Abstract
Schizophrenia is a chronic disabling mental disorder that affects about 1% population world-wide, for which there is a desperate need to develop more effective treatments. In this minireview, we summarize the findings from recent studies using induced pluripotent stem cells to model the developmental pathogenesis of schizophrenia and discuss what we have learned from these studies. We also discuss what are the important next steps and key issues to be addressed to move the field forward.
Keywords: Schizophrenia, iPSC, neural progenitor cell, differentiation
1. Introduction
Schizophrenia (SCZ) is a highly heritable [1] neurodevelopmental disorder [2] that is characterized by positive symptoms (e.g., hallucinations and delusions), negative symptoms (e.g., apathy, anhedonia) and cognitive symptoms (e.g., impairments of memory, executive functions and attention) [3]. Worldwide, 1% of the adult population suffer from this disorder, and individuals with SCZ are at high risk for substance abuse [4], suicide [5] and lifelong disability [6]. Even with antipsychotic treatment, cognitive and negative symptoms persist throughout life [7], preventing normal functioning. Furthermore, many patients discontinue antipsychotic medications due to their adverse sideeffects [8]. Given the fact that SCZ remains the seventh most costly medical disorder to our Society, it is imperative to develop novel efficacious treatments and even preventive interventions for SCZ. As SCZ is a uniquely human disorder characterized by hallucinations and thought disorder, animal models cannot recapitulate all SCZ pathophysiology, though certain aspects of SCZ pathophysiology can be replicated [9,10]. Furthermore, many therapeutics developed in animal models have failed in human clinical trials [11,12], thus it is critical to develop novel, human-specific, disease-relevant models of SCZ to develop and test novel therapeutics.
2. Developmental model for Schizophrenia
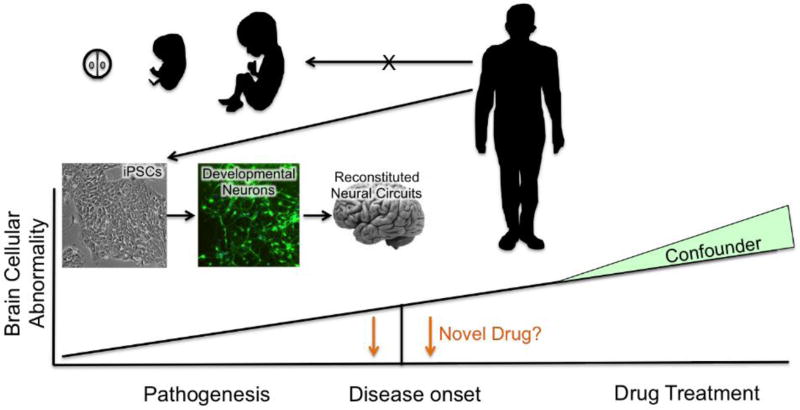
In vivo imaging and postmortem studies in SCZ have shown consistent brain abnormalities, such as reduced cortical volume, atrophic dendrites and altered gene expression, especially in cortical pyramidal neurons [13–15] and cortical interneurons [16–21], as well as abnormal structure of dopaminergic (DA) neurons [22,23] and dis-regulation in the ventral tegmental area (VTA) DA system [24]. Determining the cause of these abnormalities can be confounded by psychotropic drug treatment, malnutrition and substance abuse (Fig. 1). Since the disease process begins long before the onset of overt symptoms of psychosis [2,25], understanding disease progression during brain development could shed light on SCZ’s etiology and serve as the basis for preventive therapeutics. Many SCZ putative risk genes are expressed prenatally or early in pluripotent stem cell (PSC) differentiation [26–28], highlighting the importance of studying developmental process to clarify the etiology of SCZ. Many genetic as well as environmental models of SCZ show more pronounced SCZ-like behavioral and morphological changes with perturbations during early development, indicating the significance of developmental disturbance in generating SCZ-like abnormalities [29–32]. However, the ability to study these critical pathologic events in the developing human nervous system once seemed unimaginable since after symptom onset in young adulthood, it is not possible to go back in time to study what happened during fetal brain development (Fig. 1). Recent advances in research on iPSC technologies [33] offer the possibility of generating disease-relevant developing tissues (Fig. 1), making it possible to study developmental abnormalities in brain tissue with the exactly same genetic background as the patients diagnosed with SCZ. Thus, iPSC-derived human developmental brain cells will play a critical role to accurately recapitulate cellular pathology of SCZ and permit the screening of novel chemical agents to correct functional abnormalities or rectify the cellular pathology characteristic of SCZ.
Fig. 1. Modeling pathogenesis of SCZ using induced pluripotent stem cell technology.
