Abstract
Background
The increasing use of ketamine as a potential rapid-onset antidepressant necessitates a better understanding of its effects on blood pressure and heart rate, well-known side effects at higher doses. For the subanesthetic dose used for depression, potential predictors of these cardiovascular effects are important factors influencing clinical decisions. Since ketamine influences the sympathetic nervous system, we investigated the impact of autonomic nervous system-related factors on the cardiovascular response: a genetic polymorphism in the norepinephrine transporter and gender effects.
Methods
Blood pressure and heart rate were monitored during and following administration of a subanesthetic dose of ketamine or placebo in 68 healthy participants (mean age 26.04 ±5.562 years) in a double-blind, randomized, controlled, parallel-design trial. The influences of baseline blood pressure/heart rate, gender, and of a polymorphism in the norepinephrine transporter gene (NET SLC6A2, rs28386840 [A-3081T]) on blood pressure and heart rate changes were investigated. To quantify changes in blood pressure and heart rate, we calculated the maximum change from baseline (ΔMAX) and the time until maximum change (TΔMAX).
Results
Systolic and diastolic blood pressure as well as heart rate increased significantly upon ketamine administration, but without reaching hypertensive levels. During administration, the systolic blood pressure at baseline (TP0Sys) correlated negatively with the time to achieve maximal systolic blood pressure (TΔMAXSys, P<.001). Furthermore, women showed higher maximal diastolic blood pressure change (ΔMAXDia, P<.001) and reached this peak earlier than men (TΔMAXDia, P=.017) at administration. NET rs28386840 [T] carriers reached their maximal systolic blood pressure during ketamine administration significantly earlier than [A] homozygous (TΔMAXSys, P=.030). In a combined regression model, both genetic polymorphism and TP0Sys were significant predictors of TΔMAXSys (P<.0005).
Conclusions
Subanesthetic ketamine increased both blood pressure and heart rate without causing hypertensive events. Furthermore, we identified gender and NET rs28386840 genotype as factors that predict increased cardiovascular sequelae of ketamine administration in our young, healthy study population providing a potential basis for establishing monitoring guidelines.
Keywords: ketamine, risk factors, blood pressure, gender, norepinephrine transporter
Significance Statement
In our study, we investigated potential predictors of the cardiovascular response to single subanesthetic dose of ketamine in a healthy study population. Blood pressure and heart rate were monitored during and following administration of ketamine or placebo in 68 participants. Our findings revealed that norepinephrinetransporter (NET) genotype, gender, and baseline blood pressure are factors predicting increased cardiovascular sequelae upon ketamine administration. NET rs28386840 [T] carriers reached their maximal systolic blood pressure during ketamine administration significantly earlier than [A] homozygous carriers. Both genetic polymorphism and baseline systolic blood pressure were significant predictors of the time to achieve maximal systolic blood pressure. Additionally, women displayed higher maximal diastolic blood pressure change and reached this peak earlier compared with men at administration. The increasing application of ketamine in psychiatric therapy underlines the relevance of our work and indicates that the results presented here should be further assessed in a prospective clinical trial.
Introduction
Ketamine is widely used as an anesthetic in surgery and emergency medicine. Treatment potential at subanesthetic doses for indications such as pain or depression has been explored with studies showing promising effects including meta-analyses (Zarate et al., 2006; Mathew et al., 2012; Caddy et al., 2014; Fond et al., 2014; McGirr et al., 2015).
Based on those previous findings, ketamine has been suggested as a therapeutic option in treatment-resistant depression, administered i.v. at a low dose, such as 0.5 mg/kg over 40 minutes (Schwartz et al., 2016). High-dose ketamine administration leads to an elevation of heart rate and mean pulmonary arterial and aortal pressure (Tweed et al., 1972). These effects have been investigated in the context of anesthesia, where continuous monitoring and counteracting of adverse cardiovascular effects with other medication are available. However, little is known about the importance of these effects in a healthy population being treated with the doses used in antidepressive therapy and, moreover, whether particular individuals are at greater risk than others. Our aim was to provide baseline data to inform an individual risk assessment, estimating rapid administration effects and possible cardiovascular risk factors.
The physiological mechanisms that mediate the adverse cardiovascular effects of ketamine are thus far unclear. In general, transient changes in blood pressure are induced by the baro-reflex, which directly influences cardiac output and vascular resistance, to maintain blood pressure at nearly constant levels (Benarroch, 2008). The most important transmitter of this blood pressure regulation system is norepinephrine (NE). The NE transporter (NET) is responsible for the reuptake of NE into the presynaptic nerve cell (Schroeder and Jordan, 2012).
It has been recognized that a prominent aspect of the action of ketamine is its influence on the sympathetic nervous system (Tweed, 1972), and there is evidence in the literature that ketamine inhibits the NET. Patients undergoing minor surgical procedures showed elevated NE blood levels after ketamine administration (Baraka et al., 1973). Reuptake of NE in the heart was reduced following ketamine administration in rats (Miletich et al., 1973). Bovine NET was inhibited in vitro by ketamine via a target that also acts as the binding site for desipramine, a predominant NET inhibitor (Hara et al., 1998). Furthermore, the expression of NET is upregulated after long-term administration of ketamine (Hara et al., 2002). In human embryonic kidney cells, ketamine inhibited NET expression (Nishimura et al., 1998), and desipramine reduced the inhibitory effect of ketamine on NET, suggesting that ketamine might be a potential NET competitive antagonist (Zhao and Sun, 2008). We hypothesized that the elevation of blood pressure during ketamine administration would be at least in part mediated by inhibition of the NET following a higher NE concentration.
In the current study, we aimed to identify factors contributing to the adverse cardiovascular effects of ketamine. We proposed that factors that influence components of the autonomous nervous system would also have an impact on the adverse effects of ketamine. One such factor is already known. The alpha-2 adre nergic receptor agonist clonidine partially inhibited the blood pressure increase (Lenze et al., 2016). We postulated that other mediators of autonomous nervous system tone such as gender and genetic effects could also influence the side effects.
