Abstract
BACKGROUND
The ventral striatum (VS) and striatal network supports goal motivated behavior. Identifying how depressed patients differ in their striatal network during the processing of emotionally salient events is a step towards uncovering biomarkers for diagnosis and treatment.
METHODS
38 depressed and 30 healthy adults completed a task that examined brain activation to the anticipation and receipt of monetary rewards and losses. Data were collected using a 3T Siemens Trio scanner. Functional connectivity differences were examined with seeds in the Left or Right VS. FC estimates were regressed on specific symptoms.
RESULTS
Depressed patients displayed higher functional connectivity between the VS and midline cortical areas during loss versus reward trials. Anhedonia and depressed mood were associated to fairly similar striatal circuits but suicidality was associated to a unique VS-midline structures coupling, while depression severity was linked to higher VS to caudate and precuneus connectivity during loss versus reward trials.
CONCLUSIONS
Depression is characterized by excessive VS coupling to cognitive control and associative networks during losses versus rewards. High VS to midline cortical structures coupling may index suicidality.
Keywords: Imaging, Imaging, Depression, Adults, Functional Connectivity, Ventral Striatum, Reward Circuitry, Corticostriatal Network, Anhedonia, Suicidality, Depressed Mood
Background
Preferential processing of negative versus positive events, such as monetary losses and rewards, is a hallmark of depressive states. Theories of depression have posited that vulnerable and depressed individuals exhibit a cognitive triad comprised by rigid negative attributions regarding the self, one’s environment, and one’s future (Beck, 1972; Beck, Brown, Steer, Eidelson, & Riskind, 1987), including persistent cognitive elaboration regarding flaws, failures and negative events, i.e. rumination (Nolen-Hoeksema, 2000; Papageorgiou & Wells, 2003). These negative cognitive styles interact with stressful life events and uniquely contribute to clinical depression (Gotlib, Lewinsohn, Seeley, Rohde, & et al., 1993; Hankin, Abramson, Miller, & Haeffel, 2004), and are linked to increased depression severity (i.e., number of episodes), chronicity, and severity of individual episodes. However, some research also implicates neglect of positive information in the maintenance and onset of depression (Gotlib, 1981; Gotlib & Krasnoperova, 1998; Gotlib, Krasnoperova, Yue, & Joormann, 2004; Gotlib, et al., 1993; Papageorgiou & Wells, 2003; Papay & Hedl, 1978). Overall, these biases are notoriously difficult to change and they remain even after acute depressive symptoms elapse (Bouhuys, Geerts, & Gordijn, 1999; Dozois, 2007; Gotlib & Joormann, 2010). Critically, despite the wealth of psychological research regarding the presence of preferential processing of negative versus positive events in depression, the neurobiology and functional connection between neural areas underlying these cognitive biases is largely unknown.
The striatal network and processing of losses and rewards in depression
Aberrant reward processing and responses, commonly shown by patients with depression has drawn interest in the role of the striatal circuitry in neurobiological models of depression. The ventral striatum (VS) is dedicated to encoding and saliency of natural losses and rewards (Graybiel, 2005; O’Doherty, 2004; Zink, Pagnoni, Martin-Skurski, Chappelow, & Berns, 2004). To date, a number of fMRI studies have found reduced blood-oxygen-level dependent (BOLD) activity in the VS and other basal ganglia structures among depressed patients during processing of rewards (Delgado, 2007; Kelley, 2004; O’Doherty, Buchanan, Seymour, & Dolan, 2006) and abnormal recruitment of these substrates during the processing of positive and negative events (Canli et al., 2004; Epstein et al., 2006; Fitzgerald, Laird, Maller, & Daskalakis, 2008; Hamilton et al., 2012), highlighting its possible role in the negative affective biases associated with depression.
The striatal circuitry, including the VS, involves connections with multiple regions, and each path has some distinct involvement in behavioral responses regarding rewards and losses. The VS receives input from dopaminergic (DA) neurons in the ventral tegmental area, where it also sends DA projections to indirectly reach the PFC, amygdala and hippocampus (Volman et al., 2013). Subsequently, the medial prefrontal cortex (mPFC: BA11, 10 and 32), the anterior cingulate cortex (ACC), the amygdala and hippocampus send excitatory glutamatergic projections to the VS (Haber & Knutson, 2010). Research indicates that the VS likely encodes both rewards and losses via the activity of GABAergic neurons into the VTA and the later direct DA projections to PFC and to mid-temporal areas such as the amygdala and the hippocampus (Carlezon & Thomas, 2009; Volman, et al., 2013). VS activation is in turn modulated by excitatory projections from both mid-temporal and prefrontal cortical structures. Given that the PFC is the central brain region for associative thinking, goal setting and planning (MacLeod & Salaminiou, 2001), and that mid-temporal structures such as amygdala and hippocampus support emotion processing and memory (J. E. LeDoux, 1993; Phan, Wager, Taylor, & Liberzon, 2002), both are hypothesized to have distinct yet valuable contribution to the reward deficits and facilitated loss processing observed in depressed individuals.
Distinctions between striatal to PFC versus striatal to mid-temporal pathways are important, as they are believed to support different psychological processes. Whereas associative thinking and flexible, planned goal-oriented behavior with regards to losses and rewards are associated to PFC activation and its connections to the VS (O’Doherty, 2004), the biased affective processing (enhance emotional memory, vigilance and saliency) of negative versus positive outcomes may be associated to abnormal connectivity between the VS and mid-temporal areas, which are known to function abnormally among depressed patients (Drevets, 2000; Drevets, Price, & Furey, 2008). These two potential pathways of striatal connectivity may in turn be linked to different manifestations of biased cognition and emotion in depression.
As noted, research suggests that rumination and self-attributions for negative versus positive events is involved in the emergence and persistence of depression. While there is little information about the neurobiology of these processes, there are some suggestions that they involve the striatal to PFC network. For example, individual differences in depressive traits and rumination correlate with activity of the striatum after positive events (e.g., situations that elicit gratification), but are associated with activity in the PFC after negative ones (i.e., events that evoke regret), during a gambling task (Eryilmaz, Van De Ville, Schwartz, & Vuilleumier, 2014). Furthermore, self-attributions and rumination in depressed patients have been found to be supported by altered function in structures, such as the posterior cingulate, precuneus and extended portions of the PFC (Lemogne et al., 2009; Schiller, Minkel, Smoski, & Dichter, 2013). It is thus possible that the functional connectivity between the VS and the PFC may underlie biased self-attributions and heightened rumination for negative vs. positive events.
With regards to preferential emotional memory and vigilance for negative versus positive stimuli, a review of the literature indicates that major depressive disorder show attentional biases toward negative emotional cues (e.g. sad faces) and away from positive emotional cues such as happy faces (Leppanen, 2006). Some literature also show that depressed patients encode and remember negative stimuli more efficiently than positive ones, and have more difficulty forgetting them (Hamilton & Gotlib, 2008; Joormann, 2005; Joormann, Hertel, LeMoult, & Gotlib, 2009). This suggest possible altered connectivity between striatal areas and limbic structures within the mid-temporal lobe (i.e. hippocampus, amygdala), as the latter are involved in the encoding and remembrance of emotionally charged autobiographical events (LeDoux, 1993; Phan, Wager, Taylor, & Liberzon, 2002). For example, in a task where participants had to identify if the emotional expression (happy, sad, neutral) of a face matched that of another face shown two trials earlier, depressed adults struggled to disengage from sad faces but rapidly disengaged from happy ones based on reaction times (Levens & Gotlib, 2010). By contrast, patients without depression showed more difficulties disengaging from positive facial affect compared to neutral or negative expressions (Levens & Gotlib, 2010). In a similar vein, individuals with depression are less behaviorally driven by positive events (e.g., rewards) compared to those without depression (Levens & Gotlib, 2010). Notably, processing of faces among depressed patients is associated to hyperactivation to negative facial expressions but hypoactivity to positive facial expressions, particularly in the amygdala, insula, parahippocampal gyrus, fusiform and areas of the striatum (Stuhrmann, Suslow, & Dannlowski, 2011). It is thus possible that the functional connectivity between the VS and mid-temporal lobe areas may underlie enhanced emotional memory and vigilance for negative versus positive events.
