3% with SLN surgery. However, further analysis and examining all treatment modalities, the rate of BCRL was not different comparing patients who had SLN surgery or ALND only without chemotherapy or radiation, indicating that surgery alone is not the key driver for BCRL. Adjuvant radiation carried a higher risk of lymphedema in patients undergoing ALND but not SLN surgery. Axillary nodal radiation was associated with an increased incidence of BCRL compared to breast or chest wall radiation alone. Patients with higher BMI also had a higher incidence of BCRL. Perhaps most interestingly, our findings showed that chemotherapy, specifically the use of taxanes, contributed to the highest rates of BCRL. Correspondingly, patients with more advanced stage disease had higher rates of lymphedema in univariate analysis; however, after adjusting for treatment variables in multivariable analysis, stage was not an independent predictor of BCRL, suggesting that higher rates in more advanced stages are likely a result of more aggressive therapies.
The true incidence of BCRL is difficult to measure because the symptoms can have a delayed and variable onset and the criteria used to diagnose lymphedema are not standardized, as both subjective and objective measures are utilized.25 Reported rates of lymphedema are disproportionate, ranging from less than 5% with lumpectomy alone to more than 60% when mastectomy, ALND, and axillary radiation are combined.1–15 Historically, ALND was the primary operation to stage the axilla in all patients presenting with breast cancer. During the last two decades, SLN surgery has replaced ALND as the staging procedure in clinically node-negative patients and has led to a decrease in lymphedema. The landmark National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial randomized 5611 clinically node-negative patients to SLN surgery followed by mandatory ALND versus SLN surgery proceeding to ALND only if any of the SLNs were positive for metastatic disease.26 Morbidity results from this study showed that BCRL rates were 14% in the ALND group versus 8% in the SLN group at 36 months of follow-up.2 Another study with 10 years of follow-up of 265 patients reported lymphedema rate of 34.8% in ALND patients and 4.6% in SLN surgery patients.3 Omission of ALND in these studies is associated with much lower rates of lymphedema. In our cohort, patients who underwent ALND had increased BCRL compared to SLN surgery (HR 2.69, p<0.001). However, in the surgery-only patients (without radiation or chemotherapy), rates of BRCL were similar.
The association between axillary nodal irradiation and lymphedema is well documented in the literature. In a review by Erickson et al, lymphedema was reported in 41% of patients who underwent axillary irradiation therapy in addition to surgery as opposed to 17% of patients treated with surgery only.7 A retrospective study of 727 patients showed that the only significant risk factor for lymphedema was the addition of regional nodal irradiation (RNI).27 With a median follow-up of 72 months, the 10-year risk of developing lymphedema was 1.8% for breast radiation alone vs. 8.9% for RNI, and the extent of axillary dissection was not predictive for lymphedema. Additionally, the recently published MA.20 trial of 1832 women randomized to whole breast radiation with RNI versus whole breast radiation alone found that patients who had RNI had a significantly higher incidence of lymphedema (8.4% vs. 4.5%, p=0.001).28
Previous studies have shown that chemotherapy contributes to BCRL29–32 but other studies have not shown this correlation.33 In a recent retrospective analysis of 273 patients who all underwent ALND, 74 (27.1%) developed BCRL over a mean follow-up period of 2.67 years.34 They found that patients who had taxane-based chemotherapy were nearly three times more likely to have BCRL compared to patients who did not receive chemotherapy at all. Previous studies have suggested potential mechanisms of action whereby repeated taxane exposure induced endothelial inflammation leading to abnormal capillary permeability.35–36 These studies showed development of edema in patients receiving taxanes using capillaroscopy and capillary filtration tests involving 99mTc-labelled albumin. They concluded taxanes caused an abnormality in capillary permeability and also progressive accumulation of proteins in the interstitial space. Future studies are needed to confirm that taxanes lead to higher risks of lymphedema compared to other chemotherapeutic agents.