Since the sympathetic activity is related to the blood pressure at rest (Joyner et al., 2010), we firstly investigated whether the baseline blood pressure/heart rate would be associated with blood pressure or heart rate change after ketamine infusion.
Genetic polymorphisms in components of the noradrenergic system, like NET, also influence the sympathetic nervous system, as shown in the association between NET and blood pressure: essential hypertension occurs more often in people with a certain NET genotype (Li et al., 2013). The T-allele of a promoter region NET gene polymorphism (NET SLC6A2, rs28386840) is common in patients suffering from attention deficit hyperactivity disorder and drives decreased NET gene transcriptional expression (Kim et al., 2006, 2008). The T-allele of the rs28386840 polymorphism in particular led to elevated blood pressure on physical exercise (Kohli et al., 2011). Furthermore, the T-allele was associated with a greater heart rate elevation after medication with methylphenidate, a first-line agent for treating attention deficit hyperactivity disorder (Cho et al., 2012). We hypothesized that systolic blood pressure in at-risk subjects ([T]-carriers) would show a stronger reaction to ketamine, because of the decreased expression of NET transporter in the [T]-carriers (Kim, 2006) and thus reduced NE reuptake capacity at the effector cells.
In trials investigating the effect of gender on the sympathetic nervous system, baseline muscle sympathetic nerve activity has been shown to be higher in men than in women (Usselman et al., 2014), while a relationship between sympathetic activity and total peripheral resistance exists in men but not in women (Hart et al., 2009). Women show lower vasoconstriction in response to NE than men (Kneale et al., 2000), as well as an altered baroreflex response to carotid artery (Arteria carotis) hypertension (Kim et al., 2011). To date, little is known about gender effects of ketamine administration in humans; however, animal studies have shown that gonadal hormones may potentially facilitate the antidepressant-like effects of ketamine in female rats (Carrier and Kabbaj, 2013). The present study included both male and female subjects; therefore, a potential gender effect could be investigated.
In summary, a deeper understanding of potential influencing factors such as baseline cardiovascular response, NET rs28386840, and gender, including the mechanisms of action of ketamine and its side effects, are essential for safe ketamine administration. Our investigation focused on the low-dose application due to its antidepressive effects, and we proposed the following hypotheses.
Summary of Our Hypotheses
(1) Arterial blood pressure and heart rate increase after ketamine, even at subanesthetic doses; (2) Baseline arterial blood pressure/heart rate is associated with the arterial blood pressure or heart rate change after ketamine infusion; (3) Systolic arterial blood pressure responds more strongly to ketamine in high-risk subjects (NET SLC6A2, rs28386840 [T]-carriers) compared with low-risk subjects ([A] homozygous carriers); and (4) The effect of ketamine on arterial blood pressure differs between men and women.
Methods and Materials
Participants
The study was conducted as a double-blind, controlled, randomized, parallel-design trial. Our investigation of physiological parameters was embedded in an MRI study of a single-dose ketamine effect. A total 68 participants (38 men) were recruited by public advertisement. Mean age in the verum (ketamine) group (n=35) was 26.03 years (SD 5.90) with a mean body mass index (BMI) of 24.01 (SD 2.82). Mean age in the placebo group was 26.06 years (SD 5.26) with a mean BMI of 23.84 (SD 3.18). Participants were in a state of good general health (as determined by medical history, physical examination, blood laboratory tests, electrocardiography, and toxicology findings). Exclusion criteria included illicit drug abuse, regular medication, and excessive caffeine intake. MRI exclusion criteria included tattoos and metal implants. All subjects completed the German version 5.0.0 of the Mini International Neuropsychiatric Interview (Ackenheil et al., 1999) and underwent additional interview by the study physician, a board-certified psychiatrist (Prof. M. Walter). For group allocation of the participants, a computer-generated randomization list (Pahlke et al., 2004) was used. None of the participants, staff, or the study physician were informed about the group assignment. The study was approved by the institutional ethical review board of the University of Magdeburg, and all subjects gave written, informed consent according to the Declaration of Helsinki. The trial was registered under EudraCT number 2010-023414-31.
Procedure
Before ketamine infusion, a 60-minute baseline MRI scan was acquired, followed by collection of blood samples and insertion of an intravenous catheter. Systolic/diastolic arterial blood pressure (RR) and heart rate at baseline (TP0) were measured in the supine position with a single recording immediately prior to starting the infusion (Saegeling Invivo MAGNITUDE 3150).
Upon the beginning of the ketamine or placebo infusion in a resting, supine position, RR, and heart rate parameters were automatically measured every 5 minutes for 40 minutes. Participants were permitted to move freely afterwards. 60 minutes after baseline, the RR was measured again followed by the post-ketamine/placebo MRI scan for 60 minutes. Finally, a RR measurement was conducted 120 minutes after baseline.
Ketamine was injected with a concentration of 0.5 mg/kg body weight as ±racemate (Ketamine-ratiopharm 500 mg/10 mL, Ratiopharm). The prefilled syringes were supplied by the hospital pharmacy either as verum (ketamine) or placebo (NaCl 0.9%, Berlin-Chemie Isotone NaCl 0.9%). The 50 mL were infused over 40 minutes using a Fresenius Injectomat 2000.
Genotyping
Genomic DNA was extracted from EDTA-anticoagulated venous whole blood using the GeneMole automated DNA extraction system (Mole Genetics) according to the manufacturer’s protocol. Genotyping was performed using PCR-based allele-specific restriction fragment length analysis. The DNA sequence flanking rs28386840 in the SLC6A2 promoter region on Chr 16 was amplified using standard PCR methods (forward-primer: 5’-GCT GGG AAG TTG ACA CTC TGG GGG-3’; reverse-primer: 5’-GGA GAT AAT CCT GGA AGC AAT CGT TGG G-3’; further details available upon request) and digested with the BsrI isoschizomer BseNI (Thermo Fisher Scientific). The resulting fragments (126 bp + 104 bp for the A allele; 231 bp for the T allele) were separated on an ethidium bromide-stained agarose gel and visualized under UV light.