Determining the specific circuits within the striatal network (e.g., VS – PFC vs. VS – mid-temporal structures) that underpin cognitive and affective biases in reward processing of depressed patients may be important for guiding treatment strategies. For example, if the abnormal processing of negative versus positive events is supported primarily by VS – PFC connectivity, cognitive reframing of negative events and voluntary training of associative thinking toward positively valenced events may be an effective treatment strategy to modify biases in depression. By contrast, if VS to amygdala and hippocampus connectivity underlies such biases, emotionally charged strategies (e.g., behavioral activation or savoring of positively charged events) may be a key intervention method to change such biases (Cuijpers, van Straten, & Warmerdam, 2007; Sturmey, 2009). Consequently, the first research question addressed by this study is to determine which of the proposed circuits (VS – PFC vs. VS – mid-temporal connectivity) underlie processing biases toward negative versus positive events in depressed versus healthy adults.
Given accumulated psychological research regarding biased cognitive processing for negative vs. positive events (Beck, 2008; Disner, Beevers, Haigh, & Beck, 2011; Gotlib & Joormann, 2010; Joormann, 2009), our first hypothesis was that adult depressed patients would show greater VS – PFC connectivity during the processing of losses versus rewards compared to healthy controls, reflecting greater goal-based attention, planning, and associative thinking regarding losses. We also expected that connectivity between VS to mid-temporal structures (specifically amygdala and hippocampus) would underlie the preferential processing of negative versus positive events in depressed versus healthy adult patients.
The striatal network and key depressive symptoms
Depressive syndromes are comprised by number of key symptoms used to determine the presence of the diagnosis. Prominently, a depression diagnosis involves presence of two key symptoms: anhedonia (loss of interest in pleasurable activity) or depressed mood (sadness and irritability). Additionally, suicidal ideation leading to suicide attempts is one of the most serious outcomes of severe and treatment resistant depression (K Lönnqvist, 2000; Nock et al., 2008). Notably, the presence and saliency of certain depressive symptoms have been differentially associated with clinical outcomes, including treatment resistant depression and risk of suicide attempts. For example, high anhedonia in particular is strongly linked to suicidality and poor response to treatment (Downar et al., 2014; Spijker, de Graaf, Ten Have, Nolen, & Speckens, 2010; Winer et al., 2014). Similarly, depressed mood, including feelings of guilt and unworthiness, has been linked to suicide attempts (Nrugham, Larsson, & Sund, 2008; Oquendo et al., 2004) and completion (McGirr et al., 2007). As such, additional research on the circuitry underlying key symptoms of depression is paramount in clinical considerations of diagnosis, treatment, and prognosis.
Investigations have implicated certain aspects of the striatal network in specific depressive symptoms such as suicidality, anhedonia, and depressed mood (Der-Avakian & Markou, 2012; Drevets, et al., 2008; Marchand et al., 2012; Schlaepfer et al., 2008). However, suicidality could be supported by different circuits within the striatal network than those contributing to altered emotionality including high depressive mood and anhedonia, as suggested by recent research (Marchand, et al., 2012). Recent clinical research found hypoactivity in brain circuits mediating reward, which in turn appears to be associated with proclivity for suicide (Elman, Borsook, & Volkow, 2013). Suicidality might stem from the disruption of PFC to VS connectivity, which enables executive function, associative thinking, cognitive control and goal-directed behaviors (Marchand et al., 2012). Indeed, a recent investigation found that the striatal to anterior PFC circuit is linked to depression severity, suicidal ideation, and history of self harm (Marchand, et al., 2012). Moreover, a recent review suggests that the PFC to striatum circuitry, particularly the circuits bridging frontal lobe, ACC, and striatum, is involved in the neurobiology of suicide in depressed patients uniquely rather than in other psychiatric disorders (Zhang, Chen, Jia, & Gong, 2014). These authors further contend that among those with depression and suicide behaviors, the neural mechanisms for suicide behaviors may be distinct from depressed symptoms. By contrast, depressed mood and anhedonia might be more associated with disrupted mid-temporal to VS circuitry, as these structures have been associated with altered emotions and affective experiences (LeDoux, 2003). Specifically, the hippocampus and amygdala support cognitive and affective biases in depression, as shown by recent evidence indicating depressed relative to healthy adults yield longer and more intense amygdalar activation in response to negative stimuli (Fales et al., 2008; Siegle, Steinhauer, Thase, Stenger, & Carter, 2002) which in turn is related to self-reported rumination (Siegle, et al., 2002). However, other evidence suggests that that top-down contributions (i.e., input from PFC to the striatum) may also underlie to these symptoms. For example, high subgenual ACC in depressed patients and increased PFC activity during rewarding stimuli has been linked to higher anhedonia (Harvey, Pruessner, Czechowska, & Lepage, 2007; Mies et al., 2013). Notably, during a task that required depressed patients to reduce positive emotions, lower levels of PFC activity were linked later to greater improvements in anhedonia (Light et al., 2011).
Currently, due to the paucity of symptom-specific investigations within depression, the striatal circuits that support these characteristics remain an enigma. Particularly, it is unclear whether symptoms are due to abnormal saliency of negative vs. positive stimuli (e.g. losing money vs. earning money) driven by mid-temporal limbic structures, or to negatively biased goal-directed behavior and executive function supported by inputs from the PFC (top-down contributions to the VS), or both. It should also be noted that much of the cited evidence stems from BOLD activity associations with symptoms rather than functional connectivity estimates, raising the importance of research that addresses this gap in the scientific literature. Towards this end, as our second and exploratory research aim, we propose to explore how estimates of the coupling between striatal, mid-temporal or/and PFC areas during processing of negative versus positive events might be associated with the two key self-reported symptoms of depression (anhedonia and depressed mood), as well as with suicidality and severity of depression.
Summary of goals and hypotheses
We aimed to first better identify the striatal circuitry involved in the abnormal processing of losses versus rewards in depression, and second, to determine how such striatal circuitry (or circuits involved in receipt of monetary losses versus rewards) might be associated with core depression symptoms (anhedonia, depressed mood, and suicidality). As noted, our hypotheses were: first, higher VS coupling with structures within the PFC (e.g. ACC, BA 9, 10) would distinguish depressed versus healthy adults during receipt of monetary losses versus rewards, given past psychological research involving manifestations of associative thinking such as rumination and self-attributions during negative versus positive emotional stimuli. Second we also expected VS-mid-temporal connectivity given psychological findings regarding enhanced encoding and emotional memory for negative versus positive stimuli such as faces. Our second and exploratory goal was to test whether key diagnostic symptoms (anhedonia, depressed mood, suicidality) and depression severity would be a with specific circuits of aberrant connectivity during receipt of monetary losses versus rewards; but given the absence of prior research regarding these functional correlates, no hypotheses were posited.
Methods and Materials
Participants
Recruitment took place at the Western Psychiatric Institute and Clinic, Pittsburgh, and through advertisements. Subjects were evaluated with the Structured Clinical Interview for DSM-IV-Research-Version (SCID-P) (First, Spitzer, Gibbon, & Williams, 1995) and scanned in an MRI. Participants completed the Structured Clinical Interview for Mood Spectrum (SCI-MOODS) which yields continuous scale measures of mood symptoms such as depressed mood, anhedonia, and suicide thoughts (Rothwell & Stock, 1982), and the Hamilton Rating Scale for Depression (HAM-D) to measure severity of illness (Ho et al., 2014). Anhedonia items included endorsement of lack of interest across a number of areas of behavior such as friends, romantic relationships and previously enjoyed activities as well as loss of fun and pleasure. Depressed mood included endorsement of sadness, irritability, crying and feelings of despair. It contained also some items that overlapped with anhedonia. In the scanner, subjects completed a task paradigm (Figure 1) developed by Delgado and colleagues (2000). Participants reflected the demographics of Pittsburgh and the surrounding area (Table 1).
Figure 1.