SCZ pathogenesis can be studied by deriving patient-specific and disease-specific neural cell types using iPSC technology: this allows for the study of cell-innate abnormalities as a monoculture system (developmental neurons) and the study of circuit-based abnormalities with multi-cell type co-culture system (reconstituted neural circuit). Postmortem tissues from schizophrenia patients have been extensively used to gain understanding of schizophrenia-specific brain abnormalities. However, treatment history, substance abuse and poor nutrition can confound interpretation of postmortem findings. iPSC-derived disease-relevant tissues do not present such a problem. Observed cellular abnormalities in these model systems will provide an opportunity to develop novel therapeutics to reverse the observed phenotypes and possibly preventive interventions.
3. Strengths and limitations of iPSC modeling
Development and refinement of iPSC technology so far allows reliable and robust reprogramming into hES-like cells with most of the variance among iPSCs derived from individual differences, not clonal line differences [76,77]. Integration-mediated reprogramming methods could be a concern because of the possibility of generating copy number variants (CNVs) in iPSCs. The use of footprint-free reprogramming methods such as modified RNA methods [78] would be more suitable to study the effect of fine genetic variation of SCZ. Also, the same kind of donor cell sources should be used to minimize tissue-specific epigenetic memory effect.
One of the most critical steps in successfully utilizing iPSC-derived neural tissues with consistent and reproducible results is to overcome heterogeneity and stochasticity of differentiation, which can result in unreliable assay results. This will be overcome by the development of efficient differentiation protocols and/or isolation strategies for specific cell populations (e.g., GABAergic, dopaminergic, etc.). Single cell transcriptome analysis could help to reduce the problem of heterogeneity in differentiation, but it has a limitation in that relatively low sequencing depth could be prohibitive for novel gene discovery, especially for low abundance genes. In addition, rigorous quality control of both iPSC and derived cells will be required to generate reproducible results, avoiding any cell culture artifacts caused by abnormal karyotypes or contamination with pathogens.
While iPSCs can provide unlimited quantities of developing human neurons for disease modeling, the fact that they follow their in vivo developmental timeline during in vitro differentiation [34] could prevent the study of adult neurons (years in culture). In the case of SCZ, such a developing neuronal population could identify targets for preventive treatments in utero rather than disease-modulating targets of mature neurons after symptom onset. However, the discovery of protocols to generate mature neurons would allow us to overcome these limitations. For example, a Ngn2-based glutamatergic neuronal differentiation protocol developed by the Sudhof laboratory bypasses the embryonic precursor stage and directly generates mature neuron [35]. Thus, the field awaits protocols to efficiently generate each mature neuronal subtype to recreate the disease phenotype in mature neurons. Alternatively, directly converting from fibroblast to neurons has been shown to maintain age markers, unlike iPSCs that undergo resetting the clock back to embryonic stage [36], This could be alternative method to obtain mature neurons to recreate disease phenotype rather than disease predisposition.
While these limitations are being overcome, iPSC technology definitely provides an unprecedented opportunity to study disease cellular phenotypes, by making it possible to generate unlimited quantities of disease-relevant cells from patients for the development of novel therapeutics. For example, IPSC-based disease modeling has led to drug re-purposing in ALS [37], The investigators found hyperexcitibility of iPSC-derived ALS motor neurons that could be reversed by Retigabine, resulting in better survival of the ALS motor neurons. Retigabine was previously approved by the FDA for the treatment of epilepsy, and is now in clinical trial in ALS, encouraging the effort to use iPSC-derived progenies for development of novel therapeutics including drug screening and drug repurposing.
4. Recent iPSC-based cellular models of SCZ
4.1. Subject selection criteria and generation of tissue types for disease modeling
In recent years, an increasing number of studies have used iPSC-derived neural cells to model early developmental pathology of the SCZ brain. Subject selection criteria vary in these studies, spanning from specific genetic abnormalities like CNVs such as 22q11.2 deletion [38–40], 15q11.2 deletion [41] or CNTNAP2 deletion [42] and mutations such as DISC1 mutation [43,44] to SCZ patients with no identified genetic risk factor drawn from clinical populations [45–48]. Childhood onset SCZ (COS) [49], has also been studied since COS exhibits more severe psychopathology as compared to adult onset SCZ and thus would likely have a more robust cellular phenotype.
In light of the heterogeneity of SCZ etiology, cell lines derived from patients with highly penetrant CNVs will allow the identification of specific disease relevant abnormalities more clearly. However, knowledge gained from these subgroups of patients may not be broadly applicable to general patient population where no highly penetrant risk genes have been identified but rather where more than a hundred allelic risk variants have been identified, each of which confers a modest (<5%) risk for SCZ [50]. Stratification of subjects by sex, race, symptomatic features such as prominent negative symptoms or polygenic risk score [51] would help to minimize heterogeneity of subject populations.
