Abstract
Acute nonbacterial gastroenteritis caused by noroviruses constitutes a global public health concern and a significant economic burden. There are currently no small molecule therapeutics or vaccines for the treatment of norovirus infections. A structure-guided approach was utilized in the design of a series of inhibitors of norovirus 3CL protease that embody an oxazolidinone ring as a novel design element for attaining optimal binding interactions. Low micromolar cell-permeable inhibitors that display anti-norovirus activity have been identified. The mechanism of action, mode of binding, and structural rearrangements associated with the interaction of the inhibitors and the enzyme were elucidated using X-ray crystallography.
Graphical Abstract
1. Introduction
Human noroviruses are the primary causative agents of acute gastroenteritis and have an increasing impact on public health worldwide [1–3]. While the illness is mild and self-limiting in healthy individuals, it impacts disproportionately and most severely immunocompromised individuals, and the young and elderly [4]. There are 19–21 million norovirus (NV) infections annually in the U.S., and these are associated with high morbidity [5–7]. The toll exacted by NV infections among children <5 years old in developing countries is more acute, resulting in an estimated 71000 deaths annually [8]. The problem is further exacerbated by the highly infectious nature of noroviruses, their genetic diversity and copious virus shedding, as well as their high environmental stability.
Despite the global burden of noroviruses, there are currently no therapeutics or vaccines for the treatment of the disease [9–14], however, recent advances in norovirus pathobiology [15–17], including the identification of proteinaceous receptors for murine norovirus entry into cells [18–19], the nuanced interplay of norovirus pathogenesis and the gut microbiome [20–21], and the development of new in vitro culture systems [22] and animal models [23], have greatly illuminated our understanding of norovirus binding and entry, as well as cell tropism, and have begun to lay a solid foundation for exploring an array of conceptually-sound approaches toward the development of anti-norovirus therapeutics.
Human noroviruses are single-stranded, positive sense RNA viruses belonging to the family Caliciviridae [24]. Of the seven genogroups (GI-GVII) in the genus Norovirus, genogroups I, II and IV are known to infect humans. The norovirus genome (7–8 kb) consists of three opening reading frames that encode a 200 kDa polyprotein (ORF1), a major capsid protein VP1 (ORF2), and a small basic protein VP2 (ORF3) [25–26]. Co- and post-translational processing of the mature polyprotein precursor by the virus-encoded 3CL protease (NV 3CLpro) generates six mature non-structural proteins.
Norovirus 3CL protease (NV 3CLpro) plays a pivotal role in the life cycle of norovirus through the cleavage of the viral polyprotein and is essential for viral replication. NV 3CL pro is therefore an attractive target for the development of norovirus therapeutics. NV 3CLpro is a chymotrypsin-like cysteine protease with an active site comprised of a prototypical catalytic triad (Cys139-His30-Glu54). The protease functions as an induced fit enzyme and has an extended binding cleft [27–29]. The substrate specificity of the protease is for a P1 Gln [30] residue (or Gln surrogate) that engages in critical H-bonding interactions with Thr134 and His157 located in close proximity to the active site.
2. Results and Discussion
2.1 Inhibitor design rationale
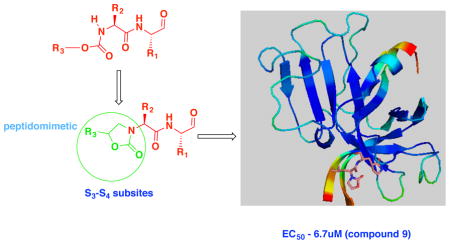
NV 3CLpro has been the focus of exploratory investigations by us [31–36] and others [29, 37] as a potential druggable target for the development of anti-norovirus small molecule drugs. Inhibitors of NV 3CLpro reported by us include peptidyl and macrocyclic transition state (TS) inhibitors and TS mimics shown to be effective in enzyme and cell-based assays, as well as efficacious in the mouse model of murine norovirus infection [31]. Reduction of the peptidyl character of an inhibitor typically enhances proteolytic stability, cellular permeability, and oral bioavailability. Depeptidization of an inhibitor can be accomplished via the construction of a macrocyclic inhibitor or a peptidomimetic capable of orienting recognition elements in a specific vector relationship, thereby exploiting binding interactions with active site residues. We hypothesized that the transformation of a peptidyl inhibitor to peptidomimetic (I) (Figure 1) can be accomplished using a functionalized heterocyclic ring (an oxazolidinone). The presence of a ring chiral center was furthermore anticipated to provide directional control for optimal interactions between the S3-S4 subsites and recognition element R3. Since previous studies [31] showed that NV 3CLpro has a strong preference for a cyclohexylalanine (Cha) or Leu as the P2 residue and a Gln or Gln surrogate [38] as the P1 residue, these recognition elements were incorporated in the structure of inhibitor (I). The design, synthesis, and evaluation of a series of oxazolidinone-derived inhibitors of NV 3CL protease are described herein.
Fig. 1.
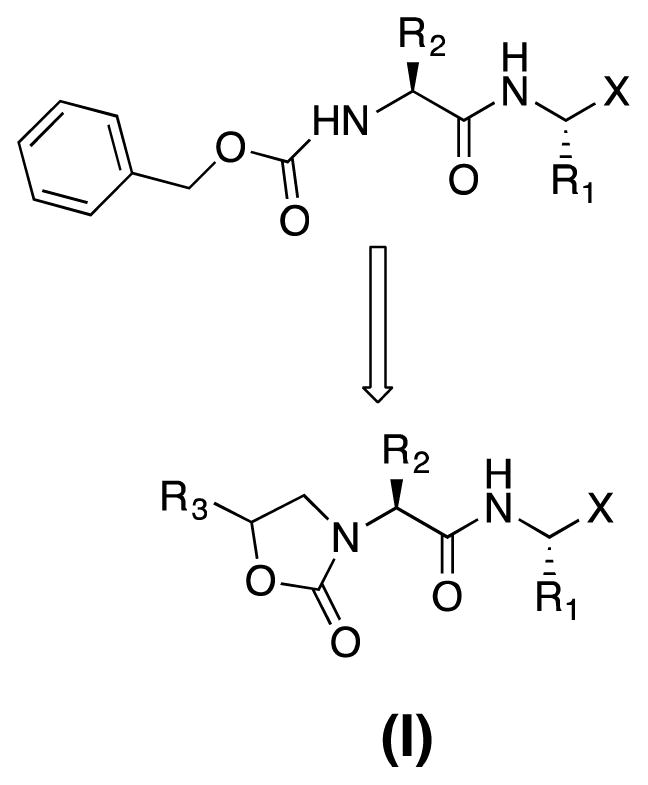
Design and general structure of inhibitor (I)
2.2 Chemistry
Compounds 2a–c were synthesized via the epoxidation of the precursor alkenes with m-chloroperbenzoic acid to yield the corresponding epoxide which was then treated with (L) Leu-OCH3 or (L) Cha-OCH3 in trifluoroethanol (TFE) to yield compounds 3a–d (Scheme 1). Reaction with carbonyl diimidazole (CDI) in dry THF yielded oxazolidinone derivatives 4a–d which were hydrolyzed with LiOH in aqueous THF to yield the corresponding acids 5a–d. Compounds 5a–d were coupled with a glutamine surrogate [38] using EDCI/HOBt/DIEA/DMF to yield compounds 6a–d which, upon reduction with lithium borohydride, generated the corresponding alcohols 7a–d. Oxidation of the alcohols using Dess-Martin periodinane yielded aldehydes 8–11. The corresponding bisulfite adducts 12–15 were readily prepared by treating the precursor aldehydes 8–11 with NaHSO3/EtOAc/EtOH/H2O. The final compounds are listed in Table 1.
Scheme 1.
a) NaH/allyl bromide/DMF; b) MCPBA/DCM; c) (L)Leu-OCH3 or (L)Cha-OCH3/TFE; d) CDI/THF; e) LiOH/aq.THF; f) EDCI/HOBt/DIEA/DMF; g) 2M LiBH4/THF/CH3OH; h) Dess-Martin periodinane/DCM; h) NaHSO3/EtOAc/CH3CH2OH/H2O
Table 1.
Inhibitory activity of compounds 8–15.
| Compound | R3 | R2 | X | IC50 (μM) | EC50 (μM) | CC50 (μM) |
|---|---|---|---|---|---|---|
| 8A | m-Cl phenyl | Leu | CHO | 7.3 | 11.5 | > 100 |
| 12A | CH(OH)SO3Na | 8.2 | 12.3 | > 100 | ||
| 8B | m-Cl phenyl | Leu | CHO | 15.3 | 15.3 | > 100 |
| 12B | CH(OH)SO3Na | 13.2 | 17.5 | > 100 | ||
| 9a | m-Cl phenyl | Cha | CHO | 13.5 | 6.7 | 55.2 |
| 13 | CH(OH)SO3Na | 15.1 | 8.1 | 46.1 | ||
| 10a | m-Cl phenoxy methyl | Leu | CHO | 3.8 | 8.2 | > 100 |
| 14a | CH(OH)SO3Na | 8.5 | 7.6 | > 100 | ||
| 11a | m-Cl benzyloxy methyl | Leu | CHO | 8.1 | 14.8 | > 100 |
| 15a | CH(OH)SO3Na | 10.6 | 17.3 | > 100 |
screened as mixtures of diastereomers
2.3 Biochemical studies
The inhibitory activity of the synthesized compounds against NV 3CLpro and their anti-norovirus activity in a cell-based replicon system were evaluated as described in the experimental section and the IC50, EC50, and CC50 values, are listed in Table 1. These are the average of at least two determinations.
Inspection of the results shown in Table 1 reveals that oxazolidinone derivatives are permeable and, furthermore, some of them display single digit potency (Table 1, compounds 9, 13, 10, and 14). Considering that these compounds were screened as mixtures of diastereomers, a further gain in potency may be realized by separating and screening the individual diastereomers. Compounds with a P2 Cha were about 2-fold more potent than those with a P2 Leu, confirming previous findings [31]. The position of the phenyl ring, and by extension of the ring substituent, which are probably accommodated in the S4 subsite of the enzyme, appear to impact potency moderately (vide infra).