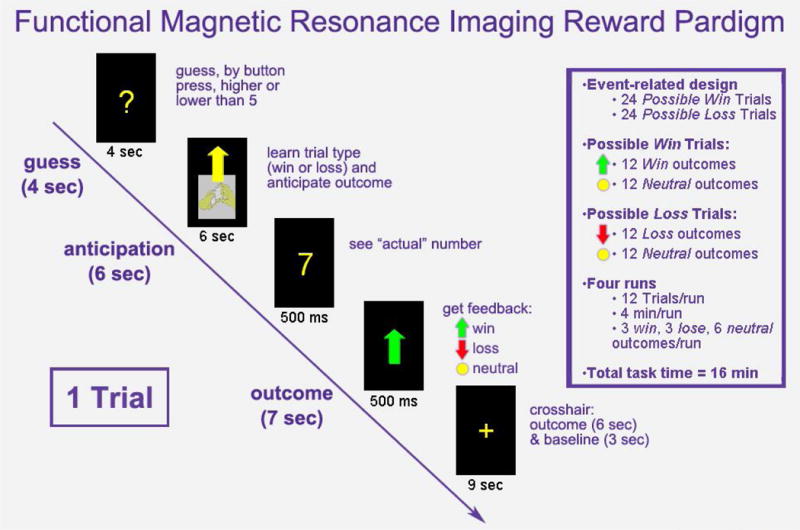
Card guessing task
Table 1.
| MDD Depressed (n=38) | Healthy Control (n=30) | Statistic | p value | |||
|---|---|---|---|---|---|---|
| Age at Scan m, SD | 30.68 | 7.73 | 31.98 | 6.11 | t(66) = 0.76 | 0.45 |
| Sex n, % | x2(1)=1.52 | 0.218 | ||||
| Female | 27 | 71.05% | 17 | 56.67% | ||
| Male | 11 | 28.95% | 13 | 43.33% | ||
| IQ m, SD | 112.04 | 9.24 | 112.82 | 7.2 | t(66) = 0.38 | 0.7 |
| Level of Education* m, SD | 6 | 1.34 | 6.43 | 1.31 | t(66) = 1.34 | 0.18 |
| HAM-D | 26.84 | 5.67 | 1.8 | 2.43 | – | – |
| Age of Illness Onset m, SD | 18.03 | 7.09 | – | – | – | – |
| Illness Duration (months) m, SD | 12.65 | 7.42 | – | – | – | – |
| Number of Psychotropics m, SD | 1.53 | 1.31 | – | – | – | – |
| Total-Medication Load m, SD | 2.18 | 2 | – | – | – | – |
| Lifetime Presence of Anxiety Disorder n, % | 24 | 63.16% | – | – | – | – |
| Lifetime Presence of Eating Disorder n, % | 2 | 5.26% | – | – | – | – |
| Lifetime Presence of Somatoform Disorder n, % | 1 | 2.63% | – | – | – | – |
| Prior History of Alcohol/Drug Abuse or Dependence n, % | 14 | 36.84% | – | – | – | – |
| Antidepressants n, % | 28 | 73.68% | – | – | – | – |
| Benzodiazepines n, % | 10 | 26.32% | – | – | – | – |
| Antipsychotics n, % | 3 | 7.89% | – | – | – | – |
| Mood Stabilizers n, % | 3 | 7.89% | – | – | – | – |
Level of Education: 1= Less than 7th grade, 6= technical school or associate degree, 8= graduate or professional degree.
All participants were right-handed and native English speaking. Exclusion criteria included a history of head injury, systemic medical illness, cognitive impairment [score <24 Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975)], premorbid IQ estimate [<85-National Adult Reading Test (Blair & Spreen, 1989)], and Axis-II borderline personality disorder. Depressed participants were symptomatic at the time of the scanning. Control participants had no previous personal or family history of psychiatric illness. Depressed participants were free from substance abuse disorders for a minimum of seven-months prior to study participation and were in remission from alcohol/substance abuse or dependence for an average of 7.9 years prior to study participation.
Data Acquisition
Neuroimaging data were collected using a 3 Tesla Siemens TIM Trio MRI scanner (Erlangen, Germany) at the University of Pittsburgh Medical Center. Structural three-dimensional axial MPRAGE images were acquired in the same session [echo time (TE) = 3.29 msec; repetition time (TR) = 2200 msec; flip angle = 9°; field of view (FOV) = 256 × 192 mm; slice thickness = 1 mm; matrix = 256 × 256; 192 continuous slices]. Mean blood oxygen level-dependent (BOLD) images were then acquired using a gradient echo EPI sequence with 39 axial slices (3.1 mm thick; TR/TE = 2000/28 msec; FOV = 205 × 205 mm; matrix 64 × 64; flip angle = 90°, 8 min).
Experiment
The fMRI paradigm (Figure 1) was an adaptation of a card guessing paradigm designed by Delgado et al. (2000) to test striatal response to feedback associated with monetary reward. Each trial consisted of an anticipation period and an outcome period followed by a win, loss, or no-change in money amount feedback. Participants were led to believe that their performance would determine the amount of money to be received after the scan. Trials were presented in pseudorandom order and had predetermined outcomes. Using a button response, participants guessed whether the value of a card (possible value of 1–9) would be greater or less than 5. During each 16 sec trial, participants had 4 sec to guess the card value (guess) followed by a visual presentation of the trial type (high likelihood of a win - indicated by an upward arrow, or loss - indicated by a downward arrow) for 6 sec (anticipation). After the anticipation, the actual card value was shown (500 msec) followed by the feedback (a green upward arrow for win, a red downward arrow for loss, or a yellow circle for neutral; 500 msec) and a crosshair presented for 9 sec allowed time to process the results (outcome; 6 sec) and provide baseline (3 sec).
Guessing incorrectly resulted in a loss of $0.50, guessing correctly lead to a win of $1.00, and neutral outcomes resulted in no change in earnings regardless of guess. During anticipation the subjects believed the visual cue displayed indicated their likelihood of winning (anticipation win; 12 trials) or losing money (anticipation loss; 12 trials) based on their guess. For example, if they guessed the card value to be less than 5 and the anticipation showed high likelihood of winning, they expected the “actual” card number to be less than 5 when revealed and to win $1.00. Four possible outcomes are classified as: “Win” (anticipated win + outcome win; 6 trials); “Loss” (anticipated loss + outcome loss; 6 trials); “Disappointment” (anticipated win + outcome neutral; 6 trials); or “Relief” (anticipated loss + outcome neutral; 6 trials). Baseline between trials (3 sec) presented no task related efforts.
Data Analysis
Data were preprocessed and analyzed with Statistical Parametric Mapping software, Version-8 (SPM8:http://www.fil.ion.ucl.ac.uk/spm). Data for each participant were realigned to the first volume in the time series to correct for head motion. Realigned images were then coregistered with the subject’s anatomical image, segmented, normalized to a standard Montreal Neurological Institute (MNI) template, and spatially smoothed with a Gaussian kernel of 8mm full-width at half-maximum (FWE). A first-level fixed-effect model was constructed for each participant producing a statistical image for 4 contrasts reflecting each possible anticipation: “Anticipation Win”, “Anticipation Loss” and outcomes condition relative to baseline: “Win”, “Loss, “Disappointment”, and “Relief”. Loss and Disappointment were considered negative outcomes and Win and Relief were considered positive outcomes.
Not Significant BOLD Activation Differences
First level activation maps were submitted to 2nd level flexible random effects GLM analyses comparing depressed and healthy controls in BOLD activity, but no significant effects of group or group by condition (2 anticipations or 4 outcomes) interactions were found in both whole brain and region of interest analyses. However, there were effects of task conditions (for both anticipation and outcomes) across both depressed and control participants.
Region-of-Interest (ROI) Seed Definitions
The seed regions for connectivity analyses were 8 mm spheres placed in the left (−9, 9, −8) or right (9, 9, −8) VS. The coordinates for the seed regions were selected based on prior studies with this task as well as meta-analyses of the VS anatomy (Di Martino et al., 2008; Mooij, Smeets, & de Wit, 2011).
Functional Connectivity (FC) Analyses
Psychophysiological interaction (PPI) was employed to explain activity changes in a target region in terms of the interaction between a psychological condition (i.e. conditions of the task) and activity in the seed area (Cisler, Bush, & Steele, 2014); for example, to explain how brain activity in a target region changes during the processing of an expected monetary loss (Loss) versus been spared a monetary loss (Relief). Specifically, we tested task-dependent contributions of the VS activity and functional coupling with brain regions that responded differentially between groups to outcomes in the task (Friston et al., 1997). The PPI physiological variable was computed by extracting the signal time course in left or right VS ROI’s (Effects of Interest, p < 0.01) for each individual participant and convolving it with the six contrasts of interest (see Table 2), resulting in PPI activation maps for each participant. The six contrasts of interests employed (Table 2) reflected the contrasts for the four outcomes (Win, Loss, Disappointment, Relief).
Table 2.