In these studies, the neural populations generated, range from neural progenitor cells (NPC) [41–43,45,46,48,49,52] to mixed neural populations [38–40,47,53] and to more specific neural subtypes such as highly enriched glutamatergic neurons [44]. These iPSC-derived neural populations recapitulate the normal developmental time frame and more closely resemble fetal neural progenitors and neuronal populations, suitable for analysis of developmental abnormalities. As discussed above, in light of the heterogeneity and stochastic behavior of iPSC differentiation in general, generating well-defined and homogeneous differentiated progenies is critical for reliable and reproducible outcomes for cellular modeling of SCZ. Recent advances in iPSC differentiation protocols to generate highly homogeneous cell populations [54–56] will help achieve this goal.
4.2. Disease-relevant cellular abnormalities
Disease-relevant cellular phenotypes have been observed in these iPSC-derived neural cells. For NPCs derived from idiopathic SCZ cohort, increased expression of the Wnt signaling pathway [52], abnormal nuclear FGFR1 pathway [48], increased protein translation [45], neuronal migration deficits and oxidative stress [46] have been reported. Topol et al. described a molecular substrate of disrupted neuronal migration, decreased mir9 expression, and found that transfection of mir9 reversed the migration deficit [49]. It remains to be determined how the NPC migration deficit contributes to the pathogenesis of SCZ and whether correction of the migration deficit could be a novel preventive therapeutic target for SCZ. Disruption of gene pathways that are critical for neurodevelopment [48,52] comports well with the developmental hypothesis of SCZ pathogenesis.
In mixed neuron cultures from iPSCs derived from patients with 22q11.2 deletion syndrome, altered miRNA expression, consistent with postmortem findings, was observed [40]. In addition, genes involved in cell cycle, apoptosis and MAPK pathway were affected [38]. Consistent with this finding, another group also observed disruption of the MAPK pathway in 22q11.2 deletion syndrome in mixed neuronal populations [39], though there was differences in miRNA findings between these two studies [39,40]. This discrepancy may have resulted from small sample size, heterogeneous differentiation or the influence of gene variants not within the CNV. Controls that better match the genetic background (sibling or isogenic control) and more homogeneous differentiation could reduce such discrepancies. The disrupted neurogenesis presumably resulting from dysregulation of the MAPK pathway was partially reversed by treating with the p38 inhibitor, SB203580 [39].
Two studies with iPSCs harboring the DISC1 mutation utilized isogenic control lines with identical genetic background. Srikanth et al [32] reported that DISC1 NPCs show an abnormal forebrain specification and high levels of Wnt signaling accompanied by increased neural proliferation, which could be reversed by early inhibition of Wnt. Wen et al. [41] examined the mature neuronal phenotype and observed a synaptic release deficit using 90% homogeneous glutamatergic neuronal population, which was reversed by isogenic correction of DISC1 mutation. It will be important to compare the similarities and differences between this robust isogenic experimental system and the results from idiopathic SCZ patients to determine the degree to which the result from single gene mutation could be used as a platform to develop novel treatments for idiopathic SCZ.
Overall, it is encouraging that now we can make neurons (or other relevant tissues that are specifically affected by the disease) from patients with the exact same genetic makeup as their living brain using this technology. This is especially so, considering the fact that the complex genetics of SCZ, with over a hundred risk alleles, each having modest effect, are difficult to replicate in an experimental animal. Patient-derived neurons generated from iPSCs faithfully reproduce their complex genetics, thereby permitting the study of pharmacologic interventions that could affect disease progression as well as symptoms. Encouragingly, some of the disease relevant phenotypes in patient iPSC-derived neurons have been shown to be reversed by targeted pharmacologic treatments. However, the preliminary findings, albeit promising, are based on quite small numbers of patients (2 to 14). Given the genetic heterogeneity of SCZ, the observed phenotypes need to be validated in much larger samples of patients and controls to determine how extensively these final common pathways of neural pathology map onto the SCZ phenotype. Nevertheless, patient subgroup-specific phenotypes could be utilized to develop personalized treatments, once the underlying mechanism of the abnormal phenotype is elucidated and effective interventions could be identified.
5. Where do we go from here?
5.1. Disease-relevant tissue types to unravel SCZ pathogenesis
One of the most consistently affected neuronal types in SCZ is the medial ganglionic eminence (MGE)-derived parvalbumin (PV+) or somatostatin (SST+) expressing GABAergic interneurons, as shown in numerous post-mortem studies [57]. In accordance with these findings, experimental evidence suggests a role for altered GABA neurotransmission in SCZ disrupting cortical gamma oscillations and thus causing cognitive deficits in patients [58]. Consistent with these findings, GABAA agonists were shown to restore gamma band activity in SCZ patients, accompanied by improved cognitive functions [59], whereas blocking of GABAA in prefrontal cortex resulted in SCZ-like cognitive, behavioral, and dopaminergic abnormalities [60]. Furthermore, interneuron-specific developmental disturbances result in a SCZ-like phenotype, including deficits in dopaminergic systems in adult mice [29], suggesting a role for impaired cortical interneuron development in SCZ pathogenesis. By default, most of the mixed neuronal populations generated from iPSCs have excitatory glutamatergic neurons as a major component, and thus suitable for studying glutamatergic pathology but are not optimal for the study of other SCZ-relevant cell types. Recent advances in protocols for generating homogeneous population of interneurons from iPSCs [34,56] will help to study interneuron-specific pathogenic mechanisms. This is currently the focus of the research in the authors’ laboratory.