Our study showed that patients who received taxane-based chemotherapy had the highest rates of BCRL, and significantly higher than non-taxane based regimens. Chemotherapy has been underappreciated as a risk factor for lymphedema. Recognition that this can be a significant and independent contributor of BCRL can lead to greater informed risk-benefit discussions with patients when consideration of chemotherapy is needed.
Patients with more advanced disease typically receive more aggressive locoregional and systemic therapies. In our study, advanced stage was associated with higher rates of BCRL; however this was not significant on multivariable analysis, indicating that the higher risk with advanced stage is linked to the greater extent of axillary surgery and use of radiation and systemic treatments. These findings are in keeping with a previous report of 455 patients with median follow-up of 53 months, which showed that the incidence of lymphedema in patients with stage I–II breast cancer was lower than in patients with stage III (24% and 35.3%, respectively, p=0.018).37
Higher body mass index (BMI) is known to be associated with higher BCRL risk and our study findings did show obesity was associated with higher rates of BCRL. NSABP B-04, which randomized 1665 patients to radical mastectomy, total mastectomy with radiation, and total mastectomy alone, showed that increasing BMI had a significant correlation with increasing incidence of lymphedema.4 Multiple studies have correlated obesity to be independent risk factor for development of BCRL.37–39
ALND, nodal radiation, and chemotherapy are treatment-related contributors to lymphedema, and obesity is a patient-related factor. Recognition that certain patients are more prone to develop lymphedema can allow individualized survivorship plans and lead to earlier detection and treatment, which leads to improved outcomes and better overall quality of life.23,40–43
Limitations of the current study include its retrospective nature and method of determination of lymphedema. Diagnosis of BCRL in our cohort was made by chart review, which is less reliable compared to a uniform objective measurement in a prospective cohort of patients. To ensure the most comprehensive assessment for lymphedema, trained nurse abstractors were used for review of clinical notes across all specialities to assess for the development of lymphedema, however, we acknowledge the lack of arm measurements and prospective study is a significant limitation. In addition, breast cancer treatment is multimodal in nature and many patients with BCRL undergo a combination of therapies. Determining which treatment has the greatest impact to the development of BCRL can be challenging.
BCRL is a significant morbidity affecting many breast cancer survivors, resulting in a reduced quality of life. Extent of axillary surgery has long been implicated as the dominant factor causing lymphedema, and locoregional therapy as the sole etiology. However, BCRL risk is a consequence of multimodal breast cancer treatment and is associated with axillary surgery, axillary radiation and chemotherapy with the highest risk occurring in patients with advanced disease requiring all treatment modalities. Higher BMI is an independent patient-related factor associated with increased risk for developing BCRL.
Synopsis.
BCRL is a sequelae of multimodal breast cancer treatment and risk is multifactorial. BCRL rates are higher in patients receiving chemotherapy, radiation, ALND, more advanced disease stage, and higher BMI.