Areas and Contrasts for which Depressed versus Healthy Adults show Higher Connectivity with the VS.
| Contrast for Outcome Conditions and Areas of Higher Activity | Cluster Size (K) | p(K) | Hemispher es of areas of FC with the VS | MNI | p | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | F/t | |||||
| Main Effect of Group | ||||||||
| Precuneus, BA7 | 206 | 0 | Right | 21 | −48 | 45 | 33.06 | 0.000 |
| Middle & Superior Frontal Gyrus, ACC, BA8 | 1310 | 0 | Right | 30 | 39 | 45 | 31.38 | 0.001 |
| Caudate, Mid-Cingulate, BA32, 8, 24 | 257 | 0 | Left | −15 | 0 | 33 | 25.52 | 0.012 |
| Loss versus Relief | ||||||||
| ACC, BA32, 24 | 7781 | 0 | Right and Left | −21 | 33 | 24 | 6.4 | 0 |
| Middle & Superior Frontal Gyrus, BA9, 10 | 30 | 45 | 24 | 6.2 | 0 | |||
| Loss versus Win | ||||||||
| Caudate, Mid-Cingulate, BA24 | 974 | 0 | Right and Left | −15 | 0 | 33 | 5.1 | 0.005 |
| Putamen | 2536 | 0 | Right | 24 | 9 | 15 | 4.9 | 0.021 |
| Inferior Frontal Gyrus, BA9 | 42 | −3 | 24 | 4.9 | 0.024 | |||
| Middle & Superior Frontal Gyrus, BA10 | 30 | 45 | 24 | 4.8 | 0.031 | |||
| Middle & Inferior Frontal Gyrus, BA10 | 335 | 0.008 | Left | −30 | 36 | 12 | 4.9 | 0.053 |
| Disappointment versus Win | ||||||||
| Precuneus, BA7 | 243 | 0.034 | Right | 18 | −51 | 45 | 5.3 | 0.005 |
| Mid-Cingulate | 413 | 0.003 | Right | 18 | 9 | 33 | 5.3 | 0.006 |
| Loss versus Disappointment | ||||||||
| Mid-Insula, BA13 | 423 | 0.002 | Right | 48 | 0 | 15 | 5.2 | 0.008 |
| Anterior Insula & Inferior Frontal Gyrus, BA47 | 39 | 18 | −18 | 4.8 | 0.038 | |||
| Relief versus Win | ||||||||
| Caudate | 368 | 0.004 | Right and Left | 24 | 0 | 24 | 5.2 | 0.009 |
| Left Insula | 555 | 0 | Right and Left | −21 | −42 | 33 | 4.7 | 0.044 |
| Cingulate | −30 | −30 | 24 | 4.7 | 0.053 | |||
The first level PPI activation maps for the 6 contrasts were then submitted to a 2nd level flexible random effects GLM with between group effects, 2 Group (Depressed vs. Controls) by within group effects represented by the 6 Contrasts (“Loss vs. Win”, “Loss vs. Relief”, “Disappointment vs. Win”, “Loss vs. Disappointment”, “Relief vs. Win”, “Disappointment vs. Relief”).
To clarify a significant omnibus group effect, follow-up separate 2nd level flexible random effects GLM analyses were conducted to investigate diagnostic group and VS differences in FC for each contrast, e.g. 2 Group (Depressed vs. Controls) by 1 within group effect contrast “Loss vs. Win”. The resulting significant brain activity would show neural areas with high or low neural activity coupled with the VS. PPI analyses were also conducted by contrasting the anticipation phases (anticipation win versus anticipation loss; see Figure 1) but no significant differences in FC were found between diagnostic groups for the anticipation condition.
FC estimates during positive versus negative outcomes were compared between depressed and controls. The areas of differential FC with the VS for controls versus depressed were the same as listed in Table 2 but significant in the opposite direction (i.e., control more than depressed). They are not presented for parsimonious reporting. Significant clusters or/and coordinates for which peak BOLD signal was statistically significant (p<0.05) are reported. Additionally, to correct for multiple comparisons, we calculated the whole-brain, voxel-wise and cluster extent thresholds via Monte Carlo simulations using the program 3dClustSim in AFNI with estimates of the smoothness of the noise from the groups analyses of 13.8323 18.6951 17.0676 in AFNI v_16.3.08. For the 2 groups by 6 contrasts GLM, given a voxel-wise threshold of p < 0.01 FWE corrected, a cluster-extent threshold of k = 183 was used as the cut off for all significant results. This joint magnitude-extent threshold was used in tables and figures, which show significant results.
Depression Symptoms and Functional Connectivity (FC) of the VS
To determine which striatal circuits supported key symptoms of depression, FC estimates centered in coordinates of activity for the main effect of group were extracted. See Table 2 for the areas listed as significantly different between the groups across all contrasts. These extractions were 8 mm spheres of activity (p<0.05), which resulted in 6 independent variables corresponding to the 6 clusters listed in Table 2 for the main effect of group. These variables then were used as predictors in regressions. Note that these FC estimates were averaged across the 6 contrasts as this main effect of group was for averaged activity across contrasts.
First, the FC estimates for left and right VS coupled with each of the following clusters: 1. Right Middle and Superior Frontal Gyrus, 2. Left Caudate, and 3. Right Precuneus, yielded by the main effect of group (Table 2) and entered in order of cluster size, were used in a hierarchical stepwise forward regression. Specifically these 3 clusters each had 2 FC estimates, one with the left and one with the right VS for a total of 6 variables entered in the regressions. These analyses were conducted with the whole sample to predict each symptom separately: anhedonia, depressive mood and suicidality reported via the SCI-MOODS, and depression severity measured by the HAM-D. These 4 regression models took place for the whole sample of both depressed and control participants (N= 68). Age, gender, IQ, and level of education were included in regressions conducted with the whole sample. However, illness factors had not enough variance to be included for the control participants so they were not predictors in these regressions. Results are reported in Table 3. Supplementary analyses were conducted just for the depressed participants to examine illness related variables. See Table 3.2 and online supplementary analyses and results. For all regression models, indexes of collinearity were within acceptable values.
Table 3.
Neurobiological Substrates of Depression Symptoms: Neural Areas Coupled with the Ventral Striatum during Loss versus Reward Events (N=68)
| Symptom | R2 | Predictor: Neural Area of FC with the VS | Hemis phere of VS Seed | MNI of Target Area of high connectivity with the VS | Std. β | Unst-SE | t | p | Tolera nce | VIF |
|---|---|---|---|---|---|---|---|---|---|---|
| Depressive Mood | 0.249 | Caudate, Mid Cingulate | L | −15, 0, 33 | 0.305 | 12.785 | 2.455 | 0.017 | 0. 798 | 1.253 |
| Precuneus, BA7 | L | 21, 48, 45 | 0.281 | 9.08 | 2.261 | 0.027 | ||||
| Anhedonia | 0.155 | Caudate, Mid Cingulate | L | −15, 0, 33 | 0.393 | 0.507 | 4.37 | 0.001 | 1 | 1 |
| Suicidality | 0.305 | Middle & Superior Frontal Gyrus, ACC, BA8 | L | 30, 39, 45 | 0.384 | 1.332 | 3.205 | 0.002 | 0.792 | 1.262 |
| Precuneus, BA7 | R | 21, 48, 45 | 0.58 | 2.169 | 2.155 | 0.035 | ||||
| Depression Severity | 0.288 | Precuneus, BA7 | L | 21, 48, 45 | 0.315 | 12.017 | 2.643 | 0.010 | 0.786 | 1.273 |
| Caudate, Mid Cingulate | L | −15, 0, 33 | 0.312 | 16.203 | 2.670 | 0.010 |
Results
Corticostriatal Functional Connectivity in Depressed versus Healthy Adults
Table 2 and Figure 2 shows a main effect of group yielded by the 2 (group) by 6 (contrasts) GLM, which showed that compared to controls, depressed patients had large neural areas and significant peaks of BOLD signal coupled to the left and right VS during all negative versus all positive outcomes. These areas of increased coupling with the VS were primarily right midline cortical structures including the precuneus, and areas within the PFC (i.e., ACC and BA10). Increased VS to left caudate and mid-cingulate coupling for negative versus positive outcomes were also present in depressed relative to control participants.
Figure 2.