The allele distribution in our cohort of participants was as follows: [AA] (n=33), T carriers ([AT]+[TT], n=31); placebo group: [AA] (n=17), T carriers (n=13); ketamine group: [AA] (n=16) and T carriers (n=18). A previous study of rs28386840 showed a nearly 1:1 distribution of [A] homozygous ([AA]) to T carriers ([AT], [TT]) in a European-American population (Kohli, 2011).
Statistics
All statistical analyses were carried out with SPSS version 20. The Shapiro-Wilk test was used to test for normality of the sample distribution, and the Levene’s test was applied to verify homogeneity of variances to ensure the validity of analysis of variance calculations.
To examine the effect of ketamine on systolic/diastolic blood pressure and heart rate, 3 separate MANOVAs were carried out to compare the ketamine with the placebo group (hypothesis I). We used the measurement points 0 minute to 40 minutes because of the constant measurement conditions (supine, resting participants). Follow-up ANOVAs for single timepoints within this observation period were corrected for multiple comparisons with Holm’s sequential Bonferroni method. The between-group effects for measurement points 60 minutes and 120 minutes were tested using separate 1-way ANOVAs. Due to missing measurements at TP0, TP5 was chosen to provide a baseline for the heart rate analysis. Too few heart rate measurements were documented at TP60 and TP120, resulting in necessary exclusion of these timepoints.
To elucidate whether blood pressure/heart rate changed significantly from baseline, we additionally computed mixed-design ANOVAs with the 2 factors time (0 minutes, 40 minutes) and group (ketamine, placebo), applying the Greenhouse-Geisser correction followed by simple effect analysis (Bonferroni-corrected). For heart rate analysis, we used timepoints 5 and 40 minutes. This method allowed us not only to reveal a difference between the 2 treatment groups but also to show an increase/decrease within the groups compared with the initial values before treatment.
To identify factors influencing the physiological parameters, we defined 2 variables to describe the blood pressure and heart rate curves yielding a single value for each participant. The maximal change in the curve during the observation period (ΔMAX) and the time to achieve this maximal change (TΔMAX). Both have unique clinical predications and have been described in the literature as providing a reliable statistical analysis of serial measurements (Matthews et al., 1990) (supplementary Figure 1).
Pearson’s correlation was calculated to study the relationship between the baseline (TP0) of systolic/diastolic blood pressure and heart rate and the ΔMAX and TΔMAX (hypothesis II).
To investigate the effect of the NET polymorphism, a mixed-design ANOVA was carried out (group factor: ketamine, placebo; genetic factor: [AA], T-carrier) for the systolic blood pressure (ΔMAXSys and TΔMAXSys) followed by a simple effect analysis (Bonferroni-corrected). To assess potential gender related differences, we performed a mixed-design ANOVA (group factor: ketamine, placebo; gender factor: male, female; time factor: TP 0–40 minutes) for the diastolic blood pressure. Additionally, we examined ΔMAXDia and TΔMAXDia for the gender factor separately for the ketamine and placebo groups in a simple effect analysis (hypotheses III, IV).
Finally, we performed a multiple linear regression analysis with the independent factors NET polymorphism and baseline systolic blood pressure at timepoint 0 (TP0Sys) on the dependent factor TΔMAXSys.
Results
Effect of Ketamine on Blood Pressure and Heart Rate
Significant time by group interactions were observed with respect to the systolic (RRSys) and diastolic blood pressure (RRDia) and heart rate (HR) when comparing the baseline with the 40 minutes postinfusion (TP40) values (RRSys [F1,65=42.160, P<.001, part. η2=0.393], RRDia [F1,65=30.060, P<.001, part. η2=0.316], HR [F1,65=6.550, P<.013, part. η2=0.092]).
Posthoc tests showed an increase in RRSys, RRDia, and HR in the ketamine group (RRSys TP40: 132.09 mmHg; 95%CI [127.98, 136.20]; baseline: 122.97 mmHg; 95%CI [118.76, 127.19] (F1,65=24.721, P<.001, part. η2=0.276) supplementary Figure 2, RRDia TP40: 79.00 mmHg; 95%CI [76.00, 82.00] compared with baseline: 71.44 mmHg; 95%CI [67.75,75.13] (F1,65=21.38, P<.001, part. η2=0.248) supplementary Figure 3, HR TP40: 73.24, 95%CI [69.40, 77.07] compared with baseline: 67.74; 95%CI [63.66, 71.81] (F1,65=13.010, P=.001, part. η2=0.167) (supplementary Figure 4).
The ketamine group effect on systolic and diastolic arterial blood pressure was still present 60 minutes after the start of the infusion, but absent by time point 120 minutes (RRSys TP60 minutes: ANOVA, F1,60=13.621, P<.001; partial η2=0.185, RRSys TP120 minutes: ANOVA, F1,47=1.982, P=.166, partial η2=0.040, RRDia TP60: ANOVA, F1,60=11.055, P=.002; part. η2=0.156, RRDia TP120: ANOVA, F1,47=2.626, P=.112, part. η2=0.053).
Detailed information regarding the systolic and diastolic blood pressure curve, with each individual time-point of the 40-minute administration period, is provided in Figures 1 and 2. A detailed heart rate curve is available in the supplementary material (supplementary Figure 5).
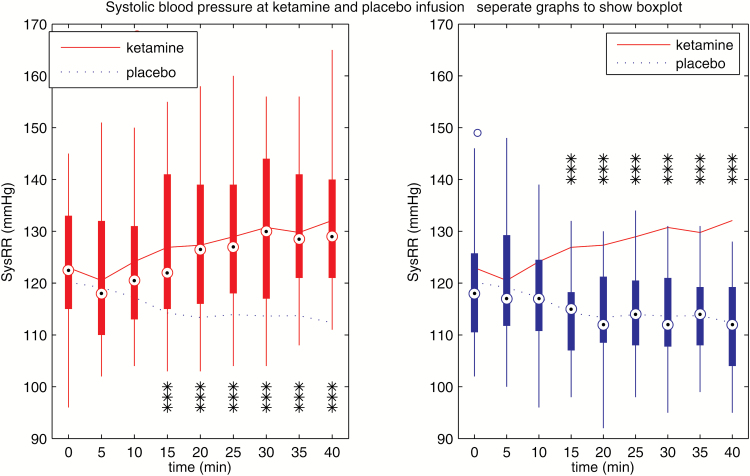
Figure 1.