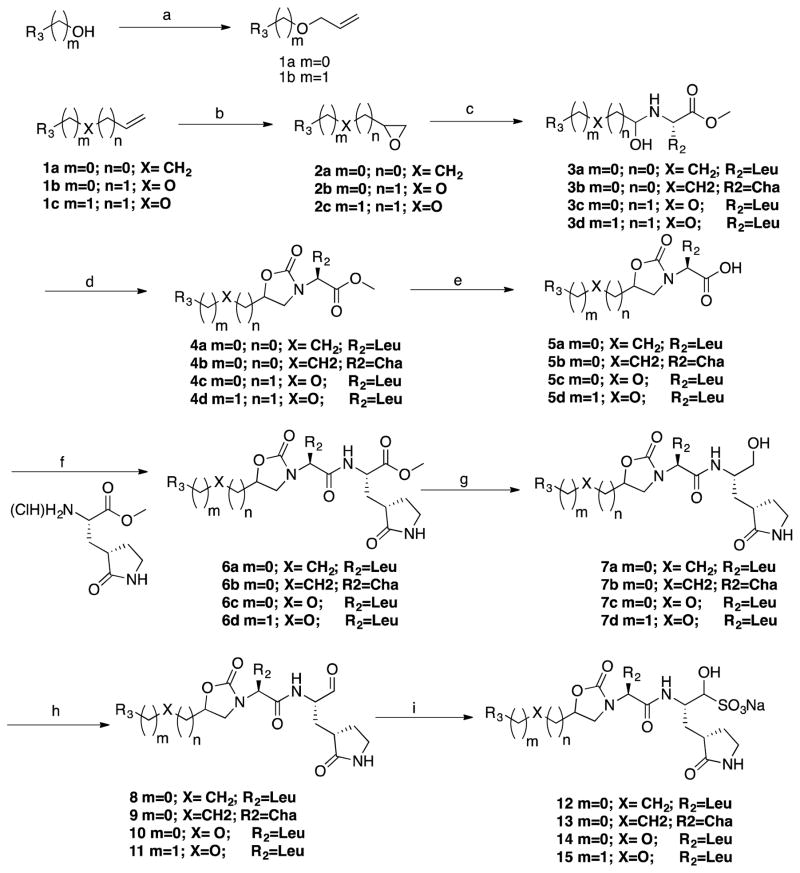
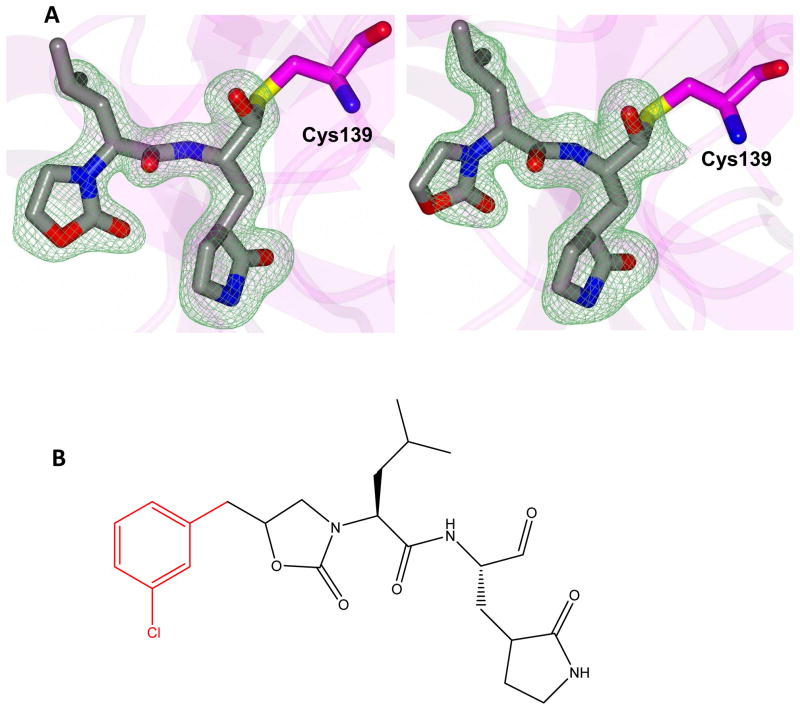
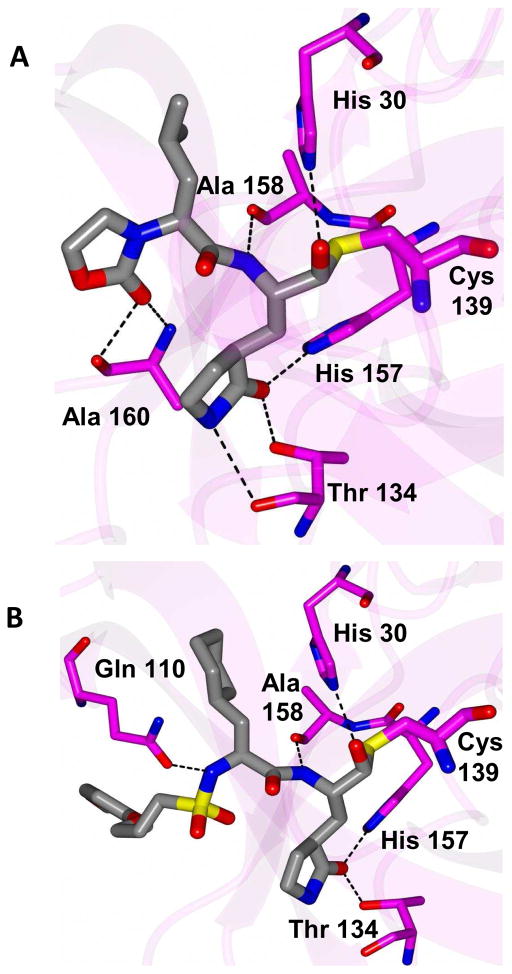
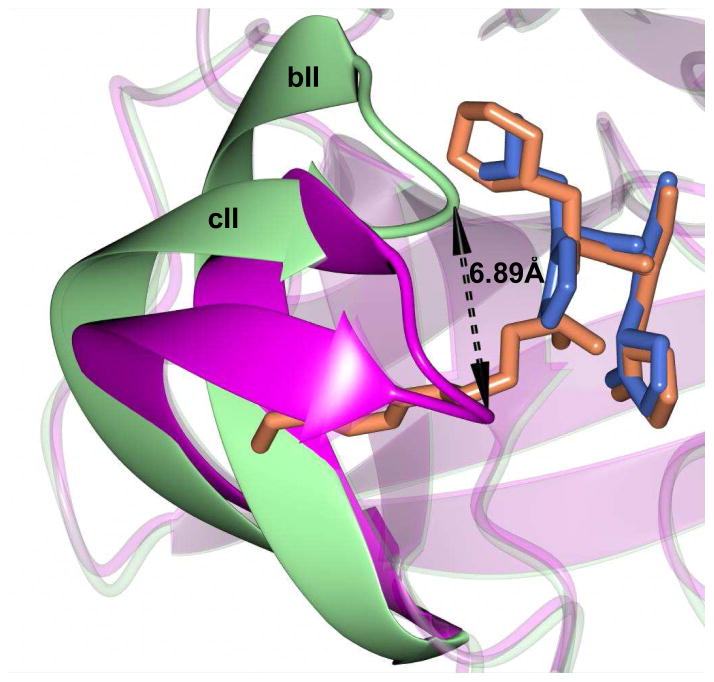
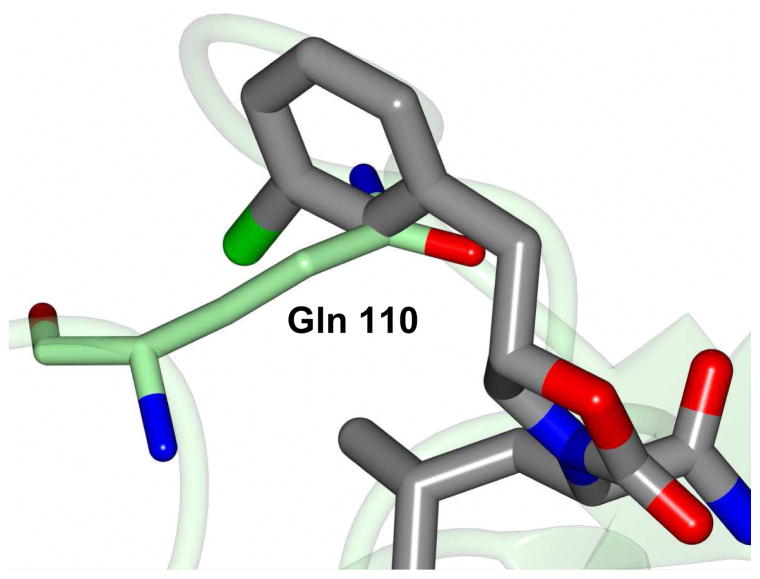
The mechanism of action and mode of binding of these compounds to the active site of NV 3CLpro were elucidated by determining a high-resolution X-ray crystal structure of inhibitor 8B bound to the enzyme. Examination of the active site revealed the presence of prominent difference electron density consistent with inhibitor 8B covalently bound to Cys 139 (Figure 2A). However, the m-chlorobenzyl group of the inhibitor (Figure 2B) was disordered and could not be modeled. The hydrogen bond interactions between NV 3CLpro and 8B are shown in Figure 3A. The expected H-bonds between the -lactam ring of the Gln surrogate with His157 and Thr134 are clearly evident. Furthermore, the backbone hydrogen bonds with Ala158 and Ala160 which serve to correctly position the inhibitor in relation to the catalytic residues and facilitate the reaction of Cys139 with the aldehyde warhead, are also evident. The negatively-charged oxygen of the tetrahedral adduct is stabilized by a hydrogen bond with His30. Dipeptidyl aldehyde inhibitors lacking an oxazolidinone ring form an additional backbone H-bond with Gln110, which is not possible with the oxazolidinone inhibitors (since the oxazolidinone ring N lacks a hydrogen) [31]. Comparison of a previously determined inhibitor bound NV 3CLpro structure of the same crystal form (5T6D) [39] revealed a high degree of similarity with an RMSD deviation of 0.71 Å (154 residues) between Cα atoms using GESAMT (Figure 3B) [40]. However, significant differences are observed in β-strands bII and cII (Gly 92 to Leu 121) which contain Gln 110. The binding of 8B results in the displacement of Gln 110 by 6.89 Å relative to the 5T6D structure (Figure 4). As noted above, the m-chlorobenzyl group of 8B was disordered and could not be modeled, however, modeling this portion of the inhibitor in an idealized position would result in a steric clash with Gln 110 (Figure 5). It is surmised that this results in the observed conformational change in the bII and cII strands. It is, however, unclear how the conformational changes induced by the oxazolidinone ring of inhibitor 8B impact potency since inhibitor 8B forms the same number of hydrogen bonds as acyclic inhibitor 5T6D (Figure 3A–B). A likely explanation for this is that the active site conformational changes induced by inhibitor 8B result in sub-optimal positioning of the inhibitor warhead for reaction with the active site Cys139.