Footnotes
Presented as an oral presentation at the American Society of Breast Surgeons annual meeting in April 2017
Disclosure: none
References
- 1.Gartner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported Arm-Lymphedema and Functional Impairment After Breast Cancer Treatment—A Nationwide Study of Prevalence and Associated Factors. Breast. 2010 Dec;19(6):506–15. doi: 10.1016/j.breast.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Ashikaga T, Krag DN, Land SR, et al. Morbidity Results from the NSABP-B-32 Trial Comparing Sentinel Lymph Node Dissection Versus Axillary Dissection. J Surg Oncol. 2010 Aug 1;102(2):111–8. doi: 10.1002/jso.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wernicke AG, Goodman RL, Turner BC, et al. A 10-year Follow-up of Treatment Outcomes in Patients With Early Stage Breast Cancer and Clinically Negative Axillary Nodes Treated With Tangential Breast Irradiation Following Sentinel Lymph Node Dissection or Axillary Clearance. Breast Cancer Res Treat. 2011 Feb;125(3):893–902. doi: 10.1007/s10549-010-1167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deutsch M, Land S, Begovic M, Sharif S. The Incidence of Arm Edema in Women With Breast Cancer Randomized on the National Surgical Adjuvant Breast and Bowel Project study B-04 to Radical Mastectomy Versus Total Mastectomy and Radiotherapy Versus Total Mastectomy Alone. Int J Radiat Oncol Biol Phys. 2008 Mar 15;70(4):1020–4. doi: 10.1016/j.ijrobp.2007.07.2376. [DOI] [PubMed] [Google Scholar]
- 5.Meric F, Buchholz TA, Mirza NQ, et al. Long-term Complications Associated With Breast-conservation Surgery and Radiotherapy. Ann Surg Oncol. 2002 Jul;9(6):543–9. doi: 10.1007/BF02573889. [DOI] [PubMed] [Google Scholar]
- 6.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a Cohort of Breast Carcinoma Survivors 20 years After Diagnosis. Cancer. 2001 Sep 15;92(6):1368–77. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm Edema in Breast Cancer Patients. J Natl Cancer Inst. 2001 Jan 17;93(2):96–111. doi: 10.1093/jnci/93.2.96. [DOI] [PubMed] [Google Scholar]
- 8.Clark B, Sitzia J, Harlow W. Incidence and Risk of Arm Oedema Following Treatment for Breast Cancer: A Three-year Follow-up Study. QJM. 2005 May;98(5):343–8. doi: 10.1093/qjmed/hci053. [DOI] [PubMed] [Google Scholar]
- 9.Coen JJ, Taghian AG, Kachnic LA, Assaad SI, Powell SN. Risk of Lymphedema After Regional Nodal Irradiation With Breast Conservation Therapy. Int J Radiat Oncol Biol Phys. 2003 Apr 1;55(5):1209–15. doi: 10.1016/s0360-3016(02)04273-6. [DOI] [PubMed] [Google Scholar]
- 10.Golshan M, Martin WJ, Dowlatshahi K. Sentinel Lymph Node Biopsy Lowers the Rate of Lymphedema When Compared With Standard Axillary Lymph Node Dissection. Am Surg. 2003 Mar;69(3):209–11. discussion 212. [PubMed] [Google Scholar]
- 11.Kosir MA, Rymal C, Koppolu P, et al. Surgical Outcomes After Breast Cancer Surgery: Measuring Acute Lymphedema. J Surg Res. 2001 Feb;95(2):147–51. doi: 10.1006/jsre.2000.6021. [DOI] [PubMed] [Google Scholar]
- 12.Paskett ED, Naughton MJ, McCoy TP, Case LD, Abbott JM. The Epidemiology of Arm and Hand Swelling in Premenopausal Breast Cancer Survivors. Cancer Epidemiol Biomarkers Prev. 2007 Apr;16(4):775–82. doi: 10.1158/1055-9965.EPI-06-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucci A, McCall LM, Beitsch PD, et al. Surgical Complications Associated With Sentinel Lymph Node Dissection (SLND) Plus Axillary Lymph Node Dissection Compared With SLND Alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007 Aug 20;25(24):3657–63. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 14.Langer I, Guller U, Berclaz G, et al. Morbidity of Sentinel Lymph Node Biopsy (SLN) Alone Versus SLN and Completion Axillary Lymph Node Dissection After Breast Cancer Surgery: A Prospective Swiss Multicenter Study on 659 Patients. Ann Surg. 2007 Mar;245(3):452–61. doi: 10.1097/01.sla.0000245472.47748.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deo SV, Ray S, Rath GK, Shukla NK, Kar M, Asthana S, Raina V. Prevalence and Risk Factors for Development of Lymphedema Following Breast Cancer Treatment. Indian J Cancer. 2004 Jan-Mar;41(1):8–12. [PubMed] [Google Scholar]