Systolic blood pressure during ketamine and placebo infusion, separately to show boxplot. Systolic blood pressure was higher in the ketamine group beginning at timepoint 15 minutes (***P<.001).
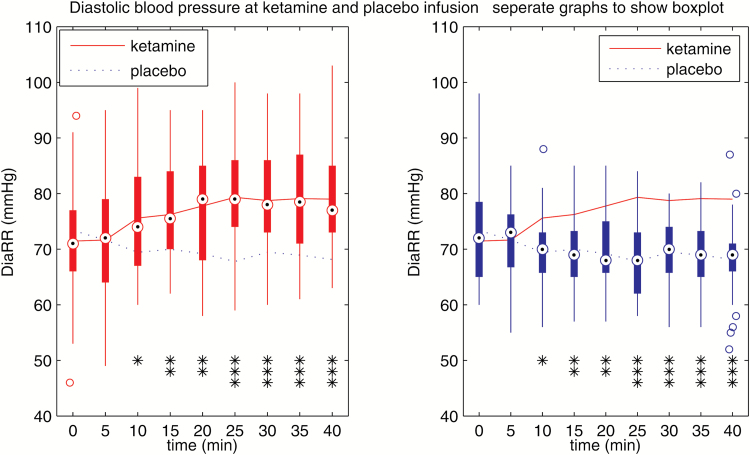
Figure 2.
Diastolic blood pressure during ketamine and placebo infusion, separately to show boxplot. Diastolic blood pressure was higher in the ketamine group beginning at timepoint 10 minutes (*P<.05, **P<.01, ***P<.001).
In summary, systolic/diastolic arterial blood pressure and heart rate increased in response to ketamine infusion. The ketamine effect was initially observed 10 minutes after the start of the infusion and was no longer present 2 hours after start of the infusion.
The maximal changes in the RRSys, RRDia, and HR curves in the observation period (ΔMAX), and the time to achieve them (TΔMAX), are summarized using mean values of ΔMAX and TΔMAX (see Table 1).
Table 1.
Means and Highest Value (Underlined) of Maximal Blood Pressure and Heart Rate Change in Participants during Ketamine/Placebo Infusion (ΔMAX) and Mean Time to the Maximal Blood Pressure and Heart Rate Change (TΔMAX)
| Ketamine | ||
|---|---|---|
| ΔMAXSys | ΔMAXDia | ΔMAXHr |
| 13.38 mmHg (27 mmHg, ± 7.30) | 12.65 mmHg (33 mmHg, ± 7.57) | 10.69 beats/min (32 beats/min, ± 6.66) |
|
Gender effect
18.54 mmHg (female) (± 6.79) 10.06 mmHg (male) (± 4.99) P<.001 | ||
| TΔMAXSys | TΔMAXDia | TΔMAXHr |
| 28.17 min (± 9.42) | 27.66 min (± 9.16) | 29.39 min (± 9.25) |
|
NET polymorphism effect
25.00 min (T) (± 8.86) 32.50 min (AA) (± 8.26) P=.030 |
Gender effect
23.85 min (female) (± 8.70) 31.67 min (male) (± 6.42) P=.017 |
|
| Placebo | ||
| ΔMAXSys | ΔMAXDia | ΔMAXHr |
| 6.33 min (± 5.16) | 4.67 min (± 5.13) | 7.10 min (± 5.00) |
| TΔMAXSys | TΔMAXDia | TΔMAXHr |
| 15.00 min (± 12.25) | 18.10 min (± 13.55) | 24.31 min (± 11.00) |
Significant gender and genetic differences shown in italics.
Baseline Effects on Blood Pressure Response
The baseline systolic blood pressure at timepoint 0 (TP0Sys) negatively correlated with the TΔMAXSys (2-sided Pearson-correlation r=-.601, n= 32, P<.001). The higher the systolic blood pressure at the baseline, the earlier the participants reached their maximum systolic blood pressure after ketamine. ΔMAXSys showed no correlation with TP0Sys. Furthermore, no baseline effects were present in the diastolic blood pressure and heart rate.
NET Polymorphism Effects on Blood Pressure Response
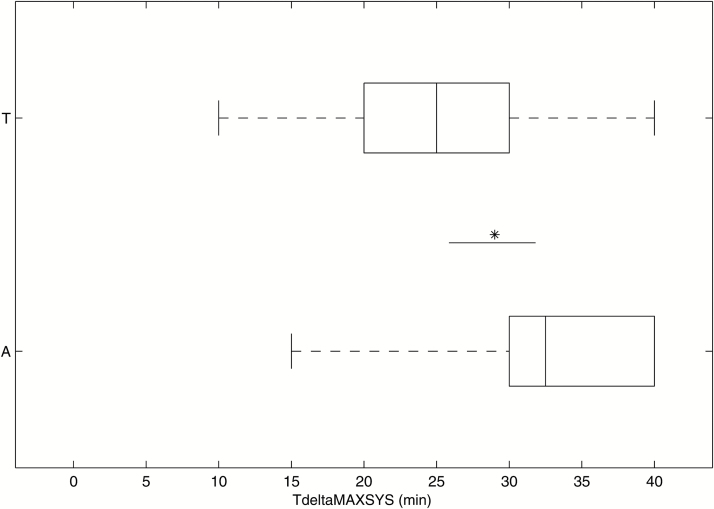
We observed an interaction between group (ketamine, placebo) and polymorphism variant ([AA], T-carrier) factors on the time to reach the maximal systolic blood pressure (TΔMAXSys) (F1,36=6.682 P=.014). In the pairwise comparison of the means with a simple effects analysis, there was a genetic effect in the ketamine group, with T-carriers reaching their maximal systolic blood pressure earlier (n=14; 25.00 minutes; 95%CI [20.33, 29.67]) than the [AA]-Carriers (n=15; 32.50 minutes; 95%CI [27.67, 37.33]) (Bonferroni-corrected F1,36=5,129, P=.030, part. η2=0.125). In the placebo group, no genetic effect was observed. The result is illustrated in Figure 3 (see supplementary Figure 6 for data curves).