Figure 2.
A) Fo-Fc polder omit map [53] of inhibitor 8B (green mesh) contoured at 3σ. The ligand associated with subunits A and B is positioned on the left and right, respectively. B) Structure of inhibitor 8B with the disordered m-chlorobenzyl group highlighted in red.
Figure 3.
Hydrogen bond interactions (dashed lines) for NV 3CLpro in complex with A) compound 8B and B) 5T6D [39].
Figure 4.
Superposition of NV 3CLpro:8B (magenta) with 5T6D (green). The inhibitors are colored blue and tan for each respective structure. The arrow indicates the positions of Gln 110 for each structure.
Figure 5.
Inclusion of the m-chorobenzyl ring of inhibitor 8B in an idealized position superimposed onto the 5T6D structure (green). The aryl ring occupies the same space and Gln 110 and results in the observed conformational change shown in Figure 4.
3. Conclusion
There is currently a pressing need for the development of norovirus-specific therapeutics and prophylactics for the management of norovirus infections. The studies described herein disclose the structure-based design of the first series of oxazolidinone-based peptidomimetic inhibitors of norovirus 3CL protease. Insights gained from these studies have laid a solid foundation for conducting further optimization of potency and PK characteristics.
4. Experimental section
4.1 General
Reagents and dry solvents were purchased from various chemical suppliers (Aldrich, Oakwood chemicals, Acros Organics, Chem Impex, TCI America, Bachem, and Fisher) and were used as obtained. Silica gel (230–450 mesh) used for flash chromatography was purchased from Sorbent Technologies (Atlanta, GA). Thin layer chromatography was performed using Analtech silica gel plates. The 1H spectra were recorded in CDCl3 or DMSO-d6 on a Varian XL-400 NMR spectrometer. High resolution mass spectra (HRMS) were performed at the University of Kansas Mass Spectrometry lab using an LCT Premier mass spectrometer (Waters, Milford, MA) equipped with a time of flight mass analyzer and an electrospray ion source or a G6230B TOF MS (Agilent Technologies, Santa Clara, CA). Visualization was accomplished using UV light and/or iodine. All final compounds were purified to ≥95% purity as assessed by HPLC and fully characterized by 1H NMR and HRMS.
4.1.1. Synthesis of compound 1 a–b. General procedure
To a solution of alcohol (35 mmol) in anhydrous DMF (45 mL) was added sodium hydride (1.09 g; 45.5 mmol) portionwise and the reaction mixture was stirred for 15 min at room temperature, followed by the addition of allyl bromide (4.23 g; 35 mmol) and stirring the reaction mixture overnight at room temperature. The solvent was removed in vacuo and the residue was partitioned between ethyl acetate (150 mL) and 5% aqueous HCl (2 × 50 mL). The layers were separated and the organic layer was washed with saturated aqueous NaHCO3 (2 × 50 mL), followed by saturated NaCl (50 mL). The organic layer was dried over anhydrous Na2SO4, filtered, and concentrated to yield an oily product.
4.1.1.1. 1-(allyloxy)-3-chlorobenzene 1a
Oil (yield 83%); 1H NMR (400 MHz, CDCl3) δ ppm 4.58–4.62 (d, J = 11.02, 2H), 5.46-5.31 (m, 2 H), 6.18-6.04 (m, 1 H), 7.35-7.03 (m, 4H). HRMS (ESI) calcd for C9H10ClO: [M+H]+: 169.0420 Found 169.0437.
4.1.1.2. 1-[(allyloxy)methyl]-3-chlorobenzene 1b
Oil (yield 84%); 1H NMR (400 MHz, CDCl3) δ ppm 4.10-4.02 (d, J = 10.12, 2H), 4.78-4.73 (s, 2H), 5.38-5.28 (m, 2 H), 6.10-6.05 (m, 1 H), 7.40-7.12 (m, 4H). HRMS (ESI) calcd for C10H12ClO: [M+H]+: 183.0577 Found 183.0595.
4.1.2. Synthesis of compounds 2a–c. General procedure
To a solution of the appropriate alkene (35 mmol) in dry DCM (100 mL) was added m-CPBA (42 mmol) at 0°C. The ice bath was removed and the reaction mixture was stirred at room temperature overnight (18 h). The consumption of starting material was monitored by TLC. The reaction mixture was cooled to 0 – 5°C and quenched with aqueous 1N NaOH solution (15 mL). The reaction mixture was partitioned between water (100 mL) and DCM (100 mL) and the layers were separated. The aqueous layer was extracted with DCM (100 mL) and the combined organic layers were washed with brine (50 mL). The organic layer was dried over anhydrous Na2SO4, filtered and concentrated to yield an oily product.
4.1.2.1. 2-(3-chlorobenzyl)oxirane 2a
Oil (yield (88%); 1H NMR (400 MHz, CDCl3) δ ppm 2.54 (dd, J = 4.93, 2.62 Hz, 1H), 2.87-2.72 (m, 1H), 3.36 (d, J = 6.77 Hz, 1H), 3.16-3.07 (m, 2H), 7.67-7.03 (m, 4H). HRMS (ESI) calcd for C9H10ClO: [M+H]+: 169.0420 Found 169.0427.
4.1.2.2. 2-[(3-chlorophenoxy) methyl] oxirane 2b
Oil (yield 85%); 1H NMR (400 MHz, CDCl3) δ ppm 2.75 (dd, J = 4.90, 2.64 Hz, 1H), 3.00-2.89 (m, 1H), 3.35 (tdd, J = 5.70, 4.10, 2.86, 2.86 Hz, 1H), 3.93 (dd, J = 11.02, 5.73 Hz, 1H), 4.22 (dd, J = 11.02, 3.04 Hz, 1H), 7.35-7.03 (m, 4H). HRMS (ESI) calcd for C9H10ClO2: [M+H]+: 185.0369 Found 185.0377.
4.1.2.3. 2-{[(3-chlorobenzyl) oxy] methyl} oxirane 2c
Oil (yield 84%); 1H NMR (400 MHz, CDCl3) δ ppm 2.61 (dd, J = 4.90, 2.64 Hz, 1H), 2.95-2.85 (m, 1H), 3.30 (tdd, J = 5.70, 4.10, 2.86, 2.86 Hz, 1H), 3.97 (dd, J = 11.02, 5.73 Hz, 1H), 4.25 (dd, J = 11.02, 3.04 Hz, 1H), 4.52 (s, 2H), 7.35-7.03 (m, 4H). HRMS (ESI) calcd for C10H12ClO2: [M+H]+: 199.0526 Found 199.0534.
4.1.3. Synthesis of compounds 3a–d. General procedure
To a solution of compound 2 (29.65 mmol) in trifluoroethanol (50 mL) was added the corresponding free amino acid ester (29.65 mmol) at room temperature. The reaction mixture was refluxed for 6 h with stirring. The progress of the reaction was monitored by TLC. The reaction mixture was cooled to room temperature and the solvent was removed. The crude residue was purified by flash chromatography to yield a colorless oil.
4.1.3.1. Methyl [3-(3-chlorophenyl)-2-hydroxypropyl]-L-leucinate 3a
Oil (yield 70%); 1H NMR (400 MHz, CDCl3) δ ppm 1.06-0.89 (d, 6H), 1.53-1.49 (m, 1H), 1.77-1.70 (m, 2H), 3.30-2.44 (m, 4H), 3.71 (s, 3H), 4.12 (tt, J = 7.15, 7.15, 3.57, 3.57 Hz, 1H), 4.85-4.48 (m, 1H), 5.70—5-60 (br. s, 1H), 7.53-7.01 (m, 4H). HRMS (ESI) calcd for C16H25ClNO3: [M+H]+: 314.1523 Found 314.1532.
4.1.3.2. Methyl (2S)-2-{[3-(3-chlorophenyl)-2-hydroxypropyl] amino}-3-cyclohexylpropanoate 3b
Oil (yield 75%); 1H NMR (400 MHz, CDCl3) δ ppm 1.93-0.76 (m, 13H), 2.71 (m, 4H), 3.52-3.47 (m, 1H), 3.70-3.60 (s, 3H), 3.95 (m, 1H), 5.18-5.04 (m, 1H), 7.49-7.03 (m, 4H). HRMS (ESI) calcd for C19H29ClNO3: [M+H]+: 354.1836 Found 354.1845.
4.1.3.3. Methyl [3-(3-chlorophenoxy)-2-hydroxypropyl]-L-leucinate 3c
Oil (yield 72%); 1H NMR (400 MHz, CDCl3) δ ppm 0.96-0.89 (d, 6H), 1.52-1.49 (m, 1H), 1.78-1.72 (m, 2H), 2.84-2.61 (m, 3H), 3.68 (s, 3H), 4.24-3.98 (m, 3H), 4.85-4.78 (m, 1H), 5.70—5-60 (br. s, 1H), 7.53-7.01 (m, 4H). HRMS (ESI) calcd for C16H25ClNO4: [M+H]+: 330.1472 Found 330.1523.
4.1.3.4. Methyl {3-[(3-chlorobenzyl) oxy]-2-hydroxypropyl}-L-leucinate 3d
Oil (yield 71%); 1H NMR (400 MHz, CDCl3) δ ppm 0.99-0.87 (d, 6H), 1.55-1.48 (m, 1H), 1.77-1.73 (m, 2H), 2.77-2.54 (m, 3H), 3.62-3.73 (m, 3 H), 3.71 (s, 3H), 4.64 (s, 2H), 5.25-5.18 (br. s, 1H), 5.70—5-60 (br. s, 1H), 7.53-7.01 (m, 4H). HRMS (ESI) calcd for C19H27ClNO4: [M+H]+: 344.1629 Found 344.1639.