In addition to abnormalities in glutamatergic neurons and GABAergic interneurons, DA neurons have long been implicated in the pathophysiology of SCZ [61]. Neuronal populations enriched with DA neurons were used to model SCZ [53,62,63]. These studies employed different DA induction protocols that generated a maximum 30% enrichment of DA neurons (15–25% of total cells for [53], 10–30% of total neurons for [62] and undefined minor % in [63]), resulting in inconsistent result among studies. Thus, development of more robust phenotype specification protocol for midbrain DA neurons, especially VTA DA neurons over substantia nigra (SN) DA neurons (reviewed in [64]) or development of protocols to isolate desired cell populations after iPSC differentiation [65] will help to generate more robust data that can be better compared across different laboratories. In addition to the neuronal subtypes, glial cells are also implicated in SCZ pathology [66]. With the protocol available to generate astrocytes [67] or oligodendrocytes [68] from human iPSCs, it will be possible to study cell-intrinsic and autonomous schizophrenia pathogenic mechanism on these disease-relevant tissues.
Finally, generation of homogeneous, well-controlled specific neural subtypes, that are critical for robust analysis of cell intrinsic mechanism, can be also essential to reconstitute specific circuit-dependent pathology. Strategically co-culturing relevant neural components together in a 3D system, would circumvent the limitations of the organoid approach, which tends to be cellularly heterogeneous and stochastic [69]. The creation of well-defined neural circuits constructed from homogeneous components would ensure reproducibility of the modeling of circuit-based abnormalities.
5.2. Connection with genetic studies
SCZ is a disorder of complex genetics, where many genes with small effects interact together with environmental risk factors to bring about the pathogenesis. Thus, it would be essential to identify common gene networks underlying heterogeneous interactions of diverse risk factors to gain further insights into SCZ pathogenesis and identify novel and common therapeutic targets. A recent genome wide association study of the risk for SCZ identified 108 genomic loci with genome-wide significance (P< 5×10−8), opening up the potential to identify such SCZ-selective risk gene networks [50]. However, the functional effects of many of these loci have not yet been elucidated. More than 99% of genetic variants lie outside coding regions, presumably in gene-regulatory sites [70]. Thus, it is essential to identify their functional effects during SCZ pathogenesis in disease-relevant brain tissues, which is generally not feasible from postmortem tissues, but now is attainable through iPSC technology. One caveat for this strategy to find the genetic link in iPSC-derived neural population is that any given risk gene presumably has small effects and thus, it will require large number of subjects to achieve needed statistical power [71]. This is quite different from gene knock-out methods with which can readily yield significant differences with small number of samples.
Minimizing heterogeneity of the differentiated progeny will be important to reduce unnecessary variance. To further reduce variance, identification of groups of subjects with several shared or overlapping risk genes by genotyping would likely yield more robust results. In addition, taking advantage of publicly available large data sets such as commonmind portal (https://www.commonmind.org) or GTEx portal (http://www.gtexportal.org) for eQTL analysis can compensate for small sample size inherent for iPSCs studies. They aid in the identification of eQTLs of risk genes and the association of these eQTLs with schizophrenia risk in larger data sets. However, results from these postmortem adult brain studies may not always comport with the developmental tissues from iPSCs. Recently, with the aim of studying phenotype effect of genotypes with iPSC-derived specific cell types, the NextGen consortium was formed [72]. Their initial proof of principle studies showed that indeed iPSC-derived specific cell types can be used for this purpose, accurately recreating in vivo phenotype [73]. This type of collaborative large-scale study could help reach greater statistical power needed for eQTL studies.
5.3. Cell-environment interaction
Although SCZ is a highly heritable disease, environmental risk factors (e.g., maternal immune activation, folate deficiency and cannabis use) are also important for SCZ pathogenesis, as shown by both epidemiological and animal model studies [74,75]. Thus, more a complete understanding of SCZ pathogenesis will require further elucidation of the interaction of risk genes with environmental risk factors. However, testing the effect of each environmental factor on specific schizophrenia-relevant cell types cannot be performed in living human brains. iPSC technologies do provide such well-defined disease-relevant developmental cell populations to analyze the cell intrinsic effect of each environmental factor. By facilitating the molecular identification of disturbances in gene expression pathways by environmental factors in well-defined system, the PSCs provide excellent tools for biochemical and mechanistic studies. Such an environmental analysis could be further performed in healthy control as compared to schizophrenia neuronal subtypes, to study possible gene-environmental interactions.