Figure 3.
Time to reach the maximal systolic blood pressure (TΔMAXSys) in the ketamine group for [A] homozygous carriers and [T] carriers. T-carriers achieved their maximal systolic blood pressure earlier than [A] homozygous carriers (P =.030) (*P<.05).
There was no difference in the value of maximal systolic blood pressure change after ketamine (ΔMAXSys) as a function of the polymorphism.
At baseline (TP0Sys), there was no effect of the polymorphism variant on the systolic blood pressure.
Gender Effects on Blood Pressure Response
In the mixed-design ANOVA of the raw diastolic blood pressure, there was a time (TP0-40, 9 timepoints) by group (ketamine, placebo) interaction (F8,63=17.041, P<.001, part. η2=0.213) and a time by gender interaction (F8,63=3.403, P=.001, part. η2=0.051) (supplementary Figure 7). To reveal what drives the time by gender interaction, we split the sample according to group. There was a highly significant time by gender interaction in the ketamine group (F5.520,176.630=3.279, Greenhouse-Geisser correction applied, P=.005, part. η2=0.093) that was not present in the placebo group. This finding indicates an influence of gender on the increase of the diastolic blood pressure after the ketamine infusion.
ΔMAXDia showed higher values in women (18.54 mmHg; 95%CI [15.41, 21.67], maximum 33 mmHg) than in men (10.06 mmHg; 95%CI [7.40, 12.72], maximum 19 mmHg) in the ketamine group (Bonferroni-corrected F1,40=17.403, P<.001, partial η2=0.303). There was no difference in the placebo group.
Women reached their maximal diastolic blood pressure change (ΔMAXDia) earlier (23.85 minutes; 95%CI [18.99, 28.70]) compared with men (31.67 minutes; 95%CI [27.54, 35.79]) in the ketamine group (Bonferroni-corrected F1,40=6.156, P=.017, part. η2=0.133). There was no difference in the placebo group.
No gender by group or by time interaction was found in the systolic blood pressure. For a summary of the gender and genetic effects on the blood pressure see Table 1.
Genetic Polymorphism and Cardiovascular Values at TP0 as Predictors of TΔMAXSys
We calculated a multiple linear regression to predict the time to the maximal systolic blood pressure (TΔMAXSys) according to the NET polymorphism and TP0Sys in the ketamine group. A significant regression equation was found (F2,26=13.001, P<.0005, R2=0.500, Durbin-Watson 2.167). TΔMAXSys in our participants was modeled by equation 81.480-0.402(TP0Sys)-7.039(genetic), where TP0Sys is given in mmHg and the genetic factor is denoted as 0=[A]-homozygous and 1=T-carriers. TΔMAXSys decreased 0.40 minutes for each mmHg at TP0Sys, and for a further 7.04 minutes for T carriers.
Both TP0Sys (P<.001) and NET variant (P<.01) were significant predictors for TΔMAXSys. The baseline systolic RR had a slightly stronger effect on TΔMAXSys than the genetic polymorphism (standardized coefficients: -0.575[TP0Sys] and -0.387[genetic]). For an illustration see supplementary Figure 8.
Discussion
We provide detailed information about the arterial blood pressure and heart rate rise following a subanesthetic dose of ketamine administered over 40 minutes. Based on the hypothesis that the sympathetic nervous system plays an important role in blood pressure regulation during ketamine administration, we were able to identify 3 factors predictive of the degree or of the time course of blood pressure change: the baseline systolic blood pressure, NET rs28386840 and gender.
Ketamine increased the arterial blood pressure over a duration of 60 minutes and by a mean maximum degree of 13 mmHg both systolic and diastolic in our participants. The increase was visible only within 2 hours after ketamine administration, reflecting the time period within which ketamine-related side effects must be expected. This observation is consistent with previous findings (Diazgranados et al., 2010; Valentine et al., 2011; Ibrahim et al., 2012; Luckenbaugh et al., 2014). An elevated blood pressure arises to a risk factor at a certain degree. Hypertension is commonly defined as blood pressure of 140/90 mmHg or higher (Mancia et al., 2014). While ketamine-related elevation in blood pressure and heart rate in our participants was within a moderate range, with no outliers reaching concerning levels, our participant group comprised healthy individuals under the age of 35 years. The impact is potentially greater, however, when treating patients with initially high blood pressure. Furthermore, the triggering of a hypertensive crisis is not implausible and should be kept in mind (Aggarwal, 2006).
As predictors for the ketamine-induced blood pressure rise, we firstly identified baseline systolic blood pressure as a predictor for further blood pressure increase. Our results show that the higher the systolic blood pressure at baseline, the earlier the participants reached their maximum systolic blood pressure after ketamine. In a study in which ketamine was used for general anesthesia, the increases in blood pressure and heart rate were greater and occurred earlier during ketamine infusion (Lilburn et al., 1978). Moreover, individuals in the prehypertension range (120–129 mm/Hg systolic) are at increased risk of developing hypertension (>140 mm Hg systolic) (Pickering, 2007).
Hypertension may be further exacerbated in the surgical environment due to noxious stimuli such as intubation (Marlow et al., 1991). We note that the systolic blood pressure recorded in the present study was not equal to the clinical blood pressure and only represented the physiological condition at a certain time point, but nevertheless subjects with a higher baseline systolic blood pressure may have had higher stress levels before ketamine administration, which could potentially explain a higher sensitivity to ketamine. The blood pressure response following NET inhibitors is described as a consequence of increased NE levels in the periphery that predominate over a simultaneous centrally mediated lowered sympathetic tone (Esler et al., 1991). The participants with high baseline blood pressure may generally possess reduced central counter mechanisms apparent in a higher blood pressure in an acute psychological stress condition (Carroll et al., 2001) and may thus be more susceptible for the peripheral NE rise.