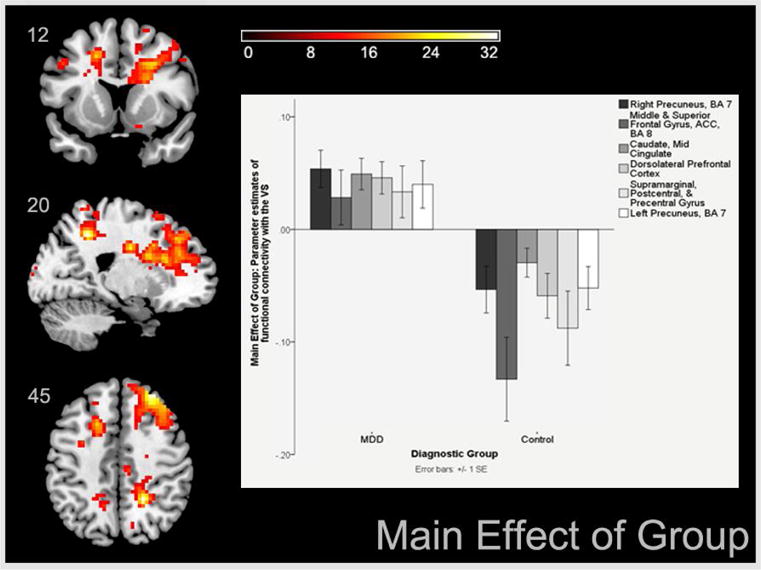
Areas of increased functional connectivity with the ventral striatum in depressed versus control participants across all negative versus positive outcomes
When follow up analyses examined group differences for each of the 6 contrasts, they showed that the group effects observed in midline cortical structures were often bilateral and mostly due to the Loss versus Relief, Loss versus Win and Disappointment versus Win contrasts (Table 2, Figure 3, Panels A, B and C). During these contrasts, depressed patients showed high bilateral VS coupling with the anterior and mid cingulate cortices (BA24 and 32) which extended into PFC areas including superior and mid frontal gyri, BA9 and 10 (Figure 3, Panels A, B and C). Additionally, within positive outcomes such as relief or win (Figure 3, Panel E), depressed patients showed VS coupling with the bilateral caudate and left insula to Relief versus Win. This activity extended to a significant cluster that included the mid-cingulate, though the peaks of functional connectivity were at the caudate and insula. This suggests that the effects of group observed for the caudate, were due to higher connectivity between the VS and the caudate for depressed participants during Relief versus Win outcomes. Within negative outcomes such as disappointment or loss (Figure 3, Panel D) depressed showed greater VS coupling with high right insula activity and with the dorsolateral prefrontal cortex (BA47) during Loss versus Disappointment. While the main group effects in the dorsolateral prefrontal cortex (DLPFC) were not statistically significant, it is suggestive of greater connectivity between this area and the VS during negative outcomes. Note also, that regions of the DLPFC are also more active for the Loss versus Win contrast (Figure 3, Panel B), suggesting that depressed participants engage the VS to DLPFC circuitry predominantly for loss outcomes. Finally, controls showed low VS coupling with the same areas during loss versus reward outcomes (See Figure 3 bar graphs). There were no effects due to hemisphere of VS seed or seed hemisphere by group interactions and the disappointment versus relief outcomes yielded no significant clusters. Illness factors (duration, number of depressive episodes) were not linked to FC estimates, and one of the contrasts (disappointment versus relief) yielded short of significant effects and is thus not reported.
Figure 3.
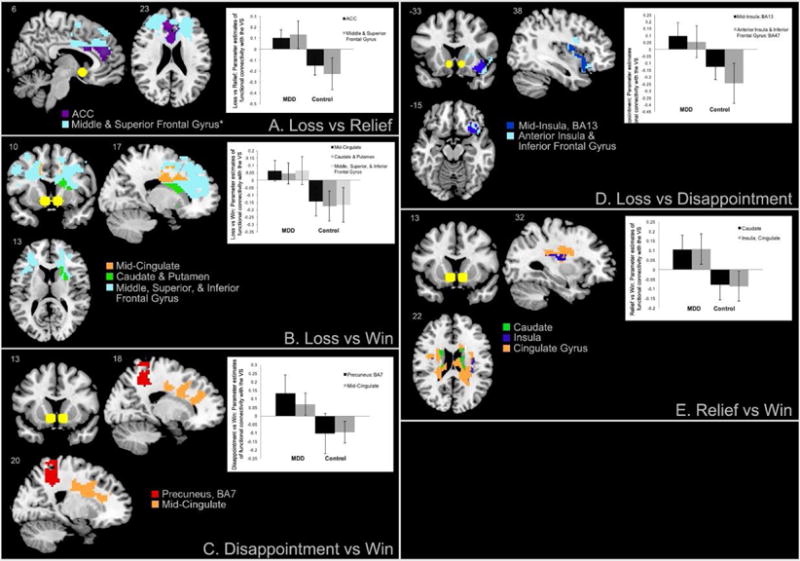
Areas of increased functional connectivity with the ventral striatum (yellow spheres) in depressed versus control participants during the receipt of distinct negative versus positive outcomes.
Corticostriatal circuits of depression symptoms
Anhedonia was associated to higher connectivity within striatal structure, specifically with left VS to left caudate as well as to mid-cingulate for loss versus rewarding outcomes (Table 3). This suggests that 15% of the variance in anhedonia may be due to connections within the striatum itself (i.e. VS- left caudate coupling) as well as with the mid-cingulate in the whole sample. However, in addition to left VS- left caudate-mid cingulate coupling, 25% of the depressed mood variance was predicted by a left VS- right precuneus coupling for losses versus rewards. Suicidality however, was predicted by a different circuitry. Specifically, 31% of the suicidality variance in the whole sample was associated to higher left VS to the right mid and superior PFC functional coupling, including the anterior cingulate cortex (ACC), as well as right VS coupling with the right precuneus. Finally, 29% of the variance in depression severity in the whole sample was associated to left VS to right precuneus and left caudate and mid-cingulate connectivity. Supplemental online figures 4.1–4.4 depict scatterplots that show associations between estimates of functional connectivity in the x axis (coupled with the VS during negative versus positive events) and symptoms in the y axis. A supplemental online table also shows that the connectivity of the left VS with the largest cluster that differed between depressed and controls (middle & superior frontal gyrus, ACC, BA8 that also predicted suicidality in the entire sample) explained 24% of the suicidality variance when examined only among depressed participants.
Effects of Medication
Table 1 shows that 73% of patients were taking antidepressants among other medications, yet their effects on the VS connectivity were modest. Comparisons of 28 medicated versus 10 unmedicated depressed participants yielded no significant effects of medication on functional connectivity, Furthermore, regression analyses showed no modifications in the significant VS functional connectivity estimates when controlling for antidepressant use.
Discussion
This study compared the striatal network of depressed patients versus healthy control adults during the processing of positive and negative monetary outcomes. It also examined whether the strength of connectivity in the striatal network was associated with self-report ratings of anhedonia, depressed mood, suicidality and severity of depression.
Our first hypothesis, which predicted higher PFC-VS coupling for losses versus earnings among depressed participants, was confirmed. Our results (Figure 2, Table 2) show significant group effects, reflecting higher cortical to VS connectivity (particularly midline cortical structures to the VS) in depressed patients compared to healthy controls. Group effects are chiefly due to higher mid-cortical to VS connectivity during processing of loss or disappointment versus relief or win. Additionally, higher VS connectivity to caudate and mid-cingulate is present in all depressed versus control adults.
The insular cortex is primarily coupled with the VS during the processing of distinct but negative valenced outcomes (i.e., loss versus disappointment), while higher caudate, mid-cingulate gyrus and insula to VS coupling characterized group differences for distinct positive outcomes (i.e., relief versus win). Finally, our second hypothesis pertaining to higher VS to mid-temporal coupling for negative versus positive events in depressed compared to healthy individuals was not confirmed and no significant clusters were found for disappointment versus relief.
Our second goal was to explore associations between the striatal network and key depression symptoms. The results suggest that they are associated to higher left VS-caudate/mid cingulate coupling during monetary losses vs. rewards, an intra-striatal network that was present for all symptoms except for suicidality. Instead, for suicidality, there may be a distinct circuitry (i.e., bilateral VS-mid/superior frontal gyrus and precuneus coupling) for losses versus earnings, observed both in the whole sample and among depressed participants only.
Midline Cortical to VS Connectivity in Depression
As noted in the introduction, the VS receives excitatory projections from both PFC and mid-temporal limbic areas such as the amygdala and the hippocampus, but it does not directly project to the PFC; instead its contribution to these areas takes place through the ventral tegmental area (VTA). Given our results, it is possible that experiences of loss alter the PFC inputs to the VS via the VTA (Friston, et al., 1997). However, it may be difficult to detect changes in VTA activity given its deep location in the brain. For now, our results suggest predominant midline cortical inputs to the VS in depressed versus healthy adults during processing of losses versus rewards, but no differences in mid-temporal inputs to the VS across groups. Further research is needed to determine the directionality of effects (e.g., output from PFC to VS versus input from VTA to PFC) that undergird key depressive symptoms.
With excitatory projections from both mid-temporal and PFC regions, the VS integrates information regarding emotional salience as well as executive/motor plans, in order to facilitate goal directed behavior. Previous literature suggests that limbic and PFC inputs to the VS compete to control behavioral outcomes (Sturup, Kristiansson, & Lindqvist, 2011). The behavioral outcomes during which the limbic areas coordinate with the VS appear to be distinct from PFC related events. Specifically, limbic-VS coupling supports learning of basic reward directed behaviors and memory of predictors of rewards (Wimmer, Daw, & Shohamy, 2012). In particular, signals of DA neurons during unexpected rewards (or stimuli that predict them) are used for learning simple behavioral response strategies, yet, such responses are relatively inflexible. By contrast, input from the PFC to the VS allows for behavioral flexibility of learned responses to adapt to changes in the value of reward cues or of internal motivational states (Sturup, et al., 2011). In other words, limbic-VS connections facilitate learning of basic emotion guided behaviors, and PFC-VS connections allow for flexible deployment and adaptation of behavior to changing circumstances.