5.4. Conclusion (Future Direction)
iPSC-derived neural tissues provide unprecedented opportunity to study SCZ pathogenesis using neural cultures comprised of cells with the same genetic background as the human patients from whom they have been derived. An immediate next step would be to develop highly enriched, well-defined neuronal cell lines to characterize the cellular pathology of SCZ. This will require either using more robust differentiation protocols and/or isolation of specific progenies by using cell surface markers or reporter expression. Obvious candidates include excitatory neurons as well as other SCZ-relevant cell types such as interneurons, DA neurons and oligodendrocytes. These pure populations of well-defined cells will provide disease-relevant phenotypes at transcriptomic level and at the cellular level, providing a platform for determining whether re-purposed or new chemical entities can reverse the phenotype. The next step will be systematic reconstitution of well-defined cellular components to recreate circuit-based phenotypes, which are now beginning to be explored [79]. Reconstitution of neural circuit from defined components will overcome the variability and the poorly controlled cellular heterogeneity of organoids.
By utilizing patient derived iPSCs, we are beginning to identify pathogenic mechanisms of SCZ during brain development. But to have meaningful impact on treatment development, we will need to considerably expand the number of subjects studied in order to identify how broadly represented are genetically and environmentally determined final common pathways for the pathogenesis for SCZ. Considering the immense cost of iPSC studies, collaborative efforts bringing together multiple laboratories and/or the creation of a public iPSC repository will greatly facilitate reaching this goal.
Table 1.
Summary of recent iPSC-based SCZ disease modeling studies
| Reference | Subjects | Generated cell types |
Phenotypes | Recovery |
|---|---|---|---|---|
| Lin et al., 2016. BMC Syst. Biol. [28] | 7 CON 8 SCZ and SAD (all 22q11.2 del) | mixed Neurons | Disrupted mRNA expression in cell cycle, survival and MAPK pathways | |
| Toyoshima et al., 2016. Transl Psychiatry [29] | 3 CON, 2 SCZ (22q11.2 del) | NPCs and Neurons | Neural differentiation and migration ↓ miR-17/92 and miR-106a/b ↓ p38α ↑ | p38α inhibitor |
| Zhao et al., 2015. PLoS One [30] | 6 CON, 1 SCZ, 3 SAD, 2 COS (all 22q11.2 del) | mixed Neurons | miRNA disruption consistent with postmortem study | |
| Yoon et al., 2014. Cell Stem Cell [31] | 3 CON, 3 COS (15q11.2 del) | NPCs | CYFIP1 and WAVE2 ↓ Disrupted Adherent junctions and apical polarity | CYFIP1 expression |
| Lee et al., 2015. NPJ Schizophr [32] | 6 CON, Trio (mother, carrier Father, SCZ daughter with CNTNAP2 del) | mixed NPCs | Exon 14–15 CNTNAP2 mRNA ↓ Neurosphere migration ↓ | |
| Srikanth et al., 2015. Cell Rep [33] | 1 CON (WT and isogenic DISC1 mutant) | mixed NPCs | Canonical WNT signaling and proliferation ↑ Disturbed NPC fate decision | WNT antagonist |
| Wen et al., 2014. Nature [34] | 1 CON, Family (2 CON, 2 DISC1 mutation) | Glut neurons | Synaptic vesicle release ↓ | Isogenic gene correction |
| Topol et al., 2015. Transl Psychiatry [35] | 6 CON, 4 SCZ | mixed NPCs | Protein synthesis ↑ in NPCs but not in neurons | Rapamycin |
| Brennard et al., 2015. Mol Psychiatry [36] | 6 CON, 4 SCZ | mixed NPCs | Migration ↓ Oxidative stress ↑ | |
| Roussos et al., 2016. JAMA Psychiatry [37] | 4 CON, 4 SCZ | Mixed Neurons | Activity-dependent gene expression ↓ | |
| Topol et al., 2016. Cell Rep [38] | 6 CON, 4 SCZ Replication (10 CON, 10 COS) | mixed NPCs | MiR-9 ↓ in NPCs but not in neurons Migration ↓ | miR-9 expression |
| Topol et al., 2015. Biol. Psychiatry [41] | 6 CON, 4 SCZ | mixed NPCs | Canonical WNT signaling ↑ | |
| Hartley et al., 2015. Mol Psychiatry [42] | 3 CON, 4 SCZ | mixed Neurons | DA neuron differentiation ↔ |
Highlights.
iPSCs provide SCZ-relevant tissues genetically identical to patient brain tissues.
SCZ iPSC-derived neural cells show disease-relevant phenotypes.
SCZ iPSCs provide a model system to dissect the pathogenetic mechanism of SCZ with.
Further development in technology will ensure reliable disease modeling using iPSCs.