Next, we observed that a genetic variation in the NET has an influence on the time course of systolic blood pressure change, with a faster rise associated with carriers of the rs28386840 [T] allele. This is consistent with the results of previous studies investigating the same NET genotype, which showed a stronger reaction in the systolic blood pressure or heart rate for the [T] carriers on physical exercise (Kohli, 2011) and following methylphenidate (Cho, 2012), both initiating sympathetic activation. It remains unclear whether a peripheral elevation of NE through NET inhibition or a central influence of ketamine on noradrenergic (Schroeder, 2012) or glutamatergic transmitter systems (Boyer et al., 1998; Berman et al., 2000; Duman and Aghajanian, 2012) predominantly influences the cardiovascular side effects. But in line with our hypothesis, we interpreted the observed rapid blood pressure response as being a consequence of higher NE levels at the peripheral effector cells caused by the reduced expression of NET in [T] carriers (Kim, 2006) and consequently impaired reuptake capacity. Another explanation could be that differences in the central NET expression lead to reduced central counter mechanisms for the blood pressure rise in [T] carriers (Okamoto et al., 2012; Sigurdardottir et al., 2016). The information about this genetic difference contributes to future individual tailoring of patient medication. Furthermore, the finding highlights the importance of the noradrenergic pathway for the adverse effects and the role of the NET in influencing their degree.
Finally, clinicians should take into consideration that women and men respond to ketamine differently. We found a faster increase in diastolic blood pressure, accompanied by almost double the increase in elevation in women compared with men. A study of ketamine discontinuation symptoms found when investigating a large sample greater anxiety, drowsiness, and tremor reported by women compared with men (Chen et al., 2014). Women are more sensitive to cocaine and methylphenidate (Dafny and Yang, wi 2006), both drugs that affect the autonomous nervous system (Rothman et al., 2001; Hodgkins et al., 2012). However, there is still a lack of research regarding gender differences, and differentiating the underlying mechanisms is not trivial, because drug metabolism, hormonal influences, and central nervous system disparity all represent partial aspects (Franconi et al., 2007). Based on our results, we suggest close monitoring in women, particularly those with baseline hypertension, during ketamine administration. Furthermore, particular attention should be paid if men show a faster rise in diastolic blood pressure than expected based on the provided data.
The particular clinical relevance of this study in the treatment of depression is evident when considering the fact that depression may be associated with hypertension. Studies investigating a relationship between depression and hypertension have yielded mixed results, including a positive association between these 2 conditions (Jonas et al., 1997; Rutledge and Hogan, 2002; Carroll et al., 2010; Nabi et al., 2011; Almas et al., 2014; Kuehl et al., 2016), a slight increase in blood pressure in depressed individuals (Delaney et al., 2010), no association (Yan et al., 2003), and an association between depression and low blood pressure (Hildrum et al., 2007; Scuteri, 2008; Licht et al., 2009). These studies involved diverse populations, which likely accounts for their differing findings, highlighting the need for identifying individual risk factors for a ketamine-induced increase in blood pressure when considering this treatment in depressed patients.
This study has some limitations. Firstly, ketamine was administered to young, healthy individuals. The average onset age of depression is in a similar range, but older individuals may react differently with a possibly less stable response. Secondly, they may already be receiving antihypertensive drugs (Tisdale, 2004; Lenze, 2016). Previous administration of antidepressant medication can also have an impact on the NET, potentially resulting in a different reaction to ketamine. Special attention must be given when combining ketamine with other noradrenaline reuptake inhibitors, for example venlafaxine or duloxetine, depending on their receptor affinity (Stahl et al., 2005), especially in patients with cardiovascular disease. Nevertheless, no serious cardiovascular adverse events were reported when combining antidepressants with synergistic action on the NE system (McGrath et al., 2006) (Hannan et al., 2007). Thirdly, our detailed observation period was 40 minutes during administration, followed by 20 minutes without measurement. We suggest a longer observation period in the resting position in further studies. Furthermore, the physiological data were not measured using invasive arterial blood pressure monitoring, which would give a more detailed curve, but the general risks of invasive methods were deemed unacceptable in a normal participant population, who did not require invasive monitoring on clinical grounds. Nevertheless, it would be interesting to replicate these results in a clinical setting and also in anesthetic doses.
By revealing the influence of baseline blood pressure, NET polymorphism, and gender on ketamine-induced cardiovascular side effects, our findings contribute to the estimation and predictability of the general and individual risks associated with ketamine administration. Our parameters and their predictive value should be further assessed in a prospective clinical trial including a psychiatric patient study population to create monitoring guidelines for safe administration. The increasing application of ketamine in psychiatric therapy underlines the importance of this future research.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Funding
T. Liebe was supported by a thesis scholarship from the University of Magdeburg, Medical Faculty. Professor M. Walter and Dr. B. Schott received support from the German Research Foundation (SFB 779/A06 to Professor Walter, SFB 779/A08 to Dr. Schott, and DFG Wa 2673/4-1 to Professor Walter), the Centre for Behavioural Brain Sciences (CBBS NN05 for Professor Walter), and Leibniz Association (Park für Forschung und Innovation to Professor Walter). L. Colic received a scholarship from the German Research Foundation (SFB 779 2013-2016).
Statement of Interest
Professor Walter has received research support from HEEL and Janssen Pharmaceutical Research.
Supplementary Material
Acknowledgments
We thank Jörg Stadler, Renate Blobel-Lüer, Claus Tempelmann, and Andreas Fügner for their help and technical advice during data acquisition. We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tübingen.