- 16.Tobin MB, Lacey HJ, Meyer L, Mortimer PS. The Psychological Morbidity of Breast Cancer-Related Arm Swelling. Psychological Morbidity of Lymphoedema. Cancer. 1993 Dec 1;72(11):3248–52. doi: 10.1002/1097-0142(19931201)72:11<3248::aid-cncr2820721119>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Passik S, Newman M, Brennan M, Holland J. Psychiatric Consultation for Women Undergoing Rehabilitation for Upper-Extremity Lymphedema Following Breast Cancer Treatment. J Pain Symptom Manage. 1993 May;8(4):226–33. doi: 10.1016/0885-3924(93)90132-f. [DOI] [PubMed] [Google Scholar]
- 18.Velanovich V, Szymanski W. Quality of Life of Breast Cancer Patients With Lymphedema. Am J Surg. 1999 Mar;177(3):184–7. doi: 10.1016/s0002-9610(99)00008-2. discussion 188. [DOI] [PubMed] [Google Scholar]
- 19.Kwan W, Jackson J, Weir LM, Dingee C, McGregor G, Olivotto IA. Chronic Arm Morbidity After Curative Breast Cancer Treatment: Prevalence and Impact on Quality of Life. J Clin Oncol. 2002 Oct 15;20(20):4242–48. doi: 10.1200/JCO.2002.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Armer JM, Stewart BR. Post-Breast Cancer Lymphedema: Incidence Increases From 12 to 30 to 60 Months. Lymphology. 2010 Sep;43(3):118–27. [PMC free article] [PubMed] [Google Scholar]
- 21.Rockson SG. Precipitating Factors in Lymphedema: Myths and Realities. Cancer. 1998 Dec 15;83(12 Suppl):2814–16. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2814::aid-cncr31>3.3.co;2-5. American. [DOI] [PubMed] [Google Scholar]
- 22.DiSipio T, Rye S, Newman B, Hayes S. Incidence of Unilateral Arm Lymphoedema After Breast Cancer: A Systematic Review and Meta-analysis. Lancet Oncol. 2013 May;14(6):500–15. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 23.Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative Assessment Enables the Early Diagnosis and Successful Treatment of Lymphedema. Cancer. 2008 Jun 15;112(12):2809–19. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 24.Bob Gray. cmprsk: Subdistribution Analysis of Competing Risks. 2013 R package version 2.2-6 http://CRAN.R-project.org/package=cmprsk.
- 25.Shah C, Vicini FA. Breast Cancer-Related Arm Lymphedema: Incidence Rates, Diagnostic Techniques, Optimal Management and Risk Reduction Strategies. J Radiat Oncol Biol Phys. 2011 Nov;81(4):15. 907–14. doi: 10.1016/j.ijrobp.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 26.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-Lymph-Node Resection Compared With Conventional Axillary-Lymph-Node Dissection in Clinically Node-Negative Patients With Breast Cancer: Overall Survival Findings From the NSABP B-32 Randomised Phase 3 Trial. Lancet Oncol. 2010 Oct;11(10):927–33. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell SN, Taghian AG, Kachnic LA, Assaad SI, Powell SN. Risk of Lymphedema After Regional Nodal Irradiation With Breast Conservation Therapy. Int J Radiat Oncol Biol Phys. 2003 Apr 1;55(5):1209–15. doi: 10.1016/s0360-3016(02)04273-6. [DOI] [PubMed] [Google Scholar]
- 28.Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med. 2015 Jul 23;373(4):307–16. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanton AW, Badger C, Sitzia J. Non-invasive Assessment of the Lymphedematous Limb. Lymphology. 2000 Sep;33(3):122–35. [PubMed] [Google Scholar]