4.1.4. Synthesis of compounds 4a–d. General procedure
To a solution of compound 3 (21 mmol) in anhydrous THF (75 mL) kept at room temperature was added carbonyl diimidazole (42 mmol) and the reaction mixture was refluxed for 5 h. The disappearance of the starting material was monitored by TLC. The reaction mixture was cooled to room temperature and the solvent was removed in vacuo. The residue was partitioned between EtOAc (250 mL) and 5% aq HCl (100 mL). The layers were separated and the organic layer was sequentially washed with 5% aq HCl, saturated NaHCO3 (2×100 mL) and brine (100 mL). The organic layer was dried over anhydrous Na2SO4, filtered and concentrated to yield a crude product which was purified by flash chromatography.
4.1.4.1. Methyl (2S)-2-[5-(3-chlorobenzyl)-2-oxooxazolidin-3-yl]-4-methylpentanoate 4a
Oil (yield 45%); 1H NMR (400 MHz, CDCl3) δ ppm 7.38-7.06 (m, 4H), 0.98-0.79 (d, 6H), 1.85-1.53 (m, 3H), 3.15-2.81 (m, 2H), 3.50-3.30 (m, 2H), 3.72 (s, 3H), 4.60-4.50 (m, 1H), 4.82-4.75 (m, 1H). HRMS (ESI) calcd for C17H23ClNO4: [M+H]+: 340.1316 Found 340.1321.
4.1.4.2. Methyl (2S)-2-[5-(3-chlorobenzyl)-2-oxooxazolidin-3-yl]-3-cyclohexylpropanoate 4b
Oil (yield 45%); 1H NMR (400 MHz, CDCl3) δ ppm 1.90-0.74 (m, 13H), 3.09-2.84 (m, 2H), 3.82-3.63 (m, 2H), 4.85-4.55 (m, 2H), 7.37-7.05 (m, 4H). HRMS (ESI) calcd for C20H27ClNO4: [M+H]+: 380.1629 Found 380.1642.
4.1.4.3. Methyl(2S)-2-{5-[(3-chlorophenoxy) methyl]-2-oxooxazolidin-3-yl}-4-methylpentanoate 4c
Oil (yield 44%); 1H NMR (400 MHz, CDCl3) δ ppm 1.03-0.86 (d, 6H), 1.85-1.78 (m, 1H), 1.50-1.47 (m, 1H), 3.05-2.88 (m, 4H), 3.76 (s, 3H), 3.80-3.70 (m, 1H), 4.60-4.50 (m, 1H), 7.45-7.14 (m, 4H). HRMS (ESI) calcd for C17H23ClNO5: [M+H]+: 356.1265 Found 356.1284.
4.1.4.4. Methyl(2S)-2-(5-{[(3-chlorobenzyl) oxy] methyl}-2-oxooxazolidin-3-yl)-4-methylpentanoate 4d
Oil (yield 40%); 1H NMR (400 MHz, CDCl3) δ ppm 1.05-0.76 (d, 6H), 1.87-1.77 (m, 1H), 1.50 (m, 1H), 2.95-2.85 (m, 4H), 3.73 (s, 3H), 3.77-3.60 (m, 1H), 4.59-4.50 (m, 1H), 4.76-4.68 (s, 3H), 7.42-7.16 (m, 4H). HRMS (ESI) calcd for C18H25ClNO5: [M+H]+: 370.1421 Found 370.1437.
4.1.5. Synthesis of compounds 5a–d. General procedure
A solution of ester 4 (9 mmol) in tetrahydrofuran (25 mL) was treated with 1M aqueous LiOH (25 mL). The reaction mixture was stirred for 3 h at room temperature while monitoring the disappearance of the ester by TLC. Most of the solvent was evaporated off and the solution was acidified to pH ~3 using 5% hydrochloric acid (10 mL). The aqueous layer was extracted with ethyl acetate (2 × 100 mL) and the combined organic layers were washed with brine (50 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated to yield compound 5.
4.1.5.1. (2S)-2-[5-(3-chlorobenzyl)-2-oxooxazolidin-3-yl]-4-methylpentanoic acid 5a
White solid (yield 93%); mp 129–130°C, 1H NMR (400 MHz, CDCl3) δ ppm 7.57-6.98 (m, 4H), 5.06-4.67 (m, 1H), 4.77-4.42 (m, 1H), 3.34-2.72 (m, 3H), 3.48 (d, J = 7.94 Hz, 1H), 1.96-1.37 (m, 5H), 1.05-0.83 (d, 6H). HRMS (ESI) calcd for C16H20ClNNaO4: [M+Na]+: 348.0979 Found 348.0995.
4.1.5.2. (2S)-2-[5-(3-chlorobenzyl)-2-oxooxazolidin-3-yl]-3-cyclohexylpropanoic acid 5b
Viscous oil (yield 94%); 1H NMR (400 MHz, CDCl3) δ ppm 1.03-0.74 (m, 6H), 1.26 (t, J = 7.16, 7.16 Hz, 2H), 1.85-1.39 (m, 5H), 2.50-2.25 (m, 2H), 3.00-2.75 (m, 2H), 3.80-3.65 (m, 1H), 4.54-4.39 (m, 1H), 4.87-4.76 (m, 1H), 7.38-7.01 (m, 4H). HRMS (ESI) calcd for C19H25ClNNaO4: [M+Na]+: 388.1292 Found 388.1309.
4.1.5.3. 2-{5-[(3-chlorophenoxy) methyl]-2-oxooxazolidin-3-yl}-4-methylpentanoic acid 5c
Viscous oil (yield 95%); 1H NMR (400 MHz, CDCl3) δ ppm 1.03-0.86 (d, 6H), 1.85-1.78 (m, 1H), 1.50-1.47 (m, 1H), 3.05-2.88 (m, 4H), 3.80-3.70 (m, 1H), 4.60-4.50 (m, 1H), 7.45-7.14 (m, 4H). HRMS (ESI) calcd for C16H21ClNO5: [M+H]+: 342.1108 Found 342.1125.
4.1.5.4. 2-(5-{[(3-chlorobenzyl) oxy] methyl}-2-oxooxazolidin-3-yl)-4-methylpentanoic acid 5d
Viscous oil, yield (95%), 1H NMR (400 MHz, CDCl3) δ ppm 1.05-0.76 (d, 6H), 1.87-1.77 (m, 1H), 1.50 (m, 1H), 2.95-2.85 (m, 4H), 3.77-3.60 (m, 1H), 4.59-4.50 (m, 1H), 4.76-4.68 (s, 3H), 7.42-7.16 (m, 4H). HRMS (ESI) calcd for C17H23ClNO5: [M+H]+: 356.1265 Found 356.1278.
4.1.6. Synthesis of compounds 6a–d. General procedure
To a solution of 5 (8.2 mmol) in dry DMF (20 mL) was added EDCI (10.6 mmol, 1.3 eq.) and HOBt (10.6 mmol, 1.3 eq) and the mixture was stirred for 30 minutes at room temperature. In a separate flask, a solution of boc-deprotected glutamine surrogate (8.2 mmol) in dry DMF (20 mL) cooled to 0–5°C was treated with diisopropylethylamine (32.8 mmol, 4.0 eq) and the solution was stirred for 30 minutes. It was then added to the solution above and the reaction mixture was stirred for 16 h while monitoring the reaction by TLC. The solvent was removed in vacuo and the residue was partitioned between ethyl acetate (200 mL) and 10% citric acid (75 mL). The layers were separated and the organic layer was washed with saturated aqueous NaHCO3 (2 × 75 mL), followed by saturated NaCl (75 mL). The organic layer was dried over anhydrous Na2SO4, filtered, and concentrated. The crude residue was purified by flash chromatography to yield the product as a white solid.
4.1.6.1. Methyl (2S)-2-{(2S)-2-[5-(3-chlorobenzyl)-2-oxooxazolidin-3-yl]-4-methylpentanamido}-3-[(S)-2-oxopyrrolidin-3-yl] propanoate 6a
diastereomers A and B were separated using flash chromatography. (A) White solid (yield 32%); mp 47–48°C, 1H NMR (400 MHz, CDCl3) δ ppm 0.93 (ddd, J = 25.59, 17.70, 6.33 Hz, 6H), 1.63 (td, J = 14.14, 7.53, 7.53 Hz, 1H), 1.85 (ddd, J = 13.52, 7.86, 2.99 Hz, 2H), 2.37 (m, 4H), 2.89 (dd, J = 14.02, 6.52Hz, 1H), 3.12 (dd, J = 14.09, 7.06 Hz, 1H), 3.50-3.31 (m, 2H), 3.69-3.50 (m, 4H), 3.77-3.67 (s, 3H), 4.33 (d, J = 6.05 Hz, 1H), 4.88-4.54 (m, 1H), 6.63 (d, 1H), 7.49-7.12 (m, 4H), 8.34 (d, J = 4.87Hz, 1H). HRMS (ESI) calcd for C24H32ClN3NaO6: [M+Na]+: 516.1877 Found 516.1981.
(B) White solid (yield 25%); mp 51–52°C, 1H NMR (400 MHz, CDCl3) δ ppm 0.95 (d, J = 20.12 Hz, 6H), 1.72-1.66 (td, J = 14.14, 7.53, 7.53 Hz, 1H), 1.90-1.83 (dd, J = 11.14, 7.12 Hz, 2H), 2.50-2.37 (m, 4H), 2.90-2.79 (dd, J = 14.02, 6.52Hz, 1H), 3.26-3.11 (d, 1H), 3.45-3.32 (m, 2H), 3.68-3.52 (m, 4H), 3.72-3.68 (s, 3H), 4.51 (d, J = 6.25 Hz, 1H), 4.90-4.85 (m, 1H), 6.83 (d, 1H), 7.51-7.22 (m, 4H), 8.34 (d, J = 4.82 Hz, 1H). HRMS (ESI) calcd for C24H32ClN3NaO6: [M+Na]+: 516.1877 Found 516.1862.