References
- Ackenheil M, Stotz-Ingenlath G, Dietz-Bauer R, Vossen A (1999) Mini international neuropsychiatric interview (German Version 5.0.0, DSM-IV). München: Psychiatrische Universitätsklinik. [Google Scholar]
- Aggarwal M, Khan IA (2006) Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin 24:135–146. [DOI] [PubMed] [Google Scholar]
- Almas A, Patel J, Ghori U, Ali A, Edhi AI, Khan MA (2014) Depression is linked to uncontrolled hypertension: a case-control study from Karachi, Pakistan. J Ment Health Abingdon Engl 23:292–296. [DOI] [PubMed] [Google Scholar]
- Baraka A, Harrison T, Kachachi T (1973) Catecholamine levels after ketamine anesthesia in man. Anesth Analg 52:198–200. [PubMed] [Google Scholar]
- Benarroch EE. (2008) The arterial baroreflex: functional organization and involvement in neurologic disease. Neurology 71:1733–1738. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Boyer PA, Skolnick P, Fossom LH (1998) Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. J Mol Neurosci MN 10:219–233. [DOI] [PubMed] [Google Scholar]
- Caddy C, Giaroli G, White TP, Shergill SS, Tracy DK (2014) Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy. Ther Adv Psychopharmacol 4:75–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M (2013) Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70:27–34. [DOI] [PubMed] [Google Scholar]
- Carroll D, Phillips AC, Gale CR, Batty GD (2010) Generalized anxiety and major depressive disorders, their comorbidity and hypertension in middle-aged men. Psychosom Med 72:16–19. [DOI] [PubMed] [Google Scholar]
- Carroll D, Smith GD, Shipley MJ, Steptoe A, Brunner EJ, Marmot MG (2001) Blood pressure reactions to acute psychological stress and future blood pressure status: a 10-year follow-up of men in the Whitehall II study. Psychosom Med 63:737–743. [DOI] [PubMed] [Google Scholar]
- Chen W- Y, Huang M- C, Lin S- K (2014) Gender differences in subjective discontinuation symptoms associated with ketamine use. Subst Abuse Treat Prev Policy 9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S- C, Kim B- N, Cummins TDR, Kim J- W, Bellgrove MA (2012) Norepinephrine transporter -3081(A/T) and alpha-2A-adrenergic receptor MspI polymorphisms are associated with cardiovascular side effects of OROS-methylphenidate treatment. J Psychopharmacol Oxf Engl 26:380–389. [DOI] [PubMed] [Google Scholar]
- Dafny N, Yang PB (2006) The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Res Bull 68:393–405. [DOI] [PubMed] [Google Scholar]
- Delaney JAC, Oddson BE, Kramer H, Shea S, Psaty BM, McClelland RL (2010) Baseline depressive symptoms are not associated with clinically important levels of incident hypertension during two years of follow-up: the multi-ethnic study of atherosclerosis. Hypertens Dallas Tex 1979 55:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr (2010) A randomized add-on trial of an N-methyl-d-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler MD, Wallin G, Dorward PK, Eisenhofer G, Westerman R, Meredith I, Lambert G, Cox HS, Jennings G (1991) Effects of desipramine on sympathetic nerve firing and norepinephrine spillover to plasma in humans. Am J Physiol 260:R817–823. [DOI] [PubMed] [Google Scholar]
- Fond G, Loundou A, Rabu C, Macgregor A, Lançon C, Brittner M, Micoulaud-Franchi JA, Richieri R, Courtet P, Abbar M, Roger M, Leboyer M, Boyer L (2014) Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl) 231:3663–3676. [DOI] [PubMed] [Google Scholar]
- Franconi F, Brunelleschi S, Steardo L, Cuomo V (2007) Gender differences in drug responses. Pharmacol Res 55:81–95. [DOI] [PubMed] [Google Scholar]
- Hannan N, Hamzah Z, Akinpeloye HO, Meagher D (2007) Venlafaxine-mirtazapine combination in the treatment of persistent depressive illness. J Psychopharmacol Oxf Engl 21:161–164. [DOI] [PubMed] [Google Scholar]
- Hara K, Yanagihara N, Minami K, Ueno S, Toyohira Y, Sata T, Kawamura M, Brüss M, Bönisch H, Shigematsu A, Izumi F (1998) Ketamine interacts with the noradrenaline transporter at a site partly overlapping the desipramine binding site. Naunyn Schmiedebergs Arch Pharmacol 358:328–333. [DOI] [PubMed] [Google Scholar]
- Hara K, Minami K, Ueno S, Toyohira Y, Tsutsui M, Shigematsu A, Yanagihara N (2002) Up-regulation of noradrenaline transporter in response to prolonged exposure to ketamine. Naunyn Schmiedebergs Arch Pharmacol 365:406–412. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ (2009) Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildrum B, Mykletun A, Stordal E, Bjelland I, Dahl AA, Holmen J (2007) Association of low blood pressure with anxiety and depression: the Nord-Trøndelag Health Study. J Epidemiol Community Health 61:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkins P, Shaw M, McCarthy S, Sallee FR (2012) The pharmacology and clinical outcomes of amphetamines to treat ADHD: does composition matter? CNS Drugs 26:245–268. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA Jr (2012) Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 37:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas BS, Franks P, Ingram DD (1997) Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Fam Med 6:43–49. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Charkoudian N, Wallin BG (2010) The sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension 56:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Deo SH, Vianna LC, Balanos GM, Hartwich D, Fisher JP, Fadel PJ (2011) Sex differences in carotid baroreflex control of arterial blood pressure in humans: relative contribution of cardiac output and total vascular conductance. Am J Physiol Heart Circ Physiol 301:H2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Hahn MK, Joung Y, Anderson SL, Steele AH, Mazei-Robinson MS, Gizer I, Teicher MH, Cohen BM, Robertson D, Waldman ID, Blakely RD, Kim KS (2006) A polymorphism in the norepinephrine transporter gene alters promoter activity and is associated with attention-deficit hyperactivity disorder. Proc Natl Acad Sci U S A 103:19164–19169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C- H, Waldman ID, Blakely RD, Kim K- S (2008) Functional gene variation in the human norepinephrine transporter: association with attention deficit hyperactivity disorder. Ann N Y Acad Sci 1129:256–260. [DOI] [PubMed] [Google Scholar]
- Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM (2000) Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36:1233–1238. [DOI] [PubMed] [Google Scholar]