- 30.Stanton AW, Northfield JW, Holroyd B, Mortimer PS, Levick JR. Validation of an Optoelectronic Limb Volumeter (Perometer) Lymphology. 1997 Jun;30(2):77–97. [PubMed] [Google Scholar]
- 31.Sackey H, Johansson H, Sandelin K, Lillegren G, MacLean G, Frisell J, Brandberg Y. Self-Perceived, But Not Objective Lymphoedema is Associated With Decreased Long-Term Health-Related Quality of Life After Breast Cancer Surgery. Eur J Surg Oncol. 2015 Apr;41(4):577–84. doi: 10.1016/j.ejso.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Pain SJ, Purushotham AD. Lymphoedema Following Surgery for Breast Cancer. Br J Surg. 2000 Sep;87(9):1128–41. doi: 10.1046/j.1365-2168.2000.01569.x. [DOI] [PubMed] [Google Scholar]
- 33.Ozcinar B, Guler SA, Kocaman N, Ozkan M, Gulluoglu BM, Ozmen V. Breast Cancer Related Lymphedema in Patients With Different Loco-regional Treatments. Breast. 2012 Jun;21(3):361–5. doi: 10.1016/j.breast.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Cariati M, Bains SK, Grootendorst MR, et al. Adjuvant Taxanes and the Development of Breast Cancer-Related Arm Lymphedema. Br J Surg. 2015 Aug;102(9):1071–8. doi: 10.1002/bjs.9846. [DOI] [PubMed] [Google Scholar]
- 35.Semb KA, Aamdal S, Oian P. Capillary protein leak syndrome appears to explain fluid retention in cancer patients who receive docetaxel treatment. J Clin Oncol. 1998;16:3426–3432. doi: 10.1200/JCO.1998.16.10.3426. [DOI] [PubMed] [Google Scholar]
- 36.Béhar A, Pujade-Lauraine E, Maurel A, et al. The pathophysiological mechanism of fluid retention in advanced cancer patients treated with docetaxel, but not receiving corticosteroid comedication. Br J Clin Pharmacol. 1997;43:653–658. doi: 10.1046/j.1365-2125.1997.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ugur S, Arıcı C, Yaprak M, et al. Risk Factors of Breast Cancer-Related Lymphedema. Lymphat Res Biol. 2013 Jun;11(2):72–5. doi: 10.1089/lrb.2013.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erdogan Iyigun Z, Duymaz T, Ilgun AS, et al. Preoperative Lymphedema-Related Risk Factors in Early-Stage Breast Cancer. Lymphat Res Biol. 2017 Mar;:27. doi: 10.1089/lrb.2016.0045. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Helyer LK, Varnic M, Le LW, et al. Obesity is a Risk Factor for Developing Postoperative Lymphedema in Breast Cancer Patients. Breast J. 2010 Jan-Feb;16(1):48–54. doi: 10.1111/j.1524-4741.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- 40.Torres Lacomba M, Yuste Sánchez MJ, Zapico Goñi A, Prieto Merino D, Mayoral de Moral O, Cerezo Téllez E, Minayo Mogollón E. Effectiveness of Early Physiotherapy to Prevent Lymphoedema After Surgery for Breast Cancer: Randomised, Single Blinded, Clinical Trial. BMJ. 2010 Jan 12;340:b5396. doi: 10.1136/bmj.b5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Box RC, Reul-Hirche HM, Bullock-Saxton JE, Furnival CM. Physiotherapy After Breast Cancer Surgery: Results of a Randomized Controlled Study to Minimise Lymphedema. Breast Cancer Res Treat. 2002 Sep;75(1):51–64. doi: 10.1023/a:1016591121762. [DOI] [PubMed] [Google Scholar]
- 42.Stout NL, Pfalzer L, Levy E, et al. Five Year Preliminary Outcomes of a Prospective Surveillance Model to Reduce Upper Extremity Morbidity Related to Breast Cancer Treatment. Cancer Res. 2011;71(24 Suppl) Abstract nr P4-12-08. [Google Scholar]
- 43.Shah C, Arthur DW, Wazer D, Khan A, Ridner S, Vicini F. The Impact of Early Detection and Intervention of Breast Cancer-Related Lymphedema: A Systematic Review. Cancer Med. 2016 Jun;5(6):1154–62. doi: 10.1002/cam4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]