Acknowledgments
This study was supported by NIH Grants MH107884 and MH051290
Abbreviation
- CON
healthy control
- SCZ
schizophrenia
- COS
child onset schizophrenia
- NPC
neural progenitor cell
- DISC1
disrupted in Schizophrenia 1
- CYFIP1
cytoplasmic FMR1 interacting protein 1
- CNTNAP2
contactin-associated protein-like 2
- DA
dopaminergic
- SAD
schizoaffective disorder
- MAPK
mitogen-activated protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosures
The authors declare no conflict of interests or any commercial associations
References
- 1.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter WT, Buchanan RW. Schizophrenia. N. Engl. J. Med. 1994;330:681–690. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- 4.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL. Comorbidity of mental disorders with alcohol other drug abuse Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 5.Radomsky ED, Haas GL, Mann JJ, Sweeney JA. Suicidal behavior in patients with schizophrenia and other psychotic disorders. Am J Psychiatry. 1999;156:1590–1595. doi: 10.1176/ajp.156.10.1590. [DOI] [PubMed] [Google Scholar]
- 6.Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- 7.Elvevåg B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- 8.Bellack AS. Scientific consumer models of recovery in schizophrenia: concordance contrasts and implications. Schizophr Bull. 2006;32:432–442. doi: 10.1093/schbul/sbj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stachowiak MK, Kucinski A, Curl R, Syposs C, YANG Y, Narla S, Terranova C, Prokop D, Klejbor I, Bencherif M, Birkaya B, Corso T, Parikh A, Tzanakakis ES, Wersinger S, Stachowiak EK. Schizophrenia: a neurodevelopmental disorder--integrative genomic hypothesis and therapeutic implications from a transgenic mouse model. Schizophr Res. 2013;143:367–376. doi: 10.1016/j.schres.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J of Pharmacol. 2011;164:1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr Pharm Des. 2010;16:323–343. doi: 10.2174/138161210790170094. [DOI] [PubMed] [Google Scholar]
- 12.Franco R, Cedazo-Minguez A. Successful therapies for Alzheimer's disease: why so many in animal models and none in humans? Front Pharmacol. 2014;5:146. doi: 10.3389/fphar.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal glial and somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia Huntington disease. Arch. Gen. Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 14.Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- 15.Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J. Neurol. Neurosurg. Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalus P, Bondzio J, Federspiel A, Müller TJ, Zuschratter W. Cell-type specific alterations of cortical interneurons in schizophrenic patients. Neuroreport. 2002;13:713–717. doi: 10.1097/00001756-200204160-00035. [DOI] [PubMed] [Google Scholar]
- 17.Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch. Gen. Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 18.Volk D, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds GP, Abdul-Monim Z, Neill JC, Zhang Z-J. Calcium binding protein markers of GABA deficits in schizophrenia--postmortem studies and animal models. Neurotox Res. 2004;6:57–61. doi: 10.1007/BF03033297. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J. Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Nature. 2010;468:194–202. doi: 10.1038/nature09569. [DOI] [PubMed] [Google Scholar]
- 22.Bogerts B, Häntsch J, Herzer M. A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biological Psychiatry. 1983;18:951–969. [PubMed] [Google Scholar]
- 23.Ikemoto K. Striatal D-neurons: in new viewpoints for neuropsychiatric research using post-mortem brains. Fukushima J Med Sci. 2008;54:1–3. doi: 10.5387/fms.54.1. [DOI] [PubMed] [Google Scholar]
- 24.Laruelle M. Imaging dopamine transmission in schizophrenia A review and meta-analysis. Q J Nucl Med. 1998;42:211–221. [PubMed] [Google Scholar]
- 25.Millan MJ, Andrieux A, Bartzokis G, Cadenhead K, Dazzan P, Fusar-Poli P. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15:485–515. doi: 10.1038/nrd.2016.28. [DOI] [PubMed] [Google Scholar]
- 26.Gilman SR, Chang J, Xu B, Bawa TS, Gogos JA, Karayiorgou M. Diverse types of genetic variation converge on functional gene networks involved in schizophrenia. Nature. 2012;15:1723–1728. doi: 10.1038/nn.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu B, Ionita-Laza I, Roos JL, Boone B, Woodrick S, Sun Y. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat Genet. 2012;44:1365–1369. doi: 10.1038/ng.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin M, Pedrosa E, Shah A, Hrabovsky A, Maqbool S, Zheng D. RNA-Seq of human neurons derived from iPS cells reveals candidate long non-coding RNAs involved in neurogenesis and neuropsychiatric disorders. PLoS ONE. 2011;6:e23356. doi: 10.1371/journal.pone.0023356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguilar-Valles A, LuheshiG N. Alterations in cognitive function and behavioral response to amphetamine induced by prenatal inflammation are dependent on the stage of pregnancy. Psychoneuroendocrinology. 