- Kohli U, Hahn MK, English BA, Sofowora GG, Muszkat M, Li C, Blakely RD, Stein CM, Kurnik D (2011) Genetic variation in the presynaptic norepinephrine transporter is associated with blood pressure responses to exercise in healthy humans. Pharmacogenet Genomics 21:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl LK, Muhtz C, Hinkelmann K, Dettenborn L, Wingenfeld K, Spitzer C, Otte C (2016) Association between major depression and cardiovascular risk: the role of antidepressant medication. Psychopharmacology (Berl) 233:3289–3295. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Farber NB, Kharasch E, Schweiger J, Yingling M, Olney J, Newcomer JW (2016) Ninety-six hour ketamine infusion with co-administered clonidine for treatment-resistant depression: a pilot randomised controlled trial. World J Biol Psychiatry 17:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zheng L, Zeng D, Hao Y, Wu B, Sun Y (2013) Investigation of the correlation between norepinephrine transporter gene polymorphisms and essential hypertension. Mol Med Rep 7:105–109. [DOI] [PubMed] [Google Scholar]
- Licht CMM, Geus EJC de, Seldenrijk A, Hout HPJ van, Zitman FG, Dyck R van, Penninx BW. (2009) Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertens Dallas Tex 1979 53:631–638. [DOI] [PubMed] [Google Scholar]
- Lilburn JK, Dundee JW, Moore J (1978) Ketamine infusions. Observations on technique, dosage and cardiovascular effects. Anaesthesia 33:315–321. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, Guevara S, Zarate CA (2014) Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord 159:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G, et al. (2014) 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press 23:3–16. [DOI] [PubMed] [Google Scholar]
- Marlow R, Reich DL, Neustein S, Silvay G (1991) Haemodynamic response to induction of anaesthesia with ketamine/midazolam. Can J Anaesth J Can Anesth 38:844–848. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, Murrough JW (2012) Ketamine for treatment-resistant unipolar depression. CNS Drugs 26:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P (1990) Analysis of serial measurements in medical research. BMJ 300:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW (2015) A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med 45:693–704. [DOI] [PubMed] [Google Scholar]
- McGrath PJ, Stewart JW, Fava M, Trivedi MH, Wisniewski SR, Nierenberg AA, Thase ME, Davis L, Biggs MM, Shores-Wilson K, Luther JF, Niederehe G, Warden D, Rush AJ (2006) Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry 163:1531–1541; quiz 1666. [DOI] [PubMed] [Google Scholar]
- Miletich DJ, Ivankovic AD, Albrecht RF, Zahed B, Ilahi AA (1973) The effect of ketamine on catecholamine metabolism in the isolated perfused rat heart. Anesthesiology 39:271–277. [DOI] [PubMed] [Google Scholar]
- Nabi H, Chastang JF, Lefèvre T, Dugravot A, Melchior M, Marmot MG, Shipley MJ, Kivimäki M, Singh-Manoux A (2011) Trajectories of depressive episodes and hypertension over 24 years: the Whitehall II Prospective Cohort Study. Hypertension 57:710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, Tohyama M (1998) Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology 88:768–774. [DOI] [PubMed] [Google Scholar]
- Okamoto LE, Shibao C, Gamboa A, Choi L, Diedrich A, Raj SR, Black BK, Robertson D, Biaggioni I (2012) Synergistic effect of norepinephrine transporter blockade and alpha-2 antagonism on blood pressure in autonomic failure. Hypertension 59:650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlke F, König I, Ziegler A (2004) Randomization In Treatment Arms (RITA): Ein Randomisierungs-Programm für klinische Studien. Inform Biom Epidemiol Med Biol 35:1–22. [Google Scholar]
- Pickering TG. (2007) The natural history of hypertension: prehypertension or masked hypertension? J Clin Hypertens Greenwich Conn 9:807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synap N Y N 39:32–41. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Hogan BE (2002) A quantitative review of prospective evidence linking psychological factors with hypertension development. Psychosom Med 64:758–766. [DOI] [PubMed] [Google Scholar]
- Schroeder C, Jordan J (2012) Norepinephrine transporter function and human cardiovascular disease. AJP Heart Circ Physiol 303:H1273–H1282. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Murrough JW, Iosifescu DV (2016) Ketamine for treatment-resistant depression: recent developments and clinical applications. Evid Based Ment Health 19:35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri A. (2008) Depression and cardiovascular risk: does blood pressure play a role? J Hypertens 26:1738–1739. [DOI] [PubMed] [Google Scholar]
- Sigurdardottir HL, Kranz GS, Rami-Mark C, James GM, Vanicek T, Gryglewski G, Kautzky A, Hienert M, Traub-Weidinger T, Mitterhauser M, Wadsak W, Hacker M, Rujescu D, Kasper S, Lanzenberger R (2016) Effects of norepinephrine transporter gene variants on NET binding in ADHD and healthy controls investigated by PET. Hum Brain Mapp 37:884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM, Grady MM, Moret C, Briley M (2005) SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr 10:732–747. [DOI] [PubMed] [Google Scholar]
- Tisdale JE, Huang MB, Borzak S (2004) Risk factors for hypertensive crisis: importance of out-patient blood pressure control. Fam Pract 21:420–424. [DOI] [PubMed] [Google Scholar]
- Tweed WA, Minuck M, Mymin D (1972) Circulatory responses to ketamine anesthesia. Anesthesiology 37:613–619. [DOI] [PubMed] [Google Scholar]
- Usselman CW, Gimon TI, Nielson CA, Luchyshyn TA, Coverdale NS, Van Uum SH, Shoemaker JK (2014) Menstrual cycle and sex effects on sympathetic responses to acute chemoreflex stress. Am J Physiol Heart Circ Physiol 308:H664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G (2011) The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [1H]-MRS. Psychiatry Res 191:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LL, Liu K, Matthews KA, Daviglus ML, Ferguson TF, Kiefe CI (2003) Psychosocial factors and risk of hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA 290:2138–2148. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Sun L (2008) Antidepressants modulate the in vitro inhibitory effects of propofol and ketamine on norepinephrine and serotonin transporter function. J Clin Neurosci Off J Neurosurg Soc Australas 15:1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.