In the context of those competing roles, our results might mean that depressed patients show preponderant PFC inputs to the VS during losses, but similar VS coupling with mid-temporal limbic structures (e.g., amygdala or hippocampus) relative to healthy controls given that VS coupling to these structures did not differ between the groups. Thus, the balance of limbic versus PFC input to the VS may be tilted toward VS-PFC coupling in depressed patients during losses to the detriment of rewards. Higher VS-PFC (BA9, 10) coupling suggests engagement of the executive control network during receipt of losses in depressed patients (Figure 3, Panels A, B, C). By contrast, healthy controls engage midline cortical structures-VS circuits more for rewards than for losses (Table 2). This might mean preferential planning and self-referential processing specifically for losses in depressed adults relative to healthy adults (Ehrensaft & Cohen, 2012; Sylvester et al., 2003). Finally, stronger VS-precuneus and to mid-cingulate coupling among depressed patients during disappointment versus win was observed. The precuneus, with direct connections to the basal ganglia (Cavanna, 2007), sustains visuo-spatial imagery, episodic memory retrieval and self-referential processing and consciousness (Dammann et al., 2011). Depressed individuals may engage in increased self-processing (Cavanna, 2007) during thwarted rewards, rather than attributing positive outcomes (win or relief) to the self. In a similar vein, depressed patients have been documented to show reduced activity in lingual gyri, cuneus, precuneus and PCC when processing positive valenced faces (Fu et al., 2007). Further, their altered functionality of midline cortical substrates has been associated with increased self-focus and negative self-attribution (Grimm et al., 2008). Thus stronger VS-precuneus coupling during losses versus earning an expected gain might represent self-attribution of negative results, which might perpetuate dysfunctional self-cognitions. Comparing functional connectivity during planning versus self-attributions of negative and positive events would clarify what specific cognitive functions are associated with striatal-PFC or striatal-limbic networks among depressed patients.
Ventral Striatum Connectivity to Limbic Structures in Depression
During negative outcomes, we noted higher VS-insula and inferior frontal gyrus connectivity (Figure 3, Panel D, loss versus disappointment). The insula supports awareness of interoceptive states and processing of salient emotional events (Menon & Uddin, 2010). Depressed individuals might experience stronger negative interoceptive sensations during losses than healthy controls. Additionally, high ACC-VS coupling to loss versus relief in depressed patients (Figure 3, Panel A) may implicate motivational processes and/or higher engagement of cognitive effort regarding negative outcomes in depressed patients (Beer, Lombardo, & Bhanji, 2010; Gilbert et al., 2006; Leshikar & Duarte, 2012; Ramnani, Elliott, Athwal, & Passingham, 2004). Dorsal ACC inputs to the VS enable planning of responses to future losses (Downar, Crawley, Mikulis, & Davis, 2002) by predicting cognitive demands that optimize future behavioral responses (Shenhav, Botvinick, & Cohen, 2013; Sheth et al., 2012). Thus planning future behavior might be exacerbated for negative events in depressed patients. However, as noted before, the ACC, insula and midline cortical structures enable a variety of functions, therefore future research would need to delve into which of those functions is affected during processing of negative versus positive events.
Regarding positive outcomes (i.e. relief and win), depressed patients showed increased VS coupling with limbic areas including the caudate, insula, and mid-cingulate gyrus (Figure 3, Panel E: relief versus win). The caudate subserves goal-directed behavior (Delgado, et al., 2000; Grahn, Parkinson, & Owen, 2008; Tricomi, Delgado, & Fiez, 2004). Given combined coupling between VS and these limbic and limbic cortex structures, it is possible that depressed patients experience relatively less positive outcomes as more salient, which in turn could either reduce behavioral preparedness (i.e. eliciting less planning) for positive outcomes or enhanced behavioral preparedness for potentially negative outcomes. Notably, VS-cortical coupling, which was strongly engaged during losses versus rewards among depressed patients, did not differ between depressed and control participants during receipt of positive outcomes, i.e., during relief or win. Thus, higher functions during receipt of good news might be comparable across healthy controls and depressed patients. However, heightened caudate and insula to VS connectivity for relief vs. win among depressed adults suggests that they may be more behaviorally prepared for relatively less positive outcomes.
Associations between abnormal corticostriatal network and depression symptoms
As Table 3 shows, suicidality was linked to stronger VS to middle and superior frontal gyrus coupling, including the ACC, and VS-precuneus coupling during losses versus rewards, with 31% of the variance in suicidality accounted by the variables in the regression model. The middle and superior frontal gyrus and ACC are dedicated to working memory, self monitoring, error detection, and executive planning (Brandi, Wohlschlager, Sorg, & Hermsdorfer, 2014; Kim, 2013). The more dorsal ACC controls behavioral responses to stimuli that demand effortful cognitive control or that bear conflicting emotional information (Gasquoine, 2013). The latter region of the ACC is the one showing higher functional connectivity with the VS in depressed patients in the present study (Figure 3, Panels A and B), suggesting that predominant cognitive effort and planning dedicated to negative versus positive outcomes might be a risk factor for suicide thoughts or behaviors. VS connectivity with the precuneus also suggests the involvement of autobiographical memory and self-referential processing in suicide thoughts.
Our results also show that high left VS-caudate and mid-cingulate regions coupling during negative versus positive outcomes predicted higher anhedonia and depressive mood symptoms (which also involved VS-precuneus coupling) across all participants. These findings again suggest that that behavioral planning and goal-oriented cognitions for negative outcomes may underlie these two key symptoms of depression. As noted, the caudate subserves goal-directed behavior (Grahn, et al., 2008), thus depressive mood and anhedonia might be associated to greater behavioral preparedness for negative versus positive outcomes. Finally, severity of depression in the whole sample was associated with greater VS coupling to caudate, mid cingulate and precuneus, similar to depressive mood. It is notable that with the exception of suicidality, the left VS seed was the predominant source of connectivity associated to symptoms of depression (Table 3). This might indicate that predominant PFC and striatal inputs to the left VS are associated to more severe depression symptoms.
Conclusions
The striatal circuitry, which evolved to process natural rewards (Carlezon & Thomas, 2009; Volman, et al., 2013) shows abnormally heightened midline cortical input to the VS (with notable contributions of the cingulate, the PFC and insula) during processing of losses in depressed patients. Our results replicate reviews regarding the typical VS functional connectivity (Cardinal, Parkinson, Hall, & Everitt, 2002; Postuma & Dagher, 2006), while also showing biased stimuli representations that mediate choice behavior in depressed patients. Heightened connectivity between the VS and midline cortical areas in depressed patients, suggests biased executive function, monitoring, self-referential and attention processes toward losses and away from positive stimuli.
Depressed patients showed no difference in VS coupling with excitatory mid-temporal structures (amygdala, hippocampus) or with the VTA, compared to controls. Instead the striatal circuitry of depressed patients appears to be shifted toward higher cortical versus limbic contributions to the VS, indicating that depression, in this sample, is notable for exaggerated attention, greater allocation of cognitive resources, and/or increased self-attributions for losses over rewards. Finally, while processing relief and win the striatal network of depressed patients is biased toward processing of relatively least positive among available outcomes, i.e. more for relief than for winning. In summary, during depressive episodes, the striatal circuitry might be exquisitely attuned toward preferential processing of punishments and losses even in the context of positive events.
Cardinal symptoms of depression (anhedonia, depressed mood and suicide thoughts) are partially explained by heightened VS coupling to the caudate, mid-cingulate, ACC and precuneus during negative versus positive events (Table 3). Treatments aimed at changing negatively biased corticostriatal circuitry may effectively alleviate several core symptoms of depression, given their shared neurobiology. Findings also suggest that diminished cortical input to the VS during negative stimuli may represent a prognostic marker for less suicidality.