4.1.6.2. Methyl (2S)-2-{(2S)-2-[5-(3-chlorobenzyl)-2-oxooxazolidin-3-yl]-3-cyclohexylpropanamido}-3-[(S)-2-oxopyrrolidin-3-yl] propanoate 6b
White solid (yield 58%); mp 88–89°C, 1H NMR (400 MHz, CDCl3) δ ppm 1.34-0.77 (m, 11H), 1.67 (m, 2H), 3.11-2.66 (m, 4H), 2.68-2.27 (m, 4H), 3.54-3.25 (m, 4H), 3.72 (s, 3H), 5.01-4.36 (m, 2H), 7.43-7.08 (m, 4H), 9.85-9.45 (br. s, 1H). HRMS (ESI) calcd for C27H36ClN3NaO6: [M+Na]+: 556.2190 Found 556.2204.
4.1.6.3. Methyl (2S)-2-[(2S)-2-{5-[(3-chlorophenoxy) methyl]-2-oxooxazolidin-3-yl}-4-methylpentanamido]-3-{(S)-2-oxopyrrolidin-3-yl} propanoate 6c
White solid (yield 65%); mp 45–46°C,1H NMR (400 MHz, CDCl3) δ ppm 1.08-0.89 (d, 6H), 1.53-1.47 (m, 1H) 1.93-1.71 (m, 2H), 2.51-2.25 (m, 2H), 3.37 (dd, J = 6.18, 3.43 Hz, 2H), 3.57 (dd, J = 8.95, 6.07 Hz, 2H), 3.79-3.71 (s, 3H), 4.12 (dtd, J = 14.73, 10.42, 10.40, 5.24 Hz, 2H), 4.68 (t, J = 7.77, 7.77 Hz, 1H), 4.89 (m, 1H), 7.01-6.65 (m, 2H), 7.31-7.12 (m, 2H), 8.44 (d, J = 6.05 Hz, 1H). HRMS (ESI) calcd for C24H32ClN3NaO7: [M+Na]+: 532.1826 Found 532.1730.
4.1.6.4. Methyl (2S)-2-[(2S)-2-(5-{[(3-chlorobenzyl) oxy] methyl}-2-oxooxazolidin-3-yl)-4-methylpentanamido]-3-{(S)-2-oxopyrrolidin-3-yl} propanoate 6d
White solid (yield 68%); mp 47–48°C, 1H NMR (400 MHz, CDCl3) δ ppm 1.04-0.79 (d, 6H), 1.91-1.53 (m, 3H), 2.48-2.13 (m, 4H), 3.42-3.18 (m, 4H), 3.81-3.60 (m, 2H), 3.79-3.71 (s, 3H), 4.12 (d, J = 7.14 Hz, 1H), 4.42-4.31 (m, 2H), 4.65-4.49 (s, 2H), 6.36 (br.s, 1H), 7.36-7.13 (m, 4H), 8.15 (d, J = 6.48 Hz, 1H). HRMS (ESI) calcd for C25H34ClN3NaO7: [M+Na] +: 546.1983 Found 546.1894.
4.1.7. Synthesis of compounds 7a–d. General procedure
To a solution of ester (1.87 mmol) in anhydrous THF (10 mL) was added dropwise lithium borohydride (2M in THF, 2.8 mL, 5.61 mmol) followed by anhydrous methanol (10 mL), and the reaction mixture was stirred at room temperature overnight. The reaction mixture was then acidified by adding 5% aqueous HCl until the pH of the solution was ~3. Removal of the solvent left a residue which was taken up in ethyl acetate (100 mL). The organic layer was washed with brine (25 mL), dried over anhydrous sodium sulfate, filtered, and concentrated to yield the desired alcohol.
4.1.7.1. (2S)-2-[5-(3-chlorobenzyl)-2-oxooxazolidin-3-yl]-N-{(S)-1-hydroxy-3-[(S)-2-oxopyrrolidin-3-yl] propan-2-yl}-4-methylpentanamide 7a
(A) White solid (yield 93%); mp 59–60°C, 1H NMR (400 MHz, CDCl3 δ 1.02-0.71 (d, 6H), 2.06-1.38 (m, 4H), 2.67-2.17 (m, 4H), 2.98-2.75 (m, 4H), 3.55-3.05 (m, 4H), 3.74-3.45 (m, 1H), 3.87 (t, J = 8.75, 8.75 Hz, 1H), 4.36 (m, 1H), 5.06-4.63 (m, 1H), 6.41-5.91 (d, 1H), 7.51-6.93 (m, 4H), 8.20-7.78 (d, 1H). HRMS (ESI) calcd for C23H32ClN3NaO5: [M+Na]+: 488.1928 Found 488.1835.
(B) White solid (yield 95%); mp 65–66°C, 1H NMR (400 MHz, CDCl3) δ 0.94-0.85 (d, 6H), 2.36-1.72 (m, 4H), 2.57-2.25 (m, 4H), 3.02-2.85 (m, 4H), 3.60-3.15 (m, 4H), 3.75-3.45 (m, 1H), 3.92-3.88 (t, J = 8.68, 8.68 Hz, 1H), 4.52 (m, 1H), 5.12-4.78 (m, 1H), 6.45-6.12 (d, 1H), 7.55-7.03 (m, 4H), 8.01-7.94 (d, 1H). HRMS (ESI) calcd for C23H32ClN3NaO5: [M+Na]+: 488.1928 Found 488.1835.
4.1.7.2. (2S)-2-[5-(3-chlorobenzyl)-2-oxooxazolidin-3-yl]-3-cyclohexyl-N-{(S)-1-hydroxy-3-[(S)-2-oxopyrrolidin-3-yl] propan-2-yl} propanamide 7b
White solid (yield 94%); mp 133–134°C, 1H NMR (400 MHz, DMSO-d6) δ ppm 1.20-0.76 (m, 9H), 1.81-1.53 (m, 6H), 2.78-2.68 (m, 4H), 3.42-3.13 (m, 4H), 3.93-3.78 (m, 4H), 4.46-4.26 (m, 1H), 4.95-4.75 (m, 1H), 7.17-7.09 (s, 1H), 7.27-7.14 (m, 3H), 7.97-7.88 (br. s, 1H), 9.80-9.65 (br. s, 1H). HRMS (ESI) calcd for C26H36ClN3NaO5: [M+Na]+: 528.2241 Found 528.22321.
4.1.7.3. (2S)-2-{5-[(3-chlorophenoxy) methyl]-2-oxooxazolidin-3-yl}-N-[(S)-1-hydroxy-3-{(S)-2-oxopyrrolidin-3-yl} propan-2-yl]-4-methylpentanamide 7c
White solid (yield 93%); mp 58–59°C, 1H NMR (400 MHz, CDCl3 δ 1.03-0.82 (d, 6H), 1.55-1.50 (m, 2H), 2.65-2.22 (m, 6H), 3.30-3.21 (m, 2H), 3.80-3.50 (m, 4H), 4.39-4.05 (m, 2H), 5.02-4.76 (m, 1H), 7.07-6.90 (m, 1H), 7.46-7.13 (m, 3H), 8.21-8.01 (br. s, 1H). HRMS (ESI) calcd for C23H32ClN3NaO6: [M+Na]+: 504.1877 Found 504.1804.
4.1.7.4. (2S)-2-(5-{[(3-chlorobenzyl) oxy] methyl}-2-oxooxazolidin-3-yl)-N-[(S)-1-hydroxy-3-{(S)-2-oxopyrrolidin-3-yl} propan-2-yl]-4-methylpentanamide 7d
White solid (yield 93%); 1H NMR (400 MHz, CDCl3 δ 1.14-0.79 (d, 6H), 1.50 (dd, J = 28.65, 20.93 Hz, 2H), 2.71-2.20 (m, 6H), 3.34 (m, 2H), 3.82-3.45 (m, 4H), 4.36-3.85 (m, 2H), 4.52 (s, 2H), 5.13-4.78 (m, 1H), 7.07-6.70 (m, 1H), 7.40-7.10 (m, 3H), 8.20-7.93 (br. s, 1H). HRMS (ESI) calcd for C24H33ClN3O6: [M−H]+: 494.2058 Found 494.2402.
Synthesis of aldehydes 8–11. General procedure
A representative alcohol (2.07 mmol) was dissolved in anhydrous dichloromethane (30 mL) under a nitrogen atmosphere and cooled to 0°C. Dess-Martin periodinane (1.75 g, 4.14 mmol, 2.0 eq.) was added to the reaction mixture with stirring. The ice bath was removed and the reaction mixture was stirred at room temperature for 3 h (monitoring by TLC indicated complete disappearance of the starting material). A solution of 40 mM sodium thiosulfate in saturated aqueous NaHCO3 (50 mL) was added and the solution was stirred for another 15 minutes. The aqueous layer was removed and the organic layer was washed with sodium bicarbonate (25 mL), water (2 × 25 mL) and brine (25 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated. The crude aldehyde was purified by flash chromatography (silica gel/methylene chloride/ethyl acetate/methanol).
(2S)-2-[5-(3-chlorobenzyl)-2-oxooxazolidin-3-yl]-4-methyl-N-[(S)-1-oxo-3-{(S)-2-oxopyrrolidin-3-yl} propan-2-yl] pentanamide (8)
(A) White solid, mp 60–61°C, yield (75%), 1H NMR (400 MHz, CDCl3) δ ppm 1.05-0.81 (d, 6H), 1.32-1.27 (m, 1H), 1.75-1.49 (m, 2H), 2.38-2.12 (m, 4H), 2.74-2.54 (m, 4H), 3.21-3.13 (m, 2H), 3.40 (d, J = 4.42 Hz, 1H), 3.61 (d, J = 39.11 Hz, 1H), 4.29-4.20 (m, 1H), 6.16-5.99 (m, 1H), 7.34-7.09 (m, 4H), 8.55-8.45 (d, 1H), 9.48 (s, 1H), 9.78-9.76 (br. s, 1H). HRMS (ESI) calcd for C23H29ClN3O5: [M−H]+: 462.1796 Found 462.2080.