2011;36:634–648. doi: 10.1016/j.psyneuen.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PLoS ONE. 2009;4:e6354. doi: 10.1371/journal.pone.0006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piper M, Beneyto M, Burne TH, Eyles DW, Lewis DA, McGrath JJ. The neurodevelopmental hypothesis of schizophrenia: convergent clues from epidemiology and neuropathology. Psychiatr Clin North Am. 2012;35:571–584. doi: 10.1016/j.psc.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 34.Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D. Functional Maturation of hPSC-Derived Forebrain Interneurons Requires an Extended Timeline and Mimics Human Neural Development. Cell Stem Cell. 2013;12:573–586. doi: 10.1016/j.stem.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S. Rapid Single-Step Induction of Functional Neurons from Human Pluripotent Stem Cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mertens J, Paquola ACM, Ku M, Hatch E, Böhnke L, Ladjevardi S. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell. 2015;17:705–718. doi: 10.1016/j.stem.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wainger BJ, Kiskinis E, Mellin C, Wiskow O, Han SSW, Sandoe J. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014;7:1–11. doi: 10.1016/j.celrep.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin M, Pedrosa E, Hrabovsky A, Chen J, Puliafito BR, Gilbert SR. Integrative transcriptome network analysis of iPSC-derived neurons from schizophrenia and schizoaffective disorder patients with 22q11.2 deletion. BMC Syst Biol. 2016;10:105. doi: 10.1186/s12918-016-0366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toyoshima M, Akamatsu W, Okada Y, Ohnishi T, Balan S, Hisano Y. Analysis of induced pluripotent stem cells carrying 22q11.2 deletion. Transl Psychiatry. 2016;6:e934. doi: 10.1038/tp.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao D, Lin M, Chen J, Pedrosa E, Hrabovsky A, Fourcade HM. MicroRNA Profiling of Neurons Generated Using Induced Pluripotent Stem Cells Derived from Patients with Schizophrenia Schizoaffective Disorder and 22q11.2 Del. PLoS ONE. 2015;10:e0132387–24. doi: 10.1371/journal.pone.0132387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon K-J, Nguyen HN, Ursini G, Zhang F, Kim N-S, Wen Z. Modeling a Genetic Risk for Schizophrenia in iPSCs and Mice Reveals Neural Stem Cell Deficits Associated with Adherens Junctions and Polarity. Stem Cell. 2014;15:79–91. doi: 10.1016/j.stem.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee IS, Carvalho CMB, Douvaras P, Ho S-M, Hartley BJ, Zuccherato LW. Characterization of molecular and cellular phenotypes associated with a heterozygous CNTNAP2 deletion using patient-derived hiPSC neural cells. NPJ Schizophr. 2015;1:15019–5. doi: 10.1038/npjschz.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srikanth P, Han K, Callahan DG, Makovkina E, Muratore CR, Lalli MA. Genomic DISC1 Disruption in hiPSCs Alters Wnt Signaling and Neural Cell Fate. Cell Rep. 2015;12:1414–1429. doi: 10.1016/j.celrep.2015.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, Kim NS, Yoon KJ, Shin J, Zhang C, Makri G, Nauen D, Yu H, Guzman E, Chiang CH, Yoritomo N, Kaibuchi K, Zou J, Christian KM, Cheng L, Ross CA, Margolis RL, Chen G, Kosik KS, Song H, Ming GL. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature. 2014:1. doi: 10.1038/nature13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topol A, English JA, Flaherty E, Rajarajan P, Hartley BJ, Gupta S. Increased abundance of translation machinery in stem cell-derived neural progenitor cells from four schizophrenia patients. Transl Psychiatry. 2015;5:e662. doi: 10.1038/tp.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brennand K, Savas JN, Kim Y, Tran N, Simone A, Hashimoto-Torii K. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry. 2015;20:361–368. doi: 10.1038/mp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roussos P, Guennewig B, Kaczorowski DC, Barry G, Brennand KJ. Activity-Dependent Changes in Gene Expression in Schizophrenia Human-Induced Pluripotent Stem Cell Neurons. JAMA Psychiatry. 2016;73:1180–1188. doi: 10.1001/jamapsychiatry.2016.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narla ST, Lee Y-W, Benson CA, Sarder P, Brennand KJ, Stachowiak EK. Common developmental genome deprogramming in schizophrenia - Role of Integrative Nuclear FGFR1 Signaling (INFS) Schizophr Res. 2017 doi: 10.1016/j.schres.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Topol A, Zhu S, Hartley BJ, English J, Hauberg ME, Tran N. Dysregulation of miRNA-9 in a Subset of Schizophrenia Patient-Derived Neural Progenitor Cells. Cell Rep. 2016;15:1024–1036. doi: 10.1016/j.celrep.2016.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, Holmans PA. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chatterjee N, Wheeler B, Sampson J, Hartge P, Chanock SJ, Park J-H. Projecting the performance of risk prediction based on polygenic analyses of genome-wide association studies. Nat Genet. 