Limitations
The participants in this study have had depressive episodes for several years, thus it is possible that biases in VS to cortical areas coupling represent the effects of chronicity, and not a signature of depression or of the symptoms investigated. However, length of illness was not a significant covariate or predictor of functional connectivity. Additionally, a number of participants had a prior history of substance use, which could also confound the results and increase the difficulty in discerning the specific effects of depression. The self-report questionnaire employed to survey the scales symptoms of depression is a relatively new tool, it would be desirable to replicate the links between symptomatology and corticostriatal circuitry with better established questionnaires or structured interviews. The task employed had 6 trials per outcome condition, lasting 20 seconds each, although this task has been extensively used to detect BOLD activity differences; this is the first time that is used to estimate functional connectivity with PPI. While, there is at least one paper that set 5 trials or less per condition as the limit to exclude participants from PPI analyses (Hutchinson, Uncapher, & Wagner, 2015) it will be important to replicate our findings with this task and in other populations to ensure that the length of the trials did not diminish the power to detect functional connectivity estimates. Finally, the analyses that explored how striatal circuitry supported self-reported symptoms (suicidality, anhedonia, depressive mood and severity of depression) were conducted without controlling for each of the other symptoms because symptom scales were derived from the same instrument and were highly correlated. In particular anhedonia and depressed mood were highly correlated and had some items that comprised the scales that were common to both. Thus it is possible that there was not enough divergent validity for these scales to differentiate these symptoms dimensions. As such, these results should be taken with caution and viewed as exploratory in nature. Future studies ought to conduct these analyses while controlling for symptoms derived from different instruments to increase the likelihood that circuits are specific and unique to symptoms such as suicidality, depressed mood or anhedonia. This would also confirm or disprove whether suicidality, anhedonia and depressive mood are in fact sustained by similar circuitry.
Supplementary Material
Highlights.
We examined the striatal network, which encodes natural rewards, in depressed and healthy adults.
Depressed adults show high striatal connectivity with neocortical areas during losses versus rewards.
Suicidality, anhedonia, and depressive mood are associated with higher VS connectivity to the Prefrontal Cortex.
Acknowledgments
This research was supported by a NIMH, K01MH092601 to the first author. We are very grateful to Drs. Tom Zeffiro and Kathleen Thomas: key mentors and supporters of the first author’s K award application and progress.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: None of the authors report biomedical financial interests or potential conflicts of interest.
References
- Beck AT. Depression; causes and treatment. Philadelphia: University of Pennsylvania Press; 1972. [Google Scholar]
- Beck AT. The Evolution of the Cognitive Model of Depression and Its Neurobiological Correlates. American Journal of Psychiatry. 2008;165(8):969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beck AT, Brown G, Steer RA, Eidelson JI, Riskind JH. Differentiating anxiety and depression: a test of the cognitive content-specificity hypothesis. J Abnorm Psychol. 1987;96(3):179–183. doi: 10.1037//0021-843x.96.3.179. [DOI] [PubMed] [Google Scholar]
- Beer JS, Lombardo MV, Bhanji JP. Roles of medial prefrontal cortex and orbitofrontal cortex in self-evaluation. J Cogn Neurosci. 2010;22(9):2108–2119. doi: 10.1162/jocn.2009.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid IQ: A revision of the national adult reading test. Clinical Neuropsychology. 1989;3:129–136. [Google Scholar]
- Bouhuys AL, Geerts E, Gordijn MC. Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. J Nerv Ment Dis. 1999;187(10):595–602. doi: 10.1097/00005053-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Brandi ML, Wohlschlager A, Sorg C, Hermsdorfer J. The neural correlates of planning and executing actual tool use. J Neurosci. 2014;34(39):13183–13194. doi: 10.1523/JNEUROSCI.0597-14.2014. doi: 10.1523/JNEUROSCI.0597-14.201434/39/13183 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Sivers H, Thomason ME, Whitfield-Gabrieli S, Gabrieli JD, Gotlib IH. Brain activation to emotional words in depressed vs healthy subjects. Neuroreport. 2004;15(17):2585–2588. doi: 10.1097/00001756-200412030-00005. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. doi: S0149763402000076 [pii] [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. doi: 10.1016/j.neuropharm.2008.06.075S0028-3908(08)00268-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE. The precuneus and consciousness. CNS Spectr. 2007;12(7):545–552. doi: 10.1017/s1092852900021295. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Bush K, Steele JS. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage. 2014;84:1042–1052. doi: 10.1016/j.neuroimage.2013.09.018. doi: 10.1016/j.neuroimage.2013.09.018S1053-8119(13)00954-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin Psychol Rev. 2007;27(3):318–326. doi: 10.1016/j.cpr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Dammann G, Hugli C, Selinger J, Gremaud-Heitz D, Sollberger D, Wiesbeck GA, Walter M. The self-image in borderline personality disorder: an in-depth qualitative research study. J Pers Disord. 2011;25(4):517–527. doi: 10.1521/pedi.2011.25.4.517. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84(6):3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Milham MP. Functional Connectivity of Human Striatum: A Resting State fMRI Study. Cerebral Cortex. 2008;18(12):2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol. 2002;87(1):615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, Giacobbe P. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry. 2014;76(3):176–185. doi: 10.1016/j.biopsych.2013.10.026. doi: 10.1016/j.biopsych.2013.10.026S0006-3223(13)01034-2 [pii] [DOI] [PubMed] [Google Scholar]
- Dozois DJ. Stability of negative self-structures: a longitudinal comparison of depressed, remitted, and nonpsychiatric controls. J Clin Psychol. 2007;63(4):319–338. doi: 10.1002/jclp.20349. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrensaft MK, Cohen P. Contribution of family violence to the intergenerational transmission of externalizing behavior. Prev Sci. 2012;13(4):370–383. doi: 10.1007/s11121-011-0223-8. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, Volkow ND. Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol. 2013;109:1–27. doi: 10.1016/j.pneurobio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163(10):1784–1790. doi: 10.1176/ajp.2006.163.10.1784. doi: 163/10/1784 [pii]10.1176/appi.ajp.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Eryilmaz H, Van De Ville D, Schwartz S, Vuilleumier P. Lasting impact of regret and gratification on resting brain activity and its relation to depressive traits. J Neurosci. 2014;34(23):7825–7835. doi: 10.1523/JNEUROSCI.0065-14.2014. doi: 10.1523/JNEUROSCI.0065-14.2014 34/23/7825 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, Sheline YI. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63(4):377–384. doi: 10.1016/j.biopsych.2007.06.012. doi: S0006-3223(07)00565-3 [pii]10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29(6):683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. doi: S1053-8119(97)90291-3 [pii]10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Brammer MJ, Suckling J, Kim J, Cleare AJ, Bullmore ET. Neural responses to happy facial expressions in major depression following antidepressant treatment. Am J Psychiatry. 2007;164(4):599–607. doi: 10.1176/ajp.2007.164.4.599. [DOI] [PubMed] [Google Scholar]
- Gasquoine PG. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci Biobehav Rev. 2013;37(3):340–348. doi: 10.1016/j.neubiorev.2013.01.002. doi: 10.1016/j.neubiorev.2013.01.002S0149-7634(13)00003-1 [pii] [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Gotlib IH. Self-reinforcement and recall: differential deficits in depressed and nondepressed psychiatric inpatients. J Abnorm Psychol. 1981;90(6):521–530. doi: 10.1037//0021-843x.90.6.521. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E. Biased information processing as a vulnerability factor for depression. Behavior Therapy. 1998;29(4):603–617. Fal 1998. [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113(1):121–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Lewinsohn PM, Seeley JR, Rohde P, et al. Negative cognitions and attributional style in depressed adolescents: An examination of stability and specificity. J Abnorm Psychol. 1993;102(4):607–615. doi: 10.1037//0021-843x.102.4.607. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86(3):141–155. doi: 10.1016/j.pneurobio.2008.09.004. doi: 10.1016/j.pneurobio.2008.09.004S0301-0082(08)00101-9 [pii] [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15(6):638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Hell D, Boeker H, Northoff G. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. doi: 10.1038/npp.2009.129npp2009129 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. American Journal of Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry. 2008;63(12):1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Miller N, Haeffel GJ. Cognitive vulnerability-stress theories of depression: Examining affective specificity in the prediction of depression versus anxiety in three prospective studies. Cognitive Therapy and Research. 2004;28(3):309–345. [Google Scholar]
- Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry. 2007;12703(8):767–775. doi: 10.1038/sj.mp.4002021. doi: 4002021 [pii]10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- Ho TC, Yang G, Wu J, Cassey P, Brown SD, Hoang N, Yang TT. Functional connectivity of negative emotional processing in adolescent depression. J Affect Disord. 2014;155:65–74. doi: 10.1016/j.jad.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Wagner AD. Increased functional connectivity between dorsal posterior parietal and ventral occipitotemporal cortex during uncertain memory decisions. Neurobiol Learn Mem. 2015;117:71–83. doi: 10.1016/j.nlm.2014.04.015. doi: 10.1016/j.nlm.2014.04.015 S1074-7427(14)00083-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J. Inhibition, rumination, and mood regulation in depression. Cognitive limitations in aging and psychopathology: Attention, working memory, and executive functions. 2005:275–312. [Google Scholar]
- Joormann J. Cognitive aspects of depression. Handbook of depression. 2009;2:298–321. [Google Scholar]
- Joormann J, Hertel PT, LeMoult J, Gotlib IH. Training forgetting of negative material in depression. J Abnorm Psychol. 2009;118(1):34–43. doi: 10.1037/a0013794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- K Lönnqvist J. Psychiatric aspects of suicidal behaviour: depression. The international handbook of suicide and attempted suicide. 2000:107–120. [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27(8):765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kim SI. Neuroscientific model of motivational process. Front Psychol. 2013;4:98. doi: 10.3389/fpsyg.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. Emotion circuits in the brain. 2003 doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory systems in the brain. Behav Brain Res. 1993;58(1–2):69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- Lemogne C, le Bastard G, Mayberg H, Volle E, Bergouignan L, Lehericy S, Fossati P. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Soc Cogn Affect Neurosci. 2009 doi: 10.1093/scan/nsp008. doi: nsp008 [pii]10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006;19(1):34–39. doi: 10.1097/01.yco.0000191500.46411.00. doi: 10.1097/01.yco.0000191500.46411.0000001504-200601000-00007 [pii] [DOI] [PubMed] [Google Scholar]
- Leshikar ED, Duarte A. Medial prefrontal cortex supports source memory accuracy for self-referenced items. Soc Neurosci. 2012;7(2):126–145. doi: 10.1080/17470919.2011.585242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens SM, Gotlib IH. Updating positive and negative stimuli in working memory in depression. J Exp Psychol Gen. 2010;139(4):654–664. doi: 10.1037/a0020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light SN, Heller AS, Johnstone T, Kolden GG, Peterson MJ, Kalin NH, Davidson RJ. Reduced right ventrolateral prefrontal cortex activity while inhibiting positive affect is associated with improvement in hedonic capacity after 8 weeks of antidepressant treatment in major depressive disorder. Biol Psychiatry. 2011;70(10):962–968. doi: 10.1016/j.biopsych.2011.06.031. doi: 10.1016/j.biopsych.2011.06.031S0006-3223(11)00738-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod AK, Salaminiou E. Reduced positive future-thinking in depression: Cognitive and affective factors. Cognition & Emotion. 2001;15(1):99–107. [Google Scholar]
- Marchand WR, Lee JN, Johnson S, Thatcher J, Gale P, Wood N, Jeong EK. Striatal and cortical midline circuits in major depression: implications for suicide and symptom expression. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36(2):290–299. doi: 10.1016/j.pnpbp.2011.10.016. doi: 10.1016/j.pnpbp.2011.10.016S0278-5846(11)00313-7 [pii] [DOI] [PubMed] [Google Scholar]
- McGirr A, Renaud J, Seguin M, Alda M, Benkelfat C, Lesage A, Turecki G. An examination of DSM-IV depressive symptoms and risk for suicide completion in major depressive disorder: a psychological autopsy study. J Affect Disord. 2007;97(1–3):203–209. doi: 10.1016/j.jad.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mies GW, Van den Berg I, Franken IH, Smits M, Van der Molen MW, Van der Veen FM. Neurophysiological correlates of anhedonia in feedback processing. Front Hum Neurosci. 2013;7:96. doi: 10.3389/fnhum.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooij T, Smeets E, de Wit W. Multi-level aspects of social cohesion of secondary schools and pupils’ feelings of safety. Br J Educ Psychol. 2011;81(Pt 3):369–390. doi: 10.1348/000709910X526614. [DOI] [PubMed] [Google Scholar]
- Nock MK, Borges G, Bromet EJ, Alonso J, Angermeyer M, Beautrais A, Williams D. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. Br J Psychiatry. 2008;192(2):98–105. doi: 10.1192/bjp.bp.107.040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 2000;109(3):504–511. [PubMed] [Google Scholar]
- Nrugham L, Larsson B, Sund AM. Predictors of suicidal acts across adolescence: influences of familial, peer and individual factors. J Affect Disord. 2008;109(1–2):35–45. doi: 10.1016/j.jad.2007.11.001. doi: S0165-0327(07)00389-8 [pii]10.1016/j.jad.2007.11.001. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49(1):157–166. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, Mann JJ. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. American Journal of Psychiatry. 2004;161(8):1433–1441. doi: 10.1176/appi.ajp.161.8.1433. [DOI] [PubMed] [Google Scholar]
- Papageorgiou C, Wells A. An empirical test of a clinical metacognitive model of rumination and depression. Cognitive Therapy and Research. 2003;27(3):261–273. [Google Scholar]
- Papay JP, Hedl JJ., Jr Psychometric characteristics and norms for disadvantaged third and fourth grade children on the state-trait anxiety inventory for children. J Abnorm Child Psychol. 1978;6(1):115–120. doi: 10.1007/BF00915787. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal Ganglia Functional Connectivity Based on a Meta-Analysis of 126 Positron Emission Tomography and Functional Magnetic Resonance Imaging Publications. Cerebral Cortex. 2006;16(10):1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Elliott R, Athwal BS, Passingham RE. Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage. 2004;23(3):777–786. doi: 10.1016/j.neuroimage.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. Effects of early overnutrition and undernutrition in rats on the metabolic responses to overnutrition in later life. J Nutr. 1982;112(3):426–435. doi: 10.1093/jn/112.3.426. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Minkel J, Smoski MJ, Dichter GS. Remitted major depression is characterized by reduced prefrontal cortex reactivity to reward loss. J Affect Disord. 2013;151(2):756–762. doi: 10.1016/j.jad.2013.06.016. doi: 10.1016/j.jad.2013.06.016S0165-0327(13)00496-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2):368–377. doi: 10.1038/sj.npp.1301408. doi: 1301408 [pii]10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. doi: 10.1016/j.neuron.2013.07.007S0896-6273(13)00607-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Eskandar EN. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488(7410):218–221. doi: 10.1038/nature11239. doi: 10.1038/nature11239nature11239 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. doi: S0006322302013148 [pii] [DOI] [PubMed] [Google Scholar]
- Spijker J, de Graaf R, Ten Have M, Nolen WA, Speckens A. Predictors of suicidality in depressive spectrum disorders in the general population: results of the Netherlands Mental Health Survey and Incidence Study. Soc Psychiatry Psychiatr Epidemiol. 2010;45(5):513–521. doi: 10.1007/s00127-009-0093-6. [DOI] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1(1):10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturmey P. Behavioral activation is an evidence-based treatment for depression. Behav Modif. 2009;33(6):818–829. doi: 10.1177/0145445509350094. [DOI] [PubMed] [Google Scholar]
- Sturup J, Kristiansson M, Lindqvist P. Violent behaviour by general psychiatric patients in Sweden - validation of Classification of Violence Risk (COVR) software. Psychiatry Res. 2011;188(1):161–165. doi: 10.1016/j.psychres.2010.12.021. doi: 10.1016/j.psychres.2010.12.021S0165-1781(10)00789-4 [pii] [DOI] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41(3):357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41(2):281–292. doi: 10.1016/s0896-6273(03)00848-1. doi: S0896627303008481 [pii] [DOI] [PubMed] [Google Scholar]
- Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, Lobo MK. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J Neurosci. 2013;33(45):17569–17576. doi: 10.1523/JNEUROSCI.3250-13.2013. doi: 10.1523/JNEUROSCI.3250-13.201333/45/17569 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Daw ND, Shohamy D. Generalization of value in reinforcement learning by humans. Eur J Neurosci. 2012;35(7):1092–1104. doi: 10.1111/j.1460-9568.2012.08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer ES, Nadorff MR, Ellis TE, Allen JG, Herrera S, Salem T. Anhedonia predicts suicidal ideation in a large psychiatric inpatient sample. Psychiatry Res. 2014;218(1–2):124–128. doi: 10.1016/j.psychres.2014.04.016. doi: 10.1016/j.psychres.2014.04.016S0165-1781(14)00294-7 [pii] [DOI] [PubMed] [Google Scholar]
- Zhang H, Chen Z, Jia Z, Gong Q. Dysfunction of neural circuitry in depressive patients with suicidal behaviors: a review of structural and functional neuroimaging studies. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:61–66. doi: 10.1016/j.pnpbp.2014.03.002. doi: 10.1016/j.pnpbp.2014.03.002S0278-5846(14)00046-3 [pii] [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42(3):509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