(B) White solid, mp 54–55°C, yield (70%), 1H NMR (400 MHz, CDCl3) δ ppm 0.98-0.77 (d, 1H), 1.60 (ddd, J = 15.25, 9.74, 5.00 Hz, 1H), 1.84 (ddd, J = 6.61, 5.82, 3.56 Hz, 2H), 2.60-2.40 (m, 5H), 2.99 (dd, J = 7.47, 5.78 Hz, 2H), 3.20 (dd, J = 8.77, 6.54 Hz, 2H), 3.40-3.27 (m, 2H), 3.96-3.66 (m, 1H), 4.29-3.97 (m, 1H), 4.52 (dd, J = 10.39, 5.38 Hz, 1H), 6.34 (s, 1H), 7.19-7.09 (m, 1H), 7.39-7.16 (m, 3H), 8.64 (s, 1H), 9.44 (s, 1H). HRMS (ESI) calcd for C23H29ClN3O5: [M−H]+: 462.1796 Found 462.1825.
(2S)-2-[5-(3-chlorobenzyl)-2-oxooxazolidin-3-yl]-3-cyclohexyl-N-[(S)-1-oxo-3-{(S)-2-oxopyrrolidin-3-yl} propan-2-yl] propanamide (9)
White solid, mp 76–77°C, yield (70%),1H NMR (400 MHz, CDCl3) δ ppm 1.15-0.75 (m, 9H), 2.17-1.37 (m, 6H), 2.51-2.31 (m, 2H), 3.09-2.89 (m, 4H), 3.47-3.24 (m, 2H), 3.94-3.67 (m, 2H), 4.27-4.09 (m, 1H), 4.71-4.38 (m, 1H), 5.02-4.71 (m, 1H), 7.37-7.05 (m, 4H), 8.75-8.57 (d, 1H), 9.50-9.46 (s, 1H). HRMS (ESI) calcd for C26H33ClN3O5: [M−H]+: 502.2109 Found 502.2212.
(2S)-2-{5-[(3-chlorophenoxy) methyl]-2-oxooxazolidin-3-yl}-4-methyl-N-[(S)-1-oxo-3-{(S)-2-oxopyrrolidin-3-yl} propan-2-yl] pentanamide (10)
White solid, mp 53–54°C, yield (65%), 1H NMR (400 MHz, CDCl3) δ ppm 1.08-0.89 (d, 6H), 1.53-1.47 (m, 1H) 1.93-1.71 (m, 2H), 2.51-2.25 (m, 2H), 3.37 (dd, J = 6.18, 3.43 Hz, 2H), 3.57 (dd, J = 8.95, 6.07 Hz, 2H), 3.79-3.71 (s, 3H), 4.12 (dtd, J = 14.73, 10.42, 10.40, 5.24 Hz, 2H), 4.68 (t, J = 7.77, 7.77 Hz, 1H), 4.89 (m, 1H), 7.01-6.65 (m, 2H), 7.31-7.12 (m, 2H), 8.44 (d, J = 6.05 Hz, 1H). HRMS (ESI) calcd for C23H29ClN3O6: [M−H]+: 478.1745 Found 478.2065.
(2S)-2-(5-{[(3-chlorobenzyl) oxy] methyl}-2-oxooxazolidin-3-yl)-4-methyl-N-[(S)-1-oxo-3-{(S)-2-oxopyrrolidin-3-yl} propan-2-yl] pentanamide (11)
White solid, mp 45–46°C, yield (55%), 1H NMR (400 MHz, CDCl3) δ ppm 1.14-0.81 (d, 6H), 1.26 (t, J = 7.15, 7.15 Hz, 1H), 1.80 (ddd, J = 25.33, 24.66, 17.19 Hz, 2H), 2.41-2.10 (m, 5H), 3.43-3.20 (m, 6H), 4.62-4.43 (m, 2H), 4.70 (s, 2H), 5.70-5.58 (m, 1H), 5.94-5.86 (m, 1H), 7.44-7.11 (m, 4H), 8.35-8.25 (d, 1H), 9.42 (s, 1H). HRMS (ESI) calcd for C24H31ClN3O6: [M−H]+: 492.1901 Found 492.2206.
Synthesis of aldehyde bisulfite salts 12–15. General procedure
To a solution of aldehyde 8–11 (0.20 mmol) in dry ethyl acetate (1.0 mL) was added absolute ethanol (0.5 mL) with stirring, followed by a solution of sodium bisulfite (0.20 mmol) in water (0.20 mL). The reaction mixture was stirred for 3 h at 50 °C, allowed to cool to room temperature, and then vacuum filtered. The solid was thoroughly washed with absolute ethanol and the filtrate was dried over anhydrous sodium sulfate, filtered, and concentrated to yield a yellowish oil. The oily product was treated with ethyl ether (2 × 5 mL) to form a white solid. The white solid was sequentially stirred with ethyl ether (3 mL) and ethyl acetate (1.5 mL) for 5 minutes. Careful removal of the solvent using a pipette left behind the product as a white solid.
Sodium (2S)-2-{(2S)-2-[5-(3-chlorobenzyl)-2-oxooxazolidin-3-yl]-4-methylpentan amido}-1-hydroxy-3-[(S)-2-oxopyrrolidin-3-yl] propane-1-sulfonate (12)
(A) White solid, mp 99–100°C, yield (67%),1H NMR (400 MHz, DMSO-d6) δ ppm 0.90-0.83 (d, 6H), 1.75-1.32 (m, 4H), 2.28-2.06 (m, 2H), 2.94-2.80 (m, 3H), 3.83-3.67 (m, 4H), 3.99-3.91 (m, 2H), 4.35-3.96 (m, 2H), 4.99-4.73 (m, 2H), 7.35 (d, J = 11.88 Hz, 1H), 7.75-7.62 (m, 3H), 8.50-8.47 (br. s, 1H). HRMS (ESI) calcd for C23H31ClN3O8S: [M−] +: 544.1526 Found 544.1537.
(B) White solid, mp 103–104°C, yield (70%),1H NMR (400 MHz, DMSO-d6) δ ppm 0.92-0.85 (d, 6H), 1.74-1.43 (m, 4H), 2.28-2.02 (m, 2H), 2.98-2.81 (m, 3H), 3.85-3.72 (m, 4H), 3.99-3.95 (m, 2H), 4.32-4.06 (m, 2H), 5.01-4.79 (m, 2H), 7.36 (d, 1H), 7.75-7.63 (m, 3H), 8.52-8.49 (br. s, 1H). HRMS (ESI) calcd for C23H31ClN3O8S: [M−] +: 544.1526 Found 544.1529.
Sodium (2S)-2-{(2S)-2-[5-(3-chlorobenzyl)-2-oxooxazolidin-3-yl]-3-cyclohexylprop anamido}-1-hydroxy-3-[(S)-2-oxopyrrolidin-3-yl] propane-1-sulfonate (13)
White solid, yield (67%), mp 123–124°C, 1H NMR (400 MHz, DMSO-d6) δ ppm 1.29-0.96 (m, 4H), 1.79-1.46 (m, 5H), 2.29-2.05 (m, 5H), 3.17-2.90 (m, 2H), 3.78-3.66 (m, 3H), 4.35-4.27 (m, 1H), 4.96-4.82 (br. s, 1H), 5.67-5.56 (m, 1H), 7.35 (d, J = 10.46 Hz, 1H), 7.66-7.63 (m, 3H), 8.47-8.40 (br. s, 1H). HRMS (ESI) calcd for C26H35ClN3O8S: [M−]+: 584.1839 Found 584.1858.
Sodium (2S)-2-[(2S)-2-{5-[(3-chlorophenoxy)methyl]-2-oxooxazolidin-3-yl}-4-methyl pentanamido]-1-hydroxy-3-[(S)-2-oxopyrrolidin-3-yl] propane-1-sulfonate (14)
White solid, yield (68%), mp 69–70°C, 1H NMR (400 MHz, DMSO-d6) δ ppm 0.92-0.82 (d, 6H), 1.21-1.02 (m, 1H), 2.36-1.88 (m, 7H), 3.21-2.94 (m, 5H), 4.06-3.64 (m, 2H), 4.55-4.10 (m, 2H), 5.06-4.86 (m, 2H), 7.03 (d, J = 9.48 Hz, 1H), 7.34-7.22 (m, 1H), 7.73-7.60 (m, 2H), 9.52-9.47 (br. s, 1H). HRMS (ESI) calcd for C23H31ClN3O9S: [M−] +: 560.1475 Found 560.1502.
Sodium (2S)-2-[(2S)-2-(5-{[(3-chlorobenzyl)oxy]methyl}-2-oxooxazolidin-3-yl)-4-methylpentanamido]-1-hydroxy-3-[(S)-2-oxopyrrolidin-3-yl]propane-1-sulfonate (15)
White solid, mp 40–41°C, yield (67%),1H NMR (400 MHz, DMSO-d6 δ 0.90-0.81 (dd, J = 18.13, 7.78 Hz, 6H), 1.71-1.40 (m, 1H), 2.21-2.00 (m, 6H), 3.20-2.90 (m, 2H), 3.64-3.54 (m, 3H), 3.78-3.64 (m, 2H), 4.55 (m, 1H), 4.85-4.75 (s, 2H), 7.39 (m, 2H), 7.71-7.63 (m, 2H), 8.51-8.46 (m, 1H). HRMS (ESI) calcd for C24H33ClN3O9S: [M−] +: 574.1632 Found 574.1658.
4.2 X-ray crystallographic studies. Crystallization and data collection
Purified norovirus 3CL protease (NV 3CLpro) (10 mg/mL) in 100 mM NaCl, 50 mM PBS buffer, pH 7.2, and 1 mM dithiothreitol (DTT) was used for the preparation of the enzyme:8B complex. A stock solution of 100 mM inhibitor 8B was prepared in DMSO and the NV 3CLpro:inhibitor complex was prepared by mixing 7 μL of inhibitor 8B (3mM) with 243 μL (0.49 mM) of NV 3CLpro and incubating on ice for 1 h. The buffer was exchanged with 100 mM NaCl, 20 mM Tris pH 8.0 using a Zeba spin desalting column (MWCO=7 kDa, Life Technologies) and the sample was concentrated to 11.0 mg/mL for crystallization screening. All crystallization experiments were conducted with Compact Jr. (Rigaku Reagents) sitting drop vapor diffusion plates at 20 °C using equal volumes of protein and crystallization solution equilibrated against 75 μL of the latter. Crystals that displayed a prismatic morphology were obtained in 2–3 days from the Index HT screen (Hampton Research) condition G2 (25% (w/v) polyethylene glycol 3350, 100 bis-Tris pH 5.5, 200 mM Li2SO4). Samples were transferred to a fresh drop composed of 80% crystallization solution and 20% (v/v) PEG 200 and stored in liquid nitrogen. X-ray diffraction data were collected at the Advanced Photon Source beamline 17-ID using a Dectris Pilatus 6M pixel array detector.