2013;45:400–5. doi: 10.1038/ng.2579. 405e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Topol A, Zhu S, Tran N, Simone A, Fang G, Brennand KJ. Altered WNT Signaling in Human Induced Pluripotent Stem Cell Neural Progenitor Cells Derived from Four Schizophrenia Patients. Biol. Psychiatry. 2015;78:e29–e34. doi: 10.1016/j.biopsych.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartley BJ, Tran N, Ladran I, Reggio K, Brennand KJ. Dopaminergic differentiation of schizophrenia hiPSCs. Mol Psychiatry. 2015;20:549–550. doi: 10.1038/mp.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahn S, Kim T-G, Kim K-S, Chung S. Differentiation of human pluripotent stem cells into Medial Ganglionic Eminence vs. Caudal Ganglionic Eminence cells. Methods. 2016;101:103–112. doi: 10.1016/j.ymeth.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim T-G, Yao R, Monnell T, Cho J-H, Vasudevan A, Koh A. Efficient specification of interneurons from human pluripotent stem cells by dorsoventral and rostrocaudal modulation. Stem Cells. 2014;32:1789–1804. doi: 10.1002/stem.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 58.Volk DW, Lewis DA. Early developmental disturbances of cortical inhibitory neurons: contribution to cognitive deficits in schizophrenia. chizophr Bull. 2014;40:952–957. doi: 10.1093/schbul/sbu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activxity induces cognitive behavioral dopaminergic abnormalities that resemble schizophrenia. Biol. Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 61.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hook V, Brennand KJ, Kim Y, Toneff T, Funkelstein L, Lee KC. Human iPSC neurons display activity-dependent neurotransmitter secretion: aberrant catecholamine levels in schizophrenia neurons. Stem Cell Reports. 2014;3:531–538. doi: 10.1016/j.stemcr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robicsek O, Karry R, Petit I, Salman-Kesner N, ller F-JMU, Klein E. Abnormal neuronal differentiation and mitochondrial dysfunction in hair follicle-derived induced pluripotent stem cells of schizophrenia patients. Mol Psychiatry. 2013;18:1067–1076. doi: 10.1038/mp.2013.67. [DOI] [PubMed] [Google Scholar]
- 64.Arenas E, Denham M, Villaescusa JC. How to make a midbrain dopaminergic neuron. Development. 2015;142:1918–1936. doi: 10.1242/dev.097394. [DOI] [PubMed] [Google Scholar]
- 65.Chung S, Moon J-I, Leung A, Aldrich D, Lukianov S, Kitayama Y. ES cell-derived renewable and functional midbrain dopaminergic progenitors. Proc Natl Acad Sci U S A. 2011;108:9703–9708. doi: 10.1073/pnas.1016443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bernstein H-G, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr Res. 2015;161:4–18. doi: 10.1016/j.schres.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 67.Krencik R, Weick JP, Liu Y, Zhang Z-J, Zhang S-C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu B-Y, Du Z-W, Li X-J, Ayala M, Zhang S-C. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. 2009;136:1443–1452. doi: 10.1242/dev.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2014;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moreau Y, Tranchevent LC. Computational tools for prioritizing candidate genes: boosting disease gene discovery. Nat Rev Genet. 2012;13:523–536. doi: 10.1038/nrg3253. [DOI] [PubMed] [Google Scholar]
- 71.Fromer M, Roussos P, Sieberts SK, Johnson JS, Kavanagh DH, Perumal TM. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci. 2016 doi: 10.1038/nn.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warren CR, Jaquish CE, Cowan CA. The NextGen Genetic Association Studies Consortium: A Foray into In Vitro Population Genetics. Cell Stem Cell. 2017;20:431–433. doi: 10.1016/j.stem.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 73.Warren CR, O'Sullivan JF, Friesen M, Becker CE, Zhang X, Liu P. Induced Pluripotent Stem Cell Differentiation Enables Functional Validation of GWAS Variants in Metabolic Disease. Cell Stem Cell. 2017;20:547–557.e7. doi: 10.1016/j.stem.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 74.van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 75.Brown AS. Progress in Neurobiology. Prog Neurobiol. 2011;93:23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rouhani F, Kumasaka N, de Brito MC, Bradley A, Vallier L, Gaffney D. Genetic background drives transcriptional variation in human induced pluripotent stem cells. PLoS Genet. 2014;10:e1004432. doi: 10.1371/journal.pgen.1004432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi J, Lee S, Mallard W, Clement K, Tagliazucchi GM, Lim H. A comparison of genetically matched cell lines reveals the equivalence of human iPSCs and ESCs. Nat Biotechnol. 2015;33:1173–1181. doi: 10.1038/nbt.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Warren L, Manos PD, Ahfeldt T, Loh Y-H, Li H, Lau F. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]