4.2.1 Structure Solution and Refinement
Intensities were integrated using XDS [41-k42] via Autoproc [43] and the Laue class analysis and data scaling were performed with Aimless [44]. Structure solution was conducted by molecular replacement with Phaser [45] using a previously determined isomorphous structure of inhibitor bound NV 3CLpro (PDB: 5T6D) [39] as the search model. Structure refinement and manual model building were conducted with Phenix [46] and Coot [47], respectively. Disordered side chains were truncated to the point for which electron density could be observed. Structure validation was conducted with Molprobity [48] and figures were prepared using the CCP4MG package [49].
4.3 FRET protease assays
The FRET protease assay was performed by preparing stock solutions of the substrate (Edans-DFHLQ/GP-Dabcyl) and inhibitor in DMSO and diluting into assay buffer which was comprised of 20 mM HEPES buffer, pH 8, containing NaCl (200 mM), 0.4 mM EDTA, glycerol (60%), and 6 mM dithiothreitol (DTT). The protease was mixed with serial dilutions of each compound up to 100 μM or with DMSO in 25 μL of assay buffer and incubated at 37 °C for 30 min, followed by the addition of 25 μL of assay buffer containing substrate. Fluorescence readings were obtained using an excitation wavelength of 360 nm and an emission wavelength of 460 nm on a fluorescence microplate reader (FLx800; Biotec, Winoosk, VT) for 1 h following the addition of substrate. Relative fluorescence units (RFU) were determined by subtracting background values (substrate-containing well without protease) from the raw fluorescence values, as described previously [31–36]. The dose-dependent FRET inhibition curves were fitted with a variable slope using GraphPad Prism software (GraphPad, La Jolla, CA) in order to determine the IC50 values of the inhibitors.
4.4 Cell-based inhibition assays
The effects of each inhibitor on virus replication were examined against NV in the NV replicon harboring cells (HG23 cells) [31]. Briefly, confluent and semi-confluent cells were incubated with medium containing DMSO (<0.1%) or each compound (up to 100 μM) for 48 h. After the incubation, total RNA was extracted and viral genome was quantitated with real-time quantitative RT-PCR (qRT-PCR). The EC50 values were determined by GraphPadPrism software [31].
4.5 Nonspecific cytotoxic effects
The cytotoxic dose for 50% cell death (CC50) for each compound was determined for HG23 cells. Confluent cells grown in 96-well plates were treated with various concentrations (1–100 μM) of each compound for 72 h. Cell cytotoxicity was measured using a CytoTox 96 nonradioactive cytotoxicity assay kit (Promega, Madison, WI) and crystal violet staining. The in vitro therapeutic index was calculated by dividing the CC50 by the EC50.
Supplementary Material
Table 2.
Crystallographic data for norovirus 3CL protease in complex with inhibitor 8B.
| NVPro:8B | |
|---|---|
| Data Collection | |
| Unit-cell parameters (Å, °) | a=b=59.56, c=357.55 |
| Space group | P6122 |
| Resolution (Å)a | 49.56-1.95 (2.00-1.95) |
| Wavelength (Å) | 1.0000 |
| Temperature (K) | 100 |
| Observed reflections | 542,337 |
| Unique reflections | 29,026 |
| <I/σ(I)>a | 18.1 (2.2) |
| Completeness (%)a | 100 (100) |
| Multiplicitya | 18.7 (19.6) |
| Rmerge (%)a,b | 13.4 (182.5) |
| Rmeas (%)a,d | 13.8 (187.0) |
| Rpim (%)a,d | 3.2 (41.7) |
| CC1/2 a,e | 0.999 (0.775) |
| Refinement | |
| Resolution (Å)a | 36.29-1.95 |
| Reflections (working/test) | 27,471/1,400 |
| Rfactor/Rfree (%)c | 18.8/22.9 |
| No. of atoms (Protein/Ligand/Water) | 2,430/48/147 |
| Model Quality | |
| R.m.s deviations | |
| Bond lengths (Å) | 0.010 |
| Bond angles (°) | 1.020 |
| Average B-factor (Å2) | |
| All Atoms | 35.1 |
| Protein | 34.7 |
| Ligand | 37.2 |
| Water | 41.5 |
| Coordinate error(maximum likelihood) (Å) | 0.18 |
| Ramachandran Plot | |
| Most favored (%) | 97.9 |
| Additionally allowed (%) | 2.1 |
Values in parenthesis are for the highest resolution shell.
Rmerge = ΣhklΣi |Ii(hkl) − <I(hkl)>|/ΣhklΣi Ii(hkl), where Ii(hkl) is the intensity measured for the ith reflection and <I(hkl)> is the average intensity of all reflections with indices hkl.
Rfactor = Σhkl ||Fobs (hkl) | − |Fcalc (hkl) ||/Σhkl |Fobs (hkl)|; Rfree is calculated in an identical manner using 5% of randomly selected reflections that were not included in the refinement.
The structure-guided design of a series of novel oxazolidinone-based inhibitors of norovirus 3CL protease inhibitors is reported.
Cell-permeable low micromolar inhibitors have been identified.
The mechanism of action and mode of binding of the inhibitors were unraveled using X-ray crystallography.
The interaction of the inhibitors with the enzyme is associated with structural rearrangements that impact pharmacological activity.
Acknowledgments
The generous financial support of this work by the National Institutes of Health (AI109039) is gratefully acknowledged. Use of the University of Kansas Protein Structure Laboratory was supported by a grant from the National Institute of General Medical Sciences (P30GM110761) of the National Institutes of Health. Use of the IMCA-CAT beamline 17-ID at the Advanced Photon Source was supported by the companies of the Industrial Macromolecular Crystallography Association through a contract with Hauptman-Woodward Medical Research Institute. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-06CH11357.
ABBREVIATIONS USED
- PK
pharmacokinetics
- DMF
N,N-dimethyl formamide
- DCM
dichloromethane
- CDI
carbonyl diimidazole
- TLC
thin layer chromatography
- EDCI
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
- HOBt
N-hydroxybenzotriazole
- DIEA
diisopropylethylamine
- DTT
dithiothreitol
- DMSO
dimethyl sulfoxide
- IC50
50% inhibitory concentration in the enzyme assay
- EC50
50% effective concentration in cell culture
- CC50
50% cytotoxic concentration in cell-based assays
- GESAMT
general efficient structural alignment of macromolecular targets
- rmsd
root mean-square deviation
- XDS
X-ray detector software
- ESI
electron spray ionization
- RFU
relative fluorescence units
Appendix A. Supplementary data
Supplementary data related to this article can be found at …….
Footnotes
Accession codes
Coordinates and structure factors were deposited to the Worldwide Protein Data Bank (wwPDB) with the accession code: 5WEJ
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koo HL, Ajami N, Atmar RL, DuPont HL. Noroviruses: the leading cause of gastroenteritis worldwide. Discov Med. 2010;10:61–70. [PMC free article] [PubMed] [Google Scholar]
- 2.Bartsch SM, Lopman BA, Ozawa S, Hall AJ, Lee BY. Global economic burden of norovirus gastroenteritis. PLoS One. 2016;11(4):e0151219. doi: 10.1371/journal.pone.0151219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopman BA, Steele D, Kirkwood CD, Parashar UD. The vast and varied global burden of norovirus: prospects for prevention and control. PLoS Medicine. 2016;13:e:1001999. doi: 10.1371/journal.pmed.1001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bok K, Green KY. Norovirus gastroenteritis in immunocompromised patients. New Engl J Med. 2012;367:2126–2132. doi: 10.1056/NEJMra1207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall AJ, Lopman BA, Payne DC, Patel MM, Gastanaduy PA, Vinje J, Parashar UD. Norovirus disease in the United States. Emerg Infect Dis. 2013;19:1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Center for Disease Control and Prevention/Norovirus/Prevent the spread of Norovirus. [access on June 5, 2017]; https://www.cdc.gov/features/norovirus/index.html.
- 7.Robilotti E, Deresinski S, Pinsky BA. Noroviruses. Clin Microbiol Rev. 2015;28:134–164. doi: 10.1128/CMR.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MKJ, Black RE. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8:e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y, Galasiti Kankanamalage AC, Chang K-O, Groutas WC. Recent advances in the discovery of norovirus therapeutics. J Med Chem. 2015;58:9438–9450. doi: 10.1021/acs.jmedchem.5b00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galasiti Kankanamalage AC, Weerawarna PM, Kim Y, Chang K-O, Groutas WC. Anti-norovirus therapeutics: a patent review (2010–2015) Expert Opin Ther Pat. 2016;26:297–308. doi: 10.1517/13543776.2016.1153065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkataraman Prasad BV, Shanker S, Muhaxhiri Z, Deng L, Choi J-M, Estes MK, Song Y, Palzkill T, Atmar RL. Antiviral targets of human noroviruses. Curr Opin Virol. 2016;18:117–125. doi: 10.1016/j.coviro.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weerasekara S, Prior AM, Hua DH. Current tools for norovirus drug discovery. Expert Opin Drug Discov. 2016;11:529–541. doi: 10.1080/17460441.2016.1178231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kocher J, Yuan L. Norovirus vaccines and potential anti-norovirus drugs: recent advances and future perspectives. Future Virol. 2015;10:899–913. doi: 10.2217/fvl.15.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha-Pereira J, Neyts J, Jochmans D. Norovirus: targets and tools in antiviral drug discovery. Biochem Pharmacol. 2014;91:1–11. doi: 10.1016/j.bcp.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karst SM, Tibbetts SA. Recent advances in understanding norovirus pathogenesis. J Med Virol. 2016;88:1837–1843. doi: 10.1002/jmv.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartnicki E, Cunha JB, Kolawole AO, Wobus CE. Recent advances in understanding noroviruses. F1000Research. 2017;6:79-. doi: 10.12688/f1000research.10081.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karst SM, Wobus CE. A working model of how noroviruses infect the intestine. PLoS Pathogens. 2015;11:e:1004626. doi: 10.1371/journal.ppat.1004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orchard RC, Wilen CB, Doench JG, Baldridge MT, McCune BT, Lee Y-CJ, Lee S, Pruett-Miller SM, Nelson CA, Fremont DH, Virgin HW. Discovery of a proteinaceous cellular receptor for a norovirus. 2016;353:933–936. doi: 10.1126/science.aaf1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haga K, Fujimoto A, Yakai-Todaka R, Miki M, Doan YH, Murakami K, Yokoyama M, Murata K, Nakanishi A, Katayama K. Functional receptor molecules CD300lf and CD300ld within the CD300 family enable murine noroviruses to infect cells. Proc Natl Acad Sci USA. 2016;113:E6248–E6255. doi: 10.1073/pnas.1605575113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karst SM. The influence of commensal bacteria on infection with enteric viruses. Nat Rev Microbiol. 2016;14:197–204. doi: 10.1038/nrmicro.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karst SM. Identification of a novel cellular target and a co-factor for norovirus infection – B cells and commensal bacteria. Gut Microbes. 2015;6:266–271. doi: 10.1080/19490976.2015.1052211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H, Hasan NM, In JG, Estes MK, Kovbasnjuk O, Zachos NC, Donowitz M. The contributions of human mini-intestines to the study of intestinal physiology and pathophysiology. Annu Rev Physiol. 2017;79:291–312. doi: 10.1146/annurev-physiol-021115-105211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taube S, Kolawole AO, Hohne M, Wilkinson JE, Handley SA, Perry JW, Thackray LB, Akkina R, Wobus CE. A mouse model for human norovirus. MBio. 2013;4:e00450–13. doi: 10.1128/mBio.00450-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KY. Caliciviridae: The Noroviruses. In: Knipe DM, editor. Field’s Virology. 6. Vol. 1. Lippincott Williams & Wilkins; Philadelphia, PA: 2013. pp. 582–608. [Google Scholar]
- 25.Thorne LG, Goodfellow IG. Norovirus gene expression and replication. J Gen Virol. 2014;95:278–291. doi: 10.1099/vir.0.059634-0. [DOI] [PubMed] [Google Scholar]
- 26.Karst SM, Wobus CE, Goodfellow IG, Green KY, Virgin HW. Advances in norovirus biology. Cell Host Microbe. 2014;15:668–680. doi: 10.1016/j.chom.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussey RJ, Coates L, Gill RS, Erskine PT, Coker SF, Mitchell E, Cooper JB, Broadbridge R, Clarke IN, Lambden PR, Shoolingin-Jordan PM. Structural study of norovirus 3C specificity: binding of a designed active site-directed peptide inhibitor. Biochemistry. 2011;50:240–249. doi: 10.1021/bi1008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardy ME, Crone TJ, Brower JE, Ettayebi K. Substrate specificity of the Norwalk virus 3C-like proteinase. Virus Res. 2002;89:29–39. doi: 10.1016/s0168-1702(02)00114-4. [DOI] [PubMed] [Google Scholar]
- 29.Muhaxhiri Z, Deng L, Shanker S, Sankaran B, Estes MK, Palzkill T, Song Y, Venkataram Prasad BV. Structural basis of substrate specificity and protease inhibition in Norwalk virus. J Virol. 2013;87:4281–4292. doi: 10.1128/JVI.02869-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomenclature used is that of L. Schechter. A Berger, Biochem Biophys Res Comm. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. where S1, S2, S3, …. Sn and S1′, S2′, S3′, …. Sn′ correspond to the enzyme subsites on the N-terminus and C-terminus side, respectively, of the scissile bond. Each subsite accommodates a corresponding amino acid residue side chain designated P1, P2, P3,…..Pn and P1′, P2′, P3′,…..Pn′ of a substrate or inhibitor. P1 is the primary substrate specificity residue and P1-P1′ is the scissile bond. [DOI] [PubMed] [Google Scholar]
- 31.Galasiti Kankanamalage AC, Kim Y, Weerawarna PM, Uy RA, Damalanka VC, Mandadapu SR, Alliston KR, Mehzabeen N, Battaille KP, Lovell S, Chang K-O, Groutas WC. Structure-guided design and optimization of norovirus 3CL protease. Structure-activity relationships and biochemical, X-ray crystallographic, cell-based and in vivo studies. J Med Chem. 2015;58:3144–3155. doi: 10.1021/jm5019934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damalanka VC, Kim Y, Alliston KR, Weerawarna PM, Galasiti Kankanamalage AC, Lushington GH, Mehzabeen N, Battaille KP, Lovell S, Chang K-O, Groutas WC. Oxadiazole-based cell permeable macrocyclic transition state inhibitors of norovirus 3CL protease. J Med Chem. 2016;59:1899–1913. doi: 10.1021/acs.jmedchem.5b01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weerawarna PM, Kim Y, Galasiti Kankanamalage AC, Damalanka VC, Lushington GH, Alliston KR, Mehzabeen N, Battaille KP, Lovell S, Chang K-O, Groutas WC. Structure-based design and synthesis of triazole-based macrocyclic inhibitors of norovirus protease: structural, biochemical, spectroscopic, and antiviral studies. Eur J Med Chem. 2016;119:300–318. doi: 10.1016/j.ejmech.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, Lovell S, Tiew KC, Mandadapu SR, Alliston KR, Battaille KP, Groutas WC, Chang KO. Broad-spectrum antivirals against 3CL or 3C-like proteases of picornaviruses. noroviruses and coronaviruses. J Virol. 2012;6:1754–1762. doi: 10.1128/JVI.01348-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galasiti Kankanamalage AC, Kim Y, Rathnayake AD, Alliston KR, Butler MM, Cardinale SC, Bowlin TL, Groutas WC, Chang CO. Design, synthesis and evaluation of novel prodrugs of transition state inhibitors of norovirus 3CL protease. J Med Chem. 2017;60:6239–6248. doi: 10.1021/acs.jmedchem.7b00497. [DOI] [PubMed] [Google Scholar]
- 36.Damalanka VC, Kim Y, Galasiti Kankanamalage AC, Lushington GH, Mehzabeen N, Battaille KP, Lovell S, Chang KO, Groutas WC. Design, synthesis and evaluation of a novel series of macrocyclic inhibotors of norovirus 3CL protease. Eur J Med Chem. 2017;127:41–61. doi: 10.1016/j.ejmech.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng L, Muhaxhiri Z, Estes MK, Palzkill T, Venkataram Prasad BV, Song Y. Synthesis, activity, and structure-activity relationship of noroviral protease inhibitors. MedChemComm. 2013;4:1354–1359. doi: 10.1039/C3MD00219E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webster SE, Okano K, Little TL, Reich SH, Xin Y, Fuhrman SA, Matthews DA, Love RA, Hendrickson TF, Patick AK, Meador JW, Ferre RA, Brown EL, Ford CE, Binford SL, Worland ST. Tripeptide aldehyde inhibitors of human rhinovirus 3C protease: design, synthesis, biological evaluation, and cocrystal structure solution of P1 glutamine isosteric replacements. J Med Chem. 1998;41:2786–2805. doi: 10.1021/jm980071x. [DOI] [PubMed] [Google Scholar]
- 39.Galasiti Kankanamalage AC, Kim Y, Rathnayake AD, Damalanka VC, Weerawarna PM, Doyle ST, Alsoudi AF, Padmasankha Dissayanake DM, Lushington GH, Mehzabeen N, Battaille KP, Lovell S, Chang KO, Groutas WC. Structure-based exploration and exploitation of the S4 subsite of norovirus 3CL protease in the design of potent and permeable inhibitors. Eur J Med Chem. 2017;126:502–516. doi: 10.1016/j.ejmech.2016.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krissinel E. Enhanced fold recognition using efficient short fragment clustering. J Mol Biochem. 2012;1:76–85. [PMC free article] [PubMed] [Google Scholar]
- 41.Kabsch W. Automatic Indexing of Rotation Diffraction Patterns. J Appl Crystallogr. 1988;21:67–72. [Google Scholar]
- 42.Kabsch W. Xds Acta Crystallogr D Biol Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vonrhein C, Flensburg C, Keller P, Sharff A, Smart O, Paciorek W, Womack T, Bricogne G. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt4):293–302. doi: 10.1107/S0907444911007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans PR. An introduction to data reduction: space-group determination, scaling and intensity statistics. Acta Crystallogr D Biol Crystallogr. 2011;67:282–292. doi: 10.1107/S090744491003982X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn M, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Cryst. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams PD, Afonine PV, Bunkoczi G, Chen VB, David IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallogr Sect D: Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr Sect D: Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr Sect D: Biol Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Potterton L, McNicholas S, Krissinel E, Gruber J, Cowlan K, Emlsey P, Murshudov GN, Cohen S, Perrakis A, Noble M. Developments in the CCP4 molecular graphics project. Acta Crystallogr Sect D: Biol Crystallogr. 2004;60:2288–2294. doi: 10.1107/S0907444904023716. [DOI] [PubMed] [Google Scholar]
- 50.Evans P. Scaling and assessment of data quality. Acta Crystallogr Sect D: Biol Crystallogr. 2006;62:72–82. doi: 10.1107/S0907444905036693. [DOI] [PubMed] [Google Scholar]
- 51.Diederichs K, Karplus PA. Improved R-factors for diffraction data analysis in macromolecular crystallography. Nat Struct Biol. 1997;4:269–275. doi: 10.1038/nsb0497-269. [DOI] [PubMed] [Google Scholar]
- 52.Weiss MS. Global indicators of X-ray data quality. J Appl Crystallogr. 2001;34:130–135. [Google Scholar]
- 53.Karplus PA, Diederichs K. Linking crystallographic model and data quality. Science. 2012;336:1030–1033. doi: 10.1126/science.1218231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans P. Biochemistry. Resolving some old problems in protein crystallography. Science. 2012;336:986–987. doi: 10.1126/science.1222162. [DOI] [PubMed] [Google Scholar]
- 55.Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov N, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD. Towards automated crystallographic structure refinement with phenix. refine. Acta Crystallogr. 2012;60:352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.